Submitted:
21 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
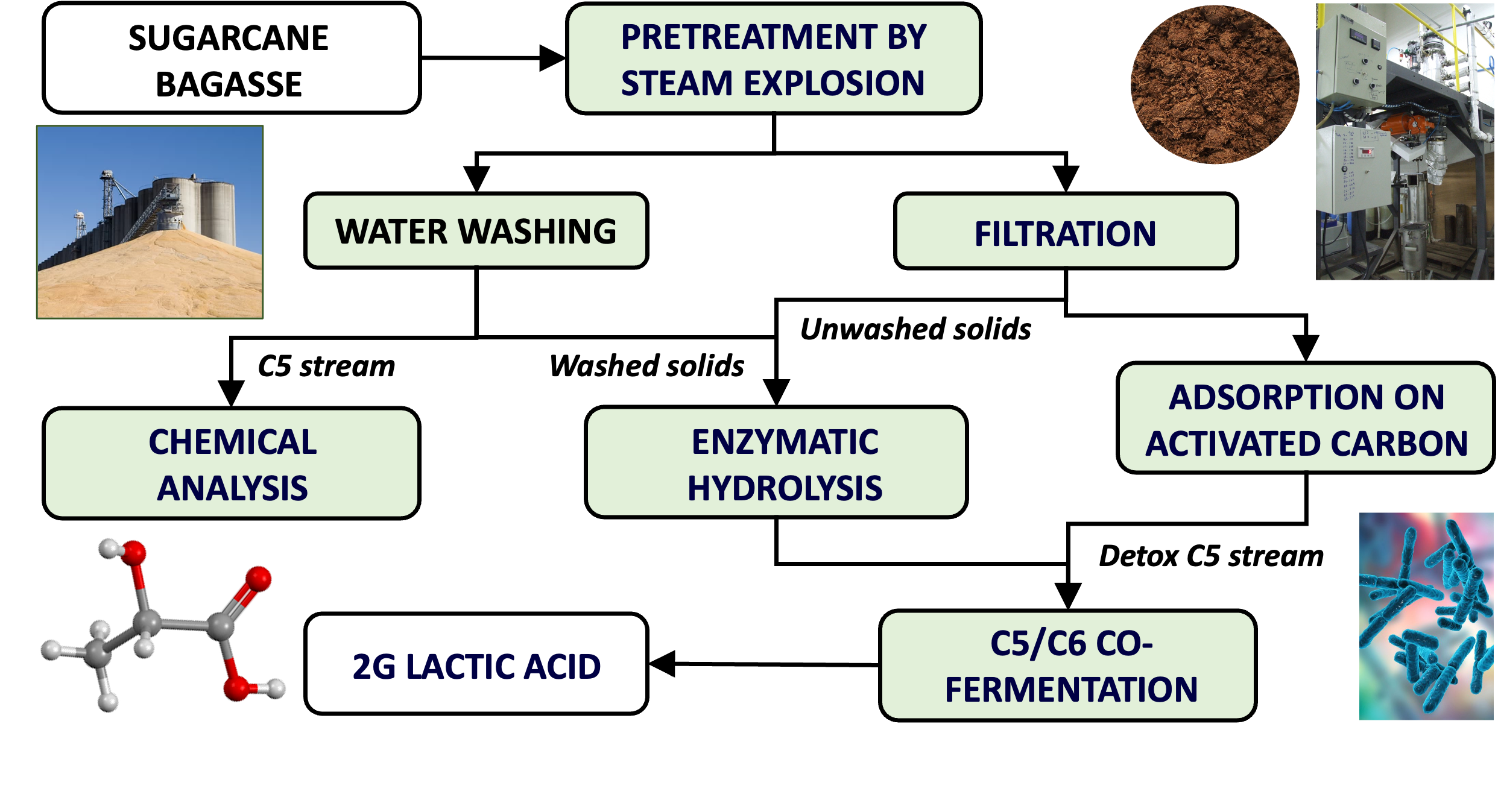
2. Material and Methods
2.1. Sugarcane Bagasse (SCB) Pretreatment and Characterization
2.2. Enzymatic Hydrolysis
2.3. Microorganism and Fermentation
2.4. Chromatographic Analysis
2.5. Statistical Analysis
3. Results
3.1. SCB Pretreatment and Characterization
3.2. Chemical Composition of Pretreatment Liquors before and after Detoxification
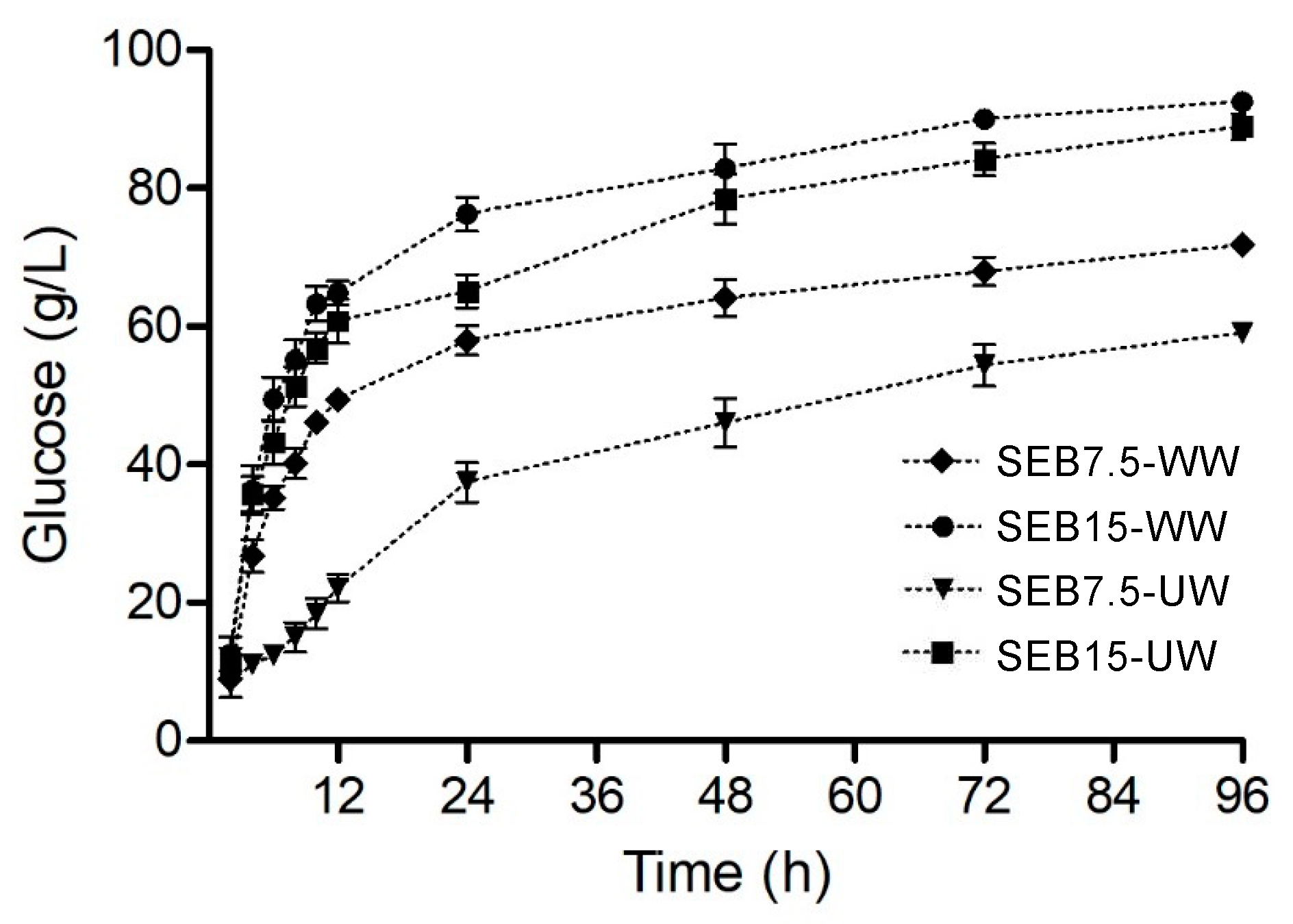
3.3. Enzymatic Hydrolysis
3.4. Fermentation of SEB Pretreatment Liquors
3.5. Fermentation of SEB-UW and SEB-WW Enzymatic Hydrolysates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alsaheb, R. A. A.; Aladdin, A.; Othman, N. Z.; Malek, R. A.; Leng, O. M.; Aziz, R.; Enshasy, H. A. El. Lactic Acid Applications in Pharmaceutical and Cosmeceutical Industries. J. Chem. Pharm. Res. 2015, 7, 729–735. [Google Scholar]
- Abedi, E.; Hashemi, S. M. B. Lactic Acid Production – Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Lactic acid market size, share & trends analysis report by raw material (sugarcane, corn, cassava), by application (PLA, food & beverages, personal care, pharmaceuticals), by region, and segment forecasts, 2022–2030. 2021.
- Singhvi, M.; Joshi, D.; Adsul, M.; Varma, A.; Gokhale, D. D-(-)-Lactic Acid Production from Cellobiose and Cellulose by Lactobacillus lactis Mutant RM2-24. Green Chem. 2010, 12, 1106–1109. [Google Scholar] [CrossRef]
- Eş, I.; Mousavi Khaneghah, A.; Barba, F. J.; Saraiva, J. A.; Sant’Ana, A. S.; Hashemi, S. M. B. Recent Advancements in Lactic Acid Production - a Review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Shah, S. R.; Walker, J. L.; Mikos, A. G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering, Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Tsuji, H.; Fukui, I. Enhanced Thermal Stability of Poly(Lactide)s in the Melt by Enantiomeric Polymer Blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Komesu, A.; de Oliveira, J. A. R.; Martins, L. H. da S.; Maciel, M. R. W.; Maciel Filho, R. Lactic Acid Production to Purification: A Review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Reddy, L. V.; Park, J.; Wee, Y. Homofermentative Production of Optically Pure L -Lactic Acid from Sucrose and Mixed Sugars by Batch Fermentation of Enterococcus faecalis RKY1. Biotechnol. Bioprocess Eng. 2015, 1105, 1099–1105. [Google Scholar] [CrossRef]
- Nwamba, M. C.; Sun, F.; Mukasekuru, M. R.; Song, G.; Harindintwali, J. D.; Boyi, S. A.; Sun, H. Trends and Hassles in the Microbial Production of Lactic Acid from Lignocellulosic Biomass. Environ. Technol. Innov. 2021, 21, 101337. [Google Scholar] [CrossRef]
- Pleissner, D.; Demichelis, F.; Mariano, S.; Fiore, S.; Gutiérrez, I. M. N.; Schneider, R.; Venus, J. Direct production of lactic acid based on simultaneous saccharification and fermentation of mixed restaurant food waste. J. Clean. Prod. 2011, 143, 615–623. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.; Cai, D.; Wang, B.; Qin, P.; Wang, Z.; Tan, T. Improvement of L-Lactic Acid Productivity from Sweet Sorghum Juice by Repeated Batch Fermentation Coupled with Membrane Separation. Bioresour. Technol. 2016, 211, 291–297. [Google Scholar] [CrossRef]
- Krishna, B. S.; Sai, S.; Gantala, N.; Tarun, B. Industrial Production of Lactic Acid and Its Applications. Int. J. Biotech Res. 2018, 1, 42–54. [Google Scholar]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Bondesson, P.-M.; Galbe, M.; Zacchi, G. Ethanol and Biogas Production after Steam Pretreatment of Corn Stover with or without the Addition of Sulphuric Acid. Biotechnol. Biofuels 2013, 6, 11. [Google Scholar] [CrossRef]
- Carrasco, C.; Baudel, H. M.; Sendelius, J.; Modig, T.; Roslander, C.; Galbe, M.; Hahn-Hägerdal, B.; Zacchi, G.; Lidén, G. SO2-Catalyzed Steam Pretreatment and Fermentation of Enzymatically Hydrolyzed Sugarcane Bagasse. Enzyme Microb. Technol. 2010, 46, 64–73. [Google Scholar] [CrossRef]
- Wee, Y.; Yun, J.; Kim, D.; Ryu, H. Batch and repeated batch production of L-(þ)-lactic 2254 acid by RKY1 using wood hydrolyzate and corn steep liquor Enterococcus faecalis, J. Ind. Microbiol. Biotechnol. 2006, 33, 431–435. [Google Scholar] [CrossRef]
- Palmqvist, E.; Grage, H.; Meinander, N. Q. Main and Interaction Effects of Acetic Acid, Furfural, and p-Hydroxybenzoic Acid on Growth and Ethanol Productivity of Yeasts. Biotechnol. Bioeng. 1999, 63, 46–55. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Cai, L.-Y.; Ma, Y.-L. Study on Inhibitors from Acid Pretreatment of Corn Stalk on Ethanol Fermentation by Alcohol Yeast. RSC Adv. 2020, 10, 38409–38415. [Google Scholar] [CrossRef]
- Fockink, D. H.; Urio, M. B.; Sa, J. H.; Ramos, L. P. Enzymatic Hydrolysis of Steam-Treated Sugarcane Bagasse: Effect of Enzyme Loading and Substrate Total Solids on Its Fractal Kinetic Modeling and Rheological Properties. Energy Fuels 2017, 31, 6211–6220. [Google Scholar] [CrossRef]
- Neves, P. V; Pitarelo, A. P.; Ramos, L. P. Production of Cellulosic Ethanol from Sugarcane Bagasse by Steam Explosion: Effect of Extractives Content, Acid Catalysis and Different Fermentation Technologies. Bioresour. Technol. 2016, 208, 184–194. [Google Scholar] [CrossRef]
- Xue, S.; Uppugundla, N.; Bowman, M. J.; Cavalier, D.; Da, L.; Sousa, C.; Dale, B. E.; Balan, V. Sugar Loss and Enzyme Inhibition Due to Oligosaccharide Accumulation during High Solids - Loading Enzymatic Hydrolysis. Biotechnol. Biofuels 2015, 8, 195. [Google Scholar] [CrossRef]
- Fockink, D. H.; Sánchez, J. H.; Ramos, L. P. Comprehensive Analysis of Sugarcane Bagasse Steam Explosion Using Autocatalysis and Dilute Acid Hydrolysis (H3PO4 and H2SO4) at Equivalent Combined Severity Factors. Ind. Crop. Prod. 2018, 123, 563–572. [Google Scholar] [CrossRef]
- Horváth, I. S.; Sjöde, A.; Nilvebrant, N. O.; Zagorodni, A.; Jönsson, L. J. Selection of Anion Exchangers for Detoxification of Dilute-Acid Hydrolysates from Spruce. Appl. Biochem. Biotechnol. 2004, 114, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Comparison of Different Detoxification Methods for Steam-Exploded Poplar Wood as a Substrate for the Bioproduction of Ethanol in SHF and SSF. Process Biochem. 2004, 39, 1533–1542. [Google Scholar] [CrossRef]
- Larsson, S.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L. J. Comparison of Different Methods for the Detoxification of Lignocellulose Hydrolyzates of Spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–104. [Google Scholar] [CrossRef]
- Pitarelo, A. P.; Fonseca, C. S.; Chiarello, L. M.; Gírio, F. M.; Ramos, L. P. Ethanol Production from Sugarcane Bagasse Using Phosphoric Acid-Catalyzed Steam Explosion. J. Braz. Chem. Soc. 2016, 27, 1889–1898. [Google Scholar] [CrossRef]
- Jurado, M.; Prieto, A.; Martínez-Alcalá, Á.; Martínez, Á. T.; Martínez, M. J. Laccase Detoxification of Steam-Exploded Wheat Straw for Second Generation Bioethanol. Bioresour. Technol. 2009, 100, 6378–6384. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C.; Lu, M.; Tu, M. Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Abdel-Rahman, M. A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Qi, B.; Luo, J.; Shen, F.; Su, Y.; Khan, R.; Wan, Y. Improving Lactic Acid Productivity from Wheat Straw Hydrolysates by Membrane Integrated Repeated Batch Fermentation under Non-Sterilized Conditions. Bioresour. Technol. 2014, 163, 160–166. [Google Scholar] [CrossRef]
- Ye, L.; Hudari, M. S. Bin; Li, Z.; Wu, J. C. Simultaneous Detoxification, Saccharification and Co-Fermentation of Oil Palm Empty Fruit Bunch Hydrolysate for L-Lactic Acid Production by Bacillus coagulans JI12. Biochem. Eng. J. 2014, 83, 16–21. [Google Scholar] [CrossRef]
- Malacara-Becerra, A.; Melchor-Martínez, E. M.; Sosa-Hernández, J. E.; Riquelme-Jiménez, L. M.; Mansouri, S. S.; Iqbal, H. M. N.; Parra-Saldívar, R. Bioconversion of Corn Crop Residues: Lactic Acid Production through Simultaneous Saccharification and Fermentation. Sustainability 2022, 14, 11799. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; Venus, J.; González-Fernández, C.; Tomás-Pejó, E. Assessment of different Bacillus coagulans strains for L-lactic acid production from defined media and gardening hydrolysates: Effect of lignocellulosic inhibitors, J. Biotechnol. 2020, 323, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M.L. PLA nano- and microparticles for drug delivery: An overview of the methods of preparation. Macromol. Biosci. 2007, 7, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsapekos, P.; Alvarado-Morales, M.; Zhu, X.; Zervas, A.; Jacobsen, C.S.; Angelidaki, I. Enhanced fermentative lactic acid production from source-sorted organic household waste: Focusing on low-pH microbial adaptation and bio-augmentation strategy. Sci. Total Environ. 2022, 808, 152129. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Rostro Alanís, M. J.; de la Cruz Quiroz, R.; Ruiz, H. A.; Iqbal, H. M. N.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Gauss, W. F.; Suzuki, S.; Takagi, M. Manufacture of Alcohol from Cellulosic Materials Using Plural Ferments. U.S. Patent No. 399 0944, 1976. [Google Scholar]
- Michelson, T.; Kask, K.; Jõgi, E.; Talpsep, E.; Suitso, I.; Nurk, A. L. (+)-Lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii ssp. lactis DSM 20073. Enzyme and microbial technology 2006, 39, 861–867. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples, Laboratory Analytical Procedure (LAP), Technical Report NREL/TP-510-42621. Natl. Renew. Energy Lab. 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass, Laboratory Analytical Procedure (LAP), Technical Report NREL/TP-510-42622. Natl. Renew. Energy Lab. 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass, Laboratory Analytical Procedure (LAP), Technical Report NREL/TP-510-42619. Natl. Renew. Energy Lab. 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure (LAP), Technical Report NREL/TP-510-42618. Natl. Renew. Energy Lab. 2008. [Google Scholar]
- Candido, J. P.; Claro, E. M. T.; de Paula, C. B. C.; Shimizu, F. L.; de Oliveria Leite, D. A. N.; Brienzo, M.; de Angelis, D. D. F. Detoxification of sugarcane bagasse hydrolysate with different adsorbents to improve the fermentative process. World J Microbiol Biotechnol. 2020, 36, 43. [Google Scholar] [CrossRef]
- RStudio, T. RStudio: Integrated Development for R. RStudio, Inc. Available from: http://www.rstudio.com/.2015.
- Andrade, L. P.; Crespim, E.; de Oliveira, N.; de Campos, R. C.; Teodoro, J. C.; Galvão, C. M. A.; Maciel Filho, R. Influence of sugarcane bagasse variability on sugar recovery for cellulosic ethanol production. Bioresour. Technol. 2017, 241, 75–81. [Google Scholar] [CrossRef]
- Wang, G.; Chen, H. Carbohydrate elimination of alkaline-extracted lignin liquor by steam explosion and its methylolation for substitution of phenolic adhesive. Ind. Crops Prod. 2014, 53, 93–101. [Google Scholar] [CrossRef]
- Antal Jr, M. J.; Mok, W. S.; Richards, G. N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr Res. 1990, 199, 91–109. [Google Scholar] [CrossRef]
- Brown, D. W.; Floyd, A. J.; Kinsman, R. G.; Roshanhyphen; Ali, Y. Dehydration reactions of fructose in non-aqueous media. J Chem Technol Biotechnol. 1982, 32, 920–924. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn-Hagerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microbial Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Ramos, L. P. The chemistry involved in the pretreatment of lignocellulosic materials. Química Nova 2003, 26, 863–871. [Google Scholar] [CrossRef]
- Du, B.; Sharma, L. N.; Becker, C.; Chen, S. F.; Mowery, R. A.; van Walsum, G. P.; Chambliss, C. K. Effect of varying feedstock–pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol. Bioeng. 2010, 107, 430–440. [Google Scholar] [CrossRef]
- Lu, C.; Dong, J.; Yang, S. T. Butanol Production from wood pulping hydrolysate in an integrated fermentation-gas stripping process. Bioresour. Tech. 2013, 143, 467–475. [Google Scholar] [CrossRef]
- Miura, M.; Suzuki, T.; Aoyama, M. Detoxification of Japanese white birch wood hemicellulose hydrolysate with a carbonaceous sorbent prepared from birch wood hydrolysis residue. Cellulose Chem and Tech. 2016, 50, 265–268. [Google Scholar]
- Sukumaran, R.K.; Singhania, R.R.; Pandey, A. Microbial cellulases production, applications and challenges. J. Sci. Ind. Res. 2005, 64, 832–844. [Google Scholar]
- Mathew, G.M.; Sukumaran, R.K.; Singhania, R.R.; Pandey, A.; 2008. Progress in research on fungal cellulases for lignocellulose degradation. J. Sci. Ind. Res. 2008, 67, 898–907. [Google Scholar]
- Kristensen, J.B.; Felby, C.; Jørgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels. 2009, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R. A.; Schneider, R.; Rossel, C.E.V.; Maciel Filho, R.; Venus, J. Polymer grade L-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour. Technol. Reports 2019, 2019, 26–31. [Google Scholar] [CrossRef]
- Ahorsu, R.; Cintorrino, G.; Medina, F.; Constantí, M. Microwave processes: a viable technology for obtaining xylose from walnut shell to produce lactic acid by Bacillus coagulans. J. Cleaner Production 2019, 231, 1171–1181. [Google Scholar] [CrossRef]
- van der Pol, E.; Springer, J.; Vriesendorp, B.; Weusthuis, R.; Eggink, G. Precultivation of Bacillus coagulans DSM2314 in the presence of furfural decreases inhibitory effects of lignocellulosic by-products during l(+)-lactic acid fermentation. Appl. Microbiol. Biotechnol. 2016, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feron, V. J.; Til, H. P.; De Vrijer, F.; Woutersen, R. A.; Cassee, F. R.; Van Bladeren, P. J. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutation Research 1991, 259, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Allen, S. A.; Clark, W.; McCaffery, J.; M. ; Cai, Z.; Lanctot, A.; Slininger, P.J.; Lewis Liu, Z.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisae. Biotechnol. Biofuels 2010, 2, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Ye, L.; Hudari, M. S. B.; Zhou, X.; Zhang, D.; Li, Z.; Wu, J. C. Conversion of acid hydrolysate of oil palm empty fruit bunch to L-lactic acid by newly isolated Bacillus coagulans JI12. App. Microbiol. Biotechnol. 2013, 97, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Trček, J.; Mira, N. P.; Jarboe, L. R. Adaptation and tolerance of bacteria against acetic acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive fermentation of lactic acid in lactic acid bacteria cultivation: a review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef]
- van der Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of L(+)-lactic acid from pretreated sugarcane bagasse using Bacillus coagulans DSM2314 in a simultaneous saccharification and fermentation strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Han, X.; Gao, C.; Ma, C.; Tao, F.; Xu, P. Kinetic characteristics of long-term repeated fed-batch (LtRFb) l-lactic acid fermentation by a Bacillus coagulans strain. Eng. Life Sci. 2020, 20, 562–570. [Google Scholar] [CrossRef]
| Component | Untreated | 195°C, 7.5 min | 195°C, 15 min | |||
| Content | Recovery | Content | Recovery | |||
| Glucans1 | 37.8 ± 0.7 | 54.7 ± 2.4 | 89.7 ± 1.8 | 55.5 ± 0.7 | 82.5 ± 1.1 | |
| Xylans2 | 21.0 ± 0.8 | 3.0 ± 0.4 | 5.9 ± 0.1 | 1.2 ± 0.1 | 3.8 ± 0.1 | |
| Arabinosyl residues2 | 1.3 ± 0.3 | bdl3 | - | - | - | |
| Acetyl groups2 | 2.6 ± 0.1 | bdl3 | - | bdl3 | - | |
| Hexoses identified as 5-HMF4 | 1.1 ± 0.2 | bdl3 | - | bdl3 | - | |
| Pentoses identified as furfural4 | 0.6 ± 0.1 | bdl3 | - | bdl3 | - | |
| Total lignin5 | 22.8 ± 0.7 | 30.5 ± 0.8 | 92.2 ± 1.9 | 31.2 ± 0.2 | 96.6 ± 0.3 | |
| Acid-soluble lignin | 5.0 ± 0.1 | 5.7 ± 0.2 | 70.4 ± 1.5 | 5.9 ± 0.2 | 78.5 ± 0.2 | |
| Acid-insoluble lignin | 19.1 ± 0.6 | 27.8 ± 0.8 | 96.3 ± 0.9 | 28.9 ± 0.1 | 101.4 ± 0.3 | |
| Ash | 4.0 ± 0.1 | 6.1 ± 1.5 | 93.7 ± 1.9 | 6.8 ± 1.1 | 113.8 ± 0.3 | |
| Total | 98.2 | 94.3 | 94.7 | |||
| Component (g·L-1) | SEB7.51 | SEB151 | |||
| Untreated | Detoxified | Untreated | Detoxified | ||
| Glucose | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | |
| Xylose | 11.2 ± 0.2 | 11.1 ± 0.2 | 4.3 ± 0.1 | 4.3 ± 0.2 | |
| Arabinose | 0.6 ± 0.1 | 0.60 ± 0.04 | 0.5 ± 0.1 | 0.4 ± 0.1 | |
| HMF2 | 1.6 ± 0.2 | 0.02 ± 0.02 | 1.6 ± 0.1 | 0.03 ± 0.01 | |
| Furfural2 | 0.8 ± 0.1 | 0.04 ± 0.02 | 1.4 ± 0.3 | 0.03 ± 0.01 | |
| Acetic acid3 | 9.7 ± 0.3 | 4.4 ± 0.5 | 12.1 ± 0.5 | 5.2 ± 0.2 | |
| Substrate | Equation | R2 |
| SEB7.5-WW | 0.9986 | |
| SEB15-WW | 0.9982 | |
| SEB7.5-UW | 0.9969 | |
| SEB15-UW | 0.9998 |
| Substrate | Conditions | Degrees of freedom | RSR* | Adjusted R2 | F-value | p-value |
| SEB-WW | 195°C, 7.5 min | 10 | 0.5219 | 0.9986 | 1702 | 1.459 x 10-14 |
| 195°C, 15 min | 10 | 0.8164 | 0.9969 | 779 | 7.208 x 10-13 | |
| SEB-UW | 195°C, 7.5 min | 10 | 0.9194 | 0.9982 | 1337 | 4.867 x 10-14 |
| 195°C, 15 min | 10 | 0.9317 | 0.9980 | 1207 | 8.156 x 10-14 |
| Parameter | SEB-WW1 | SEB-UW2 | |||
| CTec3 | CTec3/HTec3 | CTec3 | CTec3/HTec3 | ||
| Steam explosion at 195 ºC for 7.5 min | |||||
| Glucose concentration (g·L-1) | 46.17 ± 0.13 | 59.65 ± 0.33 | 45.61 ± 0.30 | 59.50 ± 0.37 | |
| Cellobiose concentration (g·L-1) | 1.50 ± 0.12 | 2.52 ± 0.04 | 1.53 ± 0.12 | 2.25 ± 0.16 | |
| Glucan conversion (%) | 39.26 ± 0.07 | 51.23 ± 0.19 | 38.27 ± 0.17 | 50.15 ± 0.22 | |
| Xylose concentration (g·L-1) | 2.59 ± 0.15 | 3.00 ± 0.09 | 6.24 ± 0.12 | 6.89 ± 0.11 | |
| Xylan conversion | 40.52 ± 0.32 | 46.9 ± 0.19 | 97.5 ± 0.16 | 97.7 ± 0.25 | |
| Steam explosion at 195 ºC for 15 min | |||||
| Glucose concentration (g·L-1) | 78.25 ± 0.28 | 84.52 ± 0.31 | 63.72 ± 0.13 | 75.14 ± 0.11 | |
| Cellobiose concentration (g·L-1) | 3.09 ± 0.12 | 3.40 ± 0.06 | 1.51 ± 0.11 | 2.63 ± 0.12 | |
| Glucan conversion (%) | 67.02 ± 0.16 | 74.44 ± 0.20 | 52.93 ± 0.08 | 63.15 ± 0.10 | |
| Xylose concentration (g·L-1) | 1.78 ± 0.09 | 2.16 ± 0.12 | 2.88 ± 0.07 | 3.30 ± 0.02 | |
| Xylan conversion | 27.8 ± 0.17 | 33.7 ± 0.11 | 45.0 ± 0.31 | 51.6 ± 0.47 | |
| Parameter | SEB7.5 liquor | SEB15 liquor | |||
| Non-detoxified | Detoxified | Non-detoxified | Detoxified | ||
| Initial Xyl (g·L-1)1 | 11.2 ± 0.2 | 11.1 ± 0.2 | 4.3 ± 0.2 | 4.3 ± 0.1 | |
| Initial Glc (g·L-1)1 | 2.2 ± 0.2 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.2 | |
| Initial Ara (g·L-1)1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.4 ± 0.1 | |
| Lactic acid (g·L-1)2 | 2.3 ± 0.6 | 13.4 ± 0.5 | 1.7 ± 0.4 | 6.6 ± 0.6 | |
| Yp/s (g·g-1)3 | 0.17 ± 0.03 | 0.96 ± 0.02 | 0.25 ± 0.10 | 0.97 ± 0.12 | |
| Yx/s (g·g-1)4 | 0.19 ± 0.03 | 0.16 ± 0.04 | 0.09 ± 0.05 | 0.11 ± 0.07 | |
| PLA (g·L-1·h-1)5 | < 0.01 | 0.54 ± 0.02 | < 0.01 | 0.24 ± 0.03 | |
| OD6006 | 1.0 ± 0.3 | 5.9 ± 0.1 | 1.6 ± 0.4 | 3.8 ± 0.6 | |
| Parameter | SEB7.5-WW | SEB7.5-UW | SEB15-WW | SEB15-UW |
| Initial Xyl (g·L-1)1 | 2.1 ± 0.1 | 6.4 ± 0.3 | 0.9 ± 0.7 | 4.9 ± 0.9 |
| Initial Glc (g·L-1)1 | 71.0 ± 0.4 | 59.8 ± 0.3 | 93.0 ± 1.8 | 89.9 ± 1.6 |
| Residual Xyl (g·L-1)1 | 0.2 ± 0.1 | 2.1 ± 0.3 | 0.13 ± 0.07 | 2.4 ± 0.8 |
| Residual Glc (g·L-1)1 | 17.1 ± 1.2 | 14.3 ± 1.6 | 34.6 ± 1.7 | 42.0 ± 1.1 |
| Lactic acid (g·L-1)2 | 64.2 ± 1.3 | 48.8 ± 0.5 | 56.9 ± 1.2 | 52.4 ± 0.8 |
| Yp/s (g·g-1)3 | 0.88 ± 0.01 | 0.74 ± 0.01 | 0.61 ± 0.01 | 0.55 ± 0.02 |
| Yx/s (g·g-1)4 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.3 |
| PLA (g·L-1·h-1)5 | 2.68 ± 0.08 | 2.03 ± 0.03 | 2.37 ± 0.07 | 2.19 ± 0.05 |
| OD6006 | 14.6 ± 0.8 | 15.3 ± 0.9 | 14.1 ± 0.3 | 15.9 ± 0.4 |
| Parameter | SEB7.5WW hydrolysates containing: | SEB15WW hydrolysates containing: | |||
| Non-detoxified C5 | Detoxified C5 | Non-detoxified C5 | Detoxified C5 | ||
| Initial Xyl (g·L-1)1 | 6.9 ± 0.7 | 6.4 ± 0.3 | 8.9 ± 0.9 | 5.5 ± 0.6 | |
| Initial Glc (g·L-1)1 | 54.4 ± 0.9 | 59.8 ± 0.3 | 87.2 ± 0.9 | 91.0 ± 1.4 | |
| Residual Xyl (g·L-1)1 | 4.4 ± 0.9 | 1.1 ± 0.5 | 6.2 ± 0.6 | 1.2 ± 0.3 | |
| Residual Glc (g·L-1)1 | 51.9 ± 0.3 | 3.8 ± 0.7 | 85.7 ± 0.4 | 26.4 ± 0.2 | |
| Lactic acid (g·L-1)2 | 4.6 ± 0.3 | 61.4 ± 1.8 | 4.1 ± 0.5 | 76.7 ± 1.4 | |
| Yp/s (g·g-1)3 | 0.07 ± 0.02 | 0.93 ± 0.02 | 0.04 ± 0.02 | 0.78 ± 0.02 | |
| Yx/s (g·g-1)4 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.01 | |
| PLA (g·L-1·h-1)5 | 0.19 ± 0.06 | 2.55 ± 0.11 | 0.17 ± 0.03 | 3.20 ± 0.08 | |
| OD6006 | 3.7 ± 0.2 | 16.2 ± 0.4 | 3.3 ± 0.2 | 16.0 ± 0.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





