1. Introduction
Currently, soybean activity is widely spread in the world context, due to its varied forms of use in different segments and consequently for having great exposure in the international market, being considered a commodity. Brazil, followed by the US, currently stands out as the world’s largest producers of this oilseed [
1], with soybean being one of the main agricultural products that is part of the export agenda of these countries.
With the technological advances of brazilian agriculture, seed companies are adopting new techniques that maximize crop yields, for example, the creation of an industrial seed treatment process [
2,
3], called industrial seed treatment (TIS), in which seed lots are treated during the processing phase, and then bagged and stored until sowing [
4,
5,
6,
7,
8,
9,
10]. Through this innovative technique, new formulations containing fungicides, insecticides, nematicides, stimulants, micronutrients, inoculants can be used in the same treatment [
11,
12,
13], which can maximize the efficiency of products, helps protect the applicator and avoid environmental contamination.
Mean while, the anticipation of chemical treatment can reduce the physiological quality of seeds during packaging, due to possible phytotoxic effects that some active ingredients of the products may have on the seeds. This phytotoxic effect reduces germination, vigor and emergence of seedlings and impairs plant establishment and crop productivity of soybean [
14].
Apparently the most toxic effects to seeds are promoted by the treatment involving the use of insecticides in isolation, but also in mixtures with other chemicals such as fungicide, micronutrients, biostimulants, etc. [
15] testing the mixture of the insecticide thiamethoxam, with a complex of nutrients, involving macronutrients and micronutrients (dimicron TMSp), and the biostimulant containing indolbutyric acid, kinetin and gibberellic acid (stimulate®), it was possible to verify that the treatment that showed the best result in the initial performance of soybean seeds was the nutrient complex, followed by the plant growth regulator with biostimulating effect. The insecticide had a negative effect on seed germination and seedling development. In study conducted by [
8] it was possible to ascertain that the use of the fungicide derosal plus® and the insecticide cruiser, in isolation or in mixture, impaired the quality and vigor of seeds of three soybean cultivars (M7110 IPRO, RR-8473RSF e M7739 IPRO), being that the seeds kept stored with chemical treatment kept within the marketing standards (>80%) until 60 days.
As for inoculants, the way widely used by soybean farmers in Brazil for supplying N to the crop, modern products known as long-life have recently been launched on the markets, which achieve not only high efficiency in the supply of rhizomes to the plant, but also make possible the use of this technique in combination with the application of fungicides and insecticides in advance of the planting time via TIS [
16], such as Granouro® and HICOAT S30® products from Basf that can be used in seeds up to 45 days before sowing, if stored in suitable conditions. However, research results on this subject are incipient.
The understanding of the factors that contribute to the variability in the responses of soybeans to the inoculation of long-life rhizobia and their mixtures with agricultural defensives such as fungicide and insecticide applied via TIS, is a step of paramount importance for the development of sustainable agricultural systems, and that really contribute to the increase in soybean production with lower costs [
17].
Hence, the purpose of this study was to evaluate the effect of application of chemical common defensives used for seed treatments and long-life inoculant in soybeans, isolated or in mixtures, on the plant nodulation process and physiological quality of soybean seeds during storage for two months.
2. Material and Methods
Greenhouse experiment
A completely randomized design was used, in an 8 x 2 factorial scheme, with three repetitions. The treatments consisted of eight combinations of seed treatments carried out via TIS, using common chemical pesticides used for seed treatment (fungicide/insecticide) and long-life inoculants, either in isolation or in mixtures, being: {T1–control; T2–fungicide (MaximAdvanced); T3–insecticide (Fortenza); T4–long-life inoculant (Nitragin CT 200); T5–mixture of products fungicide/insecticide (MaximAdvanced + Fortenza); T6–fungicide/long-life inoculant (MaximAdvanced + Nitragin CT 200); T7–insecticide/long-life inoculant (Fortenza + Nitragin CT 200); T8–mixture of products fungicide/insecticide/long-life inoculant (MaximAdvanced + Fortenza + NitragiN CT 200)}, which were sown in pots containing soils from two cultivation areas (area of first year of cultivation and area with several years of cultivation).
For the treatments performed on the seeds, the following products and their respective recommended doses were used:Fortenza (insecticide) 60mL 100kg-1;Polymer (adhesive–insecticide)100mL 100kg-1; Maxim Advanced (fungicide) 100mL 100kg-1; CRI (additive–fungicide)80mL 100kg-1; Nitragin CTS 200–Semia 5079 and Semia 5080 (inoculants–peat) 400g100kg-1; Nitragin Power 200 (additive–inoculant) 350mL 100kg-1.
The soybean seeds were treated and sown immediately in pots with a capacity of 5 L. Five seeds of the soybean cultivar 73170 RSE IPRO were sown, and then the thinning of the seedlings was carried out at 10 days after emergence (DAE) leaving two plants per pot. Irrigations were made according to the water needs of the crop.
The analysis were performed in stage V5 (full flowering), sampling the two plants in the pot, with the presence of the aerial part and the root. The root system was washed in running water for removal of soil, and aerial part if necessary. Then, the plants were sectioned in the neck region in order to make the evaluations of the aerial part and the root system, in an individualized way.
The following morphophysiological characteristics of plants were evaluated:–Chlorophyll content: The chlorophyll content was evaluated with a portable chlorofiLOG – CFL 1030 m, and 10 fully expanding leaves of mature branches were evaluated, randomly chosen in the middle portion of each plant;–Viable, Non-viable and Total Nodules: The number of total nodules, number of viable nodules and number of non-viable nodules were evaluated. These determinations were performed on the intact roots, after separation and washing when all the nodules found were removed, but only the nodules with 2 mm or more in diameter were evaluated; for the determinations of viability, these were sectioned in half with stylet and the pinkish coloring was identified;–Dry mass of nodules:After the count of nodules and identification of the percentage of viables, the samples were taken for drying in greenhouse at 65ºC until constant weight was obtained, and then the dry mass of nodules was determined [
18].
Lab experiment
A completely randomized design was used, in an 8 x 6 factorial scheme, with four repetitions. The treatments consisted of eight combinations of applications carried out via TIS, employing chemical pesticides (fungicide/insecticide) and long-life inoculants, in isolation or in mixtures, being: {T1–control, T2–fungicide (MaximAdvanced); T3–insecticide (Fortenza); T4 – long-life inoculant (Nitragin CT 200); T5–mixture of products fungicide/insecticide (MaximAdvanced + Fortenza); T6–fungicide/long-life inoculant (MaximAdvanced + Nitragin CT 200); T7 – insecticide/long-life inoculant (Fortenza + Nitragin CT 200); T8–mixture of products fungicide/insecticide/long-life inoculant (MaximAdvanced + Fortenza + Nitragin CT 200)}, and stored for two months, with evaluations every ten days (10; 20; 30; 40; 50 and 60 days).
The treated seeds were packed in raffia sacks in cold chamber, kept at temperature and relative humidity (±12ºC and ± 45%, respectively), controlled in order to minimize their metabolic activities and, consequently, the loss of viability and vigor of the seeds. The evaluations were carried out according to the intervals cited above, employing the following tests:
- Water content: It was determined by the greenhouse method, with forced ventilation, at 105 ± 3ºC for 24 hours, with the use of two subsamples of 25 seeds for each plot, according to the Rules for Seed Analysis–RAS [
19];
- Germination: it was carried out with four sub-samples of 50 seeds, arranged in germitest type paper substrate, moistened with distilled water in an amount corresponding to 2.5 times the mass of the dry paper. 50 seeds/repetition were placed, wrapped and packed in germinator under temperature of 25 ± 2ºC. The readings were taken on the eighth day after sowing, calculating the percentages of normal, abnormal seedlings and dead seeds [
19];
- First germination count: it was conducted along with the germination test, and the evaluation was performed on the fifth day of the germination test assembly. The percentage of normal seedlings was computed [
19];
- Accelerated aging: For accelerated aging, 250 seeds/repetition were distributed on the surface of a metal screen fixed and suspended inside a plastic box–gerbox, with 40 mL of water, and was maintained at 41ºC and 100% relative humidity, for 48 hours in a germinator [
20]. After this period, the seeds were submitted to the germination test, previously described, and the percentage of normal seedlings was determined on the 5th (fifth) day after the assembly of the test.
Statistical analysis
The obtained data were submitted to variance analysis (p≤0.05) and, when significant, were submitted to Tukey’s mean comparison test for qualitative factors and regression analysis for quantitative factors. Statistical analysis was obtained using R software, Version 4.2.
3. Results and Discussion
Greenhouse experiment: Chlorophyll index and nodulation of plants
By the result of the analysis of variance, it can be ascertained that all the variables analyzed were influenced by the seed treatment factor. On the other hand, both the interaction and the soil factor showed no significant difference for any of the variables studied.
The characteristic chlorophyll index was significant at 0.01 probability for seed treatment, being influenced by the different mixtures evaluated. The treatments that presented the highest chlorophyll index were T8 (association between fungicide/insecticide/inoculant) with 44.9 ICF and T4 (only inoculant) with 44.3 ICF, which differed statistically from the others (
Table 1). The other treatments presented chlorophyll values higher than the control, however not statistically different from this one.
In the work developed by [
21] in order to study the different vias, forms and doses of application of
Bradyrhizobium japonicum in soybean crop, it was found the influence of the application of the inoculant in relation to the control, which presented higher chlorophyll values when compared to the control. Averagely, the value found was 40.2 ICF, which corroborates the value found in this study, which obtained a mean of 42.1 ICF and presented the highest chlorophyll indices for the treatments that had in their mixture the use of the inoculant
B. japonicum.
Evaluating the inoculation of different doses of
Bradyrhizobium by cover and its effect on soybean crop [
22], obtained values close to that found in the study, obtaining a mean of 38.9 FCI, however the effect of the inoculant applied via foliar resulted in values of inversely proportional chlorophyll index when applied via seed. They also found that the control presented a more satisfactory result of chlorophyll index, so the application via seed when it comes to the evaluation of chlorophyll index has higher efficiency results when applied via seed. It is noteworthy that in the literature the main pigment responsible for the capture of light energy used in the photosynthesis process is chlorophyll, in addition, it is one of the main factors related to the photosynthetic efficiency of the plant, directly influencing the adaptability of the plant and its growth [
21].
There was no negative effect of the association of these products with respect to nodulation (
Table 2), as well as the results obtained by [
22]. The highest values in relation to viable nodules and total nodules were obtained in the treatments T4 (only inoculant) and T8 (association between fungicide/insecticide/inoculant), thus showing that the strain of the bacterium used is susceptible to mixtures with pesticides, corroborating the results of [
23].
Regarding the means found for the evaluation of nodulation, viable nodules, and total nodules, with respective values of 37.7 and 37.8, the results obtained are consistent with those obtained by [
18], which evaluated the influence of different treatments in soybeans obtained values for nodulation close to those found, with a total nodulation mean of 42.1. [
21] also found close means for nodulation evaluation by evaluating different doses of inoculation, and it can be inferred that the use of association had no negative influence on soybean nodulation. In addition to these authors, [
24] found that the number of nodules and mass of nodules in the BR86 bacterium strain were not affected by insecticides. According to the authors, the reduction of nodulation depends on the strain of the bacteria used, thus, it can be inferred that the strain used in the present study was susceptible to mixtures with pesticides.
The general mean dry mass of nodules - 0.80 g (
Table 3), is above the values found by [
18] who obtained a mean of 0.30 g in studies evaluating respectively ways of application of inoculant and its effects on nodulation and effects of the addition of bioregulator to industrial treatment on the quality of soybean seeds, this difference can be justified due to the time of evaluation of the nodules being different, and in the flowering period the nodules are at their apex of development thus presenting a greater mass of nodules.
The lowest dry mass values of nodules (
Table 3) were verified in treatments that had insecticide or fungicide, similar results were observed by [
25], which described 70% reduction in soybean plant biomass when fungicide (carboxin+thiram) and inoculant was used compared to inoculation without fungicides. [
26], also reported reduction of dry mass of aerial part when seeds were treated with insecticides.
Lab experiment: Physiological seed quality
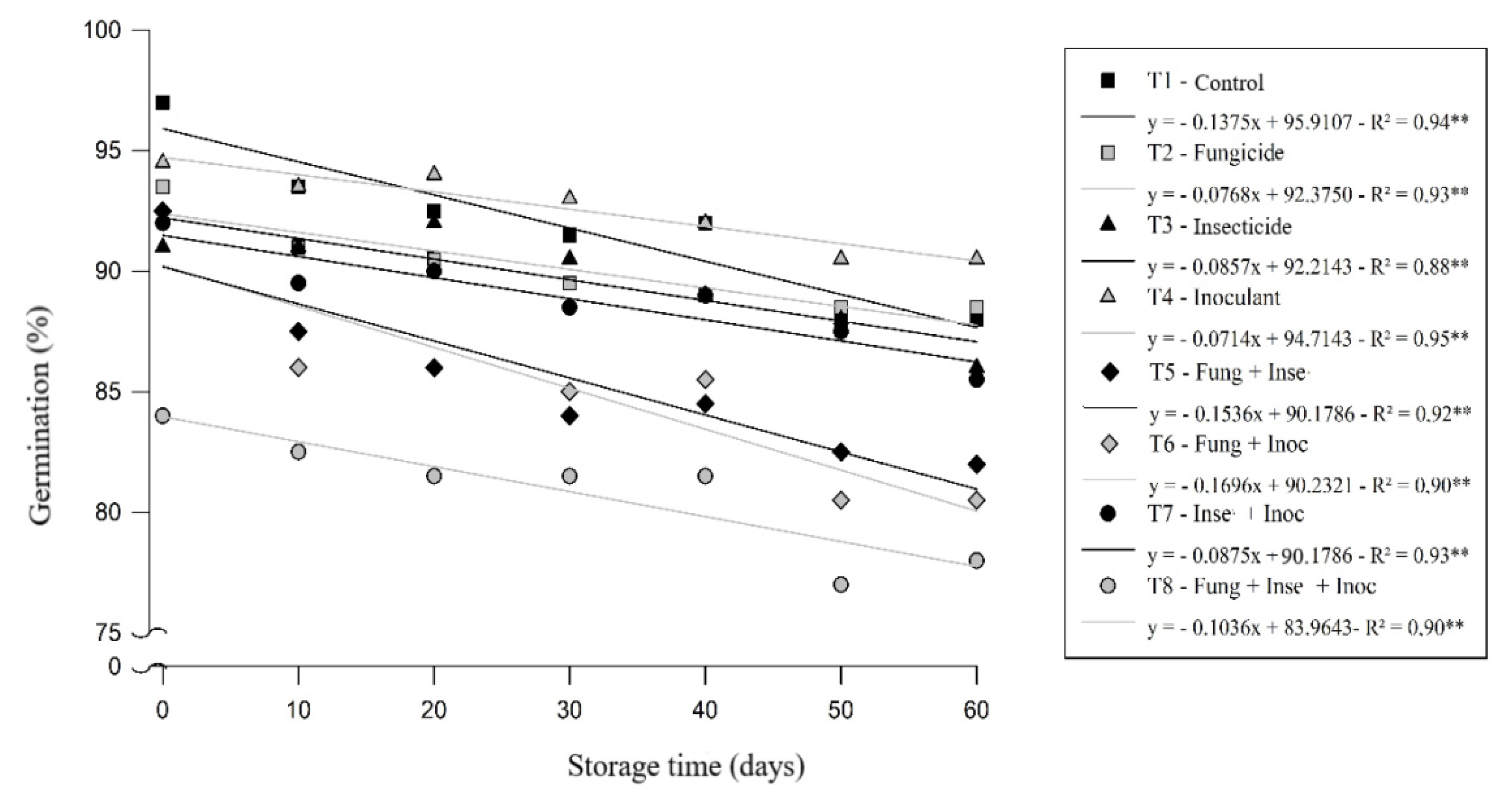
From the result of the analysis of variance all the tests performed were influenced by the interaction of factors seed treatments and storage periods. The soybean seeds evaluated differed as to the viability of seeds according to the treatments applied during storage (
Figure 1). The highest percentage values of germination were obtained at the beginning of storage in T4 treatments (long-life inoculant) followed by T1 (control), above 92 and 90% respectively, suffering a decrease in the number of normal plants at the time of the evaluations, as well as the other treatments. In the T8 treatment (mixture of fungicide/insecticide/long-life inoculant products), the lower percentage of germination was observed compared to the other treatments, reaching an average of 81% at the end of 60 days of storage.
The use of a long-life inoculant stands out as a potential for use in soybean, confirming reports by [
17], considering that the use of long-life inoculants makes it practical for the producer to apply the product via seed, in addition to efficiency in the supply of N. Furthermore, the results obtained indicate that, at least in the laboratory, seed treatments with fungicide, insecticides, or inoculant, in mixtures or not, significantly interfered with the initial development of seeds and provided physiological changes in seeds.
It is noteworthy that none of the treatments tested showed mean seed germination lower than the minimum standard required for the marketing of soybean seeds, i.e. 80% [
19], at the end of the storage period studied (60 days), even the T8 treatment that presented seeds with lower seed viability.
In a study conducted by [
27], it was verified in the absence of chemicals, during 45 days of storage, the highest proportion of normal soybean seedlings, however, the use of chemicals did not affect germination until 40 days of storage, but the seeds presented germination percentage below the recommended for commercialization [
19].
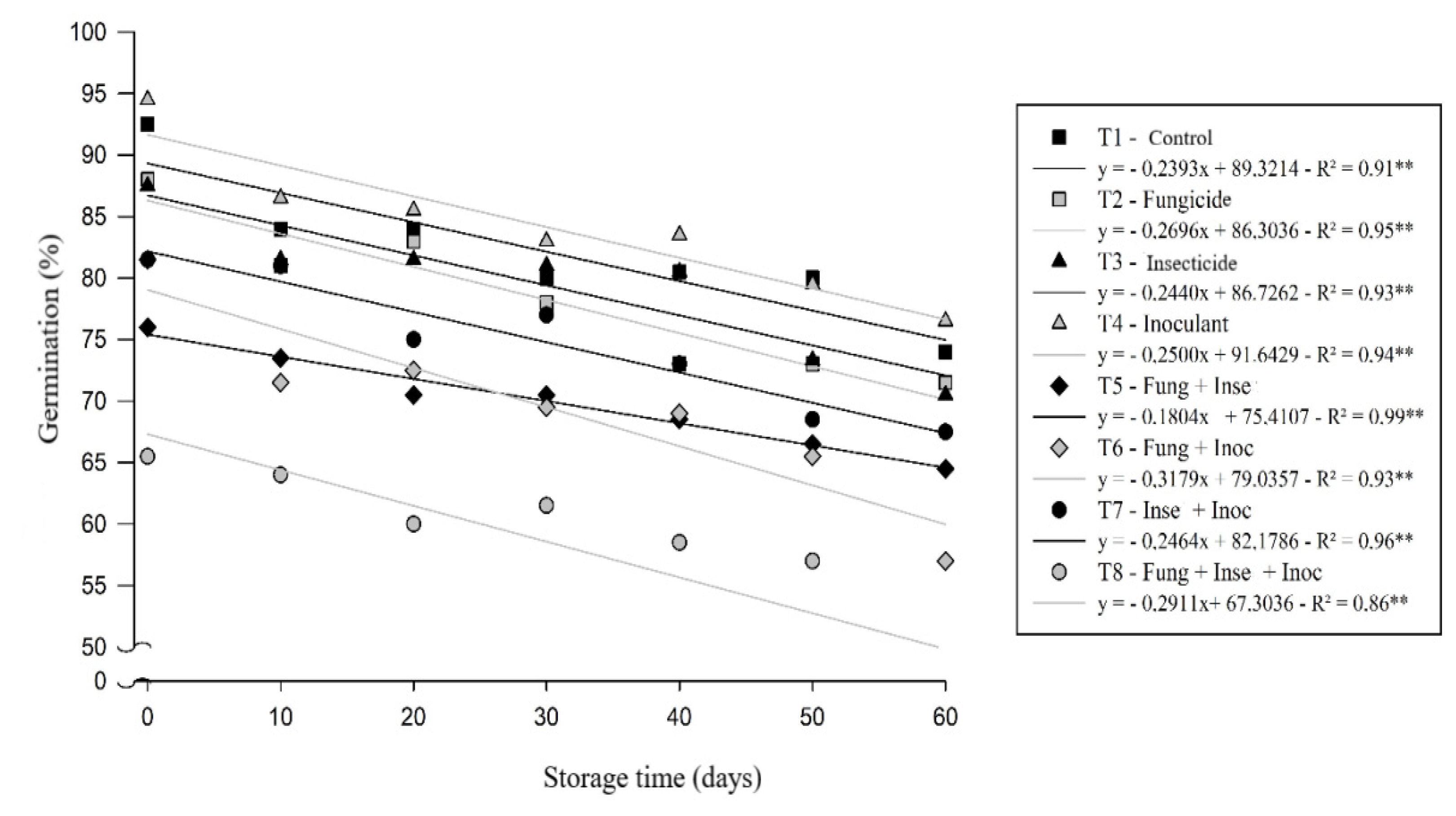
The soybean seeds evaluated differed in seed vigor depending on the treatments applied during storage. The highest values of percentages of normal seedlings were verified at the beginning of storage suffering decrease over the subsequent evaluations, the two highest percentages that differed statistically, were obtained in the absence of chemicals throughout the storage period, showing that the performance of seed treatment with chemicals, promoted damage to seed vigor from the initial period of chemical treatment. [
4], when studying the use of insecticides in the quality of soybean seeds during storage, concluded that the treatment of seeds with these products promoted negative effects under germination and first count of germination during storage for a period of 30 days, corroborating with the results found in the present study.
The T4 (inoculant only) and T1 (control) treatments showed the least influence on physiological quality throughout storage (
Figure 2). These same treatments, from 60 days of storage, still showed a good percentage of germination, being above 80%, a result that corroborates with those found by [
8], evaluating the quality of soybean seeds subjected to different chemical treatment technologies.
On the other hand, the T8 treatment, in which the mixture of fungicide/insecticide/inoculant products was used in the evaluations, it was found that these were detrimental to the vigor of the seeds, immediately after the procedure of the chemical treatments and during the entire storage period, presenting percentage values below 70% since the first evaluation, and reaching the lowest percentage at the end of storage–59% compared to the other treatments. These results are consistent with those verified for the germination test cited above.
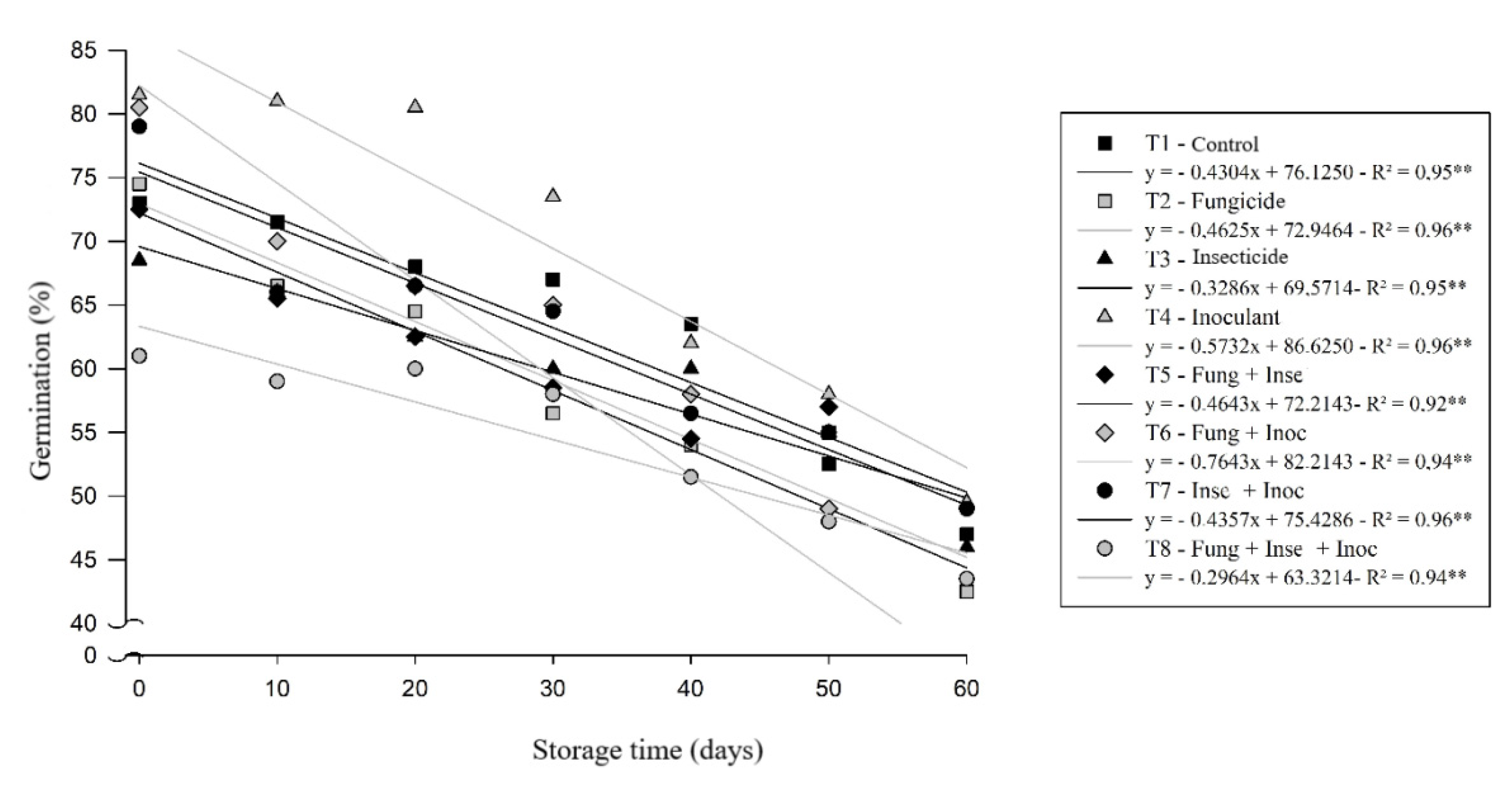
As in previous evaluations, the result of the accelerated aging test was no different, because the test follows the same behavior, however, before its assembly process, the seeds are exposed to a stress of humidity and temperature [
28], and after this process is performed the assembly of the germination test with the aged seeds. This fact contributes to the mean vigor of seedlings evaluated by the accelerated aging test being lower when compared to the values obtained in the germination and first count tests.
Despite this, the seeds treated with inoculant (T4) showed the highest average percentage of germination (69%), which differs significantly from the treatment T1 (control), that presented the second highest percentage of germination (63%) at the end of storage, all of which differed statistically from the other treatments (
Figure 3), corroborating the results of the tests of germination and first count. [
29] also observed similar numbers to those obtained in this study for the test in question.
4. Conclusions
Soybean seed treatment with fungicide/insecticide/inoculant, applied in isolation or not, does not influence the soybean nodulation process, both in soil of first year of cultivation and several years of cultivation. The use of chemicals (fungicide), (insecticide) and their combinations, negatively affects the physiological quality of soybean seeds.Soybean seeds, regardless of the type of treatment received with fungicide/insecticide/long-life inoculant products, in mixtures or in isolation, may be stored for a period of 40 days for commercial purposes.
Author Contributions
Author Contributions: Conceptualization, R.S.A. and I.R.T.; Methodology, R.S.A., G.C.S., and I.R.T.; Investigation, R.S.A., B.M.S., G.R.S. and I.R.T.; Formal Analysis, R.S.A., B.M.S., G.R.S.and M.E.V.A.; Funding Acquisition, G.C.S and I.R.T.; Supervision, G.C.S. and I.R.T.; Writing—Original Draft, R.S.A.; Writing—Review and Editing, I.A.D., M.E.V.A. and P.C.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are available upon request to contact author.
Acknowledgments
The authors are thankful for the Research Foundation of the State of Goias (FAPEG) provided partial funding for this research.
Conflicts of Interest
No conflicts of interest were declared.
References
- Fao–Food and Agriculture Organization of the United Nations. ETo Calculator. Version 3.2. 2020,Rome.
- Peske, S.T. Dinâmica do mercado de sementes no Brasil. Seed News, 2017, 5, 12–15. [Google Scholar]
- Krzyzanowski, F.C.; França-Neto, J.B.; Henning, A.A. A altaqualidade de sementes de soja: fatorimportante para a produção da cultura.1.ed. Londrina: Embrapa Soja. 2018, 24.
- Dan, L.G.M.; Braccini, A.L.; Barroso, A.L.L.; Dan, H.A.; Piccinin, G.G.; Varoniak, J.M. Physiological potential of soybean seeds treated with thiamethoxam and submitted to storage. Agricultural Sciences 2013, 4, 19–25. [Google Scholar] [CrossRef]
- Nunes, J.C.S. Tratamento de sementes de soja como um processo industrial no Brasil. Seed News, 2016, 20, 26–32. [Google Scholar]
- Pereira, L.C.; Matera, T.C.; Braccini, A.L.; Pereira, R.C.; Marteli, D.C.V.; Suzukawa, A.K.; Piana, S.C.; Ferri, G.C.; Correia, L.V. Addition of biostimulant to the industrial treatment of soybean seeds: physiological quality and yield after storage. Journal of Seed Science, 2018, 40, 442–449. [Google Scholar] [CrossRef]
- Santos, F.S.; Carvalho, E.R.; Rocha, D.K.; Nascimento, R.M. Constituições e volumes de calda no tratamento industrial de sementes de soja e a qualidadefisiológicadurante o armazenamento. Journal of Seed Science, 2018, 40, 67–74. [Google Scholar] [CrossRef]
- Silva, I.L.; Camargo, F.R.T.; Souza, R.T.G.; Teixeira, I.R.; Kikuti, H. Storage of soybean seeds treated with chemicals. Semina – CiênciasAgrárias, 2019, 40, 2961–2972. [Google Scholar] [CrossRef]
- Abati, J.; Brzezinski, C.R.; Bertuzzi, E.C.; Henning, F.A.; Zucareli, C. Physiological response ofsoybeanseedsto spray volumes of industrial chemicaltreatmentandstorage in differentenvironments. Journal of Seed Science, 2020, 42, e202042002. [Google Scholar] [CrossRef]
- Santos, V.M.; Oliveira, T.C.; Mendes, M.G.; Yamanaka, C.H.; Macedo, W.R. Soybean seed chemical treatment associated with inoculants: physiological and agronomical analyses. Plant Physiology Reports, 2021, 26, 247–255. [Google Scholar] [CrossRef]
- Dan, L.G.M.; Dan, H.A.; Braccini, A.L.; Barroso, A.L.L.; Ricci, T.T.; Piccinin, G.G.; Scapim, C.A. Insecticide treatment and physiological quality of seeds. In: Perveen, F. Insecticides: advances in integrated pest management. 2012, 327- 342.
- Brzezinski, C.R.; Henning, A.A.; Abati, J.; Henning, F.A.; França-Neto, J.B.; Krzyzanowski, F.C.; Zucareli, C. Seeds treatment times in the establishment and yield performance of soybean crops. Journal of Seed Science, 2015, 37, 147–153. [Google Scholar] [CrossRef]
- Bertuzzi, E.C.; Meneghello, G.E.; Lemes, E.; Aguiar, C.E. Emergência de milhoemfunção do tratamento das sementes com inseticida, fungicida e bioestimulante. In Produçãotécnicocientíficaemsementes; Meneghello, G.E., Almeida, A.S., Villela, F.A., Tunes, L.V.M., Eds.; Santa Cruz: Pelotas-RS, Brazil, 2017; pp. 23–38. [Google Scholar]
- Sartori, F.F.; Engroff, T.D.; Sanches, T,H.G.; Soave, J.M.; Pessotto, M.V.; Felisberto, G.; Hilgemberg Jr., V.E.; Reis, A.F.B.; Hungria, M.; Nogueira, M.A.; Jaccoud-Filho, D.S.; Andreote, F.D.; Dourado-Neto, D. Potentially harmful effects of seed treatment and pre-inoculation on soybean biological nitrogen fixation and yield. European Journal of Agronomy, 2023, 142, 126660. [CrossRef]
- Binsfeld, J.A.; Barbieri, A.P.C.; Huth, C.; Cabrera, I.C.; Henning, L.M.M. Uso de bioativador, bioestimulante e complexo de nutrientes em sementes de soja. Pesquisa Agropecuária Tropical, 2014, 44, 88–94. [Google Scholar] [CrossRef]
- Ronner, E.; Franke, A.C.; Vanlauwe, B.; Dianda, M.; Edeh, E.; Ukem, B.; Bala, A.; Van Heerwaarden, J.; Giller, K.E. Understanding variability in soybean yield and response to P-fertilizer andrhizobium inoculants on farmers' fields in northern Nigeria. Field Crop Research, 2016, 186, 133–145. [Google Scholar] [CrossRef]
- Alves Neto, A.J.; Lana, M.C.; Lorenzetti, E.; Henkemeier, N.P.; Schimiloski, S.; Ritter, G. Conventional inoculant sand biological protector, co-inoculation and nitrogen fertilization in soybean. Revista Scientia Agrária Paranaensis, 2020, 19, 187–195. [Google Scholar] [CrossRef]
- Vieira Neto, A.S.; Pires, F.R.; Menezes, C.C.E.; Menezes, J.F.S.; Silva, A.G.; Silva, G.P.; Assis, R.L. Formas de aplicação de inoculante e seus efeitos sobre a nodulação da soja. Revista Brasileira de Ciências do Solo, 2008, 32, 861–870. [Google Scholar] [CrossRef]
-
RAS–Regras para análises de sementes; Mapa: Brasília, Brazil, 2009.
- Krzyzanowski, F.C.; Vieira, R.D. (1999). Deterioração controlada. In: Krzyzanowski, F.C.; Vieira, R.D.; França Neto, J.B. (Ed.). Vigor de sementes: conceitos e testes. Abrates: Londrina, Brazil, 1999, pp. 6–8.
- Pereira, L.C.; Garcia, M.M.; Braccini, A.L.; Piana, S.C.; Ferri, C.G.; Matera, T.C.; Felber, P.H.; Marteli, D.C.V. Efeito da adição de biorregulador ao tratamento industrial sobre a qualidade de sementes de soja (Glycine max (L.) Merr.) ao ssessenta dias de armazenamento convencional. Revista Colombiana de Investigaciones Agroindustriales, 2016, 2, 15–22. [Google Scholar] [CrossRef]
- Zago, L.F.; Lima, C.R.; Cruz, R.M.S.; Alberton, O. Inoculação de diferentes doses de Bradyrhizobium porcobertura e seu efeitonacultura da soja. Arquivos de Ciências Veterinárias e Zoologia da Unipar, 2018, 21, 65–69. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e desenvolvimento vegetal, 6 ed.; Artmet: Porto Alegre, Brazil, 2016. [Google Scholar]
- Pereira, C.E.; Oliveira, J.Á.; Costa Neto, J.; Moreira, F.M.S.; Viera, A.R. Tratamentos inseticidas, peliculização e inoculação de sementes de soja com rizobio. RevistaCeres, 2010, 57, 653–658. [Google Scholar] [CrossRef]
- Zilli, J.E.; Campo, R.J.; Hungria, M. Eficácia da inoculação de Bradyrhizobium empré-semeadura da soja. Pesquisa Agropecuária Brasileira, 2010, 45, 335–338. [Google Scholar] [CrossRef]
- Pereira, C.E.; Oliveira, J.Á.; Costa Neto, J.; Moreira, F.M.S.; Viera, A.R. Tratamentosi nseticidas, peliculização e inocula ção de sementes de soja com rizobio. Revista Ceres, 2010, 57, 653–658. [Google Scholar] [CrossRef]
- Dan, L.G.M.; Dan, H.A.; Barroso, A.L.L.; Braccini, A.L. Qualidade fisiológica de sementes de soja tratadas com inseticidas sob efeito do armazenamento. Revista Brasileira de Sementes, 2010, 32, 131–139. [Google Scholar] [CrossRef]
- Marcos Filho, J. Teste de envelhecimento acelerado. In Vigor de sementes: conceitos e testes; Krzyzanowski, F.C., Vieira, R.D., França Neto, J.B., Eds.; Abrates: Londrina, Brazil, 1999; pp. 32–34. [Google Scholar]
- Pádua, G.P.; Zito, R.K.; Arantes, N.E.; FrançaNeto, J.B. Influência do tamanho da semente na qualida de fisiológica e na produtividade da cultura da soja. Revista Brasileira de Sementes, 2010, 32, 9–16. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).