Submitted:
25 July 2023
Posted:
26 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Experimental Details
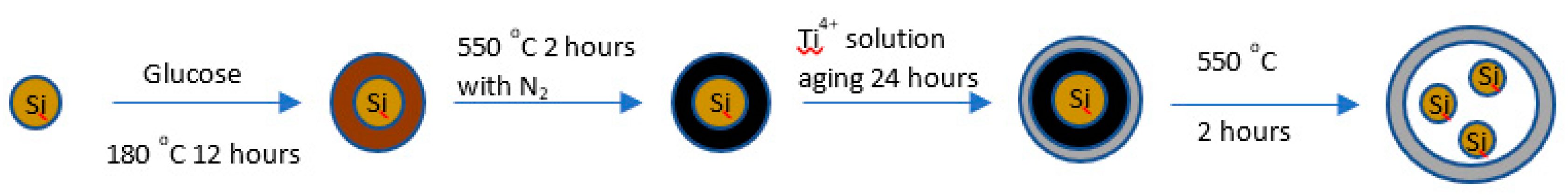
Material fabrication
Material characterisation
Electrochemical measurements
Results and discussion
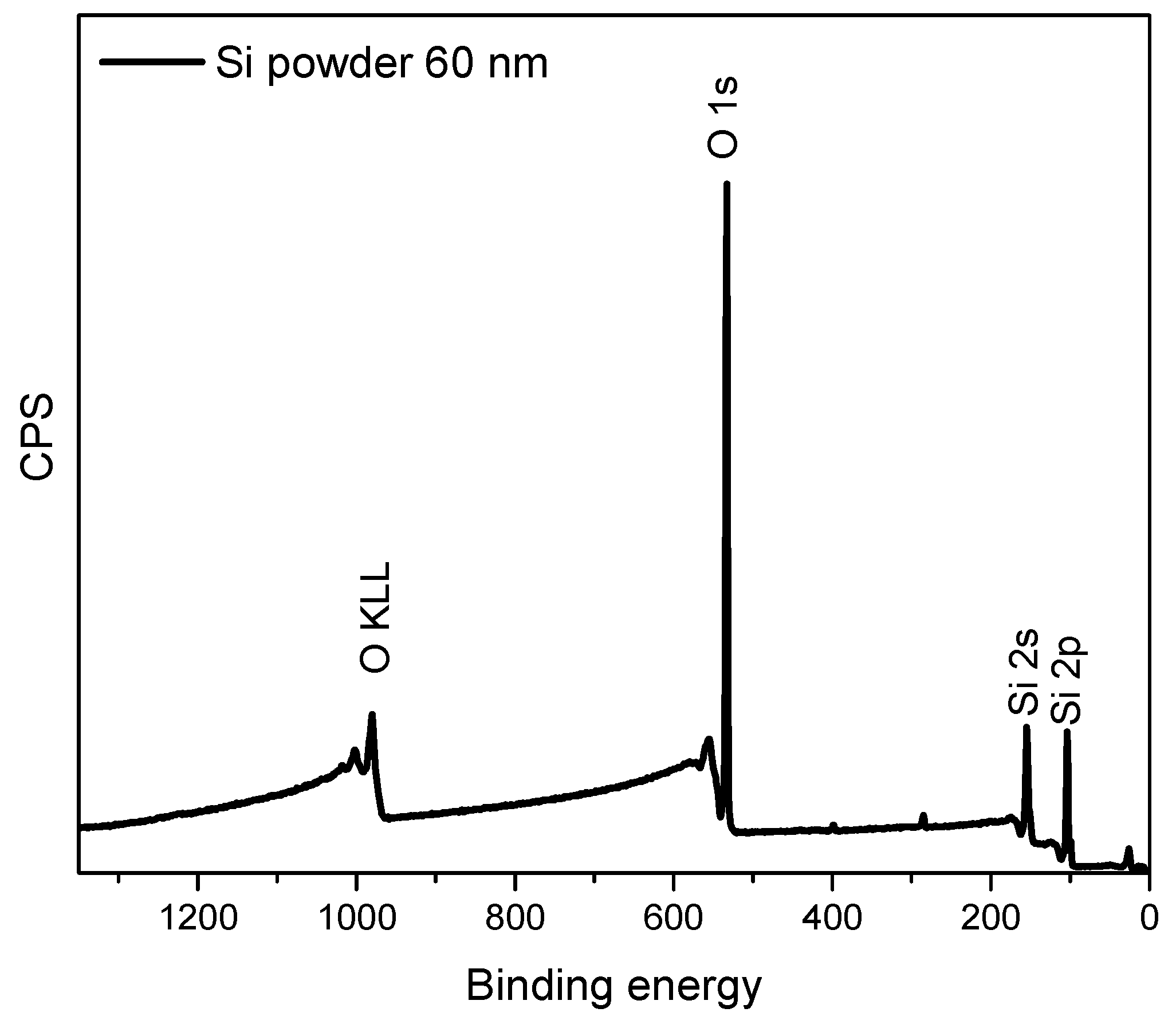
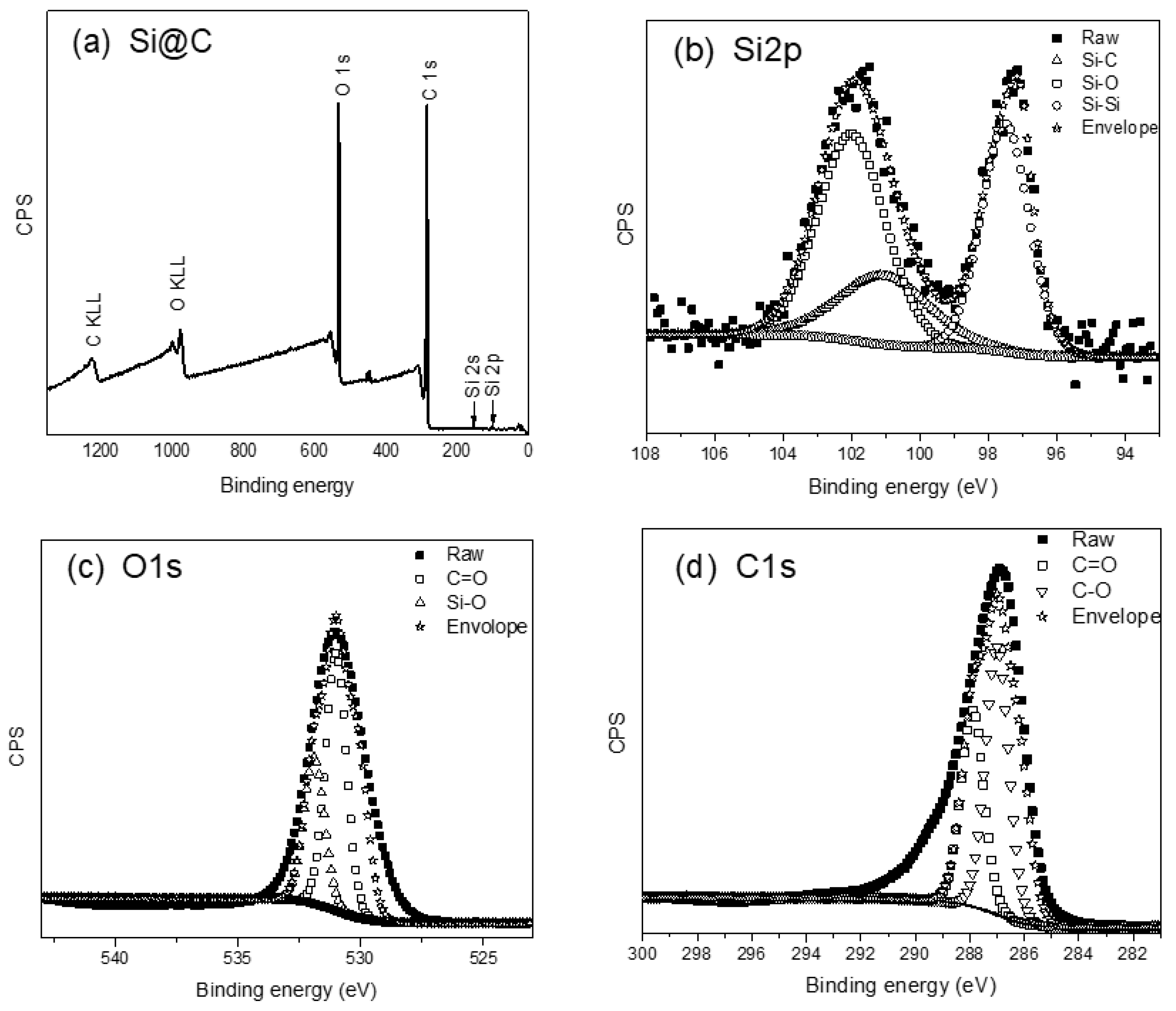
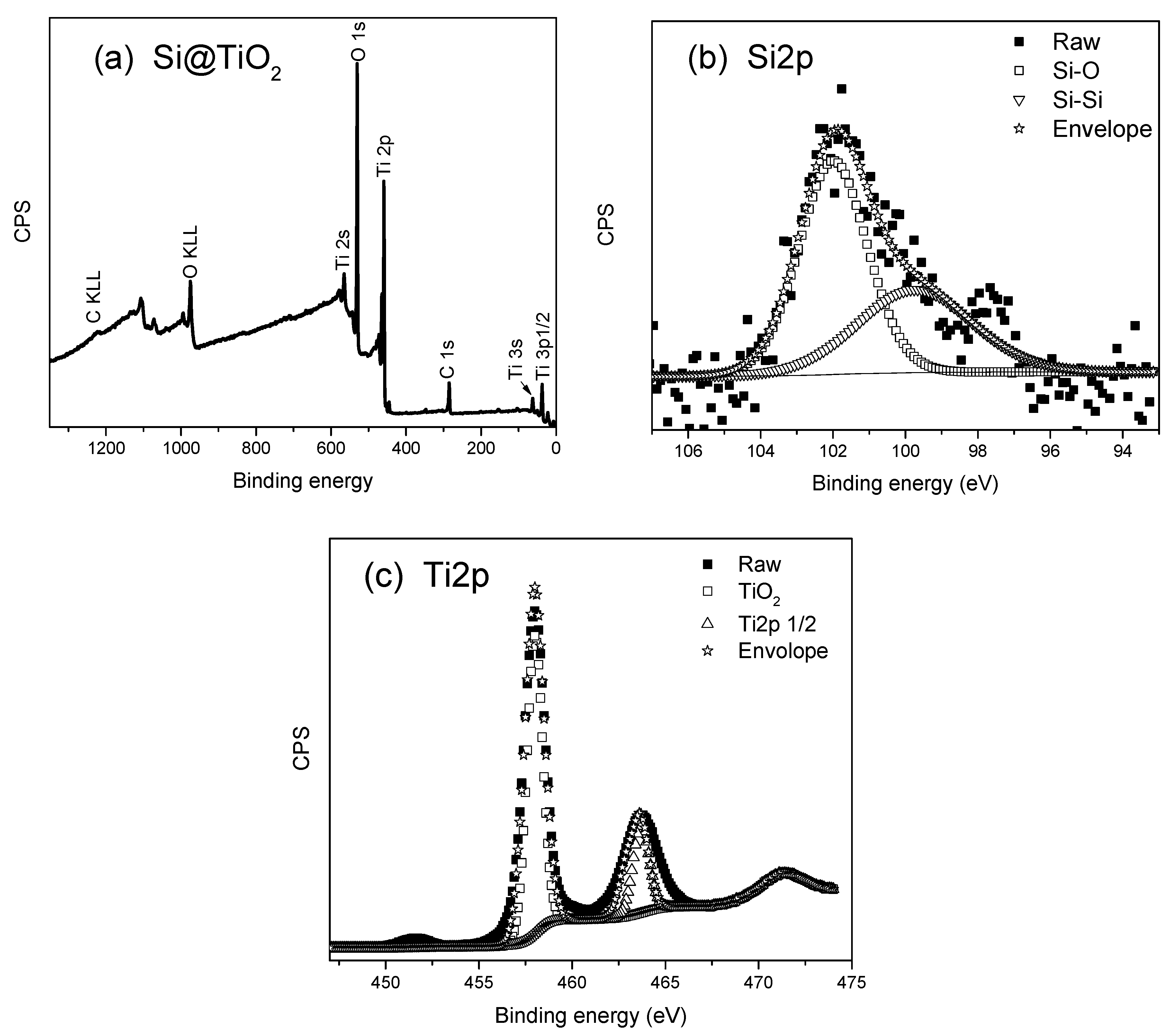
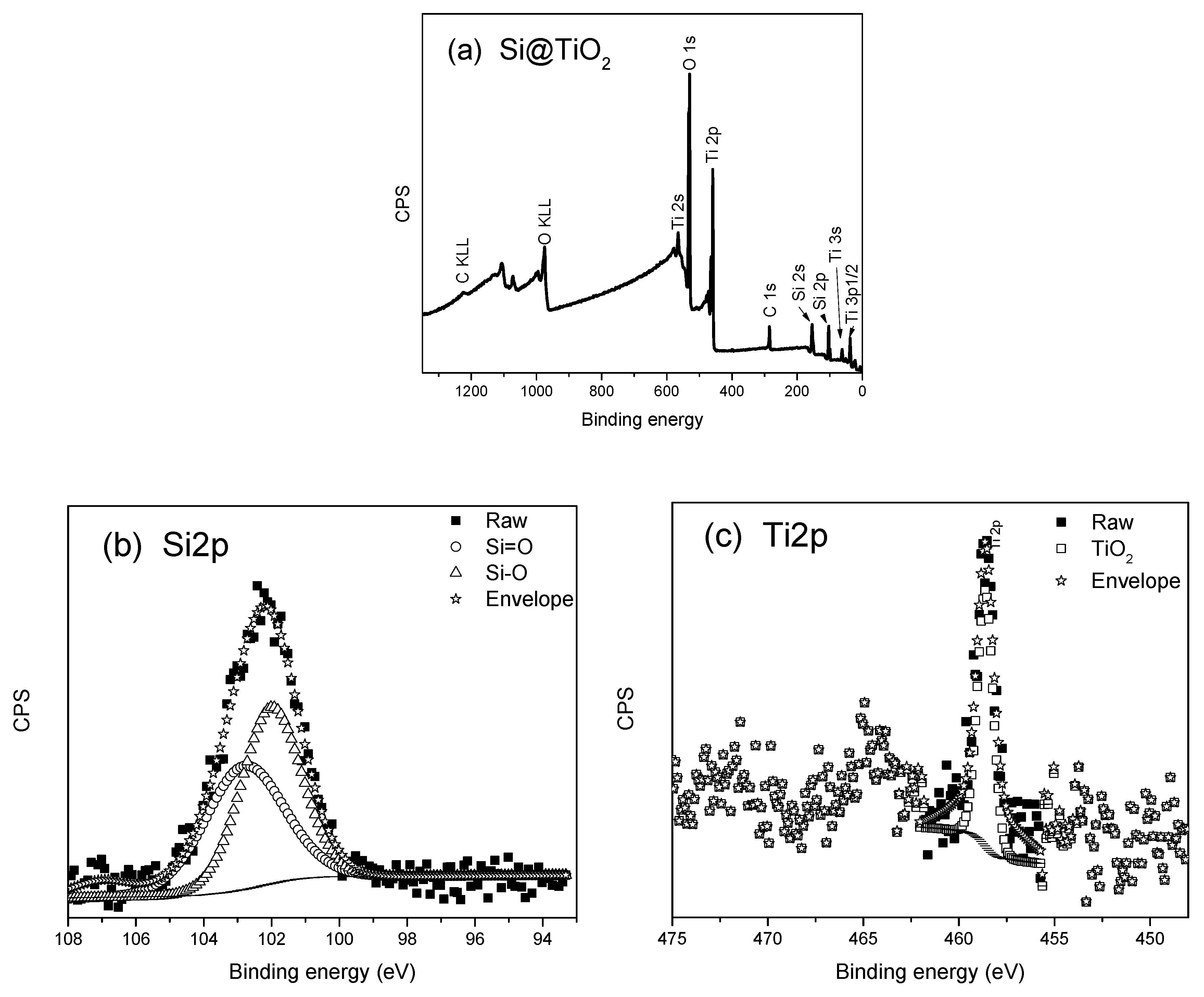
X-ray Photoelectron Spectroscopy and
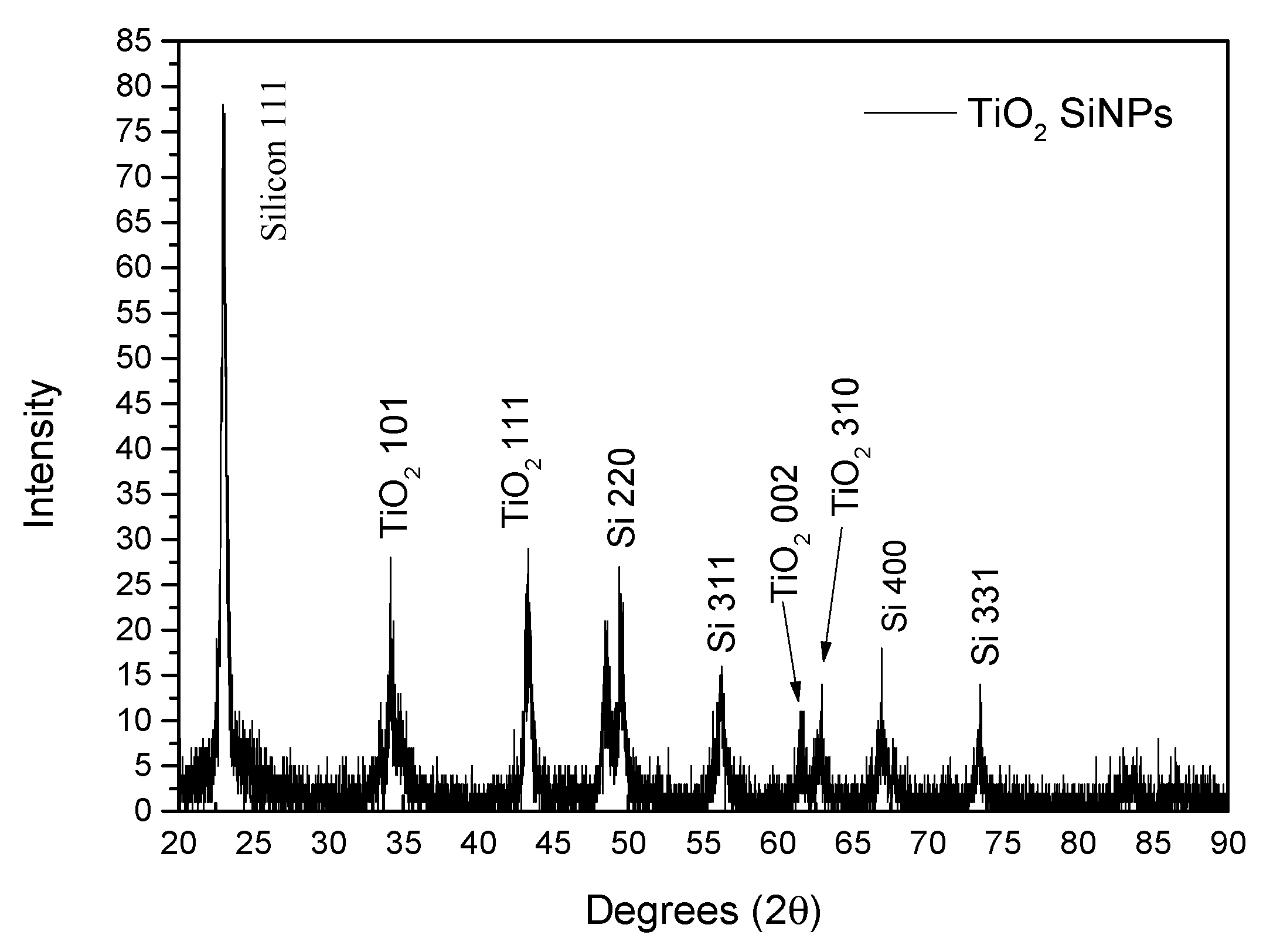
X-ray Powder Diffraction
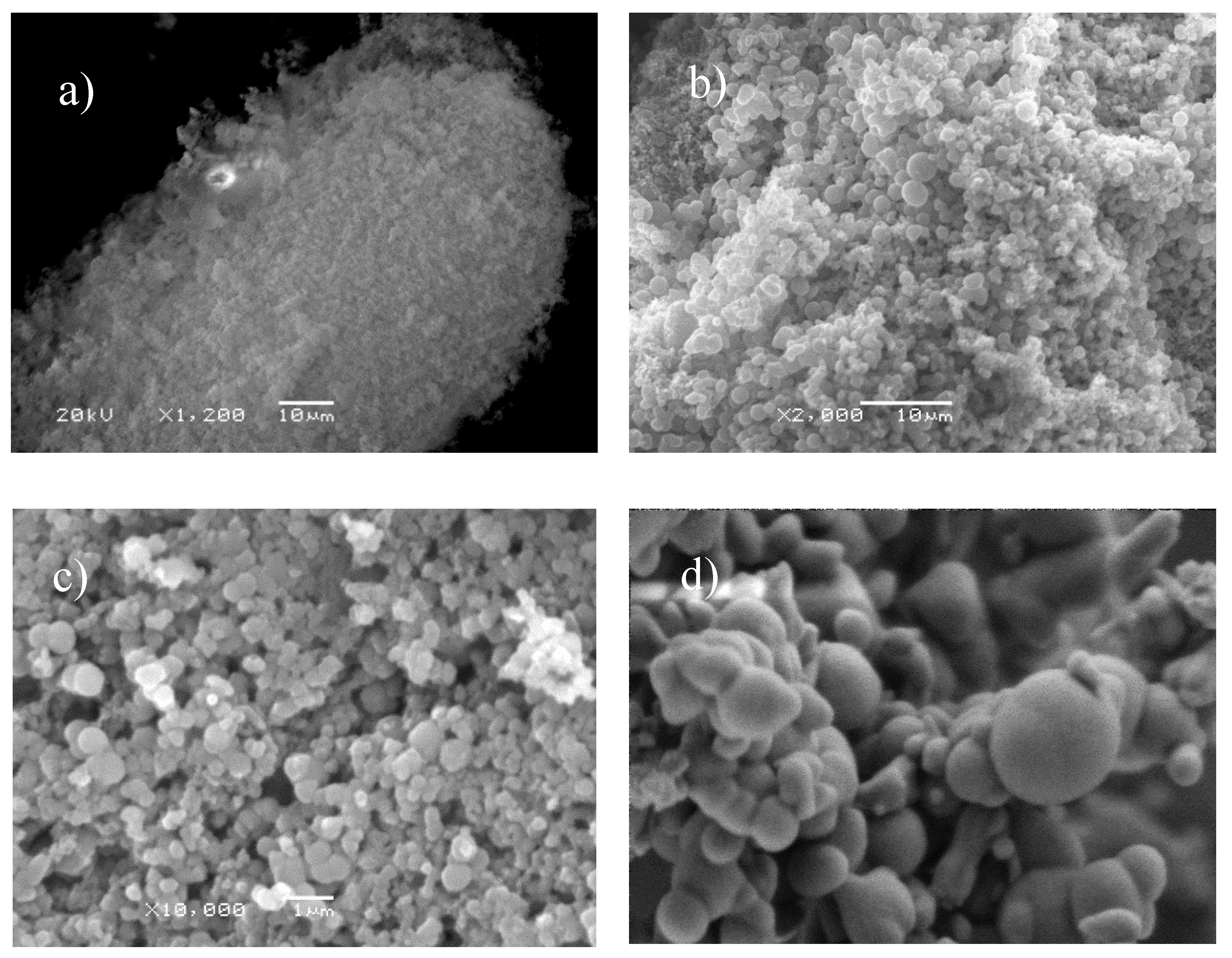
Scanning Electron Microscope
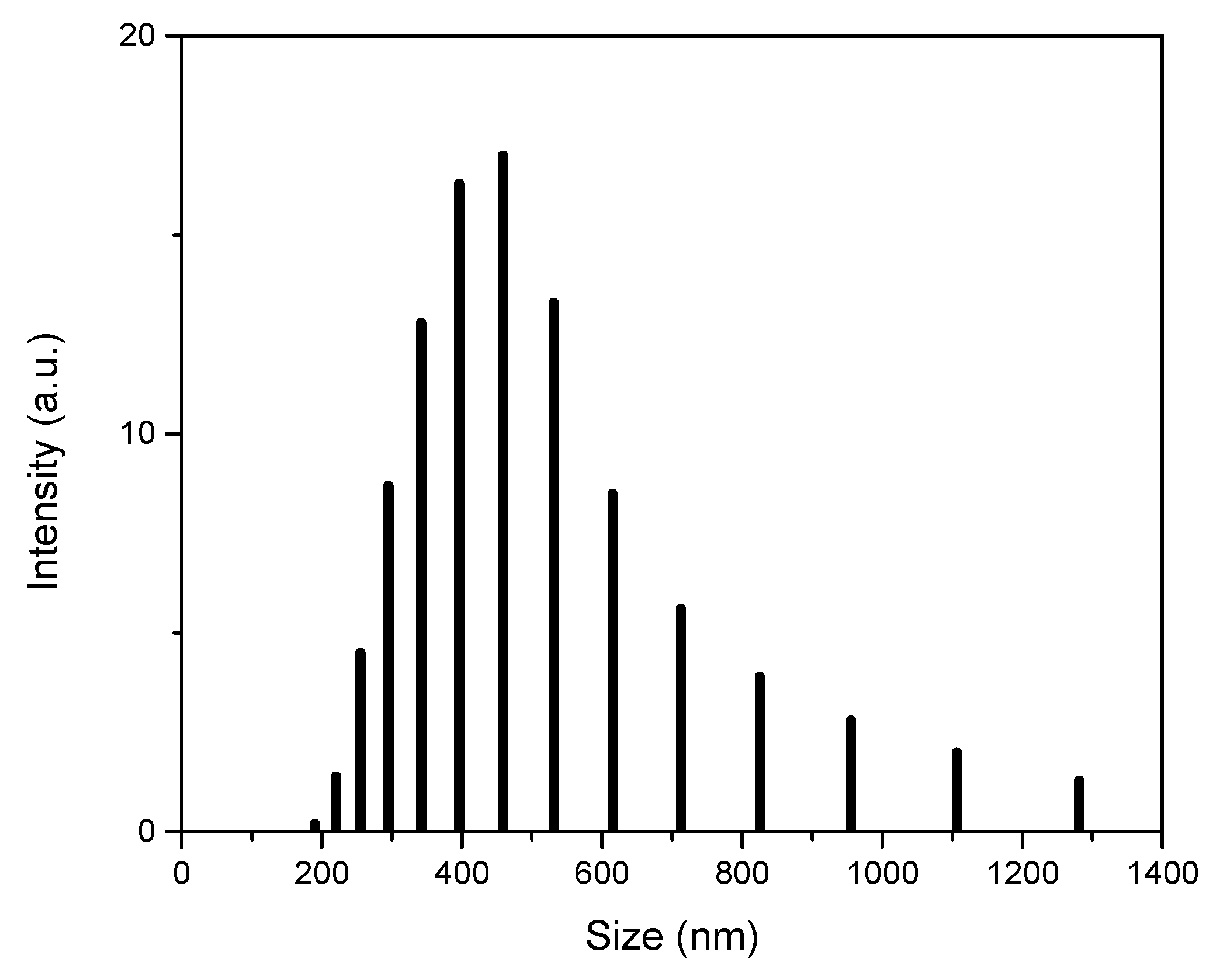
Dynamic Light Scattering
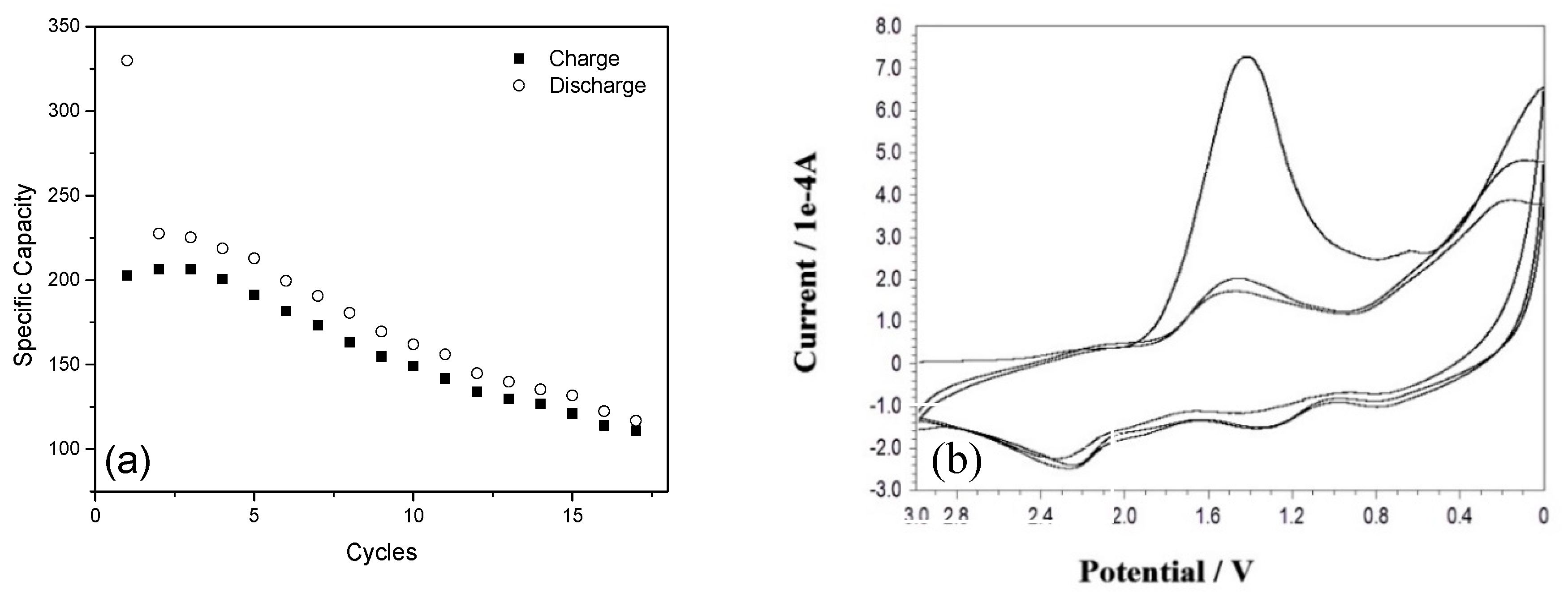
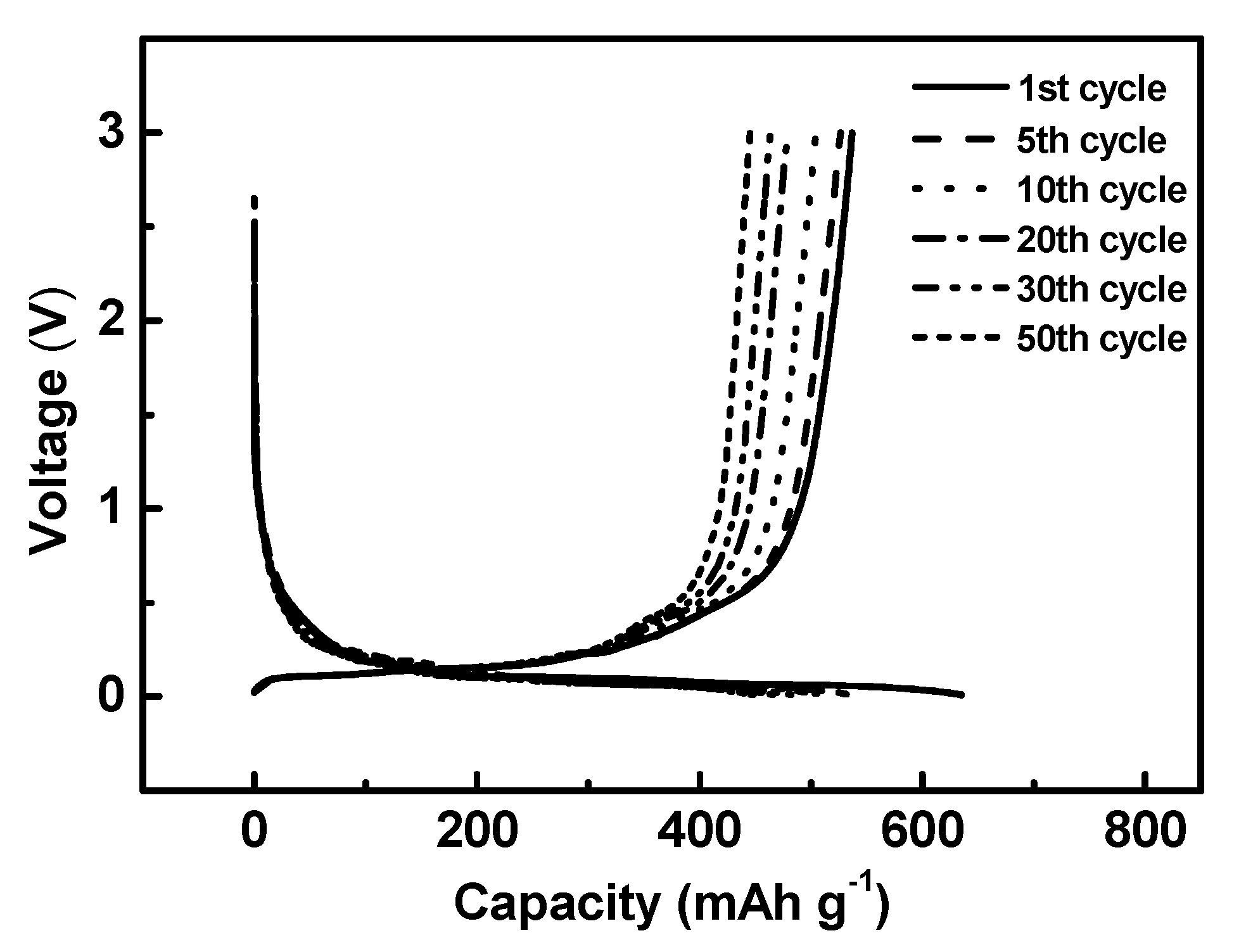
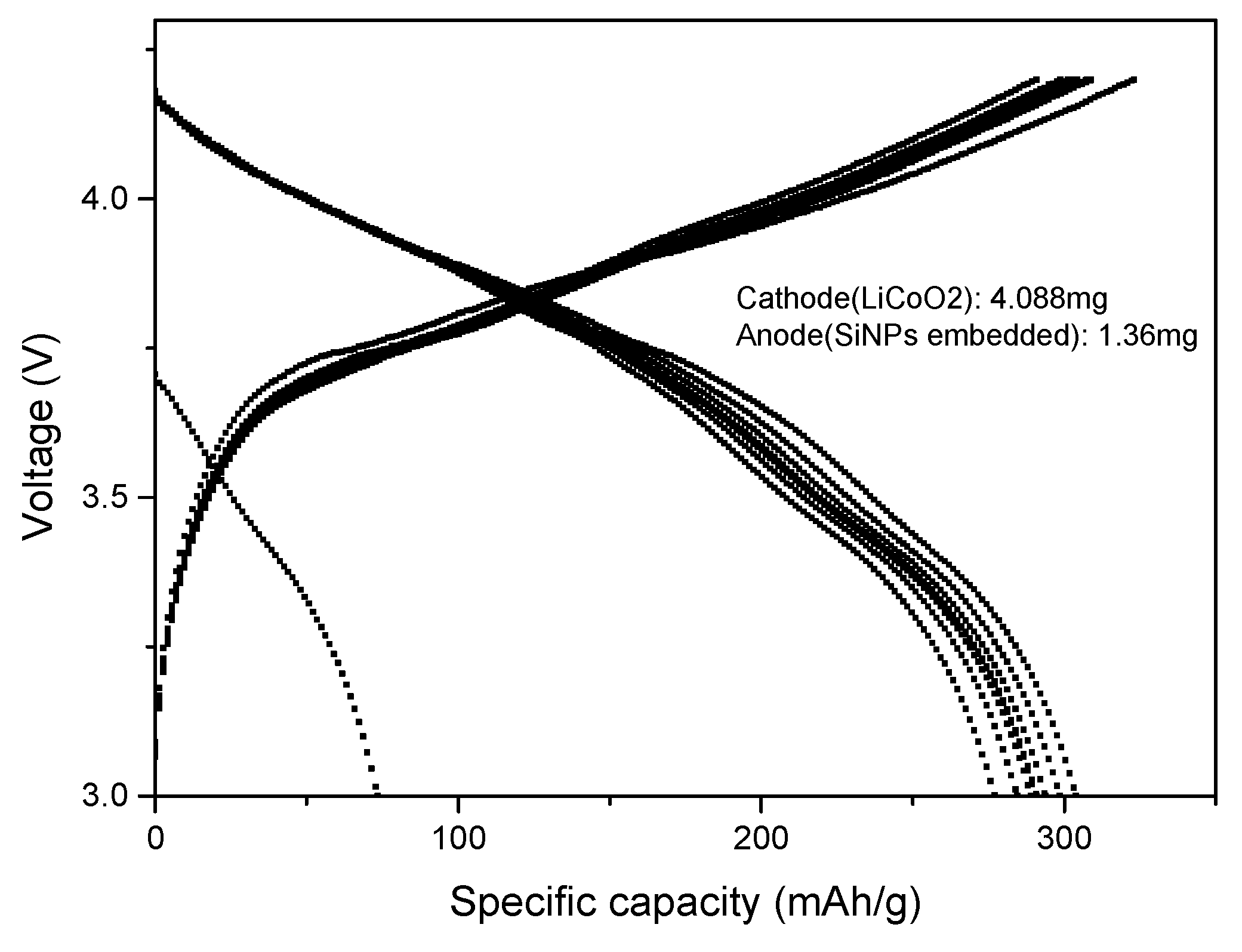
Half-cell and full-cell cycling test
Conclusion
References
- Franco, A.A., et al., Boosting Rechargeable Batteries R&D by Multiscale Modeling: Myth or Reality? Chemical Reviews, 2019. 119(7): p. 4569-4627. [CrossRef]
- Kalair, A., et al., Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage, 2020. 3(1): p. e135. [CrossRef]
- Ma, D., et al., Robust SnO2−x Nanoparticle-Impregnated Carbon Nanofibers with Outstanding Electrochemical Performance for Advanced Sodium-Ion Batteries. Angewandte Chemie International Edition, 2018. 57(29): p. 8901-8905. [CrossRef]
- Ward, J.D. and W.P. Nel, Comment on Fossil-fuel constraints on global warming by A. Zecca and L. Chiari [Energy Policy 38 (2010) 1–3]. Energy Policy, 2011. 39(11): p. 7464-7466. [CrossRef]
- Knobloch, F., et al., Net emission reductions from electric cars and heat pumps in 59 world regions over time. Nat Sustain, 2020. 3(6): p. 437-447. [CrossRef]
- Heydarian, A., et al., Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. Journal of Power Sources, 2018. 378: p. 19-30. [CrossRef]
- Xiao, F., et al., Large-scale production of holey graphite as high-rate anode for lithium ion batteries. Journal of Energy Chemistry, 2020. 48: p. 122-127. [CrossRef]
- Zhang, F., et al., Interfacial electrostatic self-assembly in water-in-oil microemulsion assisted synthesis of Li4Ti5O12/Graphene for lithium-ion-batteries. Journal of Alloys and Compounds, 2020. 819: p. 153018. [CrossRef]
- Tian, Q., et al., Reducing the excessive interior space of SnO2@C nanotubes by encapsulating SnO2 nanowires for high lithium storage. Journal of Alloys and Compounds, 2020. 820: p. 153404. [CrossRef]
- Tan, J., X. Qi, and J. Mao, A novel Al@TiO2-MCMB dual-ion battery with excellent cycling performance at high current rate. Journal of Alloys and Compounds, 2020. 818: p. 152853. [CrossRef]
- Xu, C., et al., Embedding Silicon in Pinecone-Derived Porous Carbon as a High-Performance Anode for Lithium-Ion Batteries. ChemElectroChem, 2020. 7: p. 152853. [CrossRef]
- Sun, F., et al., A rationally designed composite of alternating strata of Si nanoparticles and graphene: a high-performance lithium-ion battery anode. Nanoscale, 2013. 5(18). [CrossRef]
- Sun, F., et al., A rationally designed composite of alternating strata of Si nanoparticles and graphene: a high-performance lithium-ion battery anode. Nanoscale, 2013. 5(18): p. 8586-8592. [CrossRef]
- Han, L., et al., Toward Superb Perovskite Oxide Electrocatalysts: Engineering of Coupled Nanocomposites. Small, 2022. 18(50): p. 2204784. [CrossRef]
- Liu, X.H., et al., Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano, 2012. 6(2): p. 1522-1531. [CrossRef]
- Chen, J., et al., Facile fabrication of Si mesoporous nanowires for high-capacity and long-life lithium storage. Nanoscale, 2013. 5(21): p. 10623-10628. [CrossRef]
- Wu, H., et al., Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nature Nanotechnology, 2012. 7(5): p. 310-315. [CrossRef]
- Liu, N., et al., A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nature Nanotechnology, 2014. 9(3): p. 187-192. [CrossRef]
- Luo, W., et al., Critical thickness of phenolic resin-based carbon interfacial layer for improving long cycling stability of silicon nanoparticle anodes. Nano Energy, 2016. 27: p. 255-264. [CrossRef]
- Yang, J., et al., Yolk-shell silicon-mesoporous carbon anode with compact solid electrolyte interphase film for superior lithium-ion batteries. Nano Energy, 2015. 18: p. 133-142. [CrossRef]
- Wu, Y.-J., et al., Small highly mesoporous silicon nanoparticles for high performance lithium ion based energy storage. Chemical Engineering Journal, 2020. 400: p. 125958. [CrossRef]
- Liu, H., et al., Mesoporous TiO2-B Microspheres with Superior Rate Performance for Lithium Ion Batteries. Advanced Materials, 2011. 23(30): p. 3450-3454. [CrossRef]
- Li, J., et al., Synthesis of Si/TiO2core–shell nanoparticles as anode material for high performance lithium ion batteries. Journal of Materials Science: Materials in Electronics, 2016. 27(12): p. 12813-12819. [CrossRef]
- Jiang, J., et al., Auto-adjustment of structure and SnO2 content of SnO2/TiO2 microspheres for lithium-ion batteries. Chemical Engineering Journal, 2019. 359: p. 746-754. [CrossRef]
- Jin, Y., et al., Self-healing SEI enables full-cell cycling of a silicon-majority anode with a coulombic efficiency exceeding 99.9%. Energy and Environmental Science, 2017. 10(2): p. 580-592. [CrossRef]
- Deng, D., Li-ion batteries: basics, progress, and challenges. Energy Science & Engineering, 2015. 3(5): p. 385-418. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




