Submitted:
25 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
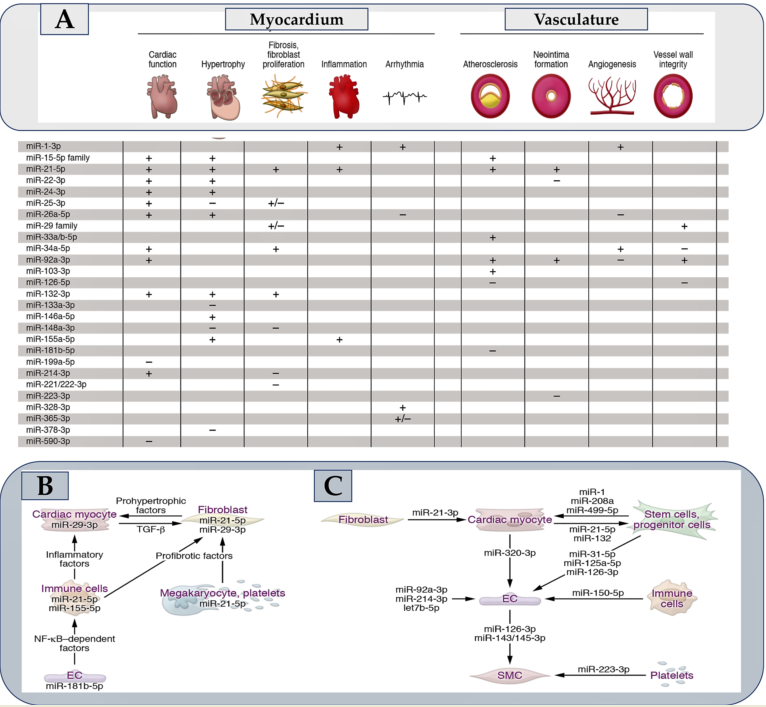
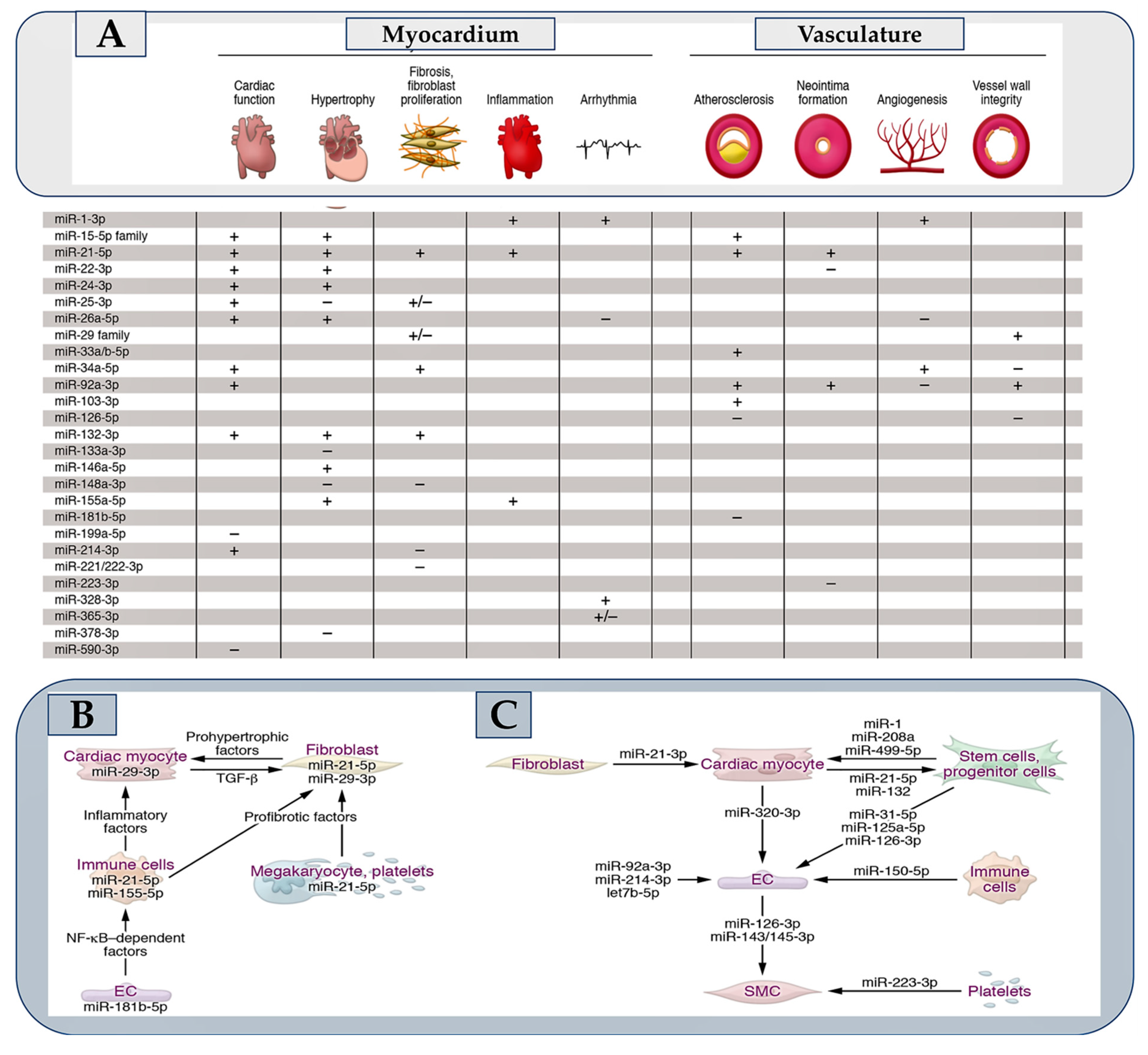
Keywords:
1. Introduction
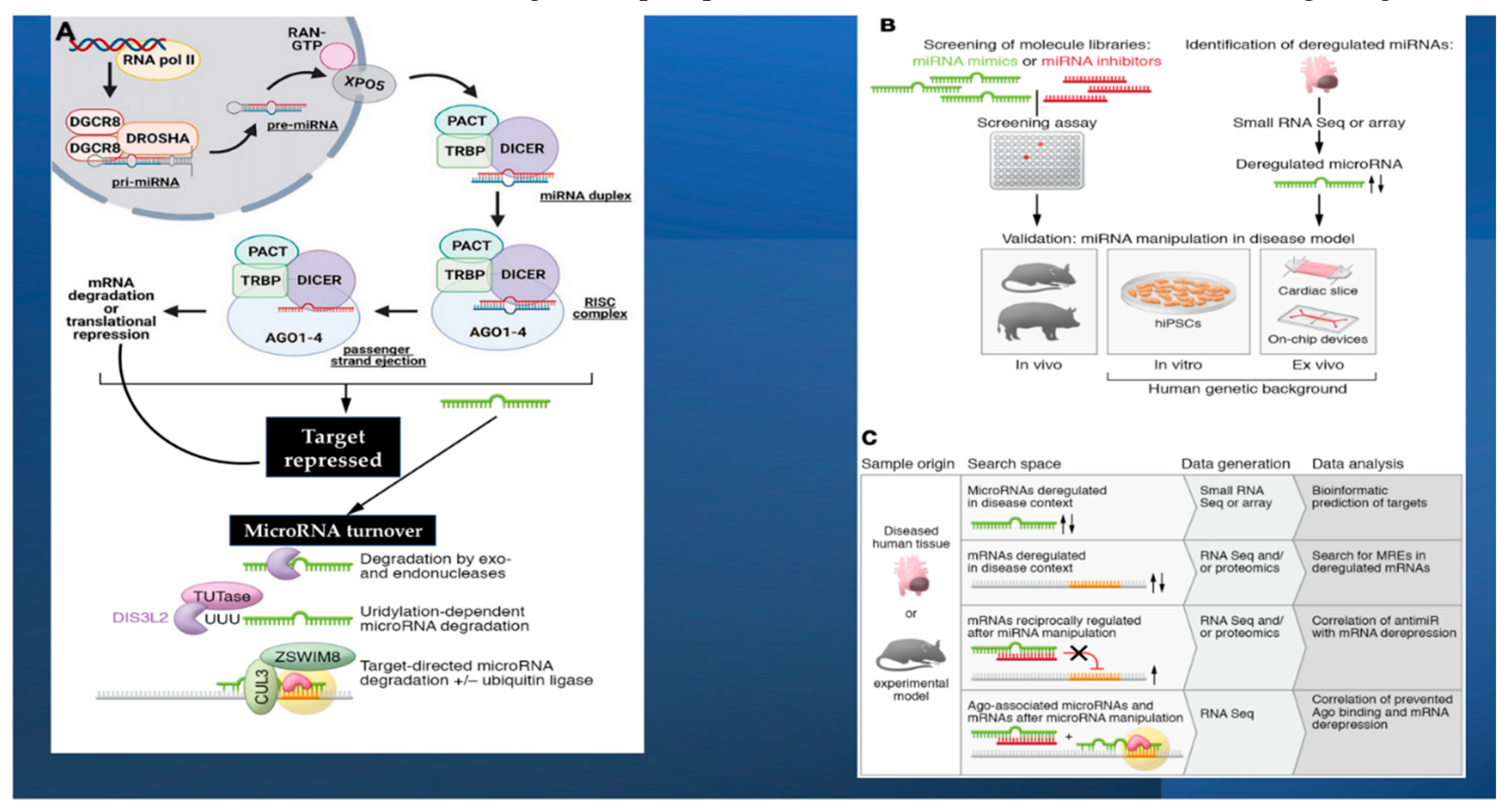
2. MicroRNAs Pathophysiologic Surroundings: Insight on Biogenesis, Stability, and Strand Bias of microRNAs
3. Searching Roles of microRNAs in the Cardiovascular System
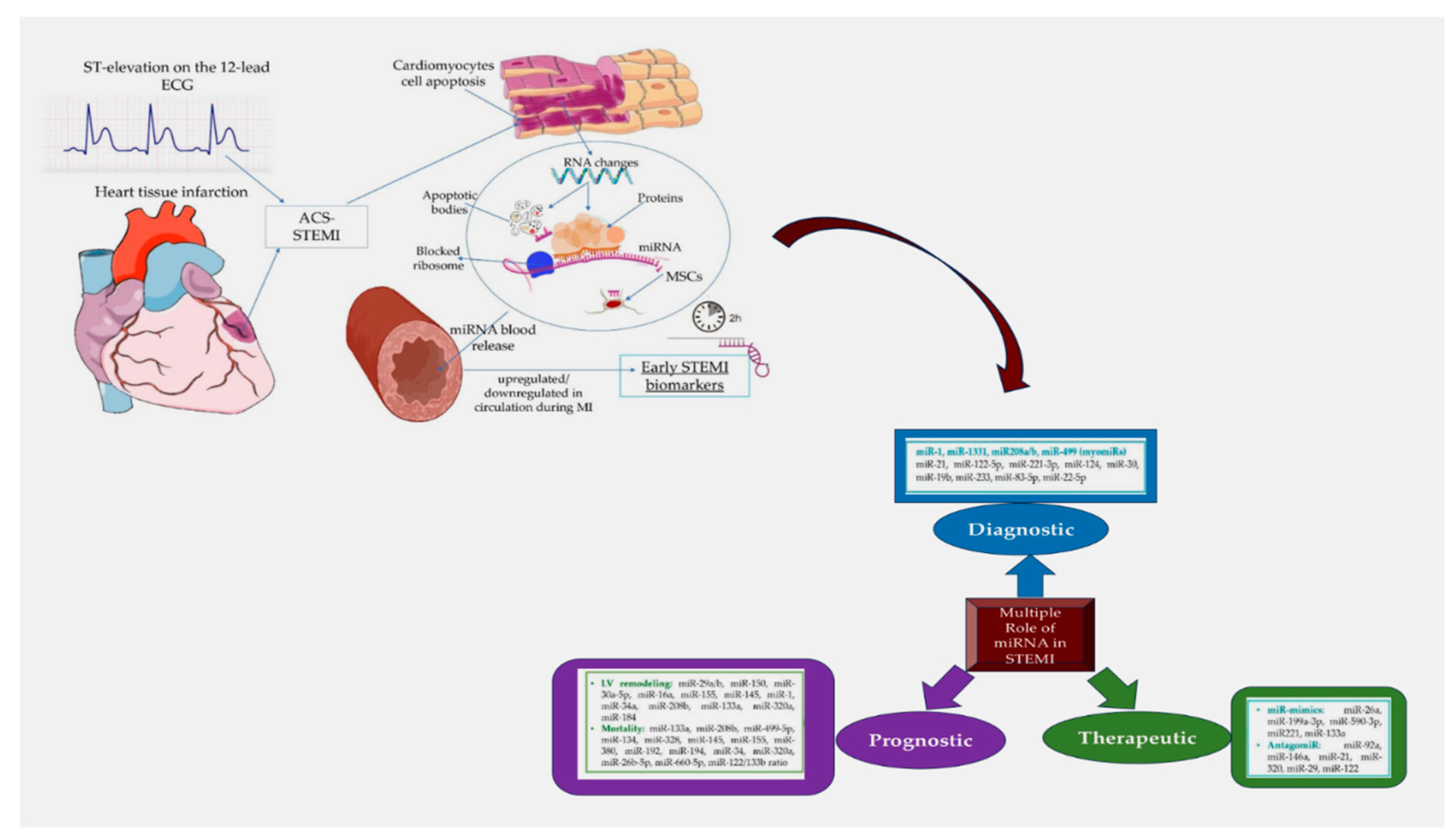
4. MicroRNAs as Circulating Biomarkers in Acute Coronary Syndromes
5. Insight into the Diagnostic and Prognostic Value of Circulating microRNAs in Coronary Artery Disease. Evaluation of the New Disease Diagnosis Guide for Stable CAD and Acute Coronary Syndrome
5.1. MicroRNAs and Coronary Artery Disease
5.2. MicroRNAs and Acute Coronary Syndrome
6. Implication of miRNA in Stent Restenosis
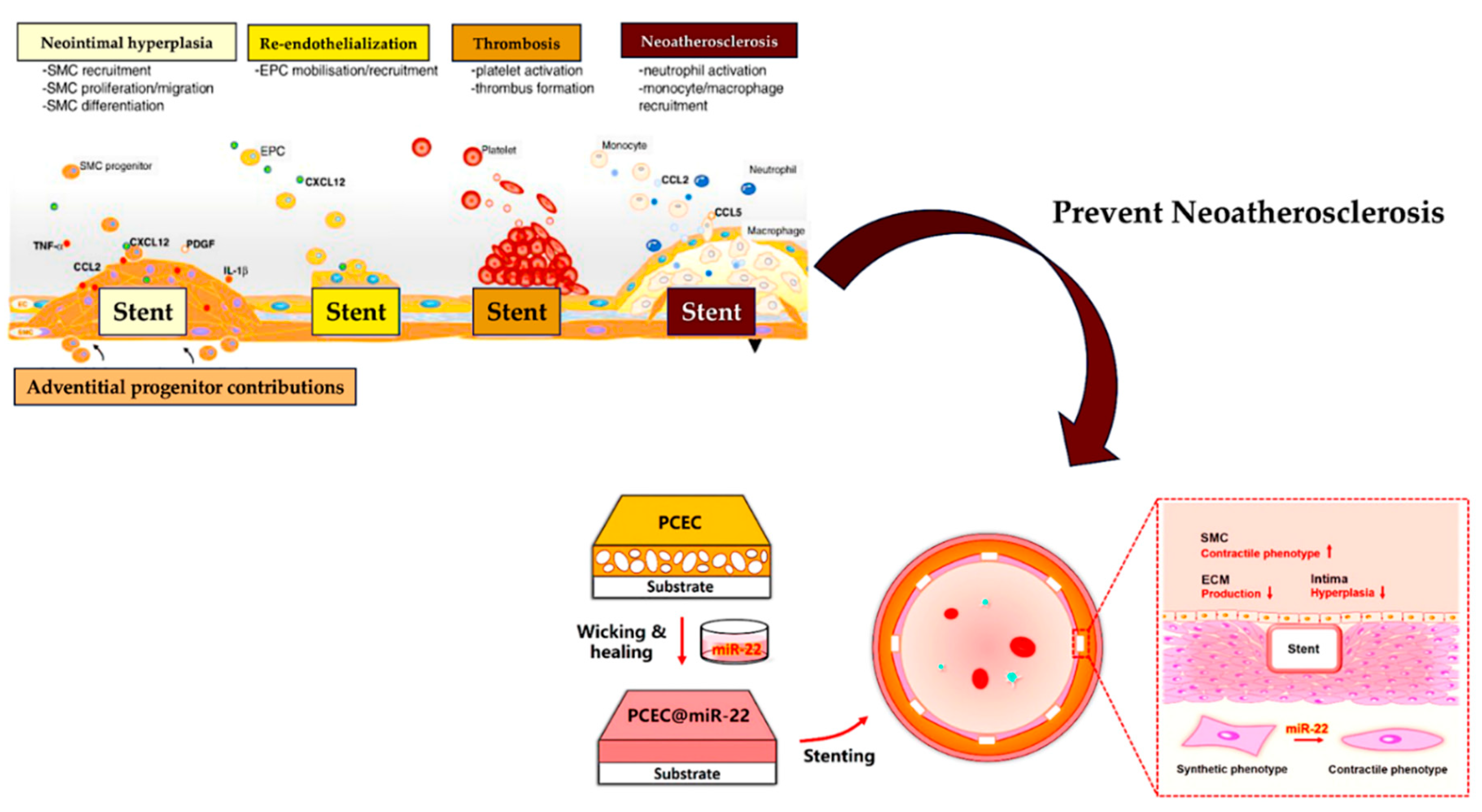
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochron ic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993, 75, 843–854. [CrossRef]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 medi- ates temporal pattern formation in C. elegans. Cell. 1993, 75, 855–862.
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. https://doi.org/10.1101/gr.082701.108. [CrossRef]
- Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47(d1): D155–D162. https://doi.org/10.1093/nar/gky1141. [CrossRef]
- Fromm B, Domanska D, Høye E, Ovchinnikov V, Kang W, Aparicio-Puerta E, Johansen M, Flatmark K, Mathelier A, Hovig E, Hackenberg M, Friedländer MR, Peterson KJ. MirGeneDB 2.0: the metazo- an microRNA complement. Nucleic Acids Res. 2020, 48, 132–141.
- Kim K, Baek SC, Lee YY, Bastiaanssen C, Kim J, Kim H, Kim VN. A quantitative map of human primary microRNA processing sites. Mol Cell. 2021, 81, 3422–3439. https://doi.org/10.1016/j.molcel.2021.07.002. [CrossRef]
- Matsui M, Prakash TP, Corey DR. Argonaute 2-dependent reg- ulation of gene expression by single-stranded miRNA mimics. Mol Ther. 2016, 24, 946–955.
- Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012, 492, 376–381. [CrossRef]
- Hinkel R, Ramanujam D, Kaczmarek V, Howe A, Klett K, Beck C, Dueck A, Thum T, Laugwitz KL, Maegdefessel L, Weber C, Kupatt C, Engelhardt S.AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J Am Coll Cardiol. 2020, 75, 1788–1800. [CrossRef]
- Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, Leierseder S, Loyer X, Giacca M, Zentilin L, Thum T, Laggerbauer B, Engelhardt S.MiR-378 controls cardiac hyper- trophy by combined repression of mitogen- activated protein kinase pathway factors. Circula- tion. 2013, 127, 2097–2106.
- Sassi Y, Avramopoulos P, Ramanujam D, Grüter L, Werfel S, Giosele S, Brunner AD, Esfandyari D, Papadopoulou AS, De Strooper B, Hübner N, Kumarswamy R, Thum T, Yin X, Mayr M, Laggerbauer B, Engelhardt S. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activat- ing Wnt signaling. Nat Commun. 2017, 8, 1614.
- Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008, 456, 980–984. [CrossRef]
- Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res.2007, 100, 1579–1588. https://doi.org/10.1161/circresaha.106.141986. [CrossRef]
- Ramanujam D, Sassi Y, Laggerbauer B, Engelhardt S. Viral vector-based targeting of miR-21 in cardiac nonmyocyte cells reduces pathologic remodeling of the heart. Mol Ther. 2016, 24, 1939–1948. https://doi.org/10.1038/mt.2016.166. [CrossRef]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR- 29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008, 105, 13027–13032.
- Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011, 109, 1115–1119. https://doi.org/10.1161/circresaha.111.255737. [CrossRef]
- Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of micro- RNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012, 122, 497–506. [CrossRef]
- McDonald RA, White KM, Wu J, Cooley BC, Robertson KE, Halliday CA, McClure JD, Francis S, Lu R, Kennedy S, George SJ, Wan S, van Rooij E, Baker AH. miRNA-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. Eur Heart J. 2013, 34, 1636–1643. [CrossRef]
- Eken SM, Christersdottir T, Winski G, Sangsuwan T, Jin H, Chernogubova E, Pirault J, Sun C, Simon N, Winter H, Backlund A, Haghdoost S, Hansson GK, Halle M, Maegdefessel L. miR-29b mediates the chronic inflammatory response in radiotherapy- induced vascular disease. JACC Basic Transl Sci. 2019, 4, 72–82.
- Zeng Z, Xia L, Fan X, Ostriker AC, Yarovinsky T, Su M, Zhang Y, Peng X, Xie Y, Pi L, Gu X, Chung SK, Martin KA, Liu R, Hwa J, Tang WH. Platelet-derived miR-223 promotes a phenotypic switch in arterial injury repair. J Clin Invest. 2019, 129, 1372–1386. [CrossRef]
- Hinkel R, Penzkofer D, Zühlke S, Fischer A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C, Dimmeler S. Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model. Circulation. 2013, 128, 1066–1075.
- Foinquinos A, Batkai S, Genschel C, Viereck J, Rump S, Gyöngyösi M, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Weber N, Zlabinger K, Hašimbegović E, Winkler J, Fiedler J, Dangwal S, Fischer M, de la Roche J, Wojciechowski D, Kraft T, Garamvölgyi R, Neitzel S, Chatterjee S, Yin X, Bär C, Mayr M, Xiao K, Thum T. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat Commun. 2020, 11, 633.
- Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SL, Hazebroek M, van Leeuwen R, Gijbels MJ, Wijnands E, Biessen EA, De Winther MP, Stassen FR, Carmeliet P, Kauppinen S, Schroen B, Heymans S. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res. 2012, 111, 415–425. [CrossRef]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S.MicroRNA-92a controls angio- genesis and functional recovery of ischemic tissues in mice. Science. 2009, 324, 1710–1713.
- Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regu- lates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012; 3:1078.
- Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stöger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schürmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, de Windt LJ, Lutgens E, Wouters K, de Winther MP, Zacchigna S, Giacca M, van Bilsen M, Papageorgiou AP, Schroen B. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013, 128, 1420–1432. [CrossRef]
- Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, Lu LF, Rudensky AY, Baltimore D. An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Commun. 2017, 8, 851.
- Pankratz F, Bemtgen X, Zeiser R, Leonhardt F, Kreuzaler S, Hilgendorf I, Smolka C, Helbing T, Hoefer I, Esser JS, Kustermann M, Moser M, Bode C, Grundmann S. MicroRNA-155 exerts cell- specific antiangiogenic but proarteriogenic effects during adaptive neovascularization. Circulation. 2015, 131, 1575–1589. [CrossRef]
- Loyer X, Potteaux S, Vion AC, Guérin CL, Boulkroun S, Rautou PE, Ramkhelawon B, Esposito B, Dalloz M, Paul JL, Julia P, Maccario J, Boulanger CM, Mallat Z, Tedgui A. Inhibition of microRNA-92a pre- vents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014, 114, 434–443.
- 30. Daniel JM, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, Bonauer A, Boon RA, Fischer A, Bauersachs J, van Rooij E, Dimmeler S, Sedding DG. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014, 103, 564–572. [CrossRef]
- Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomy- opathy. J Clin Invest. 2013, 123, 2143–2154.
- Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP; MICU Registry; Blackwell TS, Baron RM, Feinberg MW. MicroRNA-181b regulates NF-κB– mediated vascular inflammation. J Clin Invest. 2012, 122, 1973–1990.
- da Costa Martins PA, Salic K, Gladka MM, Armand AS, Leptidis S, el Azzouzi H, Hansen A, Coenen-de Roo CJ, Bierhuizen MF, van der Nagel R, van Kuik J, de Weger R, de Bruin A, Condorelli G, Arbones ML, Eschenhagen T, De Windt LJ. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. 2010, 12, 1220–1227. https://doi.org/10.1038/ncb2126. [CrossRef]
- Duygu B, Poels EM, Juni R, Bitsch N, Ottaviani L, Olieslagers S, de Windt LJ, da Costa Martins PA. miR-199b-5p is a regulator of left ventricular remodeling following myocardial infarction. Noncoding RNA Res. 2017, 2, 18–26. https://doi.org/10.1016/j.ncrna.2016.12.002. [CrossRef]
- van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress- responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006, 103, 18255–18260. https://doi.org/10.1073/pnas.0608791103. [CrossRef]
- Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012, 122, 1222–1232. https://doi.org/10.1172/jci59327. [CrossRef]
- Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise- induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. https://doi.org/10.1016/j.cmet.2015.02.014. [CrossRef]
- Verjans R, Peters T, Beaumont FJ, van Leeuwen R, van Herwaarden T, Verhesen W, Munts C, Bijnen M, Henkens M, Diez J, de Windt LJ, van Nieuwenhoven FA, van Bilsen M, Goumans MJ, Heymans S, González A, Schroen B. MicroRNA-221/222 family counteracts myocardial fibrosis in pressure overload-induced heart failure. Hypertension. 2018, 71, 280–288.
- Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007, 13, 613–618.
- Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Zhang S, Hajjar RJ, Lebeche D. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload- induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc. 2013, 2, 17–19. https://doi.org/10.1161/jaha.113.000078. [CrossRef]
- Besser J, Malan D, Wystub K, Bachmann A, Wietelmann A, Sasse P, Fleischmann BK, Braun T, Boettger T. MiRNA-1/133a clusters regulate adrenergic control of cardiac repolarization. PLoS One. 2014, 9, e113449.
- Tijsen AJ, van der Made I, van den Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, Alekseev S, Fluiter K, Schroen B, Goumans MJ, van der Velden J, Duncker DJ, Pinto YM, Creemers EE. The microRNA-15 family inhibits the TGFβ-pathway in the heart. Cardiovasc Res. 2014, 104, 61–71. [CrossRef]
- Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 pro- tects against cardiac ischemic injury. Circ Res. 2012, 110, 71–81.
- Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013, 110, 187–192. doi:10.1073/pnas.1208863110. [CrossRef]
- Fiedler J, Stöhr A, Gupta SK, Hartmann D, Holzmann A, Just A, Hansen A, Hilfiker-Kleiner D, Eschenhagen T, Thum T. Functional microRNA library screening identifies the hypoxamir miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid Redox Signal. 2014, 21, 1167–1176. [CrossRef]
- Meloni M, Marchetti M, Garner K, Littlejohns B, Sala-Newby G, Xenophontos N, Floris I, Suleiman MS, Madeddu P, Caporali A, Emanueli C. Local inhibition of micro- RNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013, 21, 1390–1402. [CrossRef]
- Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Müller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013, 495, 107–110. [CrossRef]
- Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, Milano G, Brambilla F, Saccu C, Bianchi ME, Pompilio G, Capogrossi MC, Raucci A. miR-34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (sirtuin 1) and AXL (AXl receptor tyro- sine kinase). Arterioscler Thromb Vasc Biol. 2018, 38, 2079–2090.
- Griesemer D, Xue JR, Reilly SK, Ulirsch JC, Kukreja K, Davis JR, Kanai M, Yang DK, Butts JC, Guney MH, Luban J, Montgomery SB, Finucane HK, Novina CD, Tewhey R, Sabeti PC. Genome-wide functional screen of 3′UTR variants uncovers causal vari- ants for human disease and evolution. Cell. 2021, 184, 5247–5260.
- Ramanujam D, Schön AP, Beck C, Vaccarello P, Felician G, Dueck A, Esfandyari D, Meister G, Meitinger T, Schulz C, Engelhardt S. MicroRNA-21-dependent macrophage-to-fibroblast signaling determines the cardiac response to pressure overload. Circu- lation. 2021, 143, 1513–1525. https://doi.org/10.1161/circulationaha.120.050682. [CrossRef]
- Huang CK, Kafert-Kasting S, Thum T. Preclinical and clinical development of noncoding RNA therapeutics for cardio- vascular disease. Circ Res. 2020, 126, 663–678.
- Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast–derived micro- RNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014, 124, 2136–2146. https://doi.org/10.1172/jci70577. [CrossRef]
- Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, Li Q, Xiang C, Zhang L, Wu R, Zhu W, Yu H, Hu X, Wang J. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med. 2017, 6, 209–222. https://doi.org/10.5966/sctm.2015-0386. [CrossRef]
- Cheng M, Yang J, Zhao X, Zhang E, Zeng Q, Yu Y, Yang L, Wu B, Yi G, Mao X, Huang K, Dong N, Xie M, Limdi NA, Prabhu SD, Zhang J, Qin G. Circulating myocardial micro- RNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun. 2019, 10, 959. [CrossRef]
- Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y, Zhu Z, Li D, Wang T, Liu K. The role of exosomes and exosomal microRNA in cardiovascular disease. Front Cell Dev Biol. 2021; 8:1–15. https://doi.org/10.3389/fcell.2020.616161.
- Kesidou D, da Costa Martins PA, de Windt LJ, Brittan M, Beqqali A, Baker AH. Extracellular vesicle miRNAs in the promotion of cardiac neovascularisation. Front Physiol. 2020; 11:579892. doi:10.3389/fphys.2020.579892. [CrossRef]
- Ottaviani L, Sansonetti M, da Costa Martins PA. Myocardial cell-to-cell communication via microRNAs. Noncoding RNA Res. 2018, 3, 144–153. [CrossRef]
- Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009, 2, ra81. doi:10.1126/scisignal.2000610. [CrossRef]
- Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011 Jan 20;469(7330):336-42. https://doi.org/10.1038/nature09783. [CrossRef]
- Condorelli G, Dimmeler S. MicroRNAs: components of an integrated system controlling cardiac development, physiology, and disease pathogenesis. Cardiovasc Res. 2008 Sep 1;79(4):551-2. [CrossRef]
- Pratt AJ, MacRae IJ. The RNA-induced silencing complex: a versatile gene-silencing machine. J Biol Chem. 2009 Jul 3;284(27):17897-901. https://doi.org/10.1074/jbc.r900012200. [CrossRef]
- Kobayashi H, Tomari Y. RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochim Biophys Acta. 2016 Jan;1859(1):71-81. https://doi.org/10.1016/j.bbagrm.2015.08.007. [CrossRef]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009 Jan 23;136(2):215-33. https://doi.org/10.1016/j.cell.2009.01.002. [CrossRef]
- Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3’ untranslated region in mammalian mRNAs. Nat Struct Mol Biol. 2009 Feb;16(2):144-50.
- Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014 Jan;15(1):1-19.
- Bartel DP. Metazoan microRNAs. Cell. 2018, 173, 20–51.
- Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective micro- RNA target sites in mammalian mRNAs. Elife. 2015; 4:1–38. https://doi.org/10.7554/elife.05005. [CrossRef]
- Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE. Pairing beyond the seed sup- ports MicroRNA targeting specificity. Mol Cell. 2016, 64, 320–333.
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007, 27, 91–105. [CrossRef]
- Gebert LFR, MacRae IJ. Regulation of micro- RNA function in animals. Nat Rev Mol Cell Biol. 2019, 20, 21–37. https://doi.org/10.1038/s41580-018-0045-7. [CrossRef]
- Yang A, Bofill-De Ros X, Shao TJ, Jiang M, Li K, Villanueva P, Dai L, Gu S. 3′ Uridylation confers miRNAs with non-canonical target repertoires. Mol Cell. 2019, 75, 511–522.e4. https://doi.org/10.1016/j.molcel.2019.05.014. [CrossRef]
- van der Kwast RVCT, Parma L, van der Bent ML, van Ingen E, Baganha F, Peters HAB, Goossens EAC, Simons KH, Palmen M, de Vries MR, Quax PHA, Nossent AY. Adenosine-to-inosine editing of vasoactive microRNAs alters their targetome and function in ischemia. Mol Ther Nucleic Acids. 2020; 21:932–953. https://doi.org/10.1016/j.omtn.2020.07.020. [CrossRef]
- McGahon MK, Yarham JM, Daly A, Guduric-Fuchs J, Ferguson LJ, Simpson DA, Collins A. Distinctive profile of IsomiR expression and novel microRNAs in rat heart left ventricle. PLoS One. 2013, 8, e65809. https://doi.org/10.1371/journal.pone.0065809. [CrossRef]
- van der Kwast RVCT, van Ingen E, Parma L, Peters HAB, Quax PHA, Nossent AY. Adenosine-to-inosine editing of MicroRNA-487b alters target gene selection after ischemia and promotes neovascu- larization. Circ Res. 2018, 122, 444–456.
- van der Kwast RVCT, Woudenberg T, Quax PHA, Nossent AY. MicroRNA-411 and its 5′-isomiR have distinct targets and functions and are differentially regulated in the vasculature under ischemia. Mol Ther. 2020, 28, 157–170.
- Kingston ER, Bartel DP. Global analyses of the dynamics of mammalian microRNA metabolism. Genome Res. 2019, 29, 1777–1790. https://doi.org/10.1101/gr.251421.119. [CrossRef]
- Marzi MJ, Ghini F, Cerruti B, de Pretis S, Bonetti P, Giacomelli C, Gorski MM, Kress T, Pelizzola M, Muller H, Amati B, Nicassio F. Degradation dynamics of micro- RNAs revealed by a novel pulse-chase approach. Genome Res. 2016, 26, 554–565. https://doi.org/10.1101/gr.198788.115. [CrossRef]
- Han J, LaVigne CA, Jones BT, Zhang H, Gillett F, Mendell JT. A ubiquitin ligase mediates target-directed microRNA decay inde- pendently of tailing and trimming. Science. 2020, 370, eabc9546.
- Shi CY, Kingston ER, Kleaveland B, Lin DH, Stubna MW, Bartel DP. The ZSWIM8 ubiquitin ligase medi- ates target-directed microRNA degradation. Science. 2020, 370, eabc9359.
- Bitetti A, Mallory AC, Golini E, Carrieri C, Carreño Gutiérrez H, Perlas E, Pérez-Rico YA, Tocchini-Valentini GP, Enright AJ, Norton WHJ, Mandillo S, O’Carroll D, Shkumatava A. MicroRNA degradation by a con- served target RNA regulates animal behavior. Nat Struct Mol Biol. 2018, 25, 244–251.
- Kalayinia S, Arjmand F, Maleki M, Malakootian M, Singh CP. MicroRNAs: roles in cardiovascular development and disease. Cardiovasc Pathol. 2021 Jan-Feb; 50:107296. doi:10.1016/j.carpath.2020.107296. [CrossRef]
- Sayed D, Abdullatif M. MicroRNAs in development and disease. Physiol Rev. 2011 Jul;91(3):827-87.
- Ma Q, Zhang L, Pearce WJ. MicroRNAs in brain development and cerebrovascular pathophysiology.Am J Physiol Cell Physiol. 2019 Jul 1;317(1):C3-C19. https://doi.org/10.1152/ajpcell.00022.2019. [CrossRef]
- Motshwari DD, Matshazi DM, Erasmus RT, Kengne AP, Matsha TE, George C. MicroRNAs Associated with Chronic Kidney Disease in the General Population and High-Risk Subgroups-A Systematic Review. Int J Mol Sci. 2023 Jan 16;24(2):1792. [CrossRef]
- Cheng Y, Du Y, Wang Q, Lv Q, Xue Y, Zhou W, Zhang C, Chen X, Wang D. Human cytomegalovirus-encoded microRNAs expression profile in plasma of patients with aortic dissection. J Cardiothorac Surg. 2023 Jan 18;18(1):39. [CrossRef]
- Quiat D, Olson EN. MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest. 2013 Jan;123(1):11-8. [CrossRef]
- Wu J, Song J, Wang C, Niu D, Li H, Liu Y, Ma L, Yu R, Chen X, Zen K, Yang Q, Zhang C, Zhang CY, Wang J. Identification of serum microRNAs for cardiovascular risk stratification in dyslipidemia subjects. Int J Cardiol. 2014 Mar 1;172(1):232-4. [CrossRef]
- Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012 Apr;23(2):91-7. [CrossRef]
- Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):186-92. [CrossRef]
- Caporali A, Miscianinov V, Saif J, Emanueli C. MicroRNA transport in cardiovascular complication of diabetes. Biochim Biophys Acta. 2016 Dec;1861(12 Pt B):2111-2120. https://doi.org/10.1016/j.bbalip.2016.01.010. [CrossRef]
- Mayr M, Zampetaki A, Willeit P, Willeit J, Kiechl S. MicroRNAs within the continuum of postgenomics biomarker discovery. Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):206-14. https://doi.org/10.1161/atvbaha.112.300141. [CrossRef]
- Smith JG, Gerszten RE. Emerging Affinity-Based Proteomic Technologies for Large-Scale Plasma Profiling in Cardiovascular Disease. Circulation. 2017 Apr 25;135(17):1651-1664. https://doi.org/10.1161/circulationaha.116.025446. [CrossRef]
- Santovito D, Egea V, Bidzhekov K, Natarelli L, Mourão A, Blanchet X, Wichapong K, Aslani M, Brunßen C, Horckmans M, Hristov M, Geerlof A, Lutgens E, Daemen MJAP, Hackeng T, Ries C, Chavakis T, Morawietz H, Naumann R, von Hundelshausen P, Steffens S, Duchêne J, Megens RTA, Sattler M, Weber C. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endo- thelial protection by autophagy in atherosclerosis. Sci Transl Med. 2020, 12, 1–16.
- Li H, Zhan J, Zhao Y, Fan J, Yuan S, Yin Z, Dai B, Chen C, Wang DW. Identification of ncRNA-mediated functions of nucleus-localized miR-320 in cardiomyocytes. Mol Ther Nucleic Acids. 2020; 19:132–143.
- Elbarbary RA, Miyoshi K, Myers JR, Du P, Ashton JM, Tian B, Maquat LE. Tudor-SN-mediated endonucleolytic decay of human cell micro- RNAs promotes G1/S phase transition. Science. 2017, 356, 859–862.
- Yang A, Shao TJ, Bofill-De Ros X, Lian C, Villanueva P, Dai L, Gu S. AGO-bound mature miRNAs are oli- gouridylated by TUTs and subsequently degraded by DIS3L2. Nat Commun. 2020, 11, 1–13.
- Abplanalp WT, Fischer A, John D, Zeiher AM, Gosgnach W, Darville H, Montgomery R, Pestano L, Allée G, Paty I, Fougerousse F, Dimmeler S. Efficiency and target derepres- sion of anti-miR-92a: results of a first in human study. Nucleic Acid Ther. 2020, 30, 335–345.
- Anastasiadou E, Seto AG, Beatty X, Hermreck M, Gilles ME, Stroopinsky D, Pinter-Brown LC, Pestano L, Marchese C, Avigan D, Trivedi P, Escolar DM, Jackson AL, Slack FJ. Cobomarsen, an oligo- nucleotide inhibitor of miR-155, slows DLBCL tumor cell growth in vitro and in vivo. Clin Cancer Res. 2021, 27, 1139–1149.
- Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Zlabinger K, Hašimbegović E, Winkler J, Garamvölgyi R, Neitzel S, Gyöngyösi M, Thum T. CDR132L improves systol- ic and diastolic function in a large animal model of chronic heart failure. Eur Heart J. 2021, 42, 192–201.
- Hinkel R, Batkai S, Bähr A, Bozoglu T, Straub S, Borchert T, Viereck J, Howe A, Hornaschewitz N, Oberberger L, Jurisch V, Kozlik-Feldmann R, Freudenthal F, Ziegler T, Weber C, Sperandio M, Engelhardt S, Laugwitz KL, Moretti A, Klymiuk N, Thum T, Kupatt C.AntimiR-132 attenuates myocar- dial hypertrophy in an animal model of percu- taneous aortic constriction. J Am Col Cardiol. 2021, 77, 2923–2935.
- Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, Rode L, Weigt H, Genschel C, Lorch U, Theek C, Levin AA, Bauersachs J, Solomon SD, Thum T. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. 2021, 42, 178–188. https://doi.org/10.1093/eurheartj/ehaa898. [CrossRef]
- Underlying Cause of Death 1999-2020—CDC WONDER. https://wonder.cdc.gov/wonder/help/ucd.html.
- World Health Organization, The Top 10 Causes of Death, 2014.
- Writing Committee Members; Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, Di Maio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr, Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 Jan 18;79(2):e21-e129.
- Writing Committee, Michael C. Kontos, James A. de Lemos, Steven B. Deitelzweig, Deborah B. Diercks, M. Odette Gore, Erik P. Hess, Cian P. McCarthy, James K. McCord, Paul I. Musey, Todd C. Villines, and Leesa J. Wright. 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee J Am Coll Cardiol. 2022 Nov, 80 (20) 1925–1960.
- Writing Committee Members; Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, Diercks DB, Gentile F, Greenwood JP, Hess EP, Hollenberg SM, Jaber WA, Jneid H, Joglar JA, Morrow DA, O’Connor RE, Ross MA, Shaw LJ.2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021 Nov 30;78(22):2218-2261. https://doi.org/10.1161/cir.0000000000001030. [CrossRef]
- Avdikos G, Michas G, Smith SW. From Q/Non-Q Myocardial Infarction to STEMI/NSTEMI: Why It’s Time to Consider Another Simplified Dichotomy; a Narrative Literature Review. Arch Acad Emerg Med. 2022 Oct 1;10(1): e78.
- Miyachi H, Takagi A, Miyauchi K, Yamasaki M, Tanaka H, Yoshikawa M, Saji M, Suzuki M, Yamamoto T, Shimizu W, Nagao K, Takayama M. Current characteristics and management of ST elevation and non-ST elevation myocardial infarction in the Tokyo metropolitan area: from the Tokyo CCU network registered cohort. Heart Vessels. 2016 Nov;31(11):1740-1751. https://doi.org/10.1007/s00380-015-0791-9. [CrossRef]
- Khan AR, Golwala H, Tripathi A, Bin Abdulhak AA, Bavishi C, Riaz H, Mallipedi V, Pandey A, Bhatt DL. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2017 Nov 1;38(41):3082-3089. https://doi.org/10.1093/eurheartj/ehx418. [CrossRef]
- Mitka M. New definition of myocardial infarction puts biomarkers front and center. JAMA. 2012 Oct 17;308(15):1511-2. https://doi.org/10.1001/jama.2012.12794. [CrossRef]
- Möckel M, Giannitsis E, Mueller C, Huber K, Jaffe AS, Mair J, Plebani M, Thygesen K, Lindahl B; Biomarker Study Group of the European Society of Cardiology Acute Cardiovascular Care Association. Editor’s Choice-Rule-in of acute myocardial infarction: Focus on troponin. Eur Heart J Acute Cardiovasc Care. 2017 Apr;6(3):212-217.
- Reichlin T, Cullen L, Parsonage WA, Greenslade J, Twerenbold R, Moehring B, Wildi K, Mueller S, Zellweger C, Mosimann T, Rubini Gimenez M, Rentsch K, Osswald S, Müller C. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med. 2015 Apr;128(4):369-79. e4. https://doi.org/10.1016/j.amjmed.2014.10.032. [CrossRef]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008 Jul 29;105(30):10513-8. https://doi.org/10.1073/pnas.0804549105. [CrossRef]
- Zampetaki A, Willeit P, Drozdov I, Kiechl S, Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res. 2012 Mar 15;93(4):555-62. https://doi.org/10.1093/cvr/cvr266. [CrossRef]
- Felekkis K, Papaneophytou C. Challenges in Using Circulating Micro-RNAs as Biomarkers for Cardiovascular Diseases. Int J Mol Sci. 2020 Jan 15;21(2):561. https://doi.org/10.3390/ijms21020561. [CrossRef]
- Zhao Z, Guo N, Chen W, Wang Z. Leveraging Extracellular Non-coding RNAs to Diagnose and Treat Heart Diseases. J Cardiovasc Transl Res. 2022 Jun;15(3):456-468. https://doi.org/10.1007/s12265-022-10252-x. [CrossRef]
- Shen NN, Wang JL, Fu YP. The microRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2022 Jun 15; 9:856358. https://doi.org/10.3389/fcvm.2022.856358. [CrossRef]
- Laggerbauer B, Engelhardt S. MicroRNAs as therapeutic targets in cardiovascular disease. J Clin Invest. 2022 Jun 1;132(11): e159179. https://doi.org/10.1172/jci159179. [CrossRef]
- Yan B, Wang H, Tan Y, Fu W. microRNAs in Cardiovascular disease: Small Molecules but Big Roles. Curr. Top Med. Chem. 2019, 19, 1918–1947. [CrossRef]
- Lucas T, Bonauer A, Dimmeler S. RNA Therapeutics in Cardiovascular Disease. Circ Res. 2018 Jul 6;123(2):205-220. https://doi.org/10.1161/circresaha.117.311311. [CrossRef]
- Parizadeh SM, Ferns GA, Ghandehari M, Hassanian SM, Ghayour-Mobarhan M, Parizadeh SMR, Avan A. The diagnostic and prognostic value of circulating microRNAs in coronary artery disease: A novel approach to disease diagnosis of stable CAD and acute coronary syndrome. J Cell Physiol. 2018 Sep ;233(9):6418-6424. https://doi.org/10.1002/jcp.26324. [CrossRef]
- Hang P, Guo J, Sun C, Du Z. MicroRNAs as Candidate Drug Targets for Cardiovascular Diseases. Curr Drug Targets. 2017, 18, 463–472. https://doi.org/10.2174/1389450117666160301101221. [CrossRef]
- Zeller T, Keller T, Ojeda F, Reichlin T, Twerenbold R, Tzikas S, Wild PS, Reiter M, Czyz E, Lackner KJ, Munzel T, Mueller C, Blankenberg S Assessment of microRNAs in patients with unstable angina pectoris. Eur Heart J. 2014 Aug 14;35(31):2106-14.
- D’Alessandra Y, Carena MC, Spazzafumo L, Martinelli F, Bassetti B, Devanna P, Rubino M, Marenzi G, Colombo GI, Achilli F, Maggiolini S, Capogrossi MC, Pompilio G.Diagnostic potential of plasmatic MicroRNA signatures in stable and unstable angina. PLoS One. 2013 Nov 15;8(11): e80345. https://doi.org/10.1371/journal.pone.0080345. [CrossRef]
- Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010 Sep 3;107(5):677-84. https://doi.org/10.1161/circresaha.109.215566. [CrossRef]
- Ren J, Zhang J, Xu N, Han G, Geng Q, Song J, Li S, Zhao J, Chen H. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One. 2013 Dec 5;8(12): e80738. https://doi.org/10.1371/journal.pone.0080738. [CrossRef]
- Lindsell CJ, Anantharaman V, Diercks D, Han JH, Hoekstra JW, Hollander JE, Kirk JD, Lim SH, Peacock WF, Tiffany B, Wilke EK, Gibler WB, Pollack CV Jr; EMCREG-International i*trACS Investigators. The Internet Tracking Registry of Acute Coronary Syndromes (i*trACS): a multicenter registry of patients with suspicion of acute coronary syndromes reported using the standardized reporting guidelines for emergency department chest pain studies. Ann Emerg Med. 2006 Dec;48(6):666-77, 677.e1-9.
- Su M, Niu Y, Dang Q, Qu J, Zhu D, Tang Z, Gou D. Circulating microRNA profiles based on direct S-Poly(T)Plus assay for detection of coronary heart disease. Cell Mol Med. 2020 Jun;24(11):5984-5997. https://doi.org/10.1111/jcmm.15001. [CrossRef]
- Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans, Eur. Heart J. 2010 Mar;31(6):659-66. https://doi.org/10.1093/eurheartj/ehq013. [CrossRef]
- Devaux Y, Vausort M, Goretti E, Nazarov PV, Azuaje F, Gilson G, Corsten MF, Schroen B, Lair ML, Heymans S, Wagner DR. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem. 2012 Mar;58(3):559-67. https://doi.org/10.1373/clinchem.2011.173823. [CrossRef]
- Li YQ, Zhang MF, Wen HY, Hu CL, Liu R, Wei HY, Ai CM, Wang G, Liao XX, Li X. Comparing the diagnostic values of circulating microRNAs and cardiac troponin T in patients with acute myocardial infarction. Clinics (Sao Paulo). 2013 Jan;68(1):75-80. https://doi.org/10.6061/clinics/2013(01)oa12. [CrossRef]
- Cheng C, Wang Q, You W, Chen M, Xia J. MiRNAs as biomarkers of myocardial infarction: a meta-analysis. PLoS One. 2014 Feb 12;9(2): e88566. https://doi.org/10.1371/journal.pone.0088566. [CrossRef]
- Ward JA, Esa N, Pidikiti R, Freedman JE, Keaney JF, Tanriverdi K, Vitseva O, Ambros V, Lee R, McManus DD. Circulating Cell and Plasma microRNA Profiles Differ between Non-ST-Segment and ST-Segment-Elevation Myocardial Infarction. Fam Med Med Sci Res. 2013 Oct 1;2(2):108. https://doi.org/10.4172/2327-4972.1000108. [CrossRef]
- Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011 Nov;51(5):872-5. https://doi.org/10.1016/j.yjmcc.2011.07.011. [CrossRef]
- Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW, Sluijter JP Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med. 2012 Nov;4(11):1176-85.
- Bai R, Yang Q, Xi R, Li L, Shi D, Chen K. miR-941 as a promising biomarker for acute coronary syndrome. BMC Cardiovasc Disord. 2017 Aug 22 ;17(1) :227. [CrossRef]
- Wang F, Long G, Zhao C, Li H, Chaugai S, Wang Y, Chen C, Wang DW. Atherosclerosis-related circulating miRNAs as novel and sensitive predictors for acute myocardial infarction. PLoS One. 2014 Sep 3;9(9):e105734. [CrossRef]
- Wang A, Kwee LC, Grass E, Neely ML, Gregory SG, Fox KAA, Armstrong PW, White HD, Ohman EM, Roe MT, Shah SH, Chan MY. Whole blood sequencing reveals circulating microRNA associations with high-risk traits in non-ST-segment elevation acute coronary syndrome. Atherosclerosis. 2017 Jun; 261:19-25. https://doi.org/10.1016/j.atherosclerosis.2017.03.041. [CrossRef]
- Kaur A, Mackin ST, Schlosser K, Wong FL, Elharram M, Delles C, Stewart DJ, Dayan N, Landry T, Pilote L. Systematic review of microRNA biomarkers in acute coronary syndrome and stable coronary artery disease. Cardiovasc Res. 2020 May 1;116(6):1113-1124. [CrossRef]
- Zhelankin AV, Stonogina DA, Vasiliev SV, Babalyan KA, Sharova EI, Doludin YV, Shchekochikhin DY, Generozov EV, Akselrod AS. Circulating Extracellular miRNA Analysis in Patients with Stable CAD and Acute Coronary Syndromes. Biomolecules. 2021 Jun 29;11(7):962. [CrossRef]
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006 Jun;151(6):1260-4. [CrossRef]
- Bainey KR, Norris CM, Graham MM, Ghali WA, Knudtson ML, Welsh RC; APPROACH investigators. Clinical in-stent restenosis with bare metal stents: is it truly a benign phenomenon? Int J Cardiol. 2008 Aug 29;128(3):378-82.
- Park H, Ahn JM, Kang DY, Kim SO, Ko E, Kim TO, Lee PH, Lee SW, Park SW, Park DW, Park SJ. Very Long-term Safety and Effectiveness of Drug-Eluting or Bare-Metal Stents for Left Main Coronary Disease. [CrossRef]
- Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006 Jul 4;48(1):193-202. [CrossRef]
- Nakazawa G, Finn AV, Virmani R. Vascular pathology of drug-eluting stents. Herz. 2007 Jun;32(4):274-80. [CrossRef]
- 8 May; Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, Gold HK, Virmani R. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007 May 8;115(18):2435-41. [CrossRef]
- Montelione N, Catanese V, Nenna A, Jawabra M, Verghi E, Loreni F, Nappi F, Lusini M, Mastroianni C, Jiritano F, Serraino GF, Mastroroberto P, Codispoti FA, Chello M, Spinelli F, Stilo F. The Diagnostic Value of Circulating Biomarkers and Role of Drug-Coated Balloons for In-Stent Restenosis in Patients with Peripheral Arterial Disease. Diagnostics (Basel). 2022 Sep 12;12(9):2207. https://doi.org/10.3390/diagnostics12092207. [CrossRef]
- Nusca A, Viscusi MM, Piccirillo F, De Filippis A, Nenna A, Spadaccio C, Nappi F, Chello C, Mangiacapra F, Grigioni F, Chello M, Ussia GP. In Stent Neo-Atherosclerosis: Pathophysiology, Clinical Implications, Prevention, and Therapeutic Approaches. Life (Basel). 2022 Mar 8;12(3):393. [CrossRef]
- Wang D, Deuse T, Stubbendorff M, Chernogubova E, Erben RG, Eken SM, Jin H, Li Y, Busch A, Heeger CH, Behnisch B, Reichenspurner H, Robbins RC, Spin JM, Tsao PS, Schrepfer S, Maegdefessel L. Local MicroRNA Modulation Using a Novel Anti-miR-21-Eluting Stent Effectively Prevents Experimental In Stent Restenosis. Arterioscler Thromb Vasc Biol. 2015 Sep ;35(9) :1945-53.
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infec- tion by targeting microRNA. N Engl J Med. 2013, 368, 1685–1694.
- Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY, Brenner AJ, Park K, Lee JL, Kim TY, Shin S, Becerra CR, Falchook G, Stoudemire J, Martin D, Kelnar K, Peltier H, Bonato V, Bader AG, Smith S, Kim S, O’Neill V, Beg MS. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020, 122, 1630–1637.
- Lee EC, Valencia T, Allerson C, Schairer A, Flaten A, Yheskel M, Kersjes K, Li J, Gatto S, Takhar M, Lockton S, Pavlicek A, Kim M, Chu T, Soriano R, Davis S, Androsavich JR, Sarwary S, Owen T, Kaplan J, Liu K, Jang G, Neben S, Bentley P, Wright T, Patel V. Discovery and preclinical evalua- tion of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat Commun. 2019, 10, 1–14.
- van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey DL, Cooper WA, Kritharides L, Ridley L, Pattison ST, MacDiarmid J, Brahmbhatt H, Reid G. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, openlabel, dose-escalation study. Lancet Oncol. 2017, 18, 1386–1396.
- Deng Y, Campbell F, Han K, Theodore D, Deeg M, Huang M, Hamatake R, Lahiri S, Chen S, Horvath G, Manolakopoulos S, Dalekos GN, Papatheodoridis G, Goulis I, Banyai T, Jilma B, Leivers M. Randomized clinical trials towards a single-visit cure for chronic hepatitis C: Oral GSK2878175 and injectable RG-101 in chronic hepatitis C patients and long-acting injectable GSK2878175 in healthy participants. J Viral Hepat. 2020, 27, 699–708. [CrossRef]
- van der Ree MH, van der Meer AJ, de Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ, Rodriguez-Torres M, Kupcova V, Wiercinska-Drapalo A, Hodges MR, Janssen HL, Reesink HW. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral Res. 2014; 111:53–59. 199.
- van der Ree MH, van der Meer AJ, van Nuenen AC, de Bruijne J, Ottosen S, Janssen HL, Kootstra NA, Reesink HW. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016, 43, 102–113. [CrossRef]
- van der Ree MH, de Vree JM, Stelma F, Willemse S, van der Valk M, Rietdijk S, Molenkamp R, Schinkel J, van Nuenen AC, Beuers U, Hadi S, Harbers M, van der Veer E, Liu K, Grundy J, Patick AK, Pavlicek A, Blem J, Huang M, Grint P, Neben S, Gibson NW, Kootstra NA, Reesink HW. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double- blind, randomised controlled trial. Lancet. 2017, 389, 709–717. https://doi.org/10.1016/s0140-6736(16)31715-9. [CrossRef]
- Kilikevicius A, Meister G, Corey DR. Reexamining assumptions about miRNA-guided gene silencing. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022, 50, 617–634.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




