Submitted:
22 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. The effects of TUDCA during IVC of bovine embryos
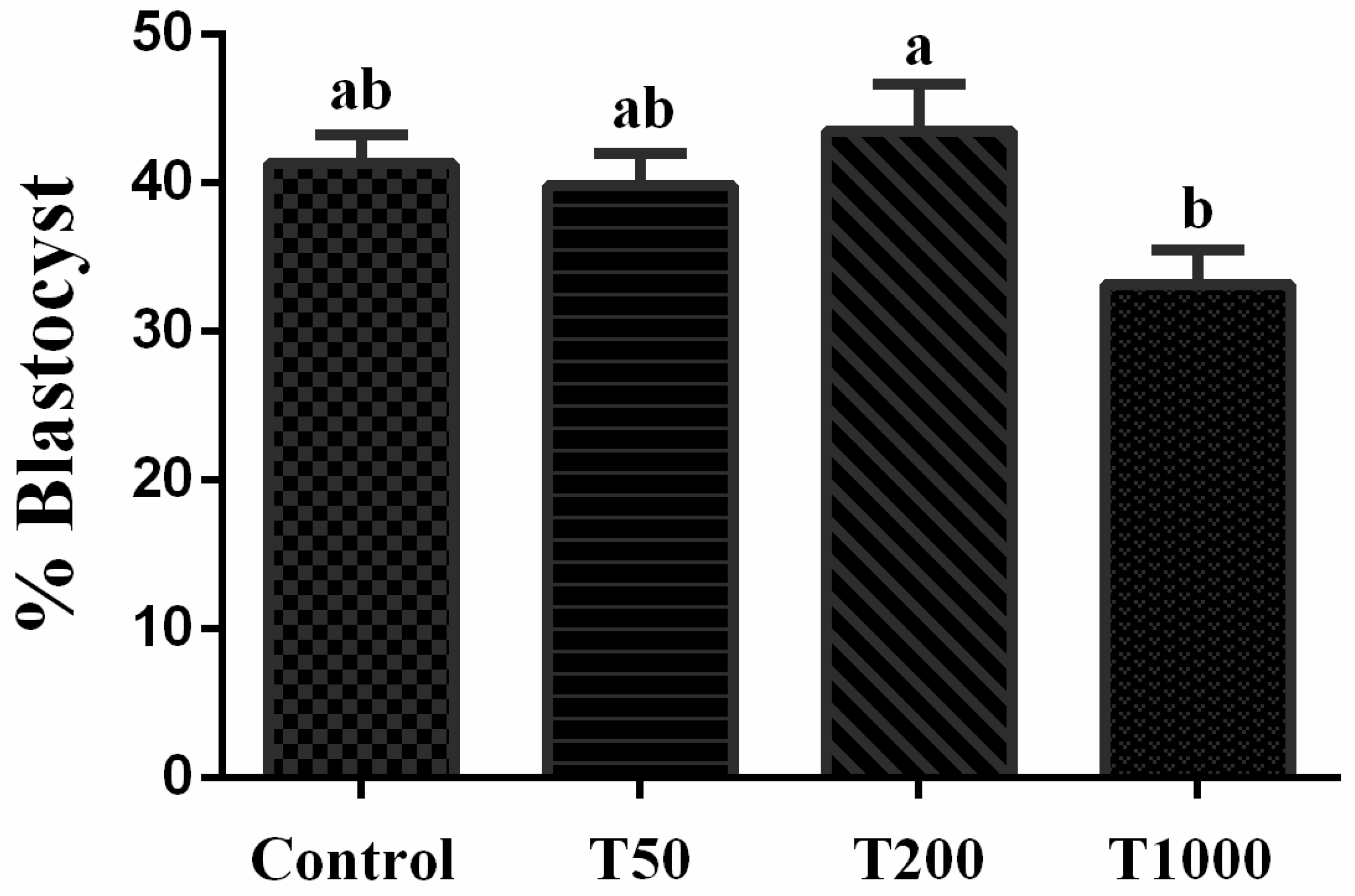
2.1.1. Developmental competence
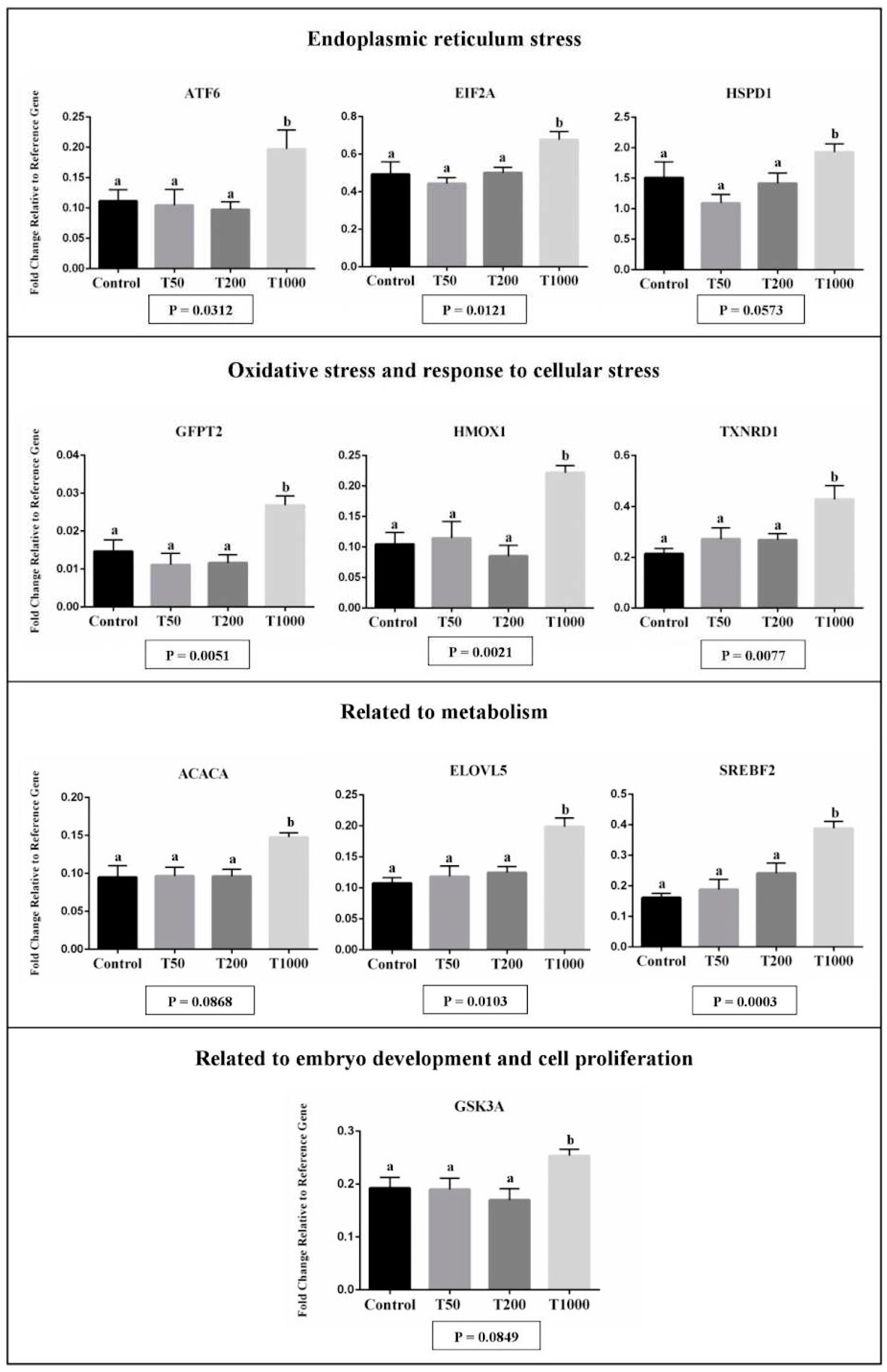
2.1.2. Gene expression
2.2. The effects of TUDCA during IVC on post-warmed vitrified blastocysts
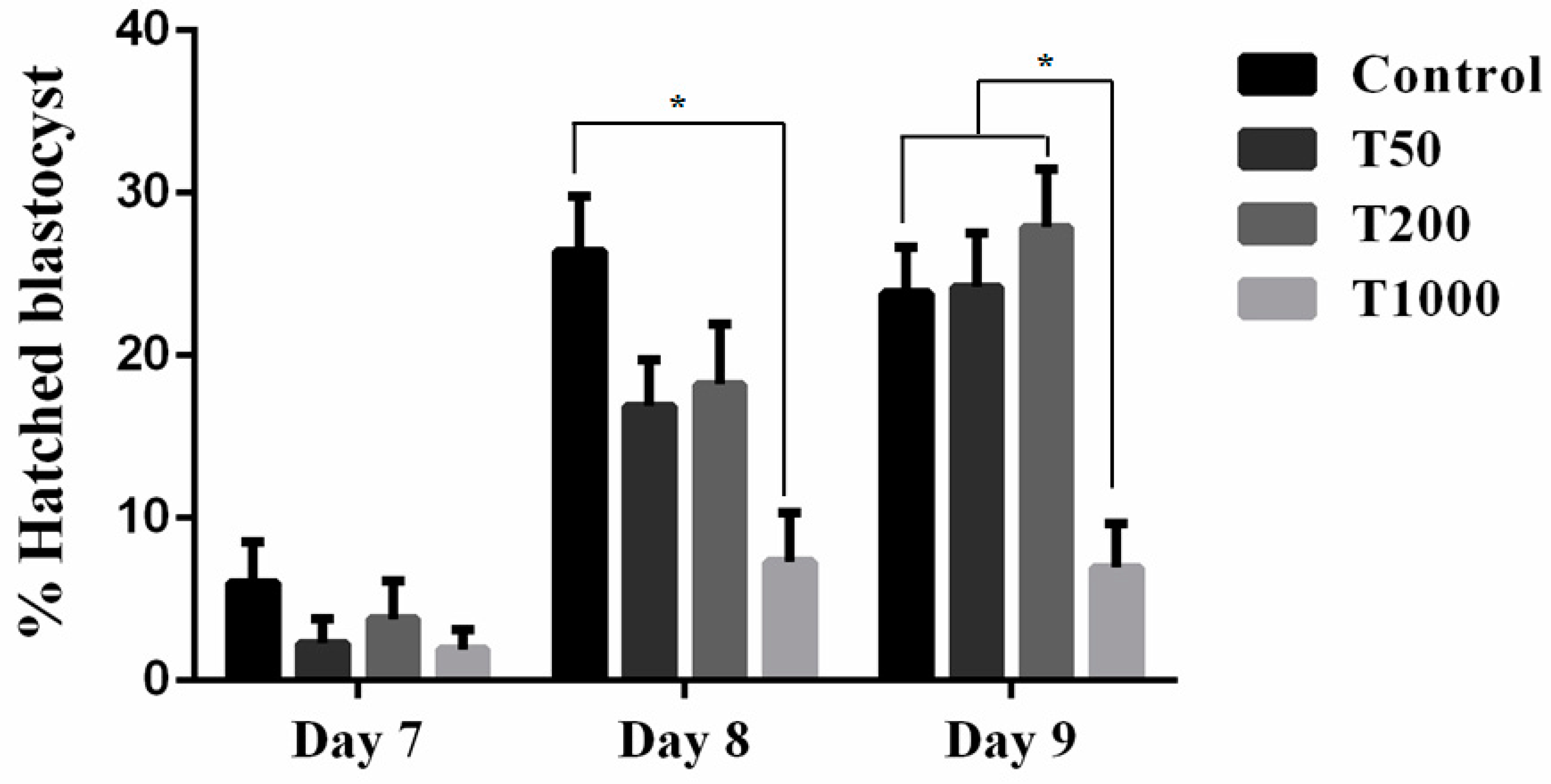
2.2.1. Developmental competence
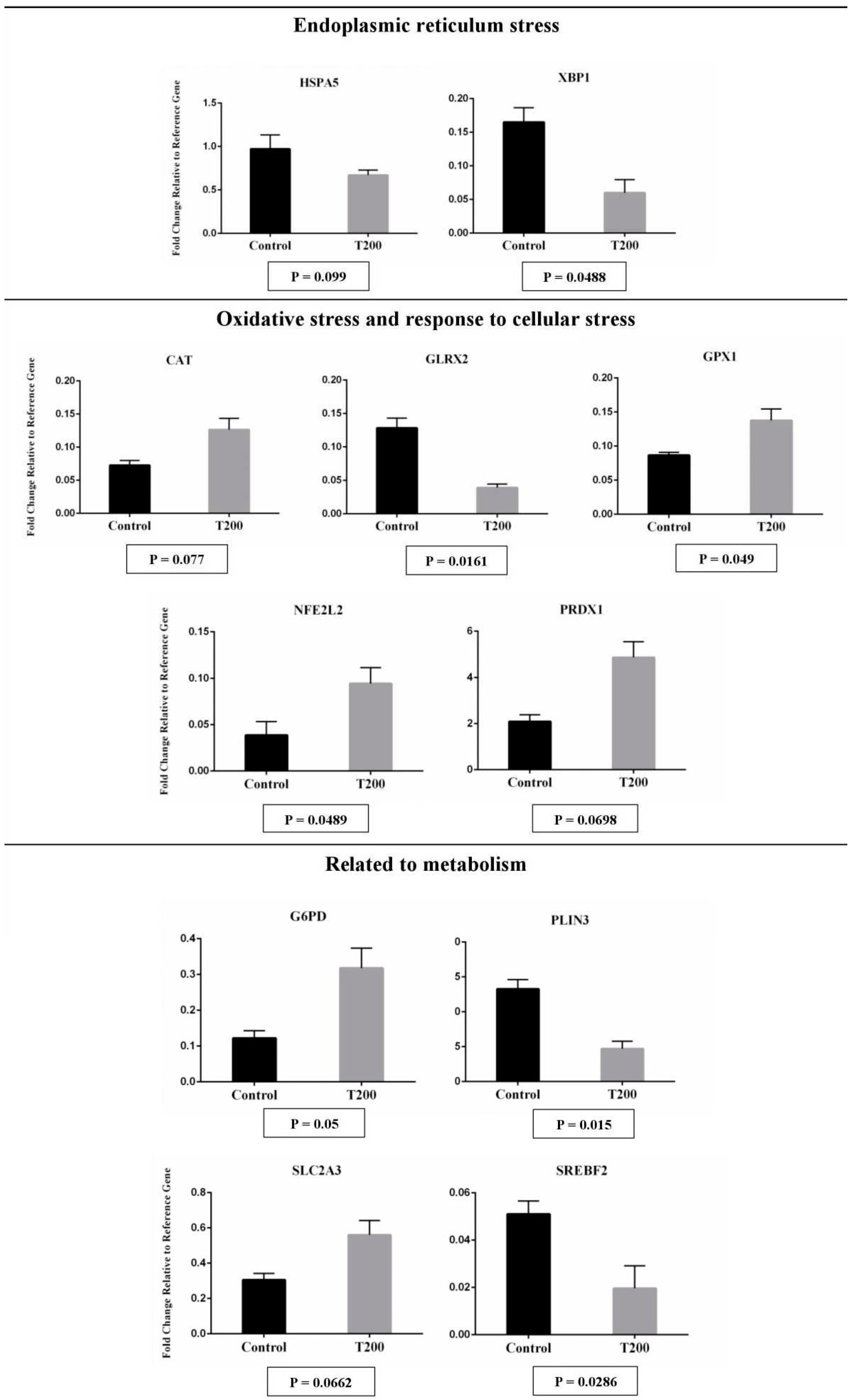
2.2.2. Gene expression
3. Discussion
4. Materials and Methods
4.1. In vitro Production
4.2. Chemical Treatment
4.3. Target-Transcripts Relative Quantitation: RT-qPCR
4.3.1. RNA isolation and reverse transcription
4.3.2. Preamplification and quantitative polymerase chain reaction
4.4. Vitrification of embryos
4.4.1. Embryo freezing
4.4.2. Warming and culture of cryopreserved embryos
4.5. Experimental Design
4.5.1. Experiment 1: The effects of TUDCA during IVC on developmental competence and gene expression of embryos
4.5.2. Experiment 2: The effects of TUDCA during IVC on developmental competence and gene expression of post-warmed vitrified blastocysts
4.6. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chipurupalli S, Kannan E, Tergaonkar V, D’Andrea R, Robinson N. Hypoxia Induced ER Stress Response as an Adaptive Mechanism in Cancer. Int. J. Mol. Sci. 2019, 20, 749. [Google Scholar] [CrossRef] [PubMed]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi R, Battaglia-Hsu SF, Arnold C. Endoplasmic Reticulum Stress in Metabolic Disorders. Endoplasmic Reticulum Stress in Metabolic Disorders. Cells 2018, 7. [Google Scholar]
- Shen XH, Zhang KZ, Kaufman RJ. The unfolded protein response—A stress signaling pathway of the endoplasmic reticulum. J. Chem. Neuroanat. 2004, 28, 79–92. [Google Scholar] [CrossRef]
- Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 2004, 10, 98–102. [Google Scholar] [CrossRef]
- Van Schadewijk A, Van’t Wout EF, Stolk J, Hiemstra PS. A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress. Chaperones 2011, 17, 275–279. [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Van der Kallen CJ, Van Greevenbroek MM, Stehouwer, CD, Schalkwijk, CG. Endoplasmic reticulum stress-induced apoptosis in the development of diabetes: Is there a role for adipose tissue and liver? Apoptosis 2009, 14, 1424–34. [Google Scholar] [CrossRef]
- Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev. Biol. 2005, 283, 40–57. [Google Scholar] [CrossRef]
- Paria BC, Dey SK. Preimplantation embryo development in vitro: Cooperative interactions among embryos and role of growth fac¬tors. Proc. Natl. Acad. Sci. USA 1990, 87, 4756–60. [Google Scholar] [CrossRef]
- O’Neill, C. Evidence for the requirement of autocrine growth fac¬tors for development of mouse preimplantation embryos in vitro. Biol. Reprod. 1997, 56, 229–37. [Google Scholar] [CrossRef] [PubMed]
- Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: Network of hormones, cytokines, and growth factors. J. Endocrinol. 2011, 210, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Michalak M, Gye MC. Endoplasmic reticulum stress in periimplantation embryos. Clin. Exp. Reprod. Med. 2015, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Latham KE Stress signaling in mammalian oocytes and embryos: A basis for intervention and improvement of outcomes. Cell Tissue Res. 2016, 363, 159–67. [CrossRef] [PubMed]
- Song BS, Yoon SB, Sim BW, Kim YH; et al. Valproic acid enhances early development of bovine somatic cell nuclear transfer embryos by alleviating endoplasmic reticulum stress. Reprod. Fertil. Dev. 2014, 26, 432–40. [Google Scholar] [CrossRef]
- Zhang JY, Diao YF, Kim HR, Jin DI. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS ONE. 2012, 7, e40433. [Google Scholar]
- Kim JS, Song BS, Lee KS, Kim DH; et al. Tauroursodeoxycholic acid enhances the pre-implantation embryo development by reducing apoptosis in pigs. Reprod. Domest. Anim. 2012, 47, 791–798. [Google Scholar] [CrossRef]
- Lee YY, Hong SH, Lee YJ, Chung SS; et al. Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem. Biophys. Res. Commun. 2010, 397, 735–39. [Google Scholar] [CrossRef]
- Yoon SB, Choi SA, Sim BW, Kim JS; et al. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol. Reprod. 2014, 90, 104. [Google Scholar]
- Khatun H, Ihara Y, Takakura K, Egashira K; et al. Role of endoplasmic reticulum stress on developmental competency and cryo-tolerance in bovine embryos. Therio. 2020, 142, 131–37. [Google Scholar] [CrossRef] [PubMed]
- Papis K, Shimizu M, Izaike Y. Factors affecting the survivability of bovine oocytes vitrified in droplets. Therio. 2000, 54, 651–58. [Google Scholar] [CrossRef] [PubMed]
- Abe H, Yamashita S, Satoh T, Hoshi H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Develop. 2002, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sudano MJ, Paschoal DM, da Silva Rascado T, Crocomo LF; et al. Crucial surviving aspects for vitrified in vitro-produced bovine embryos. Zygote. 2014, 22, 124–31. [Google Scholar] [CrossRef]
- Botigelli RC, Razza EM, Pioltine EM, Fontes PK; et al. Supplementing in vitro embryo production media by NPPC and sildenafil affect the cytoplasmic lipid content and gene expression of bovine cumulus-oocyte complexes and embryos. Reprod. Biol. 2018, 18, 66–75. [Google Scholar] [CrossRef]
- Seneda MM, Esper CR, Garcia JM, de Oliveira JA, Vantini R. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim. Reprod. Sci. 2001, 67, 37–43. [Google Scholar] [CrossRef]
- Vajta G, Holm P, Greve T; et al. The Submarine Incubation System, a new tool for in vitro embryo culture. A technique report. Therio. 1997, 48, 1379–85. [Google Scholar] [CrossRef]
- Zhang JY, Diao YF, Oqani RK, Han RX, Jin DI. Effect of endoplasmic reticulum stress on porcine oocyte maturation and parthenogenetic embryonic development in vitro. Biol. Reprod. 2012, 27, 128. [Google Scholar]
- Park HJ, Park JY, Kim JW, Yang SG, Jung JM, Kim MJ, Park JJ, Koo DB. Regulation of the Endoplasmic Reticulum Stress by BIP/GRP78 is involved in Meiotic Maturation of Porcine Oocytes In Vitro. Dev. Reprod. 2017, 21, 407–15. [Google Scholar] [CrossRef]
- Mochizuki M, Miyagi K, Kishigami S. Optimizing treatment of tauroursodeoxycholic acid to improve embryonic development after in vitro maturation of cumulus-free oocytes in mice. PLoS ONE. 2018, 27, 13–e0202962. [Google Scholar]
- Takahashi M, Keicho K, Takahashi H, Ogawa H; et al. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Therio. 2000, 54, 137–45. [Google Scholar] [CrossRef] [PubMed]
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol. Reprod. Dev. 2007, 74, 1287–94. [Google Scholar] [CrossRef]
- Lin T, Zhang JY, Diao YF, Kang JW; et al. Effects of sorbitol on porcine oocyte maturation and embryo development in vitro. Zygote 2015, 23, 297–306. [Google Scholar] [CrossRef] [PubMed]
- López-Damián EP, Jiménez-Medina JA, Alarcón MA, Lammoglia MA, Hernández A, Galina CS, Fiordelisio T. Cryopreservation induces higher oxidative stress levels in Bos indicus embryos compared with Bos taurus. Therio 2020, 143, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Song BS, Kim JS, Yoon SB, Lee KS; et al. Inactivated Sendai-virus-mediated fusion improves early development of cloned bovine embryos by avoiding endoplasmic-reticulum-stress-associated apoptosis. Reprod. Fertil. Dev. 2011, 23, 826–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma A, Agrawal H, Mullani N, Sandhu A; et al. Supplementation of tauroursodeoxycholic acid during IVC did not enhance in vitro development and quality of buffalo IVF embryos but combated endoplasmic reticulum stress. Therio. 2015, 84, 200–07. [Google Scholar] [CrossRef] [PubMed]
- Lin T, Lee JE, Oqani RK, Kim SY; et al. Tauroursodeoxycholic acid improves pre-implantation development of porcine SCNT embryo by endoplasmic reticulum stress inhibition. Reprod. Biol. 2016, 16, 269–78. [Google Scholar] [CrossRef]
- Wang J, Lee J, Liem D, Ping P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene. 2017, 618, 14–23. [Google Scholar] [CrossRef]
- Campanella C, Pace A, Caruso Bavisotto C, Marzullo P; et al. Heat shock proteins in Alzheimer’s disease: Role and targeting. Int. J. Mol. Sci. 2018, 19, 2603. [Google Scholar] [CrossRef]
- Harding HP, Zhang YH, Bertolotti A, Zeng HQ, et at. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Landau G, Kodali VK, Malhotra JD, Kaufman RJ. Detection of oxidative damage in response to protein misfolding in the endoplasmic reticulum. Methods Enzymol. 2013, 526, 231–50. [Google Scholar]
- Van der Vlies D, Makkinje M, Jansens A, Braakman I; et al. Oxidation of er resident proteins upon oxidative stress: Effects of altering cellular redox/antioxidant status and implications for protein maturation. Antioxid. Redox Signal. 2003, 5, 381–87. [Google Scholar] [CrossRef] [PubMed]
- Hausburg MA, Dekrey GK, Salmen JJ, Palic MR, Gardiner CS. Effects of paraquat on development of preimplantation embryos in vivo and in vitro. Reprod. Toxicol. 2005, 20, 239–46. [Google Scholar] [CrossRef] [PubMed]
- Kammoun HL,Chabanon H, Hainault, I; et al. “GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice,”. The Jour Clin Invest. 2009, 119, 1201–15. [CrossRef] [PubMed]
- Zhang K, Wang S, Malhotra J; et al. “The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis,”. The EMBO Journal. 2011, 30, 1357–75. [CrossRef]
- Lauressergues E, Bert E, Duriez P, Hum D, Majd Z; et al. Does endoplasmic reticulum stress participate in APD-induced hepatic metabolic dysregulation? Neuropharm. 2012, 62, 784–96. [CrossRef]
- Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–38. [Google Scholar] [CrossRef]
- Sudano MJ, Caixeta ES, Paschoal DM, Martins A; et al. Cryotolerance and global gene-expression patterns of Bos taurus indicus and Bos taurus taurus in vitro-and in vivo-produced blastocysts. Reprod. Fert. Develop. 2014, 26, 1129–41. [Google Scholar] [CrossRef]
- Harris D, Huang B, Rn Oback B. Inhibition of MAP2K and GSK3 signaling promotes bovine blastocyst development and epiblast-associated expression of pluripotency factors 1. Biol Reprod. 2013, 88, 74.
- Aksu DA, Agca C, Aksu S, Bagis H; et al. Gene expression profiles of vitrified in vitro- and in vivo-derived bovine blastocysts. Mol. Reprod. Dev. 2012, 79, 613–625. [Google Scholar] [CrossRef]
- Hua Y, Kandadi MR, Zhu M, Ren J, Sreejayan N. Tauroursodeoxycholic acid attenuates lipid accumulation in endoplasmic reticulum-stressed macrophages. J. Cardiovasc. Pharmacol. 2010, 55, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Cha BH, Kim JS, Ahn JC, Kim HC, Kim BS, Han DK, Park SG, Lee SH. The role of tauroursodeoxycholic acid on adipogenesis of human adipose-derived stem cells by modulation of ER stress. Biomaterials. 2014, 35, 2851–58. [Google Scholar] [CrossRef] [PubMed]
- Lee K, Tirasophon W, Shen X, Michalak M; et al. IRE-1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merges to regulate XBP1 in signaling the unfolded protein response. Genes. Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Hosoi T, Ogawa K, Ozawa K. Homocysteine induces X-box-binding protein 1 splicing in the mice brain. Neurochem. Int. 2010, 56, 216–20. [Google Scholar] [CrossRef]
- Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamyl- cysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem 1999, 274, 33627–36. [CrossRef]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA; et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J. Pharmacol. Exp. Ther. 2008, 325, 655–64. [Google Scholar] [CrossRef]
- Moreira S, Fonseca I, Nunes MJ; et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Experimental Neurology. 2017, 295, 77–87. [Google Scholar] [CrossRef]
- Yang HC; et al. Glucose 6-phosphate dehydrogenase deficiency enhances germ cell apoptosis and causes defective embryogenesis in Caenorhabditis elegans. Cell death & disease. 2013, 4, e616. [Google Scholar]
- Wu YH, Lee YH, Shih HY, Chen SH; et al. Glucose-6-phosphate dehydrogenase is indispensable in embryonic development by modulation of epithelial-mesenchymal transition via the NOX/Smad3/miR-200b axis. Cell Death Dis. 2018, 9, 10. [Google Scholar] [CrossRef]
- Purcell SH, Moley KH. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol. Metab. 2009, 20, 483–489. [Google Scholar] [CrossRef]
- Ortega MS, Kelleher AM, O`Neil E, Benne J; et al. NANOGis required to form the epiblast and maintainpluripotency in the bovine embryo. Mol. Reprod. Develop. 2020, 87, 152–160. [Google Scholar] [CrossRef] [PubMed]
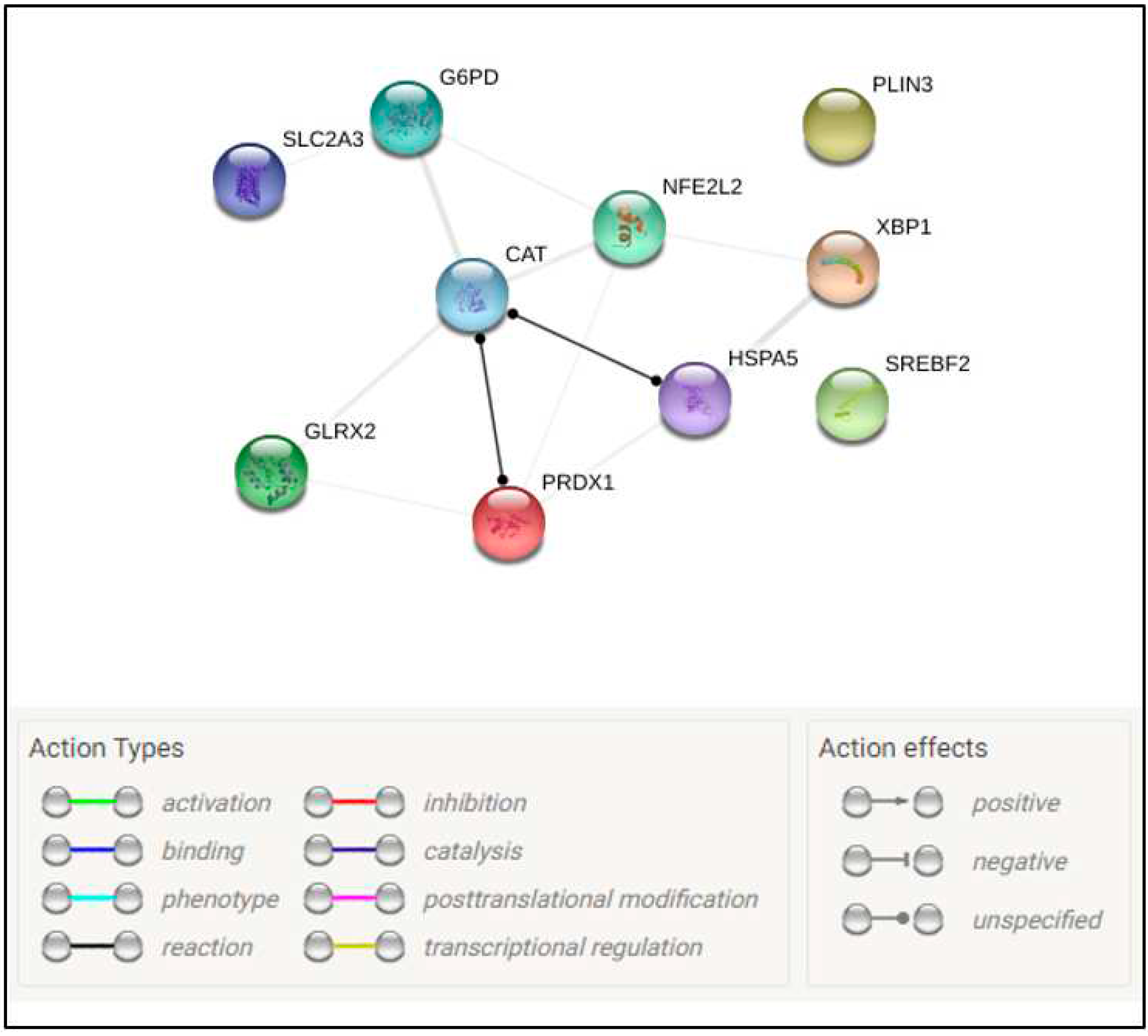
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





