1. Introduction
With the rapid development of technology, high-performance batteries such as lithium-ion [
1], sodium-ion [
2,
3], potassium-ion [4-6], zinc-ion [
7,
8], and lithium-sulfur [
9,
10] batteries have been widely used in different areas, bringing great convenience to human daily life. The battery is always constructed by electrode, electrolyte, separator, etc., among which the electrode material is the critical direction of battery property. The current battery anode materials are mainly carbon materials [
11], lithium metal [
12], lithium alloy [
13], silicon-based [
14], tin-based [
15], nitride [
16], and other materials. However, the standard battery electrodes are often subject to high cost, have a significant environmental pollution and other disadvantages. Therefore, developing new electrode materials with environmental friendliness is urgently needed.
Thanks to abundant raw materials and low-cost and easy-processing advantages, biomass has begun to be used in security [
17,
18], medicine [
19], energy and other fields. In the field of energy biomass carbon materials present the most significant potential [
20] for commercialized electrode materials. Especially hard carbon materials which contain diverse structures, large layer spacing, a high number of micropores and defects may be capable of becoming high-quality electrodes. Hard carbon materials [
21] are mainly derived from biomass, natural macromolecules [
22], artificial macromolecular polymers [
23] and organic carbon-rich materials, such as wood [
24], algae [
25], leaves [
26,
27], peel waste [
28], and plant residues [
29]. Compared with traditional electrode materials, biomass carbon electrodes have cost advantages and can generate substantial economic benefits, while effectively reducing environmental hazards and energy problems.
Thus, this paper aims to summarize the recent development of electrode materials based on biomass carbon materials, including the preparation methods of common biomass carbon materials and the applications and enhancement mechanisms in different batteries. Finally, in-depth views and perspectives on the current problems of biomass carbon material electrode development will also be presented.
2. Preparation of bio-based carbon
Biomass materials are widely distributed in nature with constituent elements of carbon, accompanied by sulfur, nitrogen and phosphorus. Different biomass-derived carbon materials exhibit unique physical and chemical structures, such as large specific surface areas [
30] and large microporous or mesoporous structures conducive to the diffusion and transfer of ions [
31]. Commonly used methods for preparing biomass-derived carbon materials include high-temperature carbonization [
32], hydrothermal [
33], template [
34], and physical [
35]/chemical [
36] activation methods.
2.1. High-temperature carbonization
High-temperature pyrolysis is the high-temperature cracking (between 400-1200°C) of organic matter [
37] or biomass into carbon materials under an inert gas atmosphere. This method is the simplest way to prepare carbon materials from biomass, but the pyrolysis temperature strongly influences the yield and performance of carbon materials. The yield of low temperature pyrolysis is high, while the crystallinity of carbon produced by pyrolysis is lower than that of high temperature cracking. In addition, the heating rate and the type of inert gas will affect the carbonization of biomass materials. Therefore, finding the right pyrolysis conditions is crucial for the performance of carbon materials. The pyrolysis process also causes the loss of nitrogen and sulfur elements, which can affect the application of the obtained biomass carbon in batteries and may lead to a decrease in electrical capacity. Biomass carbon is often prepared to improve the property by combining two or more methods. Song et al. [
38] utilized corn cob as raw material to prepare biomass carbon at different pyrolysis temperatures (1000 °C, 1200 °C, 1400 °C, and 1600 °C), the prepared sample showed higher graphitization and an increase in the number of layers as the pyrolysis temperature increased. Luca et al. [
39] used a heating rate of 10°C/min to pyrolyze the corn cob at 950 °C. The biomass carbon was used as the anode material for the battery, while carboxymethyl cellulose extracted from the corn cob chemically be used as a binder in the battery preparation. The corn cob-derived electrode provides a charge/discharge capacity of 264 mAh g
-1 at 1C (300 mAh g
-1) and good capacity retention. Most biomass materials can be carbonized by high-temperature pyrolysis, a simple process. However, industrial production of biomass carbon inevitably consumes much energy, and high-temperature carbonization will have by-products such as methane, ethylene, acetylene, aromatic compounds, and hydrocarbons, which will pollute the environment with emissions and increase the cost of sorting and disposal, which are the problems that high-temperature pyrolysis of biomass carbon will face in the future.
Although the high-temperature pyrolysis process is simple and convenient, the prepared carbon materials will receive low porosity, thus limiting the further application of biomass carbon materials in battery electrodes.
2.2. Hydrothermal method
Hydrothermal carbonization is a simple method of preparing biomass carbon materials with mild reaction conditions. The carbonization is usually carried out in a confined space with water as the medium by increasing the temperature and pressure. Compared with high temperature cracking, the hydrothermal method can complete the carbonization process at a relatively low temperature. The hydrothermal method can significantly enrich the structure of biomass carbon materials by adjusting the type of reactants to change the surface group or atomic doping kind of carbon materials. The carbon materials prepared by the hydrothermal method generally have very high oxygen content, which can be reduced by heat treatment, and the oxygen-containing functional groups, when heat treated will further enhance the porosity, specific surface area, and electrical conductivity of the carbon materials. Chen et al. [
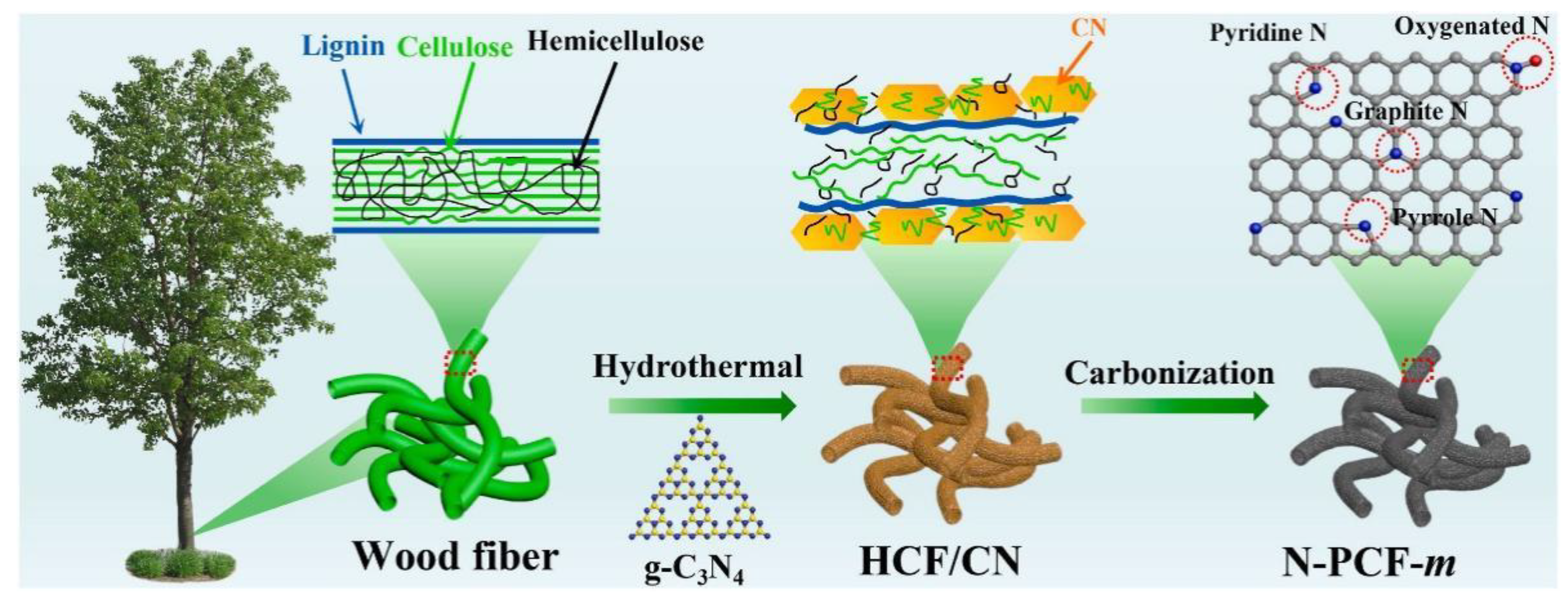
40] prepared nitrogen-doped carbonized porous carbon fibers (N-PCF) by an in situ sacrificial template-assisted hydrothermal method using graphitic carbon nitride (g-C
3N
4) as a template. The preparation process is shown in Fig.1. The hydrothermal treatment resulted in an excellent specific surface area and atomic doping of N-PCF, which enabled N-PCF-2 to have a capacity of 434 mAh g
-1 at 200 mA g
-1 after 300 cycles in a lithium-ion battery. After 300 cycles in sodium-ion batteries, it has a capacity of 266 mA g
-1 at 200 mA g
-1, showing excellent stability and specific capacity. Chen et al. [
41] used a two-step hydrothermal method to construct porous carbon microspheres based on camellia shells. The unique pores allow for excellent cycling stability to sodium and potassium ions, and the excellent Na
+/K
+ storage capacity enables it to have a specific capacity of 250 Similarly and 264.5 Similarly at a current density of 100 mAg
-1 in sodium and potassium ion batteries, respectively.
Figure 1.
Schematic diagram of the synthetic procedure for N-PCF-m. Reprinted with permission from Ref [
39], Copyright 2022 Elsevier.
Figure 1.
Schematic diagram of the synthetic procedure for N-PCF-m. Reprinted with permission from Ref [
39], Copyright 2022 Elsevier.
Hydrothermal is a very widely used carbonization method for common biomass feedstocks such as cellulose, fruit peels, animal shells, glucose, etc. can be effectively carbonized to prepare biomass-derived carbon materials. Its controlled reaction products and by-products can be quickly recovered and the products' structure, morphology and surface groups can be targeted and controlled, which may be the best method for preparing biomass-derived carbon materials.
2.3. Methods for activating biomass-derived carbon
Activation is a pretreatment process that uses different activators or gases to act on the biomass carbon material to create other structures such as microporous, mesoporous, and microporous. The purpose of activation is to create porous structures [
42], which can increase both the porosity and the pore size of the carbon material. The steps of activation can be divided into two steps, carbonization and activation. Carbonization is the conversion of biomass precursors into carbon materials through high temperatures. At the same time other elements in biomass can be converted into small volatile, which escape from the carbon material and thus form pores inside the carbon material. Activation is an integral part of the morphology control process, and the methods generally include physical activation and chemical activation.
Physical activation: Physical activation can also be divided into two stages. The first stage is the thermal decomposition of the biomass precursor, in which the biomass precursor is entirely or partially carbonized and the non-carbon elements somewhat disappear. The second stage is activating the carbon material using water vapor, carbon dioxide or air. The formation of pores originates from the volatilization and expansion of carbon vapor, causing a dramatic increase in specific surface area. The pore size can be controlled by controlling the activation temperature, gas flow rate, and activation time to prepare biomass carbon materials with particular pore size. Farma et al. [
43] used controlled activation temperature to activate biomass carbon electrodes derived from rubber seed shells (RSS) with sample codes RSS-650, RSS-750, and RSS-850 refer to the activation process at temperatures of 650 °C, 750 °C and 850 °C, respectively, where RSS-750 had the highest specific surface area with a uniform distribution of mesoporous structures and RSS-750 had the best electrochemical performance. Physical activation improves the specific surface area and pore size of carbon materials, but it isn’t easy to control precisely because of the high requirements of experimental conditions. In addition, although the physical activation is easy, it can reduce biomass carbon yield, affecting the large-scale use of biomass carbon materials.
Chemical activation: Unlike physical activation, chemical activation requires the action of chemical reagents. In the chemical activation process, the activator penetrates inside the biomass precursor and a cross-linking condensation reaction occurs between the internal structure of the biomass feedstock and the activator. Its dehydration and oxidation occur during the carbonization process, resulting in a microporous structure on the surface. Chemical activation allows for a wider variety of activators and the introduction of specific functionalized atoms. Similarly, to physical activation, factors affecting the activation properties of carbon materials include temperature, amount of activator, and property of the activation agent. Hernández-Rentero et al. [
44] investigated the properties of carbon materials prepared by de-activating cherry nuclei with phosphoric acid and KOH, respectively. Cherry kernels were treated with KOH and phosphoric acid before carbonization, dried and annealed under a nitrogen atmosphere for 2 h, followed by washing with hydrochloric acid to obtain the activated biomass carbon materials. The results showed that the carbon materials treated with phosphoric acid had 40% higher specific surface area than those treated with KOH and were micro/mesoporous, not micro/macroporous. Both materials exhibited very stable and reversible capacities with a regular capacity of 200 mA h g
-1, and the full cell assembled with both materials provided very desirable capacities close to the theoretical specific capacity with a cycle life of up to 200 cycles and an initial Coulomb efficiency close to 100%. The higher efficiency of chemical activation requires gentler conditions than physical activation, but the excessive use of the same chemicals poses some contamination, equipment damage, and safety concerns.
3. Factors affecting the application of biomass carbon materials in batteries
The natural structure of biomass is graded and ordered, and the composition is diversified, making it an available raw material for preparing electrode materials with controlled morphology and excellent performance. Biomass-derived carbon materials can be divided into hard carbon, soft carbon and hybridized carbon in terms of structure; nitrogen-doped carbon and other atomic doping in terms of elemental composition; and 0D, 1D, 2D and 3D morphology in terms of morphology. The pore can be divided into microporous, mesoporous and macroporous carbon materials. Different structures and morphologies bring other performance. For different cells, choose to regulate the proper morphology to obtain the biomass-derived carbon material electrode with the best performance.
3.1. Structure and morphology
The precursors types can be divided into plant- and animal-based biomass. The main components of plant bodies are cellulose, hemicellulose, lignin, starch, pectin, glucose, etc. Animal biomass generally refers to animal shells, wastes, etc. Its composition is quite complex, including rich proteins, amino acids, minerals, as well as oil and grease components. The structure and morphology of biomass-derived carbon materials depend mainly on the type of precursor materials[
45]. Their morphology is derived chiefly from the morphology of the precursors themselves. The high-temperature carbonization process tends to maintain the precursor morphology of carbon materials. Hence, a suitable choice of biomass precursor type is one of the ways to obtain the ideal morphology. For example, 0D carbon materials are often in the form of carbon nanospheres. Yu et al. [
46] used corn stover as raw material and combined with chemical activation to prepare carbon nanospheres, which exhibited a porous structure and excellent electrochemical properties, resulting in high capacity and stability. The carbon nanospheres have a reversible ability of 546 mA h g
-1 after 100 cycles at 0.2C. 1D carbon materials are often prepared from tubular [
47] and fibrous precursors, such as plant roots and leaves with unique tubular structures. The high aspect ratio and effective permeable inner surface of tubular carbon materials facilitate the adsorption of sodium ions and fast electron transport. The electrolyte can penetrate from the surface to the interior of the electrode through the tubular structure. The thinnest microtubules (carbonized at 1600°C) can be prepared, which reduces the diffusion energy barrier of Na
+ and enhances the reversibility of Na
+ storage. The prepared carbon material has an initial Coulomb efficiency of 90%, a capacity retention of 89.4% and excellent multiplicative performance after more than 100 cycles at 100 mAg
-1. 2D biomass carbon materials have substantial specific surface area [
48] and tunable layer spacing [
49]. 2D nanomaterials can both wrap (Yin et al. [
50]) anode nanoparticles to enhance the conductivity of electrodes and improve the multiplicative performance and cycle life of batteries. For example, Asfaw et al. [
51] prepared hard carbon nanosheets from oxidized cork. The authors found that the increase in synthesis temperature could enhance the specific surface area and (002) layer spacing (~4.0-3.7 Å) of the hard carbon material, further increasing the Coulomb efficiency from 72% to 88%. The carbon nanosheets carbonized at high temperatures have excellent electrochemical properties with a reversible capacity of 276 mA h g
-1 at 20 mA g
-1 and an iCE(initial Coulombic Efficiency) of ~88%. Although the two-dimensional carbon nanomaterials have many advantages, their practical applications in batteries are not considered ideal, and the preparation strategy needs to continue to be adjusted to enhance their electrochemical performance further.
Thanks to the highly connected internal network and abundant ion/electron transport channels, 3D carbon materials tend to have excellent electrochemical properties. The 3D structure can provide continuous channels for electrolyte penetration throughout the system. Zhao et al. [
52] prepared 3D honeycomb carbon materials with large microporous structures (less than 0.7 nm) by annealing etching. The prepared materials were used in sodium-sulfur batteries with excellent initial capacity at 0.2 C (1413 mA h g
-1), excellent cycling stability (822 mA h g
-1 after 100 cycles at 0.2 C), and excellent multiplicative performance (capacity at 3.0 C 483 mA h g
-1). This shows that the design of the structure of the carbon material for different needs is crucial at every moment, starting from the precursor type, and a reasonable design can bring excellent electrochemical performance.
3.2. Porosity
The porosity and pore configuration of a material can affect the bulk density, strength, water absorption, impermeability, frost resistance, thermal conductivity, and other properties of the material. For biomass carbon materials, porosity is undoubtedly a crucial influencing factor [
53]. The number, size and shape of the pores affect the storage capacity [
54] of ions and the diffusion rate. The storage mechanism of ions in biomass carbon materials consists of three components: absorption/desorption, insertion/extraction between carbon layers [
55], and filling of nanopores [
56] except for the ion embedding space provided by the carbon material itself. According to Yan et al. [
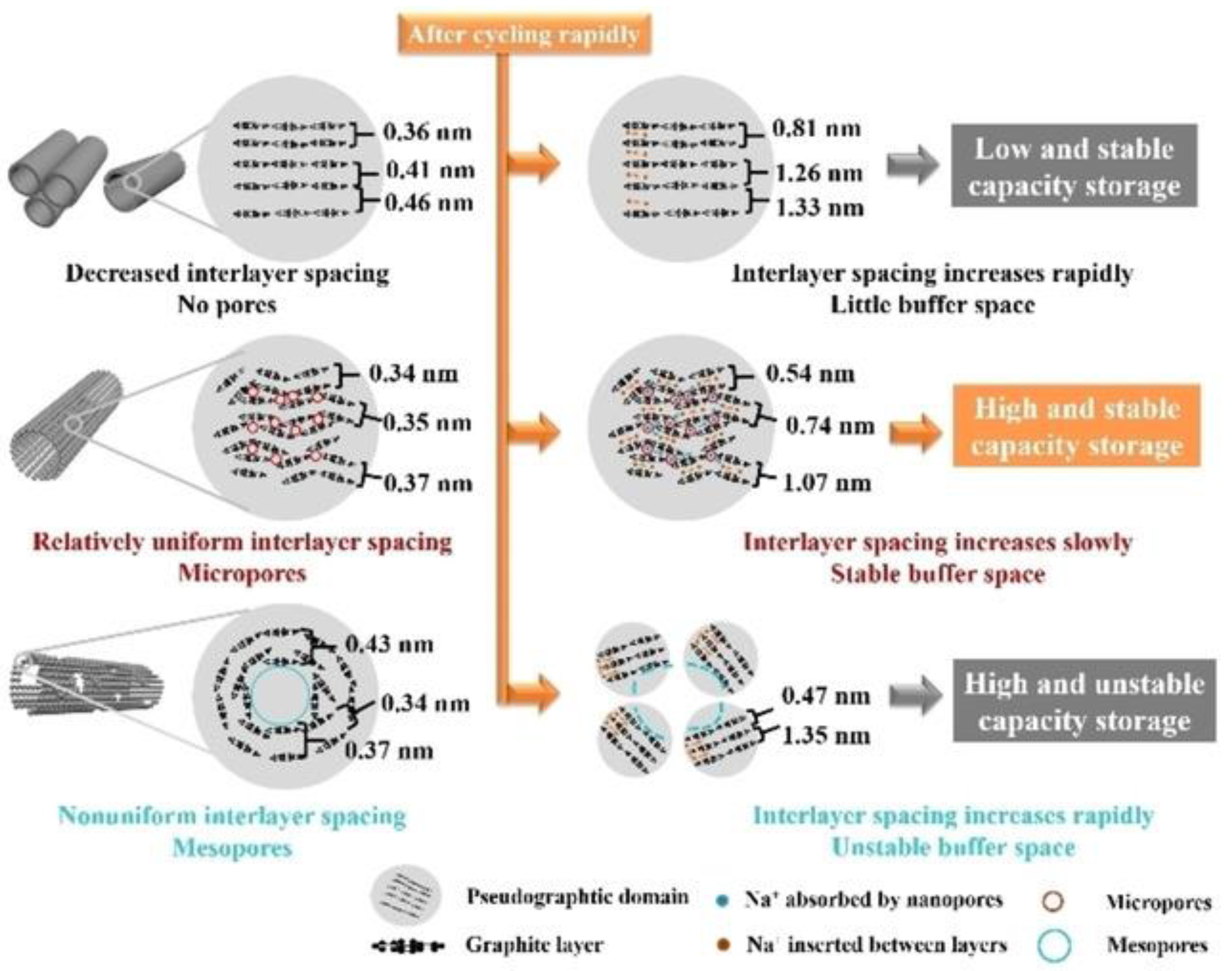
57], different pore sizes have other labor divisions. Where micropores mainly provide adsorption/desorption sites for electrolyte ions, mesopores accelerate ion transport and diffusion macropores usually serve as storage pools for electrolytes. Understanding the functions of various-sized pores will enable a more targeted structural design of biomass-derived carbon materials to achieve their excellent performance in batteries. Wang et al. [
58] prepared biomass carbon materials with different size pores using three other methods. The BET results showed that the specific surface area of carbon materials with microporous pores was as high as 1453 cm
2 g
-1 and 844 cm
2 g
-1 for carbon materials with mesoporous structures. In comparison, carbon nanotubes had a specific surface area of only 10 cm
2 g
-1. There are other differences between the three carbon materials, as shown in Fig. 2.
Figure 2.
Sodium-ion intercalation mechanism with different pore structures. Reprinted with permission from Ref [
57], Copyright 2022 John Wiley and Sons.
Figure 2.
Sodium-ion intercalation mechanism with different pore structures. Reprinted with permission from Ref [
57], Copyright 2022 John Wiley and Sons.
The carbon nanotubes with microporous structure exhibited the best overall performance in the electrochemical test. Microporous carbon tubes have more uniform layer spacing and uniform size distribution of microporous structure than the other two, giving the best multiplicative performance. This means that the smaller the pore size is, the better the cell performance is, but also the uniformity of the pores and the uniformity of the layer spacing. Carbon materials with a predominantly microporous structure have more abundant ion adsorption/desorption sites and a higher initial specific capacity, but do not have enough ion transport channels and the particular capacity decays rapidly with increasing current density. Although ions are rapidly transferred in carbon-material mesopores, the lack of ion adsorption/desorption sites leads to low initial specific capacity. In addition, although the large pores can act as a buffer pool for ions, reducing the diffusion distance of ions, its limited increase in capacity and reduce the material's vibrational density. Yu et al. [
59] used buckwheat hull as a raw material and calcium chloride as an activator via carbonization to prepare porous carbon. The porous carbon and buckwheat hull morphology consistent with the calcium chloride treatment has more and larger pores, resulting in enhanced capacity. It is confirmed that the size and distribution of pores can affect the specific ability of carbon materials. In summary, it is necessary to develop carbon materials with well-layered and uniform pore structures by rationalizing nanopore size and pore ratio design.
3.3. Defect
The defect is an effective means [
60] to enhance the performance of carbon materials as electrodes. The introduction of heterogeneous doping elements or atoms in the nanostructure of carbon materials exposes many unsaturated sites on the surface [
61] or inside the carbon material, increasing the adsorption capacity and storage capacity for ions. At the same time, the large number of defects can increase the diffusion rate of ions and charge transfer rates [
62]. In recent years, scholars have found that the presence of defects [
63] can improve electrical conductivity, cycling stability, and multiplicative performance. Doping atoms tend to form many defects inside carbon materials, and doping nitrogen, oxygen, phosphorus, and sulfur atoms is the most commonly used method to enhance the performance of biomass carbon materials. Zhang et al. [
64] prepared graphitic nanocarbon (GNC) with controlled defect concentration by annealing nickel ethylenediaminetetraacetic acid coordination compounds. GNC was obtained by nitrogen doping with a large number of surfaces GNC exhibits excellent performance in potassium ion batteries, with a stable specific capacity of 280 mA h g
-1 at a current density of 50 mA g
-1 and excellent multiplicative performance and long cycle life. Besides, a high capacity of 189 mA h g
-1 at a current density of 200 mAg
-1 remains with a long cycle time of over 200 cycles. The defect or elemental doping in biomass carbon-derived materials can be derived from elements it possesses or doped using exotic elements. Meng Zhang[
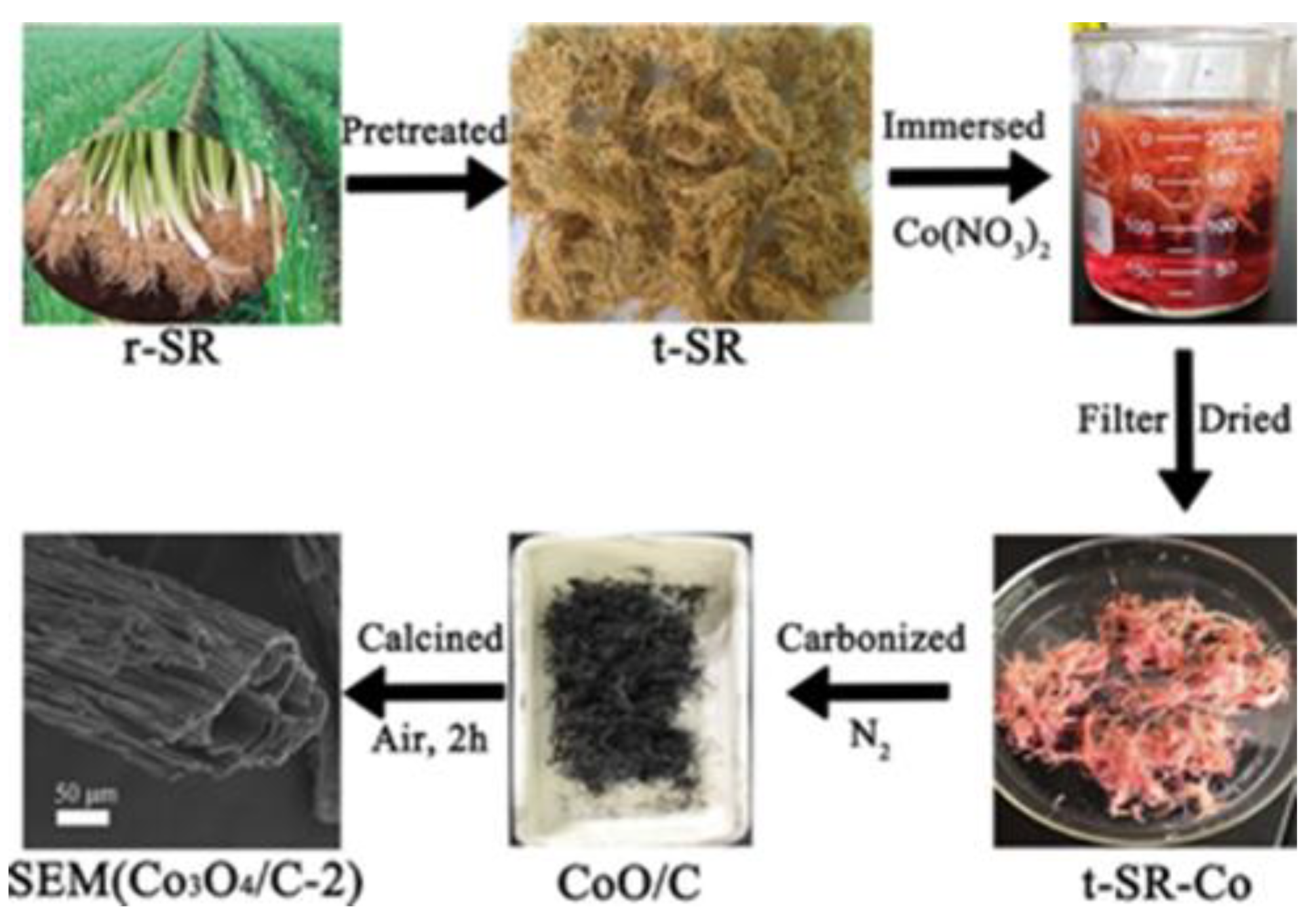
65] et al. prepared graphitic carbon-doped mesoporous Co
3O
4 nanocomposites from waste onion root by simple cobalt salt impregnation, carbonization and low-temperature oxidation, and the biomass-derived carbon materials were cross-linked with Co
3O
4 nanoparticles to form high-density oxygen vacancies in lithium-ion batteries, as shown in Fig.3. The presence of oxygen vacancies can create a built-in electric field in the carbon material, which can act as a carrier for ion transport, accelerate the rapid migration of ions and electrons, and increase the active sites on the material surface and the pseudocapacitance behavior. The pseudocapacitance behavior effectively enhances the electrochemical performance of Co
3O
4/C electrodes. Nkongolo Aristote et al. [
66] studied carbon materials derived from camphor. They doped them with a series of diatomic and triatomic doping with a combination of phosphorus-nitrogen, phosphorus-sulfur, nitrogen-sulfur and phosphorus-nitrogen-sulfur, respectively. The authors found that the phosphorus-nitrogen-sulfur co-doped carbon materials exhibited excellent initial Coulomb efficiency (70.74%), multiplicative performance and cycling stability. This is attributed to the synergistic effect of nitrogen-phosphorus-sulfur atomic doping that widens the interlayer spacing of the carbon material, thus increasing the rate of sodium ion desorption/adsorption and the storage capacity of sodium ions. At the same time, sulfur can react reversibly with sodium ions, hindering the irreversible consumption of sodium ions and increasing the stability of battery capacity.
Figure 3.
Synthesis procedure of hard carbon nanosheets from cork and chemical analyses. Reprinted with permission from Ref [
64], Copyright 2021 American Royal Society.
Figure 3.
Synthesis procedure of hard carbon nanosheets from cork and chemical analyses. Reprinted with permission from Ref [
64], Copyright 2021 American Royal Society.
Doping with non-metallic elements is one of the more commonly used methods for modifying carbon materials. Currently, it performs well in the battery field, but the current doping of non-metallic elements has limited potential to improve the electrochemical performance of the material. The doping of metal atoms has a more significant potential for application. The doping of carbon materials with metal atoms [
67] has been proven to be feasible in the field of catalysis, and may also have great potential in the area of batteries. obtain excellent electrochemical properties.
4. Specific applications of biomass-derived carbon in batteries
4.1. Individual biomass carbon materials for battery applications
Biomass carbon exhibits high specific capacity, good cycling performance and high first discharge efficiency when used as battery electrode material. Sun et al. [
68] prepared a carbon material with unique mechanical and surface structure by treating silk waste with a simple high-temperature carbonization method, avoiding using binders and organic solvents. The initial Coulomb efficiency in sodium-ion batteries was 75.6% and the capacity retention after 100 cycles was 100%. The high capacity and stability can be attributed to the additional adsorption of sodium ions by the nitrogen/oxygen active sites. In addition, the interlayer spacing of the carbon material increases and the number of defects increases positively with the carbonization temperature. Dong Wang[
69] et al. used the biomass material chitosan to prepare a large-size flexible carbon film with a unique honeycomb structure and nitrogen-doped structure, which is also used as a standalone electrode for various batteries without binder and conductive agent. This biomass carbon film has excellent performance in potassium and sodium ion batteries with a reversible capacity of 146 mA h g
-1 (2 A g
-1 after 500 cycles) and 236 mA h g
-1 (after 70 cycles), respectively. The honeycomb pores and pyridine N doping improved the adsorption capacity of potassium ions to sodium ions and its electrical conductivity, and the surface structure was decisive for the performance of biomass-derived carbon materials. Wang et al. [
70] used a two-step method of hydrothermal and high-temperature carbonization to convert reed straw into hard carbon. They applied it to sodium-ion batteries, and the hard carbon material obtained by carbonization at 1300 °C had an excellent reversible. The hard carbon material obtained by carbonization at 1300 °C has a unique reversible capacity of 372.0 mA h g
-1, an initial coulombic efficiency of 77.03 %, and excellent cycling stability. The outstanding performance is due to the high order of magnitude of microporosity, defect, and high interlayer spacing. The micropores and defect increase the ion storage capacity, and the high interlayer spacing increases the diffusion rate of sodium ions. In addition, Ren et al. [
71] prepared peanut shell-derived slatted hard carbon materials using a two-step hydrothermal method with high-temperature carbonization and direct carbonization, respectively, and compared their properties. It was found that hydrothermal treatment has a significant influence on the morphology, lattice spacing, and defect concentration of carbon materials, which can increase the sites for sodium ion adsorption and increase the sodium ion transport efficiency. The best overall performance was obtained for carbon materials subjected to hydrothermal treatment for 4 h, with a reversible capacity of up to 256 ± 5 mA h g
-1 at a current of 0.1 C and a capacity retention rate of 97 ± 2 % after 100 cycles. 100 mA h g
-1 at 5.0 C. Although biomass carbon materials alone have a high specific capacity, they often face problems such as poor stability and structural design difficulties.
4.2. Atom-doped biomass carbon materials for battery applications
To further enhance the performance of biomass carbon materials, atomic doping has become one of the most widely used methods. Atomic doping can modulate the active surface sites [
72] of biomass carbon materials, with oxygen [
73] nitrogen [
74] and phosphorus [
75,
76] being the most used atomic species. In a battery, electrolyte ions in the vicinity of the heteroatoms will interact with the electrodes in a Faraday interaction, which in turn enhances the pseudo-capacitive effect of the material. Nie et al. [
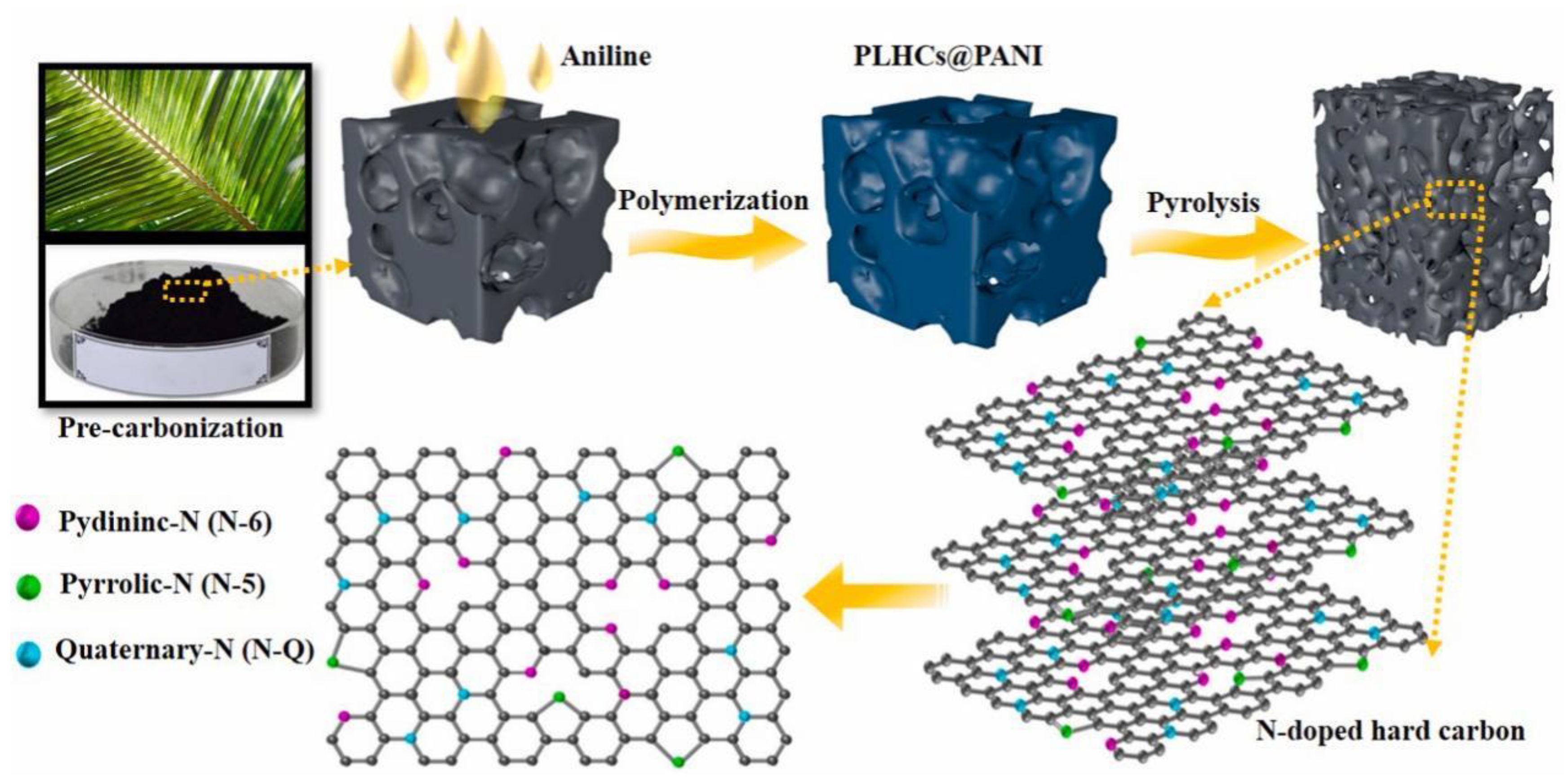
77] synthesized a high-rate and long-lived palm leaf-based hard carbon (PLHC-N) anode using palm leaf as the carbon source and polyaniline as the nitrogen source by in situ polymerization, and the preparation process is shown in Fig. 4. Thanks to the natural pore structure, PLHC-N has good Na
+ storage capacity, a large number of ion transfer channels and a good bulk buffer. Meanwhile, the N-doped material can improve the ion and electron transferability, creating more active sites for sodium storage. The PLHC-N sample has an adsorption-jack-filling storage mechanism with an ultra-high reversible capacity of 373 mA h g
-1 at 25 mA g
-1 and 200 mA g
-1 with long-term cycling stability (95.0% capacity retention at 1000 cycles). In addition, the entire cell with Na
3V
2(PO
4)
2F
3 as the cathode had good cycling capacity of 112 mA h g
-1 after 100 cycles at 0.5C (1C = 128 mA g
-1) and good capacity retention of 90.1%). Kesavan et al. [
78] showed a good capacity retention of 90.1% by Nitrogen-doped two-dimensional carbon nanomaterials (NCNS) were prepared from palm tree by high temperature carbonization and chemical activation with large surface area of 1297 m
2 g
-1, high pore volume of 0.68 cm
3 g
-1 and unique layered nitrogen-doped carbon nanosheets NCNS as anode material with specific capacities of 477 mA h g
-1 and 414 mA h g
-1 at 0.1 and 0.2 C multiplicity, respectively. 414 mA h g
-1, reaching 100% coulombic efficiency (CE) after 100 cycles.
Figure 4.
Schematic diagram for the preparation and the bonding configurations of nitrogen functionalities in the PLHC-N. Reprinted with permission from Ref [
76], Copyright 2022 Elsevier.
Figure 4.
Schematic diagram for the preparation and the bonding configurations of nitrogen functionalities in the PLHC-N. Reprinted with permission from Ref [
76], Copyright 2022 Elsevier.
The superior electrochemical properties of NCNS are attributed to its high surface area, two-dimensional nanosheet morphology, layered port structure, and good electronic conductivity after n-doping. Guo et al. [
79] used a template method to convert soymilk into ultrathin carbon nanosheets with a nitrogen-doped structure, which, when used as an anode for lithium-ion batteries, had an initial reversible specific capacity at 50 mA g
-1 1334 mA h g
-1 (1212, 555 and 336 mA h g
-1 for 0.05, 2Ag
-1,5Ag
-1, respectively) and good cycling stability (1Ag
-1 355 mAhg-1 after 1000 cycles). Xiaomin Yuan[
80] et al. prepared hierarchical nanostructured and heteroatom-doped three-dimensional porous biomass chicken bone by direct pyrolysis of carbon scaffolds (3D-HPCS) as potassium ion battery anodes, achieving excellent multiplicative capacity and cycle life. The initial capacity is 470 mA h g
-1 and 113 mA h g
-1 at a 2.0 C multiplicity. With a high capacity of 205 mA h g
-1 after 450 cycles at 0.2 C multiplicity, 3D-HPCS demonstrates excellent multiplicity performance and cycling stability with K
+ insertion/deconfinement. The superb performance results from the increased layer spacing, the synergistic effect between the active heteroatom doping and the complex porous structure, which enhances the electronic conductivity and ion diffusion rate. Atomic doping causes lattice distortion [
19] in carbon materials, producing defects or vacant sites, which increases the number of active sites, which are often preferred for ion migration, and the increase of active sites represents the increase of ion storage, which means the increase of specific capacity. In addition, doping with P and N atoms will cause changes in the built-in electric field of carbon materials. P and N atoms can be used as electron donors to promote rapid charge transfer and enhance the electrochemical activity of carbon materials. However, the concentration of P and N atoms should not be too high; if it is too high, it will cause the collapse of the structure of the carbon material and the explosive growth of the number of absences, which will reduce the electrical conductivity of the material.
4.3. Biomass Carbon Composites for Battery Applications
Although biomass-derived carbon materials have performed well in batteries, there is still much room for improving their capacity and stability, and the composite with other materials [
81,
82] is one of the ways to improve them. Zhu et al. [
83] prepared biomass-derived carbon materials from biomass corn husks and then grew CuCo
2O
4 nanowires in situ on the surface of the carbon materials to form a carbon-based composite. Nanowires to form a carbon-based composite, which was applied in a lithium-ion battery with a capacity of 887 mA h g
-1 after 250 cycles at 0.2 Ag
-1 and a coulombic efficiency of more than 99.7 % at -10 ◦C and 45 ◦C. After 120 cycles, the capacities were 726 and 700 mA h g
-1, respectively, without substantial decay. In addition, Zhang et al. [
84] used a simple hydrothermal method to anchor NiCo
2O
4 on biomass-derived carbon sheets derived from the carbonization of grapefruit peel. The composite has a high specific surface area and mesoporous structure, which is beneficial for reducing the volume expansion of the cell and enhancing ion transport simultaneously. The composite can provide a reversible capacity of 473.7 mA h g
-1 after 210 cycles at a current density of 500 mA g-1. The excellent multiplicative performance can be attributed to the uniform distribution of NiCo
2O
4 on the biomass carbon sheet and the high specific surface area, where the interface between NiCo
2O
4 and the carbon sheet accelerates the electron transfer and the curved carbon sheet attenuates the volume expansion of the cell. The vanadium oxide/biomass-derived carbon composites were prepared by using a combined salt- and ball-mill-assisted strategy and applied to lithium and sodium-ion batteries, which still provided 461.9 and 377.2 mA h g
-1 after 120 cycles at 0.5 and 1.0 Ag
-1 in lithium ion batteries with high reversible capacity and exhibited stable capacity retention in long cycle and multiplier tests [
85]. Also in sodium ion batteries with good cycling stability, 0.2 A g
-1 still has a specific capacity of 253.3 mA h g
-1 after 160 cycles. The excellent performance in both batteries is the layered porous structure brought by ball milling and salt. The layered porous structure contributes a large specific surface area and many ion transport channels, and reduces the volume expansion of the cell during practical use. Chen et al. [
86] prepared SnS
2/C and FeS
2/C anode materials from crab shells using a hydrothermal method.SnS
2/C and FeS
2/C provided capacities at current densities of 0.1 Ag
-1. It shows that the biomass carbon source has the advantages of low cost, high porosity and large specific surface area. Combining with the multiple active sites possessed by transition metal sulfides, the performance of the composites in the battery can be effectively enhanced.
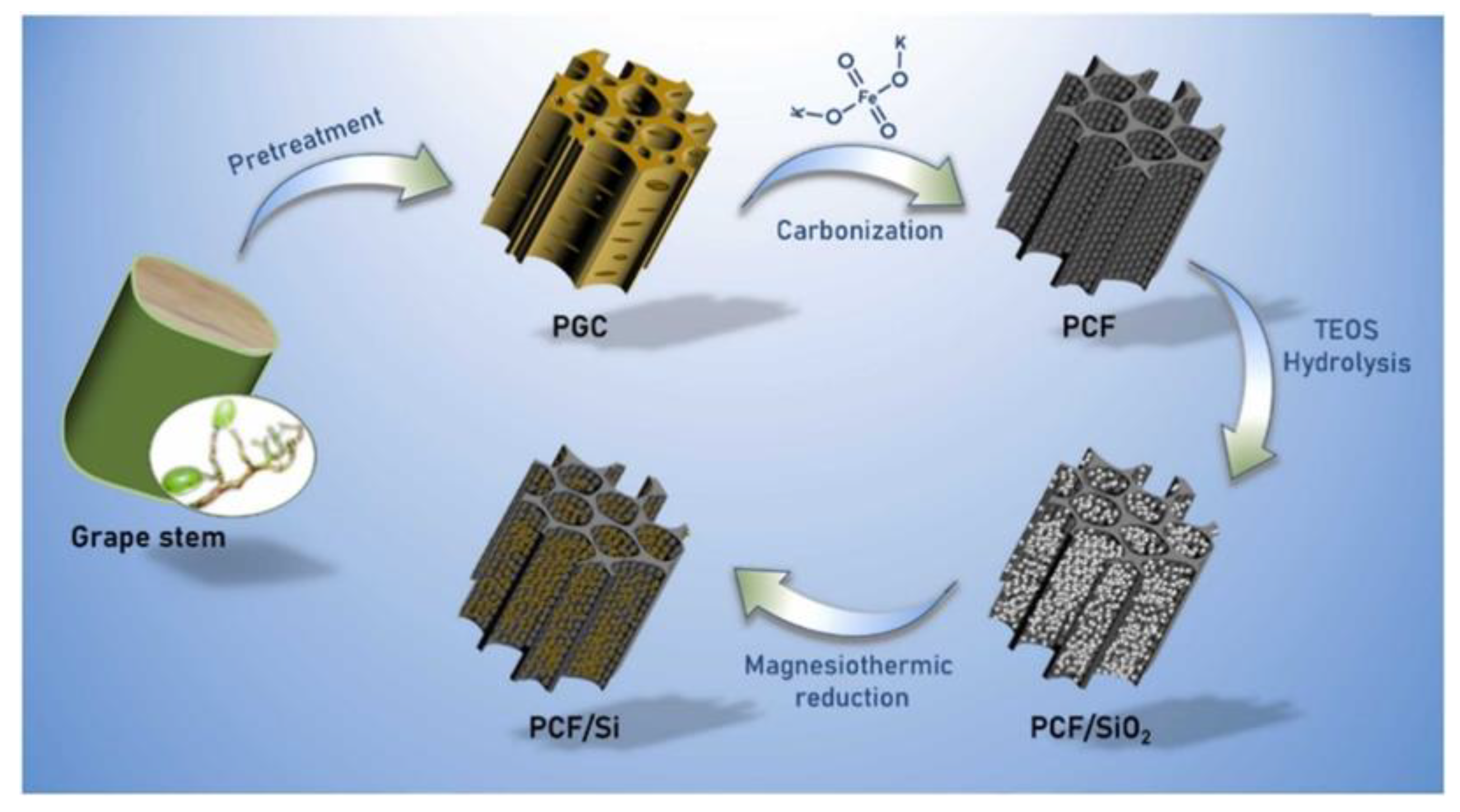
In addition to metal-based materials, silicon with ultra-high theoretical specific capacity is also a potential material in batteries. Still, silicon is limited in its application in storms because of its intrinsic insulation and susceptibility to volume expansion. In order to apply it in batteries, it can be combined with biomass carbon materials, both of which are the most abundant materials on earth and can significantly reduce the manufacturing cost of batteries. He et al. [
87] then prepared porous carbon framework (PCF) using grape stems and used it to support silicon in the process shown in Fig.5. The porous structure of this composite is an essential factor to overcome the volume expansion while providing more adsorption active sites, and the highly conductive PCF facilitates the electrolyte penetration and electron transfer. The material exhibits excellent charge/discharge capacity in Li-ion batteries, with a capacity of 1006 mA h g
-1 at a current density of 0.2Ag
-1, and the capacity remains at 891 mA h g
-1 after 400 cycles. biomass carbon materials can be used as electrodes alone or equally Biomass carbon materials can be used as electrodes alone, but also as auxiliary materials to help other materials to realize the application in batteries. In addition, biomass carbon materials can also be used in the process of material preparation to improve the overall performance of the material in the battery. Yang et al. [
88] prepared dragon fruit peels as oxygen-doped carbon materials (PCarbons) and obtained Nb
2C-PCarbons composites by ultrasonic treatment, which have dual functions. On the one hand, a high specific capacitance of up to 465.6 F g
-1 was acquired in a three-electrode system with a potassium hydroxide (6M) electrolyte. The prepared symmetric supercapacitors (SSCs) exhibited a high energy density (126.9 Wh kg
-1) in ionic liquid electrolytes and a long cycle life after 10,000 cycles of long cycle life. Meanwhile, the discharge-specific capacity in aqueous zinc ion batteries reached 239.7 mA h g
-1 with good cycling stability.
Figure 5.
Schematic illustration of the synthesis of the PCF/Si composites. Reprinted with permission from Ref [
86], Copyright 2022 Elsevier.
Figure 5.
Schematic illustration of the synthesis of the PCF/Si composites. Reprinted with permission from Ref [
86], Copyright 2022 Elsevier.
Table 1.
Summary of the properties of biomass char and its composites prepared by different strategies when used as electrode materials.
Table 1.
Summary of the properties of biomass char and its composites prepared by different strategies when used as electrode materials.
| Materials |
Biomass source |
Dopant/composite |
Battery type |
Reversible
capacity
(mA h g-1) |
Cyclic
stability
(capacity retention, %) |
Ref |
| 3D-PNC@CNTs |
Probiotics |
N/CNTs |
K+
|
458 (100 mA g-1) |
83 after 5 cycles |
[89] |
| S-BC/E-MoS2@N-C carbon core-shell nanospheres |
Bifidobacterium |
S/N/MoS2
|
K+
|
538.9 (200 mA g-1) |
68.9 after 200 cycles (200 mAg-1) |
[90] |
| HCNB |
Hyphae balls of Rhizopus |
Pt/MWNCT |
Li-S |
0.1 C 9.8 mA h cm-2
|
77 after 400 cycles |
[91] |
| GL800 |
Ganoderma |
N/O |
Li-S |
1367.8 (0.1C) |
72.3 after 300 cycles |
[92] |
| Fe2O3@C |
Peanut shell |
Fe2O3
|
Li+
|
1000.8(200 mA g-1) |
98.5 after 100 cycles |
[93] |
| NGF |
Hemp |
- |
Li+
|
806.6
(30 mA g-1
)429.2
(2000 mA g-1
|
85.0 after 45 cycles
(30 mA g-1) |
[94] |
| ZnO@PC |
Absorbent cotton |
ZnO |
Li+
|
611.0
(200 mA g-1
) |
74.85 after 880 cycles
( full-battery capacity) |
[95] |
| CCDHC |
Corn cob |
Si |
Li+
|
690 (200 mA g-1) |
Average
capacity
retention
87 |
[96] |
| PTA-700 |
Poly tannic acid |
- |
Li+
|
218(100 mA g-1) |
- |
[97] |
| HCl-1400 |
Hazelnut shell |
- |
Na+
|
306
(20 mA g-1) |
91 after 100 cycles |
[98] |
WHH-Derived
Hard Carbons. |
Waste Hemp |
|
Na+
|
267 and 79 at 0.03 and 1 A g−1
|
96 after 300 cycles
(2A g−1) |
[99] |
| SBNPk |
Sugarcane bagasse |
N/P |
Na+
|
304.1(25 mA g-1) |
96.5 after 1000 cycles
(500mA g-1) |
[100] |
| SC-800 |
Sugarcane |
- |
Na+
|
189(100 mA g-1) |
≈100 after 2000 cycles |
[101] |
| SnO2/NC |
Chitosan |
N/SnO2
|
Na+/Li+
|
557.1
(Li+) (50 mA g-1)
320
(Na+) (1.0 A g-1) |
97.7 after 300 cycles
(Li+) |
[102] |
| S-Cmph |
Camphor tree |
S |
Na+
|
145.6
(2000 mA g-1 over 500 cycles) |
93 after 100 cycles |
[103] |
5. Prospects
Biomass is an inevitable choice to take the path of sustainable development in the field of energy storage due to its abundant reserves, species, and environmentally friendly characteristics. Besides, different preparation processes endow the different properties of biomass carbon material-based electrode. The biomass carbon materials prepared by high temperature cracking always exhibited high crystallinity, but they are also accompanied by the loss of elements such as P and S and low porosity which may lower the capacity of ion battery. Also, the release of harmful gases and large consume of energy limit the development of carbon material. Physical activation and chemical activation process always need combine the different method to achieve the optimal property of the biomass material. Thus, we believe the hydrothermal method is the optimal solution for the preparation of biomass carbon materials since the process is easy to manipulate and can effectively prepare biomass carbon with target morphology.
Besides, individual biomass carbon materials were restricted to low specific capacity, poor cycling stability, difficulty resisting volume expansion, etc. To solve these problems, the emergence biomass carbon composites may be a good choice[
104], since the introduction of heteroatoms causes lattice distortion and increases the number of active sites to improve the battery's performance. Combining the advantages of other materials to make up for the shortcomings of biomass carbon materials can also effectively reduce the production cost and reduce the damage to the environment. Sustainable development is the future of social development, of which biomass is an even more important part. Research and development of biomass carbon materials, green synthesis methods, optimizing the preparation process, improving the utilization rate of biomass, and industrial production are the most important priorities for the future development of biomass carbon materials.
6. Conclusions
Carbon materials from biomass have a variety of natural structures and can meet development needs beyond the energy storage field. In this review, we concluded the recent progress of biomass carbon materials from multiple perspectives, with special attention to the effects of microstructure and macrostructure on the electrochemical properties of biomass carbon materials, and summarize the modification strategies for biomass carbon materials from the preparation process. Finally, we believe that the hydrothermal method will be the most promising method for preparing the biomass carbon materials. In addition, to achieve optimal performance, biomass carbon often needs to be complexed with other atoms, which can synergize with other atoms to achieve optimal performance in ion batteries. The ion batteries can be optimized by the synergistic effect of biomass carbon.
Author Contributions
Qiankun Zhou: Writing - Original draft preparation, Conceptualization. Wenjie Yang: Writing - Review & Editing. Lili Wang: Supervision, Funding acquisition. Chunxiang Wei: Writing - Review & Editing. Hongdian Lu: Writing - Review & Editing. Hong-Dian Lu: Writing - Review & Editing. Wei Yang: Supervision, Project administration, Funding acquisition.
Funding
This work was co-financed by Anhui Provincial Natural Science Foundation for Distinguished Young Scholar (2008085J26), Natural Science Foundation in University of Anhui Province (KJ2021ZD0119 and 2022AH040251), Excellent Scientific Research and Innovation Team in University of Anhui Province (2022AH010096), Startup Fund for Distinguished Scholars in Hefei University (20RC37), Anhui Provincial Natural Science Foundation (2108085QB47) and Research Grant Council of the Hong Kong Special Administrative Region, China (Project No. CityU 11214221).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We also appreciated the support and valuable suggestions from Richard Kwok Kit Yuen who is the supervisor of Wen-Jie Yang at the City University of Hong Kong.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, G.; Zhang, W.; Li, H.; Xie, Y.; Hu, H.; Xiao, Y.; Liang, Y.; Liu, Y.; Liu, W.R.; Zheng, M. Non-tubular-biomass-derived nitrogen-doped carbon microtubes for ultrahigh-area-capacity lithium-ion batteries. J Colloid Interface Sci 2020, 580, 638–644. [Google Scholar] [CrossRef]
- Zhu, J.; Roscow, J.; Chandrasekaran, S.; Deng, L.; Zhang, P.; He, T.; Wang, K.; Huang, L. Biomass-Derived Carbons for Sodium-Ion Batteries and Sodium-Ion Capacitors. ChemSusChem 2020, 13, 1275–1295. [Google Scholar] [CrossRef]
- Darjazi, H.; Bottoni, L.; Moazami, H.R.; Rezvani, S.J.; Balducci, L.; Sbrascini, L.; Staffolani, A.; Tombesi, A.; Nobili, F. From waste to resources: transforming olive leaves to hard carbon as sustainable and versatile electrode material for Li/Na-ion batteries and supercapacitors. Materials Today Sustainability 2023, 21, 100313. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, B.; Feng, J.; Wang, C.; Cai, X.; Qin, R. Recent advance of biomass-derived carbon as anode for sustainable potassium ion battery. Chemical Engineering Journal 2021, 405, 126897. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, E.; Wang, J.; Liu, Z.; Ge, J.; Yu, X.; Yang, H.; Lu, B. Potato derived biomass porous carbon as anode for potassium ion batteries. Electrochimica Acta 2019, 293, 364–370. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Zhao, S.; Feng, J.; Wang, R.; Parkin, I.P.; He, G. Robust Biomass-Derived Carbon Frameworks as High-Performance Anodes in Potassium-Ion Batteries. Small 2023, 19, e2206588. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, M.; Zhang, B.; Shao, G.; Zhao, Y. One-Pot Synthesis of Nitrogen-Doped Porous Carbon Derived from the Siraitia grosvenorii Peel for Rechargeable Zinc–Air Batteries. Energy & Fuels 2023, 37, 5412–5420. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, M.; Jia, X.; Liu, F.; Yao, J.; Hu, R.; Jiang, X.; Yu, P.; Yang, H. Application of Biomass Materials in Zinc-Ion Batteries. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cui, Y.; Huang, X.; Wu, X.; Sun, H.; Tang, S. Dual-Defect Engineering of Bidirectional Catalyst for High-Performing Lithium-Sulfur Batteries. Small 2023, e2301545. [Google Scholar] [CrossRef] [PubMed]
- Kumar Nema, P.; Mohanty, K.; Thangavel, R. Bio-mass derived hierarchically porous and high surface area carbon as an efficient sulfur host for lithium-sulfur batteries. Journal of Industrial and Engineering Chemistry 2023, 121, 235–241. [Google Scholar] [CrossRef]
- Ahmed, F.; Almutairi, G.; Hasan, P.M.Z.; Rehman, S.; Kumar, S.; Shaalan, N.M.; Aljaafari, A.; Alshoaibi, A.; AlOtaibi, B.; Khan, K. Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries. Micromachines (Basel) 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, L.; Peng, C.; Feng, W. Gas-phase fluorination of conjugated microporous polymer microspheres for effective interfacial stabilization in lithium metal anodes. Carbon Energy 2023. [Google Scholar] [CrossRef]
- Kong, L.L.; Wang, L.; Ni, Z.C.; Liu, S.; Li, G.R.; Gao, X.P. Lithium–Magnesium Alloy as a Stable Anode for Lithium–Sulfur Battery. Advanced Functional Materials 2019, 29, 1808756. [Google Scholar] [CrossRef]
- Majeed, M.K.; Iqbal, R.; Hussain, A.; Majeed, M.U.; Ashfaq, M.Z.; Ahmad, M.; Rauf, S.; Saleem, A. Silicon-based anode materials for lithium batteries: recent progress, new trends, and future perspectives. Critical Reviews in Solid State and Materials Sciences 2023, 1–33. [Google Scholar] [CrossRef]
- Il Yong Choi, C.J. , Won-Gwang Lim, Jong-Chan Han, Byeong-Gyu Chae, Chan Gyung Park, Jinwoo Lee, and Jong Kyu Kim. Amorphous Tin Oxide Nanohelix Structures Based Electrode for Highly Reversible Na-Ion Batteries. ACS Nano, 2019. [Google Scholar]
- Xiong, T.; Li, J.; Chandra Roy, J.; Koroma, M.; Zhu, Z.; Yang, H.; Zhang, L.; Ouyang, T.; Balogun, M.S.; Al-Mamun, M. Hetero-interfacial nickel nitride/vanadium oxynitride porous nanosheets as trifunctional electrodes for HER, OER and sodium ion batteries. Journal of Energy Chemistry 2023, 81, 71–81. [Google Scholar] [CrossRef]
- Gao, T.-Y.; Wang, F.-D.; Xu, Y.; Wei, C.-X.; Zhu, S.-E.; Yang, W.; Lu, H.-D. Luteolin-based epoxy resin with exceptional heat resistance, mechanical and flame retardant properties. Chemical Engineering Journal 2022, 428, 131173. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.-J.; Zhou, Q.-K.; Gao, T.-Y.; Xu, G.-M.; Tai, Q.-L.; Zhu, S.-E.; Lu, H.-D.; Yuen, R.K.K.; Yang, W.; et al. Highly thermo-stable resveratrol-based flame retardant for enhancing mechanical and fire safety properties of epoxy resins. Chemical Engineering Journal 2022, 450, 138475. [Google Scholar] [CrossRef]
- Li, Y.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Liposomes modified with bio-substances for cancer treatment. Biomater Sci 2020, 8, 6442–6468. [Google Scholar] [CrossRef]
- He, H.; Zhang, R.; Zhang, P.; Wang, P.; Chen, N.; Qian, B.; Zhang, L.; Yu, J.; Dai, B. Functional Carbon from Nature: Biomass-Derived Carbon Materials and the Recent Progress of Their Applications. Adv Sci (Weinh) 2023, 10, e2205557. [Google Scholar] [CrossRef]
- Moon, H.; Innocenti, A.; Liu, H.; Zhang, H.; Weil, M.; Zarrabeitia, M.; Passerini, S. Bio-Waste-Derived Hard Carbon Anodes Through a Sustainable and Cost-Effective Synthesis Process for Sodium-Ion Batteries. ChemSusChem 2023, 16, e202201713. [Google Scholar] [CrossRef]
- Zhu, X.; Jing, Y. Natural quinone molecules as effective cathode materials for nonaqueous lithium-ion batteries. Journal of Power Sources 2022, 531, 231291. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, Q.; Pan, W.; Zhu, S.; Wei, C.; Lu, H.; Yang, W.; Yuen, A.C.Y. Synthesis of vanillin-based porphyrin for remarkably enhancing the toughness, UV-resistance and self-extinguishing properties of polylactic acid. Chemical Engineering Journal 2023, 469, 143935. [Google Scholar] [CrossRef]
- Zhai, S.; Abraham, A.M.; Chen, B.; Fan, Z.; Hu, J.; Cai, Z.; Thangadurai, V. Abundant Canadian pine with polysulfide redox mediating ZnS/CuS nanocomposite to attain high-capacity lithium sulfur battery. Carbon 2022, 195, 253–262. [Google Scholar] [CrossRef]
- Senthil, C.; Park, J.W.; Shaji, N.; Sim, G.S.; Lee, C.W. Biomass seaweed-derived nitrogen self-doped porous carbon anodes for sodium-ion batteries: Insights into the structure and electrochemical activity. Journal of Energy Chemistry 2022, 64, 286–295. [Google Scholar] [CrossRef]
- Murugesan, C.; Senthilkumar, B.; Barpanda, P. Biowaste-Derived Highly Porous N-Doped Carbon as a Low-Cost Bifunctional Electrocatalyst for Hybrid Sodium–Air Batteries. ACS Sustainable Chemistry & Engineering 2022, 10, 9077–9086. [Google Scholar] [CrossRef]
- Deng, Y.; Lei, T.; Feng, Y.; Zhang, B.; Ding, H.; Lu, Q.; Tian, R.; Mushtaq, M.; Guo, W.; Yao, M.; et al. Biomass fallen leaves derived porous carbon for high performance lithium sulfur batteries. Ionics 2023, 29, 1029–1038. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.; Zheng, Y.; Yang, X.; Liu, C.; Di, M.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. Journal of the Taiwan Institute of Chemical Engineers 2019, 95, 217–226. [Google Scholar] [CrossRef]
- Qatarneh, A.F.; Dupont, C.; Michel, J.; Simonin, L.; Beda, A.; Matei Ghimbeu, C.; Ruiz-Villanueva, V.; da Silva, D.; Piégay, H.; Franca, M.J. River driftwood pretreated via hydrothermal carbonization as a sustainable source of hard carbon for Na-ion battery anodes. Journal of Environmental Chemical Engineering 2021, 9, 106604. [Google Scholar] [CrossRef]
- Wang, P.F.; Sui, B.B.; Sha, L.; Gong, Z.; Zhang, Y.H.; Wu, Y.H.; Zhao, L.N.; Shi, F.N. Nitrogen-rich Graphite Flake from Hemp as Anode Material for High Performance Lithium-ion Batteries. Chem Asian J 2023, 18, e202300279. [Google Scholar] [CrossRef]
- Venkatachalam, C.D.; Sekar, S.; Sengottian, M.; Ravichandran, S.R.; Bhuvaneshwaran, P. A critical review of the production, activation, and morphological characteristic study on functionalized biochar. Journal of Energy Storage 2023, 67, 107525. [Google Scholar] [CrossRef]
- Sarma, H.R.; Sun, J.; Hora, Y.; Forsyth, M.; Byrne, N. Effect of Carbonization Behaviour of Cotton Biomass in Electrodes for Sodium-Ion Batteries. ChemElectroChem 2023. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.-W.; Kim, H.-J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass and Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Luo, J.D.; Qi, X.T.; Yu, J.; Cai, J.X.; Yang, Z.Y. Molten-Salt-Assisted Synthesis of Hierarchical Porous MnO@Biocarbon Composites as Promising Electrode Materials for Supercapacitors and Lithium-Ion Batteries. ChemSusChem 2019, 12, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, W.; Yang, H.; Chen, Y.; Xia, S.; Xia, M.; Tu, X.; Chen, H. Mechanism of biomass activation and ammonia modification for nitrogen-doped porous carbon materials. Bioresour Technol 2019, 280, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Balahmar, N.; Al-Jumialy, A.S.; Mokaya, R. Biomass to porous carbon in one step: directly activated biomass for high performance CO2 storage. Journal of Materials Chemistry A 2017, 5, 12330–12339. [Google Scholar] [CrossRef]
- Hao-Ran Chen, W.-M.M. , Ri-Yuan Wang, Fang-Lin Chen, Tao Li,Ding-Ding Wang, Feng Wang, San-E Zhu, Chun-Xiang Wei, Hong-Dian Lu, Wei Yang Engineering highly graphitic carbon quantum dots by catalytic dehydrogenation and carbonization of Ti3C2Tx-MXene wrapped polystyrene spheres. Carbon. [CrossRef]
- Song, P.; Wei, S.; Di, J.; Du, J.; Xu, W.; Liu, D.; Wang, C.; Qiao, S.; Cao, Y.; Cui, Q.; et al. Biomass-derived hard carbon microtubes with tunable apertures for high-performance sodium-ion batteries. Nano Research 2022. [Google Scholar] [CrossRef]
- Bottoni, L.; Darjazi, H.; Sbrascini, L.; Staffolani, A.; Gabrielli, S.; Pastore, G.; Tombesi, A.; Nobili, F. Electrochemical Characterization of Charge Storage at Anodes for Sodium-Ion Batteries Based on Corncob Waste-Derived Hard Carbon and Binder. ChemElectroChem 2023, 10. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Liao, Y.; Zhang, Z.; Luo, S.; Li, L.; Wu, Y.; Qing, Y. Tuning carbonized wood fiber via sacrificial template-assisted hydrothermal synthesis for high-performance lithium/sodium-ion batteries. Journal of Power Sources 2022, 546, 231993. [Google Scholar] [CrossRef]
- Chen, S.; Tang, K.; Song, F.; Liu, Z.; Zhang, N.; Lan, S.; Xie, X.; Wu, Z. Porous hard carbon spheres derived from biomass for high-performance sodium/potassium-ion batteries. Nanotechnology 2021, 33. [Google Scholar] [CrossRef]
- Yang, X.; He, H.; Lv, T.; Qiu, J. Fabrication of biomass-based functional carbon materials for energy conversion and storage. Materials Science and Engineering: R: Reports 2023, 154, 100736. [Google Scholar] [CrossRef]
- Farma, R.; Husni, H.; Apriyani, I.; Awitdrus, A.; Taer, E. Biomass Waste-Derived Rubber Seed Shell Functionalized Porous Carbon As an Inexpensive and Sustainable Energy Material for Supercapacitors. Journal of Electronic Materials 2021, 50, 6910–6919. [Google Scholar] [CrossRef]
- Hernandez-Rentero, C.; Marangon, V.; Olivares-Marin, M.; Gomez-Serrano, V.; Caballero, A.; Morales, J.; Hassoun, J. Alternative lithium-ion battery using biomass-derived carbons as environmentally sustainable anode. J Colloid Interface Sci 2020, 573, 396–408. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.L.; Yang, Y.L.; Suo, G.; Zhang, L.; Lu, S.; Chen, Z.G. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. Advanced Functional Materials 2022, 32, 2201584. [Google Scholar] [CrossRef]
- Yu, K.; Wang, J.; Wang, X.; Liang, J.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Materials Chemistry and Physics 2020, 243, 122644. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z.; Ma, C.; Xu, M.; Luo, S.; Li, W.; Liu, S. Biomass-derived tubular carbon materials: progress in synthesis and applications. Journal of Materials Chemistry A 2021, 9, 13822–13850. [Google Scholar] [CrossRef]
- Gao, F.; Geng, C.; Xiao, N.; Qu, J.; Qiu, J. Hierarchical porous carbon sheets derived from biomass containing an activation agent and in-built template for lithium ion batteries. Carbon 2018, 139, 1085–1092. [Google Scholar] [CrossRef]
- Dong, W.-X.; Qu, Y.-F.; Liu, X.; Chen, L.-F. Biomass-derived two-dimensional carbon materials: Synthetic strategies and electrochemical energy storage applications. FlatChem 2023, 37, 100467. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Liu, N.; Sun, B.; Zhang, N. Biomass-Derived P/N-Co-Doped Carbon Nanosheets Encapsulate Cu(3)P Nanoparticles as High-Performance Anode Materials for Sodium-Ion Batteries. Front Chem 2020, 8, 316. [Google Scholar] [CrossRef]
- Asfaw, H.D.; Gond, R.; Kotronia, A.; Tai, C.-W.; Younesi, R. Bio-derived hard carbon nanosheets with high rate sodium-ion storage characteristics. Sustainable Materials and Technologies 2022, 32, e00407. [Google Scholar] [CrossRef]
- Zhao, D.; Ge-Zhang, S.; Zhang, Z.; Tang, H.; Xu, Y.; Gao, F.; Xu, X.; Liu, S.; Zhou, J.; Wang, Z.; et al. Three-Dimensional Honeycomb-Like Carbon as Sulfur Host for Sodium-Sulfur Batteries without the Shuttle Effect. ACS Appl Mater Interfaces 2022, 14, 54662–54669. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, J.M.; Yan, B.; Hong, H.; Piao, Y. Influence of flake size and porosity of activated graphene on the performance of silicon/activated graphene composites as lithium-ion battery anodes. Journal of Electroanalytical Chemistry 2020, 876, 114475. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, H.; Yoon, Y.; Park, S.; Shin, M.W. Porosity tailoring of the Zn-MOF-5 derived carbon materials and its effects on the performance as a cathode for lithium-air batteries. Microporous and Mesoporous Materials 2021, 311, 110726. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Wang, P.; Liang, C.; Yu, K. Preparation and mechanism of biomass-derived graphene-like Li+/Na+ battery anodes controlled by N/O functional groups and interlayer spacing. Journal of Alloys and Compounds 2022, 918, 165785. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, X.; Yan, Z.; Huang, Z.; Yao, Y.; Li, H.; Kuang, Y.; Zhou, H. Construction and preparation of nitrogen-doped porous carbon material based on waste biomass for lithium-ion batteries. International Journal of Hydrogen Energy 2021, 46, 17267–17281. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. Pore engineering: Structure-capacitance correlations for biomass-derived porous carbon materials. Materials & Design 2023, 229, 111904. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Li, J.; Cao, L.; Chen, Q.; Qian, C.; Chen, S. Revealing the Effect of Nanopores in Biomass-Derived Carbon on its Sodium-Ion Storage Behavior. ChemElectroChem 2020, 7, 201–211. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Z.; Liang, J.; Liang, C. Natural biomass-derived porous carbons from buckwheat hulls used as anode for lithium-ion batteries. Diamond and Related Materials 2021, 119, 108553. [Google Scholar] [CrossRef]
- Gao, Y.; Bai, Y.; Sun, R.; Qu, M.; Wang, M.; Peng, L.; Wang, Z.; Sun, W.; Sun, K. Advanced Separator Enabled by Sulfur Defect Engineering for High-Performance Lithium–Sulfur Batteries. Industrial & Engineering Chemistry Research 2022, 61, 6957–6966. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Xie, C.; Wang, D.; Zou, Y.; Chen, R.; Wang, Y.; Jia, C.; Wang, S. Defect Engineering on Electrode Materials for Rechargeable Batteries. Adv Mater 2020, 32, e1905923. [Google Scholar] [CrossRef]
- Kumaresan, T.K.; Masilamani, S.A.; Raman, K.; Karazhanov, S.Z.; Subashchandrabose, R. High performance sodium-ion battery anode using biomass derived hard carbon with engineered defective sites. Electrochimica Acta 2021, 368, 137574. [Google Scholar] [CrossRef]
- Verduzco, J.C.; Bettes, B.; Hu, Q.; Marinero, E.E. A review on defect engineering of anode materials for solid-state battery applications. Ionics 2022, 29, 439–453. [Google Scholar] [CrossRef]
- Zhang, W.; Ming, J.; Zhao, W.; Dong, X.; Hedhili, M.N.; Costa, P.M.F.J.; Alshareef, H.N. Graphitic Nanocarbon with Engineered Defects for High-Performance Potassium-Ion Battery Anodes. Advanced Functional Materials 2019, 29, 1903641. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, Z.-P.; Zhang, X.-F.; Huo, L.-H.; Gao, S. Biomass-Derived Graphitic Carbon/Co3O4 Nanocomposites with Pseudocapacitance for Lithium Storage. ACS Applied Nano Materials 2021, 4, 1340–1350. [Google Scholar] [CrossRef]
- Aristote, N.T.; Song, Z.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Effect of double and triple-doping of sulfur, nitrogen and phosphorus on the initial coulombic efficiency and rate performance of the biomass derived hard carbon as anode for sodium-ion batteries. Journal of Power Sources 2023, 558, 232517. [Google Scholar] [CrossRef]
- Gao, F.; Yue, X.-A.; Xu, X.-Y.; Xu, P.; Zhang, F.; Fan, H.-S.; Wang, Z.-L.; Wu, Y.-T.; Liu, X.; Zhang, Y. A N/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium–selenium batteries. Rare Metals 2023. [Google Scholar] [CrossRef]
- Sun, J.; Rakov, D.; Wang, J.; Hora, Y.; Laghaei, M.; Byrne, N.; Wang, X.; Howlett, P.C.; Forsyth, M. Sustainable Free-Standing Electrode from Biomass Waste for Sodium-Ion Batteries. ChemElectroChem 2022, 9. [Google Scholar] [CrossRef]
- Wang, D.; Du, G.; Han, D.; Su, Q.; Ding, S.; Zhang, M.; Zhao, W.; Xu, B. Porous flexible nitrogen-rich carbon membranes derived from chitosan as free-standing anodes for potassium-ion and sodium-ion batteries. Carbon 2021, 181, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Ren, Q.; Fan, L.; Zhang, F.; Shi, Z. Facile hydrothermal treatment route of reed straw-derived hard carbon for high performance sodium ion battery. Electrochimica Acta 2018, 291, 188–196. [Google Scholar] [CrossRef]
- Ren, X.; Xu, S.-D.; Liu, S.; Chen, L.; Zhang, D.; Qiu, L. Lath-shaped biomass derived hard carbon as anode materials with super rate capability for sodium-ion batteries. Journal of Electroanalytical Chemistry 2019, 841, 63–72. [Google Scholar] [CrossRef]
- Dutta, D.P.; Modak, B.; Ravuri, B.R. Phosphorous/Fluorine Co-doped Biomass-derived Carbon for Enhanced Sodium-ion and Lithium-ion Storage. ChemNanoMat 2023, 9. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Z.; Wang, J.; Lu, W.; Wang, W. A bean catching double pigeons: Sonication assisted modification of Nb2C MXenes composites by O-doping porous biomass-carbons for supercapacitors and zinc-ion batteries. Journal of Energy Storage 2023, 65, 107334. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J.-S. Biomass-derived N-doped porous carbon nanosheets for energy technologies. Chemical Engineering Journal 2021, 425, 129017. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, X.; Kang, B.; Wang, H.-E.; Xiong, P.; Chen, Q.; Wei, M.; Li, N.; Qian, Q.; et al. Structural engineering of tin sulfides anchored on nitrogen/phosphorus dual-doped carbon nanofibres in sodium/potassium-ion batteries. Carbon 2022, 189, 46–56. [Google Scholar] [CrossRef]
- Xu, L.; Guo, W.; Zeng, L.; Xia, X.; Wang, Y.; Xiong, P.; Chen, Q.; Zhang, J.; Wei, M.; Qian, Q. V3Se4 embedded within N/P co-doped carbon fibers for sodium/potassium ion batteries. Chemical Engineering Journal 2021, 419, 129607. [Google Scholar] [CrossRef]
- Nie, W.; Cheng, H.; Liu, X.; Sun, Q.; Tian, F.; Yao, W.; Liang, S.; Lu, X.; Zhou, J. Surface organic nitrogen-doping disordered biomass carbon materials with superior cycle stability in the sodium-ion batteries. Journal of Power Sources 2022, 522, 230994. [Google Scholar] [CrossRef]
- Thangaian Kesavan, a.M.S. Palm Spathe Derived N-doped Carbon Nanosheets as a High Performance Electrode for Li-ion Batteries and Supercapacitors. ACS Sustainable Chemistry & Engineering 2019. [CrossRef]
- Guo, S.; Chen, Y.; Shi, L.; Dong, Y.; Ma, J.; Chen, X.; Song, H. Nitrogen-doped biomass-based ultra-thin carbon nanosheets with interconnected framework for High-Performance Lithium-Ion Batteries. Applied Surface Science 2018, 437, 136–143. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, B.; Feng, J.; Wang, C.; Cai, X.; Qin, R. Biomass bone-derived, N/P-doped hierarchical hard carbon for high-energy potassium-ion batteries. Materials Research Bulletin 2021, 139, 111282. [Google Scholar] [CrossRef]
- Baskar, A.V.; Singh, G.; Ruban, A.M.; Davidraj, J.M.; Bahadur, R.; Sooriyakumar, P.; Kumar, P.; Karakoti, A.; Yi, J.; Vinu, A. Recent Progress in Synthesis and Application of Biomass-Based Hybrid Electrodes for Rechargeable Batteries. Advanced Functional Materials 2022, 33, 2208349. [Google Scholar] [CrossRef]
- Xu, X.; Wu, F.; Yang, W.; Dai, X.; Wang, T.; Zhou, J.; Wang, J.; Guo, D. Silicon@Natural Nitrogen-Doped Biomass Carbon Composites Derived from “Silicon Tofu” as Green and Efficient Anode Materials for Lithium-Ion Batteries. ACS Sustainable Chemistry & Engineering 2021, 9, 13215–13224. [Google Scholar] [CrossRef]
- Zhu, L.; Han, T.; Lin, X.; Chen, Z.; Hu, C.; Liu, J. In-situ growing nanowires on biomass corn pods as free-standing electrodes with low surface reaction barrier for Li-, Al-, and Na-ion batteries. Applied Surface Science 2023, 608, 155223. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Z.; Yang, W.; Liang, Y.; Meng, D.; He, X.; Liang, P.; Zhang, Z. NiCo2O4/biomass-derived carbon composites as anode for high-performance lithium ion batteries. Journal of Power Sources 2020, 451, 227761. [Google Scholar] [CrossRef]
- Li, Y.; Lin, W.; Xue, L.; Xie, J.; Wei, B.; Chen, G.; Chen, D. Facile preparation of V2O3/black fungus-derived carbon composite with hierarchical porosity as a promising electrode for lithium/sodium ion batteries. Journal of Alloys and Compounds 2022, 905, 164258. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Liu, A.; Zhu, H.; Ma, T. Recent progress in biomass-derived carbon materials used for secondary batteries. Sustainable Energy & Fuels 2021, 5, 3017–3038. [Google Scholar] [CrossRef]
- He, W.; Luo, H.; Jing, P.; Wang, H.; Xu, C.; Wu, H.; Wang, Q.; Zhang, Y. Embedding silicon in biomass-derived porous carbon framework as high-performance anode of lithium-ion batteries. Journal of Alloys and Compounds 2022, 918, 165364. [Google Scholar] [CrossRef]
- Yang, W.; Lu, W.; Wang, W. Synthesis of O-Doped and Hierarchical Porous Structured Carbons as Bifunctional Materials for Li-Ion Batteries and Zinc-Ion Supercapacitors. Energy & Fuels 2021, 36, 669–676. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Cheng, X.; Yu, H.; Yin, X.; Jiang, Y.; Zhang, H.; Li, S.; Huang, F. Tuning hierarchical structure of probiotics-derived porous carbon for potassium-ion batteries. Journal of Power Sources 2023, 574, 233164. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, L.; Zheng, Z.; Xie, D.; Liu, P.; Mai, Y.; Cheng, F. Synergistic S-doped biomass carbon and N-doped carbon for construction of interlayer expanded MoS2 core/shell nanospheres for potassium and sodium storage. Electrochimica Acta 2023, 461, 142626. [Google Scholar] [CrossRef]
- Han, F.; Fan, L.; Ma, X.; Lu, H.; Li, L.; Zhang, X.; Wu, L. Conversion of LiPSs Accelerated by Pt-Doped Biomass-Derived Hyphae Carbon Nanobelts as Self-Supporting Hosts for Long-Lifespan Li–S Batteries. Energy & Environmental Materials, 2023. [Google Scholar] [CrossRef]
- Cui, J.; Liu, J.; Chen, X.; Meng, J.; Wei, S.; Wu, T.; Wang, Y.; Xie, Y.; Lu, C.; Zhang, X. Ganoderma Lucidum-derived erythrocyte-like sustainable materials. Carbon 2022, 196, 70–77. [Google Scholar] [CrossRef]
- Wu, S.; Jin, Y.; Wang, D.; Xu, Z.; Li, L.; Zou, X.; Zhang, M.; Wang, Z.; Yang, H. Fe2O3/carbon derived from peanut shell hybrid as an advanced anode for high performance lithium ion batteries. Journal of Energy Storage 2023, 68, 107731. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, K.; Ye, K.; Gong, Z.; Liu, R.; Cheng, K.; Wang, G.; Yan, J.; Cao, D. Three-dimensional biomass derived hard carbon with reconstructed surface as a free-standing anode for sodium-ion batteries. J Colloid Interface Sci 2020, 561, 203–210. [Google Scholar] [CrossRef]
- Wei, X.; Deng, Y.; Hu, X.; Zhao, J.; Wei, H.; Yang, Z.; Han, G. Biomass derived fibrous porous carbon loaded zinc oxide nanoparticles as high-performance anode materials for lithium ion batteries. Journal of Energy Storage 2023, 70, 107854. [Google Scholar] [CrossRef]
- Sbrascini, L.; Staffolani, A.; Bottoni, L.; Darjazi, H.; Minnetti, L.; Minicucci, M.; Nobili, F. Structural and Interfacial Characterization of a Sustainable Si/Hard Carbon Composite Anode for Lithium-Ion Batteries. ACS Appl Mater Interfaces 2022. [Google Scholar] [CrossRef]
- Huang, G.; Kong, Q.; Yao, W.; Wang, Q. Poly tannic acid carbon rods as anode materials for high performance lithium and sodium ion batteries. J Colloid Interface Sci 2023, 629, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; He, X.; Qiao, Y.; Li, L.; Chou, S.-L. Hard carbon derived from hazelnut shell with facile HCl treatment as high-initial-coulombic-efficiency anode for sodium ion batteries. Sustainable Materials and Technologies 2022, 33, e00446. [Google Scholar] [CrossRef]
- Antorán, D.; Alvira, D.; Peker, M.E.; Malón, H.; Irusta, S.; Sebastián, V.; Manyà, J.J. Waste Hemp Hurd as a Sustainable Precursor for Affordable and High-Rate Hard Carbon-Based Anodes in Sodium-Ion Batteries. Energy & Fuels 2023, 37, 9650–9661. [Google Scholar] [CrossRef]
- Zou, X.; Dong, C.; Jin, Y.; Wang, D.; Li, L.; Wu, S.; Xu, Z.; Chen, Y.; Li, Z.; Yang, H. Engineering of N, P co-doped hierarchical porous carbon from sugarcane bagasse for high-performance supercapacitors and sodium ion batteries. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2023, 672, 131715. [Google Scholar] [CrossRef]
- Kim, M.; Fernando, J.F.S.; Li, Z.; Alowasheeir, A.; Ashok, A.; Xin, R.; Martin, D.; Kumar Nanjundan, A.; Golberg, D.V.; Yamauchi, Y.; et al. Ultra-stable sodium ion storage of biomass porous carbon derived from sugarcane. Chemical Engineering Journal 2022, 445, 136344. [Google Scholar] [CrossRef]
- Yang, L.; Lei, Y.; Liang, X.; Qu, L.; Xu, K.; Hua, Y.; Feng, J. SnO2 nanoparticles composited with biomass N-doped carbon microspheres as low cost, environmentally friendly and high-performance anode material for sodium-ion and lithium-ion batteries. Journal of Power Sources 2022, 547, 232032. [Google Scholar] [CrossRef]
- Aristote, N.T.; Liu, C.; Deng, X.; Liu, H.; Gao, J.; Deng, W.; Hou, H.; Ji, X. Sulfur-doping biomass based hard carbon as high performance anode material for sodium-ion batteries. Journal of Electroanalytical Chemistry 2022, 923, 116769. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem Soc Rev 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).