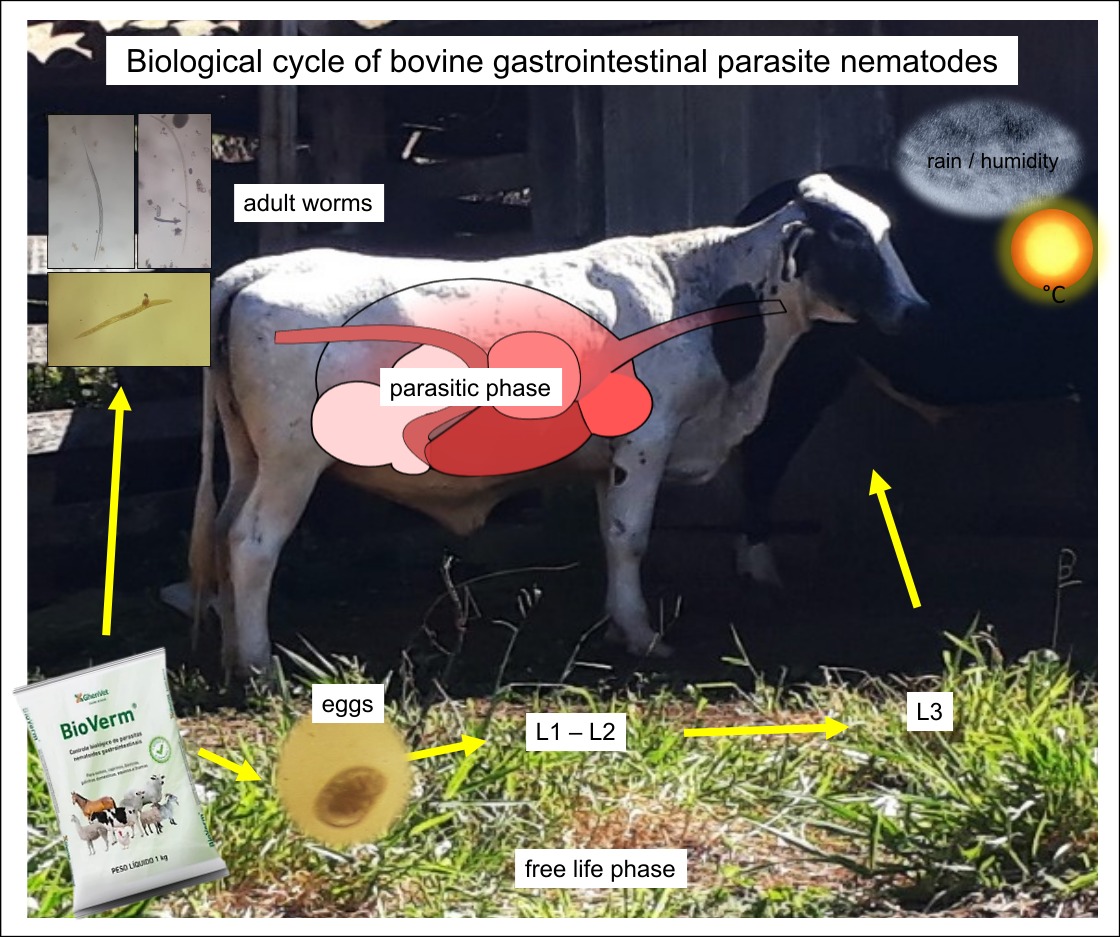
1. Introduction
Brazil has the largest commercial bovine population in the world, and the Southeast region has greatest representation, with emphasis on the state of Minas Gerais for dairy cattle breeding [
1]. The current trends of ruminant breeding are increasingly demanding increased production while also assuring the welfare of animals. The control of helminths is an important factor related to both aspects [
2,
3].
Animals become infected with helminths principally through exposure in pastures [
4]. The main form of combating these parasites is through treatment of animals with antiparasitic agents [
5,
6], but in many cases these are administered incorrectly, with excessive and indiscriminate use of therapeutic bases, increasing production costs without achieving effective control of infestations [
7], besides leaving harmful residues in products of animal origin [
8].
Control using bioproducts is an alternative for management of bovine nematodes in pastures [
9]. The use of biological control methods involving nematophagous fungi has been shown to be a safe and viable alternative [
9,
10,
11].
Promising results have been achieved with various species nematophagous fungi, in particular
D. flagrans for control of helminth larvae and
P. chlamydosporia with ovicidal action to control helminth eggs [
12,
13,
14]. Therefore, the development of biological control of helminths is an increasingly attractive option, but requires the selection of the most effective fungal isolates or their associations.
With this in mind, we evaluated the effects of two commercial bioproducts, one based on Duddingtonia flagrans (Bioverm®) and the other its association with Pochonia chlamydosporia for the reduction of eggs in animal feces and larval infestations in pastures.
2. Materials and Methods
The commercial formulations based on the fungus Duddingtonia flagrans (AC01 isolate) (Bioverm®) and on D. flagrans (AC01 isolate) associated with Pochonia chlamydosporia (VC04 isolate) were supplied by the company GhenVet Saúde Animal (Brazil).
The experiment was conducted on a farm located in the municipality of Abre Campo, state of Minas Gerais, southeastern Brazil, latitude 20º 18 '04 "S, longitude 42º 28' 39" W.
Initially, to monitor the animals, feces and pasture samples were collected and subjected to the egg count per gram of feces (EPG) according to the methods of Gordon and Whitlock [
15] and Dennis, Stone and Swanson [
16], while the pasture forage samples were analyzed by the technique described by Raynaud and Gruner [
17] and the parasites were identified according to Keith [
18].
Eighteen Holstein x Zebu crossbred cattle, aged between 12 and 15 months, with average initial weight of 150 kg, were previously treated with an anthelmintic suspension of 15% albendazole sulfoxide (Agebendazol®) by injection, in a single dose of 1 mL/44 kg of body weight. Twenty-one days after anthelmintic treatment and after confirmation of the absence of nematode eggs eliminated by feces in the EPG, a field test was conducted of the efficacy of products as described by the World Association for the Advancement of Veterinary Parasitology (WAAVP) [
19]. The animals were randomly divided into three groups of six animals each and placed in three
Brachiaria brizantha paddocks naturally infested with helminth larvae, having been previously used for grazing by young and adult animals. In treatment group 1, each animal was treated with 10 to 6 g/100 kg body weight containing the fungus
D. flagrans, administered daily with corn bran, while group 2 received 10 to 6 g of each fungus/100 kg body weight containing
D. flagrans and
P. chlamydosporia, also administered daily along with corn bran. In the control group, each animal received daily feed of corn bran without fungus. The animals were monitored fortnightly and the dose of the products was maintained by the body score and based on previous studies conducted on the same farm, with the same animals, as described by Vieira and collaborators [
14].
The experiment lasted nine months (February to October 2021), during which fecal and pasture vegetation samples were collected. The method was consistent with the guidelines of the World Association for the Advancement of Veterinary Parasitology (WAAVP) and followed the determinations for assessing the efficacy of anthelmintics in cattle and sheep described by Powers
et al. [
19] and the second edition of Wood
et al. [
20] besides the requirements of Edict 48 from Brazil’s Ministry of Agriculture and Supply [
21]. The study was also approved by the Ethics Committee on Animal Use (CEUA) of Viçosa Federal University (UFV), under reference no. 37/2020.
Every 15 days after the animals were introduced to the pastures, the body score was checked, and fecal samples were collected from all animals in each group directly from the rectum. Egg count per gram of feces (EPG) was determined by the method of Gordon and Whitlock [
15], with modifications suggested by Lima [
22]. At the same time, coprocultures were produced using 20 g of feces mixed with vermiculite and placed in biochemical oxygen demand (BOD) chamber at 26 °C for 15 days to obtain infective larvae (L3), which were subsequently identified according to Keith [
18].
Every 15 days from the start of the experiment, two samples of
Brachiaria brizantha grass (0-20 cm and 20-40 cm distance from stools) were collected from the grazing areas of the treated and control groups at six different points according to Raynaud and Gruner [
17]. Samples of 500 g of pasture forage were used to recover infective larvae (L3) following the method described by Lima [
22]. The sediment was examined under an optical microscope and the larvae were counted and identified according to the criteria established by Keith [
18]. The 500 g samples of grass that were used were placed in an oven at 100 °C to obtain dry matter. The data obtained were transformed into the number of larvae per kilogram of dry matter.
The climatic data regarding minimum, average and maximum monthly temperatures and monthly precipitation were obtained from the Agricultural Meteorological Monitoring System (Agritempo), available at the website
https://www.agritempo.gov.br/agritempo/index.jsp.
Mean egg counts per gram of feces (EPG), nematodes recovered in coproculture and L3 from pasture samples and weather data during the nine months of the experiment were converted into monthly values. Then, EPG and L3 data from pasture were log (x + 1) transformed, submitted to Levene's test of equality of variances, ANOVA and the Tukey test for EPG data, and the Chi-square test for L3 in pasture, in all cases at 5% significance levels. All were performed with the IBM SPSS Statistics 2.0 software.
3. Results
3.1. General Results
The results obtained by the initial evaluation, before starting to provide the products, verified the absence of trematode eggs according to the method of Dennis, Stone and Swanson [
16]. However, it is important to mention that Cestoda eggs and unsporulated oocysts were verified, identified by morphology according to Araújo [
23].
3.1.1. Egg Counting Techniques
The EPG results are presented in
Table 1, which shows the monthly average EPG of feces in the three groups during the period from February to October 2021. In the first month of treatment, the low EPG value was due to the result of the anthelmintic treatment administered to the animals before the beginning of the experiment. This protocol is based on the recommendations of WAVVP [
19,
20] and MAPA [
21].
EPG values were lower in treatment group 1 than in the control in March, while in April, May and July, the values were lower in both treatment groups compared to the control. In June, September and October there were no statistical differences in the mean EPG values in any of the groups, although numerically the values were lower in August and September.
3.1.2. Larva Recovery Techniques
Figure 1 shows the values of the L3 genera recovered from the stool cultures of animals in treatment 1, based on
Duddingtonia flagrans (Bioverm®), and treatment 2, based on combination of
D. flagrans and
Pochonia chlamydosporia (Association) and the control group.
The genus Haemonchus was predominant, followed by Cooperiai, in treatment 2 and control groups. No genera were observed in group 1 after the start of the treatment protocol.
The means of larvae recovered at both distances from the fecal masses in treatment 1, treatment 2 and control groups differed significantly (p≤0.05), as shown in
Table 2. Like in the coprocultures, the agents recovered in the pasture predominantly belonged to the genera
Haemonchus and
Cooperia in early stages.
3.1.3. Climate Determinations
The presentation of the minimum, average and maximum monthly temperatures, as well as the monthly precipitation during the experimental period in Abre Campo, Minas Gerais State, Brazil, are shown in
Table 3. The lowest temperatures recorded were in the months of May and July 2021. The average monthly temperatures ranged from 19.26 °C in July to 25.57 °C in March 2021. The highest precipitation rates were recorded in October, with 6.60 L/m², while in other months the rainfall ranged from zero in July to 1.46 L/m²in March.
3.1.4. Other Findings
However, it is important to emphasize that Cestoda eggs and unsporulated oocysts were verified, identified by morphology according to Araújo [
23]. The Cestoda eggs were found in the treatment group 1 in the months of May and October, and oocysts were found from the pilot phase in all groups in February, March and April.
4. Discussion
Ruminant breeding must evolve to adapt to more stringent global food management standards. Parasitosis is a factor that must be considered, and the main control measure is based on administration of anthelmintic chemicals. However, these chemicals can cause negative environmental effects as well as development of resistance of nematodes due to indiscriminate use and overdosing. These drawbacks have attracted interest in the use of alternative methods, in particular the use of nematophagous fungi for biological control of ruminant helminths. Various studies have shown the effectiveness of biological control of gastrointestinal nematodes in pastures.
According to Li
et al. [
24], for efficient worm control, it is essential to interfere in their entire life cycle, i.e. to reduce the number of nematodes in animals and in pastures at the same time. Our study is in this line, investigating the environmental action of nematophagous fungi. Many tests have been performed to determine the fungal action against gastrointestinal nematodes of ruminants. We analyzed the use of two commercial bioproducts (Bioverm® and Association), with different functional characteristics. According to Kaplan [
6], the effect of anthelmintics eliminates the adult stages and eggs in the feces, which was noted in the analysis before the start of the experiment. However, the subsequent period of natural exposure to
Brachiaria brizantha forage without chemical control, natural reinfection by the most common helminths was observed in this environment, especially in the control group (
Table 1).
Parasitic agents are found in about 95% of pastures according to epidemiological studies conducted by Kenyon
et al. [
25] and Franco
et al. [
26]. This can be reduced by the use of nematophagous fungi for control, either singly or combined. The use of the fungus
D. flagrans (Bioverm®) or the combined use of
D. flagrans and
P. chlamydosporia (Association) significantly reduced the EPG counts compared to the control group in some periods of the experiment. Similar results were found in a previous study by the same research group performed on the same farm, using different fungal bases [
14].
Oral administration of fungi is both practical and most effective, because the fungus or fungi have the ability to bind to the feces of the animals, and are released along with the eggs [
24,
27].
The fungal species
Duddingtonia flagrans forms chlamydospores and stands out for its ability to survive and spread through the gastrointestinal tract of animals [
9,
14,
24,
28,
29]. It has been shown to be an excellent way to control helminth larvae in live animals. This is the case of the commercial product based on chlamydospores of
D. flagrans (Bioverm®), licensed and marketed in Brazil [
27,
30].
In addition, there are also ovicidal fungi, such as
P. chlamydosporia, which form dictyochlamydospores, with selective action against gastrointestinal helminth eggs, used for control of helminthiases [
30,
31].
The most frequent parasite genera in our coprocultures were
Haemonchus sp. and
Cooperia sp. (
Figure 1), corroborating previous studies [
12,
13,
14]. Species of these genera are prevalent in Brazil and cause economic losses due to problems such as anemia, appropriation of nutrients and even death of parasitized animals [
32,
33].
The life cycle of gastrointestinal parasites occurs inside the animal and in the environment (in pastures in extensive grazing systems) [
34]. In the present study, fungal control led to significant reductions of environmental contamination by larvae (
Table 2) as well as of EPG counts. This can be explained by the different recovery techniques and the action of different fungi on the life stages of the helminths. A previous study by our group, performed in a short interval using other control fungi, also reduced these parameters, with the residual action in the pasture, contributing to the low levels of environmental contamination and release of eggs [
14].
The EPG examinations or sedimentation methods of the animals in the present study did not indicate the presence of trematodes and other helminths such as ascarids. The can be explained by the action of the nematophagous fungus P. chlamydosporia, since helminths of the genera Haemonchus and Cooperia, have rapid life-stage transitions, and their egg phase is brief before hatching to form the first larval stage (L1). Hence, the direct action of this fungus may have been interrupted. However, Cestoda eggs (Moniezia spp.) were found in the Bioverm® group but not in the Association group, which suggests ovicidal action of P. chlamydosporia.
The grass height of the pasture varied from 15 cm to 80 cm. This variation probably destabilized the microclimate favorable to the development and survival of the larvae, in addition to interfering with the protection of the feces and their rapid degradation [
35]. Climatic conditions are important factors that influence the growth of pastures and the maintenance of infective larvae [
36]. Another factor that influenced the distribution of L3 was the average temperature recorded during the experimental period.
According to Heckler and Borges [
33], temperature variations between 13 °C and 26 °C are adequate to maintain the free-living stages of the
Haemonchus and
Cooperia genera. The maximum temperature was above that level (
Table 3). The high temperature associated with the lack of rain during the experimental phase, mainly in the months of May, June, July and August, contributed to reduce the pasture areas, and consequently increased the degradation of eggs and reduced the load of larvae in the pastures in all groups, as indicated by the EPG and pasture results in the final months of the experiment. The presence of water is essential for the migration of L3 from feces to pasture [
36,
37].
In this sense, our study showed that climatic factors were involved in the reduction of both factors (OPG counts and pasture parasite loads), but there was a significant action of the fungi D. flagrans and P. chlamydosporia, alone or in combination, reducing the number of infective larvae in pastures and the parasitic load of cattle by counting with reduction of eggs from animals raised in pastures.
Extensive grazing of ruminants is the most widespread and productive method in Brazil, therefore the availability of bioproducts to control parasites will contribute immensely to minimize the negative impacts resulting from helminth infections and the indiscriminate use of drugs in animal production systems [
30].
5. Conclusions
Knowledge about sustainable products is important for greater adherence to the use of alternative practices by livestock breeders. In our study, we showed the effects of exposure of naturally infected cattle to the use of the nematophagous fungus D. flagrans alone (Bioverm®) or combined with P. chlamydosporia (Association), which reduced the release of eggs and the presence of larvae in pastures. Therefore, employment of these bioproducts is a promising method for the integrated control of helminths in ruminant livestock.
Author Contributions
Maria Larissa and Jackson Victor, de Araújo conceived and designed the study. Maria Larissa performed the data collection. Maria Larissa and Italo performed statistical analyses. Maria Larissa, Ítalo, Samuel, Lorena, André and Jackson Victor wrote the article.
Funding
This research was funded by Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and Research Support Foundation of the State of Minas Gerais (FAPEMIG).
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki, and approved by the or Ethics Committee on Animal Use (CEUA) of Viçosa Federal University (UFV), under reference no. 37/2020 for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the 'Conselho Nacional de Desenvolvimento Científico e Tecnológico' (CNPq) and 'Fundação de Amparo à Pesquisa do Estado de Minas Gerais' (FAPEMIG) Coordenação para o Aperfeiçoamento do Pessoal do Ensino Superior' (CAPES) for their support in this study, in the form of a PhD scholarship and the Veterinary Department of the Universidade Federal de Viçosa.
Conflicts of Interest
The authors declare that they have no competing financial interests or personal relationships that may have influenced the work reported in this article.
References
- Instituto Brasileiro de Geografia e Estatística (IBGE) Pesquisa Trimestral do leite Available, 2022. online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9209-pesquisa-trimestral-do-leite.html?edicao=27146&t=resultados (accessed on Mar 3, 2022).
- Molento, M.B. Resistência parasitária em helmintos de eqüídeos e propostas de manejo. Ciência Rural 2005, 35, 1469–1477. [Google Scholar] [CrossRef]
- Grisi, L.; Leite, R.C.; Martins, J.R. de S.; Barros, A.T.M. de; Andreotti, R.; Cançado, P.H.D.; León, A.A.P. de et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Veterinária 2014, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi-Storm, N., Moakes, S., Thuer, S. Grovermann, C., Verwer, C., Verkaik, J., et al. Parasite control in organic cattle farming: Management and farmers' perspectives from six European countries. Veterinary Parasitology: Regional Studies and Reports. 18, 2019. https://doi.org/10.1016/j.vprsr.2019.100329. [CrossRef]
- Vidal, M.L.B.; Viana, M.V.G.; Ito, M.; Trivilin, L.O.; Martins, I.V.F. Anti-helmínticos de importância veterinária no Brasil. In Tópicos especiais em ciência animal VIII; CAUFES: Alegre, 2019; pp. 273–297. [Google Scholar]
- Kaplan, R.M. Biology, Epidemiology, Diagnosis, and Management of Anthelmintic Resistance in Gastrointestinal Nematodes of Livestock. Vet. Clin. North Am. Food Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.E. da F.; Lima, W. dos S.; Cunha, A.P. da; Bello, A.C.P. de P.; Domingues, L.N.; Wanderley, R.P.B., et al. Verminoses dos bovinos: percepção de pecuaristas em Minas Gerais, Brasil. Rev. Bras. Parasitol. Veterinária, 2009, 18, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Sindicato Nacional da Indústria de Produtos para saúde animal. Mercado nacional de produtos para saúde animal. 2018. Available online: http://www.sindan.org.br/mercado-brasil-2018/ (accessed on Mar 3, 2022).
- Braga, F.R., Ferraz, C.M., Silva, E., Araújo, J. Efficiency of the Bioverm (Duddingtonia flagrans) fungal formulation to control in vivo and in vitro of Haemonchus contortus and Strongyloides papillosus in sheep. 3 Biotech, 2020, 10, 62. [CrossRef]
- Luns, F.D., Assis, R.C.L., Silva, L.P.C., Ferraz, C.M., Braga, F.R., Araújo, J.V. Coadministration of nematophagous fungi for biological control over nematodes in bovine in the South-eastern Brazil. Biomed Res Int, 2018, p.1-6. [CrossRef]
- Vilela, V.L.R, Feitosa, T.F., Braga, F.R., Vieira, V.D., Lucena, S.C., Araújo, J.V. Control of sheep gastrointestinal nematodes using the combination of Duddingtonia flagrans and levamisole hydrochloride 5%. Rev Bras Parasitol Vet, 2018, 27, 23-31. [CrossRef]
- Mendonza-de-Gives, P., López-Arellano, M.A., Aguilar-Marcelino, L., Olazarán-Jenkins, S., Reyes-Guerrero, D., Ramírez-Várgas, G., et al. The nematophagous fungus Duddingtonia flagrans reduces the gastrointestinal parasitic nematode larvae population in faeces of orally treated calves maintained under tropical conditions - Dose/response assessment, Vet Parasitol, 2018, 263, 66-72. [CrossRef]
- Oliveira, I.C., Vieira, I.S., Carvalho, L.M., Campos, A.K., Freitas, S.G., Araujo, J.M., et al. Reduction of bovine strongilides in naturally contaminated pastures in the southeast region of Brazil. Exp Parasitol, 2018, 194, 9-15. [CrossRef]
- Vieira, I.S. , Oliveira, I.C., Freitas, S.G., Campos, A.K., Araújo, J.V. (2020) Arthrobotrys cladodes and Pochonia chlamydosporia in the biological control of nematodiosis in extensive bovine production system. Parasitology, 2020, 147, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.M., Whitlock, H.V. A new technique for counting nematode eggs in sheep faeces. J Sci Ind Res, 1939, 12, 50-52.
- Dennis, W.R., Stone, W.M., Swanson, L.E. A new laboratory and field diagnostic test for fluke ova, in feces. J. Amer. Vet Med. Am., 1954, 124, 47-50.
- Raynaud, J.P., Gruner, L. Feasibility of herbage sampling in large extensive and avaibility of cattle nematode in mountain pastures. Vet Parasitol, 1982, 10, 57-64. [CrossRef]
- Keith, R.K. The differentiation on the infective larvae of some common nematode parasites of cattle. Austr Jour Zool, 1953, 1, 223-235. [CrossRef]
- Powers, K.G., Wood, I.B., Eckert, J., Gilson, T., Smith, H.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) Guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine and ovine). Vet Parasitol, 1982, 10, 265-284.
- Wood, J.B., Amaral, N.K., Bairden, K., Duncan, J.L., Kassai, T., Malone, J.B., et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet Parasitol, 1995, 58, 181-213. [CrossRef]
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Regulamento Técnico para Licenciamento e/ou Renovação de Licença de Produtos Antiparasitários de Uso Veterinário. Portaria nº 48, de 12 de maio de1997. Diário Oficial da União de 16/05/1997, Seção 1, Página 10165.
- Lima, W.S. Dinâmica das populações de parasitos gastrintestinais em bovinos de corte, alguns aspectos da relação parasito-hospedeiro e do comportamento dos estádios de vida livre na região do vale do Rio Doce, MG. Brasil. PhD thesis, 1989, Universidade Federal de Minas Gerais, Minas Gerais, Brasil.
- Araújo, J.V. Diagnóstico das helmintoses. 113 – Ciências Biológicas e da Saúde, Caderno Didático, 2014, Viçosa- UFV, 84p.
- Li, S. , Wang, D., Gong, J., Zhang, Y. (2022) Individual and Combined Application of Nematophagous Fungi as Biological Control Agents against Gastrointestinal Nematodes in Domestic Animals. Pathogens, 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, F., Greer, A.W., Coles, G.C., Cringoli, G., Papadopoulos, E., Cabaret, J., et al. (2009). The role of targeted selective treatments in the development of refugia-based approaches to the control of gastrointestinal nematodes of small ruminants. Vet. Parasitol., 2009, 164, 3–11.
- Franco, B., Alberto, F.L., Federica, S.M., Emilia, I.L., Silvina, F.A., Sara, Z., et al. Predatory effect of Duddingtonia flagrans on infective larvae of gastro-intestinal parasites under sunny and shaded conditions. Exp. Parasitol., 2018, 193, 27–32. [CrossRef]
- Rodrigues, J.A., Roque, F.L., Alvares, F.B.V., da Silva, A.L.P., de Lima, E.F., Filho, G.M.D., et al. Efficacy of a commercial fungal formulation containing Duddingtonia flagrans (Bioverm (R)) for controlling bovine gastrointestinal nematodes. Rev. Bras. Parasitol. Vet., 2021, 30, e026620. [CrossRef]
- Gronvold, J., Henriksen, S.A., Larsen, M., Nansen, P., Wolstrup, J. Biological control-Aspects of biological control-With special reference to arthropods, protozoans and helminths of domesticated animals. Vet. Parasitol., 1996, 64, 47–68. [CrossRef]
- Buzatti, A., De Paula Santos, C., Fernandes, M.A.M., Yoshitani, U.Y., Sprenger, L.K., dos Santos, C.D., et al. Duddingtonia flagrans in the control of gastrointestinal nematodes of horses. Exp. Parasitol., 2015, 159, 1–4. [CrossRef]
- Braga, F.R. , Araújo, J., Campos, A.K., Silva, A.R., Araujo, J.M., Carvalho, R.O., et al. (2008) In vitro evaluation of the effect of the nematophagous fungi Duddingtonia flagrans, Monacrosporium sinense and Pochonia chlamydosporia on Schistosoma mansoni eggs. World J. Microbiol. Biotechnol., 2008, 24, 2713–2716. [Google Scholar] [CrossRef]
- Lopez-Llorca, L.V., Olivares-Bernabeu, C., Salinas, J., Jansson, H.B., Kolattukudy, P.E. Pre-penetration events in fungal parasitismo of nematode eggs. Mycol. Res. 2002, 106, 499–506. [CrossRef]
- Girão, E.S., Leal, J.A., Girão, R.N., Medeiros, L.P. Verminose bovina. Teresina: Embrapa Meio Norte, 1999, Documentos 41, 30p. [CrossRef]
- Heckler, R.P., Borges, F.A. Climate variations and the environmental population of gastrointestinal nematodes of ruminants. Nematoda, 2016, 3. [CrossRef]
- Torres-Acosta, J.F.L., Hoste, H. Alternative or improved methods to limit gastro intestinal parastism in grazing sheep and goats. Small Ruminant Res, 2008, 77, 159-173. [CrossRef]
- Rocha, R.A., Rocha, G.P., Bricarello, P.A., Amarante, A.F.T. Recovery of Trichostrongylus colubriformis infective larvae from three grass species contaminated in summer. Rev Bras Parasitol Vet, 2008, 17, 227-234. [CrossRef]
- Quadros, D.G., Sobrinho, A.G.S., Rodrigues, L.R.A., Oliveira, G.P., Xavier, C.P., Andrade, A.P. Effect of three species of forage grasses on pasture structure and vertical distribution of infective larvae of gastrointestinal nematodes of sheep. Ci Anim Bras Goiânia, 2012, 13, 139-144. [CrossRef]
- Van Dijk, J., Morgan, E.R. The influence of water on the migration of infective trichostrongyloid larvae onto grass. Parasitology, 2011, 138,780-788. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).