Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. EOs chemical composition
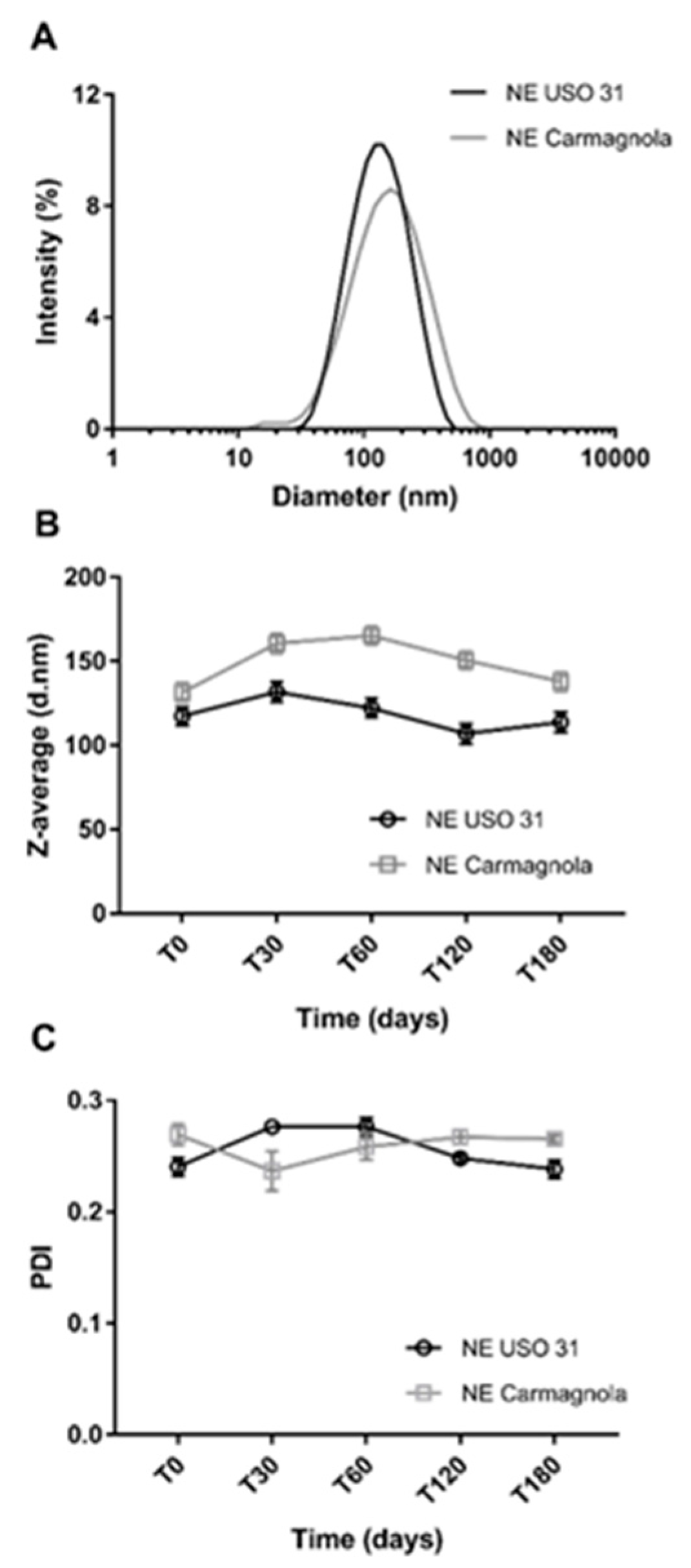
2.2. Characterization and physical stability over time of C. sativa EO-based NEs
2.3. Evaluation of C. sativa EO-based NEs safety profile in human cell lines
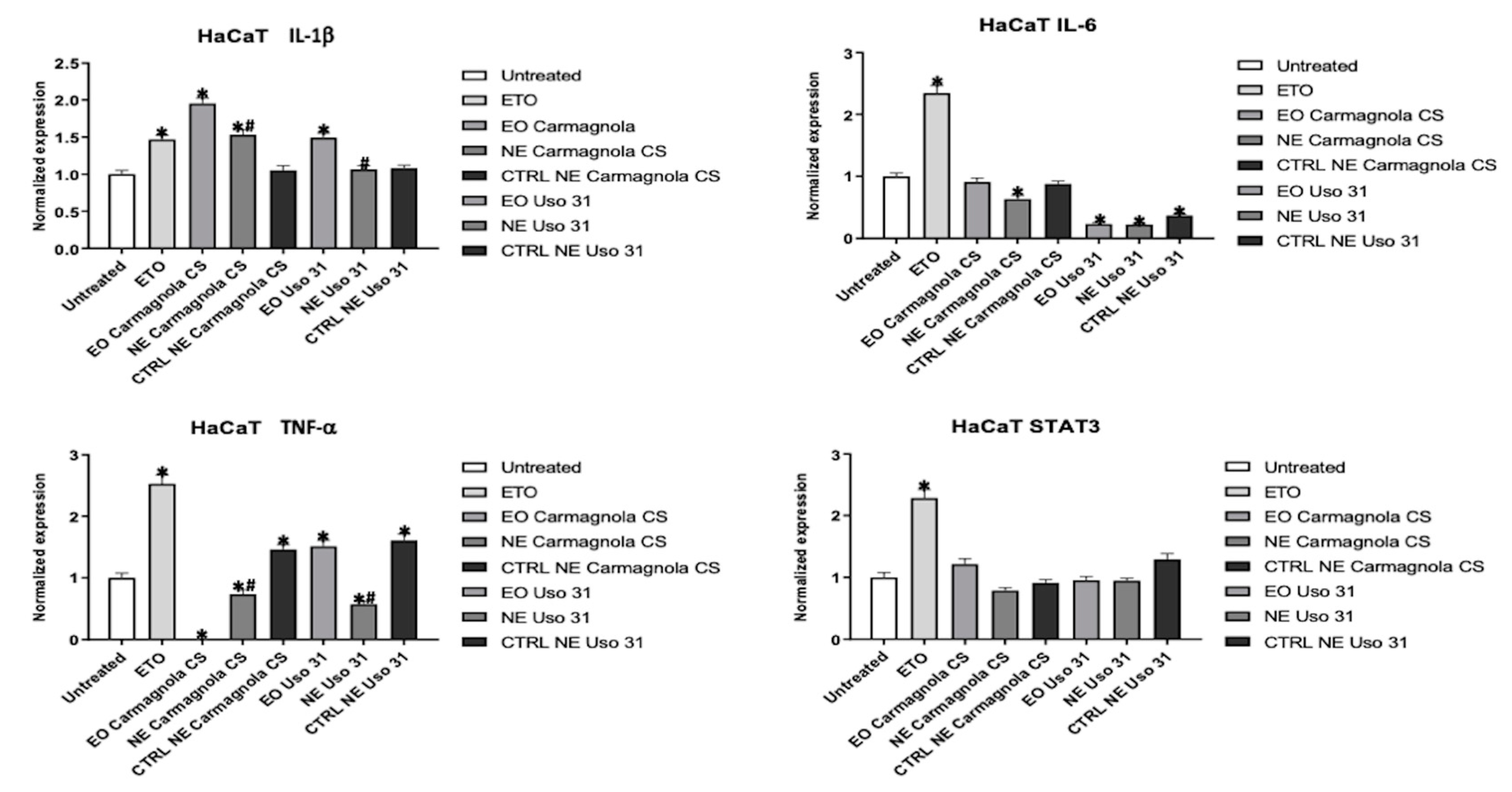
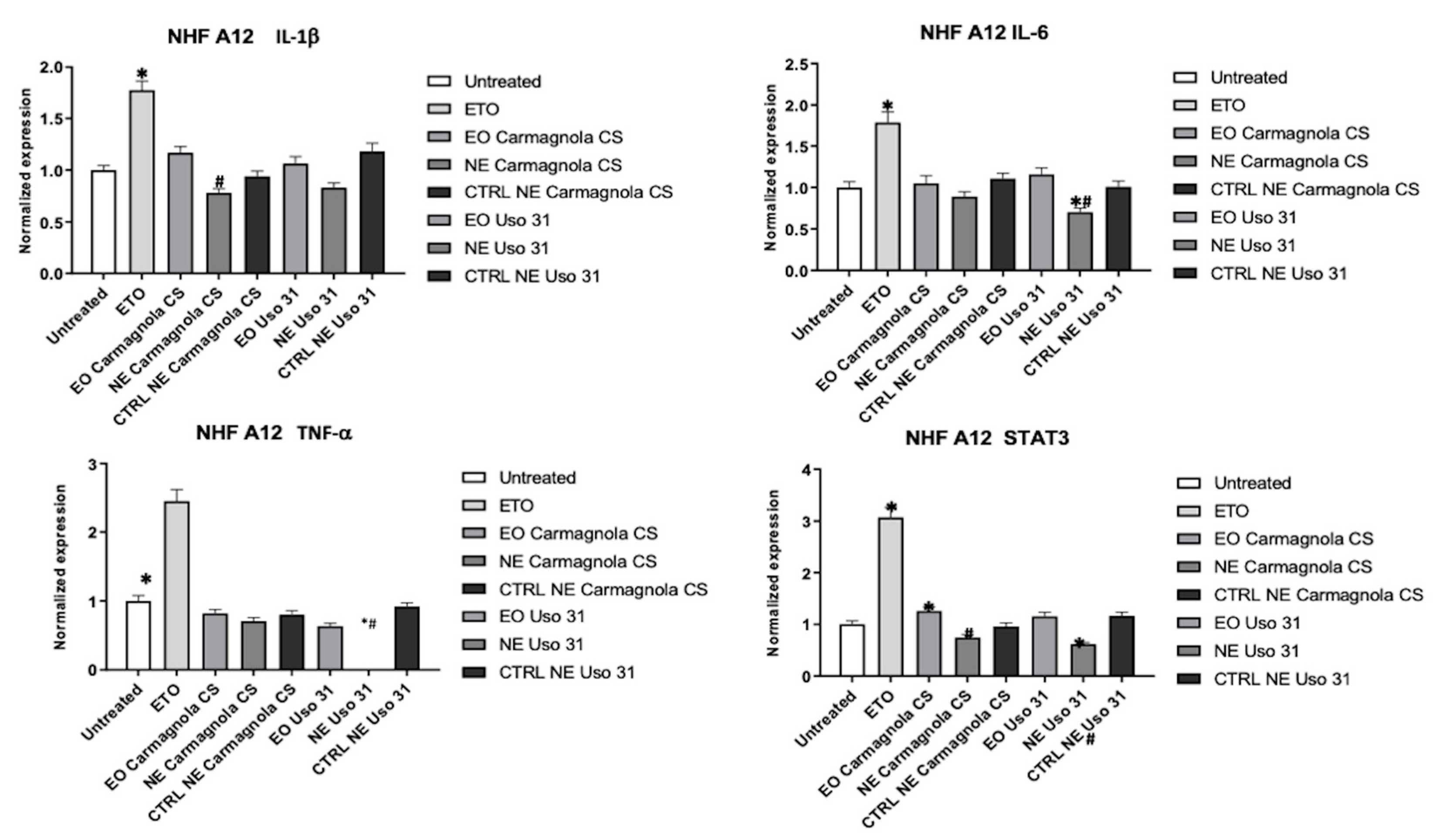
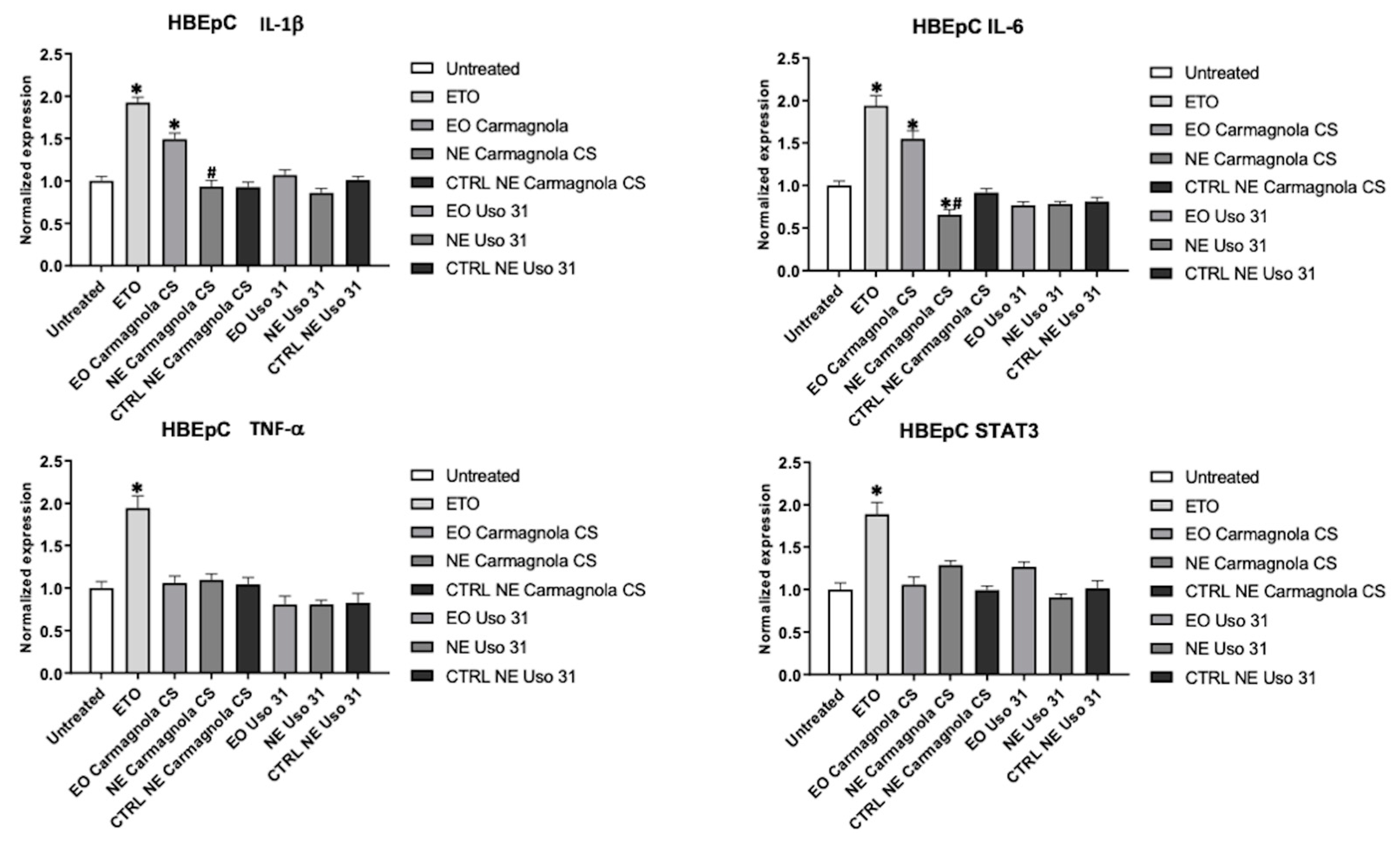
2.4. Evaluation of C. sativa EO-based NEs effect on inflammatory condition of human cell lines
3. Discussion
4. Materials and Methods
4.1. Plant material
4.2. Microwave assisted extraction (MAE)
4.3. EOs GC-MS characterization
4.4. C. sativa EO-based nanoemulsions (NEs) formulation and characterization
4.5. Cell lines
4.6. Cell viability assay
4.7. RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR and TaqMan Array
4.8. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fike, J. Industrial Hemp: Renewed Opportunities for an Ancient Crop. https://doi.org/10.1080/07352689.2016.1257842 2017, 35, 406–424. [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Dincheva, I.; Kačániová, M.; Astatkie, T.; Semerdjieva, I.B.; Latkovic, D. Industrial, CBD, and Wild Hemp: How Different Are Their Essential Oil Profile and Antimicrobial Activity? Mol. 2020, Vol. 25, Page 4631 2020, 25, 4631. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Sotto, A. Di; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Ngahang Kamte, S.L.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The crop-residue of fiber hemp cv. Futura 75: from a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. 2018, 25, 10515–10525. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Tabari, M.A.; Khodashenas, A.; Jafari, M.; Petrelli, R.; Cappellacci, L.; Nabissi, M.; Maggi, F.; Pavela, R.; Youssefi, M.R. Acaricidal properties of hemp (Cannabis sativa L.) essential oil against Dermanyssus gallinae and Hyalomma dromedarii. Ind. Crops Prod. 2020, 147, 112238. [Google Scholar] [CrossRef]
- Pagano, S.; Coniglio, M.; Valenti, C.; Federici, M.I.; Lombardo, G.; Cianetti, S.; Marinucci, L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed. Pharmacother. 2020, 132. [Google Scholar] [CrossRef]
- Cerino, P.; Buonerba, C.; Cannazza, G.; D’Auria, J.; Ottoni, E.; Fulgione, A.; Di Stasio, A.; Pierri, B.; Gallo, A. A Review of Hemp as Food and Nutritional Supplement. Cannabis Cannabinoid Res. 2021, 6, 19. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants (Basel, Switzerland) 2021, 10. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Mol. 2017, Vol. 22, Page 700 2017, 22, 700. [Google Scholar] [CrossRef]
- Martins, A.M.; Gomes, A.L.; Boas, I.V.; Marto, J.; Ribeiro, H.M. Cannabis-Based Products for the Treatment of Skin Inflammatory Diseases: A Timely Review. Pharmaceuticals 2022, 15. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. wound care 2014, 3, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elseady, W.S.; Saleh, A.; Alotaibi, K.N.; El-Sherbeni, S.A. Antibacterial, Immunomodulatory, and Lung Protective Effects of Boswelliadalzielii Oleoresin Ethanol Extract in Pulmonary Diseases: In Vitro and In Vivo Studies. Antibiot. 2021, Vol. 10, Page 1444 2021, 10, 1444. [Google Scholar] [CrossRef]
- Mamber, S.W.; Gurel, V.; Lins, J.; Ferri, F.; Beseme, S.; McMichael, J. Effects of cannabis oil extract on immune response gene expression in human small airway epithelial cells (HSAEpC): implications for chronic obstructive pulmonary disease (COPD). J. Cannabis Res. 2020 21 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Aswad, M.; Hamza, H.; Pechkovsky, A.; Zikrach, A.; Popov, T.; Zohar, Y.; Shahar, E.; Louria-Hayon, I. High-CBD Extract (CBD-X) Downregulates Cytokine Storm Systemically and Locally in Inflamed Lungs. Front. Immunol. 2022, 0, 2252. [Google Scholar] [CrossRef]
- Rossi, P.; Cappelli, A.; Marinelli, O.; Valzano, M.; Pavoni, L.; Bonacucina, G.; Petrelli, R.; Pompei, P.; Mazzara, E.; Ricci, I.; et al. Mosquitocidal and Anti-Inflammatory Properties of The Essential Oils Obtained from Monoecious, Male, and Female Inflorescences of Hemp (Cannabis sativa L.) and Their Encapsulation in Nanoemulsions. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Ciacciarelli, A.; Quassinti, L.; Bramucci, M.; Miano, A.; Casettari, L.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Properties and stability of nanoemulsions: How relevant is the type of surfactant? J. Drug Deliv. Sci. Technol. 2020, 58. [Google Scholar] [CrossRef]
- Mazzara, E.; Torresi, J.; Fico, G.; Papini, A.; Kulbaka, N.; Dall’acqua, S.; Sut, S.; Garzoli, S.; Mustafa, A.M.; Cappellacci, L.; et al. A Comprehensive Phytochemical Analysis of Terpenes, Polyphenols and Cannabinoids, and Micromorphological Characterization of 9 Commercial Varieties of Cannabis sativa L. Plants 2022, Vol. 11, Page 891 2022, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Identif. Essent. oil components by gas Chromatogr. Spectrom. 2007. [Google Scholar]
- NIST 17 Mass Spectral Library (NIST/EPA/NIH). National Institute of Standards and Technology; Gaithersburg, USA., 2017.
- FFNSC 3 Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; 3rd editio.; John Wiley & Sons, Inc.: Hoboken, NJ., 2015; ISBN 978-1-119-06984-3.
- Procaccia, S.; Lewitus, G.M.; Lipson Feder, C.; Shapira, A.; Berman, P.; Meiri, D. Cannabis for Medical Use: Versatile Plant Rather Than a Single Drug. Front. Pharmacol. 2022, 13, 1308. [Google Scholar] [CrossRef]
- Bukke, V.N.; Archana, M.; Villani, R.; Serviddio, G.; Cassano, T. Pharmacological and Toxicological Effects of Phytocannabinoids and Recreational Synthetic Cannabinoids: Increasing Risk of Public Health. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Iannone, M.; Cinque, G.; Marianelli, A.; Pistelli, L.; Flamini, G. “Hemping” the drinks: Aromatizing alcoholic beverages with a blend of Cannabis sativa L. flowers. Food Chem. 2020, 325, 126909. [Google Scholar] [CrossRef]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cappellacci, L.; Petrelli, R.; Spinozzi, E.; Aguzzi, C.; Zeppa, L.; Ubaldi, M.; et al. Encapsulation of Carlina acaulis essential oil and carlina oxide to develop long-lasting mosquito larvicides: microemulsions versus nanoemulsions. J. Pest Sci. (2004). 2021, 94, 899–915. [Google Scholar] [CrossRef]

| Componenta | RI Calc.b | RI litc | % Uso 31 | % Carmagnola CS |
|---|---|---|---|---|
| α-pinene | 926 | 932 | 11.07 | 13.45 |
| β-pinene | 968 | 974 | 3.59 | 5.45 |
| myrcene | 989 | 988 | 15.28 | 37.57 |
| limonene | 1025 | 1024 | 1.59 | 5.37 |
| (E)-β-ocimene | 1047 | 1044 | 6.71 | 1.79 |
| terpinolene | 1085 | 1086 | 5.46 | 2.97 |
| (E)-caryophyllene | 1409 | 1417 | 25.93 | 16.99 |
| α-humulene | 1443 | 1452 | 8.92 | 6.11 |
| caryophyllene oxide | 1571 | 1582 | 7.23 | 2.95 |
| Total identified (%) | 99.03 | 97.72 | ||
| Monoterpene hydrocarbons (%) | 44.24 | 67.27 | ||
| Oxygenated monoterpenes (%) | 0.43 | |||
| Sesquiterpene hydrocarbons (%) | 45.31 | 26.18 | ||
| Oxygenated sesquiterpenes (%) | 9.04 | 3.58 | ||
| Cannabinoids (%) | 0.43 | 0.23 |
| IC50 mg mL-1 | |||
| HaCaT | NHF A12 | HBEpC | |
| Carmagnola CS EO | 0.052 ± 0.002 | 0.119 ± 0.009 | 0.034 ± 0.002 |
| Carmagnola CS NE | 0.354 ± 0.018 (0.021± 0.001) |
0.448 ± 0.020 (0.023± 0.001) |
0.154 ± 0.010 (0.009± 0.001) |
| CTRL Carmagnola CS NE | 2.606 ± 0.050 | 9.666 ± 0.600 | 1.221 ± 0.500 |
| Uso 31 EO | 0.049 ± 0.002 | 0.103 ± 0.008 | 0.041 ± 0.003 |
| Uso 31 NE | 0.857 ± 0.030 (0.043± 0.002) |
1.505 ± 0.058 (0.075 ± 0.003) |
0.245 ± 0.010 (0.012 ± 0.001) |
| CTRL Uso 31 NE | 5.388 ± 0.100 | >> 10.04 | 3.255 ± 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





