1. Introduction
Dental implants, widely utilized for replacement of missing teeth, represent a stable and reliable treatment option. However, in many cases, implant placement in the appropriate location is limited due to insufficient alveolar bone volume caused by factors such as post-extraction bone resorption, periodontal diseases, and trauma. For long-term stability of implants, a minimum bone width of 1 mm is required in both the buccal and lingual aspects and if the alveolar bone width is insufficient, additional bone grafting procedures such as guided bone regeneration (GBR), horizontal ridge augmentation, ridge splitting osteotomy, and horizontal onlay block bone grafting are necessary[
1].
Autogenous bone grafts, commonly employed for bone volume augmentation, are considered the gold standard due to their osteogenic, osteoinductive, and osteoconductive properties. However, they have disadvantages such as complications at the donor site, limited availability of graft material, and unpredictable graft resorption. Therefore, alternative substitutes such as allografts, xenografts, and synthetic bone substitutes are commonly utilized. In particular, xenografts derived from bovine, porcine, or equine bone are widely used due to their advantage of abundant availability and reduced risk of complications associated with graft harvesting from the donor site and are known to have excellent osteoconductive properties[
2,
3,
4]. Extensive clinical studies have been conducted on bovine bone grafts, which are the oldest and most widely used of all xenograft materials. In cases of ridge augmentation and maxillary sinus floor augmentation using bovine bone graft materials, long-term bone stability similar to that of autogenous bone at more than 5 years has been confirmed[
5,
6,
7,
8]. However, the risk of transmitting bovine spongiform encephalopathy cannot be completely excluded from bovine bone grafts; as an alternative, porcine bone grafts have been developed and used in clinical practice[
9]. Histologically, porcine bone exhibits structural similarities to human bone and has porosity and surface area similar to that of bovine bone[
10,
11]. During a 4-month observation after tooth extraction and socket grafting, porcine bone grafts exhibited histologically and radiographically similar bone formation to bovine bone grafts[
3,
12]. In a 6-month observation after maxillary sinus floor augmentation using bone grafts, porcine bone grafts demonstrated similar bone formation and volume stability to bovine bone grafts[
3,
12,
13]. In addition, when simultaneous implant placement and GBR were performed in alveolar ridges with severe horizontal atrophy, porcine bone grafts demonstrated a similar increase in alveolar bone width after 6 months, comparable to that of bovine bone grafts[
14]. However, there is a lack of clinical studies on the long-term outcomes of implants placed with porcine bone grafting. Furthermore, most of these studies are limited to maxillary sinus floor augmentation or socket preservation, and there is a scarcity of studies investigating the long-term stability and effectiveness of porcine bone grafts in horizontal ridge augmentation[
15,
16,
17,
18].
The purpose of this retrospective study is to evaluate the clinical and radiographic results of implants placed in horizontally augmented alveolar ridges using porcine bone grafts and to investigate the long-term stability and effectiveness of the porcine bone grafts through a follow-up period longer than 5 years.
2. Materials and Methods
This study was conducted under the approval of the Bioethics Review Committee of Seoul National University Bundang Hospital (IRB: B-2208-774-114). The study was conducted on a total of 32 patients (14 males and 18 females) who underwent bone grafting procedures using porcine bone grafts (The Graft, Purgo Biologics, Seongnam, Korea) performed by a single oral and maxillofacial surgeon at Seoul National University Bundang Hospital between 2014 and 2017. All patients included in the study presented with horizontal bone defects and underwent horizontal ridge augmentation for implant placement. A total of 55 implants was placed simultaneously or delayed (mean 5.3 months) after bone grafting, and prosthetic restoration was completed at a mean of 8.8 months after implant placement. After prosthesis loading, the patients were observed at regular intervals of 1 year for an average of 67.3 months (range: 49.3-86.9 months).
The selection criteria were patients who had a follow-up period longer than 5 years after the bone grafting procedure. Two other dentists who were not involved in the surgery conducted the investigation of the research data. Using medical records and periapical radiographs, they analyzed sex, age, smoking status, location of bone grafting and implant placement, timing of implant placement after bone grafting, use of other bone graft materials, use of barrier membrane, diameter and length of implant, type of prosthesis, period from implant placement to completion of prosthesis, follow-up period after completion of prosthesis, implant stability, marginal bone loss at 1 year after prosthesis loading and at the time of final observation, survival and success rates, and complications. For a subset of 24 implants using only porcine bone grafts and simultaneous implant placement, an additional analysis was conducted to assess marginal bone loss, survival and success rates, and complications.
Implant Stability Quotient (ISQ) measured by Osstell Mentor (Osstell, Goteborg, Sweden) was used to determine the stability of the implant. Primary stability was measured immediately after implant placement, and secondary stability was measured at the time of the second surgery in which a healing abutment was connected or at the time of impression for prosthesis.
Operation-related complications, including nerve damage, infection, and maxillary sinus membrane perforation, were investigated. Also, postoperative complications, such as fever, delayed bleeding, hematoma, exudate, pain, cyst, paresthesia, exposure of barrier membrane and graft material due to wound dehiscence, postoperative infection, maxillary sinusitis, marginal bone loss, graft material loss, implant fixture exposure, implant fixture fracture, failure of osseointegration, and implant loss were investigated.
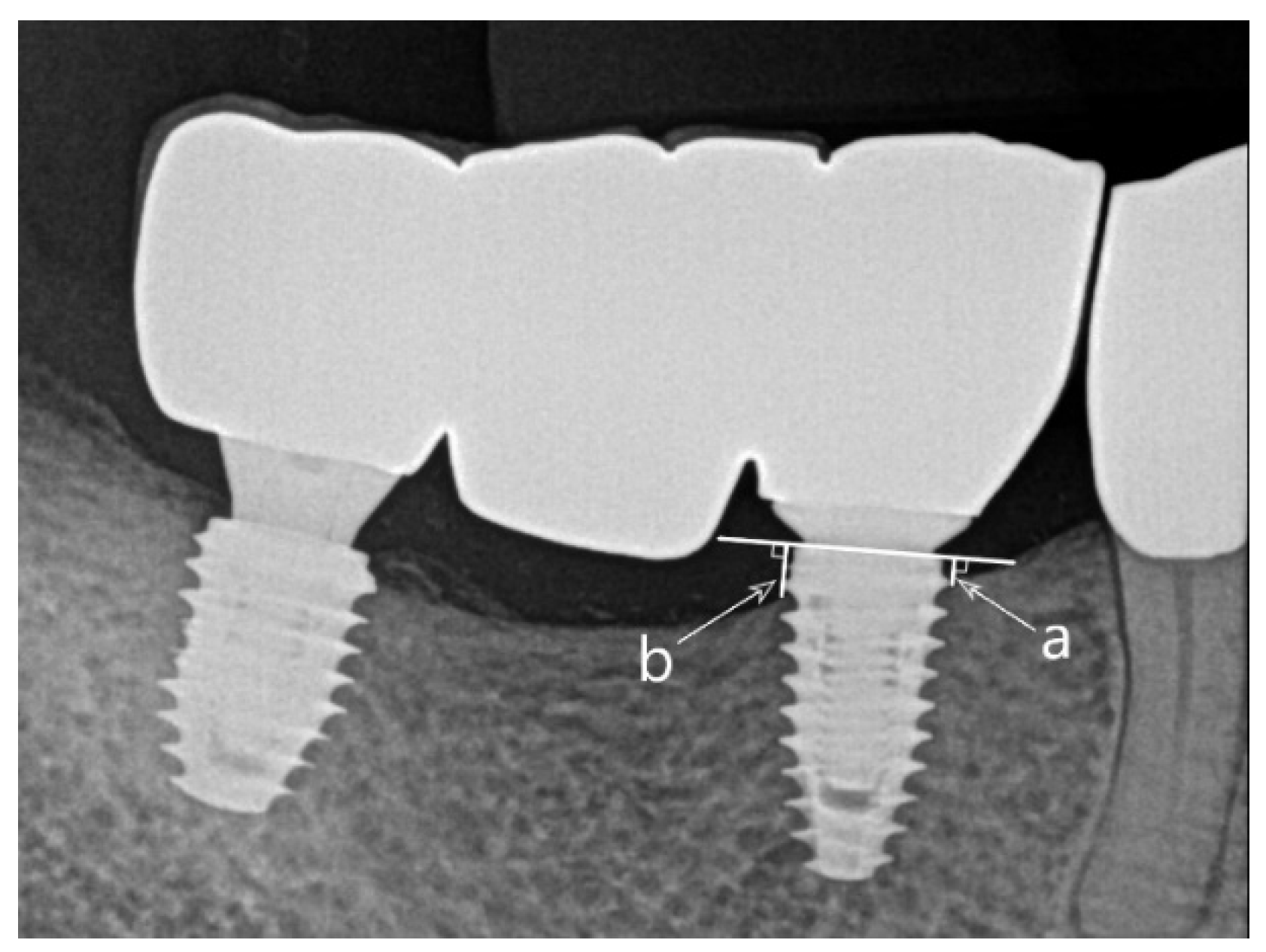
Marginal bone loss was assessed by measuring the marginal bone level on the periapical radiographs at the time of prosthesis completion, 1 year after prosthesis loading, and at the final observation and calculating the difference. The marginal bone level was measured from the implant shoulder to the mesial and distal uppermost point where the implant and bone contact, and the average value was calculated (
Figure 1). Periapical radiographs were obtained using OC100 CR (Instrumentarium Imaging, Tuusula, Finland) and Heliodent DS (Sirona, Bensheim, Germany) with parallel techniques, and the marginal bone level was measured using INFINITT PACS 3.0 software (INFINITT Healthcare Co., Ltd., Seoul, Korea).
The success of the implants was determined according to the following success criteria suggested by Albrektsson et al[
19]: 1) No movement of the implant, 2) no radiolucent lesion around the implant, 3) no symptoms such as pain, discomfort, or infection, 4) bone loss less than 0.2 mm every year after the first year of prosthesis function and of less than 1.5 mm during functional loading. Implant survival was defined as the presence of the implant in the oral cavity at the final observation, meeting criteria 1), 2), and 3) but not meeting criterion 4).
2.1. Surgical Procedure
All patients were instructed to take 2 tablets of 500mg cephalosporin (Mesexin, Hanlim, Yongin, Korea), 1 tablet of 500mg nonsteroidal anti-inflammatory drug (Naxen, ChongKunDang, Seoul, Korea), and 1 tablet of 60mg mucosal protectant (Stillen, Donga ST, Seoul, Korea) and perform oral cavity disinfection with 0.12% chlorhexidine gargle (Heaxamedine, Bukwang, Seoul, Korea) twice a day, starting one day before the surgery. Immediate preoperative intraoral disinfection was performed using chlorhexidine gargle for 2 minutes, and the surgery was performed under local anesthesia with 2% lidocaine with epinephrine (1:100,000). Through crestal and releasing incisions, a full-thickness flap was elevated and the alveolar bone was exposed. In cases where the operator judged that the width of the exposed alveolar ridge was too narrow, ridge splitting was performed selectively according to the method described in other studies[
20]. Except for 6 cases (10.9%) where initial implant fixation was deemed difficult, simultaneous bone grafting was performed with implant placement. Titanium implants were placed at the bone level according to the manufacturer's instructions, following the planned length as assessed by preoperative evaluation using cone beam computed tomography (CBCT). The buccal cortical bone was decorticated with a 2 mm round bur, and the porcine bone grafts were placed on the buccal side of the defect. When a significant amount of bone grafting was required due to severe bone defect, a combination of autogenous tooth bone graft materials (Auto BT) or allografts was used. In cases where a significant amount of bone grafts was used, all graft materials were covered with a resorbable collagen membrane at the operator's discretion. The mucoperiosteal flap was sutured without tension, and the sutures were removed 7-10 days later. The patients were instructed to take Mesexin, Naxen, and Stillen as previously described for 5 days after the surgery, maintain oral hygiene using 0.12% chlorhexidine gargle twice a day for 2 weeks, and follow a soft diet during that period. Six implants were placed using the same method described above after an average healing period of 5.3 months following horizontal ridge augmentation. After an average of 8.8 months following implant placement, crown or bridge prosthesis was delivered.
2.2. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics software (Ver 18, SPSS Inc., Chicago, IL, USA). The Mann-Whitney test was performed to examine whether there was a significant difference in marginal bone loss according to the use of barrier membrane and mixed bone grafts, and Fisher's exact test was performed to examine whether there were significant differences in survival and success rates according to the use of barrier membrane and mixed bone grafts. In 24 cases where bone grafting and implant placement were performed simultaneously using only porcine bone grafts, the independent t-test was performed to examine whether there was a significant difference in marginal bone loss according to the use of barrier membrane. A statistical significance level of 95% was used.
3. Results
In a group of 32 patients (14 males and 18 females) with a mean age of 58.6±15.7 years, bone grafting using porcine bone grafts and implant placement were performed in 55 sites. An average of 1.7±1.0 implants were placed per patient, and the mean follow-up period after prosthesis loading was 67.3±10.3 months (
Table 1).
Of the 55 sites, ridge splitting was performed in 10 sites where the alveolar ridge width was too narrow to accommodate implant placement. In 30 sites (54.5%), a combination of porcine bone grafts and other graft materials such as autogenous tooth bone graft materials (Auto BT) or allografts was used. In 36 sites (65.5%), a resorbable collagen barrier membrane was utilized. The implants were placed simultaneously with bone grafting in 49 sites (89.1%) or placed with a delay of an average of 5.3±0.7 months in 6 sites (10.9%). Among these sites, 15 implants (27.3%) were placed in the maxillary anterior region, 13 implants (23.6%) in the maxillary posterior region, 7 implants (12.7%) in the mandibular anterior region, and 20 implants (36.4%) in the mandibular posterior region. The final prosthesis was delivered an average of 8.8±3.0 months after implant placement (
Table 2).
The average primary stability of the implants was measured at 71.7±8.7 ISQ, while the average secondary stability was measured at 76.1±10.6 ISQ. Compared to the time of prosthesis delivery, there was a marginal bone loss of 0.18±0.45 mm during the first year after prosthesis loading; throughout the mean follow-up period of 67.3 months, there was a marginal bone loss of 0.37±0.70 mm. During the follow-up period of up to 86.9 months, 2 of 55 implants were lost (survival rate of 96.4%), and 4 implants survived but showed significant marginal bone loss, failing to meet Albrektsson's criteria (success rate of 89.1%). There was no significant difference in the amount of marginal bone loss according to the use of barrier membrane and mixed bone grafts (p>0.05). Additionally, correlation analysis between the use of barrier membrane and mixed bone grafts and the survival rate and success rate showed a significant correlation only between the use of mixed bone grafts and the success rate (Fisher's exact test p-value=0.027) (
Table 3).
In 6 sites in 3 of 32 patients, operation-related complications or postoperative complications occurred (
Table 4). In 5 sites among 2 patients, complications occurred during or immediately after the surgery and showed a causal relationship that led to long-term complications at the time of prosthesis loading. These complications included 2 cases of osseointegration failure caused by infection and 3 cases of marginal bone loss caused by infection. One other site, which showed normal conditions immediately after the surgery, experienced delayed infection due to postoperative barrier membrane exposure, which subsequently affected marginal bone loss. The 2 cases that were lost were complex cases involving ridge splitting, and they experienced infection leading to osseointegration failure. The 4 cases that failed with significant marginal bone loss were also associated with invasive surgery involving ridge splitting, with 3 cases experiencing infection and 1 case experiencing postoperative wound dehiscence leading to barrier membrane exposure and infection.
In 24 sites in 15 patients, simultaneous implant placement was performed along with horizontal ridge augmentation using only porcine bone grafts. Compared to the time of prosthesis delivery, there was a marginal bone loss of 0.22±0.28 mm during the first year after prosthesis loading; throughout the mean follow-up period of 65.8 months, there was a marginal bone loss of 0.40±0.36 mm. In the comparison between the group using resorbable collagen membrane (12 cases) and the group not using it (12 cases), there was slightly more marginal bone loss in the group without the membrane, but there was no statistically significant difference (p>0.05). Regardless of the use of the barrier membrane, all 24 implants survived during the follow-up period of up to 85.4 months (100% survival rate) and met the success criteria of Albrektsson et al. (100% success rate) (
Table 5).
4. Discussion
Throughout the entire sample, there was a marginal bone loss of 0.18 mm during the first year after prosthesis loading, and throughout the mean follow-up period of 67.3 months, there was a marginal bone loss of 0.37 mm. The 24 implants that were placed simultaneously with horizontal ridge augmentation using only porcine bone grafts and no other bone graft material showed a marginal bone loss of 0.22 mm during the first year after prosthesis loading and 0.40 mm of marginal bone loss throughout the mean follow-up period of 65.8 months, indicating a similar amount of marginal bone loss to the previous outcomes. According to our investigation, while no previous studies have specifically evaluated long-term outcomes of implants placed in horizontally augmented ridges using porcine bone grafts, other studies using a mixture of autogenous bone and bovine bone grafts in horizontal ridge augmentation reported marginal bone loss ranging from 0.2-0.3 mm during 1-2 years of follow-up and 0.3 mm during a 3-year follow-up period after implant placement[
21,
22]. This confirmed the similar levels of marginal bone loss observed in this study. In most cases, no significant amount of marginal bone loss was observed, indicating that porcine bone grafts are predictable grafting materials for horizontal ridge augmentation.
Throughout the mean follow-up period of 67.3 months, only 2 of 55 implants were lost (survival rate of 96.4%) and 4 implants survived but exhibited significant marginal bone loss, failing to meet the success criteria of Albrektsson et al. (success rate of 89.1%). In other studies, a mixture of autogenous bone and bovine bone grafts was used in horizontal ridge augmentation for implant placement, and the survival rate ranged from 95.9% to 100% over 3 years of follow-up[
22,
23]. Additionally, Le et al. reported a survival rate of 98.1% after 3 years of follow-up after bone grafting in buccal bone defects using allografts, and Mordenfeld et al. reported a survival rate of 94.4% to 100% and a success rate of 91.7% to 97.1% over 2 years of follow-up after horizontal ridge augmentation using autogenous bone and bovine bone grafts[
21,
24]. Considering the longer follow-up period in this study, the use of porcine bone grafts in horizontal ridge augmentation demonstrated implant survival and success rates similar to those of other bone graft materials. In the 24 implants placed simultaneously with horizontal ridge augmentation using only porcine bone grafts and no other bone graft materials, no complications including severe marginal bone loss were observed, and all implants showed successful long-term outcomes. The higher survival and success rates in this group compared to the overall sample may be attributed to the lack of standardization in conditions and parameters across the study and the more frequent use of mixed bone grafts in complex and challenging cases that required extensive bone augmentation.
The most common complication observed in this study was infection. In 3 of 32 patients, infections occurred in 6 sites, serving as the cause for the failure or loss of all affected implants. All 6 sites were challenging cases where ridge splitting was performed and mixed bone graft materials were used due to severely constricted alveolar ridges. It is possible that the infection was caused by contamination during the surgical procedure, wound dehiscence due to lack of intact primary closure, or inadequate postoperative oral hygiene management rather than by the type of bone graft material. Despite the use of chlorhexidine irrigation and antibiotic treatment, postoperative infection occurred in one implant where the barrier membrane was exposed, leading to subsequent marginal bone loss. This finding is consistent with other studies that suggest that exposure of barrier membrane has a negative effect on bone regeneration[
25,
26]. On the other hand, other studies have reported successful results without any additional complications by employing appropriate treatments such as use of antibiotics and chlorhexidine rinses after the exposure of the barrier membrane[
21,
23,
27].
In both samples of this study, no significant difference was found in marginal bone loss according to the use of barrier membranes, which is consistent with the study by Gielkens et al., reporting a lack of evidence supporting the preventive effect of barrier membranes on marginal bone loss[
28]. Although no statistically significant differences were found in the entire sample, survival and success rates were lower when barrier membranes were used and implant survival rate was lower when mixed bone grafts were used. Furthermore, when mixed bone grafts were used, the success rate was significantly lower. Barrier membrane and mixed bone graft materials were utilized in cases with extensive bone defects that required significant bone grafting, increasing the surgical invasiveness, and it is thought that this influenced the outcomes. Therefore, future studies with larger sample sizes and controlled experiments are needed to investigate the effects of barrier membranes and mixed bone graft materials in horizontal ridge augmentation on marginal bone loss, implant survival rate, and success rate. On the other hand, in samples where only porcine bone grafts were used without other mixed bone graft materials, there was no significant difference in marginal bone loss according to the use of barrier membranes, and 100% survival and success rates were observed. This highly favorable outcomes, regardless of the use of barrier membranes, can be attributed to the fact that the sole use of porcine bone grafts was primarily employed in small defects.
This study has several limitations as it is a retrospective observational study. First, it was not a completely controlled study, and conditions such as use of mixed bone graft materials, use of barrier membranes, types of implant, length and diameter of implant, and timing of implant placement were not unified, and the sample size for each condition was insufficient. In addition, this study targeted patients who underwent horizontal ridge augmentation; however, it had a limitation in that only vertical bone loss was measured as a radiographic parameter and changes in horizontal bone width were not examined. To assess changes in horizontal bone width, CBCT imaging would be necessary, but since the radiation dose could not be justified for CBCT in annual observations, the evaluation of horizontal changes was substituted with clinical assessment of buccolingual soft tissue contour changes. Last, the change in depth of the periodontal pocket could not be assessed as only cases with a probing depth exceeding 5 mm were recorded according to the implant success criteria of Karoussis et al.[
29].
Nevertheless, this study holds significance as it is, to the best of our knowledge, the first investigation to assess the long-term outcomes of implants placed in horizontally augmented ridges using porcine bone grafts, with a follow-up period longer than 5 years. In this study, the stability and efficacy of porcine bone grafts in horizontal ridge augmentation were confirmed, indicating their potential as an alternative to autogenous bone and other bone graft materials. The most significant cause of implant failure/loss was infection, highlighting the importance of preventive measures and management of infections. Unless complex cases requiring significant bone augmentation are involved, long-term stable outcomes can be achieved using porcine bone grafts alone without barrier membranes.
5. Conclusions
With careful attention paid to infection prevention, porcine bone grafts can be successfully used for long periods of time in horizontal ridge augmentation for implant placement in horizontally narrow alveolar ridges. Even with the use of porcine bone grafts alone in simultaneous horizontal ridge augmentation and implant placement, a small amount of bone loss and long-term stable prognosis are shown regardless of the use of barrier membrane.
Author Contributions
Conceptualization, P.Y.Y. and Y.K.K.; data curation, J.W.C., S.S.H., and Y.K.K.; formal analysis, J.W.C.; methodology, J.W.C., S.S.H., and Y.K.K.; resources, Y.K.K; supervision, Y.K.K.; validation, S.S.H., P.Y.Y., and Y.K.K.; writing—original draft preparation, J.W.C; writing—review and editing, S.S.H. and Y.K.K.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board at Seoul National University Bundang Hospital (IRB no: B-2208-774-114).
Informed Consent Statement
The Seoul National University Bundang Hospital IRB approved the use of the anonymized data without need of consent from patients.
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esposito, M.; Worthington, H.V.; Coulthard, P.; Thomsen, P. Maintaining and re-establishing health around osseointegrated oral implants: A Cochrane systematic review comparing the efficacy of various treatments. Periodontol. 2000 2003, 33, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Candrlic, M.; Tomas, M.; Karl, M.; Malesic, L.; Vcev, A.; Peric Kacarevic, Z.; Matijevic, M. Comparison of Injectable Biphasic Calcium Phosphate and a Bovine Xenograft in Socket Preservation: Qualitative and Quantitative Histologic Study in Humans. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.H.; Song, Y.W.; Cha, J.K.; Jung, U.W.; Kim, C.S.; Lee, J.S. Histologic analysis following grafting of damaged extraction sockets using deproteinized bovine or porcine bone mineral: A randomized clinical trial. Clin. Oral. Implant. Res. 2020, 31, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Addis, A.; Canciani, E.; Campagnol, M.; Colombo, M.; Frigerio, C.; Recupero, D.; Dellavia, C.; Morroni, M. A New Anorganic Equine Bone Substitute for Oral Surgery: Structural Characterization and Regenerative Potential. Mater. (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Raustia, A.M.; Kubilius, R. A 5-year follow-up study on one-stage implants inserted concomitantly with localized alveolar ridge augmentation. J. Oral. Rehabil. 2007, 34, 781–789. [Google Scholar] [CrossRef]
- Beretta, M.; Cicciu, M.; Poli, P.P.; Rancitelli, D.; Bassi, G.; Grossi, G.B.; Maiorana, C. A Retrospective Evaluation of 192 Implants Placed in Augmented Bone: Long-Term Follow-Up Study. J. Oral. Implant. 2015, 41, 669–674. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Anorganic bovine bone (ABB) vs. autologous bone (AB) plus ABB in maxillary sinus grafting. A prospective non-randomized clinical and histomorphometrical trial. Clin. Oral. Implant. Res. 2015, 26, 1043–1050. [Google Scholar] [CrossRef]
- Lutz, R.; Berger-Fink, S.; Stockmann, P.; Neukam, F.W.; Schlegel, K.A. Sinus floor augmentation with autogenous bone vs. a bovine-derived xenograft - a 5-year retrospective study. Clin. Oral. Implant. Res. 2015, 26, 644–648. [Google Scholar] [CrossRef]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef]
- Holzer, A.; Pietschmann, M.F.; Rosl, C.; Hentschel, M.; Betz, O.; Matsuura, M.; Jansson, V.; Muller, P.E. The interrelation of trabecular microstructural parameters of the greater tubercle measured for different species. J. Orthop. Res. 2012, 30, 429–434. [Google Scholar] [CrossRef]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal Implant. Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cha, J.K.; Kim, C.S. Alveolar ridge regeneration of damaged extraction sockets using deproteinized porcine versus bovine bone minerals: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, H.K.; Yun, J.H.; Cho, K.S. Randomized Clinical Trial of Maxillary Sinus Grafting using Deproteinized Porcine and Bovine Bone Mineral. Clin. Implant. Dent. Relat. Res. 2017, 19, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Agop-Forna, D.; Törok, R.; Törok, B.; Dragomir, R.; Ehrenfest, D.M.D.; Dascălu, C.; Stelea, C.G. Postoperative Study of Bone Gain in Mandibular Alveolar Bone Reconstructed with Screw-Guided Bone Regeneration Technique and Porcine-Derived Xenograft in 42 Edentulous Patient Candidates for Implant-Prosthetic Therapy. Appl. Sci. 2021, 11. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, A.; Assenza, B.; Quaranta, A.; Perrotti, V.; Piattelli, M.; Iezzi, G. Porcine bone used in sinus augmentation procedures: A 5-year retrospective clinical evaluation. J. Oral. Maxillofac. Surg. 2010, 68, 1869–1873. [Google Scholar] [CrossRef]
- Roberto, C.; Paolo, T.; Giovanni, C.; Ugo, C.; Bruno, B.; Giovanni-Battista, M.F. Bone remodeling around implants placed after socket preservation: A 10-year retrospective radiological study. Int. J. Implant. Dent. 2021, 7, 74. [Google Scholar] [CrossRef]
- Marconcini, S.; Giammarinaro, E.; Derchi, G.; Alfonsi, F.; Covani, U.; Barone, A. Clinical outcomes of implants placed in ridge-preserved versus nonpreserved sites: A 4-year randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 906–914. [Google Scholar] [CrossRef]
- Barone, A.; Orlando, B.; Cingano, L.; Marconcini, S.; Derchi, G.; Covani, U. A randomized clinical trial to evaluate and compare implants placed in augmented versus non-augmented extraction sockets: 3-year results. J. Periodontol. 2012, 83, 836–846. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral. Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Tolstunov, L.; Hicke, B. Horizontal augmentation through the ridge-split procedure: A predictable surgical modality in implant reconstruction. J. Oral. Implant. 2013, 39, 59–68. [Google Scholar] [CrossRef]
- Mordenfeld, A.; Aludden, H.; Starch-Jensen, T. Lateral ridge augmentation with two different ratios of deproteinized bovine bone and autogenous bone: A 2-year follow-up of a randomized and controlled trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Hellem, S.; Astrand, P.; Stenstrom, B.; Engquist, B.; Bengtsson, M.; Dahlgren, S. Implant treatment in combination with lateral augmentation of the alveolar process: A 3-year prospective study. Clin. Implant. Dent. Relat. Res. 2003, 5, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Meloni, S.M.; Jovanovic, S.A.; Urban, I.; Baldoni, E.; Pisano, M.; Tallarico, M. Horizontal ridge augmentation using GBR with a native collagen membrane and 1:1 ratio of particulate xenograft and autologous bone: A 3-year after final loading prospective clinical study. Clin. Implant. Dent. Relat. Res. 2019, 21, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; Borzabadi-Farahani, A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J. Craniomaxillofac Surg. 2014, 42, 552–559. [Google Scholar] [CrossRef]
- Machtei, E.E. The effect of membrane exposure on the outcome of regenerative procedures in humans: A meta-analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Meraw, S.J.; Lee, E.J.; Giannobile, W.V.; Wang, H.L. Comparative analysis of collagen membranes for the treatment of implant dehiscence defects. Clin. Oral. Implant. Res. 2003, 14, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ehmke, B.; Rudiger, S.G.; Hommens, A.; Karch, H.; Flemmig, T.F. Guided tissue regeneration using a polylactic acid barrier. J. Clin. Periodontol. 2003, 30, 368–374. [Google Scholar] [CrossRef]
- Gielkens, P.F.; Bos, R.R.; Raghoebar, G.M.; Stegenga, B. Is there evidence that barrier membranes prevent bone resorption in autologous bone grafts during the healing period? A systematic review. Int. J. Oral. Maxillofac. Implant. 2007, 22. [Google Scholar]
- Karoussis, I.K.; Brägger, U.; Salvi, G.E.; Bürgin, W.; Lang, N.P. Effect of implant design on survival and success rates of titanium oral implants: A 10-year prospective cohort study of the ITI® Dental Implant System. Clin. Oral. Implant. Res. 2004, 15, 8–17. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).