1. Introduction
Oak mortality is among the most observed phenomenon in oak forests in Eastern U.S. [
1,
2,
3,
4]. The mortality starts with the oak tree browning of leaf, turning black, curl-up, and finally falling to the ground [
5,
6,
7,
8,
9] Factors responsible for the oak decline are suspected long-term predisposing, short-term inciting, and contributing factors [
7,
9]. Predisposing factors are related to stand longevity and maturity, which are responsible for a tree's natural ability to growth inhibition and lead to injury-inducing agents. The inciting factors are related to physical or biological conditions, which are related to defoliating insects, hail, frost, and drought. Reports from inciting factors showed typical crown dieback, browning, and new leaf emergence in dying trees, which eventually leads to deaths [
10]. The contributing factors are related to pathogenic fungi and boring insects that ultimately kill the trees. This decline has grappled with notable drought outbreaks, late spring frost, the emergence of saprophytic fungus due to climate change [
11], and oak borer attacks on the most vulnerable sites.
Typically, oak mortality has been targeted in red oak group species. More recently, white oak mortality (WOM) becomes a prominent target across the eastern US. It seems that the spatial distribution pattern of WOM is not uniform. Poor resource sites [
12] such as droughty, poor drainage, and soil nutrient deficiency are more prone to WOM. It is because low resources had led to declining and widespread regeneration failure. Scientists also reported WOM in higher-quality mesic sites where the forest has gone through a high stand density and maturity stage [
13,
14]. The mortality is more prevalent in the self-thinning stage, as the tree species under high stand density struggle to utilize maximum resources [
15]. WOM has been observed in different topographies, from low-lying lands to valley floors (Abrams, 2003). North-facing slopes where sunlight is low are more prone to high oak mortality [
16]. Besides, ecological stressors such as browsing, heavy shade, and disturbances have influenced white oaks in many parts of eastern hardwood forests [
17].
Soil properties play an important role in tree species' survival. The study on tree species at local scales may not represent an actual pattern that can be best reflected at the regional level [
18]. While numerous factors influence white oak's spatial pattern and survival, soil properties emerged as significant factors that influence forest ecosystem dynamics [
10]. Eastern US covers a diverse range of soil types and landscapes, which offers a unique opportunity to study the influence of soil properties on WOM patterns. By examining the spatial heterogeneity of soil types and their interactions with other environmental factors, we can understand how soil properties contribute to the spatial patterns of WOM across a broad scale [
19,
20].
Variations in soil properties, such as texture, soil moisture, and organic matter can influence white oaks' survival and establishment, and susceptibility to various stressors and disturbance factors. Therefore, it is necessary to incorporate data from multiple sites from the eastern US and study regional variations in soil types that influence the spatial pattern of WOM [
21].
The aim of this study is (1) to assess the spatial distribution pattern of WOM rate across the eastern US and (2) to evaluate the influence of soil properties (soil texture, organic matter, and total available water) on spatial distribution patterns of WOM rate. The hypothesis was that the observed spatial pattern of the WOM rate is random across the eastern US.
2. Materials and Methods
2.1. Study Area
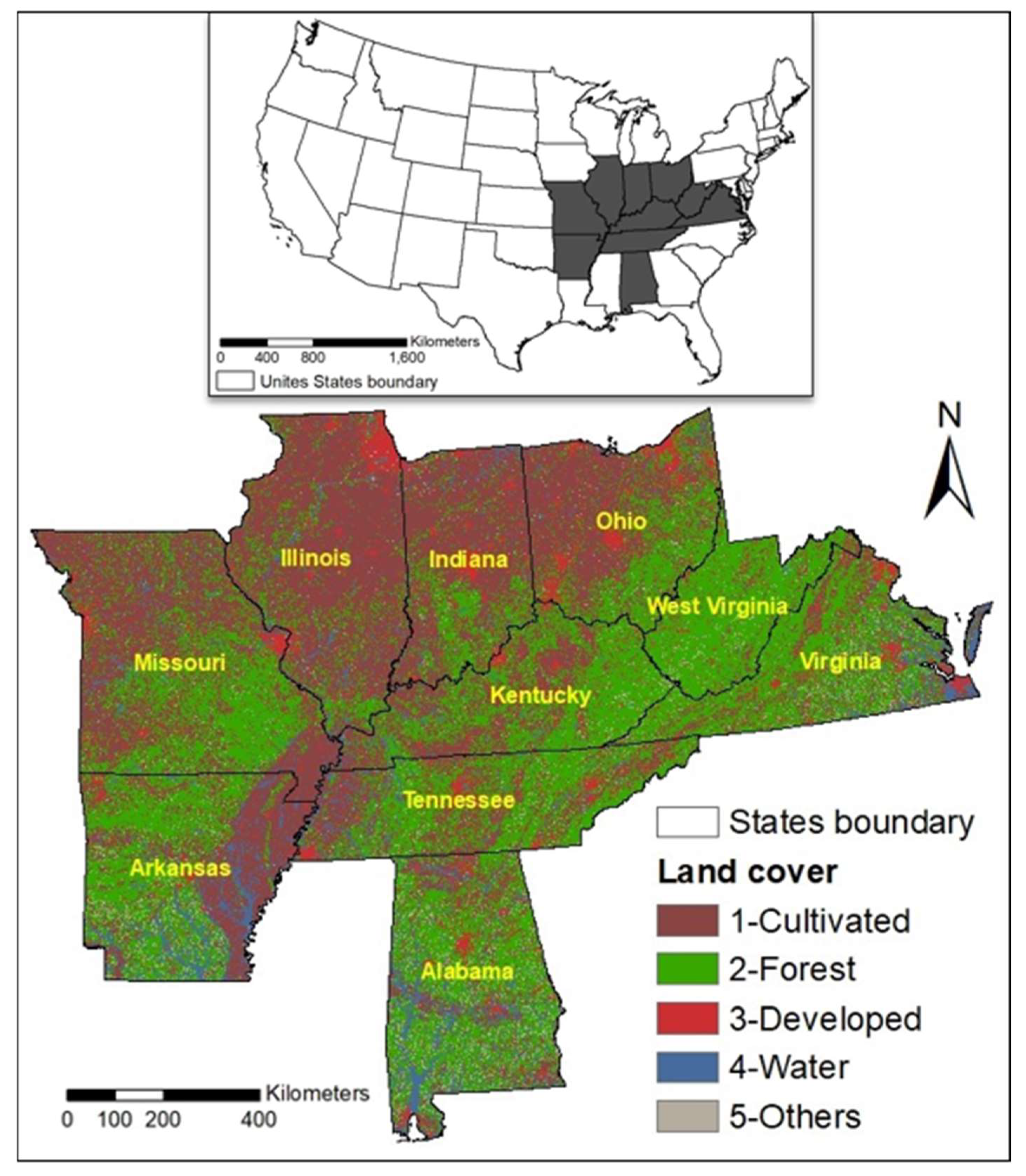
We selected the eastern United States as our study area, which predominantly covers oak forests [
22]. It is comprised of ten states that cover a land area of 1,273,420,251.33 hectares (
Figure 1). It consists of varied tree species, and a wide range of climate, soil, and terrains [
23,
24,
25]. The land structure in the eastern part of the study area is significantly Appalachian plateaus with low mountains, narrow valleys, and sharp edges. On the western portion, the area varies from highly flat central till plains to interior low plateau along with Ozark highlands. The area consists of a variety of ecological regions such as the Ouachita Mountains of Arkansas to the Northern Cumberland Mountains in West Virginia. In the south, central till plains Oak-Hickory of Illinois to Interior Low Plateau Highland Riff of Alabama.
The climate in this region is long, hot summers and cool winters. The mean annual temperature ranges from 4 degrees to 18 degrees Celsius in the east-west gradient with warmer temperatures in the south. The precipitation ranges from 500 mm in the northwest to 1,650 mm in the southeast. Some Appalachian Mountains precipitation goes up to 2,000 mm during spring and fall. The soil types are mostly mollisols, inceptisols, alfisol, and ultisols. These areas consist of xeric gradient characterized by thin, rocky soils, exposed south, southwest, and wet slopes [
26]. Our study area was historically dominated by oaks, hickories, and pines. Today, most of the forest areas have been replaced by agriculture and rapid urbanization [
27]. In our study area, there are deciduous types of forests such as oaks (
Quercus spp.), hickory (
Carya spp.), american beech (
Fagus grandifolia), ash (
Fraxinus spp.), and maple (
Acer spp.).
Figure 1.
Study area showing ten states of the eastern US [
68].
Figure 1.
Study area showing ten states of the eastern US [
68].
2.2. Data Acquisition and Processing
2.2.1. Forest Inventory and Analysis Program
FIA program has been monitoring national forest resources across all ownerships in the US [
28,
29]. The inventory data are collected and processed based on a relational database and categorized into multiple phases. Stratified estimations are used to estimate population parameters for most of the variables depending on the scale and level of information [
30]. The inventoried data have an advantage over other databases as the plots are evenly distributed without any geospatial bias [
31]. FIA plots are recorded with at least one forested condition, which is remeasured every five years in the eastern US. Each plot measures key attributes of all tree species including plots, surveyed years, and others [
32]. The plot designs in FIA are permanent samples with a fixed radius. The sampling plots are designed with the hexagonal grid having one plot per 2,428 ha (6,000 ac). This plot is based on phase 2 and 3 ground plots, which are clusters of four points. Point 1,2,3,4 is in central, 0 degree, 120 degrees, and 240 degrees azimuth, respectively away from point 1.
FIA also records condition-level information from the sampled data. A condition is based on changes in land use or vegetation that fall inside a plot. Furthermore, it is also determined by the ten percent crown or canopy cover of live tally trees [
33]. A condition is designed in such a way that arbitrary numbers are assigned and defined using discrete variables such as forest type, stand size, species composition, stand structure, stand origin, ownership group, and disturbance history [
33].
The periodic and annual inventory is recorded approximately every ten and five years, respectively. However, both periodic and annual inventories contained differences in plot design, sampling, and measurement protocols. Hence, it is not possible to combine both periodic and annual data with dissimilar samples. For instance, our farthest annual data goes back to 1998 from Virginia, which is the oldest annual inventory among ten states. Also, most of the plot inventories are fully recorded until 2019. That is why we chose our timeframe from 1998 to 2019.
2.2.2. Plot Level and White Oak Data
Phase 2 and 3 ground plot information from USDA Forest Service DataMart [
69], were acquired and have been assigned to a stratum from ten states of the eastern US (
Table 1). A stratum refers to a set of plots that have similar classifications captured from remotely sensed imagery. Within the estimation unit of plot sampling, the weight of the stratum is based on the proportion of the stratum [
28,
33]. In the same set of plot data, geographic coordinates (latitudes and longitudes) were recorded that captured a 0.40 ha (1 acre) sample area but not for all trees. The plot recorded by the Forest Service was located within one mile of the original location. It is because landowners prefer not to disclose their property publicly due to security reasons associated with it [
33,
34]. We utilized sampled i.e., forested conditions for the plot data which captured most of the hardwood tree species including white oaks in ten states.
The white oak tree tables were extracted from USDA Forest Service in which we used attributes such as inventory year (INVYR), county code (COUNTYCD), plot number (PLOT), species code (SPCD), tree status code (STATUSCD), trees per acre unadjusted (TPA_UNADJ), cycles (CYCLE), and current diameter (DIA). In it, we utilized plot numbers to connect plot, tree, and condition level data to extract appropriate white oak trees from respective plots.
2.2.3. Soil Properties Data
We acquired soil variables from Gridded National Soil Survey Geographic Database (gNATSGO) data provided by USDA Natural Resources Conservation Service [
71]. The soil texture classifications from SSURGO tabular data were combined with the spatial soil data provided with GIS shapefiles. Some regions were null for soil texture classification, which was addressed by obtaining information from the FAO dataset [
70]. We used Soil Texture Triangle as a reference to fill all null values for soil texture by placing SSURGO and FAO spatial soil data into the ArcGIS [
35]. By doing this, we obtained eighteen types of soil texture classification (including inland water) that were common in our study area (
Table 2). We also calculated soil organic matter and total available water using soil horizon thickness; and classified them into five classes.
2.3. Data Analysis
2.3.1. Plot, Condition-Level, and White Oak Data Analysis
Annualized data from 1998 to 2019 were processed to acquire 2,220 white oak plots across ten states. Our annual data were grouped into multicycles based on a five-survey-year cycle. For instance, Missouri had cycle 5 which consisted of inventory years grouped from 1999 to 2003 (usually year-wise data). Similarly, other cycles had a set of inventory years that goes until 2019 which was grouped as cycle 6 (2004 to 2008), cycle 7 (2009 to 2013), and cycle 8 (2014 to 2019). We performed our data processing by selecting only forested conditions. By doing this, white oak trees from multi-cycles were extracted from tree-level data for further analysis. Tree level attributes such as DBH and density (trees per acre unadjusted) were used to calculate the total basal area (feet2/acre) in each plot. Later, we converted the basal area into the standard unit as a square meter per hectare.
2.3.2. White Oak Mortality Rate Analysis
The mortality rate was calculated by using basal areas of two inventory cycles, which were grouped into five years. We selected all the basal areas from declining plots of white oaks concerning inventory years. We calculated the yearly rate of change (m
2 ha
-1 year
-1) by dividing the white oak reduced basal area with the change in inventory years between two cycles. All those declining plots representing mortality rates were extracted. Some of the white oak plots were remeasured in the period from 1998 to 2019. Hence, we omitted those repeated plots to have accurate results and analysis. However, we summed up all those remeasured plots’ basal areas to obtain the true basal area. We calculated the mortality rate as:
We assumed oak forests in this region have mostly gone through the self-thinning stage [
36,
37,
38] and thus reduction of the white oak basal area can be attributed to WOM.
2.3.3. Spatial Distribution Analysis of WOM by univariate Ripley’s K function
We used Multi-Distance Spatial Cluster Analysis-Ripley’s K function tool [
39], which uses nearest neighbor distance to process spatial distribution patterns. ArcMap version 10.7.1 was used for Ripley's K function which defines whether our spatial pattern is cluster, random, or uniform on each analysis scale [
40,
41]. All 2,220 WOM points from 1998 to 2019 were compiled that contained point locations i.e., longitudes and latitudes. In Ripley's k function tool, the initial distance was 2,000 m and the increment distance is 20,000 m. Edge correction was done by using Ripley's edge correction formula [
39], which automatically counts and measures points inside the study area. To test our null hypothesis of complete spatial randomness (CSR), we formed upper and lower confidence envelopes of 99.9% generated from randomizations of 999 plot points using the default random generator in ArcGIS. We examined whether our theoretical curve (K
theo) deviates from CSR or not. If the observed curve (K
obs) is above the theoretical curve and upper envelope, then there is spatial clustering. If it is below the theoretical curve and lower envelope, then WOM points follow spatial dispersion (uniform). There is also a greater chance that both observed and theoretical curves could line up denoting complete spatial randomness or random pattern. We used the transformed K function (L(d)) to represent the graphical interpretation of Ripley's K function.
2.3.4. WOM Rate, Stand Density, and Size Distribution among Soil Variables
We did a log transformation for the WOM rate and stand density of white oaks so that our data distribution could achieve normality. Basal area per area was calculated for stand density from the dying plots of white oak. Soil textures were classified (18 classes in our study area) taking a reference from Food and Agriculture Organization Soils Portal based on World Reference Base (WRB) soil classification system [
70]. We interpolated soil organic matter and total available water and use the interpolated results to compare them with the spatial pattern of the WOM rate. Soil organic matter and total available water percentage were classified into five classes i.e., very low, low, medium, high, and very high using natural breaks (Jenks) from ArcGIS. Our data were analyzed using R studio [
42]. In it, we fitted a linear model (
lm) to find the relationship between WOM rate and soil variables; and between the stand density of WOM and soil variables using significance levels at α = 0.1 and α = 0.05, respectively.
3. Results
3.1. White Oak Mortality Spatial Distribution Patterns
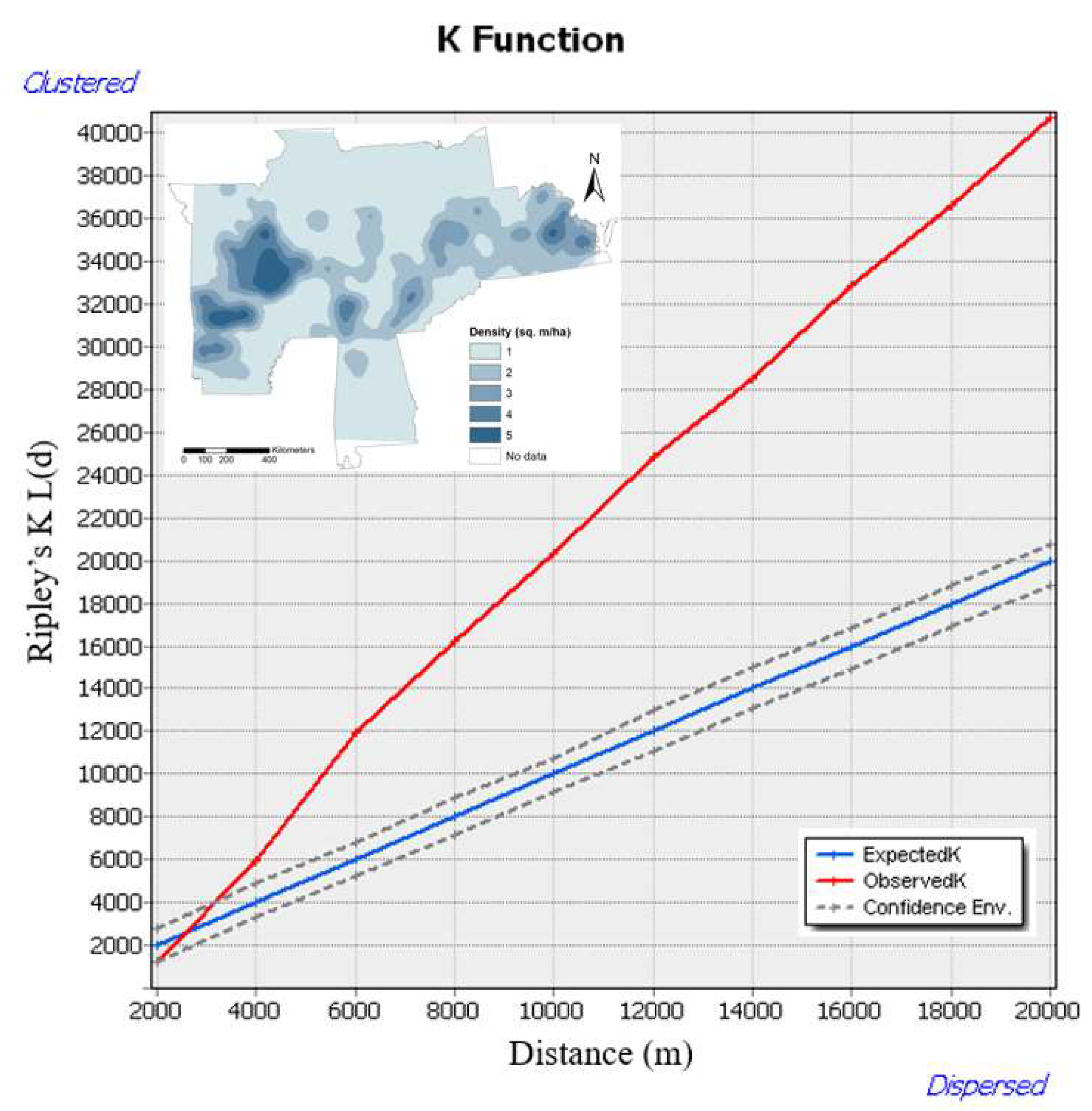
The spatial distribution pattern of the WOM rate showed random patterns up to 3000 m, as the observed K value was inside the confidence envelopes, and we accept the null hypothesis (
Figure 2). Beyond this point, we reject our null hypothesis, the spatial distribution of WOM rate showed clustered until 20,000 m is statistically significant. It is due to the spatial clustering of WOM rates, as indicated by the observed K value being significantly larger than the expected K function and falling outside the confidence envelopes. This clustering pattern of the WOM rate increases as the distance increases. Most of our pattern analysis for the WOM rate depicted clustered (non-random) patterns at a larger distance across the eastern US. Thus, our spatial distribution pattern analysis of WOM showed more clustering patterns than the random pattern as distance increases.
The kernel density maps for white oaks show the clusters spatial distribution across the declining plots of white oaks (
Figure 2). This density distribution indicated areas for higher or lower concentrations across the eastern US.
Figure 2.
Observed spatial patterns through Ripley's K L(d) as a transformed function of WOM rate across the eastern US. The red line indicates the observed K function. The blue line indicates the expected K function. The gray envelope indicates 99.9% confidence level for the CSR hypothesis.
Figure 2.
Observed spatial patterns through Ripley's K L(d) as a transformed function of WOM rate across the eastern US. The red line indicates the observed K function. The blue line indicates the expected K function. The gray envelope indicates 99.9% confidence level for the CSR hypothesis.
3.2. Relationship between WOM rate and Soil Variables
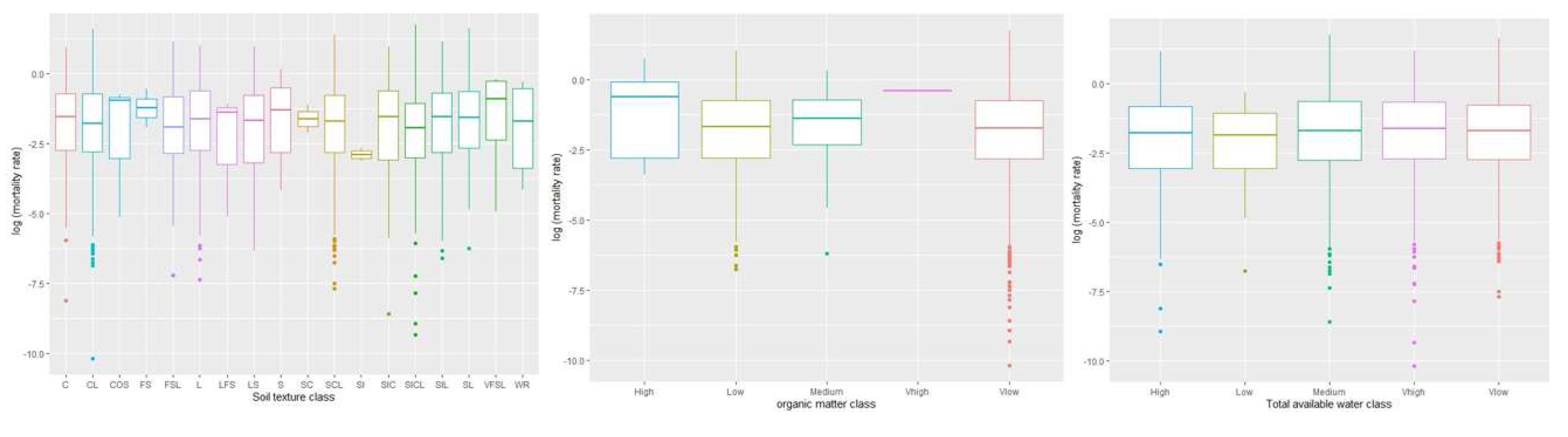
The relationship between logarithmic WOM rate and soil variables was also explained based on central tendency (
Figure 3). Results showed that there was a higher variation among soil texture classes concerning WOM rate. However, the silt (SI) had less variability as compared with other texture classes. Our results showed significant effects with a negative linear relationship between silty clay loam (SICL) class and WOM rate (β = -0.39; p = 0.06). This indicates that the content of silt clay loam is negatively related to WOM.
Results from the central tendency of total available water class showed greater variation in WOM rate. The total available water classes were more or less similar in the data distribution with respect to the WOM rate. However, there was a significant and positive linear relationship between very high total available water class and WOM rate (β = 0.20; p= 0.05) indicating that the content of total available water is positively related to WOM rate.
Our results showed that a very high class of soil organic matter had a higher variation with respect to the WOM rate. However, the data variation was much higher for the rest of the soil organic matter classes except for very high soil organic matter. Other soil variables such as very low and low organic matter classes were significant and showed a negative linear relationship with WOM rate i.e., β = -0.81, p = 0.08, and β = -0.83, p = 0.07, respectively. These indicate that the content of low and very low organic matter is negatively related to the WOM rate.
3.3. Relationship between Density Distribution of WOM and Soil Variables
The relationship between the density distribution of WOM and soil variables i.e., texture, organic matter, and total available water were highly significant (p = 0.00). In this regard, the density distribution of soil properties i.e., texture, soil organic matter, and total available water concerning WOM rate was successful in interpreting the spatial pattern of WOM across the eastern US.
The result showed a significant and a negative linear relationship between loamy fine sand and the stand density of WOM (β = -1.50; p = 0.04), indicating that the content of loamy fine sand is negatively related to the density distribution of WOM. Also, fine sandy loam and clay loam showed a significant and a negative relationship with stand density i.e., β = -0.39, p = 0.02; and β = -0.29, p = 0.04, respectively. This indicates that the content of fine sandy loam and clay loam is negatively related to the density distribution of WOM.
Our result did not show a statistically significant relationship between soil organic matter and stand density of WOM. However, there was a variation of organic matter class along with the stand density of WOM across the eastern US (
Figure 4). The central tendency analysis showed that very high organic matter class played a significant role in the stand density distribution of WOM i.e., 2 m
2/ha. However, the rest of the organic matter class i.e., high, medium, low, and very low classes were positively related to the density distribution of WOM but not quite greater in comparison to very high organic matter.
Our results showed a statistically significant linear relationship between the stand density of white oaks and very low total available water class (β = 0.28 and p = 0.001). However, results showed that a very high total available water class (β = 0.32, p = 0.00) is highly significant with the stand density of white oaks. Our central tendency analysis showed that there was a variation among total available water classes with respect to the stand density of WOM (Figure 6). Result showed that very high total available water class had a greater variation concerning the stand density of WOM. We also found that very low total available water class had significant variation in the distribution of stand density of WOM.
4. Discussion
4.1. Spatial Distribution Patterns of WOM Rate
K-function indicates that the WOM rate at site scales is random. This confirms with findings that WOM is reported at various site conditions including poor and good resources sites as well as various topographic and hydrological conditions [
41,
43,
44]. WOM rate shows increasing clustered distribution at broad scales, indicating that mid-level controls such as topography and soil, and regional-scale controls such as climate, drought in particular, may exert some dominant roles over the WOM rate [
45,
46,
47]. The method and analysis used in this study is in accordance with other similar findings that aggregation factors were responsible for the spatial distribution of WOM rate [
41,
48]. It also appears that site-scale processes such as self-thinning in white oak stands may accumulate across the region, which may result in a region-wide WOM since most white oak stands were regenerated after forest clearing and the subsequent agricultural abandonment nearly a century ago [
49,
50,
51].
The spatial pattern of WOM has been influenced by edaphic factors such as soil variables responsible for clustering. It is because soil conditions determine species establishment and can influence resource availability and forest disturbance [
52,
53]. For instance, our results found soil texture (e.g., silty clay loam) had significant effects on white oaks development, suggesting a clustered spatial pattern of WOM rate at a larger scale. This may be due to coarser soil textures such as fine sandy loam, clay loam, and loamy fine sand, across our study area, had a higher infiltration rate where water movement occurs through the soil profile resulting reduction in water retention and availability for white oaks [
54,
55]. The research found that the spatial pattern of WOM becomes more clustered with the influence of abiotic factors such as soil characteristics, climate, and topography that limit the development of white oaks [
41,
56]. This might be the probable reason that the spatial pattern of WOM is clustered across the eastern US.
Our findings align with the previous studies that have shown a strong connection between soil organic matter and tree mortality [
57,
58,
59]. Our study follows similar findings of [
60] that white oaks exhibited reduced growth and were dying in clusters across organic matter deficient areas. Another study from [
61] on tree survival and growth in mixed soil types reported that WOM rates were higher in organic matter-scarce areas where the soil was extremely dry. Our results reported low i.e., 0.01%, and very low i.e., no soil organic matter had significant impacts on shaping clustered spatial patterns of WOM rate. It is because low or no soil organic matter had combined effects such as reduced nutrient availability, increased susceptibility to insect pests and diseases, and stress from drought that may have contributed to clustered patterns [
62,
63].
Our findings show that the spatial pattern of WOM rate could be influenced by variations in soil moisture content. It is because previous studies have found that soil moisture plays a critical role in tree health due to excessive or insufficient moisture levels leading to increased stress and susceptibility to diseases and pests [
64,
65]. For instance, our findings indicated that clustered pattern of WOM rate in low moisture areas (e.g., very low class of total available water), suggesting extremely dry conditions resulting in white oak decline. However, our results also found compelling evidence suggesting elevated soil moisture levels (e.g., very high class of total available water) are associated with higher mortality rates in white oaks. Elevated soil moisture has adverse conditions on white oaks due to reduced soil aeration and increased waterlogging. [
66] and [
67] reported similar patterns of increased mortality in areas with poor drainage and high soil moisture levels.
While our study mainly focused on the relationship between soil variables and WOM rate. It is important to consider other potential factors that can contribute to white oak decline such as climate, topography, stand structure, terrains, insect pest attacks, and others. Moreover, our study was conducted on specific geographic regions, mainly the eastern US, and the conclusion drawn from our findings to other tree species should be investigated further.
5. Conclusions
Our study examined the WOM rate and its spatial patterns across the eastern United States using annual forest inventories and soil variables. The spatial pattern of the WOM rate was clustered at a larger distance and random at a shorter distance. And these clustering patterns were influenced by various sizes of soil variables. Mostly, silty clay loam, fine sandy loam, loamy fine sand, very high and low class of total available water, and very low and low class of soil organic matter played a significant role in the spatial distribution of WOM rate.
The information from WOM rate, stand density of WOM, soil texture, soil organic matter, and total available water revealed mostly clustered spatial patterns, and the patterns differed on the size of scales. Except for some soil textures and total available water, other sizes of organic matter did not show any significant role in the spatial pattern of WOM rate in our study area. We investigated that the regional pattern analysis of WOM rate had been influenced by soil characteristics across a broad scale. The observed relationship highlights the importance of various soil textures, soil organic matter, and soil moisture in determining the clustered spatial pattern of the WOM rate. However, further research is needed to investigate the specific mechanism underlying these relationships and to assess the long-term impacts on oak forest ecosystems. Future studies could explore the effects of multiple factors such as biotic and abiotic factors as well as land use practices, to obtain a more comprehensive understanding of the spatial pattern of WOM.
Author Contributions
Conceptualization, S.K.; methodology, S.K.; validation, S.K. and H.H.; writing—original draft preparation, S.K.; writing—review and editing, S.K., H.H., S.B. and A.K.; supervision, H.H.; project administration, H.H. and S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
United States Department of Agriculture/National Institute of Food and Agriculture 1890 Capacity Building Grant, Award number 2021-38821-34704
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Lincoln University College of Agriculture, environment, and Human Science for its facilities and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tainter, F.H.; Retzlaff, W.A.; Starkey, D.A.; Oak, S.W. Decline of Radial Growth in Red Oaks Is Associated with Short-Term Changes in Climate. European Journal of Forest Pathology 1990, 20, 95–105. [Google Scholar] [CrossRef]
- Mccracken, K.E.; Witham, J.W.; Hunter, M.L. Relationships between Seed Fall of Three Tree Species and Peromyscus Leucopus and Clethrionomys Gapperi during 10 Years in an Oak-Pine Forest. J Mammal 1999, 80, 1288–1296. [Google Scholar] [CrossRef]
- 3. Starkey, D.A.; Oliveria, F.; Mangini, A.; Mielke, M. NATURAL PHENOMENA, SEVERE OCCURRENCES. In Proceedings of the Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability: Fayetteville, Arkansas, October 7-10, 2002; 2004; Vol. 73, p. 217.
- Haavik, L.J.; Jones, J.S.; Galligan, L.D.; Guldin, J.M.; Stephen, F.M. Oak Decline and Red Oak Borer Outbreak: Impact in Upland Oak-Hickory Forests of Arkansas, USA. Forestry: An International Journal of Forest Research 2012, 85, 341–352. [Google Scholar] [CrossRef]
- Spetich, M.A. Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability. Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability 2002, 318.
- Heitzman, E.; Shelton, M.G.; Grell, A. Species Composition, Size Structure, and Disturbance: History of an Old Growth Bottomland Hardwood Loblolly Pine (Pinus Taeda) Forest in Arkansas, USA. Natural Areas Journal 24: 177-187 2004.
- Shifley, S.R.; Fan, Z.; Kabrick, J.M.; Jensen, R.G. Oak Mortality Risk Factors and Mortality Estimation. For Ecol Manage 2006, 229, 16–26. [Google Scholar] [CrossRef]
- Greenberg, Cathryn H; Colllins, S.B. Natural Disturbances and Historic Range of Variation. Natural Disturbances and Historic Range of Variation 2016, 32, 167–202.
- Reed, S.E.; English, J.T.; Muzika, R.M. Phytophthora Species Detected in Two Ozark Forests with Unusual Patterns of White Oak Mortality. Plant Dis 2019, 103, 102–109. [Google Scholar] [CrossRef]
- Nagle, A.M.; Long, R.P.; Madden, L.V.; Bonello, P. Association of Phytophthora Cinnamomi with White Oak Decline in Southern Ohio. Plant Dis 2010, 94, 1026–1034. [Google Scholar] [CrossRef]
- Wang, C.; He, H.S.; Kabrick, J.M. A Remote Sensing-Assisted Risk Rating Study to Predict Oak Decline and Recovery in the Missouri Ozark Highlands, USA. GIsci Remote Sens 2008, 45, 406–425. [Google Scholar] [CrossRef]
- McConnell, M.E.; Balci, Y. Phytophthora Cinnamomi as a Contributor to White Oak Decline in Mid-Atlantic United States Forests. Plant Dis 2014, 98, 319–327. [Google Scholar] [CrossRef]
- Dey, D. Dey DC 2002 Chapter 5 Oak Book for Oak Silviculture in Eastern North America. 2009.
- Aldrich, P.R.; Parker, G.R.; Romero-severson, J.; Michler, C.H. Forest : 75 Years of Data. Forest Science 2005, 51. [Google Scholar]
- Greenberg, C.H.; Keyser, T.L.; Speer, J.H. Temporal Patterns of Oak Mortality in a Southern Appalachian Forest (1991-2006). Natural Areas Journal 2011, 31, 131–137. [Google Scholar] [CrossRef]
- Balci, Y.; Long, R.P.; Mansfield, M.; Balser, D.; MacDonald, W.L. Involvement of Phytophthora Species in White Oak (Quercus Alba) Decline in Southern Ohio. For Pathol 2010, 40, 430–442. [Google Scholar] [CrossRef]
- Abrams, M.D. Where Has All the White Oak Gone? Bioscience 2003, 53, 927–939. [Google Scholar] [CrossRef]
- Dale, M.R.T. Spatial Pattern Analysis in Plant Ecology; Cambridge university press, 2000;
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol Lett 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Milbau, A.; Stout, J.C.; Graae, B.J.; Nijs, I. A Hierarchical Framework for Integrating Invasibility Experiments Incorporating Different Factors and Spatial Scales. Biol Invasions 2009, 11, 941–950. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity Influences Spatial Patterns and Demographics in Forest Stands. Journal of Ecology 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Clark, F.B.; Hutchinson, J.G. Central Hardwood Notes; US Department of Agriculture, Forest Service, North Central Forest~…, 1989.
- Fralish, J.S. The Central Hardwood Forest: Its Boundaries and Physiographic Provinces. General Technical Report - North Central Research Station, USDA Forest Service 2003, 1–20.
- Cleland, D.T.; Freeouf, J.A.; Keys, J.E.; Nowacki, G.J.; Carpenter, C.A.; McNab, W.H. Ecological Subregions: Sections and Subsections for the Conterminous United States. Gen. Tech. Rep. WO-76D 2007, 242.
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Hanberry, B.B.; Dijak, W.D. Importance of Succession, Harvest, and Climate Change in Determining Future Composition in U.S. Central Hardwood Forests. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Hanberry, B.B.; Dijak, W.D. Importance of Succession, Harvest, and Climate Change in Determining Future Composition in U.S. Central Hardwood Forests. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Stambaugh, M.C.; Knapp, B.O.; Dey, D.C. Fire Ecology and Management of Forest Ecosystems in the Western Central Hardwoods and Prairie-Forest Border; 2021; ISBN 9783030732677 .
- Bechtold, W.A.; Patterson, P.L. The Enhanced Forest Inventory and Analysis Program--National Sampling Design and Estimation Procedures; USDA Forest Service, Southern Research Station, 2005; Vol. 80;
- Tinkham, W.T.; Mahoney, P.R.; Hudak, A.T.; Domke, G.M.; Falkowski, M.J.; Woodall, C.W.; Smith, A.M.S. Applications of the United States Forest Inventory and Analysis Dataset: A Review and Future Directions. Canadian Journal of Forest Research 2018, 48, 1251–1268. [Google Scholar] [CrossRef]
- McRoberts, R.E.; Miles, P.D. United States of America. In National Forest Inventories: Assessment of Wood Availability and Use; Vidal, C., Alberdi, I.A., Hernández Mateo, L., Redmond, J.J., Eds.; Springer International Publishing: Cham, 2016; pp. 829–842. ISBN 978-3-319-44015-6. [Google Scholar]
- Smith, W.B. Forest Inventory and Analysis: A National Inventory and Monitoring Program. Environmental Pollution 2002, 116, 233–242. [Google Scholar] [CrossRef]
- Gray, A.; Brandeis, T.; Shaw, J.; McWilliams, W.; Miles, P. Forest Inventory and Analysis Database of the United States of America (FIA). Biodiversity & Ecology 2012, 4, 225–231. [Google Scholar] [CrossRef]
- Burrill, E.A.; Wilson, A.M.; Turner, J.A.; Pugh, S.A.; Menlove, J.; Christiansen, G.; Conkling, B.L.; David, W. The Forest Inventory and Analysis Database: Database Description and User Guide Version 8.0 for Phase 2. US Department of Agriculture, Forest Service 2018, 946. [Google Scholar]
- Khadka, S.; Gyawali, B.R.; Shrestha, T.B.; Cristan, R.; Banerjee, S. “Ban”; Antonious, G.; Poudel, H.P. Exploring Relationships among Landownership, Landscape Diversity, and Ecological Productivity in Kentucky. Land use policy 2021, 111, 105723. [Google Scholar] [CrossRef]
- Johnston, K.; Ver Hoef, J.M.; Krivoruchko, K.; Lucas, N. Using ArcGIS Geostatistical Analyst. Analysis 2001, 300, 300. [Google Scholar]
- Iverson, L.R.; Hutchinson, T.F.; Prasad, A.M.; Peters, M.P. Thinning, Fire, and Oak Regeneration across a Heterogeneous Landscape in the Eastern U.S.: 7-Year Results. For Ecol Manage 2008, 255, 3035–3050. [Google Scholar] [CrossRef]
- Garnas, J.R.; Ayres, M.P.; Liebhold, A.M.; Evans, C. Subcontinental Impacts of an Invasive Tree Disease on Forest Structure and Dynamics. Journal of Ecology 2011, 99, 532–541. [Google Scholar] [CrossRef]
- Yang, S.-I.; Brandeis, T.J. Estimating Maximum Stand Density for Mixed-Hardwood Forests among Various Physiographic Zones in the Eastern US. For Ecol Manage 2022, 521, 120420. [Google Scholar]
- Ripley, B.D. Spatial Statistics. 2005.
- Wehenkel, C.; Brazão-Protázio, J.M.; Carrillo-Parra, A.; Martínez-Guerrero, J.H.; Crecente-Campo, F. Spatial Distribution Patterns in the Very Rare and Species-Rich Picea Chihuahuana Tree Community (Mexico). PLoS One 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Miron, A.C.; Bezerra, T.G.; Nascimento, R.G.M.; Emmert, F.; Pereira, R.S.; Higuchi, N. Spatial Distribution of Six Managed Tree Species Is Influenced by Topography Conditions in the Central Amazon. J Environ Manage 2021, 281. [Google Scholar] [CrossRef]
- Team, R.C. ; others R Development Core Team. R: A language and environment for statistical computing 2016, 55, 275–286. [Google Scholar]
- Petroselli, A.; Vessella, F.; Cavagnuolo, L.; Piovesan, G.; Schirone, B. Ecological Behavior of Quercus Suber and Quercus Ilex Inferred by Topographic Wetness Index (TWI). Trees - Structure and Function 2013, 27, 1201–1215. [Google Scholar] [CrossRef]
- Palik, B.; Mitchell, R.J.; Pecot, S.; Battaglia, M.; Mou, P. Spatial Distribution of Overstory Retention Influences Resources and Growth of Longleaf Pine Seedlings. Ecological Applications 2003, 13, 674–686. [Google Scholar] [CrossRef]
- Schulze, R.E. Climate Change and Water Resources in Southern Africa. Water Resources 2005, 15–17. [Google Scholar]
- Seligson, K.E. The Prehispanic Maya Burnt Lime Industry: Socio-Economy and Environmental Resource Management in the Late and Terminal Classic Period Northern Maya Lowlands (650-950 CE). 2016.
- Cortner, O. Collectives to Capital : Institutional, Forest, and Farm Transitions In. 2023.
- Condit, R. Spatial Patterns in the Distribution of Tropical Tree Species. Science (1979) 2000, 288, 1414–1418. [Google Scholar] [CrossRef]
- Southgate, E.W.B.R.; Thompson, J.E. Secondary Forest Succession in a Post-Agricultural Landscape in the Hudson Valley, New York. Northeast Nat (Steuben) 2014, 21. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Dijak, W.D. Changes in Forest Biomass and Tree Species Distribution under Climate Change in the Northeastern United States. Landsc Ecol 2017, 32, 1399–1413. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Spetich, M.A.; Fraser, J.S. Effects of Species Biological Traits and Environmental Heterogeneity on Simulated Tree Species Distribution Shifts under Climate Change. Science of the Total Environment 2018, 634, 1214–1221. [Google Scholar] [CrossRef]
- Schietti, J.; Emilio, T.; Rennó, C.D.; Drucker, D.P.; Costa, F.R.C.; Nogueira, A.; Baccaro, F.B.; Figueiredo, F.; Castilho, C. V.; Kinupp, V.; et al. Vertical Distance from Drainage Drives Floristic Composition Changes in an Amazonian Rainforest. Plant Ecol Divers 2014, 7, 241–253. [Google Scholar] [CrossRef]
- Zuleta, D.; Russo, S.E.; Barona, A.; Barreto-Silva, J.S.; Cardenas, D.; Castaño, N.; Davies, S.J.; Detto, M.; Sua, S.; Turner, B.L.; et al. Importance of Topography for Tree Species Habitat Distributions in a Terra Firme Forest in the Colombian Amazon. Plant Soil 2020, 450, 133–149. [Google Scholar] [CrossRef]
- Wang, D.Y.; Yan, D.H.; Song, X.S.; Wang, H. Impact of Biochar on Water Holding Capacity of Two Chinese Agricultural Soil. Adv Mat Res 2014, 941–944, 952–955. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Zhuravel, A.; Silva, F.C.; Amaro, A.; Ben-Hur, M.; Keizer, J.J. The Influence of Biochar Particle Size and Concentration on Bulk Density and Maximum Water Holding Capacity of Sandy vs Sandy Loam Soil in a Column Experiment. Geoderma 2019, 347, 194–202. [Google Scholar] [CrossRef]
- de Almeida, L.S.; Gama, J.R.V.; Oliveira, F. de A.; de Carvalho, J.O.P.; Gonçalves, D.C.M.; Araújo, G.C. Fitossociologia e Uso Múltiplo de Espécies Arbóreas Em Floresta Manejada, Comunidade Santo Antônio, Município de Santarém, Estado Do Pará. Acta Amazon 2012, 42, 185–193. [Google Scholar] [CrossRef]
- Cahoon, D.R.; Hensel, P.; Rybczyk, J.; McKee, K.L.; Proffitt, C.E.; Perez, B.C. Mass Tree Mortality Leads to Mangrove Peat Collapse at Bay Islands, Honduras after Hurricane Mitch. Journal of Ecology 2003, 91, 1093–1105. [Google Scholar] [CrossRef]
- Bennett, J.A.; Franklin, J.; Karst, J. Plant-Soil Feedbacks Persist Following Tree Death, Reducing Survival and Growth of Populus Tremuloides Seedlings. Plant Soil 2022, 1–13. [Google Scholar]
- Song, H.; Zhou, D.; Chen, S.; Li, J.; Wang, C.; Ren, Y.; Yang, X. Disparity in the Relative Roles of Biotic and Abiotic Drivers on Tree Mortality between Warm-Temperate and Temperate Forests in China. For Ecol Manage 2023, 537, 120938. [Google Scholar]
- Ponder Jr, F. Contrasting the Effects of Organic Matter Removal and Soil Compaction on Root Biomass of 9-Year-Old Red Oak, White Oak, and Shortleaf Pine in a Missouri Ozark Forest. 17th Central Hardwood Forest Conference 2010, 78, 323. [Google Scholar]
- Lyczak, S.J.; Kabrick, J.M.; Knapp, B.O. Long-Term Effects of Organic Matter Removal, Compaction, and Vegetation Control on Tree Survival and Growth in Coarse-Textured, Low-Productivity Soils. For Ecol Manage 2021, 496, 119428. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production. 2005.
- Cairns, J.E.; Sonder, K.; Zaidi, P.H.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.K.; Das, B.; Govaerts, B.; Vinayan, M.T.; et al. Maize Production in a Changing Climate. Impacts, Adaptation, and Mitigation Strategies. Advances in Agronomy 2012, 114, 1–58. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Koocheki, A.; Leifert, C. Soil Management for Sustainable Crop Disease Control: A Review. Environ Chem Lett 2008, 6, 149–162. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C. Tudi2021.Pdf. Environmental Rsearch and public health 2021, 18, 1–23. [Google Scholar]
- Fuss, C.B.; Driscoll, C.T.; Campbell, J.L. Recovery from Chronic and Snowmelt Acidification: Long-Term Trends in Stream and Soil Water Chemistry at the Hubbard Brook Experimental Forest, New Hampshire, USA. J Geophys Res Biogeosci 2015, 120, 2360–2374. [Google Scholar] [CrossRef]
- Arellano, G.; Medina, N.G.; Tan, S.; Mohamad, M.; Davies, S.J. Crown Damage and the Mortality of Tropical Trees. New Phytologist 2019, 221, 169–179. [Google Scholar] [CrossRef]
- Multi-Resolution Land Characteristics Consortium. USGS. Available online: https://www.mrlc.gov/data?f%5B0%5D=year%3A2019 (accessed on 2 February 2023).
- USDA Forest Inventory and Analysis Dataamart. Available online: https://apps.fs.usda.gov/fia/datamart/datamart.html (accessed on 23 June 2021).
- Food and Agriculture Organization of the United Nations. FAO Soils Portal – World Reference Base Map. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/other-global-soil-maps-and-databases/en/ (accessed on 22 April 2023).
- USDA Natural Resource Conservation Service. Gridded National Soil Survey Geographic Database (gNATSGO). Available online: https://nrcs.app.box.com/v/soils/folder/191785692827 (accessed on 4 April 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).