1. Introduction
Despite improvements in standard-of-care management, infective endocarditis (IE), especially in cases of severe valvular dysfunction and perivalvular abscesses, still represents a complex disease with high risk of severe congestive heart failure and cardiac-related death [
1].
Recent advancements in antibiotic therapy have improved the clinical course of IE, however, 25% to 50% of patients with acute IE still require surgery for replacement or reconstruction of the valves and annular tissue [
2]. Mortality rates remain high, with reports of 6% to 50% in-hospital mortality in the worst-case scenario, as well as significantly reduced long-term survival ranging between 18% and 81% at five years [
1,
3].
Cardiac surgery in patients presenting with IE carries a disproportionately increased risk for peri-operative hyperinflammation and post-operative sepsis due to the dissemination of bacteria from the infected valves, and the release of cytokines and vasoactive peptides triggered by the cardiopulmonary bypass (CPB) itself [4-6].
CytoSorb® (CytoSorbents, Princeton, NJ, USA) is a CE-marked whole-blood hemoadsorption device capable of removing circulating levels of cytokines from blood. The therapy has been increasingly used in patients with hyperinflammation from various etiologies, and studies suggest a potential effectiveness in ameliorating inflammation and reducing organ injuries [7-9]. The device can be easily integrated into the CPB circuit for intraoperative use during cardiac surgery and is indicated in patients with infective endocarditis to reduce proinflammatory mediators [
10]. Recently, beneficial effects have been observed in patients developing perioperative renal failure in combination with severe hemodynamic instability [
11].
In addition to various potential clinical benefits, CytoSorb® adjunctive therapy might have relevant economic implications derived from the reduced perioperative need for vasoactive drugs and from general optimization in resource use. To date, two studies have shown a shorter ICU stay in patients treated intraoperatively with CytoSorb®, compared to patients undergoing standard surgical treatment [
10,
12].
In this study, we aimed to investigate the economic impact of the intraoperative use of CytoSorb® in IE patients undergoing cardiac surgery in the setting of the German healthcare system with specific emphasis on ICU-stay.
2. Materials and Methods
A Microsoft
® Excel-based [
13] budget impact model was developed to estimate the economic impact of adding intraoperative hemoadsorption with (one cartridge of) CytoSorb® to standard surgical treatment of severe IE in Germany. A one-year period was used to estimate the potential population eligible for surgical treatment with adjunctive CytoSorb® therapy in Germany. The patient population was calculated based on official hospital volume data sources. Only effects deriving from differences in the ICU stay were considered to assess the economic implications of the intraoperative use of the therapy. Deterministic and probabilistic sensitivity analyses were performed to test the robustness of results.
2.1. Data on resource use
Data on ICU length of stay were extrapolated from a retrospective case series comparing 39 IE patients treated with CytoSorb® hemoadsorption to an historical control group of 28 patients who did not receive intraoperative hemoadsorption [
10]. The investigators reported a difference of 2.5 days in ICU length of stay, which was equal to 5 (IQR:4 - 8) days in the treated group and 7.5 (4.5 - 10) in the historical controls (p=0.059).
2.2. Cost data
The overall cost of therapy with one CytoSorb® adsorber was calculated by taking into consideration the selling price of the device (including CPB adapters and primers) in Germany, adding 19% VAT and personnel costs (physicians) for priming, installing, monitoring, and removing the adsorber (measured in a hospital using the therapy routinely). Furthermore, in accordance with the requirements published by the German Institute for Reimbursement in Hospitals (InEK), an exemplary infrastructure surcharge was considered and added to calculate the full cost of the device [
14]. This surcharge is set individually by hospitals, therefore based on feedback from our network, we assumed it to be equal to 12% of the cost item it applied to.
The average cost of a stay of one day in ICU was extrapolated from the literature [
15] and updated according to the German Health Consumer Price Index to 2022 healthcare prices.
Details of the cost calculations are displayed in
Table 1.
2.3. Patient population
The annual patient population of surgical IE patients thought to be eligible for treatment with CytoSorb® was estimated using German DRG official volume data for the year 2021. Diagnosis and DRG data were combined with data on the use of the OPS procedure code for hemoadsorption with CytoSorb® (OPS 8-821.2 “Extracorporeal adsorption of low and medium molecular weight hydrophobic substances”) to extrapolate the number of surgical infective endocarditis episodes where CytoSorb® was used.
In 2021, 15,477 patients were diagnosed with infective endocarditis in Germany [
16]. These cases were all coded with ICD-10 codes for Infective Endocarditis (I33.0 and I33.9) as primary or secondary diagnosis. They were all grouped in 187 medical and surgical DRGs of different complexity. Four out of the top 6 most frequent DRGs were surgical DRGs, suggesting that a total of 2,489 acute IE patients (19.5%) required heart valve surgery with a heart lung machine (F03A to F03D). Among these DRGs, we selected those reporting on cases where the OPS procedure code 8-821.2 was used (634 cases). In total, 634 cases were found where the procedure code 8-821.2 was coded in surgical DRGs for heart valve surgery in IE patients. There were no other relevant surgical DRGs where the diagnosis codes for infective endocarditis were used in combination with the procedure code for hemoadsorption (see Appendix and Table A1 for details).
To account for possible overestimates – i.e. due to use of CytoSorb® in the post-operative setting only, or due to coding of other hemoadsorption devices -, the number of patients with IE, requiring heart valve surgery and receiving CytoSorb hemoadsorption therapy was rounded down to 550 patients.
2.3. Scenario analyses
We considered intraoperative installation of one cartridge of CytoSorb® hemoadsorption therapy into the CPB circuit during cardiac surgery for IE. Two hospital financing scenarios were considered. In the first scenario, the differences in costs between the two therapeutic strategies were calculated assuming that the hospital received no device-specific reimbursement for CytoSorb® therapy; in the second scenario, we assumed that sickness funds would provide full therapy reimbursement for the therapy, equal to the full cost of CytoSorb® as calculated in
Table 1. The analysis was conducted over a potential annual population of 550 patients for the whole German hospital system. Results of the economic impact of treating one patient are also presented.
2.3. Sensitivity analyses
Sensitivity analyses were conducted to assess the confidence in the results of the model. A one-way deterministic sensitivity analysis was performed by varying the base-case model inputs one at a time. A variation of ±50% was used for the daily cost of ICU stay, the infrastructure surcharge range was set to a range between 0% and 100% of the therapy cost and a variation of ± for the interquartile range (IQR) was used for the change in ICU length of stay. A tornado diagram was used to visually demonstrate the resulting reduction in costs corresponding to the altered model input and to the maximum and minimum values of the range.
To further reduce uncertainty associated with input parameters, a probabilistic sensitivity analysis was conducted. A bootstrap analysis for the input parameter ICU length of stay was conducted in order to estimate the parameter’s value in the treated and control populations. The bootstrap consisted of 1000 samples with 100 observations each and was developed in the Microsoft Excel’s visual basic environment [
13]. The resulting lengths of stay were multiplied by the daily cost of ICU stay, which was calculated using a two-parameter gamma distribution. The gamma distribution was chosen and used, being the reference probability distribution recommended for probabilistic assessment of cost variables in cost-effectiveness analysis and health decision modelling [
17].
3. Results
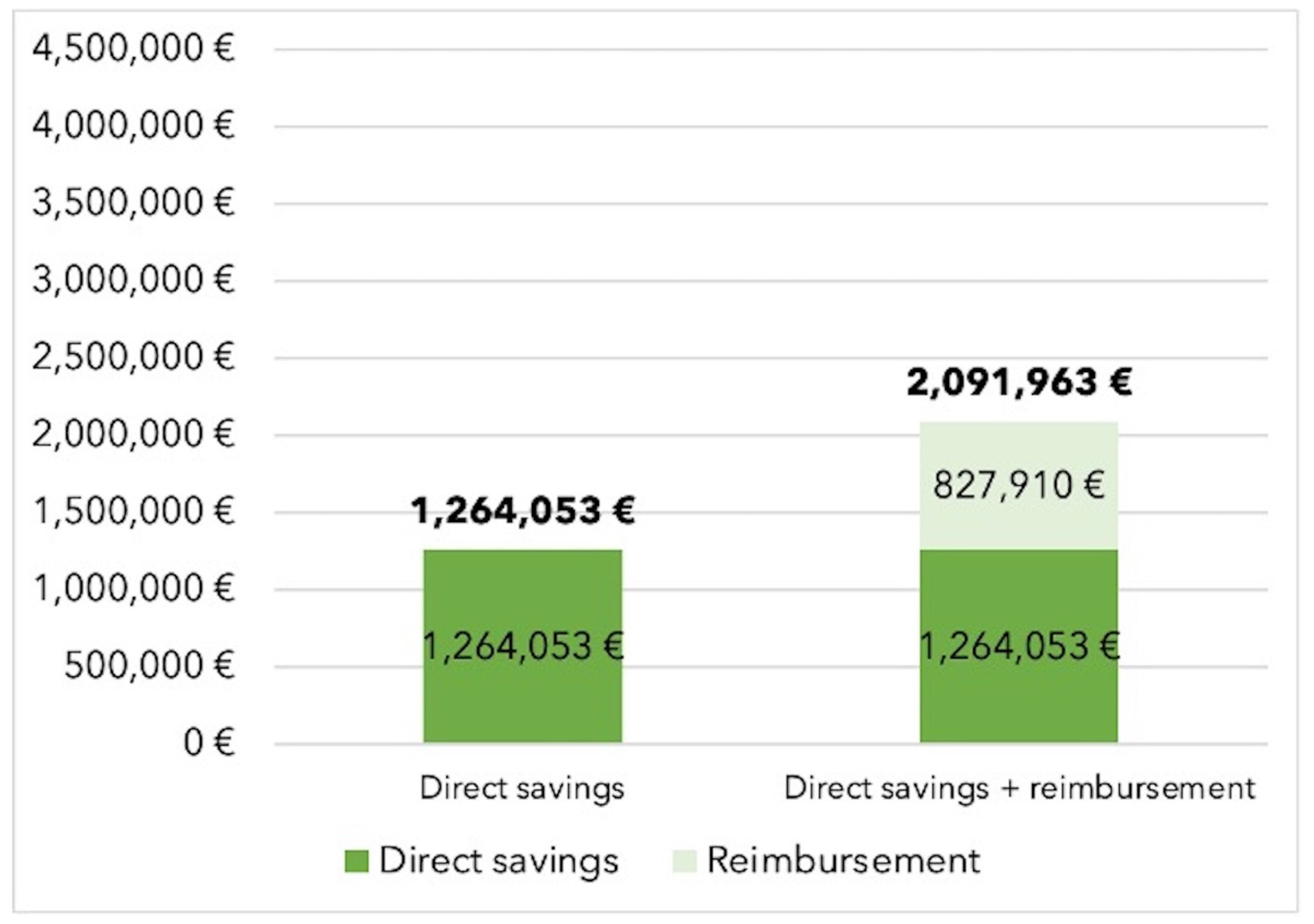
In the base-case scenario without dedicated reimbursement for hemoadsorption therapy, the additional use of CytoSorb® therapy resulted in total savings of 1,264,053€ when treating an annual potential population of 550 cases, corresponding to 2,298€ saved per patient. Savings increased to 2,091,963€ (3,804€ per patient) in cases of full therapy reimbursement (see
Figure 1).
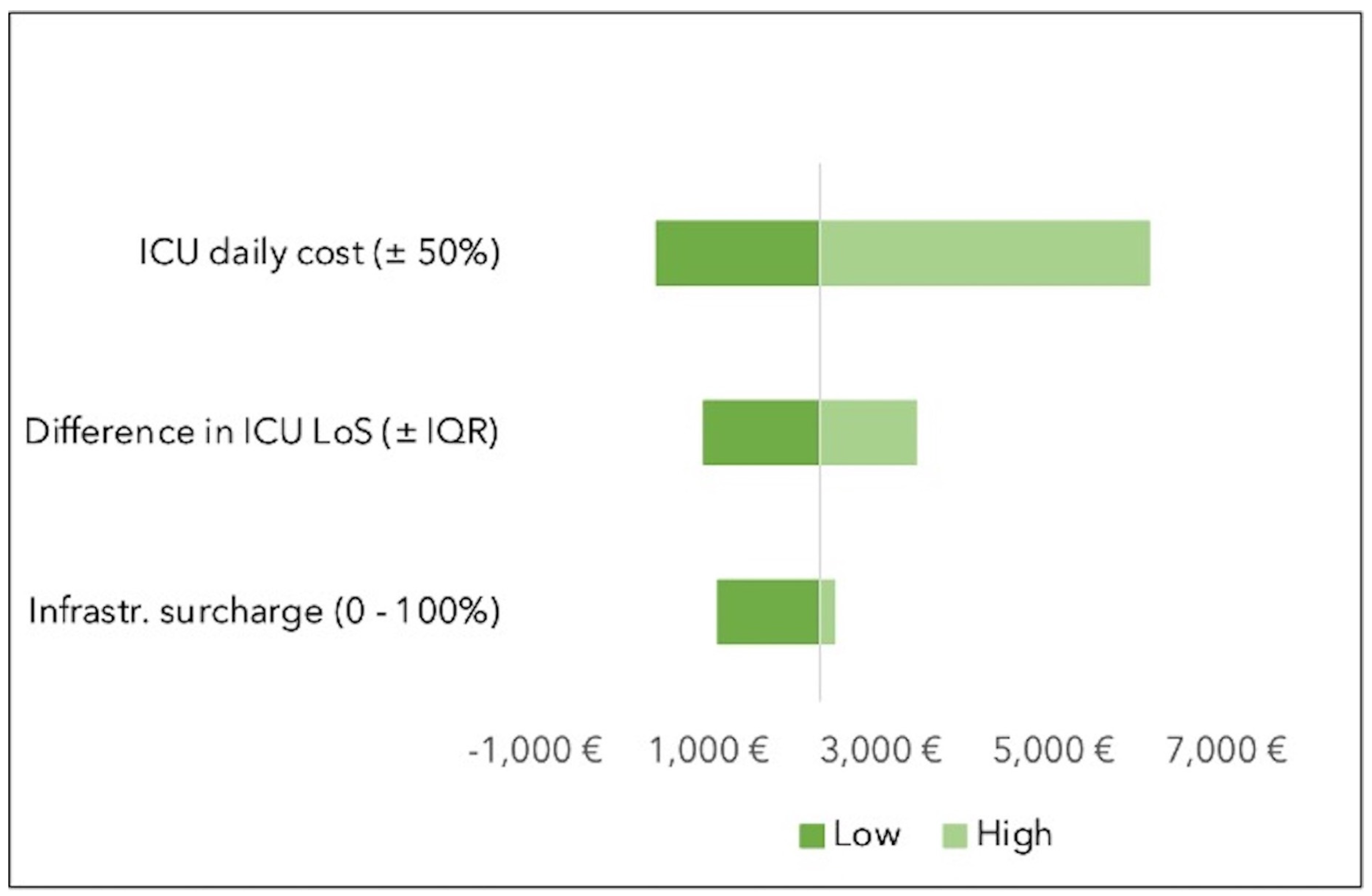
In the one-way sensitivity analysis, the variable with the highest impact was the cost of ICU stay. When varying the cost of ICU stay by ±50% of its value, savings ranged from 396€ (in the case of a 50% decrease in the cost of an ICU stay) to 6,102€ (in the case of a 50% increase in the cost of ICU occupation). The variation in infrastructure surcharge had the lowest impact on the range of savings (see
Figure 2).
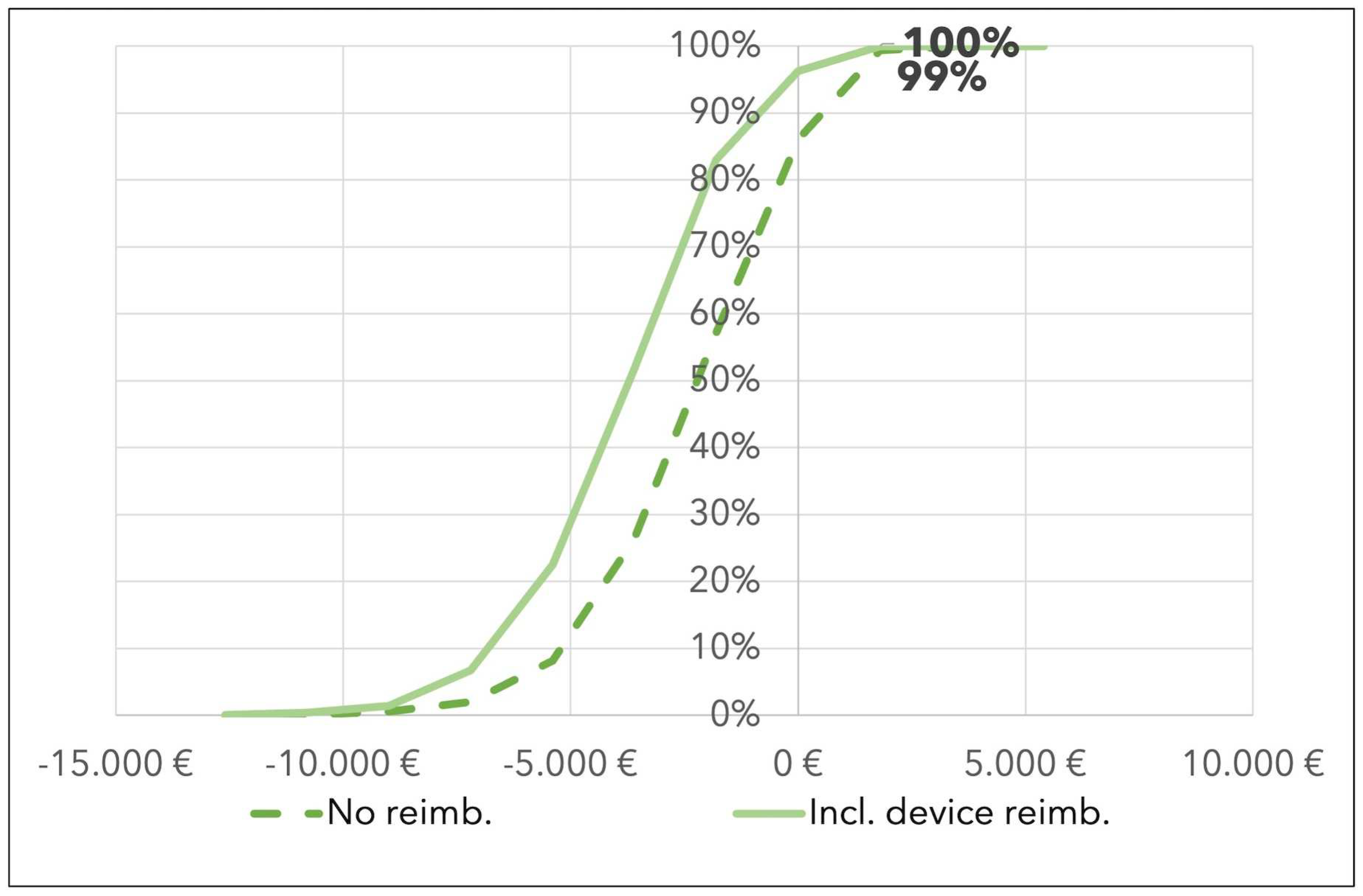
A probabilistic sensitivity analysis was conducted using the difference in the bootstrap analysis of the ICU length of stay in the two groups and the probabilistic value of the ICU daily cost (see Sensitivity Analysis in the Materials and Methods section).
These results suggest that CytoSorb® has a probability of being cost saving equal to 99% in the base case scenario, and to 100% in cases of full therapy reimbursement (see
Figure 3).
4. Discussion
This study investigated the economic implications of using intraoperative hemoadsorption with CytoSorb® in high-risk infective endocarditis patients requiring cardiac surgery in Germany. We observed that, even without reimbursement, intraoperative hemoadsorption leads to 1,264,053€ (2,298€ per patient) cost savings per annum, which increases to 2,091,963€ (3,804€ per patient) in cases of full therapy reimbursement. Daily ICU costs were the major cost driver in the postoperative period and, even after conducting sensitivity analyses and varying the daily ICU costs by +/- 50%, savings were still observed.
Currently, CytoSorb® therapy is coded in Germany through the specific procedure code OPS 8-821.2 ‘Extracorporeal adsorption of low and medium sized molecular, hydrophobic substances (including cytokine adsorption)’. The use of this code is linked to an additional reimbursement (ZE) on top of the DRG rate, which is
negotiated every year by hospitals and sickness funds [
18], and which was assumed to be in place in the second scenario of this German-specific analysis.
Of note, the findings of the present study can be generalized to other European and non-European health care systems with similar financing and cost structures. Although costs vary across countries and hospitals, and depend ultimately on the medical complexity of treated cases, the average daily costs for ICU occupation are in a similar cost range in European countries, which reflects the availability of homogeneous technologies and human resource use [
19,
20]. This implies that the lower expenditures derived from the reduced ICU stay will likely compensate for the cost of the therapy, also in other healthcare contexts. This could result in savings for both budget funded providers, with neither device nor procedure-specific reimbursement, as well as for those healthcare contexts with dedicated therapy reimbursement. Considering the latter, savings on a hospital level could even be higher.
The intraoperative use of CytoSorb® therapy in IE patients has potentially important clinical and economic effects that go beyond the perioperative time period investigated in this analysis. Recent literature on infective endocarditis patients observed a reduced need for vasoactive drugs in CytoSorb® patients compared to control patients [
12,
21], as well as a diminished incidence of post-operative sepsis and septic complications [
7]. Data also suggest a benefit when continuing postoperatively with hemoadsorption treatment in patients with infective endocarditis who develop renal failure in combination with severe hemodynamic instability [
11]. These findings have not been captured in this analysis but might have relevant consequences on patients’ morbidity and mortality, as well as on the clinical management, hospital and societal costs associated with this population. Of note, a reduced ICU stay itself is associated with improvements in patients’ quality of life and overall health outcomes [
22,
23], suggesting that not only purely financial effects should be considered when appraising the value and benefits of the therapy.
Regarding use of anti-infective therapy with hemoadsorption in IE patients, it must be acknowledged that CytoSorb® therapy, along with other extracorporeal therapies, can reduce levels of vancomycin from the blood. Recently published studies recommend a 50% increase in the vancomycin dose when treating patients with concomitant hemoadsorption therapy [
24,
25]. Additional costs derived from potential antibiotic therapy adjustment, including medicines and drug monitoring costs, were not included in this model. The reason for their exclusion is the neglectable impact of such costs on overall ICU costs, and on the overall cost of the therapy (i.e. one unit of 500 mg of Vancomycin Hydrochloride Injection IP, 500000 Iu/Vial costs 12€ to 25€ in Germany, depending on the package [
26]).
The analysis presented in this study has some limitations. First, only the costs associated to the therapy itself and to the post-operative ICU stay were considered. Other factors (i.e. antibiotic therapy adjustment, hospital length of stay, etc.) have not been incorporated in this study and might have an impact on the final calculated savings. A second limitation concerns the fact that there is uncertainty regarding the favorable effect on ICU length of stay assumed and modelled in the study. Data on ICU-stay in patients receiving intraoperative hemoadsorption is mixed and, although reporting improvements in patients’ outcomes, recent studies have not confirmed the reduction in ICU occupation observed in the study used to inform this model, and suggest that further data are needed to confirm this hypothesis [7,27-30].
5. Conclusions
Our budget impact model could show that the intraoperative use of adjunctive hemoadsorption with CytoSorb® in infective endocarditis patients has the potential to lead to important cost savings. However, these findings must be confirmed by further prospective analyses reporting benefits in terms of reduced intensive care unit stay.
Author Contributions
Conceptualization, Cristina Rao, Franziska Preissing and Daniel Wendt; Data curation, Cristina Rao and Franziska Preissing; Formal analysis, Cristina Rao; Methodology, Cristina Rao and Daniel Wendt; Project administration, Cristina Rao; Resources, Franziska Preissing and Lothar Daake; Software, Cristina Rao; Supervision, Daniel Wendt; Validation, Franziska Preissing, Matthias Thielmann, Zaki Haidari, Jurij Kalisnik and Karl Traeger; Writing – original draft, Cristina Rao, Daniel Wendt and Karl Traeger; Writing – review & editing, Matthias Thielmann, Zaki Haidari and Jurij Kalisnik. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
Cristina Rao, Franziska Preissing and Professor Daniel Wendt are employed of CytoSorbents Europe GmbH. Professor Matthias Thielmann, Doctor Zaki Haidari, Professor Jurij M. Kalisnik and Professor Karl Traeger have received honoraria and reimbursement for travel expenses by CytoSorbents Europe GmbH in the past years.
Appendix A
All surgical diagnostic related groups (DRGs) reporting both the OPS procedure code 8-821.2 ‘Extracorporeal adsorption of low and medium sized molecular, hydrophobic substances (including cytokine adsorption)’ and a primary or secondary diagnosis code for infective endocarditis (I33.0 and I33.9) were selected. By searching the database of the reimbursement.INFO platform [
16], we identified a total of 2,489 infective endocarditis patients who underwent heart valve surgery with a heart lung machine (cases grouped in DRGs from F03A to F03D). In a second step, we searched through all episodes and DRGs where the OPS procedure code 8-821.2 ‘Extracorporeal adsorption of low and medium sized molecular, hydrophobic substances (including cytokine adsorption)’ was used. In total, 16,720 coded adsorption treatments were identified and coded throughout 94 medical and surgical DRGs of various complexity. We combined data on coding of the hemoadsorption procedure with data on the four surgical DRGs for IE patents requiring heart valve surgery to select 634 cases where the procedure code 8-821.2 was used. There were no other relevant surgical DRGs where the diagnosis codes for infective endocarditis were used in combination with the procedure code for hemoadsorption.
To account for possible overestimates due to use of the therapy in the post-operative setting only or to coding of other devices, the value was rounded down to 550 patients.
| |
|
IE as primary diagnosis |
IE as secondary diagnosis |
Total |
OPS code 8.821.2 |
| DRG code |
DRG label |
n |
% |
n |
% |
n |
n |
% |
| F03D |
Heart valve surgery with HLM, age > 0 yrs., Intensive care complex treatment <197 / 185 / - points, with double surgery or congenital heart defect, without complex surgery or age < 16 yrs. or without double surgery, except congenital heart defect, age > 15 years. with implanted valve-bearing vascular prosthesis |
588 |
9.2 |
594 |
9.29 |
1182 |
178 |
1,32 |
| F03A |
Heart valve surgery with HLM, with certain complicating constellation or certain double surgery |
240 |
3.75 |
268 |
4.19 |
508 |
216 |
1,6 |
| F03C |
Heart valve surgery with HLM, age > 0 yrs., Intensive care complex treatment > 196 / 184 / - points and Intensive care complex treatment < 393 / 369 / - points with double surgery or congenital heart defect, with complex surgery or pre-existing heart valve surgery or other complicating constellation |
227 |
3.55 |
230 |
3.6 |
457 |
130 |
0,96 |
| F03B |
Heart valve surgery with heart-lung machine, with multiple surgeries or age < 1 year or surgery in deep hypothermia or Intensive care complex treatment > 392 / 368 / - points or certain other complicating constellation or pulmonary endarterectomy |
161 |
2.52 |
181 |
2.83 |
342 |
110 |
0,81 |
References
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Prendergast, B.D.; Tornos, P. Surgery for infective endocarditis: who and when? Circulation 2010, 121, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, J.J.P.; Noordermeer, D.J.; van Leeuwen, W.J.; Verkaik, N.J.; Lattwein, K.R. Native valve, prosthetic valve, and cardiac device-related infective endocarditis: A review and update on current innovative diagnostic and therapeutic strategies. Front Cell Dev Biol 2022, 10, 995508. [Google Scholar] [CrossRef]
- Boyle, E.M., Jr.; Pohlman, T.H.; Johnson, M.C.; Verrier, E.D. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg 1997, 63, 277–284. [Google Scholar] [CrossRef]
- Farag, M.; Borst, T.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Arif, R.; Beller, C.J.; Popov, A.F.; Kallenbach, K.; Ruhparwar, A.; et al. Surgery for Infective Endocarditis: Outcomes and Predictors of Mortality in 360 Consecutive Patients. Med Sci Monit 2017, 23, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Sonneville, R.; Hajage, D.; Novy, E.; Tubach, F.; Vignon, P.; Perez, P.; Lavoué, S.; Kouatchet, A.; Pajot, O.; et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur Heart J 2014, 35, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Haidari, Z.; Wendt, D.; Thielmann, M.; Mackowiak, M.; Neuhauser, M.; Jakob, H.; Ruhparwar, A.; El-Gabry, M. Intraoperative Hemoadsorption in Patients With Native Mitral Valve Infective Endocarditis. Ann Thorac Surg 2020, 110, 890–896. [Google Scholar] [CrossRef]
- Soltesz, A.; Molnar, Z.A.; Szakal-Toth, Z.; Tamaska, E.; Katona, H.; Fabry, S.; Csikos, G.; Berzsenyi, V.; Tamas, C.; Edes, I.F.; et al. Influence of Venoarterial Extracorporeal Membrane Oxygenation Integrated Hemoadsorption on the Early Reversal of Multiorgan and Microcirculatory Dysfunction and Outcome of Refractory Cardiogenic Shock. J Clin Med 2022, 11, 6517. [Google Scholar] [CrossRef] [PubMed]
- Hawchar, F.; Tomescu, D.; Trager, K.; Joskowiak, D.; Kogelmann, K.; Soukup, J.; Friesecke, S.; Jacob, D.; Gummert, J.; Faltlhauser, A.; et al. Hemoadsorption in the critically ill-Final results of the International CytoSorb Registry. PLoS One 2022, 17, e0274315. [Google Scholar] [CrossRef] [PubMed]
- Traeger, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass - a case series. Int J Artif Organs 2017, 40, 240–249. [Google Scholar] [CrossRef]
- Kuhne, L.U.; Binczyk, R.; Riess, F.C. Comparison of intraoperative versus intraoperative plus postoperative hemoadsorption therapy in cardiac surgery patients with endocarditis. Int J Artif Organs 2019, 42, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Holmen, A.; Corderfeldt, A.; Lannemyr, L.; Dellgren, G.; Hansson, E.C. Whole Blood Adsorber During CPB and Need for Vasoactive Treatment After Valve Surgery in Acute Endocarditis: A Randomized Controlled Study. J Cardiothorac Vasc Anesth 2022, 36, 3015–3020. [Google Scholar] [CrossRef] [PubMed]
- MicrosoftCorporation. MicrosoftCorporation Microsoft Excel 16. Microsoft Corporation: Redmond, WA, USA, 2019. [Google Scholar]
- IneK. Hilfestellung für die Kalkulation von Zusatzentgelten gem. § 6 Abs. 1 KHEntgG und Zusatzentgelten für neue Untersuchungs- und Behandlungsmethoden gem. § 6 Abs. 2 KHEntgG. 2005.
- Martin, J.; Neurohr, C.; Bauer, M.; Weiss, M.; Schleppers, A. [Cost of intensive care in a German hospital: cost-unit accounting based on the InEK matrix]. Anaesthesist 2008, 57, 505–512. [Google Scholar] [CrossRef]
- Reimbursement.info. Data on inpatient staya. Available online: https://reimbursement.info (accessed on 10 March).
- Briggs A., S.M., Claxton, Karl. Decision Modelling for Health Economic Evaluation; Oxford University Press: 2006.
- InEK. OPS catalogue 2023, BfARM and DRG catalogue 2023. Available online: https://www.bfarm.de/EN/Code-systems/Classifications/OPS-ICHI/OPS/_node.html (accessed on 10 March).
- Bittner, M.I.; Donnelly, M.; van Zanten, A.R.; Andersen, J.S.; Guidet, B.; Trujillano Cabello, J.J.; Gardiner, S.; Fitzpatrick, G.; Winter, B.; Joannidis, M.; et al. How is intensive care reimbursed? A review of eight European countries. Ann Intensive Care 2013, 3, 37. [Google Scholar] [CrossRef]
- Tan, S.S.; Bakker, J.; Hoogendoorn, M.E.; Kapila, A.; Martin, J.; Pezzi, A.; Pittoni, G.; Spronk, P.E.; Welte, R.; Hakkaart-van Roijen, L. Direct cost analysis of intensive care unit stay in four European countries: applying a standardized costing methodology. Value Health 2012, 15, 81–86. [Google Scholar] [CrossRef] [PubMed]
- ZHaidari, Z.; Leiler, S.; Mamdooh, H.; Fittkau, M.; Boss, K.; Tyczynski, B.; Thielmann, M.; Bagaev, E.; El Gabry, M.; Wendt, D.; et al. Effect of intraoperative haemoadsorption therapy on cardiac surgery for active infective endocarditis with confirmed Staphylococcus aureus bacteraemia. Interdiscip Cardiovasc Thorac Surg 2023, 36, ivad–010. [Google Scholar] [CrossRef]
- Winters, B.D.; Eberlein, M.; Leung, J.; Needham, D.M.; Pronovost, P.J.; Sevransky, J.E. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 2010, 38, 1276–1283. [Google Scholar] [CrossRef]
- Feemster, L.C.; Cooke, C.R.; Rubenfeld, G.D.; Hough, C.L.; Ehlenbach, W.J.; Au, D.H.; Fan, V.S. The influence of hospitalization or intensive care unit admission on declines in health-related quality of life. Ann Am Thorac Soc 2015, 12, 35–45. [Google Scholar] [CrossRef]
- Scharf, C.; Weinelt, F.; Schroeder, I.; Paal, M.; Weigand, M.; Zoller, M.; Irlbeck, M.; Kloft, C.; Briegel, J.; Liebchen, U. Does the cytokine adsorber CytoSorb((R)) reduce vancomycin exposure in critically ill patients with sepsis or septic shock? a prospective observational study. Ann Intensive Care 2022, 12, 44. [Google Scholar] [CrossRef]
- Scandroglio, A.M.; Pieri, M.; Nardelli, P.; Fominskiy, E.; Calabro, M.G.; Melisurgo, G.; Ajello, S.; Pappalardo, F. Impact of CytoSorb on kinetics of vancomycin and bivalirudin in critically ill patients. Artif Organs 2021, 45, 1097–1103. [Google Scholar] [CrossRef]
- Medizinfuchs.de. Vancomycin Dr. Eberth 500 mg, preis 10 Stk. Available online: https://www.medizinfuchs.de/preisvergleich/vancomycin-dr.-eberth-500-mg-10-st-dr.-friedrich-eberth-arzneimittel-gmbh-pzn-12472460.html?mft_package (accessed on 10 March).
- Haidari, Z.; Demircioglu, E.; Boss, K.; Tyczynski, B.; Thielmann, M.; Schmack, B.; Kribben, A.; Weymann, A.; El Gabry, M.; Ruhparwar, A.; et al. Intraoperative hemoadsorption in high-risk patients with infective endocarditis. PLoS One 2022, 17, e0266820. [Google Scholar] [CrossRef] [PubMed]
- Asch, S.; Kaufmann, T.P.; Walter, M.; Leistner, M.; Danner, B.C.; Perl, T.; Kutschka, I.; Niehaus, H. The effect of perioperative hemadsorption in patients operated for acute infective endocarditis-A randomized controlled study. Artif Organs 2021, 45, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Lehmann, T.; Bothe, W.; Akhyari, P.; Platzer, S.; Wendt, D.; Deppe, A.C.; Strauch, J.; Hagel, S.; Gunther, A.; et al. Cytokine Hemoadsorption During Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results From a Multicenter Randomized Controlled Trial. Circulation 2022, 145, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Kalisnik, J.M.; Leiler, S.; Mamdooh, H.; Zibert, J.; Bertsch, T.; Vogt, F.A.; Bagaev, E.; Fittkau, M.; Fischlein, T. Single-Centre Retrospective Evaluation of Intraoperative Hemoadsorption in Left-Sided Acute Infective Endocarditis. J Clin Med 2022, 11, 3954. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).