Submitted:
14 July 2023
Posted:
24 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Biochemical Characteristics of the Microalgae
2.2. Cytotoxic Potential of the Microalgae Extracts
2.3. Antibacterial activity of microalgae extracts
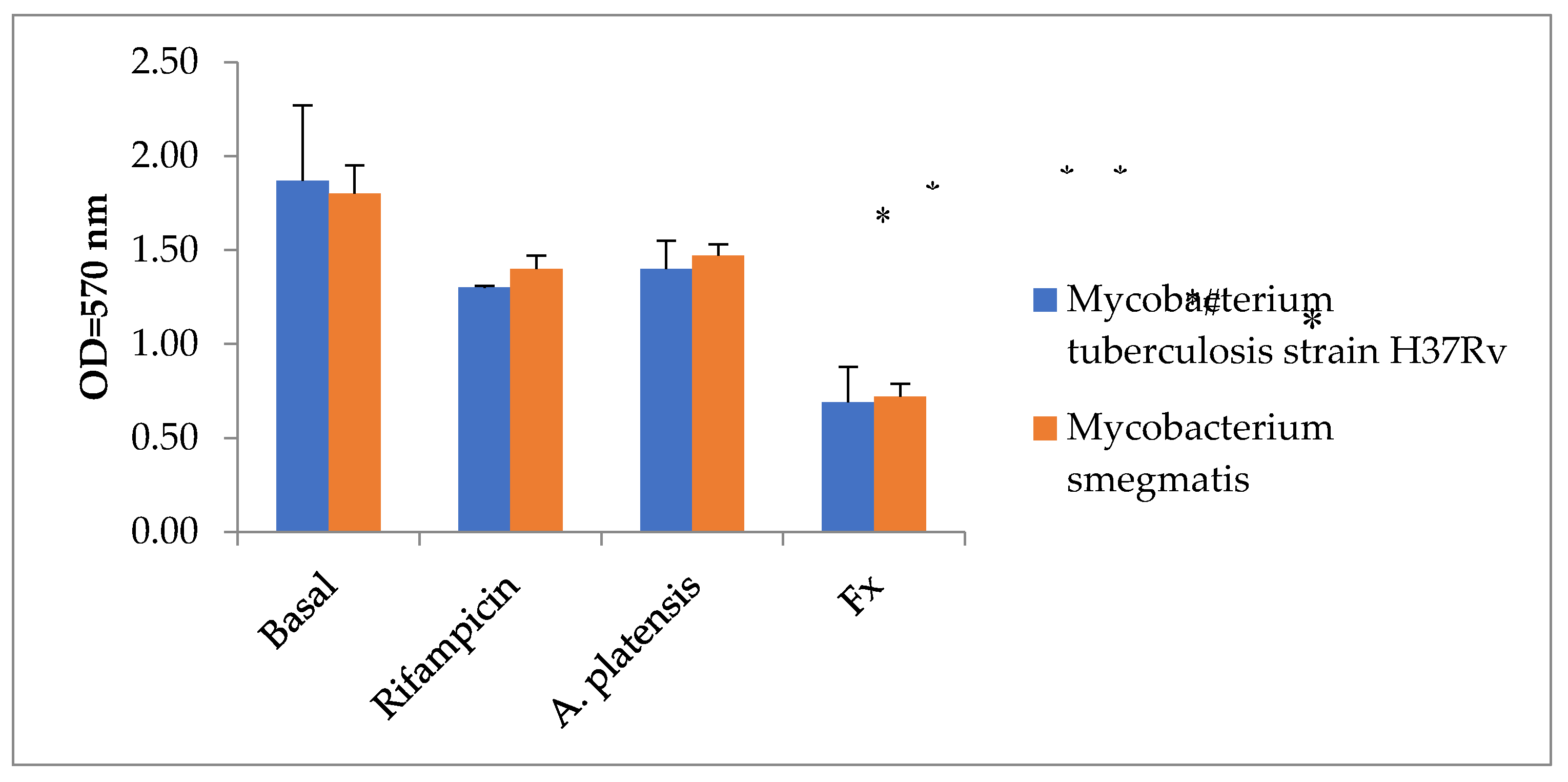
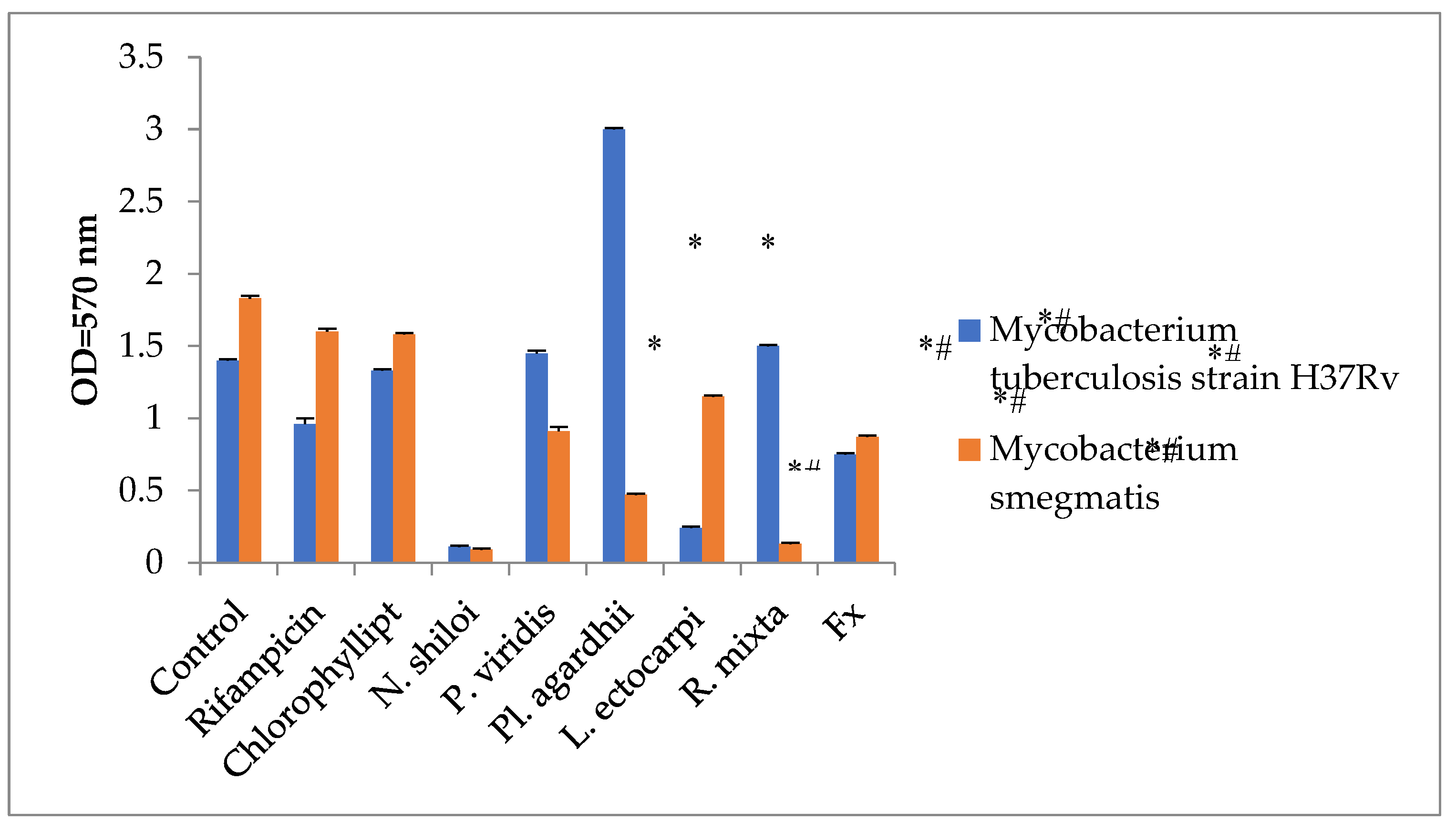
2.4. Antimycotic Potential of Microalgae
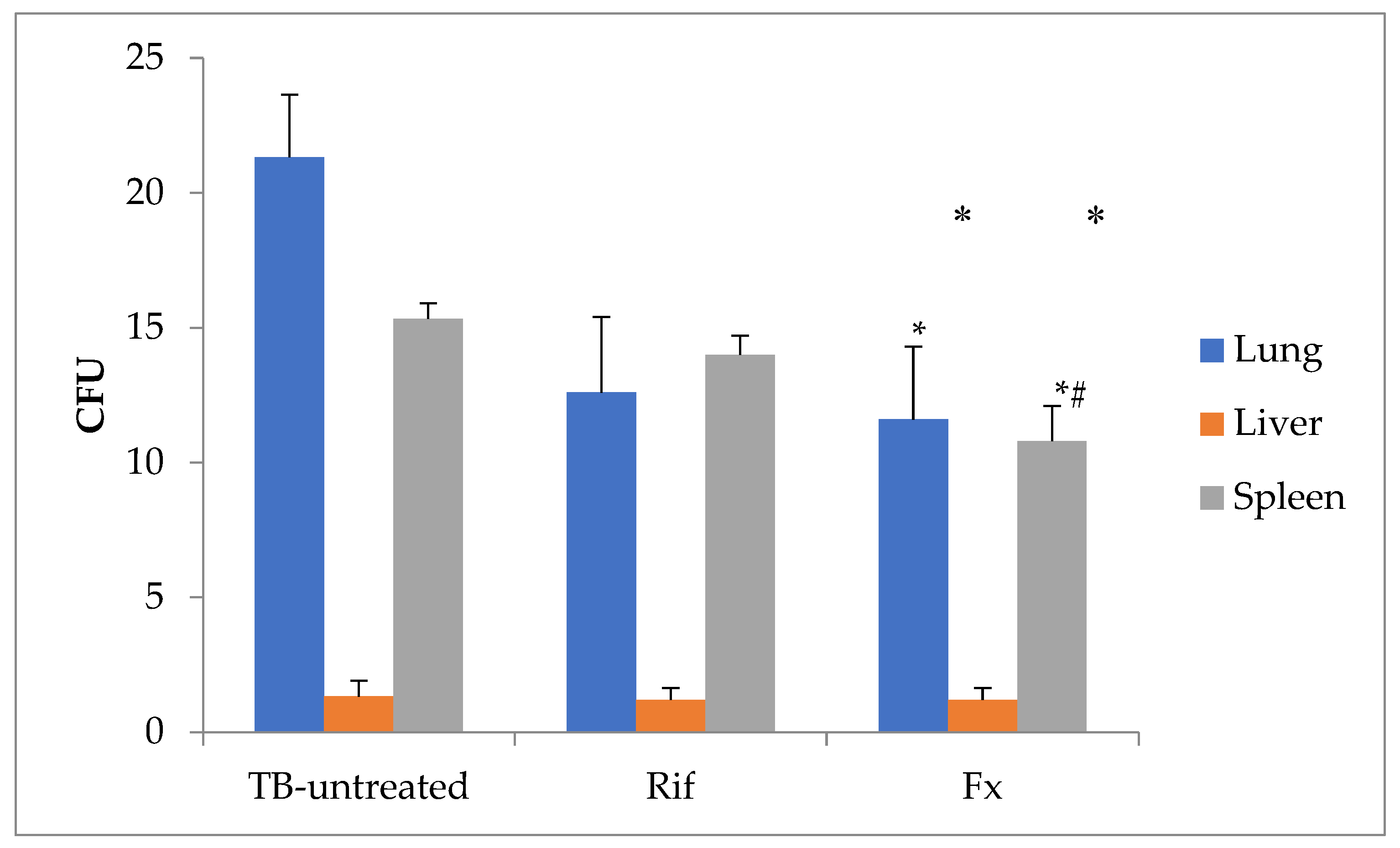
2.5. Effect of the Fucoxanthin Administration in Mice with Tuberculosis
3. Discussion
4. Materials and Methods
4.1. Microalgae
4.2. Chemical Analysis
4.3. Fucoxanthin Extraction
4.4. Microalgae extracts
4.5. Cytotoxicity Assay
4.6. Antimicrobial Assay
4.7. Mice Model of Tuberculosis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464. [Google Scholar] [CrossRef]
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Šantek, M.I. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterization of Biomass, Pigment, Lipid and Fatty Acid Composition, and Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef] [PubMed]
- Tolpeznikaite, E.; Bartkevics, V.; Ruzauskas, M.; Pilkaityte, R.; Viskelis, P.; Urbonaviciene, D.; Zavistanaviciute, P.; Zokaityte, E.; Ruibys, R.; Bartkiene, E. Characterization of Macro- and Microalgae Extracts Bioactive Compounds and Micro- and Macroelements Transition from Algae to Extract. Foods 2021, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; Gutiérrez-Del-Río, I.; López, Y.; Redondo-Blanco, S.; Gabasa, Y.; Iglesias, M.J.; Soengas, R.; Fernández-Lorenzo, A.; López-Ibáñez, S.; Villar, C.J.; et al. Microalgae and Cyanobacteria Strains as Producers of Lipids with Antibacterial and Antibiofilm Activity. Mar. Drugs 2021, 19, 675. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Zheleva-Dimitrova, D.; Rusinova-Videva, S.; Ilieva, Y.; Brachkova, A.; Balabanova, V.; Gevrenova, R.; Kim, T.C.; Kaleva, M.; Georgieva, A.; et al. Antimicrobial and Antioxidant Potential of Scenedesmus obliquus Microalgae in the Context of Integral Biorefinery Concept. Molecules 2022, 27, 519. [Google Scholar] [CrossRef]
- Kumar, T.; Josephine, A.; Sreelatha, T.; Dusthackeer, V.A.; Mahizhaveni, B.; Dharani, G.; Kirubagaran, R.; Kumar, S.R. Fatty acids-carotenoid complex: An effective anti-TB agent from the chlorella growth factor-extracted spent biomass of Chlorella vulgaris. J. Ethnopharmacol. 2019, 249, 112392. [Google Scholar] [CrossRef]
- Lauritano, C.; Martín, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First identification of marine diatoms with anti-tuberculosis activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef]
- Ramos, D.F.; Halicki, P.C.B.; Caprara, C.d.S.C.; Borges, P.; M.D'Oca, C.d.R.; Santos, M.d.F.C.; D'Oca, M.G.M.; Roselet, F.; da Silva, P.E.A.; Abreu, P.C. Chemical Profile and Antimicrobial Activity of the Marine Diatom Chaetoceros muelleri. Chem. Biodivers. 2022, 19, e202100846. [CrossRef]
- Viazau, Y.V.; Manankina, E.E.; Filipchik, E.A.; Goncharik, R.G.; Shalygo, N.V. Effectivness of repeated usage of the modified Zarrouk culture medium for cultivation of Spirulina platensis. Proc. Nat. Acad. Sci. Belarus. Biological series, 2018, 63(4), 426-436. [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria Secondary Metabolites as Biotechnological Ingredients in Natural Anti-Aging Cosmetics: Potential to Overcome Hyperpigmentation, Loss of Skin Density and UV Radiation-Deleterious Effects. Mar. Drugs 2022, 20, 183. [Google Scholar] [CrossRef]
- Vahdati, S.N.; Behboudi, H.; Tavakoli, S.; Aminian, F.; Ranjbar, R. Antimicrobial Potential of the Green Microalgae Isolated from the Persian Gulf. Iran. J. Public Heal. 2022, 51, 1134–1142. [Google Scholar] [CrossRef]
- Al Naim, H.M.; El Semary, N. Laser Treatment Increases the Antimicrobial Efficacy of Cyanobacterial Extracts against Staphylococcusaureus (SA) and Methicillin-resistantStaphylococcus aureus (MRSA). Int. J. Environ. Res. Public Heal. 2022, 19, 13305. [Google Scholar] [CrossRef] [PubMed]
- Štěpánková, T.; Ambrožová, L.; Bláha, L.; Giesy, J.; Hilscherová, K. In vitro modulation of intracellular receptor signaling and cytotoxicity induced by extracts of cyanobacteria, complex water blooms and their fractions. Aquat. Toxicol. 2011, 105, 497–507. [Google Scholar] [CrossRef]
- Abdullin, S.R.; Nikulin, V.Y.; Nikulin, A.Y.; Manyakhin, A.Y.; Bagmet, V.B.; Suprun, A.R.; Gontcharov, A.A. Roholtiella mixta sp. nov. (Nostocales, Cyanobacteria): morphology, molecular phylogeny, and carotenoid content. Phycologia 2021, 60, 73–82. [Google Scholar] [CrossRef]
- Erdoğan, A.; Karataş, A.B.; Demir, D.; Demirel, Z.; Aktürk, M.; Çopur. ; Conk-Dalay, M. Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography. Molecules 2023, 28, 1988. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, F.; Wong, G.; Román, T.; Cárdenas, C.; Alvárez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of Antimicrobial Peptides from the Microalgae Tetraselmis suecica (Kylin) Butcher and Bactericidal Activity Improvement. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef]
- Han, S.; Zhao, J.; Liu, P.; Wang, K.; Qin, S.; Zhao, Z.; Cui, Y. Two Foreign Antimicrobial Peptides Expressed in the Chloroplast of Porphyridium purpureum Possessed Antibacterial Properties. Mar. Drugs 2022, 20, 484. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Rivas, L.; Cárdenas, C.; Guzmán, F. Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules 2020, 25, 5804. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem. 2017, 235, 34–44. [Google Scholar] [CrossRef]
- Shaima, A.F.; Yasin, N.H.M.; Ibrahim, N.; Takriff, M.S.; Gunasekaran, D.; Ismaeel, M.Y. Unveiling antimicrobial activity of microalgae Chlorella sorokiniana (UKM2), Chlorella sp. (UKM8) and Scenedesmus sp. (UKM9). Saudi J. Biol. Sci. 2022, 29, 1043–1052. [Google Scholar] [CrossRef]
- de Macedo, D.M.; de Barbosa, C.Y.; Pedrosa Brandão, R.M.; das Graças, M.; Gálvez, A.O.; Souza Bezerra, R. Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci. Technol. Int. 2019, 25, 318–326. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Bioprospecting for bioactive compounds in microalgae: Antimicrobial compounds. Biotechnol. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Vornoli, A.; Grande, T.; Lubrano, V.; Vizzarri, F.; Gorelli, C.; Raffaelli, A.; Della Croce, C.M.; Baca, S.Z.; Sandoval, C.; Longo, V.; et al. In Vitro Characterization of Antioxidant, Antibacterial and Antimutagenic Activities of the Green Microalga Ettlia pseudoalveolaris. Antioxidants 2023, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.E.; Murphy, E.; Parkes, R.; Fleming, G.T.A.; Campanile, F.; Thomas, O.P.; Touzet, N. Antibacterial Activity and Amphidinol Profiling of the Marine Dinoflagellate Amphidinium carterae (Subclade III). Int. J. Mol. Sci. 2021, 22, 12196. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Prosekov, A.; Ivanova, S.; Maslennikov, P.; Andreeva, A.; Budenkova, E.; Kashirskikh, E.; Tcibulnikova, A.; Zemliakova, E.; Samusev, I.; et al. Identification of Metabolites with Antibacterial Activities by Analyzing the FTIR Spectra of Microalgae. Life 2022, 12, 1395. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Andreeva, A.; Sukhikh, S.; Kashirskikh, E.; Prosekov, A.; Ivanova, S.; Michaud, P.; Babich, O. Study of the Physicochemical and Biological Properties of the Lipid Complex of Marine Microalgae Isolated from the Coastal Areas of the Eastern Water Area of the Baltic Sea. Molecules 2022, 27, 5871. [Google Scholar] [CrossRef]
- Šudomová, M.; Shariati, M.A.; Echeverría, J.; Berindan-Neagoe, I.; Nabavi, S.M.; Hassan, S.T.S. A Microbiological, Toxicological, and Biochemical Study of the Effects of Fucoxanthin, a Marine Carotenoid, on Mycobacterium tuberculosis and the Enzymes Implicated in Its Cell Wall: A Link Between Mycobacterial Infection and Autoimmune Diseases. Mar. Drugs 2019, 17, 641. [Google Scholar] [CrossRef]
- Liu, B.; Eltanahy, E.E.; Liu, H.; Chua, E.T.; Thomas-Hall, S.R.; Wass, T.J.; Pan, K.; Schenk, P.M. Growth-promoting bacteria double eicosapentaenoic acid yield in microalgae. Bioresour. Technol. 2020, 316, 123916. [Google Scholar] [CrossRef]
- Dao, G.; Wang, S.; Wang, X.; Chen, Z.; Wu, Y.; Wu, G.; Lu, Y.; Liu, S.; Hu, H. Enhanced Scenedesmus sp. growth in response to gibberellin secretion by symbiotic bacteria. Sci. Total. Environ. 2020, 740, 140099. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Aburai, N.; Fujii, K. Microalga–bacteria Community with High Level Carbon Dioxide Acclimation and Nitrogen-fixing Ability. Protist 2023, 174. [Google Scholar] [CrossRef]
- Astafyeva, Y.; Gurschke, M.; Streit, W.R.; Krohn, I. Interplay between the microalgae Micrasterias radians and its symbiont Dyadobacter sp. HH091. Front. Microbiol. 2022, 13, 1006609. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à l'étude d'une cyanophycée. Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthèse de Spirulina maxima (Setch et Gardner) Geitler: Ph.D thesis. - Paris, 1966. - 114 p. - (A la faculté des sciences de l'université de Paris).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. j. biochem. Physiol., 1959, 37(8), 911-917.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ryabushko, V.I.; Zheleznova, S.N.; Nekhoroshev, M.V. Effect of Nitrogen on Fucoxanthin Accumulation in the Diatom Cylindrotheca closterium (Ehrenb.) Reimann et Lewin. Int. J. Algae 2017, 19, 79–84. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
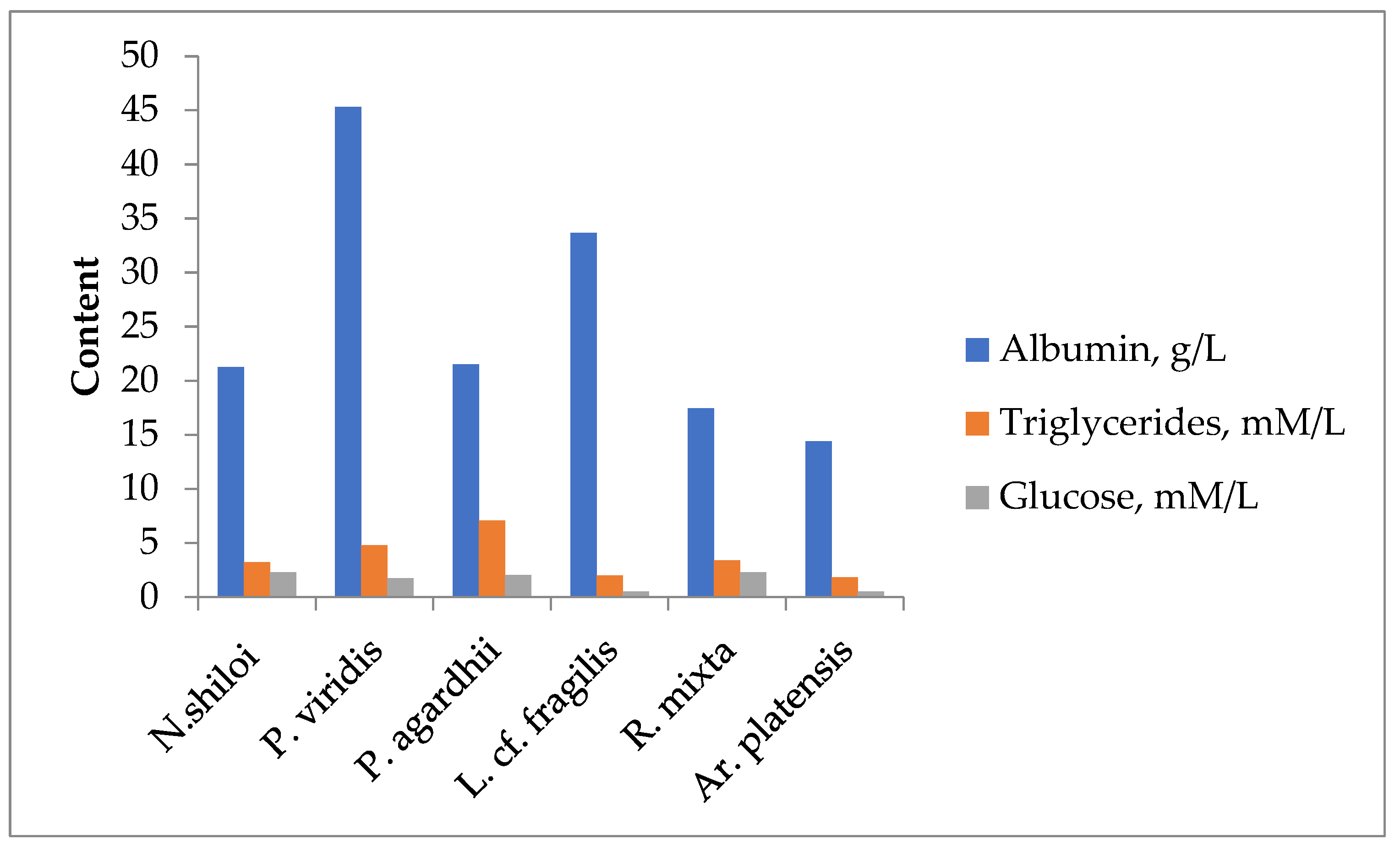

| Content, mg/g of Dry Biomass | L. cf. ectocarpi | N. shiloi | R. mixta sp. Nov. | P. agardhii | P. viridis |
|---|---|---|---|---|---|
| Proteins | 560±5 | 450±5 | 680±10 | 600±10 | 350±10 |
| Lipids | 250±5 | 280±5 | 50±5 | 100±5 | 195±6 |
| Carbohydrates | 50±3 | 70±3 | 110±5 | 180±5 | 340±5 |
| Fx | ND | 15 ±0.5 | ND | ND | ND |
| С-phycoerythrin | 78.8 ± 0.8 | ND | 35 ± 0.,8 | 20 ± 1.2 | ND |
| С-phycocyanin | 22.0 ±0.8 | ND | 140±1 | 88.8 ± 1 | ND |
| PUFA, include: | 50±1.2 | 67±1.25 | 10 ±1.,1 | 11±1.2 | 70±1.25 |
| Eicosapentaenoic acid (C20:5 ω3) | ND | 47.3±0.61 | ND | ND | 4.8±0.7 |
| Arachidonic acid (C20:4ω6) | ND | 8.5 ±0.31 | ND | ND | 0.6±0.6 |
| γ-Linolenic acid (C18:3 n-6) | 18± 0.04 | 0.22± 0.01 | 2.75 ± 0.02 | 1.5 ± 0.01 | 10.7 ± 0.05 |
| Linoleic acid (С18:2 n-6) | 25 ± 0.05 | 1.455±0.04 | 3.16 ± 0.02 | 2.1 ± 0.01 | 2.5 ± 0.02 |
| Oleic acid (C18:1 (n-9) | 0.1± 0.02 | 1.46 ±0.01 | ND | 2 ±0.01 | 0,1± 0,02 |
| α-Linolenic acid (C18:3 n-3) | 10,0 ± 0,05 | ND | ND | ND | 6,5 ± 0,05 |
| Parameters | Kl. pneumoniae (s) | Kl. pneumoniae (r) | S. aureus MSSA | S. aureus MRSA | S. aureus clinical isolate | A. baumannii (r) | E. faecalis (r) | Ps. aeruginosa (s) | Ps. aeruginosa (r) | S. pyogenes(r) |
|---|---|---|---|---|---|---|---|---|---|---|
| On Day 14 | ||||||||||
| Control | 1.18±0.08 | 1.75±0.2 | 1.1±0.09 | 1.47±0.08 | 1.46±0.16 | 0.98±0.17 | 1.08±0.09 | 1.94±0.33 | 1.37±0.33 | 1.88±0.32 |
| Cef | 0.82±0.08* | 1.24±0.04* | 1.03±0.14 | 1.32±0.1* | 1.49±0.13 | 0.7±0.1* | 2.1±0.32 | 1.29±0.03* | 1.3±0.03 | 1.53±0.27 |
| A. platensis | 0.6±0.17* | 0.8±0.05*# | 0.83±0.01*# | 0.96±0.11*# | 0.96±0.04*# | 0.68±0.11* | 1.15±0.13# | 0.49±0.15*# | 0.67±0.03*# | 1.3±0.07* |
| Fx | 0.83±0.1* | 0.66±0.05*# | 0.72±0.08*# | 0.46±0.07*# | 1.16±0.05*# | 0.68±0.14 | 2±0.26 | 0.5±0.06*# | 0.66±0.07*# | 2.07±0.29# |
| On Day 21 | ||||||||||
| Control | 2.52±0.9 | 3.66±0.39 | 4±1 | 4±0.9 | 4±1 | 3.09±1 | 4±1 | 4±0.8 | 4±1 | 4±0.9 |
| Cef | 2.15±0.9 | 3.53±0.54 | 2.07±0.45* | 3.28±0.96 | 1.23±1.03* | 4±0.9 | 4±0.8 | 3.75±0.51 | 3.23±0.97 | 2.39±1.67 |
| A. platensis | 0.54±0.24*# | 2.33±0.1*# | 1.98±0.43* | 2.07±0.51* | 2±0.65* | 0.85±0.29*# | 2.01±0.65*# | 1.99±0.69*# | 1.89±0.79* | 2±0.65* |
| Fx | 1.11±0.03*# | 2.75±0.2*# | 2.26±0.62* | 2.16±0.92 | 2.68±0.34# | 1.06±0.01*# | 2.6±0.34*# | 2.85±0.2*# | 2.89±0.1* | 2.2±0.5* |
| Parameters | N. shiloi | P. viridis | P. agardhii | L. ectoarpi | R. mixta | Fx |
|---|---|---|---|---|---|---|
| Kl. pneumoniasensitive [Control = 1.38±0.01, Ceftazidime = 0.92±0.01] | ||||||
| 1% v/v | 1.79±0.22 | 1.7±0.04 | 2.18±0.01 | 2.06±0.04 | 2.05±0.04 | 1.4±0.01 |
| 0.5% v/v | 2.11±0.08 | 2.01±0.02 | 2.33±0.02 | 1.91±0.07 | 1,74±0.05 | 1.35±0.01 |
| 0.25% v/v | 1.72±0.01 | 1.75±0.01 | 2.11±0.08 | 1.8±0.07 | 1.29±0.01* | 1.18±0.01* |
| Kl. pneumonia(ESBL) [Control = 1.83±0.02, Ceftazidime = 1.72±0.01] | ||||||
| 1% v/v | 1.3±0.01*# | 1.76±0.01* | 1.7±0.01 | 1.46±0.01 | 1.36±0.02*# | 1.18±0.01*# |
| 0.5% v/v | 1.19±0.01*# | 1.68±0. 01* | 1.68±0.01* | 1.24±0.02*# | 1.23±0.01*# | 0.83±0.01*# |
| 0.25% v/v | 1.22±0.01*# | 1.47±0.01*# | 1.1±0.01*# | 1.04±0.03*# | 0.97±0.01*# | 1.19±0.01*# |
| S. aureusMSSA [Control = 1.92±0.04, Ceftazidime = 0.43±0.01] | ||||||
| 1% v/v | 1.45±0.01* | 1.19±0.01* | 2±0.01 | 1.53±0.01* | 1.47±0.01* | 1.61±0.01* |
| 0.5% v/v | 1.08±0.01* | 1.27±0.01* | 1.47±0.01* | 1.69±0.0* | 1.49±0.01* | 1.53±0.01* |
| 0.25% v/v | 1.34±0.01* | 1.43±0.01* | 1.51±0.01* | 1.38±0.01* | 2.01±0.01 | 1.19±0.01* |
| S. aureusMRSA [Control = 2.11±0.07, Ceftazidime = 2.34±0.01] | ||||||
| 1% v/v | 1.52±0.03*# | 1.41±0.01*# | 2.01±0.02 | 1.92±0.1*# | 2.16±0.03 | 1.35±0.01*# |
| 0.5% v/v | 1.58±0.01*# | 1.08±0.01*# | 1.92±0.01*# | 1.8±0.01*# | 1.83±0.01*# | 1.25±0.01*# |
| 0.25% v/v | 1.59±0.03*# | 1.46±0.01*# | 1.31±0.01*# | 1.58±0.01*# | 1.98±0.01*# | 0.55±0.01*# |
| A. baumanniiresistant [Control = 2.09±0.03, Ceftazidime = 0.92±0.01] | ||||||
| 1% v/v | 2.92±0.01 | 2.39±0.02 | 3.62±0.08 | 2.28±0.01 | 2.23±0.01 | 1.43±0.02* |
| 0.5% v/v | 2.75±0.01 | 2.52±0.12 | 2.68±0.02 | 2.33±0.04 | 2.02±0.18 | 1.18±0.24* |
| 0.25% v/v | 2.15±0.01 | 2.53±0.06 | 2.64±0.05 | 2.18±0.01 | 2.03±0.01 | 0.9±0.1* |
| E. faecalis(VRE) [Control = 2.61±0.03, Ceftazidime = 0.92±0.03] | ||||||
| 1% v/v | 1.67±0.01* | 1.54±0.01* | 2.11±0.01* | 2.16±0.02* | 2.46±0.02* | 1.08±0.01* |
| 0.5% v/v | 1.64±0.01* | 1.82±0.01* | 2±0.01* | 2.18±0.01* | 2.4±0.01* | 1.01±0.01* |
| 0.25% v/v | 1.57±0.01* | 1.56±0. 01* | 1.38±0.01* | 1.69±0.03* | 1.61±0.01* | 0.59±0.01*# |
| Ps. aeruginosasensitive [Control = 1.77±0.01, Ceftazidime =1.4±0.05] | ||||||
| 1% v/v | 2.8±0.04 | 1.65±0.01* | 2.83±0.02 | 1.84±0.01 | 1.55±0.01* | 0.78±0,01*# |
| 0.5% v/v | 1.72±0.01 | 1.45±0.01* | 1.81±0.01 | 1.68±0.01 | 1.55±0.01* | 0.55±0.01*# |
| 0.25% v/v | 1.5±0.01 | 1.41±0.01* | 1.47±0.01* | 1.36±0.01* | 1.59±0.02* | 0.44±0.01# |
| Ps. aeruginosaresistant [Control = 1.73±0.01, Ceftazidime = 1.25±0.01] | ||||||
| 1% v/v | 2.33±0.02 | 1.35±0.01* | 2.28±0.02 | 1.46±0.01* | 0.92±0.01*# | 0.88±0.01*# |
| 0.5% v/v | 1.9±0.01 | 2.13±0.01 | 1,71±0.01 | 1.36±0.03* | 0.75±0.01*# | 0.7±0.01*# |
| 0.25% v/v | 1.64±0.01* | 1.45±0.02* | 1.63±0.01 | 1.19±0.03* | 0.67±0.01*# | 0.43±0.01*# |
| S. pyogenesresistant [Control = 0.98±0.01, Ceftazidime = 0.99±0.01] | ||||||
| 1% v/v | 0.66±0.01* | 0.84±0.01* | 1.05±0.01 | 0.91±0.01* | 0.99±0.03 | 0.47±0.04*# |
| 0.5% v/v | 0.53±0.06* | 0.79±0.03* | 0.95±0.08 | 0.28±0.02*# | 0.68±0.02*# | 0.54±0.04*# |
| 0.25% v/v | 1.55±0.07 | 0.36±0.05* | 1.14±0.06 | 0.99±0.09 | 0.78±0.08*# | 0.5±0.02*# |
| Parameters | Control | Ceftazidime | Chlorophyllipt | N.shiloi | P. viridis | P. agardhii | L. ectoarpi | R. mixta | Fx |
|---|---|---|---|---|---|---|---|---|---|
| Kl. pneumoniae | 2.27±0.05 | 0.78±0.01 | 0.98±0.01* | 1.85±0.01* | 1.53±0.01* | 1.78±0.01* | 1.93±0.04* | 1.7±0.01* | 0.77±0.01*& |
| S aureus MRSA | 3.02±0.03 | 2.44±0.01 | 0.72±0.0*# | 0.9±0.01*# | 1.2±0.01*# | 1.93±0.02*# | 0.93±0.01*# | 0.9±0.01*# | 0.6±0.01*#& |
| A. baumannii | 3.49±0.03 | 0.92±0.01 | 1.04±0.01* | 1.6±0.02* | 1.88±0.04* | 1.86±0.0* | 2.27±0.02* | 1.53±0.01* | 0.8±0.01*#* |
| E. faecalis | 2.29±0.02 | 0.92±0.01 | 0.57±0.01*# | 1.41±0.01* | 1.78±0.01* | 1.82±0.26* | 2.06±0.01* | 1.7±0.0* | 2.03±0.02* |
| Ps. aeruginosa | 1.08±0.01 | 0.93±0.01 | 0.67±0.01*# | 0.49±0.01*#& | 1.23±0.02 | 2.18±0.03 | 2.54±0.03 | 0.74±0.001*# | 0.66±0.01*# |
| Parameters | N. shiloi | P.s viridis | P. agardhii | L. ectoarpi | R. mixta | Fx |
|---|---|---|---|---|---|---|
| Mycobacterium tuberculosis strain H37Rv [Control = 1.38±0.01, Rifampicin 100 µg/mL = 0.96±0.04] | ||||||
| 1% v/v | 1.74±0.01 | 1.72±0.01 | 1.33±0.01* | 1.09±0.01* | 0.62±0.01*# | 1.18±0.01* |
| 0.5% v/v | 0.97±0.01* | 1.41±0.01 | 1.06±0.01* | 0.89±0.01*# | 0.79±0.0*#1 | 0.73±0.01*# |
| 0.25% v/v | 0.67±0.01*# | 1.18±0.01 | 0.77±0.01*# | 0.57±0.01*# | 1.47±0.01 | 2.06±0.01 |
| Mycobacterium smegmatis [Control = 1.82±0.02, Rifampicin = 1.6 ±0.02] | ||||||
| 1% v/v | 1.91±0.01 | 1.08±0.01*# | 2.76±0.01 | 1.12±0.01*# | 0.99±0.01*# | 1.43±0.01*# |
| 0.5% v/v | 0.96±0.01*# | 2.01±0.01 | 0.93±0.01*# | 1.01±0.01*# | 0.76±0.01*# | 1.13±0.01*# |
| 0.25% v/v | 1.46±0.01*# | 1.94±0.01 | 0.84±0.01*# | 0.54±0.01*# | 1.57±0.01*# | 0.99±0.01*# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





