Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
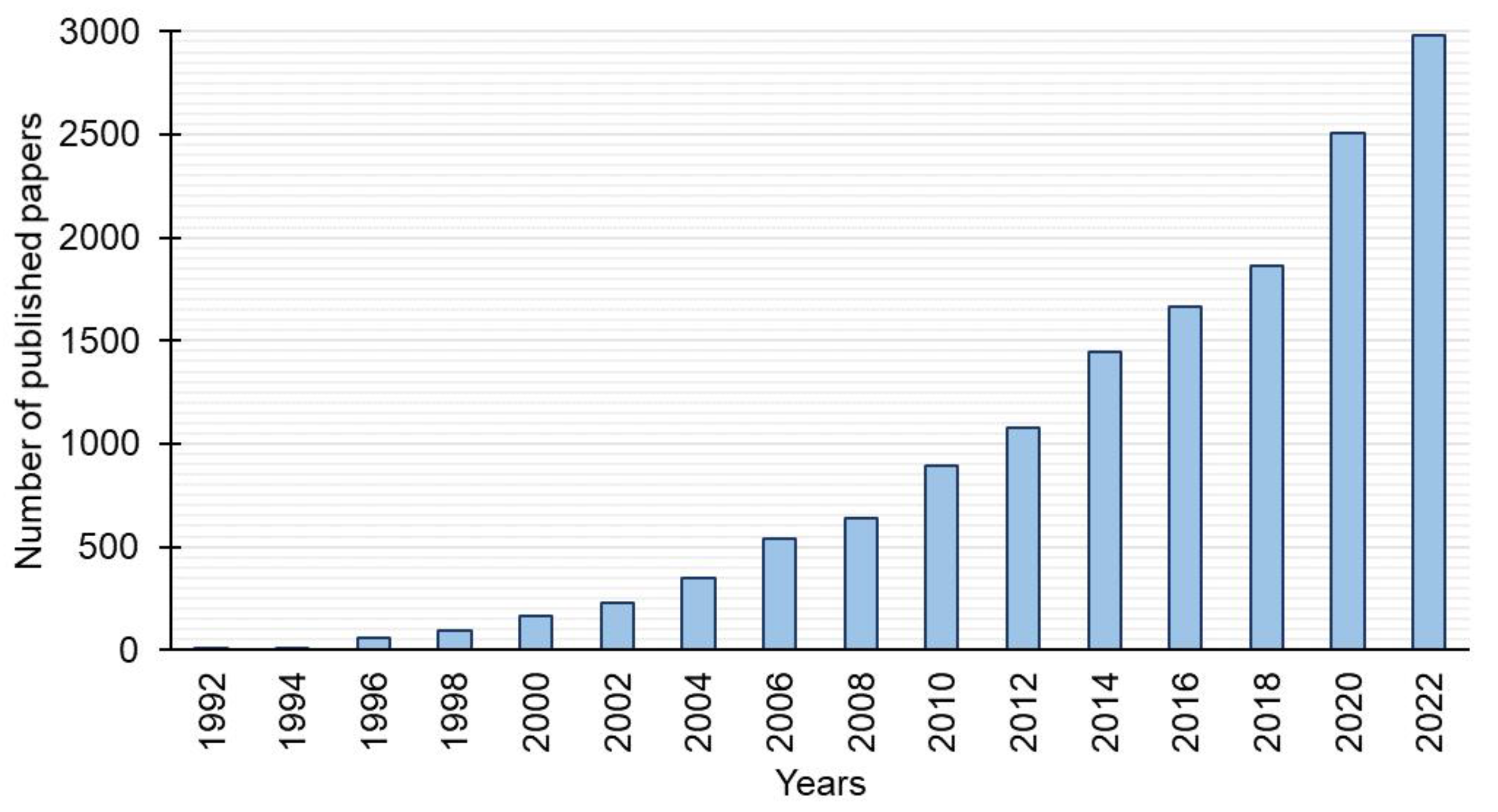
1. Introduction
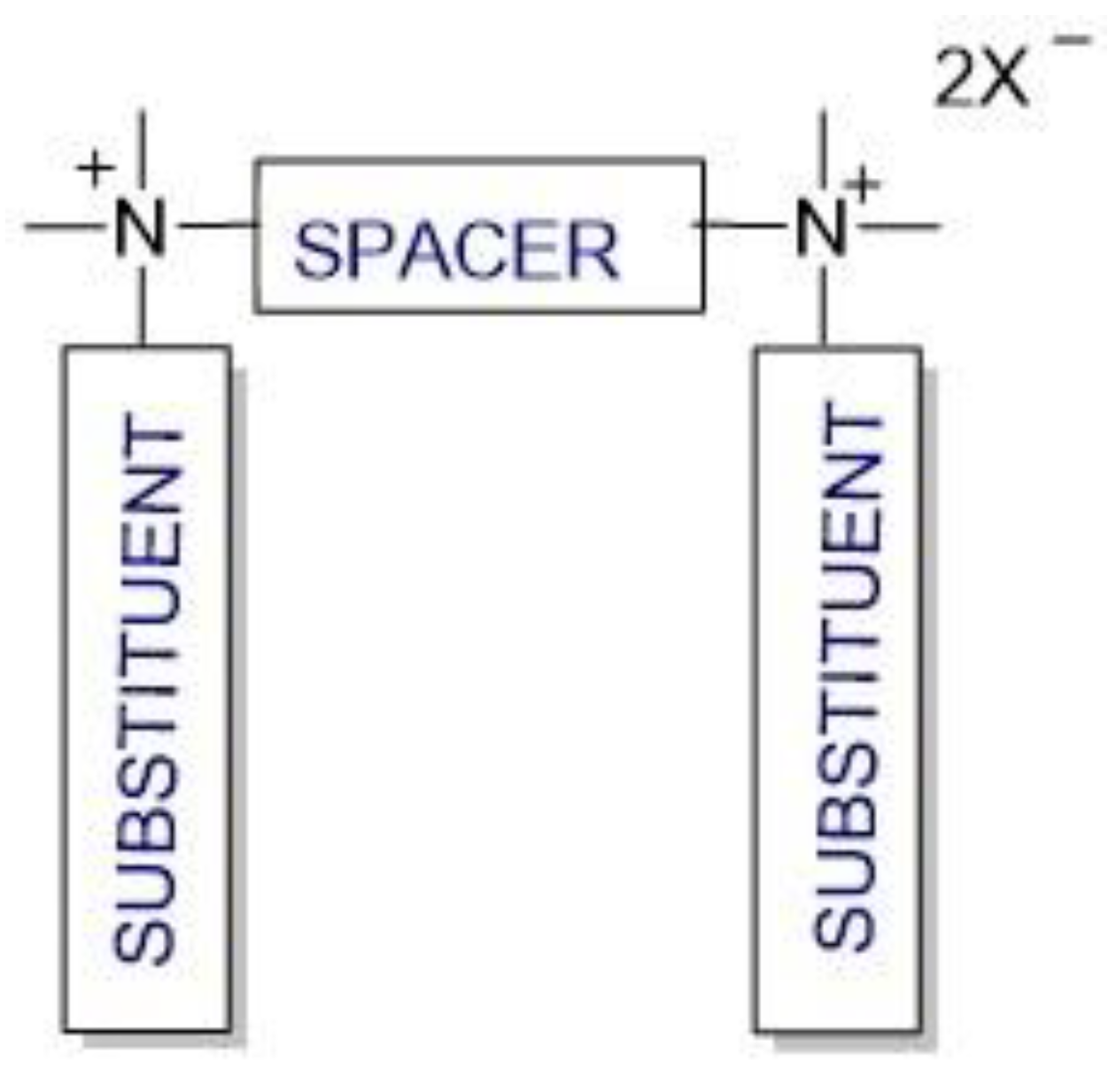
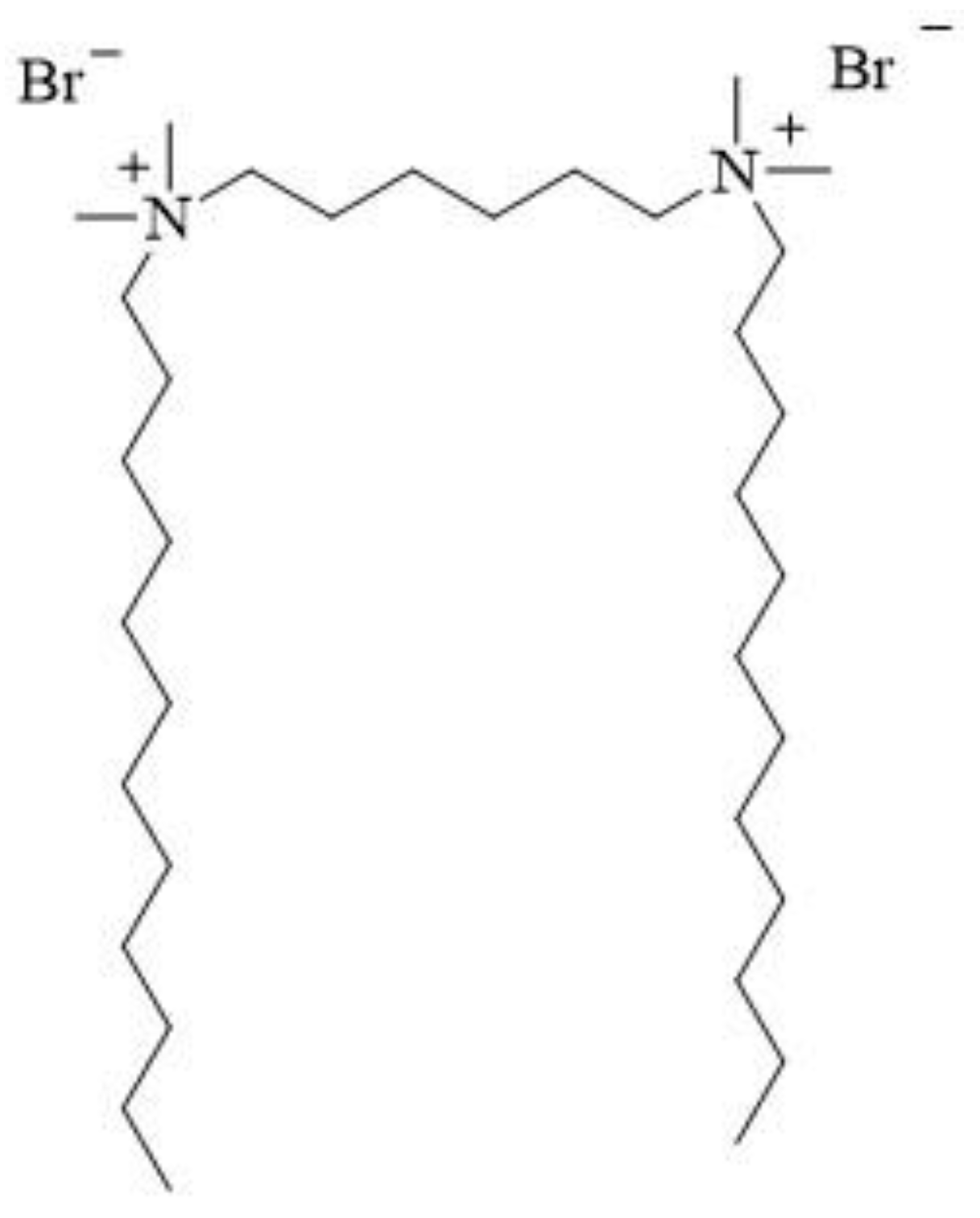
2. Structure and synthesis
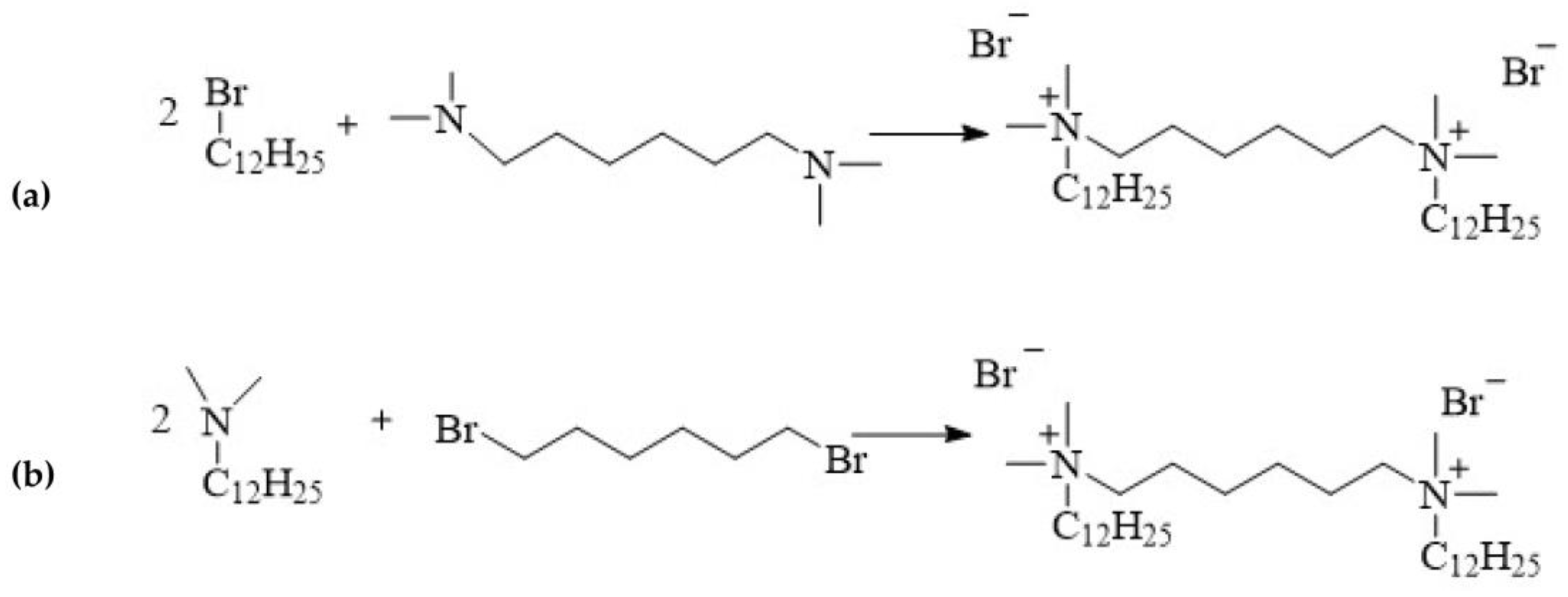
- alkylation of hexamethylene-bis(N,N-dimethylamine) with 1-dodecylbromide (Figure 4a)
3. Analysis
4. Properties
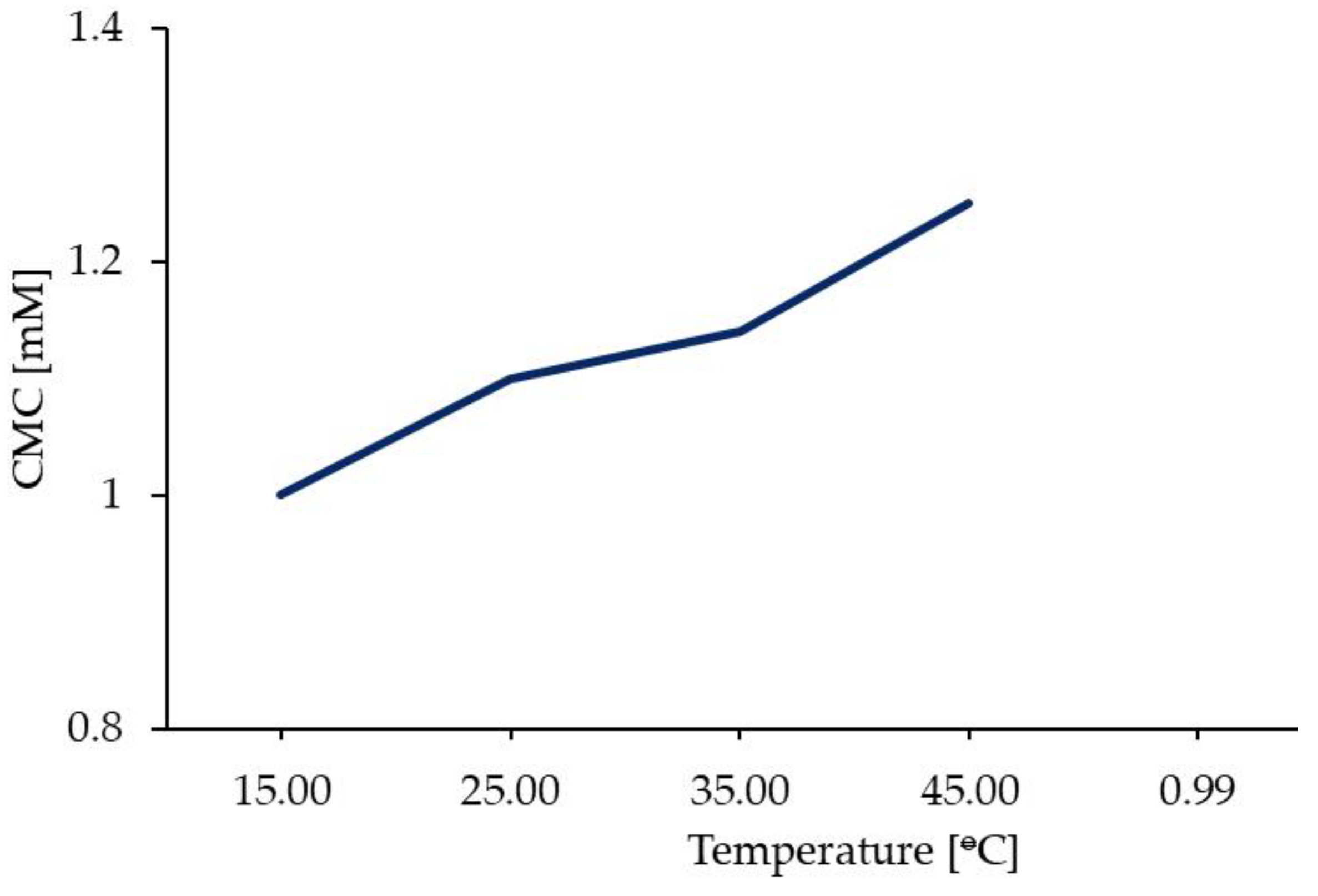
4.1. Surface activity in aqueous solutions
4.2. Antimicrobial properties
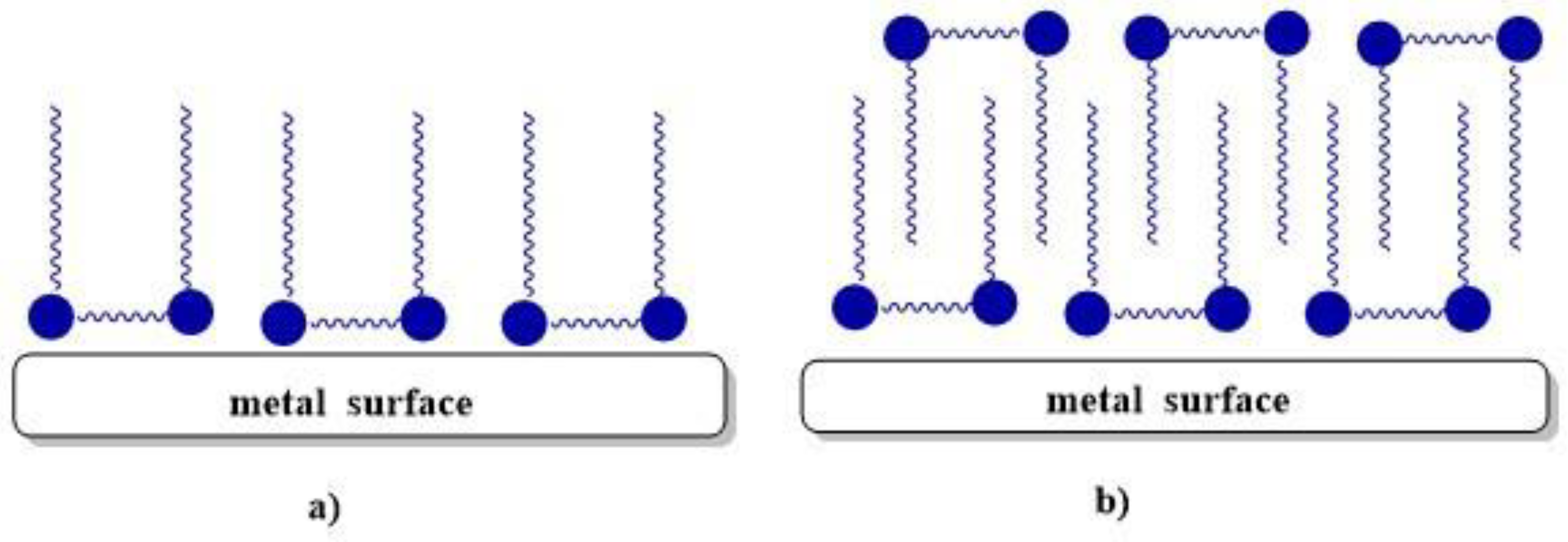
4.3. Anticorrosion properties
4.4. Interaction with macromolecules
5. Toxicity and environmental impact
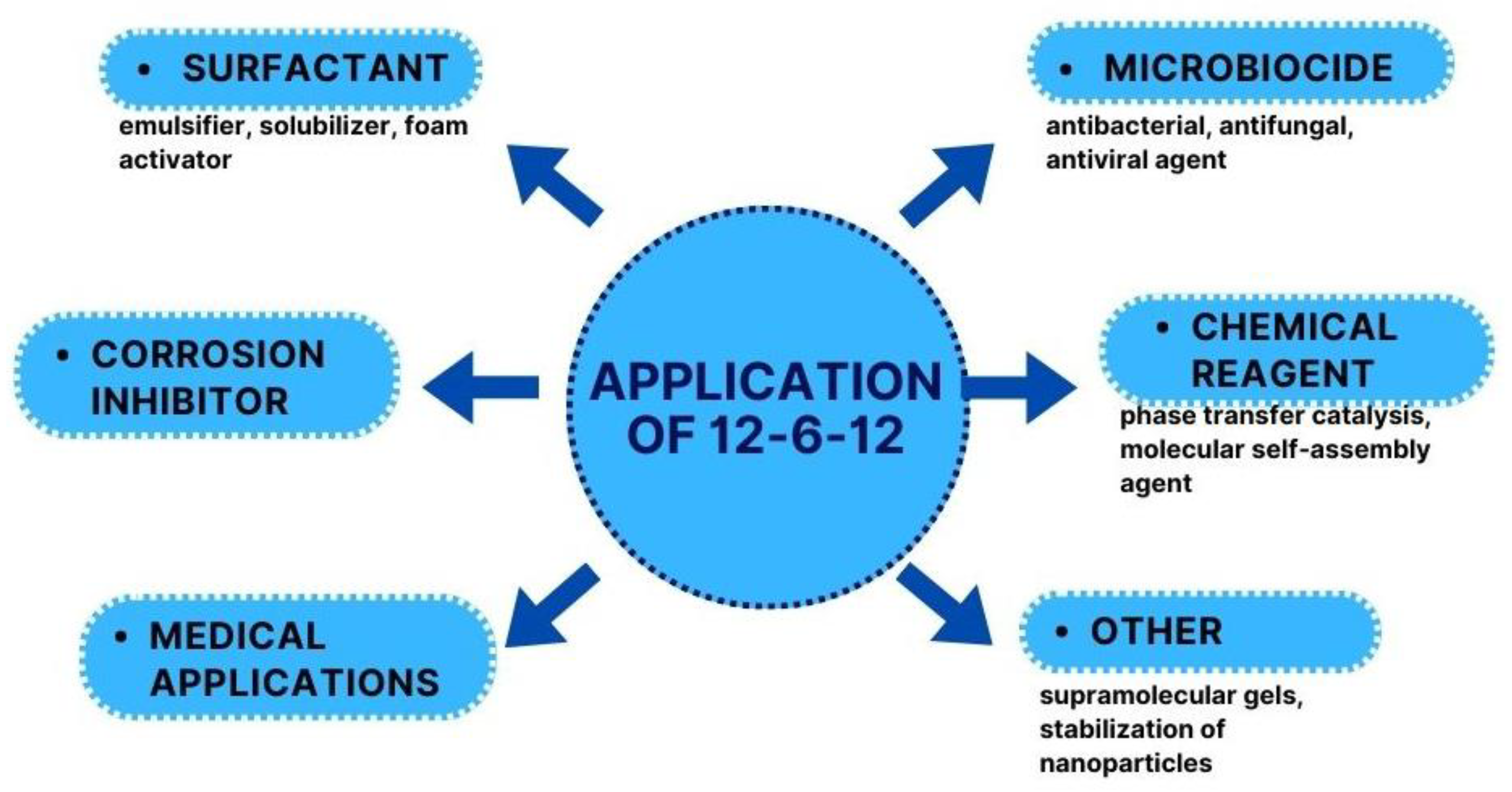
6. Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Roy, A. A Review on the Biosurfactants: Properties, Types and its Applications. J. Fundam. Renew. Energy Appl. 2018, 8, 248. [Google Scholar] [CrossRef]
- Gayathiri, E.; Prakash, P.; Karmegam, N.; Varjani, S.; Awasthi, M.K.; Ravindran, B. Biosurfactants: Potential and Eco-Friendly Material for Sustainable Agriculture and Environmental Safety—A Review. Agronomy 2022, 12, 662. [Google Scholar] [CrossRef]
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial Biosurfactant as an Alternate to Chemical Surfactants for Application in Cosmetics Industries in Personal and Skin Care Products: A Critical Review. BioMed Res. Int. 2023, 2023, 2375223. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Global Biosurfactants Market: By Type: Glycolipids, Lipopeptides, Fatty Acids, Polymerics, Others; By Application: Emulsifiers, Humectants, Preserving Agents, By End Use: Detergents, Personal Care, Food Processing, Agrochemicals; Regional Analysis; Competitive Landscape; Key Trends and Developments in the Market; 2023–2028. Available online: https://www.expertmarketresearch.com/reports/biosurfactants-market (accessed on 29 August 2023).
- Surfactants Market—Global Industry Assessment & Forecast. Available online: https://www.vantagemarketresearch.com/industry-report/surfactants-market-1671 (accessed on 29 August 2023).
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3325-4. [Google Scholar]
- Menger, F.M.; Littau, C.A. Gemini-surfactants: Synthesis and properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A.; Babkova, M. Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Appl. Sci. 2020, 11, 154. [Google Scholar] [CrossRef]
- Feizi, N.; Yamini, Y.; Moradi, M.; Karimi, M.; Salamat, Q.; Amanzadeh, H. A new generation of nano-structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal. Chim. Acta 2017, 953, 1–9. [Google Scholar] [CrossRef]
- Yadav, S.K.; Parikh, K.; Kumar, S. Solubilization potentials of single and mixed oppositely charged gemini surfactants: A case of polycyclic aromatic hydrocarbons. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 47–55. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Kolay, S.; Bal, S.; Satnami, M.L.; Quagliotto, P.; Dafonte, P.R. Effect of cationic gemini surfactants on the hydrolysis of carboxylate and phosphate esters using hydroxamate ions. Colloid Polym. Sci. 2008, 286, 293–303. [Google Scholar] [CrossRef]
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.J.M.; Söderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; García Rodríguez, C.L.; et al. Gemini Surfactants: New Synthetic Vectors for Gene Transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457. [Google Scholar] [CrossRef]
- Akram, M.; Ansari, F.; Bhat, I.A.; Din, K.U. Probing interaction of bovine serum albumin (BSA) with the biodegradable version of cationic gemini surfactants. J. Mol. Liq. 2019, 276, 519–528. [Google Scholar] [CrossRef]
- Akram, M.; Ansari, F.; Bhat, I.A.; Chaturvedi, S.K.; Khan, R.H.; Din, K.U. Analyzing the interaction between porcine serum albumin (PSA) and ester-functionalized cationic gemini surfactants. Process Biochem. 2017, 63, 145–153. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Ya Zakharova, L.; Khairutdinova, E.I.; Lukashenko, S.S.; Sinyashin, O.G. Supramolecular systems based on gemini surfactants for enhancing solubility of spectral probes and drugs in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 33–42. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Industrial Applications of Dimeric Surfactants: A Review. J. Dispers. Sci. Technol. 2014, 35, 205–214. [Google Scholar] [CrossRef]
- Sidenko, Z.S.; Limanov, V.E.; Skvortsova, E.K.; Dziomko, V.M. Synthesis and antibacterial activity of several bisammonium compounds. Pharm. Chem. J. 1968, 2, 247–250. [Google Scholar] [CrossRef]
- McLachlan, A.; Singh, K.; Piggott, E.; McAlduff, M.; MacLennan, S.; Sandre, V.; Reid, T.; Marangoni, D.G. m-s-m Cationic Gemini and Zwitterionic Surfactants–Spacer-Dependent Synergistic Interactions. J. Phys. Chem. B 2019, 123, 1855–1868. [Google Scholar] [CrossRef]
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Micellization of Cationic Gemini Surfactants with Various Counterions and Their Interaction with DNA in Aqueous Solution. J. Phys. Chem. B 2004, 108, 15385–15391. [Google Scholar] [CrossRef]
- Mivehi, L.; Bordes, R.; Holmberg, K. Adsorption of Cationic Gemini Surfactants at Solid Surfaces Studied by QCM-D and SPR: Effect of the Rigidity of the Spacer. Langmuir 2011, 27, 7549–7557. [Google Scholar] [CrossRef]
- Zana, R.; Benrraou, M.; Rueff, R. Alkanediyl-.alpha.,.omega.-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075. [Google Scholar] [CrossRef]
- Szymaniak, D.; Maćkowiak, A.; Ciarka, K.; Praczyk, T.; Marcinkowska, K.; Pernak, J. Synthesis and Characterization of Double-Salt Herbicidal Ionic Liquids Comprising both 4-Chloro-2-methylphenoxyacetate and trans-Cinnamate Anions. ChemPlusChem 2020, 85, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Junior, P.B.S.; Tiera, V.A.O.; Tiera, M.J. A fluorescence probe study of gemini surfactants in aqueous solution: A comparison between n-2-n and n-6-n series of the alkanediyl-α,ω-bis (dimethylalkylammonium bromides). Eclet. Quím. 2007, 32, 47–54. [Google Scholar] [CrossRef]
- Takács, D.; Péter, T.; Vargáné Árok, Z.; Katana, B.; Papović, S.; Gadzuric, S.; Vraneš, M.; Szilágyi, I. Structure–Stability Relationship in Aqueous Colloids of Latex Particles and Gemini Surfactants. J. Phys. Chem. B 2022, 126, 9095–9104. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Devinsky, F.; Lacko, I.; Mlynarčík, D.; Krasnec, L. Preparation and antimicrobial activity of some new bisquaternary ammonium salts. Pharmazie 1983, 38, 308–310. [Google Scholar] [CrossRef]
- Esen, I.; Yolacan, C.; Aydogan, F. Long Chain Dicationic Phase Transfer Catalysts in the Condensation Reactions of Aromatic Aldehydes in Water Under Ultrasonic Effect. Bull. Korean Chem. Soc. 2010, 31, 2289–2292. [Google Scholar] [CrossRef]
- Öge, A.; MaviŞ, M.E.; Yolaçan, Ç.; Aydoğan, F. Solvent-free Michael addition of 2-cyclohexenone under ultrasonic irradiation in the presence of long chain dicationic ammonium salts. Turk. J. Chem. 2012, 36, 137–146. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.-C.; Zhang, J.-Z.; Chai, S.-G. Resonance light scattering method for the determination of DNA with cationic methacrylate based polymer nanoparticle probes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 437–443. [Google Scholar] [CrossRef]
- Lee, H.I.; Pak, C.; Yi, S.H.; Shon, J.K.; Kim, S.S.; So, B.G.; Chang, H.; Yie, J.E.; Kwon, Y.-U.; Kim, J.M. Systematic phase control of periodic mesoporous organosilicas using Gemini surfactants. J. Mater. Chem. 2005, 15, 4711. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium Bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Zhou, L.; Wang, J.; Liu, L.; Wang, S.; Wang, Y. Soft Particles of Gemini Surfactant/Conjugated Polymer for Enhanced Anticancer Activity of Chemotherapeutics. ACS Appl. Mater. Interfaces 2018, 10, 37–41. [Google Scholar] [CrossRef]
- Singer, O.M.; Campbell, J.W.; Hoare, J.G.; Masuda, J.D.; Marangoni, G.; Singer, R.D. Improved Green Synthesis and Crystal Structures of Symmetrical Cationic Gemini Surfactants. ACS Omega 2022, 7, 35326–35330. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide). J. Mol. Liq. 2016, 221, 1086–1096. [Google Scholar] [CrossRef]
- Calas, M.; Ancelin, M.L.; Cordina, G.; Portefaix, P.; Piquet, G.; Vidal-Sailhan, V.; Vial, H. Antimalarial Activity of Compounds Interfering with Plasmodium falciparum Phospholipid Metabolism: Comparison between Mono- and Bisquaternary Ammonium Salts. J. Med. Chem. 2000, 43, 505–516. [Google Scholar] [CrossRef]
- Devinsky, F.; Pisarcik, M.; Lacko, I. Hydrodynamic size of DNA/cationic gemini surfactant complex as a function of surfactant structure. Gen. Physiol. Biophys. 2009, 28, 160–167. [Google Scholar] [CrossRef]
- Pisárčik, M.; Jampílek, J.; Lukáč, M.; Horáková, R.; Devínsky, F.; Bukovský, M.; Kalina, M.; Tkacz, J.; Opravil, T. Silver Nanoparticles Stabilised by Cationic Gemini Surfactants with Variable Spacer Length. Molecules 2017, 22, 1794. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Heteroatoms and π electrons as favorable factors for efficient corrosion protection. Mater. Corros. 2019, 70, 1099–1110. [Google Scholar] [CrossRef]
- Aubagnac, J.-L.; Gilles, I.; Calas, M.; Cordina, G.; Piquet, G.; Portefaix, P.; Giral, L. Fast atom bombardment, frit fast atom bombardment and electrospray ionization mass spectrometric study of organic salts C2+ 2X−: Matrix and anion effects. J. Mass Spectrom. 1995, 30, 985–992. [Google Scholar] [CrossRef]
- Buse, J.; Badea, I.; Verrall, R.E.; El-Aneed, A. Tandem Mass Spectrometric Analysis of the Novel Gemini Surfactant Nanoparticle Families G12-s and G18:1-s. Spectrosc. Lett. 2010, 43, 447–457. [Google Scholar] [CrossRef]
- Woch, J.; Iłowska, J.; Hordyjewicz-Baran, Z.; Arabasz, S.; Kaczmarczyk, B.; Grabowski, R.; Libera, M.; Dworak, A.; Trzebicka, B. Aqueous solution behaviour and solubilisation properties of octadecyl cationic gemini surfactants and their comparison with their amide gemini analogues. Soft Matter 2018, 14, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Akbaş, H.; Elemenli, A.; Boz, M. Aggregation and Thermodynamic Properties of Some Cationic Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 33–40. [Google Scholar] [CrossRef]
- Alami, E.; Beinert, G.; Marie, P.; Zana, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 1993, 9, 1465–1467. [Google Scholar] [CrossRef]
- Bakshi, M.S.; Kaur, G.; Yoshimura, T.; Esumi, K. Azeotropic mixing of C16peLac with different surfactants of monomeric and dimeric nature under the effect of temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 163–170. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfactants Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Hydrophilicity and flexibility of the spacer as critical parameters on the aggregation behavior of long alkyl chain cationic gemini surfactants in aqueous solution. J. Mol. Liq. 2017, 230, 453–460. [Google Scholar] [CrossRef]
- Chavda, S.; Kuperkar, K.; Bahadur, P. Formation and Growth of Gemini Surfactant (12- s -12) Micelles as a Modulate by Spacers: A Thermodynamic and Small-Angle Neutron Scattering (SANS) Study. J. Chem. Eng. Data 2011, 56, 2647–2654. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, Y.; Ye, J.; Yan, H.; Thomas, R.K. Micellization of a Series of Dissymmetric Gemini Surfactants in Aqueous Solution. J. Phys. Chem. B 2003, 107, 11428–11432. [Google Scholar] [CrossRef]
- Atkin, R.; Craig, V.S.J.; Wanless, E.J.; Biggs, S. Adsorption of 12- s -12 Gemini Surfactants at the Silica−Aqueous Solution Interface. J. Phys. Chem. B 2003, 107, 2978–2985. [Google Scholar] [CrossRef]
- Burrows, H.D.; Tapia, M.J.; Silva, C.L.; Pais, A.A.C.C.; Fonseca, S.M.; Pina, J.; Seixas de Melo, J.; Wang, Y.; Marques, E.F.; Knaapila, M.; et al. Interplay of Electrostatic and Hydrophobic Effects with Binding of Cationic Gemini Surfactants and a Conjugated Polyanion: Experimental and Molecular Modeling Studies. J. Phys. Chem. B 2007, 111, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Graciani, M.M.; Rodríguez, A.; Martín, V.I.; Fernández, G.; Moyá, M.L. Concentration and Medium Micellar Kinetic Effects Caused by Morphological Transitions. Langmuir 2010, 26, 18659–18668. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, A.; Singh, K.; McAlduff, M.; Marangoni, D.G.; Shortall, S.; Wettig, S.D. m-s-m cationic gemini and zwitterionic surfactants—A thermodynamic analysis of their mixed micelle formation. RSC Adv. 2020, 10, 3221–3232. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Yuan, H.; Yin, J.; Hu, M. Antibacterial Mechanism of Octamethylene-1,8-Bis(Dodecyldimethylammonium Bromide) Against E. coli. J. Surfactants Deterg. 2017, 20, 717–723. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.A.; Guastavino, J.F.; Nicollier, R.A.; Lancelle, M.V.; Russell-White, K.; Murguía, M.C. Synthesis and Properties of Cleavable Quaternary Ammonium Compounds. J. Oleo Sci. 2021, 70, 59–65. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Brycki, B.E.; Szulc, A.; Kowalczyk, I.; Koziróg, A.; Sobolewska, E. Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules 2021, 26, 5759. [CrossRef]
- Balgavý, P.; Devinsky, F. Cut-off effects in biological activities of surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Devinsky, F.; Lacko, I.; Mlynarčík, D.; Racansky, V.; Krasnec, L. Relationship between critical micelle concentration and minimum inhibitory concentration for some non-aromatic quaternary ammonium salts and amine oxides. Tenside Surfactants Deterg. 1985, 22, 10–15. [Google Scholar] [CrossRef]
- Ciganekova, V.; Kallova, J.; Devinsky, F.; Lacko, I. Effect of N,N’-Bis(alkyldimethyl)- -α,ω-alkanediammonium Dibromides on Bacteria of the Genus Clostridium. Folia Microbiol. 1989, 34, 202–208. [Google Scholar] [CrossRef]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents—Influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Kręgiel, D.; Brycki, B. Action of Monomeric/Gemini Surfactants on Free Cells and Biofilm of Asaia lannensis. Molecules 2017, 22, 2036. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Otlewska, A.; Brycki, B. Viability, Enzymatic and Protein Profiles of Pseudomonas aeruginosa Biofilm and Planktonic Cells after Monomeric/Gemini Surfactant Treatment. Molecules 2018, 23, 1294. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Otlewska, A.; Gapińska, M.; Michlewska, S. Influence of Gemini Surfactants on Biochemical Profile and Ultrastructure of Aspergillus brasiliensis. Appl. Sci. 2019, 9, 245. [Google Scholar] [CrossRef]
- Koziróg, A.; Brycki, B.; Pielech-Przybylska, K. Impact of Cationic and Neutral Gemini Surfactants on Conidia and Hyphal Forms of Aspergillus brasiliensis. IJMS 2018, 19, 873. [Google Scholar] [CrossRef] [PubMed]
- Winnicki, K.; Łudzik, K.; Żabka, A.; Polit, J.T.; Zawisza, A.; Maszewski, J. Anti-algal activity of the 12-5-12 gemini surfactant results from its impact on the photosynthetic apparatus. Sci. Rep. 2021, 11, 2360. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.L.; Calas, M.; Bonhoure, A.; Herbute, S.; Vial, H.J. In Vivo Antimalarial Activities of Mono- and Bis Quaternary Ammonium Salts Interfering with Plasmodium Phospholipid Metabolism. Antimicrob. Agents Chemother. 2003, 47, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- Khodsiani, M.; Kianmehr, Z.; Brycki, B.; Szulc, A.; Mehrbod, P. Evaluation of the antiviral potential of gemini surfactants against influenza virus H1N1. Arch. Microbiol. 2023, 205, 184. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H. Understanding the effect of the spacer length on adsorption of gemini surfactants onto steel surface in acid medium. Appl. Surf. Sci. 2005, 246, 1–5. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.; Leiva Garcia, R.; Moorcroft, R.; Nichol, T.; Smith, T.; Akid, R.; Brycki, B. Gemini surfactant as multifunctional corrosion and biocorrosion inhibitors for mild steel. Bioelectrochemistry 2019, 128, 252–262. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, J. Adsorption of quaternary ammonium gemini surfactants on zinc and the inhibitive effect on zinc corrosion in vitriolic solution. Colloids Surf. A 2006, 278, 246–251. [Google Scholar] [CrossRef]
- Chorro, C.; Chorro, M.; Dolladille, O.; Partyka, S.; Zana, R. Adsorption of Dimeric (Gemini) Surfactants at the Aqueous Solution/Silica Interface. J. Colloid Interface Sci. 1998, 199, 169–176. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.; He, B.; Sun, C.; Li, X.; Wu, X.; An, X.; Xie, X. Gemini quaternary ammonium salt cationic surfactant-assisted hydrothermal synthesis: An effective way to tune the textural properties of zeolite and the acidity of Beta molecular sieves. Appl. Organometal. Chem. 2018, 32, e4145. [Google Scholar] [CrossRef]
- Majchrzycka, K.; Okrasa, M.; Szulc, J.; Brycki, B.; Gutarowska, B. Time-Dependent Antimicrobial Activity of Filtering Nonwovens with Gemini Surfactant-Based Biocides. Molecules 2017, 22, 1620. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, W.; Tang, Y.; Zhang, S.; Li, Z.; Wang, Y. Coassembly of Poly(ethylene glycol)- block -Poly(glutamate sodium) and Gemini Surfactants with Different Spacer Lengths. Langmuir 2013, 29, 9316–9323. [Google Scholar] [CrossRef]
- Pi, Y.; Shang, Y.; Peng, C.; Liu, H.; Hu, Y.; Jiang, J. Phase behavior of gemini surfactant hexylene-1,6-bis(dodecyldimethylammonium bromide) and polyelectrolyte NaPAA. J. Colloid Interface Sci. 2006, 299, 410–415. [Google Scholar] [CrossRef]
- Pi, Y.; Shang, Y.; Liu, H.; Hu, Y.; Jiang, J. Salt effect on the interactions between gemini surfactant and oppositely charged polyelectrolyte in aqueous solution. J. Colloid Interface Sci. 2007, 306, 405–410. [Google Scholar] [CrossRef]
- Wang, R.; Yan, H.; Ma, W.; Li, Y. Complex formation between cationic gemini surfactant and sodium carboxymethylcellulose in the absence and presence of organic salt. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 293–300. [Google Scholar] [CrossRef]
- Wettig, S.D.; Verrall, R.E. Studies of the Interaction of Cationic Gemini Surfactants with Polymers and Triblock Copolymers in Aqueous Solution. J. Colloid Interface Sci. 2001, 244, 377–385. [Google Scholar] [CrossRef]
- Pisárčik, M.; Devínsky, F. Surface tension study of cationic gemini surfactants binding to DNA. Cent. Eur. J. Chem. 2014, 12, 577–585. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X.; Li, L.; He, Y.; Song, G. Mechanism studies on the interaction of Gemini surfactant 12-6-12 with bovine serum albumin by fluorescence method. Anal. Chem. 2011, 10, 817–824. [Google Scholar]
- Kumari, S.; Halder, S.; Aggrawal, R.; Aswal, V.K.; Sundar, G.; Saha, S.K. Refolding of protein unfolded by gemini surfactants using β-cyclodextrin and sodium dodecyl sulfate in aqueous medium: Study on role of spacer chain of surfactants. J. Mol. Liq. 2020, 300, 112238. [Google Scholar] [CrossRef]
- Almeida, J.A.S.; Faneca, H.; Carvalho, R.A.; Marques, E.F.; Pais, A.A.C.C. Dicationic Alkylammonium Bromide Gemini Surfactants. Membrane Perturbation and Skin Irritation. PLoS ONE 2011, 6, e26965. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of quaternary ammonium-based gemini surfactants: Effect of the spacer on their ecological properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef]
- Silva, S.M.C.; Sousa, J.J.S.; Marques, E.F.; Pais, A.A.C.C.; Michniak-Kohn, B.B. Structure Activity Relationships in Alkylammonium C12-Gemini Surfactants Used as Dermal Permeation Enhancers. AAPS J. 2013, 15, 1119–1127. [Google Scholar] [CrossRef]
- Brycki, B.; Waligórska, M.; Szulc, A. The Biodegradation of Monomeric and Dimeric Alkylammonium Surfactants. J. Hazard. Mater. 2014, 280, 797–815. [CrossRef]
- Bergero, M.F.; Liffourrena, A.S.; Opizzo, B.A.; Fochesatto, A.S.; Lucchesi, G.I. Immobilization of a microbial consortium on Ca-alginate enhances degradation of cationic surfactants in flasks and bioreactor. Int. Biodeterior. Biodegrad. 2017, 117, 39–44. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef]
- Yang, J.; Yun, L.; Zhao, G.; Zhang, F.; Chen, Y.; Wang, C. Fabrication of pH-responsive system based on cationic gemini surfactant/sodium octanedioate and its application on controlled release of paclitaxel. Colloids Surf. A 2018, 539, 101–108. [Google Scholar] [CrossRef]
- Wang, M.; Wu, C.; Tang, Y.; Fan, Y.; Han, Y.; Wang, Y. Interactions of cationic trimeric, gemini and monomeric surfactants with trianionic curcumin in aqueous solution. Soft Matter 2014, 10, 3432. [Google Scholar] [CrossRef]
- Wettig, S.D.; Deubry, R.; Akbar, J.; Kaur, T.; Wang, H.; Sheinin, T.; Joseph, J.W.; Slavcev, R.A. Thermodynamic investigation of the binding of dissymmetric pyrenyl-gemini surfactants to DNA. Phys. Chem. Chem. Phys. 2010, 12, 4821–4826. [Google Scholar] [CrossRef]
- Falsini, S.; Di Cola, E.; In, M.; Giordani, M.; Borocci, S.; Ristori, S. Complexation of short ds RNA/DNA oligonucleotides with Gemini micelles: A time resolved SAXS and computational study. Phys. Chem. Chem. Phys. 2017, 19, 3046–3055. [Google Scholar] [CrossRef]
- Acosta-Martínez, D.R.; Rodríguez-Velázquez, E.; Araiza-Verduzco, F.; Taboada, P.; Prieto, G.; Rivero, I.A.; Pina-Luis, G.; Alatorre-Meda, M. Bis-quaternary ammonium gemini surfactants for gene therapy: Effects of the spacer hydrophobicity on the DNA complexation and biological activity. Colloids Surf. B Biointerfaces 2020, 189, 110817. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Brycki, B.; Olejnik, K.; Wysocka-Robak, A.; Dębska-Winkler, P. Cellulose products modified with monomeric and gemini surfactants: Antimicrobial aspects. Cellulose 2019, 26, 5559–5570. [Google Scholar] [CrossRef]
- Wietecha, J.; Kopania, E.; Ciechańska, D.; Wiśniewska-Wrona, M.; Gruchała, B.; Data, M.; Brycki, B.; Kowalczyk, I. Kompozycja sanityzująca do wyrobów papierniczych. Patent PL 232698B1, 1 August 2016. [Google Scholar]
- Wang, H.; He, L.; Brycki, B.E.; Kowalczyk, I.H.; Kuliszewska, E.; Yang, Y. Electrochemical characterization of the hydrophobic microenvironment within gemini surfactant micellar-hybridized supramolecular gels. Electrochim. Acta 2013, 90, 326–331. [Google Scholar] [CrossRef]
- Gustafsson, H.; Isaksson, S.; Altskär, A.; Holmberg, K. Mesoporous silica nanoparticles with controllable morphology prepared from oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal alkylammonium surfactants: Aggregation properties and catalytic activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A. Aqueous solutions of geminal alkylammonium surfactants as a medium for reactions of long-chain amines. Russ. J. Gen. Chem. 2009, 79, 42–48. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Yackevich, E.I.; Valeeva, F.G.; Pankratov, V.A.; Zakharova, L.Y. Solubilizing and catalytic properties of supramolecular systems based on gemini surfactants. Russ. Chem. Bull. 2014, 63, 82–87. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, J.; Wen, Z. Self-Assembly of Quaternary Ammonium Gemini Surfactants in Cyclohexane upon Reinforcement by Simple Counterions. RSC Adv. 2018, 8, 18880–18888. [Google Scholar] [CrossRef] [PubMed]
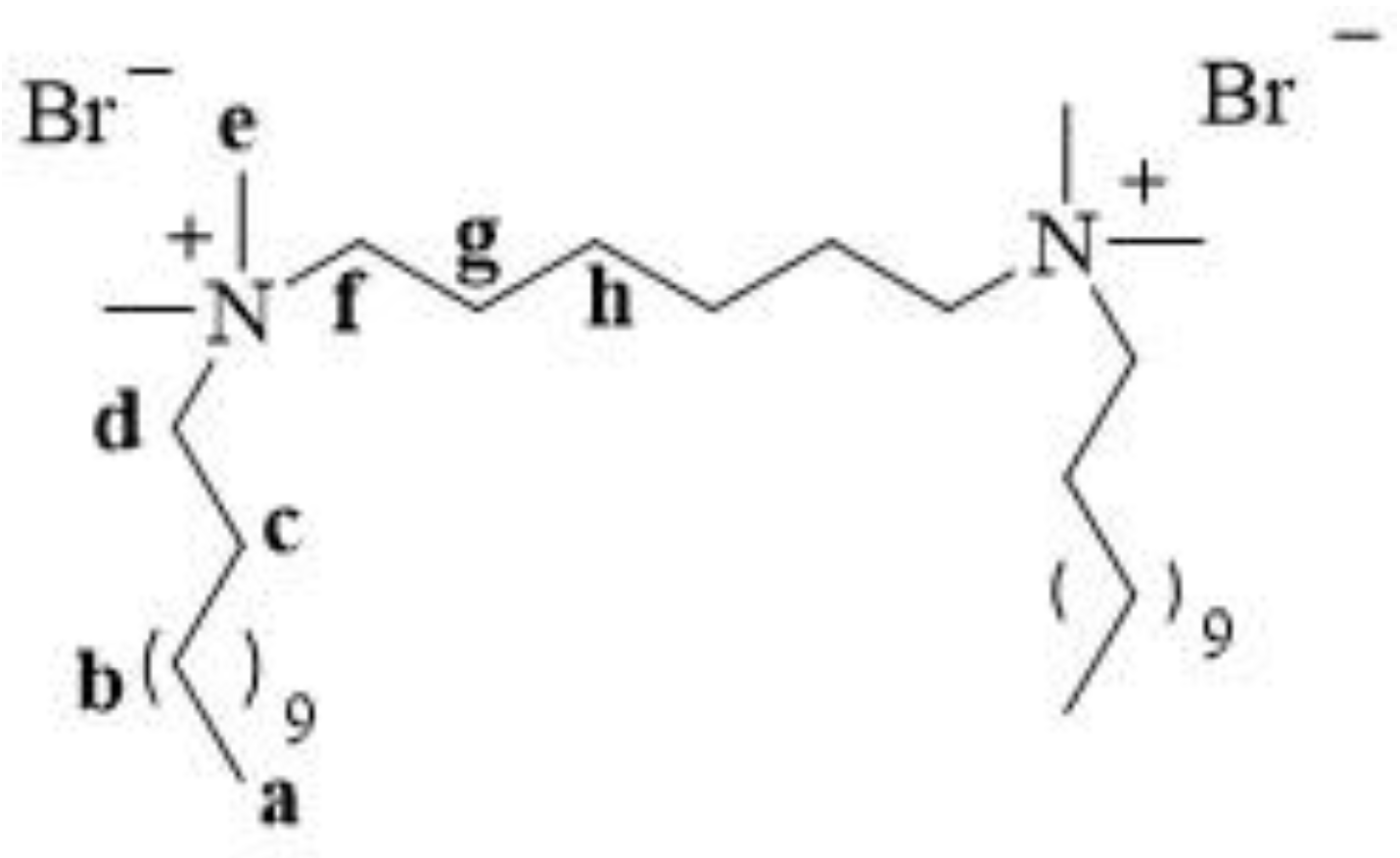
| Proton | δ | ||
|---|---|---|---|
| D2O 1 | CDCl3 2 | CD3OD 3 | |
| a (6H) | 0.86 | 0.85 | 0.86 |
| b (36H) | 1.37–1.28 | 1.33–1.19 | 1.37–1.28 |
| c (4H) | 1.77 | 1.89 | 1.77 |
| d (4H) | 3.35 | 3.68 | 3.34 |
| e (12H) | 3.12 | 3.35 | 3.12 |
| f (4H) | 3.32 | 3.42 | 3.31 |
| g (4H) | 1.71 | 1.69 | 1.71 |
| h (4H) | 1.45 | 1.35 | 1.45 |
| Carbon | δ | |
|---|---|---|
| D2O 1 | CDCl3 2 | |
| a | 15.83 | 13.84 |
| b | 33.96–31.48 | 31.60–29.17 |
| c | 24.41 | 22.38 |
| d | 64.84 | 64.38 |
| e | 53.51 | 50.73 |
| f | 64.72 | 63.81 |
| g | 23.86 | 21.51 |
| h | 27.10 | 24.42 |
| Ions | m/z |
|---|---|
| [M]2+ | 255.30 |
| [M-C12H25]+ | 341.41 |
| [M-C12H24]2+ | 171.20 |
| [M-C12H24-C12H24-C2H5N]+ | 128.12 |
| Parameters | DTAB | 12-6-12 |
|---|---|---|
| CMC (conductometric) [mM] | 15.4 1 | 0.98 2 |
| CMC (tensiometric) [mM] | 15.0 3 | 0.85 3 |
| A | 0.29 3 | 0.46 3 |
| ΔG°mic [kJ/mol] | −35.0 4 | −59.8 4 |
| γCMC [mN/m] | 37.5 3 | 37.7 3 |
| Å2 [nm2] | 62 3 | 108 3 |
| Number of molecules per nm2 | 1.61 3 | 0.93 3 |
| Anion | CMC [mM] |
|---|---|
| SO42− | 0.68 |
| NO3− | 0.89 |
| Br− | 0.98 |
| Ac− | 1.10 |
| Cl− | 1.33 |
| F− | 1.84 |
| Bacteria | MIC [mM] | |
|---|---|---|
| DTAB | 12-6-12 | |
| Gram-positive: | ||
| S. aureus | - | 0.008 1 |
| 0.044 2 | 0.0028 2 | |
| 0.252 3 | 0.0036 3 | |
| C. perfringens | - | 0.04 4 |
| Gram-negative: | ||
| E. coli | - | 0.052 1 |
| 0.36 2 | 0.0868 2 | |
| P. aeruginosa | 0.126 3 | 0.0073 3 |
| A. lannensis | 0.127 5 | 0.0073 5 |
| Fungus | MIC [mM] |
|---|---|
| A. niger | 0.12 1 |
| P. chrysogenum | 0.06 1 |
| C. albicans | 0.015 1 0.022 2 |
| A. brasiliensis | 0.12 3 |
| Concentration of Surfactant [mM] | Material | Corrosive Medium | CR [mm/year] | IE [%] | Ref |
|---|---|---|---|---|---|
| 0 | Stainless steel | 3 M HCl | 19.74 | - | [42] |
| 0.1 | 3.71 | 84 | |||
| 0.5 | 0.92 | 95 | |||
| 1 | 0.71 | 97 | |||
| 5 | 0.65 | 97 | |||
| 0 | Mild steel | 3.5% NaCl | 1.39 | - | [74] |
| 0.01 | 0.068 | 95.2 | |||
| 0.1 | 0.021 | 98.5 | |||
| 1 | 0.019 | 98.7 | |||
| 2 | 0.010 | 99.3 |
| Application | References |
|---|---|
| Enhanced drug delivery | [87,89] |
| Drug carrier | [93] |
| Stabilization of drugs | [94] |
| Detection of DNA | [33] |
| Nonviral gene delivery agent | [44,95,96,97] |
| Eradication of biofilm | [66,67] |
| Filtering nonwovens | [78] |
| Bioactive cellulose products | [98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





