Submitted:
25 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Experimental
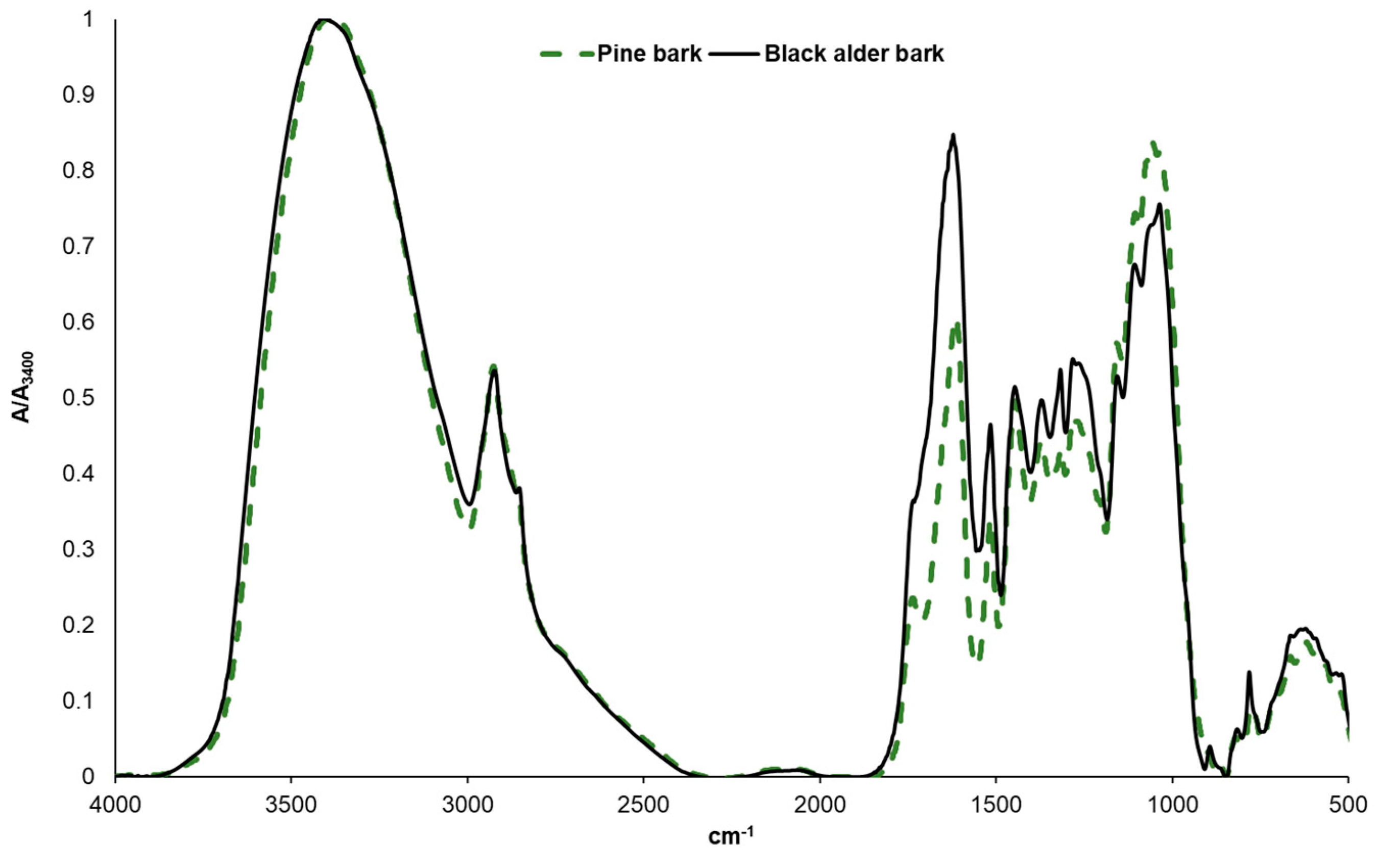
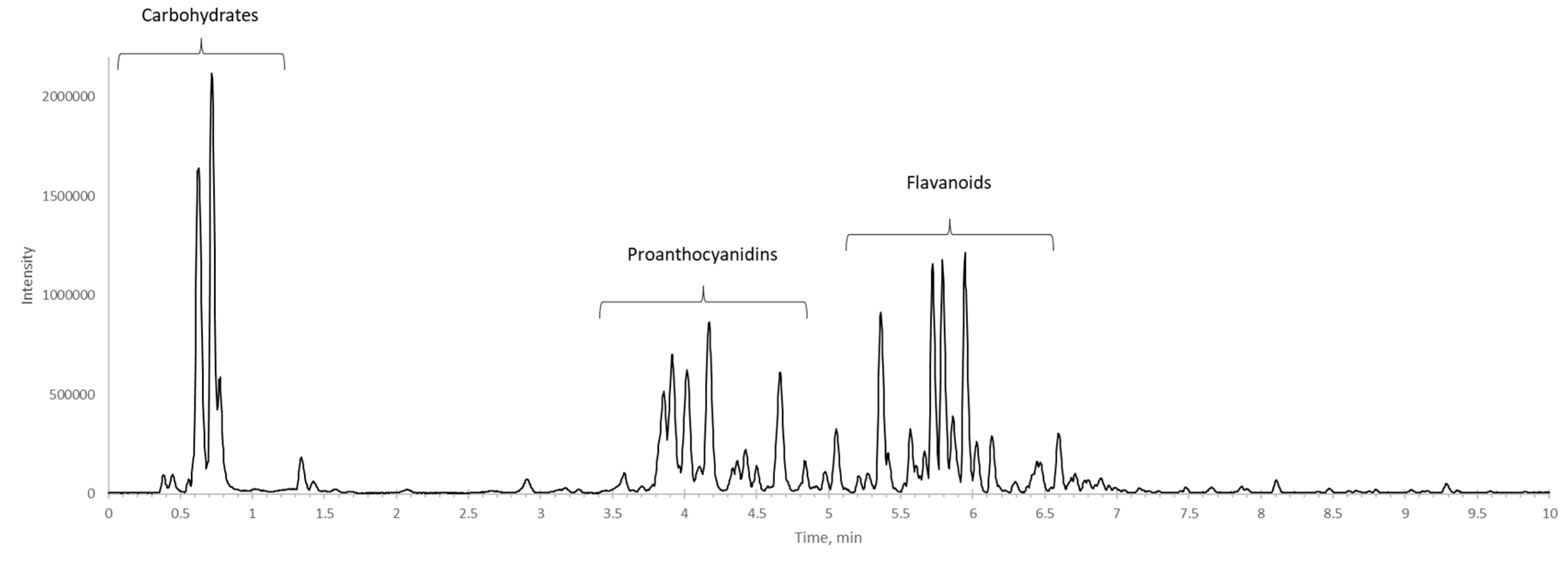
Results and Discussion
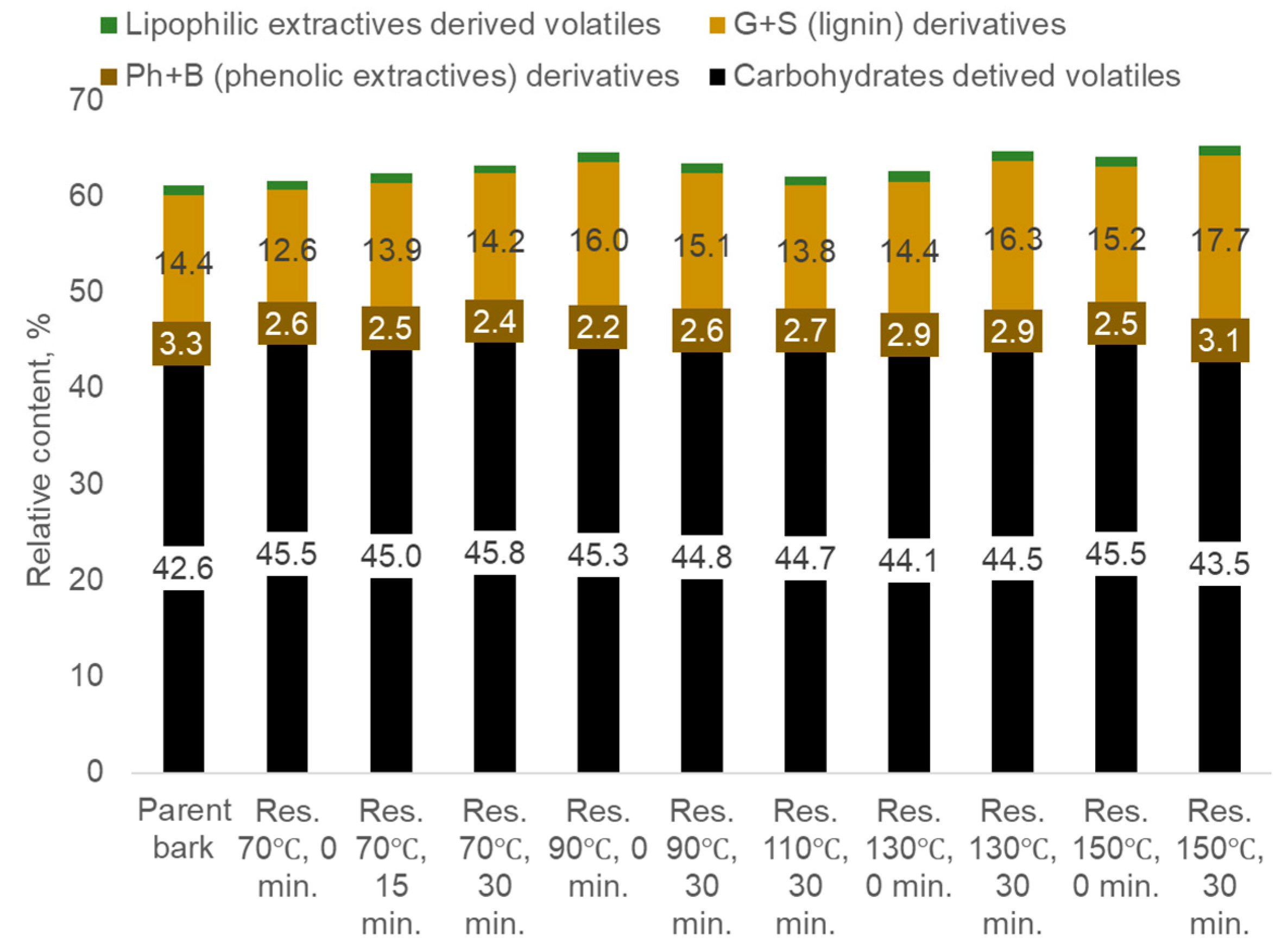
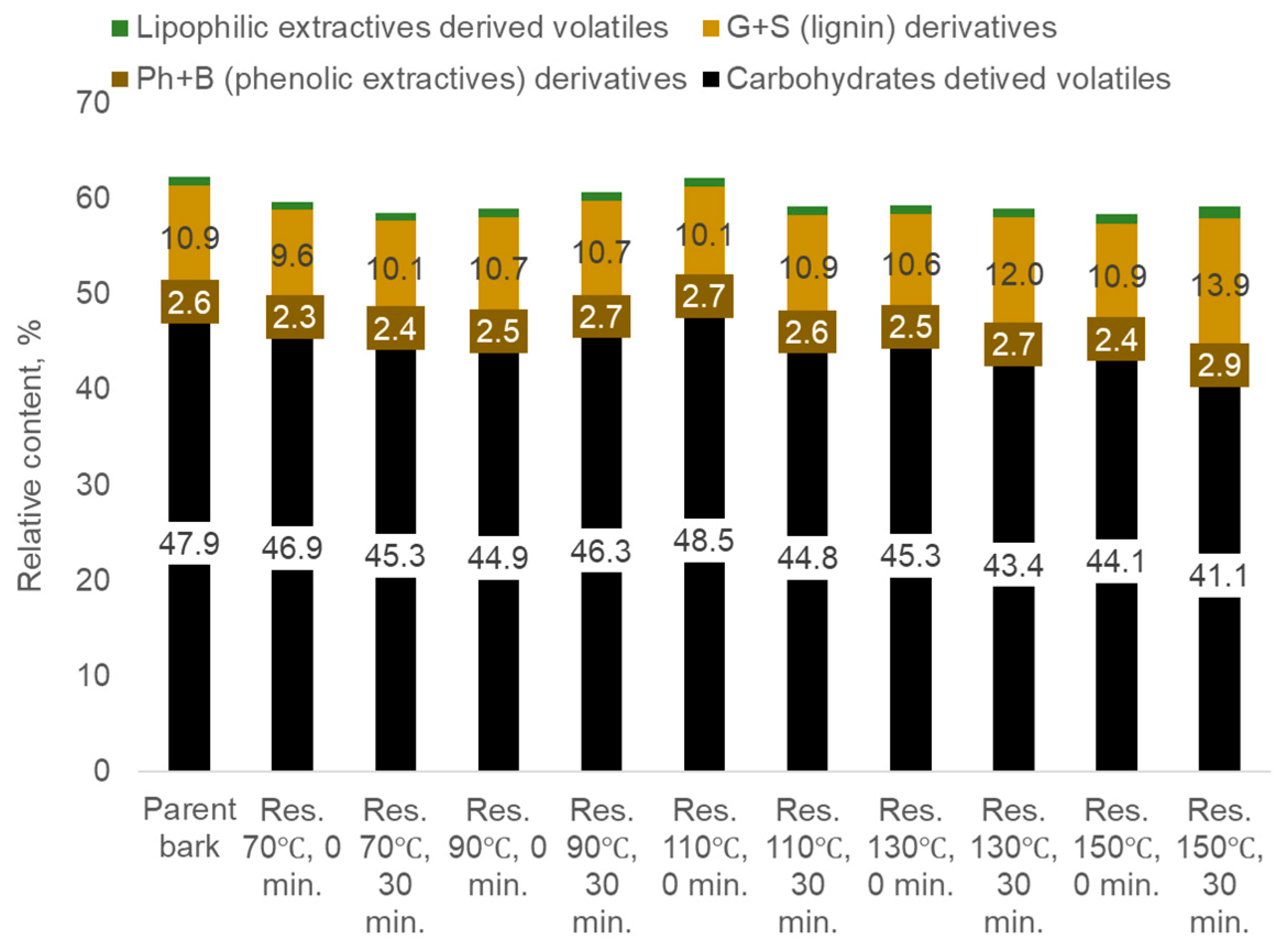
- Carbohydrate-derived compounds, primarily represented by aliphatic acids and esters, aliphatic alcohols, aliphatic aldehydes and ketones, furan and pyran derivatives, cyclopentane derivatives, and anhydro sugars.
- Non-methoxylated aromatic compounds such as phenol, 2-methyl-phenol, 3-and 4-methyl-phenol, 4-methoxy-3-methyl-phenol, 3,4-dimethyl- phenol, 3',5'-dihydroxyacetophenone, 1,2-benzenediol, 4-(4-hydroxyphenyl)-2-butanone etc. derived mainly from phenolic extractives.
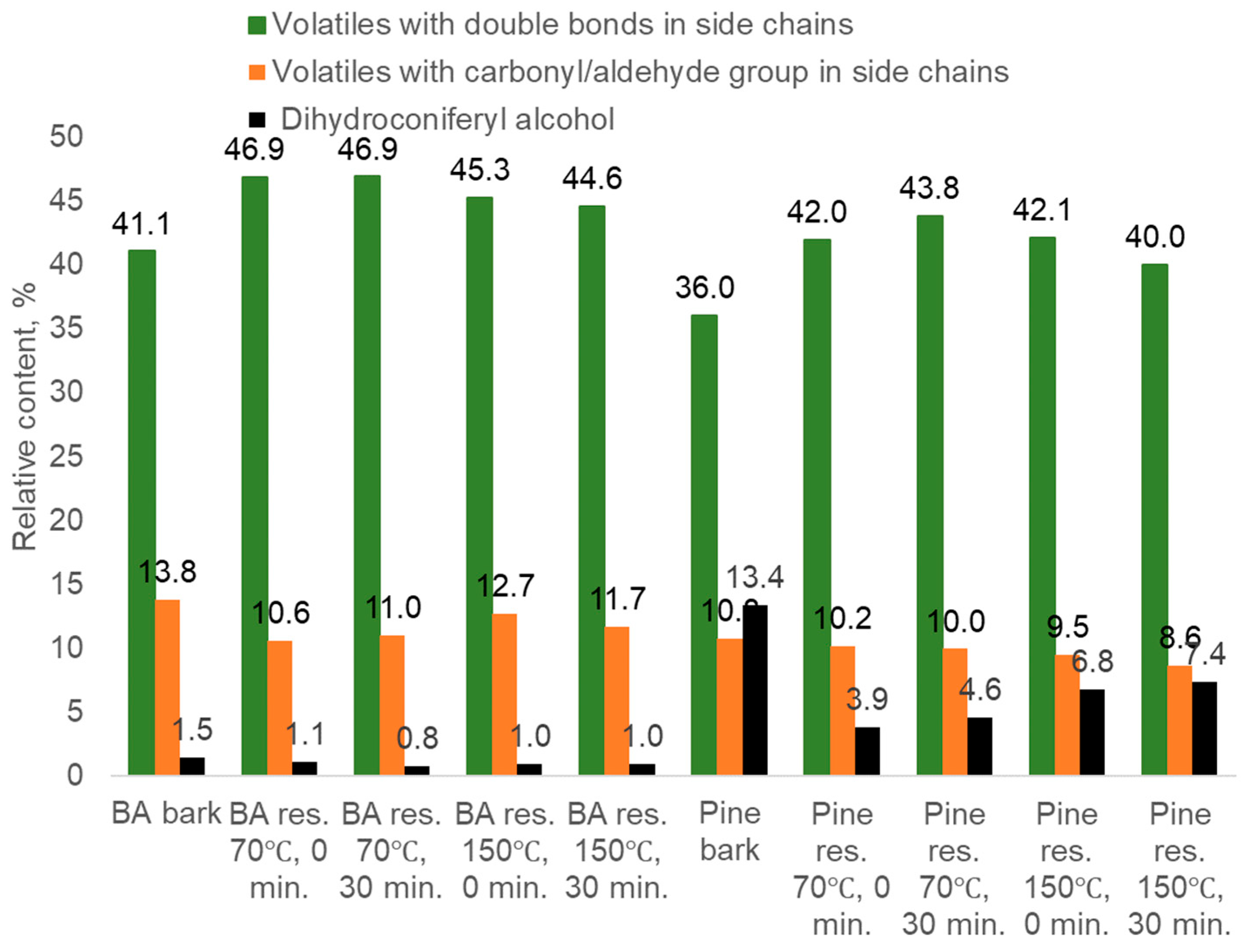
- Methoxylated phenols, guaiacyl (G-) and syringyl (S-) derivatives such as guaiacol, p-methylguaiacol, p-ethylguaiacol, p-vinylguaiacol, eugenol, p-propylguaiacol, isoeugenol, vanillin, acetoguaiacon, guaiacylacetone, propioguaiacone, dihydroconiferyl alcohol, syringol 4-methyl- syringol, 4-ethyl-syringol, 4-vinyl-syringol, 4-allyl- and 4-propyl- syringol, 4-propenyl-syringol, syringaldehyde, acetosyringone, syringylacetone derived from lignin.
- Lipophilic extractive-derived compounds such as 1,4-dioxin, 2,3-dihydro- 1-dodecene, 1,3-dioxol-2-one,4,5-dimethyl-tetradecane, 3-tetradecene, cycloundecane, 1,1,2-trimethyl-1-octadecene etc.
Conclusions
References
- Argyropoulos, D. S., Pajer, N., & Crestini, C. (2021). Quantitative 31P NMR Analysis of Lignins and Tannins. Journal of Visualized Experiments, 174. https://doi.org/10.3791/62696. [CrossRef]
- Arshanitsa, A., Ponomarenko, J., Lauberts, M., Jurkjane, V., Jashina, L., Semenischev, A., Akishin, J., & Telysheva, G. (2020). Composition of extracts isolated from black alder bark by microwave assisted water extraction. 87–94. [CrossRef]
- Blakeney, A. B., Harris, P. J., Henry, R. J., & Stone, B. A. (1983). A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research, 113(2), 291–299. [CrossRef]
- Dizhbite, T., Telysheva, G., Jurkjane, V., & Viesturs, U. (2004). Characterization of the radical scavenging activity of lignins--natural antioxidants. Bioresource Technology, 95(3), 309–317. [CrossRef]
- Hagerman, A. E. (1995). Acid butanol assay for proanthocyanidins. Tannin Analysis, 45(1983), 24–25.
- Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent (pp. 152–178). [CrossRef]
- Zakis, G. F. (viaf)276849665. (1994). Functional analysis of lignins and their derivatives. Atlanta (Ga.) : TAPPI press. http://lib.ugent.be/catalog/rug01:001647975.
| T, min | A, % | B, % |
|---|---|---|
| 0 | 95 | 5 |
| 0.5 | 95 | 5 |
| 17 | 5 | 95 |
| 18 | 5 | 95 |
| 18.5 | 95 | 5 |
| 20 | 95 | 5 |
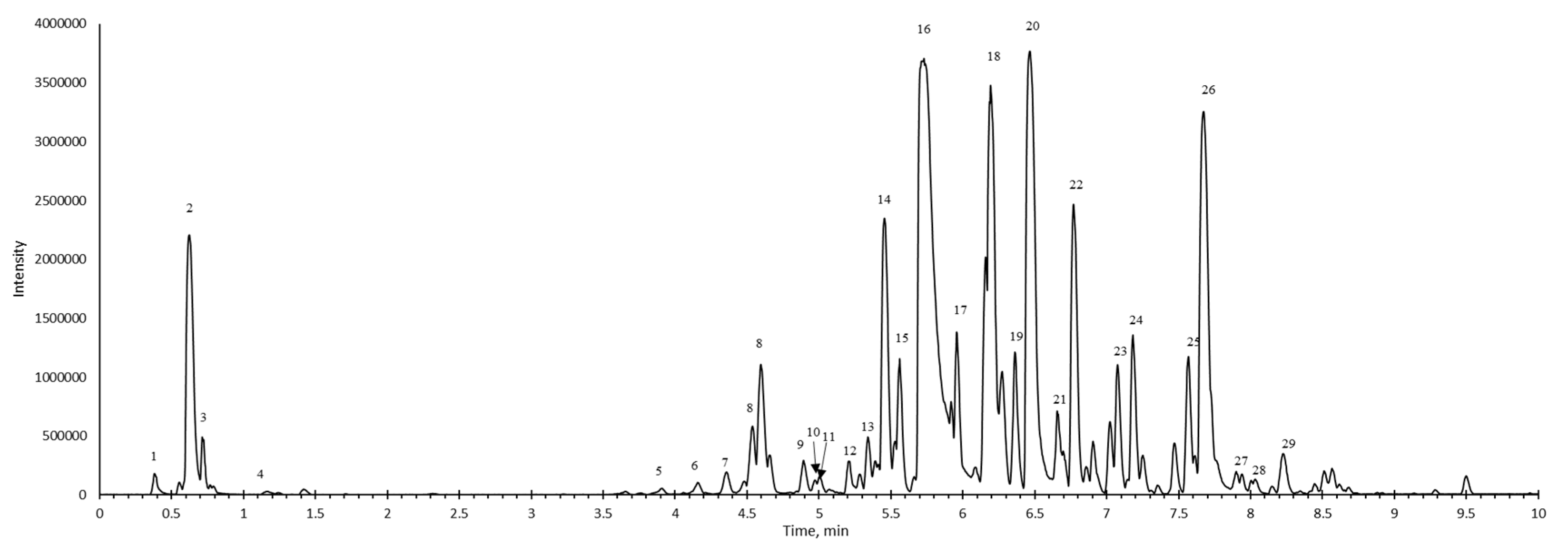
| No | Rt. min | [M-H]- | M2 | Identification | |
|---|---|---|---|---|---|
| 1 | 0.38 | 288.94 | 174.96 | 158.96 | Carbohydrates |
| 2 | 0.62 | 341.11 | 179.06 | 89.02 | Myzodendrone |
| 3 | 0.71 | 191.06 | 473.15 | 113.02 | Quinic acid |
| 4 | 1.16 | 191.02 | 111.01 | Quinic acid | |
| 5 | 3.90 | 577.14 | 407.08 | 289.07 | (Epi)catechin- (epi)catechin |
| 6 | 4.16 | 289.07 | 245.08 | Catechin | |
| 7 | 4.36 | 337.09 | 163.04 | Coumaroylquinic acid | |
| 8 | 4.60 | 577.14 | 409.11 | 289.07 | Epi)catechin- (epi)catechin |
| 9 | 4.89 | 866.00 | 407.08 | 289.06 | Epi)catechin- (epi)catechin- (epi)catechin |
| 10 | 4.98 | 337.09 | 173.05 | Coumaroylquinic acid | |
| 11 | 5.00 | 761.14 | 615.19 | 3-O-galloyl- (epi)gallocatechin- (epi)gallocatechin | |
| 12 | 5.21 | 881.19 | 509.2 | 289.07 | Epi)catechin- (epi)catechin- (epi)gallocatechin |
| 13 | 5.34 | 441.18 | 359 | 289.07 | catechin-3-O-gallate |
| 14 | 5.45 | 507.19 | 327.12 | 205.09 | Hirsutenone hexoside |
| 15 | 5.56 | 479.19 | 347.15 | Hydroxyoregonin | |
| 16 | 5.73 | 477.18 | 327.12 | 205.09 | Oregonin |
| 17 | 5.96 | 477.18 | 327.12 | 205.09 | Oregonin |
| 18 | 6.19 | 625.25 | 493.25 | 311.13 | Rubranol C |
| 19 | 6.37 | 461.18 | 493.25 | 311.13 | Aceroside VII |
| 20 | 6.46 | 493.21 | 331.16 | 311.13 | Rubranoside A |
| 21 | 6.65 | 609.25 | 493.21 | 327.12 | Hirsutenone derivative |
| 22 | 6.77 | 463.20 | 331.15 | Rubranol xyloside | |
| 23 | 7.07 | 652.22 | 331.15 | Blank | |
| 24 | 7.18 | 593.26 | 461.22 | 299.16 | Blank |
| 25 | 7.47 | 461.22 | 299.16 | 1- (4-hydroxyphenyl)-7- (3.4-dihydroxyphenyl) heptan-3-one-5-O-pentoside | |
| 26 | 7.67 | 327.13 | 205.9 | Hirsutenone | |
| 27 | 7.90 | 327.13 | 205.9 | Hirsutenone | |
| 28 | 8.03 | 327.13 | 205.9 | Hirsutenone | |
| 29 | 8.22 | 293.17 | 193.11 | Gingerol | |
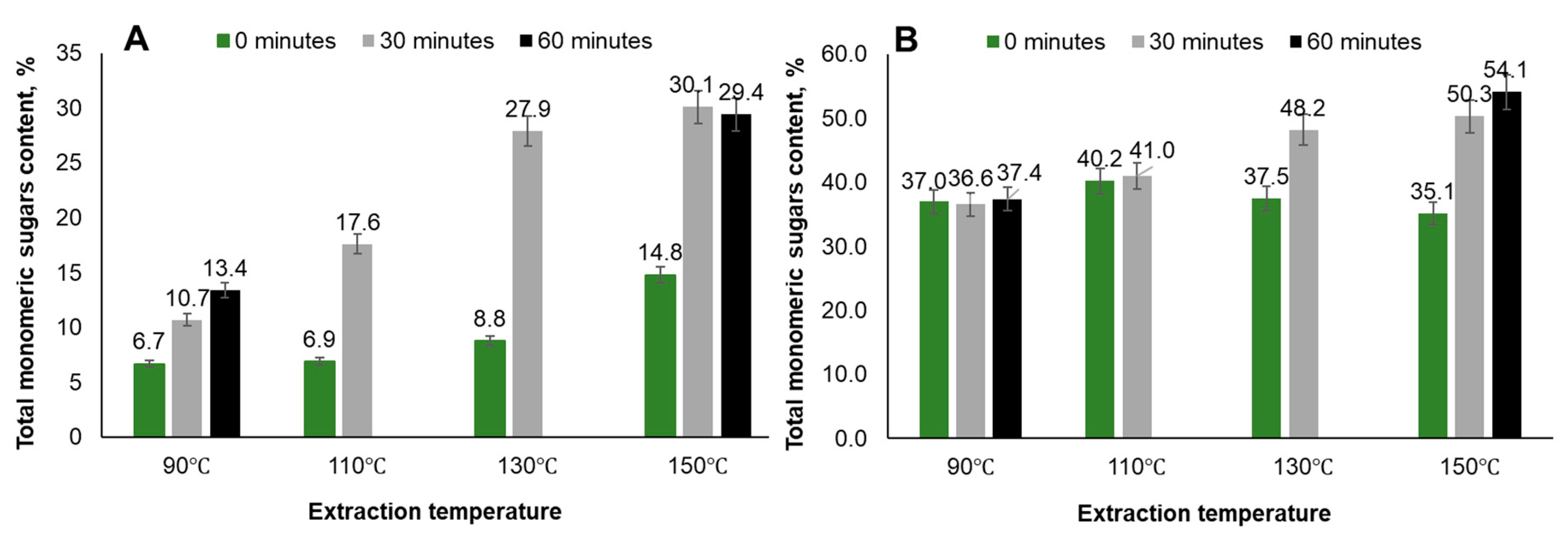
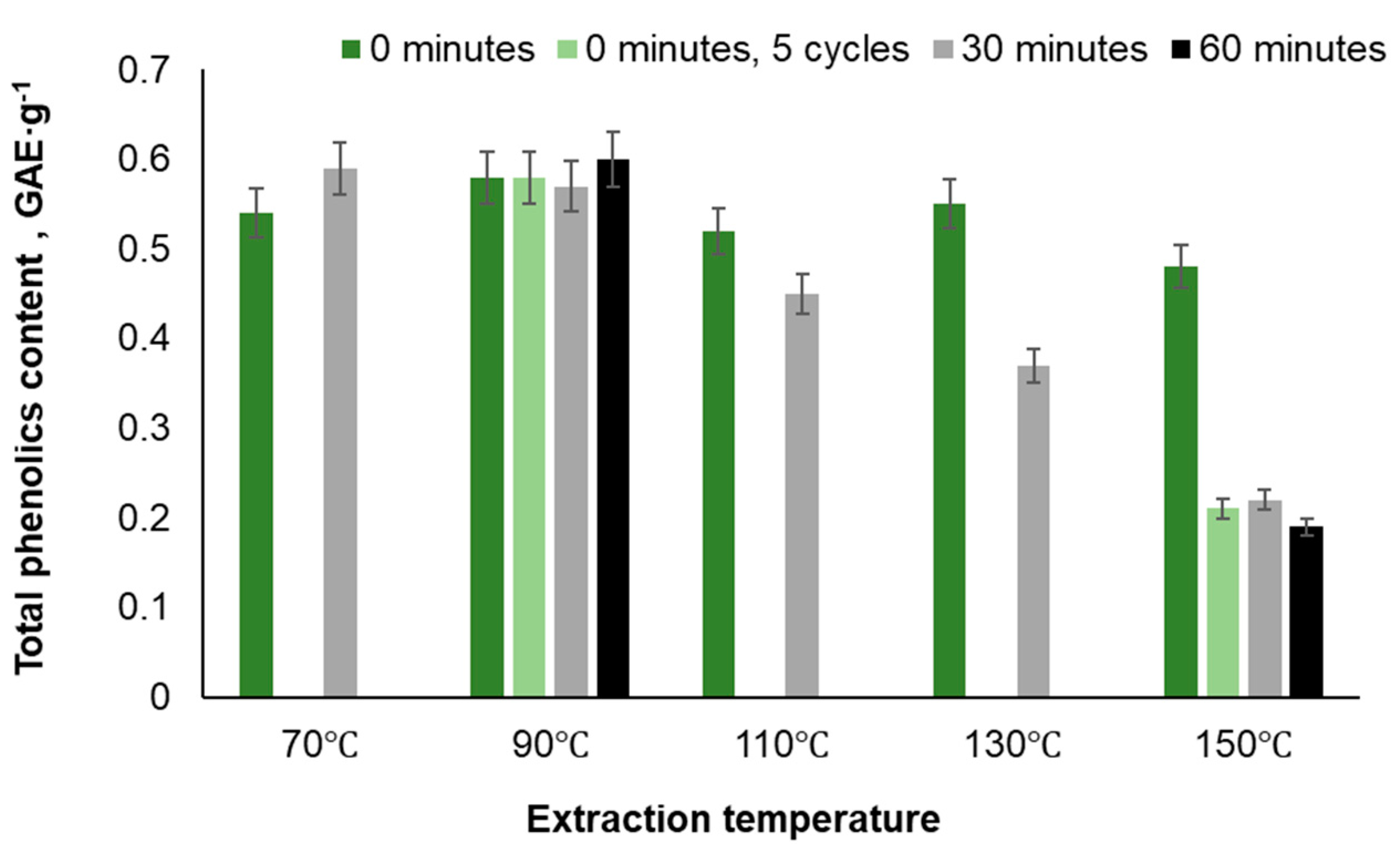
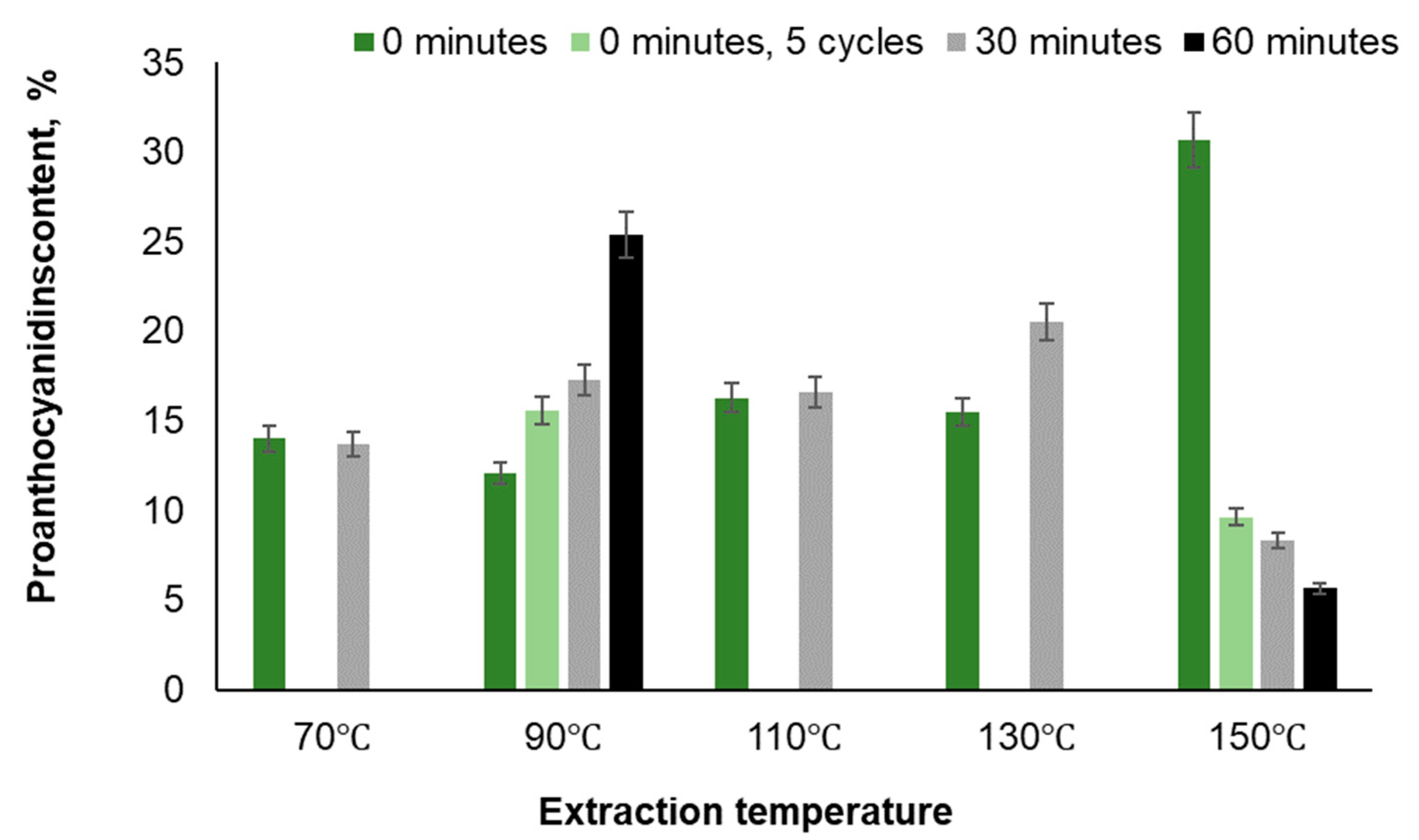
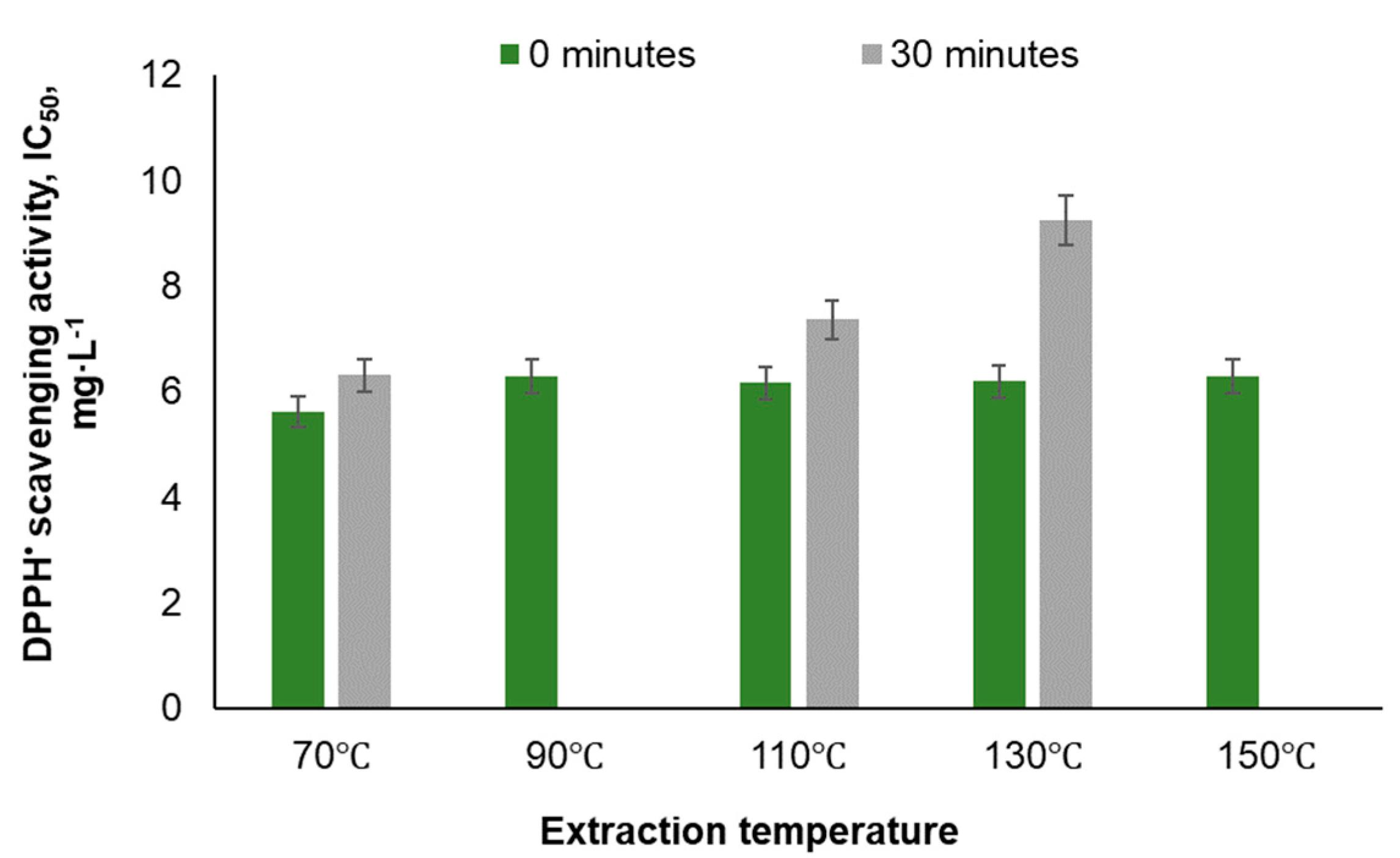
| Temperature, ℃ | Time, min | Yield, % | TPC, GAE·g-1 | PAC, % | Total sugar content, % | IC 50, mg·L-1 | |
|---|---|---|---|---|---|---|---|
| -Hydrol | +Hydrol | ||||||
| 70 | 0 | 0.34 | 77.3 | ||||
| 70 | 30 | 0.36 | 87.9 | 13 | 21.8 | 39.92 | |
| 90 | 0 | 10.5 | 0.36 | 82.9 | 11.8 | 23.6 | |
| 90 | 0 (5 cycles) | 11.94 | 0.27 | 75.19 | 9.1 | 38.7 | |
| 90 | 30 | 11.9 | 0.35 | 81.8 | 10.6 | 39.5 | |
| 90 | 60 | 12.01 | 0.27 | 83.69 | 9.6 | 34.7 | |
| 110 | 0 | 0.36 | 53.8 | 11.6 | 33.98 | ||
| 110 | 30 | 0.28 | 37.6 | 11.2 | 44.2 | ||
| 130 | 0 | 0.31 | 52.1 | 9.11 | 34.5 | ||
| 130 | 30 | 0.24 | 24.5 | 12.6 | 48.9 | ||
| 150 | 0 | 0.16 | 8.0 | 12.5 | 49.82 | 63.1 | |
| 150 | 0 (5 cycles) | 21.59 | 0.21 | 15.52 | 9.0 | 48.2 | |
| 150 | 30 | 22.40 | 0.15 | 14.63 | 15.2 | 52.5 | |
| 150 | 60 | 22.39 | 0.11 | 12.87 | 18.6 | 61.1 | |
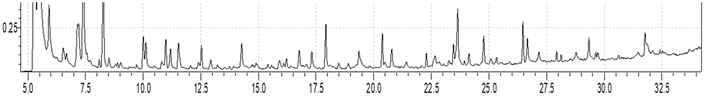 Retention time, min. Retention time, min. | |||
|---|---|---|---|
| Identified compound | MW | Retention time, min. | Normalized peak area, % from chromatogram |
| Carbon dioxide | 44 | 5.265 | 16.89 |
| Water | 18 | 5.445 | 20.5 |
| Methylglyoxal | 72 | 5.918 | 2.6 |
| Furan, 2-methyl- | 82 | 6.253 | 0.15 |
| 2,3-Butanedione | 86 | 6.525 | 0.87 |
| 2-Butanone, 1-hydroxy- | 88 | 6.675 | 0.31 |
| Acetaldehyde, hydroxy- | 60 | 7.148 | 4.17 |
| Acetic acid | 60 | 7.404 | 7.78 |
| (S)-2-Hydroxypropanoic acid | 90 | 7.555 | 0.07 |
| 2,3-Pentanedione | 100 | 7.706 | 0.07 |
| 1,4-Dioxin, 2,3-dihydro- | 86 | 8.095 | 0.35 |
| 2-Propanone, 1-hydroxy- | 74 | 8.265 | 4.51 |
| Formic acid, methyl ester | 60 | 8.52 | 0.25 |
| 1H-Pyrrole, 1-methyl- | 81 | 8.525 | 0.19 |
| Furan, 2,5-dihydro-3-methyl- | 84 | 8.796 | 0.13 |
| Propanoic acid, 2-oxo- | 88 | 8.886 | 0.18 |
| Propanoic acid | 74 | 9.023 | 0.29 |
| Furan, 3-methyl- | 82 | 9.249 | 0.03 |
| Hexanal | 100 | 9.41 | 0.07 |
| 2-Propenoic acid, methyl ester | 86 | 9.721 | 0.19 |
| Acetic acid, methyl ester | 74 | 10.001 | 1.76 |
| 2-Butanone, 1-hydroxy-, isomer | 88 | 10.116 | 0.67 |
| Pyrrole | 67 | 10.116 | 0.56 |
| 2(3H)-Furanone | 84 | 10.486 | 0.09 |
| 3(2H)-Furanone | 98 | 10.806 | 0.24 |
| Propanoic acid, 2-oxo-, methyl ester | 102 | 10.981 | 1.44 |
| Propanal and Butanedial | 58/86 | 11.18 | 1.12 |
| Furfural | 96 | 11.53 | 1.41 |
| 2-Cyclopenten-1-one | 82 | 11.575 | 0.29 |
| 3-Hexanone, 2,2-dimethyl- | 128 | 12.054 | 0.16 |
| Butanoic acid, 2-methyl- | 102 | 12.275 | 0.06 |
| 2-Furanmethanol | 98 | 12.401 | 0.25 |
| 2-Propanone, 1-(acetyloxy)- | 116 | 12.529 | 1.03 |
| 2-Cyclopenten-1-one, 2-methyl- | 96 | 12.892 | 0.1 |
| 2-Heptanone, 3-methyl- | 128 | 12.934 | 0.46 |
| Furan, 2,3-dihydro-2,5-dimethyl- | 98 | 13.111 | 0.03 |
| Acetylfuran | 110 | 13.225 | 0.17 |
| Crotonic acid vinyl ester | 112 | 13.542 | 0.13 |
| 4-Cyclopentene-1,3-dione | 96 | 13.74 | 0.13 |
| 1,2-Cyclopentanedione | 98 | 14.275 | 1.4 |
| 2,5-Hexanedione | 114 | 14.467 | 0.04 |
| Propanoic acid, 2-methylpropyl ester | 130 | 14.716 | 0.09 |
| 2-Butanone, 1-(acetyloxy)- | 130 | 14.785 | 0.06 |
| 2-Furancarboxaldehyde, 5-methyl- | 110 | 14.905 | 0.15 |
| 2-Cyclopenten-1-one, 3-methyl- | 96 | 15.415 | 0.2 |
| 2(3H)-Furanone, dihydro- | 86 | 15.567 | 0.15 |
| Hexanoic acid | 116 | 15.686 | |
| 2(5H)-Furanone | 84 | 15.915 | 0.63 |
| 4-Hydroxy-,5,6-dihydro-(2H)-pyran-2-one | 114 | 16.217 | 0.41 |
| 2,5-Furandione, 3-methyl- | 112 | 16.408 | 0.1 |
| 1-Dodecene | 168 | 16.561 | 0.05 |
| 1,2-Cyclopentanedione, 3-methyl- | 112 | 16.769 | 1.04 |
| 2(5H)-Furanone, 3-methyl- | 98 | 16.933 | 0.04 |
| Phenol | 94 | 17.313 | 0.92 |
| Guaiacol | 124 | 17.921 | 2.14 |
| 1,3-Dioxol-2-one,4,5-dimethyl- | 114 | 18.11 | 0.07 |
| Phenol, 2-methyl- | 108 | 18.485 | 0.27 |
| 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 126 | 18.906 | 0.12 |
| Methyl 2-furoate | 126 | 18.883 | 0.11 |
| 4H-Pyran-4-one, 3-hydroxy-2-methyl- | 126 | 19.242 | 0.05 |
| Phenol, 3-and 4-methyl- | 108 | 19.359 | 0.77 |
| Phenol, 4-methoxy-3-methyl- | 138 | 19.578 | 0.04 |
| 4-Methyl-5H-2-one | 98 | 19.842 | 0.13 |
| 2,5-Furandione, dihydro-3-methyl- | 114 | 20.068 | |
| Phenol, 3,4-dimethyl- | 122 | 20.409 | 0.21 |
| p-Methylguaiacol | 138 | 20.372 | 1.4 |
| Pentanal and Pentanadial | 86/100 | 20.783 | 1.12 |
| Tetradecane | 198 | 21.096 | 0.04 |
| 3-Tetradecene, (Z)- | 196 | 21.195 | 0.08 |
| Phenol, 4-ethyl- | 122 | 21.418 | 0.28 |
| 3',5'-Dihydroxyacetophenone | 152 | 21.827 | 0.05 |
| Phenol, 2,5-dimethyl- | 122 | 22.078 | 0.13 |
| p-Ethylguaiacol | 152 | 22.287 | 0.59 |
| 1-Heptanol, 2-propyl- | 158 | 22.617 | 0.16 |
| 2,4(3H,5H)-Furandione, 3-methyl- | 114 | 22.661 | 0.61 |
| 2H-Pyran-3(4H)-one, dihydro- | 100 | 22.845 | 0.08 |
| 1,4;3,6-Dianhydro-.alpha.-d-glucopyranose | 144 | 23.262 | 0.24 |
| Benzofuran, 2,3-dihydro- | 120 | 23.46 | 0.87 |
| p-Vinylguaiacol | 150 | 23.644 | 1.5 |
| 1,2-Benzenediol | 110 | 23.938 | 0.17 |
| Eugenol and p-Propylguaiacol | 164/166 | 24.131 | 0.54 |
| -D-Ribopyranoside, methyl, 3-acetate | 206 | 24.407 | 0.09 |
| Syringol | 154 | 24.773 | 1.24 |
| 1,3-Di-O-acetyl---D-ribopyranose | 234 | 25.082 | 0.31 |
| cis-isoeugenol | 164 | 25.334 | 0.26 |
| Phenol, 3-methoxy-5-methyl- | 138 | 25.537 | 0.09 |
| Phenol, 4-(2-propenyl)- | 134 | 25.891 | 0.08 |
| trans-isoeugenol | 164 | 26.474 | 1.65 |
| -D-Glucopyranoside, methyl 3,6-anhydro | 176 | 26.67 | 0.59 |
| Syringol, 4-methyl- | 168 | 26.67 | 0.6 |
| Vanillin | 152 | 27.169 | 0.33 |
| Cycloundecane, 1,1,2-trimethyl- | 196 | 27.937 | 0.3 |
| Syringol, 4-ethyl- | 182 | 28.135 | 0.3 |
| 1,2-Benzenediol, 3-methoxy- | 140 | 28.538 | 0.09 |
| Acetoguaiacon | 166 | 28.776 | 0.29 |
| Syringol, 4-vinyl- | 180 | 29.336 | 0.92 |
| Guaiacylacetone | 180 | 29.723 | 0.28 |
| Syringol, 4-allyl- and 4-propyl- | 194/196 | 29.637 | 0.28 |
| Propioguaiacone | 180 | 30.334 | 0.09 |
| Syringol, 4-propenyl-(cis) | 194 | 30.626 | 0.2 |
| Propioguaiacone, alfa-oxy- | 194 | 30.751 | 0.07 |
| 1,6-Anhydro--D-glucopyranose | 162 | 31.771 | 1 |
| 2-Butanone, 4-(4-hydroxyphenyl)- | 164 | 31.481 | 0.15 |
| Syringol, 4-propenyl-(trans) | 194 | 31.771 | 0.55 |
| Dihydroconiferyl alcohol | 182 | 32.101 | 0.21 |
| Syringaldehyde | 182 | 32.532 | 0.15 |
| 4-sec-Butoxy-2-butanone | 144 | 32.99 | 0.11 |
| Acetosyringone | 196 | 33.705 | 0.16 |
| 1-Octadecene | 252 | 34.102 | 0.11 |
| Syringylacetone | 210 | 34.393 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





