Submitted:
24 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fennel (Foeniculum vulgare Mill.)
3. Lavender (Lavandula angustifolia Mill.)
4. Thyme (Thymus vulgaris L.)
5. Echinacea (Echinacea angustifolia DC.; Echinacea purpurea L.; Echinacea pallida)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahrajabian, M.H.; Sun, W. Assessment of wine quality, traceability and detection of grapes wine, detection of harmful substances in alcohol and liquor composition analysis. Lett. Drug Des. Discov. 2023, 20. [CrossRef]
- Shahrajabian, M.H.; Sun, W. Survey on medicinal plants and herbs in traditional Iranian medicine with anti-oxidant, anti-viral, anti-microbial, and anti-inflammation properties. Lett. Drug Des. Discov. 2023, 19. [CrossRef]
- Shahrajabian, M.H.; Sun, W. Importance of thymoquinone, sulforaphane, phloretin, and epigallocatechin and their health benefits. Lett. Drug Des. Discov. 2023, 19. [CrossRef]
- Shahrajabian, M.H. Medicinal herbs with anti-inflammatory activities for natural and organic healing. Curr. Org. Chem. 2021, 25(23), 1-17. [CrossRef]
- Marovska, G.; Vasileva, I.; Petkova, N.; Ognyanov, M.; Gandova, V.; Stoyanova, A.; Merdzhanov, P.; Simitchiev, A.; Slavov, A. Lavender (Lavandula angustifolia Mill.) industrial by-products as a source of polysaccharides. Ind. Crops Prod. 2022, 188(Part B), 115678. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q.; The insight and survey on medicinal properties and nutritive components of shallot. J. Med. Plant Res. 2019, 13(18), 452-457. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Anise (Pimpinella anisum L.), a dominant spice and traditional medicinal herb for both food and medicinal purposes. Cogent Biol. 2019, 5(1673688), 1-25. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Barberry (Berberis vulgaris), a medicinal fruit and food with traditional and modern pharmaceuticals uses. Isr J Plant Sci. 2021, 68(1-2), 1-11. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Health benefits of wolfberry (Gou Qi Zi) on the basis of ancient Chinese herbalism and Western modern medicine. Avicenna J. Phytomedicine. 2021, 11(2), 109-119. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek cultivation with emphasis on historical aspects and its uses in traditional medicine and modern pharmaceutical science. Mini Rev. Med. Chem. 2021, 21(6), 724-730. [CrossRef]
- Shahrajabian, M.H.; Sun, W. Various techniques for molecular and rapid detection of infectious and epidemic diseases. Lett. Org. Chem. 2023, 20, 1-23. [CrossRef]
- Shahrajabian, M.H.; Sun, W. The importance of salicylic acid, humic acid and fulvic acid on crop production. Lett Drug Des Discov. 2023, 2023(20), 1-16. [CrossRef]
- Baby, K.C.; Ranganathan, T.V. Effect of enzyme pre-treatment on extraction yield and quality of fennel (Foeniculum vulgare) volatile oil. Biocatal Agric Biotechnol. 2016, 8, 248-256. [CrossRef]
- Ehsanipour, A.; Razmjoo, J.; Zeinali, H. Effect of nitrogen rates on yield and quality of fennel (Foeniculum vulgare Mill.) accessions. Ind Crops Prod. 2012, 35(1), 121-125. [CrossRef]
- Melfi, M.T.; Kanawati, B.; Schmitt-Kopplin, P.; Macchia, L.; Centonze, D.; Nardiello, D. Investigation of fennel protein extracts by shot-gun Fourier transform ion cyclotron resonance mass spectrometry. Food Res Int. 2021, 139, 109919. [CrossRef]
- Shojaiefar, S.; Sabzalian, M.R.; Mirlohi, A.; Tajdivand, A. Evidence for self-compatibility and variation for inbreeding depression within breeding populations of fennel (Foeniculum vulgare Mill.). J Appl Res Med Aromat Plants. 2021, 22, 100299. [CrossRef]
- Bayrami, A.; Shirdel, A.; Pouran, S.R.; Mahmoudi, F.; Habibi-Yangjeh, A.; Singh, R.; Raman, A.A.A. Co-regulative effects of chitosan-fennel seed extract system on the hormonal and biochemical factors involved in the polycystic ovarian syndrome. Mater Sci Eng C. 2020, 117, 111351. [CrossRef]
- Yaldiz, G.; Camlica, M. Variation in the fruit phytochemical and mineral composition, and phenolic content and antioxidant activity of the fruit extracts of different fennel (Foeniculum vulgare L.) genotypes. Ind Crops Prod. 2019, 142, 111852. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Molecular breeding and the impacts of some important genes families on agronomic traits, a review. Genet Resour Crop Evol. 2021, 68(3), 1709-1730. [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules. 2021, 11(5), 698. [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules. 2021, 11(6), 819. [CrossRef]
- Sirotkin, A.V.; Macejkova, M.; Tarko, A.; Fabova, Z.; Alwasel, S.; Kotwica, J.; Harrath, A.H. Ginkgo, fennel, and flaxseed can affect hormone release by porcine ovarian cells and modulate the effect of toluene. Reprod Biol. 2023, 23(1), 100736. [CrossRef]
- Sanli, A.; Ok, F.Z. Determination of optimal harvesting time for essential oil and estragole yield in bitter fennel (Foeniculum vulgare Mill.) growing in culture conditions. S Afr J Bot. 2023, 155, 98-102. [CrossRef]
- Akbari, A.; Izadi-Darbandi, A.; Bahmani, K.; Farhadpour, M.; Ebrahimi, M.; Ramshini, H.; Esmaeili, Z. Assessment of phenolic profile, and antioxidant activity in developed breeding populations of fennel (Foeniculum vulgare Mill.). Biocatal Agric Biotechnol. 2023, 48, 102639. [CrossRef]
- Hosseini, E.S.; Majidi, M.M.; Ehtemam, M.H.; Hughes, N. Characterization of fennel germplasm for physiological persistence and drought recovery: Association with biochemical properties. Plant Physiol Biochem. 2023, 194, 499-512. [CrossRef]
- Khammassi, M.; Ayed, R.B.; Loupasaki, S.; Amri, I.; Hanana, M.; Hamrouni, L.; Jamoussi, B.; Khaldi, A. Chemical diversity of wild fennel essential oils (Foeniculum vulgare Mill.): A source of antimicrobial and antioxidant activities. S Afr J Bot. 2023, 153, 136-146. [CrossRef]
- Mohammadi, M.; Pouryousef, M.; Farhang, N. Study on germination and seedling growth of various ecotypes of fennel (Foeniculum vulgare Mill.) under salinity stress. J Appl Res Med Aromat Plants. 2023, 34, 100481. [CrossRef]
- Marmitt, D.; Shahrajabian, M.H. Plant species used in Brazil and Asia regions with toxic properties. Phytother Res. 2021, 2021(2), 1-24. [CrossRef]
- Marmitt, D.; Shahrajabian, M.H.; Goettert, M.I.; Rempel, C. Clinical trials with plants in diabetes mellitus therapy: a systematic review. Expert Rev Clin Pharmacol. 2021, 14(4), 1-14. [CrossRef]
- Zali, A.G.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind Crops Prod. 2018, 111, 133-140. [CrossRef]
- Bidgeloo, M.; Kowsari, E.; Ehsani, A.; Ramakrishna, S.; Chinnappan, A. Activated carbon derived from fennel flower waste as high-efficient sustainable materials for improving cycle stability and capacitance performance of electroactive nanocomposite of conductive polymer. J Energy Storage. 2022, 55, 105793. [CrossRef]
- Feizi, H.; Kamali, M.; Jafari, L.; Moghaddam, P.R. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill.). Chemosphere. 2013, 91(4), 506-511. [CrossRef]
- Moura, L.S.; Carvalho, R.N.; Stefanini, M.B.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from fennel (Foeniculum vulgare): global yield, composition and kinetic data. J Supercrit Fluids. 2005, 35(3), 212-219. [CrossRef]
- Torres, M.; Frutos, G. Logistic function analysis of germination behaviour of aged fennel seeds. Environ Exp Bot. 1990, 30(3), 383-390. [CrossRef]
- Vella, A.; Cammilleri, G.; Pulvirenti, A.; Galluzzo, F.; Randisi, B.; Giangrosso, G.; Macaluso, A.; Gennaro, S.; Ciaccio, G.; Cicero, N.; Ferrantelli, V. High hydroxycinnamic acids contents in fennel honey produced in Southern Italy. Nat Prod Res. 2020, 35(21), 4104-4109. [CrossRef]
- Hatami, T.; Johner, J.C.F.; Meireles, M.A.A. Investigating the effects of grinding time and grinding load on content of terpenes in extract from fennel obtained by supercritical fluid extraction. Ind Crops Prod. 2017, 109, 85-91. [CrossRef]
- Lee, H.W.; Ang, L.; Kim, E.; Lee, M.S. Fennel (Foeniculum vulgare Miller) for the management of menopausal women ,s health: A systematic review and meta-analysis. Complement Ther Clin Pract. 2021, 43, 101360. [CrossRef]
- Rezaei-Chiyaneh, E.; Amirnia, E.; Machiani, M.A.; Javanmard, A.; Maggi, F.; Morshedloo, M.R. Intercropping fennel (Foeniculum vulgare L.) with common bean (Phaseolus vulgaris L.) as affected by PGPR inoculation: A strategy for improving yield, essential oil and fatty acid composition. Sci Hortic. 2020, 261, 108951. [CrossRef]
- Schurr, L.; Masotti, V.; Geslin, B.; Gachet, S.; Mahe, P.; Jeannerod, L.; Affre, L. To what extent is fennel crop dependent on insect pollination? Agric Ecosyst Environ. 2022, 338, 108047. [CrossRef]
- Levorato, S.; Dominici, L.; Fatigoni, C.; Zadra, C.; Pagiotti, R.; Moretti, M.; Villarini, M. In vitro toxicity evaluation of esteragole-containing preparations derived from Foeniculum vulgare Mill. (fennel) on HepG2 cells. Food Chem. Toxicol. 2018, 111, 616-622. [CrossRef]
- Mishra, B.K.; Meena, K.K.; Dubey, P.N.; Aishwath, O.P.; Kant, K.; Sorty, A.M.; Bitla, U. Influence on yield and quality of fennel (Foeniculum vulgare Mill.) grown under semi-arid saline soil, due to application of native phosphate solubilizing rhizobacterial isolates. Ecol. Eng. 2016, 97, 327-333. [CrossRef]
- Singh, B.; Kale, R.K. Chemomodulatory action of Foeniculum vulgare (Fennel) on skin and forestomach papillomagenesis, enzymes associated with xenobiotic metabolism and antioxidant status in murine model system. Food. Chem. Toxicol. 2008, 46(12), 3842-3850. [CrossRef]
- Tarko, A.; Fabova, Z.; Kotwica, J.; Valocky, I.; Alrezaki, A.; Alwasel, S.; Harrath, A.H.; Sirotkin, A.V. The inhibitory influence of toluene on mare ovarian granulosa cells can be prevented by fennel. Gen. Comp. Endocrinol. 2020, 295, 113491. [CrossRef]
- Hafez, D.A.; Abdelmonsif, D.A.; Aly, R.G.; Samy, W.M.; Elkhodairy, K.A.; Aasy, N.K.A. Role of fennel oil/quercetin dual nano-phytopharmaceuticals in hampering liver fibrosis: Comprehensive optimization and in vivo assessment. J Drug Deliv Sci Technol. 2022, 69, 103177. [CrossRef]
- Abdel-Wahhab, K.G.; Fawzi, H.; Mannaa, F.A. Paraoxonase-1 (PON1) inhibition by tienilic acid produces hepatic injury: Antioxidant protection by fennel extract and whey protein concentrate. Pathophysiology. 2016, 23(1), 19-25. [CrossRef]
- Hatami, T.; Johner, J.C.F.; Kurdian, A.R.; Meireles, M.A.A. A step-by-step finite element method for solving the external mass transfer control model of the supercritical fluid extraction process: A case study of extraction from fennel. J Supercrit Fluids. 2020, 160, 104797. [CrossRef]
- Marand, S.A.; Almasi, H.; Amjadi, S.; Alamdari, N.G.; Salmasi, S. Ixiolirion tataricum mucilage/chitosan based antioxdant films activated by free and nanoliposomal fennel essential oil. Int. J. Biol. Macromol. 2023, 230, 123119. [CrossRef]
- Bahmani, K.; Darbandi, A.I.; Ramshini, H.; Moradi, N.; Akbari, A. Agro-morphological and phtyochemical diversity of various Iranian fennel landraces. Ind. Crops Prod. 2015, 77, 282-294. [CrossRef]
- Rajabi, A.; Ehsanzadeh, P.; Razmjoo, J. Partial relief of drought-stressed fennel (Foeniculum vulgare Mill.) in response to foliar-applied zinc. Pedosphere 2019, 29(6), 752-763. [CrossRef]
- Rodriguez-Solana, R.; Salgado, J.M.; Dominguez, J.M.; Cortes-Dieguez, S. Characterization of fennel extracts and quantification of estragole: Optimization and comparison of accelerated solvent extraction and Soxhlet techniques. Ind Crops Prod. 2014, 52, 528-536. [CrossRef]
- Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and volatile compounds of fennel (Foeniculum vulgare) seeds and their sprouts prevent oxidative DNA damage and ameliorates CCl4-induced hepatotoxicity and oxidative stress in rats. Antioxidants. 2022, 11, 2318. [CrossRef]
- Noreen, S.; Tufail, T.; Ain, H.B.U.; Awuchi, G. Pharmacological, nutraceutical, functional and therapeutic properties of fennel (Foeniculum vulgare). Int J Food Prop. 2023, 26(1), 915-927. [CrossRef]
- Ke, W.; Zhao, X.; Lu, Z. Foeniculum vulgare seed extract induces apoptosis in lung cancer cells partly through the down-regulation of Bcl-2. Biomed Pharmacother. 2021, 135, 111213. [CrossRef]
- Calisir, F.; Urgalioglu, A.; Bilal, B.; Tok, A.; Bolcal, H.A.; Oksuz, H. The effect of lavender aromatherapy on the level of intraoperative anxiety in caesarean case under spinal anesthesia: A randomized controlled trial. Explore 2023, 19(3), 356-361. [CrossRef]
- Dogan, C.; Dogan, N.; Gungor, M.; Eticha, A.K.; Akgul, Y. Novel active food packaging based on centrifugally spun nanofibers containing lavender essential oil: Rapid fabrication, characterization. And application to preserve of minced lamb meat. Food Packag. Shelf Life. 2022, 34, 100942. [CrossRef]
- Karatopuk, S.; Yarici, F. Determining the effect of inhalation and lavender essential oil massage therapy on the severity of perceived labor pain in primiparous women: A randomized controlled trial. Explore 2023, 19(1), 107-114. [CrossRef]
- Khatri, P.K.; Paolini, M.; Larcher, R.; Ziller, L.; Magdas, D.A.; Marincas, O.; Roncone, A.; Bontempo, L. Validation of gas chromatographic methods for lavender essential oil authentication based on volatile organic compounds and stable isotope ratios. Microchem. J. 2023, 186, 108343. [CrossRef]
- Motaghi, N.; Tajadini, H.; Shafiei, K.; Sharififar, F.; Ansari, M.; Sharifi, H.; Sarhadynejad, Z.; Tavakoli-Far, F.; Kamali, H.; Amiri-Ardekani, E. Lavender improves fatigue symptoms in multiple sclerosis patients: A double-blind, randomized controlled trial. Mult Scler Relat Disord. 2022, 65, 104000. [CrossRef]
- Xu, Y.; Ma, L.; Liu, F.; Yao, L.; Wang, W.; Yang, S.; Han, T. Lavender essential oil fractions alleviate sleep disorders induced by the combination of anxiety and caffeine in mice. J Ethnopharmacol. 2023, 302(Part A), 115868. [CrossRef]
- Semeniuc, C.A.; Mandrioli, M.; Socaci, B.S.; Socaciu, M.-I.; Fogarasi, M.; Podar, A.S.; Michiu, D.; Jimborean, A.M.; Muresan, V.; Ionescu, S.R.; Toschi, T.G. Changes in lipid composition and oxidative status during ripening of Gouda-type cheese as influenced by addition of lavender flower powder. Int Dairy J. 2022, 133, 105427. [CrossRef]
- Ghavami, T.; Kazeminia, M.; Rajati, F. The effect of lavender on stress in individuals: A systematic review and meta-analysis. Complement Ther Med. 2022, 68, 102832. [CrossRef]
- Xu, S.; Zuo, C.; Sun, X.; Ding, X.; Zhong, Z.; Xing, W.; Jin, W. Enriching volatile aromatic compounds of lavender hydrolats by PDMS/ceramic composite membranes. Sep Purif Technol. 2022, 294, 121198. [CrossRef]
- Shirzad, M.; Nasiri, E.; Hesamirostami, M.H.; Akbari, H. the effect of lavender of anxiety and hemodynamic status before septorhinoplasy and rhinoplasty. J PeriAnesth Nurs. 2023, 38(1), 45-50. [CrossRef]
- Girbu, V.; Organ, A.; Grinco, M.; Cotelea, T.; Ungur, N.; Barba, A.; Kulcitki, V. Identification, quantitative determination and isolation of pomolic acid from lavender (Lavandula angustifolia Mill.) wastes. Sustain Chem Pharm. 2023, 33, 101140. [CrossRef]
- Zanotti, A.; Baldino, L.; Scognamiglio, M.; Reverchon E. Post-processing of a lavender flowers solvent extract using supercritical CO2 fractionation. J Taiwan Inst Chem Eng. 2023, 147, 104901. [CrossRef]
- Shamabadi, A.; Hazanzadeh, A.; Ahmadzade, A.; Ghadimi, H.; Gholami, M.; Akhondzadeh, S. The anxiolytic effects of Lavandula angustifolia (lavender): An overview of systematic reviews. J Herbal Med. 2023, 40, 100672. [CrossRef]
- Lorimer, H. An aid to loveliness: lavender, femininity and the affective economy of English beauty. J Hist Geogr. 2023, 79, 13-25. [CrossRef]
- Shaaban, M.M.; Kholif, A.E.; El Tawab, A.M.; Radwan, M.A.; Hadhoud, F.I.; Khattab, M.S.A.; Saleh, H.M.; Anele, U.Y. Thyme and celery as potential alternatives to ionophores use in livestock production: their effects on feed utilization, growth performance and meat quality of Barki lambs. Small Rumin Res. 2021, 200, 106400. [CrossRef]
- Shokoohi, F.; Ebadi, M.-T.; Ghomi, H.; Ayyari, M. Changes in qualitative characteristics of garden thyme (Thymus vulgaris L.) as affected by cold plasma. J Appl Res Med Aromat Plants. 2022, 31, 100411. [CrossRef]
- Kirkin, C.; Gunes, G. Quality of thyme (Thymus vulgaris L.) and black pepper (Piper nigrum L.) during storage as affected by the combination of gamma-irradiation and modified atmosphere packaging. S Afr J Bot. 2022, 150, 978-985. [CrossRef]
- Divband, K.; Shokri, H.; Khosravi, A.R. Down-regulatory effect of Thymus vulgaris L. on growth and Tri4 gene expression in Fusarium oxysporum strains. Microb Pathog. 2017, 104, 1-5. [CrossRef]
- Pavela, R.; Zabka, M.; Vrchotova, N.; Triska, J. Effect of foliar nutrition on the essential oil yield of Thyme (Thymus vulgaris L.). Ind Crops Prod. 2018, 112, 762-765. [CrossRef]
- Lagou, M.K.; Karagiannis, G.S. Obesity-induced thymic involution and cancer risk. Semin Cancer Biol. 2023, 93, 3-19. [CrossRef]
- Segawa, R.; Kyoda, T.; Yagisawa, M.; Muramatsu, T.; Hiratsuka, M.; Hirasawa, N. Hypoxia-inducible factor prolyl hydroxylase inhibitors suppressed thymic stromal lymphopoietin production and allergic responses in a mouse air-pouch-type ovalbumin sensitization model. Int Immunopharmacol. 2023, 118, 110127. [CrossRef]
- Rivera-Perez, A.; Romero-Gonzalez, R.; Frenich, A.G. Fingerprinting based on gas chromatography-Orbitrap high-resolution mass spectrometry and chemometrics to reveal geographical origin, processing, and volatile markers for thyme authentication. Food Chem. 2022, 393, 133377. [CrossRef]
- Petrusic, M.; Stojic-Vukanic, Z.; Pilipovic, I.; Kosec, D.; Prijic, I.; Leposavic, G. Thymic changes as a contributing factor in the increased susceptibility of olf Albino Oxford rats to EAE development. Exp Gerontol. 2023, 171, 112009. [CrossRef]
- Ao, Y.-Q.; Jiang, J.-H.; Gao, J.; Wang, H.-K.; Ding, J.-Y. Recent thymic emigrants as the bridge between thymoma and autoimmune diseases. Biochim Biophys Acta BBA-Rev Cancer. 2022, 1877(3), 188730. [CrossRef]
- Barros, F.A.P.; Radunz, M.; Scariot, M.A.; Camargo, T.M.; Nunes, C.F.P.; de Souza, R.R.; Gilson, I.K.; Hackbart, H.C.S.; Radunz, L.L.; Oliveira, J.V.; Tramontin, M.A.; Radunz, A.L.; Magro, J.D. Efficacy of encapsulated and non-encapsulated thyme essential oil (Thymus vulgaris L.) in the control of Sitophilus zeamais and its effects on the quality of corn grains throughout storage. Crop Prot. 2022, 153, 105885. [CrossRef]
- Arora, H.; Sharma, A.; Sharma, S. Thyme essential oil fostering the efficacy of aqueous extract of licorice against fungal phytopathogens of Capsicum annuum L. J Biosci Bioeng. 2023, 135(6), 466-473. [CrossRef]
- Posgay, M.; Greff, B.; Kapcsandi, V.; Lakatos, E. Effect of Thymus vulgaris L. essential oil and thymol on the microbiological properties of meat and meat products: A review. Heliyon. 2022, 8(10), e10812. [CrossRef]
- Silva, A.S.; Tewari, D.; Sureda, A.; Suntar, I.; Belwal, T.; Battino, M.; Nabavi, S.M.; Nabavi, S.F. The evidence of health benefits and food applications of Thymus vulgaris L. Trends Food Sci Technol. 2021, 117, 218-227. [CrossRef]
- Lashgari, S.M.; Bahlakeh, G.; Ramezanzadeh, B. Detailed theoretical DFT comuptation/molecular simulation and electrochemical explorations of Thymus vulgaris leave extract for effective mild-steel corrosion retardation in HCL solution. J Mol Liq. 2021, 335, 1155897. [CrossRef]
- Adhar, M.; HadjKacem, B.; Perino-Issartier, S.; Amor, I.B.; Feki, A.; Gargouri, J.; Gargouri, A.; Tounsi, S.; Chemat, F.; Allouche, N. Thymol-enriched extract from Thymus vulgaris L. leaves: Green extraction processes and antiaggregant effects on human platelets. Bioorg Chem. 2022, 125, 105858. [CrossRef]
- Moazeni, M.; Davari, A.; Shabanzadeh, S.; Akhtari, J.; Saeedi, M.; Mortyeza-Semnani, K.; Abastabar, M.; Nabili, M.; Moghadam, F.H.; Roohi, B.; Kelidari, H.; Nokhodchi, A. In vitro antifungal activity of Thymus vulgaris essential oil nanoemulsion. J. Herb Med. 2021, 28, 100452. [CrossRef]
- Prieto, M.C.; Camacho, N.M.; Inocenti, F.D.; Mignolli, F.; Lucini, E.; Palma, S.; Bima, P.; Grosso, N.R.; Asensio, C.M. Microencapsulation of Thymus vulgaris and Tagetes minuta essential oils: Volatile release behavior, antibacterial activity and effect on potato yield. J. Saudi Soc. Agric. Sci. 2023, 22(3), 195-204. [CrossRef]
- Lei, Y.-Y.; Chen, X.-R.; Jiang, S.; Guo, M.; Yu, C.-L.; iao, J.-H.; Cai, B.; Ai, H.-S.; Wang, Y.; Hu, K.-X. Mechanisms of thymic repair of in vitro induced precursor T cells as a haploidentical hematopoietic stem cell transplantation regimen. Transplant Cell. Ther. 2023, 29(6), 382.e1-382.e11. [CrossRef]
- Silva, C.S.; Cerqueira, M.T.; Reis, R.L.; Martins, A.; Neves, N.M. Laminin-2 immobilized on a 3D fibrous structure impacts cortical thymic epithelial cells behaviour and their interaction with thymocytes. Int J Biol Macromol. 2022, 222(Part B), 3168-3177. [CrossRef]
- Perez, N.; Altube, M.J.; Barbosa, L.R.S.; Romero, E.L.; Perez, A.P. Thymus vulgaris essential oil+tobramycin within nanostructured archaeolipid carriers: A new approach against Pseudomonas aeruginosa biofilms. Phytomedicine. 2022, 102, 154179. [CrossRef]
- Bauer, R.; Khan, I.A.; Wray, V.; Wagner, H. Two acetylenic compounds from Echinacea pallida roots. Phytochemistry. 1987, 26(4), 1199-1200. [CrossRef]
- Chicca, A.; Adinolfi, B.; Martinotti, E.; Fogli, S.; Breschi, M.C.; Pellati, F.; Benvenuti, S.; Nieri, P. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J. Ethnopharmacol. 2007, 110(1), 148-153. [CrossRef]
- Fu, R.; Zhang, P.; Deng, Z.; Jin, G.; Guo, Y.; Zhang, Y. Diversity of antioxidant ingredients among Echinacea species. Ind. Crops Prod. 2021, 170, 113699. [CrossRef]
- Sabra, A.; Daayf, F.; Renault, S. Differential physiological and biochemical responses of three Echinacea species to salinity stress. Sci. Hortic. 2012, 135, 23-31. [CrossRef]
- Bruni, R.; Brighenti, V.; Caesar, L.K.; Bertelli, D.; Cech, N.B.; Pellati, F. Analytical methods for the study of bioactive compounds from medicinally used Echinacea species. J. Pharm. Biomed. Anal. 2018, 160, 443-477. [CrossRef]
- Pellati, F.; Calo, S.; Benvenuti, S.; Adinolfi, B.; Nieri, P.; Melegari, M. Isolation and structure elucidation of cytotoxic polyacetylenes and polyenes from Echinacea pallida. Phytochemistry. 2006, 67(13), 1359-1364. [CrossRef]
- Pellati, F.; Calo, S.; Benvenuti, S. High-performance liquid chromatography analysis of polyacetylenes and polyenes in Echinacea pallida by using a monolithic reversed-phase silica column. J Chromatogr A. 2007, 1149(1), 56-65. [CrossRef]
- Thude, S.; Classen, B. High molecular weight constituents from roots of Echinacea pallida: An arabinogalactan-protein and an arabinan. Phytochemistry 2005, 66(9), 1026-1032. [CrossRef]
- Zhai, Z.; Haney, D.M.; Wu, L.; Solco, A.K.; Murphy, P.A.; Wurtele, E.S.; Kohut, M.L.; Cunnick, J.E. Alcohol extract of Echinacea pallida reverses stress-delayed wound healing in mice. Phytomedicine 2009, 16(6-7), 669-678. [CrossRef]
- Binns, S.E.; Inparajah, I.; Baum, B.R.; Arnason, J.T. Methyl jasmonate increases reported alkamides and ketoalkene/ynes in Echinacea pallida (Asteraceae). Phytochemistry 2001, 57(3), 417-420. [CrossRef]
- Dufault, R.J.; Rushing, J.; Hassell, R.; Shepard, B.M.; McCutcheon, G.; Ward, B. Influence of fertilizer on growth and marker compound of field-grown Echinacea species and feverfew. Sci. Hortic. 2003, 98(1), 61-69. [CrossRef]
- Orhan, I.; Senol, F.S.; Gulpinar, A.R.; Kartal, M.; Sekeroglu, N.; Deveci, M.; Kan, Y.; Sener, B. Acetylcholinesterase inhibitory and antioxidant properties of Cyclotrichium niveum, Thymus praecox subsp. caucasicus var. caucasicus, Echinacea purpurea and E. pallida. Food Chem. Toxicol. 2009, 47(6), 1304-1310. [CrossRef]
- Lopresti, A.L.; Smith, S.J. An investigation into the anxiety-relieving and mood-enhancing effects of Echinacea angustifolia (EP107TM): A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2021, 293, 229-237. [CrossRef]
- Barkat, M.; Bouguerra, A. Study of the antifungal activity of essential oil extracted from seeds of Foeniculum vulgare Mill, for its use as food conservative. New Biotechnol. 2012, 29, S133. [CrossRef]
- Chen, J.; Ding, J.; Li, D.; Wang, Y.; Wu, Y.; Yang, X.; Chinnathambi, A.; Salmen, S.H.; Alharbi, S.A. Facile preparation of Au nanoparticles mediated by Foeniculum Vulgare aqueous extract and investigation of the anti-human breast carcinoma effects. Arab J Chem. 2022, 15(1), 103479. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. B. Soil Plant Sci. 2019, 69(6), 546-556. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. A review of astragalus species as foodstuffs, dietary supplements, a traditional Chinese medicine and a part of modern pharmaceutical science. Appl. Ecol. Environ. Res. 2019, 17(6), 13371-13382.
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Traditional herbal medicine for the prevention and treatment of cold and flu in the autumn of 2020, overlapped with Covid-19. Nat. Prod. Commun. 2020, 15(8), 1-10. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Soleymani, A.; Cheng, Q. Traditional herbal medicines to overcome stress, anxiety and improve mental health in outbreaks of human coronaviruses. Phytother. Res. 2020, 2020(1), 1-11. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Exploring Artemisia annua L., artemisinin and its derivatives, from traditional Chinese wonder medicine science. Not Bot Horti Agrobot Cluj-Napoca. 2020, 48(4), 1719-1741. [CrossRef]
- He, G.; Sun, H.; Liao, R.; Wei, Y.; Zhang, T.; Chen, Y.; Lin, S. Effects of herbal extracts (Foeniculum vulgare and Artemisia annua) on growth, liver antioxidant capacity, intestinal morphology and microorganism of juvenile largemouth bass, Micropterus salmoides. Aquac Rep. 2022, 23, 101081. [CrossRef]
- Khazaei, M.; Dastan, D.; Ebadi, A. Binding of Foeniculum vulgare essential oil and its major compounds to double-stranded DNA: In silico and in vitro studies. Food Biosci. 2021, 41, 100972. [CrossRef]
- Akhtar, I.; Javad, S.; Ansari, M.; Ghaffar, N.; Tariq, A. Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology. J King Saud Univ Sci. 2020, 32(2), 1451-1458. [CrossRef]
- Benddine, H.; Zaid, R.; Babaali, D.; Daoudi-Hacini, S. Biological activity of essential oils of Myrtus communis (Myrtaceae, Family) and Foeniculum vulgare (Apiaceae, Family) on open fields conditions against corn aphids Rhopalosiphum maidis (Fitch, 1856) in western Algeria. J. Saud. Soc. Agric. Sci. 2023, 22(2), 78-88. [CrossRef]
- Torres, M.; Frutos, G. Analysis of germination curves of aged fennel seeds by mathematical models. Environ. Exp. Bot. 1989, 29(3), 409-415. [CrossRef]
- Boudraa, H.; Kadri, N.; Mouni, L.; Madani, K. Microwave-assisted hydrodistillation of essential oil from fennel seeds: Optimization using Plackett-Burman design and response surface methodology. J. Appl. Res. Med. Aromat Plants. 2021, 23, 100307. [CrossRef]
- Telci, I.; Demirtas, I.; Sahin, A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30(1), 126-130. [CrossRef]
- Fatima, A.; Chand, N.; Naz, S.; Saeed, M.; Khan, N.U.; Khan, R.U. Coping heat stress by crushed fennel (Foeniculum vulgare) seeds in broilers: Growth, redox balance and humoral immune response. Livestock Sci. 2022, 265, 105082. [CrossRef]
- Hashemirad, S.; Soltani, E.; Darbandi, A.I.; Alahdadi, I. Cold stratification requirement to break morphophysiology dormancy of fennel (Foeniculum vulgare Mill.) seeds varies with seed length. J Appl Res Med Aromat Plants. 2023, 35, 100465. [CrossRef]
- Mokhtari, L.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of trans-anethole from Foeniculum vulgare (fennel) seeds: Optimization of operating conditions through response surface methodology and genetic algorithm. J CO2 Util. 2019, 30, 1-10. [CrossRef]
- Abdellaoui, M.; Bouhlali, E.D.T.; Kasrati, A.; El Rhaffari, L. The effect of domestication on seed yield, essential oil yield and antioxidant activities of fennel seed (Foeniculum vulgare Mill.) grown in Moroccan oasis. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 24(1), 107-114. [CrossRef]
- Noyraksa, S.; Wichianwat, K.; Punpuk, S.; Aiemyeesun, S.; Maitip, J.; Suttiarporn, P. Optimization of microwave-assisted hydrodistillation of essential oils from fennel seeds. Materialstoday: Proceedings. 2023, 77(4), 1079-1085. [CrossRef]
- Shojaiefar, S.; Sabzalian, M.R.; Mirlohi, A.; Mirjalili, M.H. Seed yield stability with modified essential oil content and composition in self-compatible progenies of bitter fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2022, 182, 114821. [CrossRef]
- Hayat, K.; Abbas, S.; Hussain, S.; Shahzad, S.A.; Tahir, M.U. Effect of microwave and conventional oven heating on phenolic constituents, fatty acids minerals and antioxidant potential of fennel seed. Ind. Crops Prod. 2019, 140, 111610. [CrossRef]
- Rezaei, S.; Ebadi, M.-T.; Ghobadian, B.; Ghomi, H. Optimization of DBD-Plasma assisted hydro-distillation for essential oil extraction of fennel (Foeniculum vulgare Mill.) seed and spearmint (Mentha spicata L.) leaf. J Appl Res Med Aromat Plants. 2021, 24, 100300. [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. The nutritional composition of fennel (Foeniculum vulgare): shoots, leaves, stems and inflorescences. LWT-Food Sci Technol 2010, 43, 814-818.
- Mabungela, N.; Shooto, N.D.; Mtunzi, F.; Naidoo, E.B.; Mlambo, M.; Mokubung, K.E.; Mpelane, S. Multi-application of fennel (Foeniculum vulgaris) seed composites for the adsorption and photo-degradation of methylene blue in water. S Afr J Chem Eng. 2023, 44, 283-296. [CrossRef]
- Soleymani, A.; Shahrajabian, M.H.; Karimi, M. Growth behaviour of elite barley lines as influenced by planting date and plant densities. Res. on Crops. 2012, 13(2), 463-466.
- Soleymani, A.; Shahrajabian, M.H. Effects of planting dates and different levels of nitrogen on seed yield and yield components of nuts sunflower. Res. on Crops. 2012, 13(2): 521-524.
- Soleymani, A.; Shahrajabian, M.H. Response of different cultivars of fennel (Foeniculum vulgare) to irrigation and planting dates in Isfahan, Iran. Res. on Crops. 2012, 13(2), 656-660.
- Soleymani, A.; Shahrajabian, M.H.; Naranjani, L. Effect of planting dates and different levels of nitrogen on seed yield and yield components of nuts sunflower (Helianthus annuus L.). Afr J Agric Res. 2013, 8(46), 5802-5805.
- Karakus, Y.Y.; Yildirim, B.; Acemi, A. Characterization polyphenol oxidase from fennel (Foeniculum vulgare Mill.) seeds as a promising source. Int J Biol Macromol. 2021, 170, 261-271. [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind Crops Prod. 2018, 111, 92-98. [CrossRef]
- Alazadeh, M.; Azadbakht, M.; Niksolat, F.; Asgarirad, H.; Mossazadeh, M.; Ahmadi, A.; Yousefi, S.S. Effect of sweet fennel seed extract capsule on knee pain in women with knee osteoarthritis. Complement Ther Clin Pract. 2020, 40, 101219. [CrossRef]
- Machiani, M.A.; Rezaei-Chiyaneh, E.; Javanmard, A.; Maggi, F.; Morshedloo, M.R. Evaluation of common bean (Phaseolus vulgaris L.) seed yield and quali-quantitative production of the essential oils from fennel (Foeniculum vulgare Mill.) and dragonhead (Dracocephalum moldavica L.) in intercropping system under humic acid application. J Clean Prod. 2019, 235, 112-122. [CrossRef]
- Masoudzadeh, S.H.; Mohammadabadi, M.; Kherzi, A.; Stavetska, R.V.; Oleshko, V.P.; Babenko, O.I.; Yemets, Z.; Kalashnik, O.M. Effects of diets with different levels of fennel (Foeniculum vulgare) seed power on DLK1 gene expression in brain, adipose tissue, femur muscle and rumen of Kermani lambs. Small Rumin Res. 2020, 193, 106276. [CrossRef]
- Ramalho, F.D.S.; Malaquias, J.B.; Brito, B.D.D.S.; Fernandes, F.S.; Zanuncio, J.C. Assessment of the attack of Hyadaphis foeniculi (Passerini) (Hemiptera: Aphididae) on biomass, seed and oil in fennel intercropped with cotton with colored fibers. Ind Crops Prod. 2015, 77, 511-515. [CrossRef]
- Kargar, S.; Nowroozinia, F.; Kanani, M. Feeding fennel (Foeniculum vulgare) seed as potential appeptie stimulant to newborn Holstein dairy calves: Effects on meal pattern, ingestive behavior, oro-sensorial preference, and feed sorting. Anim. Feed Sci. Technol. 2021, 278, 115009. [CrossRef]
- Hajalizadeh, Z.; Dayani, O.; Khezri, A.; Tahmasbi, R.; Mohammadabadi, M.R. The effect of adding fennel (Foeniculum vulgare) seed powder to the diet of fattening lambs on performance, carcass characteristics and liver enzymes. Small Rumin. Res. 2019, 175, 72-77. [CrossRef]
- Nowroozinia, F.; Kargar, S.; Akhlaghi, A.; Fard, F.R.; Bahadori-Moghaddam, M.; Kanani, M.; Zamiri, M.J. Feeding fennel (Foeniculum vulgare) seed as a potential appetite stimulant for Holstein dairy calves: Effects on growth performance and health. J. Dairy Sci. 2021, 105, 654-664.
- Gonzalez-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innov. Food Sci. Emerg. Technol. 2016, 33, 308-318. [CrossRef]
- Rodriguez-Solana, R.; Salgado, J.M.; Dominguez, J.M.; Cortes-Dieguez, S. Estragole quantity optimization from fennel seeds by supercritical fluid extraction (carbon dioxide-methanol) using a Box-Behnken design. Characterization of fennel extracts. Ind Crops Prod. 2014, 60, 186-192. [CrossRef]
- Burkhardt, A.; Sintim, H.Y.; Gawde, A.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Schlegel, V. Method for attaining fennel (Foeniculum vulgare Mill.) seed oil fractions with different composition and antioxidant capacity. J Appl Res Med Aromat Plants. 2015, 2(3), 87-91. [CrossRef]
- Shojaiefar, S.; Mirlohi, A.; Sabzalian, M.R.; Yaghini, H. Seed yield and essential oil content of fennel influenced by genetic variation and genotype x year interaction. Ind Crops Prod. 2015, 71, 97-105. [CrossRef]
- Hazar, H.; Sevinc, H.; Sap, S. Performance and emission properties of preheated and blensed fennel vegetable oil in a coated diesel engine. Fuel 2019, 254, 115677. [CrossRef]
- Desoky, E.-S.M.; El-Maghrabu, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [CrossRef]
- Damjanovic, B.; Lepojevic, Z.; Zivkovic, V.; Tolic, A. Extraction of fennel (Foeniculum vulgare Mill.) seeds with supercritical CO2: Comparison with hydrodistillation. Food Chem. 2005, 92(1), 143-149. [CrossRef]
- Moser, B.R.; Zheljazkov, V.D.; Bakota, E.L.; Evangelista, R.L.; Gawde, A.; Cantrell, C.L.; Winkler-Moser, J.K.; Hristov, A.N.; Astatkie, T.; Jeliazkova, E. Method for obtaining three products with different properties from fennel (Foeniculum vulgare) seed. Ind Crops Prod. 2014, 60, 335-342. [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014, 35(1), 109-116. [CrossRef]
- Pavela, R.; Zabka, M.; Bednar, J.; Triska, J.; Vrchotova, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2016, 83, 275-282. [CrossRef]
- Khammassi, M.; Mighri, H.; Mansour, M.B.; Amri, I.; Jamoussi, B.; Khaldi, A. Metabolite profiling and potential antioxidant activity of sixteen fennel (Foeniculum vulgare Mill.) populations growing wild in Tunisia. S. Afr. J. Bot. 2022, 148, 407-414. [CrossRef]
- Oktay, M.; Gulcin, I.; Kufrevioglu, O.I. Determination on in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci. Technol. 2003, 36(2), 263-271. [CrossRef]
- Lee, C.-H.; Sung, B.-K.; Lee, H.-S. Acaricidal activity of fennel seed oils and their main components against Tyrophagus putrescentiae, a stored-food mite. J. Stored Prod. Res. 2006, 42(1), 8-14. [CrossRef]
- Ghasemian, A.; Al-Marzoi, A.-H.; Mostafavi, S.K.S.; Alghanimi, Y.K.; Teimouri, M. Chemical composition and antimicrobial and cytotoxic activities of Foeniculum vulgare Mill essential oils. J Gastrointest Cancer. 2020, 51(1), 260-266. [CrossRef]
- Sadrefozalayi, S.; Farokhi, F. Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna J Phytomed. 2014, 4(2), 110-117.
- Farid, A.; Kamel, D.; Montaser, S.A.; Ahmed, M.M.; El-Amir, M.; El-Amir, Z. Assessment of antioxidant, immune enhancement, and antimutagenic efficacy of fennel seed extracts in irradiated human blood cultures. J Radiat Res Appl Sci. 2020, 13(1), 260-266. [CrossRef]
- Alasalvar, H.; Yildirim, Z. Ultrasound-assisted extraction of antioxidant phenolic compounds from Lavandula angustifolia flowers using natural deep eutectic solvents: An experimental design approach. Sustain. Chem. Pharm. 2021, 22, 100492. [CrossRef]
- Ozsevinc, A.; Alkan, C. Ethylene glycol based polyurethane shell microcapsules for textile applications releasing medicinal lavender and responding to mechanical stimuli. Colloids Surf. A. Physicochem. Eng. Asp. 2022, 652, 129888. [CrossRef]
- Villalpando, M.; Gomez-Hurtado, M.A.; Rosas, G.; Saavedra-Molina, A. Ag nanoparticles synthesized using Lavandula angustifolia and their cytotoxic evaluation in yeast. Mater. Today Commun. 2022, 31, 103633. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Product of natural evolution (SARS, MERS, and SARS-CoV-2); deadly diseases, from SARS to SARS-CoV-2. Hum. Vaccines Immunother. 2020, 17(1), 62-83. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Shen, H.; Cheng, Q. Chinese herbal medicine for SARS and SARS-CoV-2 treatment and prevention, encouraging using herbal medicine for COVID-19 outbreak. Acta Agric. Scand. B Soil Plant Sci. 2020, 70(5), 437-443. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Khoshkharam, M.; Cheng, Q. Caraway, Chinese chives and cassia as functional foods with considering nutrients and health benefits. Carpathian J. Food Sci. Technol. 2021, 13(1), 101-119. [CrossRef]
- Danila, A.; Muresan, E.I.; Ibanescu, S.-A.; Popescu, A.; Danu, M.; Zaharia, C.; Turkoglu, G.C.; Erkan, G.; Staras, A.-I. Preparation, characterization, and application of polysaccharide--based emulsions incorporated with lavender essential oil for skin-friendly cellulosic support. Int. J. Biol. Macromol. 2021, 191, 405-413. [CrossRef]
- Firoozeei, T.S.; Barekatain, M.; Karimi, M.; Zargaran, A.; Akhondzadeh, S.; Rezaeizadeh, H. Lavender and dodder combined herbal syrup versus citalopram in major depressive disorder with anxious distress: A double-blind randomized trial. J. Integr. Med. 2020, 18(5), 409-415. [CrossRef]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Milani, F.; Spada, A.; Papini, A.; Flamini, G.; Fico, G. Insight into micromorphology and phytohemistry of Lavandula angustifolia Mill. from Italy. S. Afr. J. Bot. 2023, 153, 83-92. [CrossRef]
- Ozsevinc, A.; Alkan, C. Polyurethane sheel medicinal lavender release microcapsules for textile materials: An environmentally friendly preparation. Ind. Crops. Prod. 2023, 192, 116131. [CrossRef]
- Ganguly, R.; Kumar, S.; Basu, M.; Kunwar, A.; Dutta, D.; Aswal, V.K. Micellar solubilization of lavender oil in aqueous P85/P123 systems: Investigating the associated micellar structural transitions, therapeutic properties and existence of double cloud points. J Mol Liq. 2021, 338, 116643. [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int J Pharm. 2019, 569, 118590. [CrossRef]
- Perovic, A.; Stankovic, M.Z.; Veljkovic, V.B.; Kostic, M.D.; Stamenkovic, O.S. A further study of the kinetics and optimization of the essential oil hydrodistillation from lavender flowers. Chin J Chem Eng. 2021, 29, 126-130. [CrossRef]
- Fascella, G.; Mammano, M.M.; D,Angiolillo, F.; Pannico, A.; Rouphael, Y. Coniferous wood biochar as substrate component of two containerized lavender species: Effects on morpho-physiological traits and nutrients partitioning. Sci Hortic. 2020, 267, 109356. [CrossRef]
- Khatami, S.A.; Kasraie, P.; Oveysi, M.; Moghadam, H.R.T.; Ghooshchi, F. Mitigating the adverse effects of salinity stress on lavernder using biodynamic preparations and bio-fertilizers. Ind Crops Prod. 2022, 183, 114985. [CrossRef]
- Pecanha, D.A.; Freitas, M.S.M.; Vieira, M.E.; Cunha, J.M.; Jesus, A.C.D. Phosphorus fertilization affects growth, essential oil yield and quality of true lavender in Brazil. Ind Crops Prod. 2021, 170, 113803. [CrossRef]
- Hassiotis, C.N.; Tarantilis, P.A.; Daferera, D.; Polissiou, M.G. Etherio, a new variety of Lavandula angustifolia with improved essential oil production and composition from natural selected genotypes growing in Greece. Ind Crops Prod. 2010, 32(2), 77-82. [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa Officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13-21. [CrossRef]
- Shi, J.-L.; Tang, S.-Y.; Liu, C.-B.; Ye, L.; Yang, P.-S.; Zhang, F.-M.; He, P.; Liu, Z.-H.; Miao, M.-M.; Guo, Y.-D.; Shen, Q.-P. Three new benzolactones from Lavandula angustifolia and their bioactivities. J Asian Nat Prod Res. 2017, 19(8), 766-773. [CrossRef]
- Rashed, M.M.A.; Mahdi, A.A.; Ghaleb, A.D.S.; Zhang, F.R.; Huan, D.Y.; Qin, W.; Hai, Z.W. Synergistic effects of amorphous OSA-modified starch, unsaturated lipid-carrier, and sonocavitation treatment in fabricating of Lavandula angustifolia essential oil nanoparticles. Int J Biol Macromol. 2020, 151, 702-712. [CrossRef]
- Miastkowska, M.; Sikora, E.; Kulawik-Pioro, A.; Kantyka, T.; Bielecka, E.; Kalucka, U.; Kaminska, M.; Szulc, J.; Piasecka-Zelga, J.; Zelga, P.; Staniszewska-Slezak, E. Bioactive Lavandula angustifolia essential oil-loaded nanoemulsion dressing for burn wound healing. In vitro and in vivo studies. Biomater Adv. 2023, 148, 213362. [CrossRef]
- Kirimer, N.; Mokhtarzadeh, S.; Demirci, B.; Goger, F.; Khawar, K.M.; Demirci, F. Phytochemical profiling of volatile components of Lavandula angustifolia Miller propagated under in vitro conditions. Ind Crops Prod. 2017, 96, 120-125. [CrossRef]
- Stanley, P.F.; Wan, L.F.; Karim, R.A. A randomized prospective placebo-controlled study of the effects of lavender aromatherapy on preoperative anxiety in cataract surgery patients. J PeriAnesth Nurs. 2020, 35(4), 403-406. [CrossRef]
- Bensmira, M.; Jiang, B.; Nsabimana, C.; Jian, T. Effect of lavender and thyme incorporation in sunflower seed oil on its resistance to frying temperatures. Food Res Int. 2007, 40(3), 341-346. [CrossRef]
- Rivaz, M.; Rahpeima, M.; Khademian, Z.; Dabbaghmanesh, M.H. The effects of aromatherapy massage with lavender essential oil on neuropathic pain and quality of life in diabetic patients: A randomized clinical trial. Complement Ther Clin Pract. 2021, 44, 101430. [CrossRef]
- Gismondi, A.; Marco, G.D.; Redi, E.L.; Ferrucci, L.; Cantonetti, M.; Canini, A. The antimicrobial activity of Lavandula angustifolia Mill. Essential oil against Staphylococcus species in a hospital environment. J Herb Med. 2021, 26, 100426. [CrossRef]
- Muller, W.E.; Sillani, G.; Schuwald, A.; Friedland, K. Pharmacological basis of the anxiolytic and antidepressant properties of Silexan®, an essential oil from the flowers of lavender. Neurochem Int. 2021, 143, 104899. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Plant of the Millennium, caper (Capparis spinosa L.), chemical composition and medicinal uses. Bull Nat Res Cent. 2021, 45(131), 1-9. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. The importance of flavonoids and phytochemicals of medicinal plants with antiviral activities. Mini Rev Org Chem. 2022, 19(3), 293-318. [CrossRef]
- Shahrajabian, M.H.; Sun, W. The important nutritional benefits and wonderful health benefits of Cashew (Anacardium occidentale L.). Nat Prod J. 2023, 13(4), 2-10. [CrossRef]
- Shahrajabian, M.H.; Sun, W. Survey on multi-omics, and multi-omics data analysis, integration and application. Curr. Pharm. Anal. 2023, 19(4), 267-281. [CrossRef]
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the influences of microbial biostimulants on horticultural crops: Case studies and successful paradigms. Horticulturae. 2023, 9(193), 1-24. [CrossRef]
- Shahrajabian, M.H.; Marmitt, D.; Cheng, Q.; Sun, W. Natural antioxidants of the underutilized and neglected plant species of Asia and South America. Lett. Drug. Des. Discov. 2023, 19. [CrossRef]
- Ivanova, L.; Vassileva, P.; Detcheva, A. Studies on copper (II) biosorption using a material based on the plant Thymus vulgaris L. Mater. Today Proceed. 2022, 61(4), 1237-1242. [CrossRef]
- Moori, S.; Ahmadi-Lahijani, M.J. Hormopriming instigates defense mechanisms in Thyme (Thymus vulgaris L.) seeds under cadmium stress. J. Appl. Res. Med. Aromat. Plants. 2020, 19, 100268. [CrossRef]
- Ghoshal, G.; Shivani. Thyme essential oil nano-emulsion/Tamarind starch/protein concentrate novel edible films for tomato packaging. Food Control 2022, 138, 108990. [CrossRef]
- Silva, A.M.; Felix, L.M.; Teixerira, I.; Martins-Gomes, C.; Schafer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. 2021, 12, 100171. [CrossRef]
- Soleimani, M.; Arzani, A.; Arzani, V.; Roberts, T.H. Phenolic compounds and antimicrobial properties of mint and thyme. J. Herb Med. 2022, 36, 100604. [CrossRef]
- Zhang, X.; He, J.; Zhao, K.; Liu, S.; Xuan, L.; Chen, S.; Xue, R.; Lin, R.; Xu, J.; Zhang, Y.; Xiang, A.P.; Jin, H.; Liu, Q. Mesenchymal stromal cells ameliorate chronic graft-versus-host disease by boosting thymic regeneration in a CCR9-dependent manner in mice. Blood Adv. 2023. [CrossRef]
- Zhou, L.; Hao, M.; Min, T.; Bian, X.; Du, H.; Sun, X.; Zhu, Z.; Wen, Y. Kaolin incorporated with thyme essential oil for humidity-controlled antimicrobial food packaging. Food Pack. Shelf Life. 2023, 38, 101106. [CrossRef]
- Ghai, R.; Saraswat, S. Anti-hyperlipidaemic effect of thyme infused green coffee on human subjects. Hum. Nutr. Metab. 2023, 33, 200199. [CrossRef]
- Erkaya-Kotan, T.; Gurbuz, Z.; Dagdemir, E.; Sengul, M. Utilization of edible coating based on quince seed mucilage loaded with thyme essential oil: Shelf life, quality, and ACE-inhibitory activity efficiency in Kasar cheese. Food Biosci. 2023, 54, 102895. [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35-40. [CrossRef]
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 177, 517-525. [CrossRef]
- Morsy, N.F.S. Production of thymol rich extracts from ajwain (Carum copticum L.) and thyme (Thymus vulgaris L.) using supercritical CO2. Ind. Crops Prod. 2020, 145, 112072. [CrossRef]
- Sojic, B.; Tomovic, V.; Kocic-Tanackov, S.; Kovacevic, D.B.; Putnik, P.; Mrkonjic, Z.; Durovic, S.; Jokanovic, M.; Ivic, M.; Skaljac, S. Pavlic, B. Supercritical extracts of wild thyme (Thymus serpyllum L.) by-product as natural antioxidants in ground pork patties. LWT 2020, 130, 109661. [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; Baruzzi, F. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [CrossRef]
- Mrkonjic, Z.; Rakic, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslic, N.; Zekovic, Z.; Pavlic, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants. 2021, 24, 100333. [CrossRef]
- Cho, Y.; Kim, H.; Beuchat, L.R.; Ryu, J.-H. Synergistic activities of gaseous oregano and thyme thymol essential oils against Listeria monocytogenes on surfaces of a laboratory medium and radish sprouts. Food Microbiol. 2020, 86, 103357. [CrossRef]
- Shirvani, A.; Goli, S.A.H.; Shahedi, M.; Soleimanian-Zad, S. Changes in nutritional value and application of thyme (Thymus vulgaris) essential oil on microbial and organoleptic markers of Persian clover (Trifolium resupinatum) sprouts. LWT-Food Sci. Technol. 2016, 67, 14-21. [CrossRef]
- El-Newary, S.A.; Shaffie, N.M.; Omer, E.A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac. J. Trop. Med. 2017, 10(4), 361-371. [CrossRef]
- Pereira, E.; Barros, L.; Antonio, A.L.; Verde, S.C.; Santos-Buelga, C.; Ferreira, I.C.F.R. Infusion from Thymus vulgaris L. treated at different gamma radiation doses; Effects on antioxidant activity and phenolic. LWT 2016, 74, 34-39. [CrossRef]
- Rabiei, B.; Bahador, S.; Kordrostami, M. The expression of monoterpene synthase genes and their respective and products are affected by gibberellic acid in Thymus vulgaris. J. Plant Physiol. 2018, 230, 101-108. [CrossRef]
- Nadi, A.; Shirvai, A.A.; Mohammadi, Z.; Aslani, A.; Zeinalian, M. Thymus vulgaris, a natural pharmacy against COVID-19: A molecular review. J. Herb. Med. 2023, 38, 100635. [CrossRef]
- Tardugno, R.; Serio, A.; Purgatorio, C.; Savini, V.; Paparella, A.; Benevenuti, S. Thymus vulgaris L. essential oils from Emilia Romagna Apennines (Italy): phytochemical composition and antimicrobial activity on food-borne pathogens. Nat. Prod. Res. 2022, 36(3), 837-842. [CrossRef]
- Labiad, M.H.; Belmaghraoui, W.; Ghanimi, A.; El-Guezzane, C.; Chahboun, N.; Harhar, H.; Egea-Gilabert, C.; Zarrouk, A.; Tabyaoui, M. Biological properties and chemical profiling of essential oils of Thymus (vulgaris, algeriensis and broussonettii) grown in Morocco. Chem. Data. Collect. 2022, 37, 100797. [CrossRef]
- Orhan-Yanikan, E.; Gulseren, G.; Ayhan, K. Antimicrobial characteristics of Thymus vulgaris and Rose damascena oils against some milk-borne bacteria. Microchem. J. 2022, 183, 108069. [CrossRef]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 2021, 7(5), e07054. [CrossRef]
- Ben-Jabeur, M.; Vicente, R.; Lopez-Cristoffanini, C.; Alesami, N.; Djebali, N.; Gracia-Romero, A.; Serret, M.D.; Lopez-Carbonell, M.; Araus, J.L.; Hamada, W. A novel aspect of essential oils: coating seeds with thyme essential oil induces drought resistance in wheat. Plants 2019, 8(371), 1-17. [CrossRef]
- Konstantinovic, B.; Popov, M.; Samardzic, N.; Acimovic, M.; Sucur Elez, J.; Stojanovic, T.; Crnkovic, M.; Rajkovic, M. The effect of Thymus vulgaris L. hydrolate solutions on the seed germination, seedling length, and oxidative stress of some cultivated and weed species. Plants 2022, 11(1782), 1-15. [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014, 174, 88-97. [CrossRef]
- Lorenzo-Leal, A.; Palou, E.; Lopez-Malo, A. Evaluation of the efficiency allspice, thyme and rosemary essential oils on two foodborne pathogens in in vitro and on alfalfa seeds, and their effect on sensory characteristics of the sprouts. Int. J. Food Microbiol. 2019, 295, 19-24. [CrossRef]
- Majinasab, M.; Niakousari, M.; Shaghaghian, S.; Dehghani, H. Antimicrobial and antioxidant coating based on basil seed gum incorporated with Shirazi thyme and summer savory essential oils emulsions for shelf-life extension of refrigerated chicken fillets. Food Hydrocoll. 2020, 108, 106011. [CrossRef]
- Sotelo, J.P.; Oddino, C.; Giordano, D.F.; Carezzano, M.E.; Oliva, M.D.I.M. Effect of Thymus vulgaris essential oil on soybeans seeds infected with Pseudomonas syringae. Physiol. Mol. Plant Pathol. 2021, 116, 101735. [CrossRef]
- Debonne, E.; Leyn, I.D.; Verwaeren, J.; Moens, S.; Devlieghere, F.; Eeckhout, M.; Van Bockstaele, F. The influence of natural oils of blackcurant, black cumin seed, thyme and wheat germ on dough and bread technological and microbiological quality. LWT. 2018, 93, 212-219. [CrossRef]
- Khalifa, F.K.; Alkhalaf, M.I. Effects of black seed and thyme leaves dietary supplements against malathion insecticide-induced toxicity in experimental rat model. J. King Saud. Univ. Sci. 2020, 32(1), 914-919. [CrossRef]
- Zakrzewski, A.; Purkiewicz, A.; Jakuc, P.; Wisniewski, P.; Sawicki, T.; Chajecka-Wierzchowska, W.; Tanska, M. Effectiveness of various solvent-produced thyme (Thymus vulgaris) extracts in inhibiting the growth of Listeria monocytogenes in forzen vegetables. NFS J. 2022, 29, 26-34. [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Sayed, S.R.M. In vitro antimicrovial activity of Thymus vulgaris extracts against some nosocomial and food poisoning bacterial strains. Process Biochem. 2022, 115, 152-159. [CrossRef]
- Aucoin, M.; Cooley, K.; Saunders, P.R.; Care, J.; Anheyer, D.; Medina, D.N.; Cardozo, V.; Remy, D.; Hannan, N.; Garber, A. The effect of Echinacea spp. On the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Integr. Med. 2020, 7(4), 203-217. [CrossRef]
- Eldin, S.M.S.; Shawky, E.; Sallam, S.M.; El-Nikhely, N.; El-Sohafy, S.M. Metabolomics approach provides new insights into the immunomodulatory discriminatory biomarkers of the herbs and roots of Echinacea species. Ind. Crops Prod. 2021, 168, 113611. [CrossRef]
- Jedrzejczyk, I. Genome size and SCoT markers as tools for identification and genetic diversity assessment in Echinacea genus. Ind. Crops Prod. 2020, 144, 112055. [CrossRef]
- Erenturk, K.; Erenturk, S.; Tabil, L.G. A comparative study for the estimation of dynamic drying behavior of Echinacea angustifolia: regression analysis and neural network. Comput. Electron Agric. 2004, 45(1-3), 71-90. [CrossRef]
- Cui, H.; Shahrajabian, M.H.; Kuang, Y.; Zhang, H.; Sun, W. Heterologous expression and function of cholesterol oxidase: A review. Protein Pept. Lett. 2023, 30. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Lin, M. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation 2022, 8(12), 688. [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants-natural health products for human health. Molecules 2023, 28(1845), 1-47. [CrossRef]
- Molaveisi, M.; Taheri, R.A.; Dehnad, D. Innovative application of the Echinacea purpurea (L.) extract-phospholipid phytosomes embedded within Alyssum homolocarpum seed gum film for enhancing the shelf life of chicken meat. Food Biosci. 2022, 50(Part A), 102020. [CrossRef]
- Sagvand, M.; Esfahani, M.N.; Hadi, F. Pre-sowing enrichment of Echinacea angustifolia seeds with macronutrients improved germination performance and early seedling growth via stimulating the metabolism of reserves. Ind. Crops Prod. 2022, 188(Part A), 115416. [CrossRef]
- Qu, L.; Widrlechner, M.P. Reduction of seed dormancy in Echinacea pallida (Nutt.) Nutt. by in-dark seed selection and breeding. Ind. Crops Prod. 2012, 36(1), 88-93. [CrossRef]
- Oomah, B.D.; Dumon, D.; Cardador-Martinez, A.; Godfrey, D.V. Characteristics of Echinacea seed oil. Food Chem. 2006, 96(2), 304-312. [CrossRef]
- Tyub, S.; Ahmad Dar, S.; Lone, I.M.; Hussain Mir, A.; Kamili, A.N. A robust in vitro protocol for shoot multiplication of Echinacea angustifolia. Curr. Plant Biol. 2021, 28, 100221. [CrossRef]
- Cichello, S.A.; Yao, Q.; He, X.Q. Proliferative activity of a blend of Echinacea angustifolia and Echinacea purpurea root extracts in human vein epithelial, HeLa, and QBC-939 cell lines, but not in Beas-2b cell lines. J. Tradit. Complement. Med. 2016, 6(2), 193-197. [CrossRef]
- Aiello, N.; Carlini, A.; Scartezzini, F.; Fusani, P.; Berto, C.; Dall,Acqua, S. Harvest in different years of growth influences chemical composition of Echinacea angustifolia roots. Ind. Crops Prod. 2015, 76, 1164-1168. [CrossRef]
- Lucchesini, M.; Bertoli, A.; Mensuali-Sodi, A.; Pistelli, L. Establishment of in vitro tissue cultures from Echinacea angustifolia D.C. adult plants for the production of phytochemical compounds. Sci. Hortic. 2009, 122(3), 484-490. [CrossRef]
- Montanari, M.; Degl,Innocenti, E.; Maggini, R.; Pacifici, S.; Pardossi, A.; Guidi, L. Effect of nitrate fertilization and saline stress on the contents of active constituents of Echinacea angustifolia DC. Food. Chem. 2008, 107(4), 1461-1466. [CrossRef]
- Morazzoni, P.; Cristoni, A.; Di Pierro, F.; Avanzini, C.; Ravarino, D.; Stornello, S.; Zucca, M.; Musso, T. In vitro and in vivo immune stimulating effects of a new standardized Echinacea angustifolia root extract (PolinaceaTM). Fitoterapia 2005, 76(5), 401-411. [CrossRef]
- Maggini, R.; Tozzini, L.; Pacifici, S.; Raffaelli, A.; Pardossi, A. Growth and accumulation of caffeic acid derivatives in Echinacea angustifolia DC. var. angustifolia grown in hydroponic culture. Ind. Crops Prod. 2012, 35(1), 269-273. [CrossRef]
- Stefano, D.A.; Nicola, A.; Fabrizio, S.; Valentina, A.; Gabbriella, I. Analysis of highly secondary-metabolite producing roots and flowers of two Echinacea angustifolia DC. var. angustifolia accessions. Ind. Crops Prod. 2010, 31(3), 466-468. [CrossRef]
- Darvizheh, H.; Zahedi, M.; Abbaszadeh, B.; Razmjoo, J. Changes in some antioxidant enxymes and physiological indices of purple coneflower (Echinacea purpurea L.) in response to water deficit and foliar application of salicylic acid and spermine under field condition. Sci. Hortic. 2019, 247, 390-399. [CrossRef]
- Xu, W.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Effect of geographical location and environmental factors on metabolite content and immune activity of Echinacea purpurea in China based on metabolomics. Ind. Crops Prod. 2022, 189, 115782. [CrossRef]
- Ren, W.; Ban, J.; Xia, Y.; Zhou, F.; Yuan, C.; Jia, H.; Huang, H.; Jiang, M.; Liang, M.; Li, Z.; Yuan, Y.; Yin, Y.; Wu, H. Echinacea purpurea-derived homogeneous polysaccharide exerts anti-tumor efficacy via facilitating M1 macrophage polarization. Innovation 2023, 4(2), 100391. [CrossRef]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Potassium homeostasis and signaling as a determinant of Echinacea species tolerance to salinity stress. Environ. Exp. Bot. 2023, 206, 105148. [CrossRef]
- Gu, D.; Wang, H.; Yan, M.; Li, Y.; Yang, S.; Shi, D.; Guo, S.; Wu, L.; Liu, C. Echinacea purpurea (L.) Moench extract suppresses inflammaton by inhibition of C3a/C3aR signaling pathway in TNBS-induced ulcerative colitis rats. J. Ethnopharmacol. 2023, 307, 116221. [CrossRef]
- Mengoni, A.; Maida, I.; Chiellini, C.; Emiliani, G.; Mocali, S.; Fabiani, A.; Fondi, M.; Firenzuoli, F.; Fani, R. Antibiotic resistance differentiates Echinacea purpurea endophytic bacterial communities with respect to plant organs. Res. Microbiol. 2014, 165(8), 686-694. [CrossRef]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Morphological, phytochemical, and essential oil changes induced by different nitrogen supply forms and salinity stress in Echinacea purpurea L. Biocatal. Agric. Biotechnol. 2022, 43, 102396. [CrossRef]
- Al-Hakkani, M.F.; Gouda, G.A.; Hassan, S.H.A.; Nagiub, A.M. Echinacea purpurea mediated hematite nanoparticles (α-HNPs) biofabrication, characterization, physicochemical properties, and its in vitro biocompatibility evaluation. Surf. Interfaces. 2021, 24, 101113. [CrossRef]
- Waidyanatha, S.; Pierfelice, J.; Cristy, T.; Mutlu, E.; Burback, B.; Rider, C.V.; Ryan, K. A strategy for test article selection and phytochemical characterization of Echinacea purpurea extract for safety testing. Food Chem. Toxicol. 2020, 137, 111125. [CrossRef]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Perlite particle size and NO3-/NH4+ ratio affect growth and chemical composition of purple coneflower (Echinacea purpurea L.) in hydroponics. Ind. Crops Prod. 2021, 162, 113285. [CrossRef]
- Fan, M.-Z.; Wu, X.-H.; Li, X.-F.; Piao, X.-C.; Jiang, J.; Lian, M.-I. Co-cultured adventitious roots of Echinacea pallida and Echinacea purpurea inhibit lipopolysaccharide-induced inflammation via MAPK pathway in mouse peritoneal macrophages. Chin. Herb. Med. 2021, 13(2), 228-234. [CrossRef]
- Temerdashev, Z.; Vinitskaya, E.; Meshcheryakova, E.; Shpigun, O. Chromatographic analysis of water and water-alcohol extracts of Echinacea purpurea L. obtained by various methods. Microchem. J. 2022, 197, 107507. [CrossRef]
- Pill, W.G.; Crossan, C.K.; Frett, J.J.; Smith, W.G. Matric and osmotic priming of Echinacea purpurea (L.) Moench seeds. Sci. Hortic. 1994, 59(1), 37-44. [CrossRef]
- Chiu, K.Y.; Chuang, S.J.; Sung, J.M. Both anti-oxidation and lipid-carbohydrate conversion enhancements are involved in priming-improved emergence of Echinacea purpurea seeds that differ in size. Sci. Hortic. 2006, 108(2), 220-226. [CrossRef]
- Mainous, A.G. Echinacea purpurea is ineffective for upper respiratory tract infections in children. Evid. Based Healthcare. 2004, 8(3), 165-167. [CrossRef]
- Yu, T.; He, Y.; Chen, H.; Lu, X.; Ni, H.; Ma, Y.; Chen, Y.; Li, C.; Cao, R.; Ma, L.; Li, Z.; Lei, Y.; Luo, X.; Zheng, C. Polysaccharide from Echinacea purpurea plant ameliorates oxidative stress-induced liver injury by promoting Parkin-dependent autophagy. Phytomedicine 2022, 104, 154311. [CrossRef]
- Kraus, G.A.; Liu, F. The preparation of ketone constituents from Echinacea pallida. Tetrahedron 2011, 67(43), 8235-8237. [CrossRef]
- Wu, C.H.; Tang, J.; Jin, Z.X.; Wang, M.; Liu, Z.Q.; Huang, T.; Lian, M.L. Optimizing co-culture conditions of adventitious roots of Echinacea pallida and Echinacea purpurea in air-lift bioreactor systems. Biochem. Eng. J. 2018, 132, 206-216. [CrossRef]
- Morandi, S.; Pellati, F.; Benvenuti, S.; Prati, F. Total synthesis of a dienynone from Echinacea pallida. Tetrahedron 2008, 64(27), 6324-6328. [CrossRef]
- Tacchini, M.; Spagnoletti, A.; Brighenti, V.; Prencipe, F.P.; Benvenuti, S.; Sacchetti, G.; Pellati, F. A new method based on supercritical fluid extraction for polyacetylenes and polyenes from Echinacea pallida (Nutt.) Nutt. roots. J. Pharma. Biomed. Ananl. 2017, 146, 1-6. [CrossRef]
- Shahrajabian, M.H.; Sun, W. Five important seeds in traditional medicine, and pharmacological benefits. Seeds 2023, 2, 290-308. [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Petropoulos, S.A.; Shahrajabian, N. Developing sustainable agriculture systems in medicinal and aromatic plant production by using chitosan and chitin-based biostimulants. Plant 2023, 12(3), 2469. [CrossRef]
- Chuanren, D.; Bochu, W.; Wanqian, L.; Jing, C.; Jie, L.; Huan, Z. Effect of chemical and physical factors to improve the germination rate of Echinaceae angustifolia seeds. Colloids Surf. B. Biointerfaces. 2004, 37(3-4), 101-105. [CrossRef]
| Keypoints | References |
|---|---|
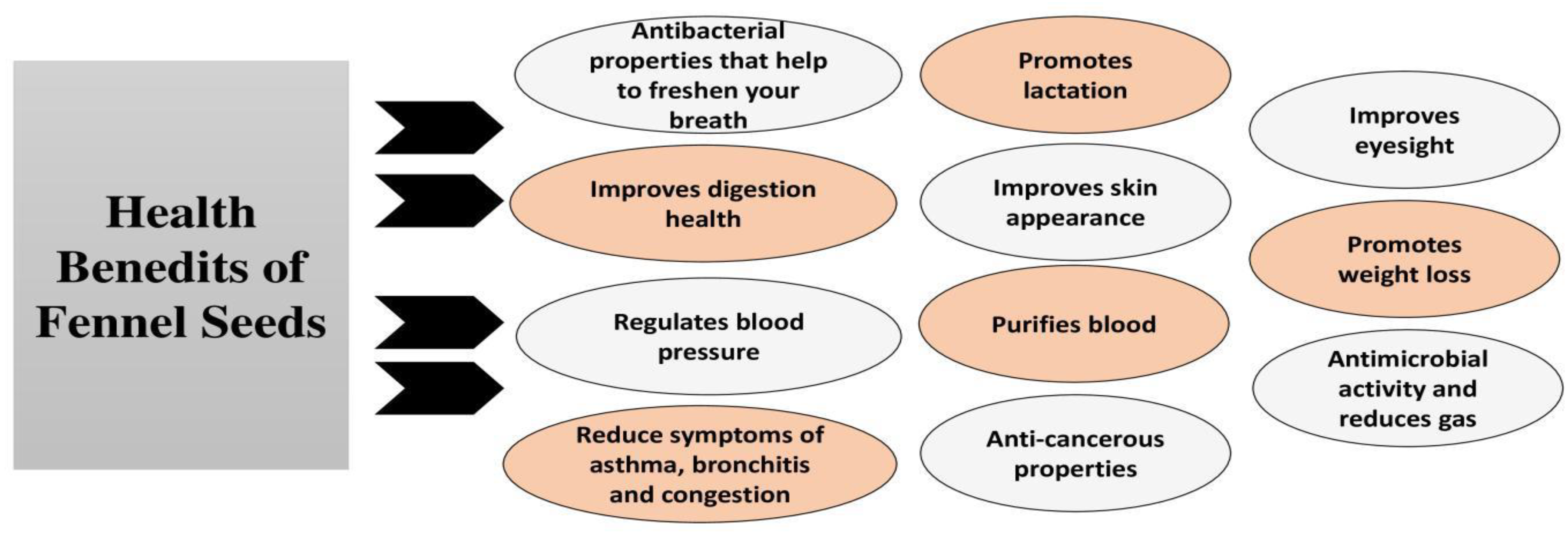
| Fennel is cultivated all over the world for its important essential oil and its utilization in different traditional medicine systems. | [14,15,16,17] |
| Fennel is a perennial or biennial herb up to two meters high and golden yellow flowers and feathery leaves. | [15,16,17,18] |
| Chemical components based on the total essential oil distilled from fennel seeds are (E)-Anethole (trans-anethole), Limonene, Fenchone, α-Pinen, (Z)-β-Ocimene, Estragole (methyl chavicol), Carvone, Myrcene, dimethyl acetal, 1,8-Cineole, p-Anisaldehyde, Sabinene, Camphor, Camphene, γ-Terpinene, (Z)-Anethole (cis-anethole), α-Phellandrene, p-Cymene, exo-Fenchyl acetate, Germacrene D, Carvacrol, β-Pinene, allo-Ocimene, and Terpinen-4-ol. | [36,37,38,39,40,141,142,143,144] |
| Fennel seed is a rich source of volatile oil, with fenchone and trans-anethoe as its main ingredients. | [36,37,38,39,40] |
| Other components of the essential oil are camphene, limonene, and alpha-pinene. | [36,37,38,39,40] |
| Fennel seed with its spicy odor and burning sweet taste has a particular usage in perfumes, condiments, and liqueurs industrial as flavoring agent. | [45,46,47] |
| The special health benefits of fennel are because of its antioxidant content. | [45,46,47] |
| Aging-related diseases like heart cancer and heart diseases can be prevented by fennel seed oils. | [36,37,38,39,40,41,42] |
| The main essential oil components of fennel are trans anethole, fenchone, methyl chavicole (estragole), and limonene. | [36,37,38,39,40,146] |
| Fennel essential oil or its natural constituents such as anethole shows various activities like antibacterial, antifungal, and insecticidal activity. | [20,21,22,23,24,25,26,27,122,123] |
| Fennel has antioxidant property, anti-inflammatory effect, prophylactic activity, anti-allergic, and antispasmodic and hepatoprotective activity. | [19,20,21,22,23,24,25,26,27,153,154] |
| In livestock industries, the notable improvement in chicks body weight and feed effectiveness are obtained by addition of fennel seed to their feed. | [45,46,47,48,49,50] |
| The phenolic molecules in fennel have been proved to possess potent antioxidant activity in a number of trials. | [48,49,50] |
| Keypoints | References |
|---|---|
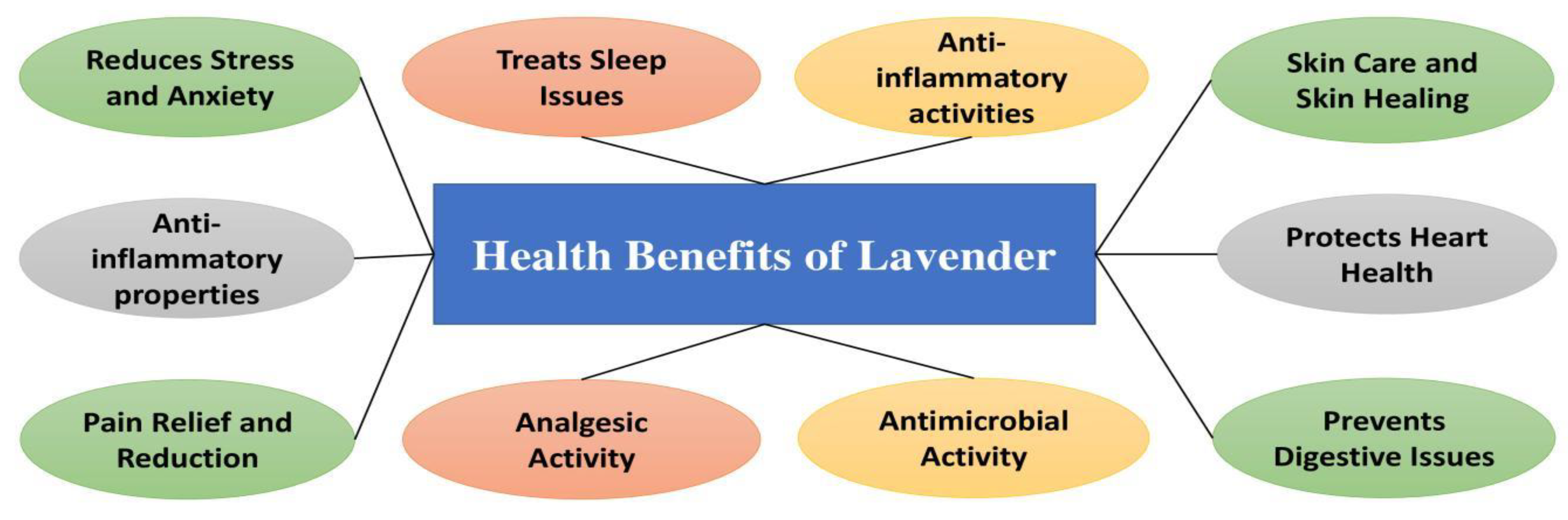
| Thyme is the main component of essential oil extracted from Thymus vulgaris belonging to the family of Lamiaceae. | [182,183,184,185,186,187] |
| Traditionally, it is used as carminative, anti-septic, stimulant, anti-spasmodic, anaesthetic, and also contains analgesic agent, and anti-oxidant properties. | [188,189] |
| The phenolic constituent of volatile oils is hydrophobic in nature, binds the bacterial proteins, breakdown and permeates the cell membrane, effectual anti-fungal component to extend the shelf life of packaged foods. | [190] |
| Thyme extracts present neuroprotective, anti-aging and antioxidant activity. | [191] |
| Thyme extract present high anti-inflammatory properties with no cytotoxicity. | [191] |
| Essential oils of thyme is used for a wide variety of applications, such as to impart fragrance and flavoring to cosmetics and spice mixtures, and as components of pesticides and repellents. | [192] |
| Phenolic components, comprising polyphenols and phenols, are the most abundant secondary metabolites in the essential oil and extract of thyme. | [192,193,194] |
| Thyme showed significant decline in weight, fasting blood, waist circumference, total cholesterol, triglycerides and low density lipoproteins. | [195] |
| Edible coating based on quince seed mucilage loaded with thyme essential oil showed good potential as a coating material for the protection of cheese shelf and quality as well as for enhancing Angiotensin-converting enzyme (ACE)-inhibitory activity. | [196] |
| Thyme essential oil has important function in controlling gray mold and Fusarium wilt and inducing systemic acquired resistance in tomato seedlings and tomato grown. | [197] |
| Thyme volatile oil loaded with chitosan nanoparticles as an edible coating has a great potential in shelf life extension of some medicinal plant ,s leaves. | [198] |
| The essential oil can be used in a variety of pharmaceutical, agro-food, and non-food applications. | [199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214] |
| The main health benefits of seeds are anti-inflammatory, antioxidant, antineoplastic, antiviral, antifungal, antibacterial and antiseptic activities. | [215,216,217,218,219,220,221,222] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





