Submitted:
25 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
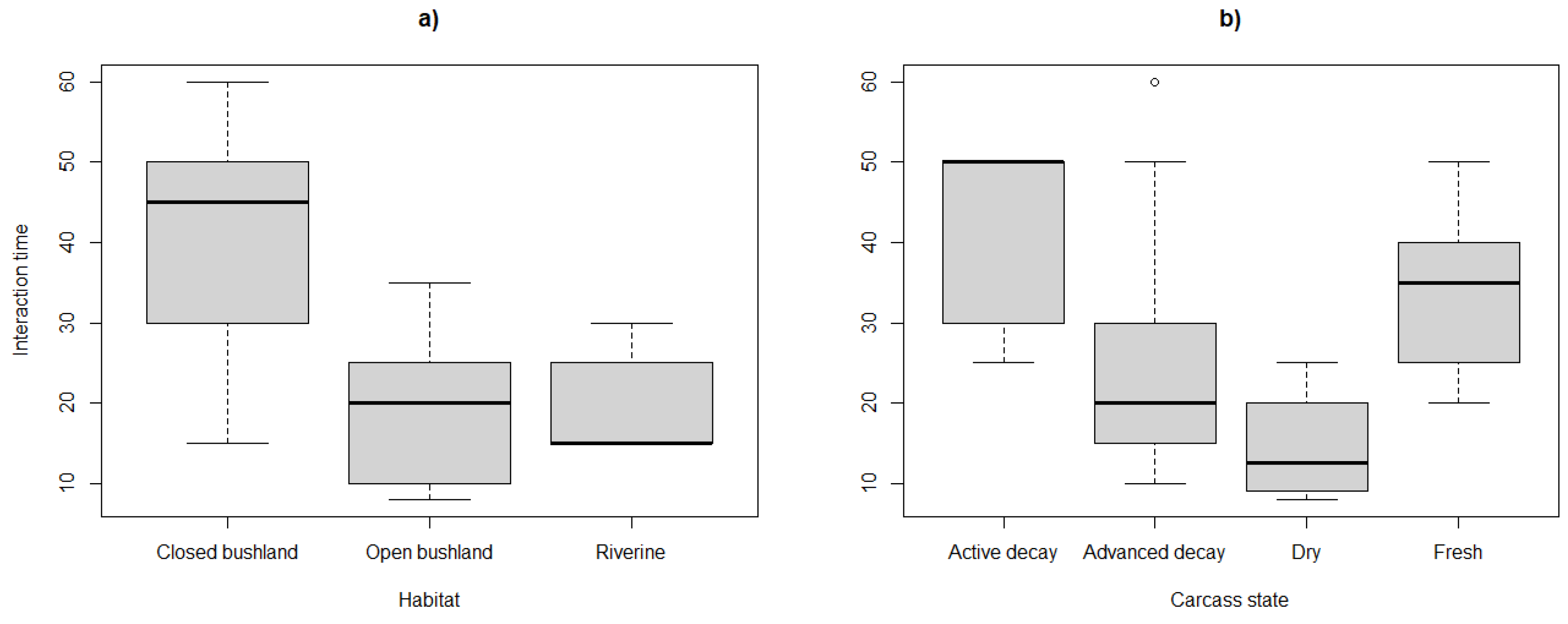
3. Results
3.1. Carcass descriptions and scavenger composition
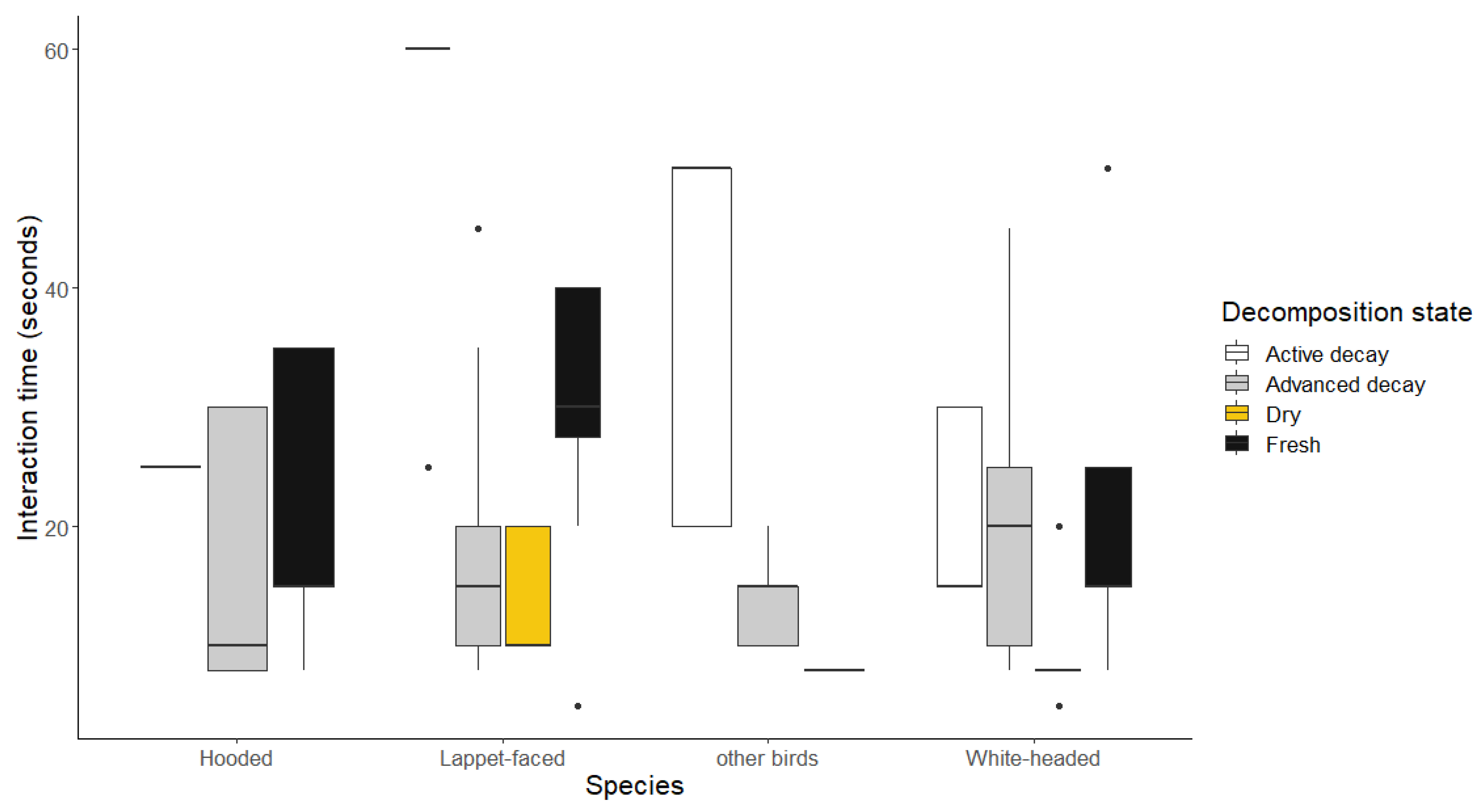
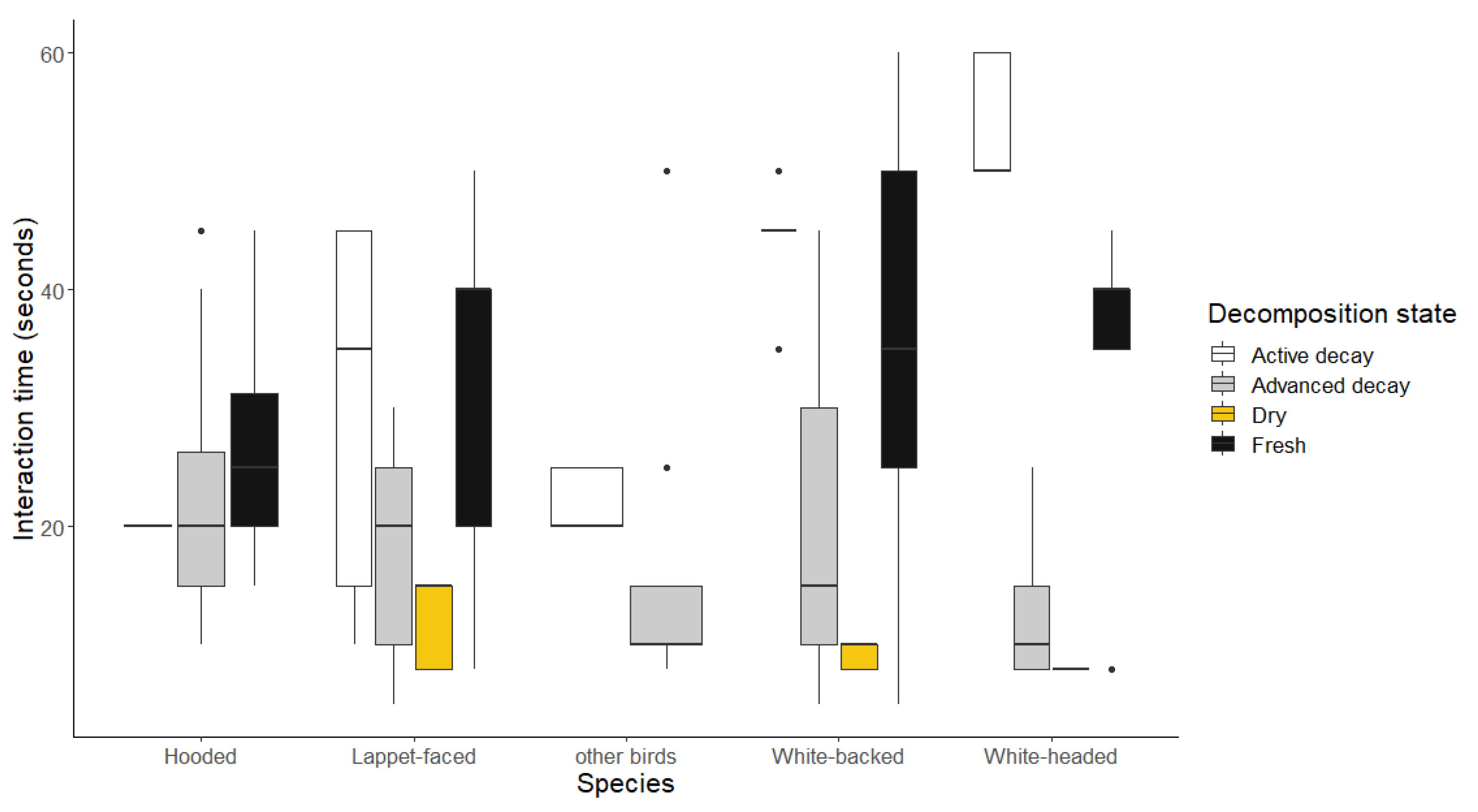
3.2. Vulture interactions
3.2.1. Cases of the Hooded Vulture dominating interactions
3.2.2. Cases of White-headed Vultures dominating interactions
3.2.3. Cases of White-backed Vultures dominating interactions
3.2.4. Cases of Lappet-faced Vultures dominating interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model | Variable | Estimate | Std. Error | z value | Pr(>|z|) |
| Hooded Vultures | Intercept | 3.63 | 0.04 | 77.895 | <0.0001 |
| Open woodland | -0.695 | 0.034 | -20.304 | < 0.0001 | |
| Riverine | -0.526 | 0.025 | -20.793 | < 0.0001 | |
| Giraffe carcass | - | - | - | - | |
| Kudu carcass | 0.581 | 0.053 | 11.485 | <0.0001 | |
| Lappet-faced Vulture | -0.333 | 0.07 | -4.716 | <0.0001 | |
| Other non-vulture birds | -0.802 | 0.128 | -6.581 | <0.0001 | |
| Advanced decay state | -0.023 | 0.048 | -0.478 | 0.633 | |
| Dry state | -0.775 | 0.113 | -6.853 | <0.0001 | |
| Fresh state | -0.558 | 0.053 | -10.584 | <0.0001 | |
| Lappet-faced Vulture: Advanced decay stage | -0.227 | 0.075 | -3.027 | 0.003 | |
| Other non-vulture birds: Advanced decay stage | 0.326 | 0.127 | 2.556 | 0.011 | |
| Lappet-faced Vulture: Dry stage | - | - | - | - | |
| Other non-vulture birds: Dry stage | - | - | - | - | |
| Lappet-faced Vulture: Fresh stage | 0.624 | 0.077 | 8.06 | <0.0001 | |
| Other non-vulture birds: Fresh stage | - | - | - | - | |
| White-headed | Intercept | 3.219 | 0.071 | 45.522 | <0.0001 |
| Open woodland | -0.36 | 0.024 | -14.864 | <0.0001 | |
| Riverine habitat | -0.563 | 0.038 | -14.752 | <0.0001 | |
| Giraffe carcass | - | - | - | - | |
| Kudu carcass | 0.285 | 0.049 | 0.0584 | <0.0001 | |
| Lappet-faced Vulture | 0.7367 | 0.0744 | 0.099 | <0.0001 | |
| Other non-vulture birds | 0.47 | 0.077 | 6.05 | <0.0001 | |
| White-headed Vulture | -0.27 | 0.079 | -3.427 | 0.001 | |
| Advanced decay stage | -0.366 | 0.076 | -4.82 | <0.0001 | |
| Dry stage | -0.392 | 0.070 | -0.056 | <0.0001 | |
| Fresh stage | -0.269 | 0.076 | -3.549 | 0.001 | |
| Lappet-faced Vulture: Advanced decay stage | -0.441 | 0.081 | -0.054 | <0.0001 | |
| Other non-vulture birds: Advanced decay stage | -0.618 | 0.089 | -0.069 | <0.0001 | |
| White-headed Vulture: Advanced decay stage | 0.722 | 0.084 | 8.573 | <0.0001 | |
| Lappet-faced Vulture: Dry stage | -0.564 | 0.091 | -0.0617 | <0.0001 | |
| Other non-vulture birds: Dry stage | -0.857 | 0.191 | -0.045 | <0.0001 | |
| White-headed Vulture: Dry stage | - | - | - | - | |
| Lappet-faced Vulture: Fresh stage | -0.361 | 0.083 | -0.048 | <0.0001 | |
| Other non-vulture birds: Fresh stage | - | - | - | - | |
| White-headed: Fresh stage | 0.404 | 0.086 | 0.047 | <0.0001 | |
| White-backed | Intercept | 2.974 | 0.057 | 52.547 | <0.0001 |
| Open woodland | -0.283 | 0.020 | -14.358 | <0.0001 | |
| Riverine habitat | 0.043 | 0.017 | 2.504 | 0.012 | |
| Giraffe carcass | - | - | - | - | |
| Kudu carcass | 0.355 | 0.040 | 8.83 | <0.0001 | |
| Lappet-faced Vulture | 0.434 | 0.062 | 6.953 | <0.0001 | |
| Other birds | 0.100 | 0.073 | 1.356 | 0.175 | |
| White-backed Vulture | 0.827 | 0.059 | 13.953 | <0.0001 | |
| White-headed Vulture | 0.991 | 0.060 | 16.444 | <0.0001 | |
| Advanced decay stage | 0.232 | 0.058 | 3.97 | <0.0001 | |
| Dry stage | -1.603 | 0.128 | -12.502 | <0.0001 | |
| Fresh stage | 0.286 | 0.060 | 4.762 | <0.0001 | |
| Lappet-faced Vulture: Advanced decay stage | -0.611 | 0.067 | -9.184 | <0.0001 | |
| Other non-vulture birds: Advanced decay stage | -0.446 | 0.081 | -5.454 | <0.0001 | |
| White-backed Vulture: Advanced decay stage | -0.864 | 0.063 | -13.65 | <0.0001 | |
| White-headed Vulture: Advanced decay stage | -1.518 | 0.066 | -22.878 | <0.0001 | |
| Lappet-faced Vulture: Dry stage | 0.962 | 0.141 | 6.841 | <0.0001 | |
| other on-vulture birds: Dry stage | - | - | - | - | |
| White-backed Vulture: Dry stage | 0.328 | 0.142 | 2.31 | 0.021 | |
| White-headed Vulture: Dry stage | - | - | - | - | |
| Lappet-faced Vulture: Fresh stage | -0.275 | 0.068 | -4.02 | 0.001 | |
| other non-vulture birds: Fresh stage | - | - | - | - | |
| White-backed Vulture: Fresh stage | -0.511 | 0.065 | -7.877 | <0.0001 | |
| White-headed Vulture: Fresh stage | -0.762 | 0.067 | -11.448 | <0.0001 | |
| Lappet-faced | Intercept | 3.805 | 0.024 | 158.556 | <0.0001 |
| Open woodland | -0.875 | 0.028 | -31.211 | <0.0001 | |
| Riverine | -0.922 | 0.034 | -27.032 | <0.0001 | |
| Giraffe carcass | - | - | - | - | |
| Kudu carcass | 0.137 | 0.066 | 2.084 | 0.037 | |
| Advanced decay stage | 0.009 | 0.029 | 0.307 | 0.759 | |
| Dry stage | -0.256 | 0.053 | -4.868 | <0.0001 | |
| Fresh stage | -0.225 | 0.031 | -7.155 | <0.0001 |
References
- Hovardas, T. and K. Korfiatis, Towards a critical re-appraisal of ecology education: Scheduling an educational intervention to revisit the ‘balance of nature’metaphor. Science and Education, 2011. 20: p. 1039-1053. [CrossRef]
- McKean, S., M. Mander, N. Diederichs, L. Ntuli, K. Mavundla, V. Williams, and J. Wakelin, The impact of traditional use on vultures in South Africa. Vulture News, 2013. 65: p. 15-36. [CrossRef]
- Thompson, L. and A. Blackmore, A brief review of the legal protection of vultures in South Africa. Ostrich, 2020. 91(1): p. 1-12. [CrossRef]
- Kendall, C., M. Virani, P. Kirui, S. Thomsett, and M. Githiru, Mechanisms of coexistence in vultures: understanding the patterns of vulture abundance at carcasses in Masai Mara National Reserve, Kenya. The Condor, 2012. 114(3): p. 523-531. [CrossRef]
- Carrete, M. and J. Donázar, Application of central-place foraging theory shows the importance of Mediterranean dehesas for the conservation of the cinereous vulture, Aegypius monachus. Biological Conservation, 2005. 126(4): p. 582-590. [CrossRef]
- Mundy, P., D. Butchart, J. Ledger, and S. Piper, The vultures of Africa. Vol. 671. 1992: Academic Press London.
- Ogada, D., P. Shaw, R. Beyers, R. Buij, C. Murn, J. Thiollay, C. Beale, R. Holdo, D. Pomeroy, and N. Baker, Another continental vulture crisis: Africa's vultures collapsing toward extinction. Conservation Letters, 2016. 9(2): p. 89-97. [CrossRef]
- Virani, M., C. Kendall, P. Njoroge, and S. Thomsett, Major declines in the abundance of vultures and other scavenging raptors in and around the Masai Mara ecosystem, Kenya. Biological Conservation, 2011. 144(2): p. 746-752. [CrossRef]
- IUCN, S., IUCN SSC Guiding principles on Creating Proxies of Extinct Species, 2016, IUCN.
- Ogada, D., The power of poison: pesticide poisoning of Africa's wildlife. Annals of the New York Academy of Sciences, 2014. 1322(1): p. 1-20. [CrossRef]
- Ottinger, M., A. Botha, R. Buij, B. Coverdale, M. Gore, R. Harrell, J. Hassell, S. Krüger, C. McClure, and J. Mullinax, A strategy for conserving Old World vulture populations in the framework of One Health. Journal of Raptor Research, 2021. 55(3): p. 374-387. [CrossRef]
- Monadjem, A., M. Anderson, S. Piper, and A. Boshoff. The vultures of southern Africa-quo vadis. in Proceedings of a workshop on vulture research and conservation in southern Africa. Bird of Prey Working Group, Endangered Wildlife Trust, Johannesburg, South Africa. 2004.
- Selva, N. and M. Fortuna, The nested structure of a scavenger community. Proceedings of the Royal Society B: Biological Sciences, 2007. 274(1613): p. 1101-1108. [CrossRef]
- Blazquez, M., J. Sanchez-Zapata, F. Botella, M. Carrete, and S. Eguía, Spatio-temporal segregation of facultative avian scavengers at ungulate carcasses. Acta Oecologica, 2009. 35(5): p. 645-650. [CrossRef]
- Kruuk, H. and M. Turner, Comparative notes on predation by lion, leopard, cheetah and wild dogs in the Serengeti area, East Africa. Mammalia, 1967.
- Kendall, C., The early bird gets the carcass: temporal segregation and its effects on foraging success in avian scavengers. The Auk: Ornithological Advances, 2014. 131(1): p. 12-19. [CrossRef]
- Brown, J. and B. Maurer, Body size, ecological dominance and Cope's rule. Nature, 1986. 324(6094): p. 248-250. [CrossRef]
- Gomez, L., D. Houston, P. Cotton, and A. Tye, The role of Greater Yellow-headed Vultures Cathartes melambrotus as scavengers in neotropical forest. Ibis, 1994. 136(2): p. 193-196. [CrossRef]
- Hunter, J., S. Durant, and T. Caro, Patterns of scavenger arrival at cheetah kills in Serengeti National Park Tanzania. African Journal of Ecology, 2007. 45(3): p. 275-281. [CrossRef]
- Harel, R., O. Duriez, O. Spiegel, J. Fluhr, N. Horvitz, W. Getz, W. Bouten, F. Sarrazin, O. Hatzofe, and R. Nathan, Decision-making by a soaring bird: time, energy and risk considerations at different spatio-temporal scales. Philosophical Transactions of the Royal Society B: Biological Sciences, 2016. 371(1704): p. 20150397. [CrossRef]
- Bamford, A., A. Monadjem, M. Anderson, A. Anthony, W. Borello, M. Bridgeford, P. Bridgeford, P. Hancock, B. Howells, and J. Wakelin, Trade-offs between specificity and regional generality in habitat association models: a case study of two species of African vulture. Journal of Applied Ecology, 2009. 46(4): p. 852-860. [CrossRef]
- Platt, S. and T. Rainwater, Noteworthy observations of foraging turkey vultures. The Wilson Journal of Ornithology, 2009: p. 839-841. [CrossRef]
- Pereira, L., N. Owen-Smith, and M. Moleón, Facultative predation and scavenging by mammalian carnivores: Seasonal, regional and intra-guild comparisons. Mammal review, 2014. 44(1): p. 44-55. [CrossRef]
- Valverde, I., S. Espín, P. María-Mojica, and A. García-Fernández, Protocol to classify the stages of carcass decomposition and estimate the time of death in small-size raptors. European Journal of Wildlife Research, 2020. 66(6): p. 93. [CrossRef]
- Rogers, C., A woody vegetation survey of Hwange National Park, Zimbabwe. Department of National Parks and Wildlife Management report, 1993.
- Oksanen, J., F.G. Blanchet, R. Kindt, P. Legendre, P.R. Minchin, R. O’hara, G.L. Simpson, P. Solymos, M. Stevens, and H. Wagner, vegan: Community Ecology Package. R package version 2.0-10. 2022.
- R Development Core Team, R: A language and environment for statistical computing. Vienna, Austria; 2014, 2022.
- Miller, C., M. Pittman, X. Wang, X. Zheng, and J. Bright, Diet of Mesozoic toothed birds (Longipterygidae) inferred from quantitative analysis of extant avian diet proxies. BMC biology, 2022. 20(1): p. 1-37. [CrossRef]
- du Toit, J., Large herbivores and savanna heterogeneity. The Kruger experience: Ecology and management of savanna heterogeneity, 2003. 4(1): p. 292-309.
- Fritz, H., P. Duncan, I.J. Gordon, and A. Illius, Megaherbivores influence trophic guilds structure in African ungulate communities. Oecologia, 2002. 131: p. 620-625. [CrossRef]
- Hill, J., T. DeVault, J. Beasley, O. Rhodes Jr, and J. Belant, Effects of vulture exclusion on carrion consumption by facultative scavengers. Ecology and Evolution, 2018. 8(5): p. 2518-2526. [CrossRef]
- Hunter, J., D. Durant, and T. Caro, Patterns of scavenger arrival at cheetah kills in Serengeti National Park Tanzania. African Journal of Ecology, 2006: p. 1-7. [CrossRef]
- Houston, D., The role of griffon vultures Gyps africanus and Gyps ruppellii as scavengers. Journal of Zoology, 1974. 172(1): p. 35-46. [CrossRef]
- Nyathi, S., J. Olowoyo, and A. Oludare, Perception of scavengers and occupational health hazards associated with scavenging from a waste dumpsite in Pretoria, South Africa. Journal of environmental and public health, 2018. 2018. [CrossRef]
- Cortés-Avizanda, A., G. Blanco, T. DeVault, A. Markandya, M. Virani, J. Brandt, and J. Donázar, Supplementary feeding and endangered avian scavengers: benefits, caveats, and controversies. Frontiers in Ecology and the Environment, 2016. 14(4): p. 191-199. [CrossRef]
- Kane, A. and C. Kendall, Understanding how mammalian scavengers use information from avian scavengers: cue from above. Journal of Animal Ecology, 2017: p. 837-846. [CrossRef]
- García Alfonso, M., Individual and environmental drivers of resource use in an endangered vulture: Integrating movement, spatial and social ecology. 2020.
- Stattersfield, A., D. Capper, G. Dutson, and T. Morrisey, Threatened birds of the world: the official source for birds on the IUCN Red List. Bird Life International.2000, Barcelona and Cambridge.
- Kasielke, S. and S. Keeper, White-backed Vulture, Gyps africanus. 2015, Berkeley: University of California Press.
- Ferguson-Lees, J. and D. Christie, Raptors of the world2001: Houghton Mifflin Harcourt.
- Duffey, E., Handbook of the birds of the world, vol. 3. Hoatzin to auks: Edited by Josep del Hoyo, Andrew Elliot and Jordi Sargatal. 1996. Lynx Editions, Barcelona. 824 pp. ISBN 84 87334 20 2. Price£ 110.00, 1998, Elsevier.
- Murn, C., Field identification of individual White-headed Vultures Trigonoceps occipitalis using plumage patterns-an information theoretic approach. Bird Study, 2012. 59(4): p. 515-521. [CrossRef]
- Shimelis, A., E. Sande, S. Evans, and P.J. Mundy, International Species Action Plan for the Lappet-faced Vulture, Torgos tracheliotus BirdLife International, 2005, BirdLife International Africa Partnership Secretariat.
- Petrides, G., Competition for food between five species of East African vultures. The Auk, 1959. 76(1): p. 104-106. [CrossRef]
- Kemp, A., G. Kirwan, D. Christie, and C. Sharpe, eds. White-headed Vulture (Trigonoceps occipitalis). In Birds of the World. 2020, Cornell Lab of Ornithology. [CrossRef]
- Portugal, S., C. Murn, and G. Martin, White-headed Vulture Trigonoceps occipitalis shows visual field characteristics of hunting raptors. Ibis, 2017. 159(2): p. 463-466. [CrossRef]
- Hockey, P., W. Dean, and P. Ryan. Roberts birds of southern Africa. 7th edn. Trustees of the John Voelcker Bird Book Fund: Cape Town. 2005.
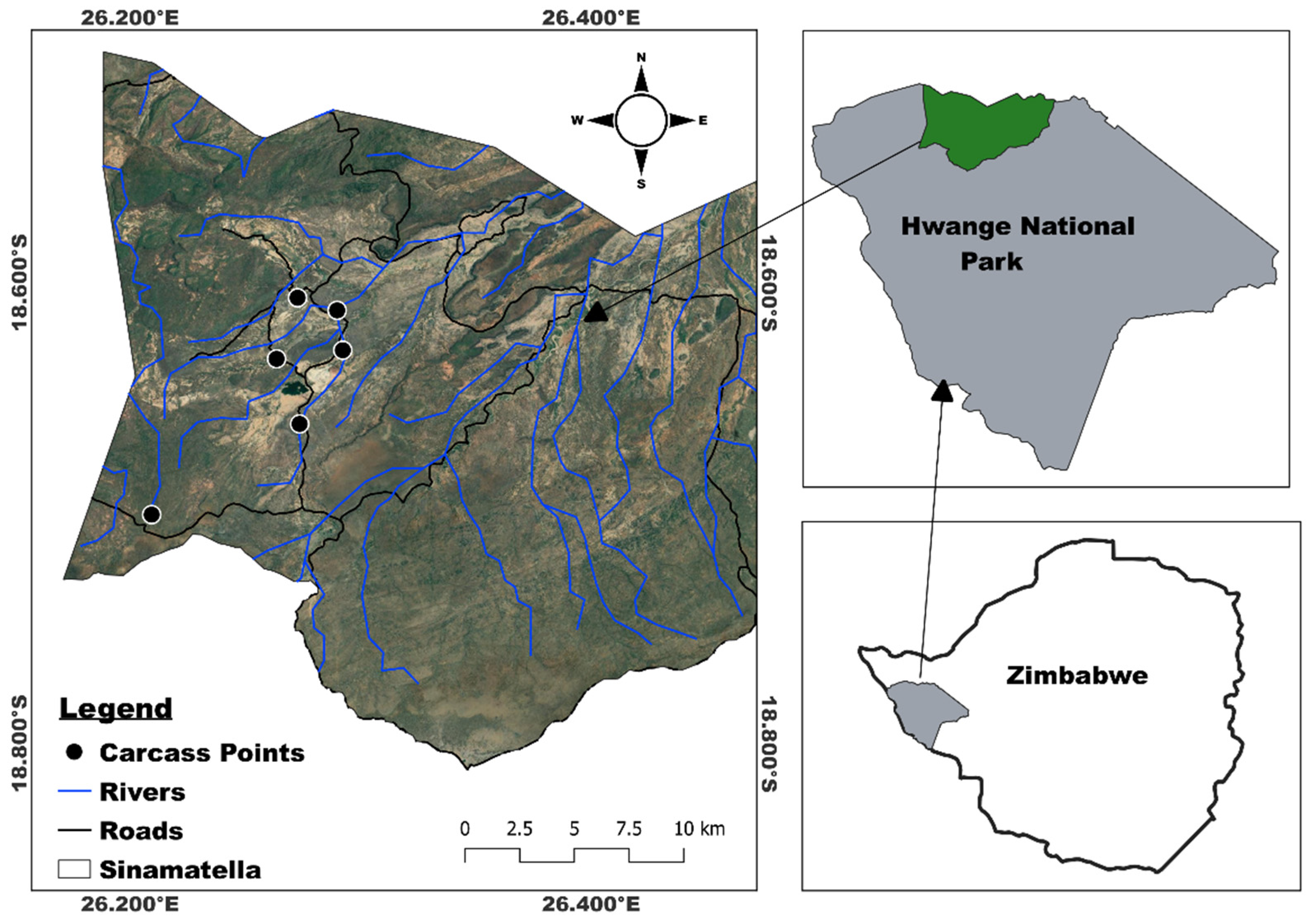
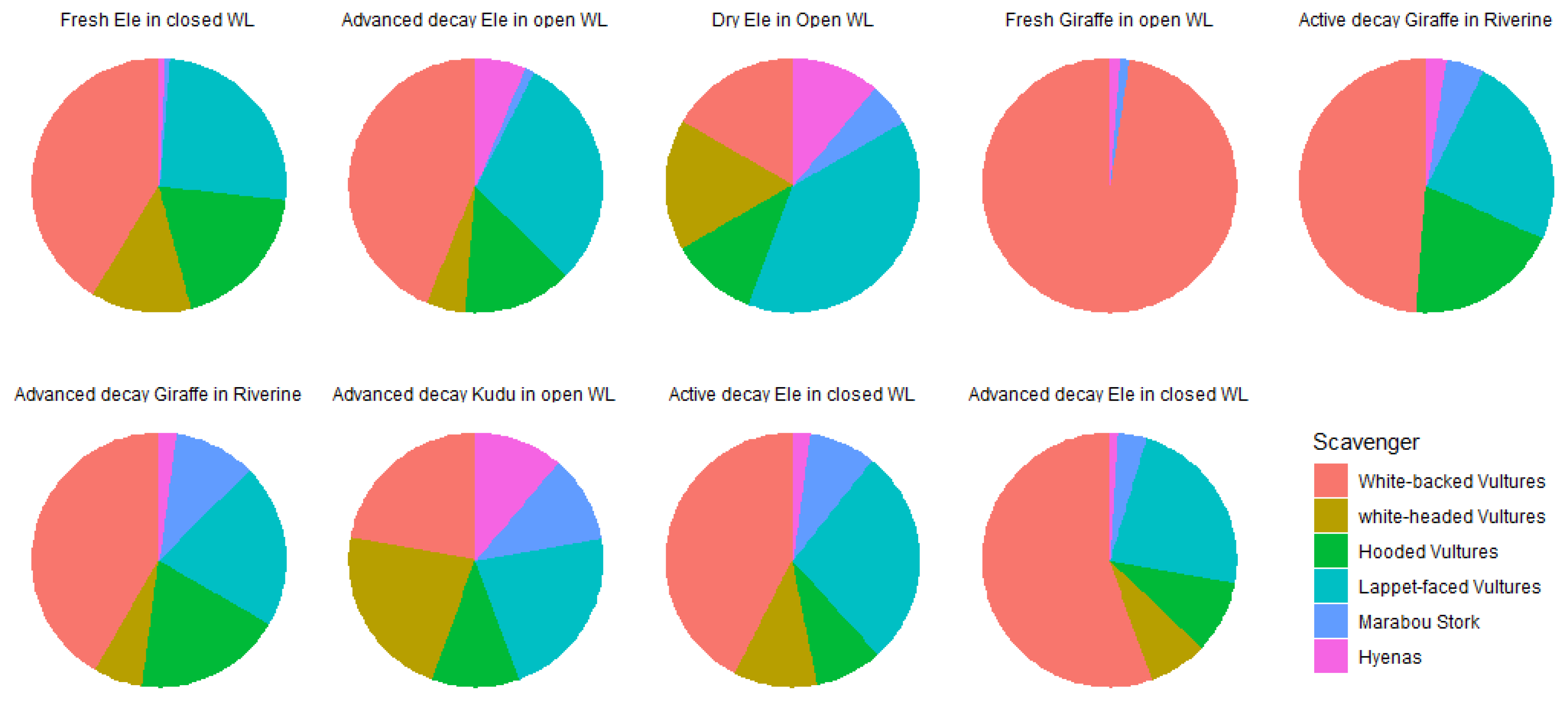
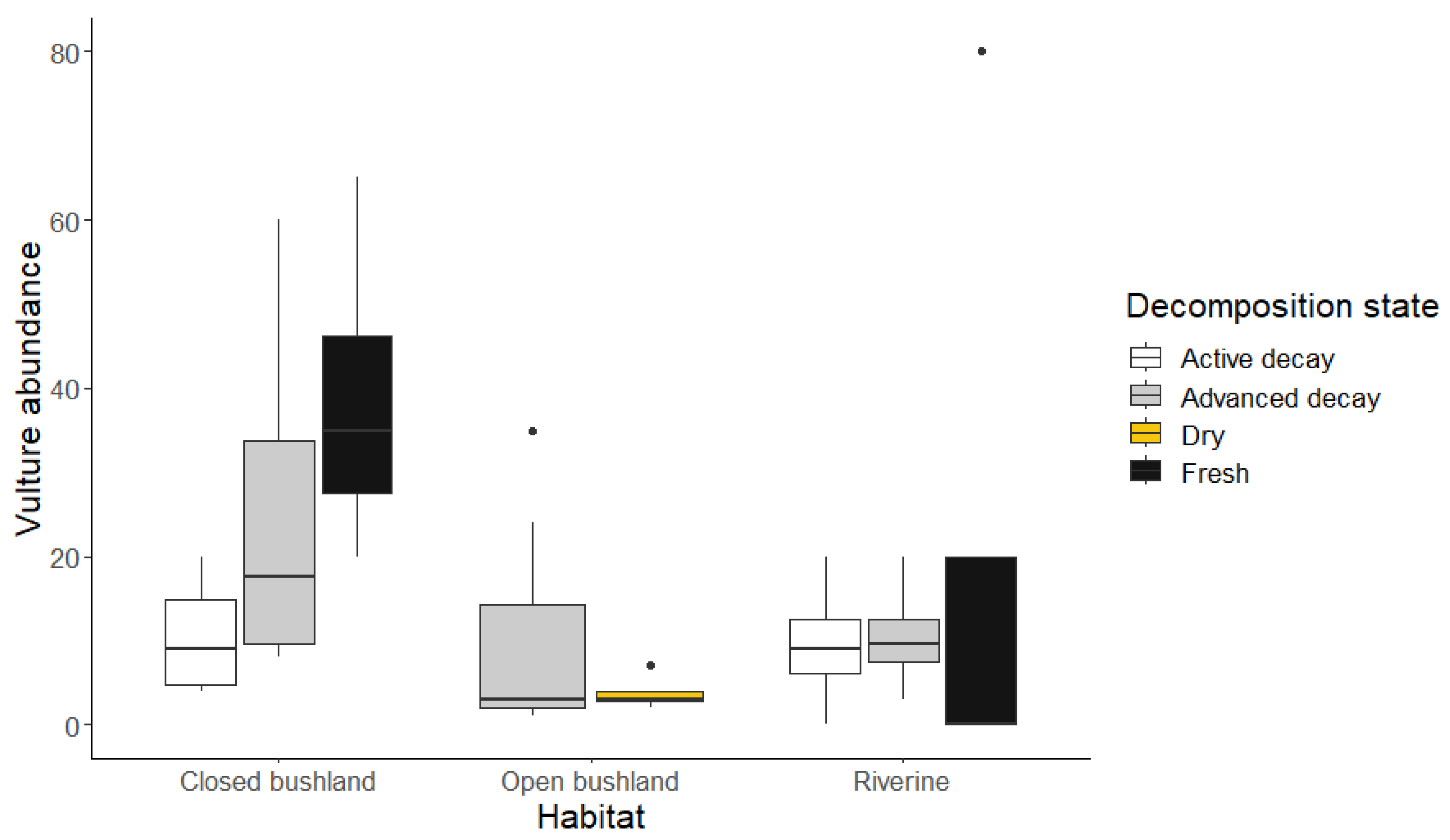
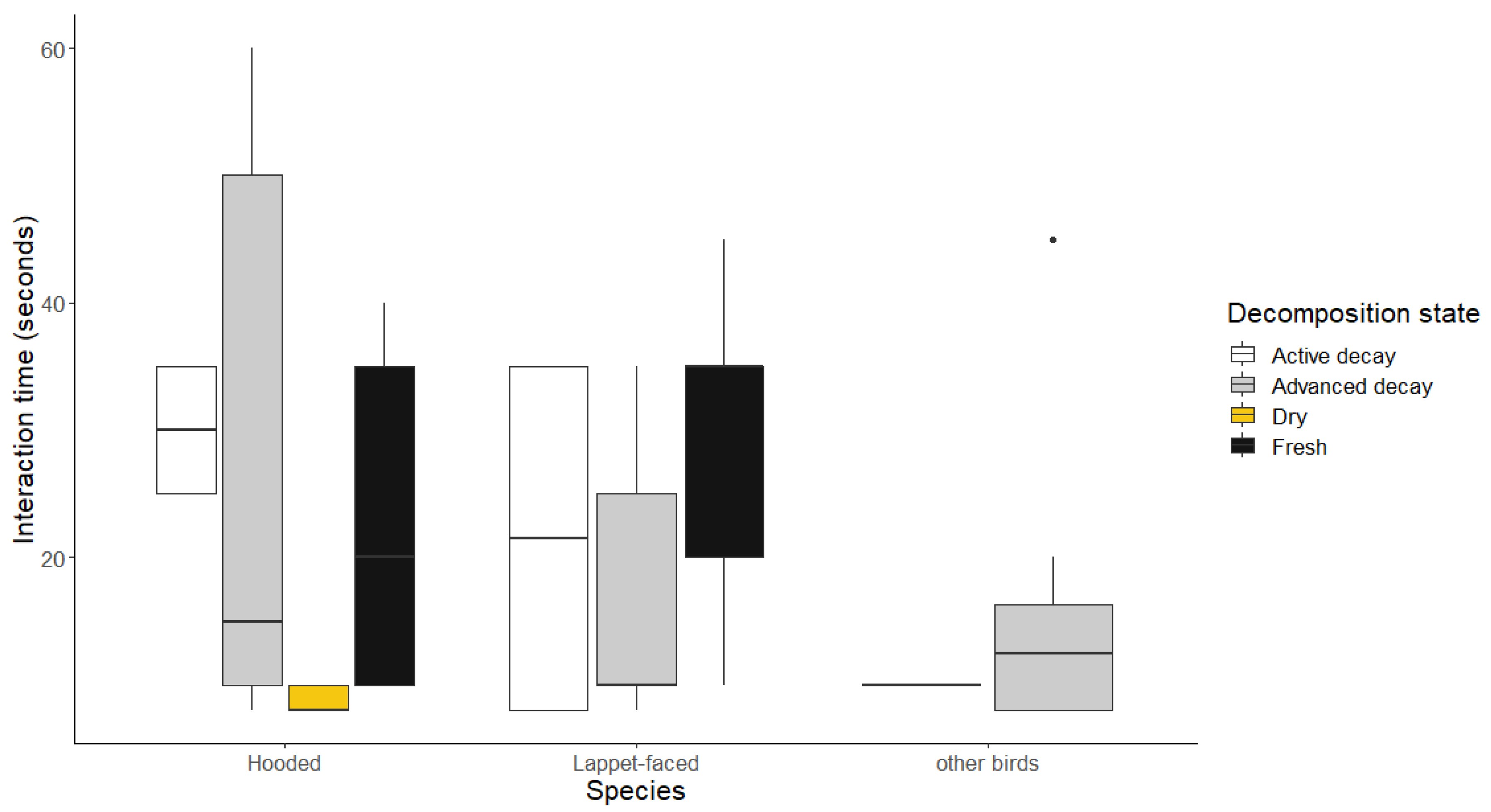
| Carcass ID | Carcass Species | Closed woodland | Open woodland | Riverine | Decomposition states monitored |
|---|---|---|---|---|---|
| 1 | Elephant | √ | i, iii | ||
| 2 | Elephant | √ | iii, iv | ||
| 3 | Giraffe | √ | i, ii, iii | ||
| 4 | Elephant | √ | Iii | ||
| 5 | Kudu | √ | Iii | ||
| 6 | Elephant | √ | Ii |
| Variable | Estimate | Std. Error | z value | p value |
|---|---|---|---|---|
| Intercept | 2.351 | 0.154 | 15.239 | < 0.0001 |
| Open woodland | -0.933 | 0.149 | -6.285 | < 0.0001 |
| Riverine | -0.1 | 0.224 | -0.447 | 0.65486 |
| Advanced decay | 0.897 | 0.183 | 4.9 | < 0.0001 |
| Dry state | -0.096 | 0.335 | -0.287 | 0.7743 |
| Fresh state | 1.306 | 0.174 | 7.506 | < 0.0001 |
| Open woodland:Advanced decay | - | - | - | - |
| Riverine:Advanced decay | -0.797 | 0.289 | -2.756 | 0.00586 |
| Open woodland:Dry | - | - | - | - |
| Riverine : Dry state | - | - | - | - |
| Open woodland : Fresh state | - | - | - | - |
| Riverine : Fresh state | -0.561 | 0.263 | -2.136 | 0.0327 |
| Vulture species | ||||||
|---|---|---|---|---|---|---|
| Hooded | Lappet-faced | White-backed | White-headed | Other birds | ||
| Vulture interaction | Hooded loosing | 16 | 16 | 0 | 0 | 0 |
| Hooded winning | 280 | 244 | 0 | 0 | 56 | |
| Lappet-faced loosing | 0 | 24 | 0 | 0 | 0 | |
| Lappet-faced winning | 0 | 404 | 0 | 0 | 0 | |
| White-backed loosing | 0 | 4 | 0 | 16 | 0 | |
| White-backed winning | 272 | 380 | 404 | 272 | 80 | |
| White-Headed winning | 176 | 260 | 0 | 372 | 88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





