Submitted:
27 July 2023
Posted:
28 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Participants
2.3. Preoperative examination
2.4. Surgical technique
2.5. Intraocular lenses
2.6. Postoperative examination
2.7. Statistical analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etya'Ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Tseng, V.L.; Yu, F.; Lum, F.; Coleman, A.L. Risk of Fractures Following Cataract Surgery in Medicare Beneficiaries. JAMA 2012, 308, 493–501. [Google Scholar] [CrossRef]
- Leske, M.; Wu, S.-Y.; Nemesure, B.; Hennis, A.; Barbados Eye Studies Group. Risk factors for incident nuclear opacities. Ophthalmology 2002, 109, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.C.; Gordon, M.O.; Beiser, J.A.; Chylack, L.T.; Lamping, K.A.; Schein, O.D.; Soltau, J.B.; Kass, M.A.; Ocular Hypertension Treatment Study (OHTS) Group. Topical Ocular Hypotensive Medication and Lens Opacification: Evidence From the Ocular Hypertension Treatment Study. Am. J. Ophthalmol. 2006, 142, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Cataract Surgery Outcomes in Glaucomatous Eyes: Results From the Veterans Affairs Ophthalmic Surgery Outcomes Data Project - ClinicalKey. https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0002939415004444?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0002939415004444%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ajo.com%2F (accessed 2023-07-02).
- Musch, D.C.; Gillespie, B.W.; Niziol, L.M.; Janz, N.K.; Wren, P.A.; Rockwood, E.J.; Lichter, P. R; Collaborative Initial Glaucoma Treatment Study Group. Cataract Extraction in the Collaborative Initial Glaucoma Treatment Study: Incidence, Risk Factors, and the Effect of Cataract Progression and Extraction on Clinical and Quality-of-Life Outcomes. Arch. Ophthalmol. 2006, 124, 1694–1700. [Google Scholar] [CrossRef]
- Bhartiya, S.; Sharma, A.; Ichhpujani, P. Premium IOLs in Glaucoma. J. Curr. Glaucoma Pr. 2013, 7, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Ichhpujani, P.; Thakur, S.; Spaeth, G.L. Contrast Sensitivity and Glaucoma. Eur. J. Gastroenterol. Hepatol. 2020, 29, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Braga-Mele, R.; Chang, D.; Dewey, S.; Foster, G.; Henderson, B.A.; Hill, W.; Hoffman, R.; Little, B.; Mamalis, N.; Oetting, T.; et al. Multifocal intraocular lenses: Relative indications and contraindications for implantation. J. Cataract. Refract. Surg. 2014, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Schallhorn, J.M.; Pantanelli, S.M.; Lin, C.C.; Al-Mohtaseb, Z.N.; Steigleman, W.A.; Santhiago, M.R.; Olsen, T.W.; Kim, S.J.; Waite, A.M.; Rose-Nussbaumer, J.R. Multifocal and Accommodating Intraocular Lenses for the Treatment of Presbyopia: A Report by the American Academy of Ophthalmology. Ophthalmology 2021, 128, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J.C.; Ahmed, I.I.K. Intraocular lens choices for patients with glaucoma. Curr. Opin. Ophthalmol. 2010, 21, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.J.; Wilson, C.W.; Shafer, B.M.; Berdahl, J.P.; Terveen, D.C. Clinical Outcomes of a Non-Diffractive Extended Depth-of-Focus IOL in Eyes with Mild Glaucoma. Clin. Ophthalmol. 2023, ume 17, 861–868. [Google Scholar] [CrossRef]
- Pedrotti, E.; Bruni, E.; Bonacci, E.; Badalamenti, R.; Mastropasqua, R.; Marchini, G. Comparative Analysis of the Clinical Outcomes With a Monofocal and an Extended Range of Vision Intraocular Lens. J. Refract. Surg. 2016, 32, 436–442. [Google Scholar] [CrossRef]
- Lee, J.; Mori, Y.; Nejima, R.; Minami, K.; Miyata, K. Influence of implantations of extended depth-of-focus on standard automated perimetry. Sci. Rep. 2020, 10, 20153. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamashiro, C.; Yoshimoto, T.; Kobayashi, Y.; Higashijima, F.; Kobayashi, M.; Hatano, M.; Ohta, M.; Nagai, T.; Teranishi, S.; et al. Influence of extended depth of focus intraocular lenses on visual field sensitivity. PLOS ONE 2020, 15, e0237728. [Google Scholar] [CrossRef] [PubMed]
- Auffarth, G.U.; Gerl, M.; Tsai, L.; Janakiraman, D.P.; Jackson, B.; Alarcon, A.; Dick, H.B. ; Quantum Study Group Clinical evaluation of a new monofocal IOL with enhanced intermediate function in patients with cataract. J. Cataract. Refract. Surg. 2020, 47, 184–191. [Google Scholar] [CrossRef]
- Wan, K.H.; Au, A.C.; Kua, W.N.; Ng, A.L.; Cheng, G.P.; Lam, N.M.; Chow, V.W. Enhanced Monofocal Versus Conventional Monofocal Intraocular Lens in Cataract Surgery: A Meta-analysis. J. Refract. Surg. 2022, 38, 538–546. [Google Scholar] [CrossRef]
- Rampat, R.; Gatinel, D. Multifocal and Extended Depth-of-Focus Intraocular Lenses in 2020. Ophthalmology 2021, 128, e164–e185. [Google Scholar] [CrossRef] [PubMed]
- Bala, C.; Poyales, F.; Guarro, M.; Mesa, R.R.; Mearza, A.M.; Varma, D.K.B.; Jasti, S.; Lemp-Hull, J.D. Multicountry clinical outcomes of a new nondiffractive presbyopia-correcting IOL. J. Cataract. Refract. Surg. 2022, 48, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Majstruk, L.; Leray, B.; Bouillot, A.; Michée, S.; Sultan, G.; Baudouin, C.; Labbé, A. Long term effect of phacoemulsification on intraocular pressure in patients with medically controlled primary open-angle glaucoma. BMC Ophthalmol. 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.; Lee, J. Measurement of macular structure-function relationships using spectral domain-optical coherence tomography (SD-OCT) and pattern electroretinograms (PERG). PLOS ONE 2017, 12, e0178004. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Eom, Y.; Park, S.Y.; Choi, S.Y.; Hwang, H.S.; Kim, J.-H.; Song, J.S.; Kim, H.M. Rainbow halos occur less following implantation of extended range of vision one-piece intraocular lenses vs diffractive bifocal intraocular lenses. Int. J. Ophthalmol. 2020, 13, 913–919. [Google Scholar] [CrossRef]
- Hood, D.C.; Kardon, R.H. A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef]
- Huh, J.; Eom, Y.; Yang, S.K.; Choi, Y.; Kim, H.M.; Song, J.S. A comparison of clinical outcomes and optical performance between monofocal and new monofocal with enhanced intermediate function intraocular lenses: a case-control study. BMC Ophthalmol. 2021, 21, 365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jung, J.W.; Lee, J.M.; Seo, K.Y.; Kim, E.K.; Kim, T.-I. Clinical Outcomes Following Implantation of Diffractive Multifocal Intraocular Lenses With Varying Add Powers. Arch. Ophthalmol. 2015, 160, 702–709. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.E.; Nuijts, R.M. Multifocal intraocular lenses in cataract surgery: Literature review of benefits and side effects. J. Cataract. Refract. Surg. 2013, 39, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Black, S. A clinical assessment of visual performance of combining the TECNIS® Symfony Extended Range of Vision IOL (ZXR00) with the +3.25 D TECNIS Multifocal 1-piece IOL (ZLB00) in subjects undergoing bilateral cataract extraction. Clin. Ophthalmol. 2018, ume 12, 2129–2136. [CrossRef]
- Rosen, E.; Alió, J.L.; Dick, B.H.; Dell, S.; Slade, S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: Metaanalysis of peer-reviewed publications. J. Cataract. Refract. Surg. 2016, 42, 310–328. [Google Scholar] [CrossRef]
- Fernández, J.; Rocha-De-Lossada, C.; Zamorano-Martín, F.; Rodríguez-Calvo-De-Mora, M.; Rodríguez-Vallejo, M. Positioning of enhanced monofocal intraocular lenses between conventional monofocal and extended depth of focus lenses: a scoping review. BMC Ophthalmol. 2023, 23, 101. [Google Scholar] [CrossRef]
- Choi, W.K.; Han, H.J.; Son, H.-S.; Khoramnia, R.; Auffarth, G.U.; Choi, C.Y. Clinical outcomes of bilateral implantation of new generation monofocal IOL enhanced for intermediate distance and conventional monofocal IOL in a Korean population. BMC Ophthalmol. 2023, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.B.; Hotchkiss, J.; Scally, A.J.; Foster, R.; Buckley, J.G. Intermediate addition multifocals provide safe stair ambulation with adequate ‘short-term’ reading. Ophthalmic Physiol. Opt. 2016, 36, 60–68. [Google Scholar] [CrossRef]
- Mencucci, R.; Cennamo, M.; Venturi, D.; Vignapiano, R.; Favuzza, E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: preliminary results. J. Cataract. Refract. Surg. 2020, 46, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Cinar, E.; Bolu, H.; Erbakan, G.; Yuce, B.; Aslan, F.; Fece, M.; Emre, S. Vision outcomes with a new monofocal IOL. Int. Ophthalmol. 2021, 41, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.S.; Szlyk, J.P.; Ardickas, Z.; Alexander, K.R.; Wilensky, J.T. Comparison of Contrast Sensitivity, Visual Acuity, and Humphrey Visual Field Testing in Patients with Glaucoma. Eur. J. Gastroenterol. Hepatol. 2003, 12, 134–138. [Google Scholar] [CrossRef]
- Korth, M.J.; Jünemann, A.M.G.; Horn, F.K.; Bergua, A.; Cursiefen, C.; Velten, I.; Budde, W.M.; Wisse, M.; Martus, P. Synopsis of various electrophysiological tests in early glaucoma diagnosis--temporal and spatiotemporal contrast sensitivity, light- and color-contrast pattern-reversal electroretinogram, blue-yellow VEP. Klin. Monatsbl. Augenheilkd. 2000, 216, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Cillino, S.; Casuccio, A.; Di Pace, F.; Morreale, R.; Pillitteri, F.; Cillino, G.; Lodato, G. One-Year Outcomes with New-Generation Multifocal Intraocular Lenses. Ophthalmology 2008, 115, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Barisić, A.; Dekaris, I.; Gabrić, N.; Bohac, M.; Romac, I.; Mravicić, I.; Lazić, R. Comparison of Diffractive and Refractive Multifocal Intraocular Lenses in Presbyopia Treatment. Coll. Antropol. 2008, 32 Suppl 2, 27–31. [Google Scholar]
- Mesci, C.; Erbil, H.H.; Olgun, A.; Aydin, N.; Candemir, B.; A Akçakaya, A. Differences in contrast sensitivity between monofocal, multifocal and accommodating intraocular lenses: long-term results. Clin. Exp. Ophthalmol. 2010, 38, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, A.; Cánovas, C.; Koopman, B.; Weeber, H.; Auffarth, G.U.; Piers, P.A. Enhancing the Intermediate Vision of Monofocal Intraocular Lenses Using a Higher Order Aspheric Optic. J. Refract. Surg. 2020, 36, 520–527. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Nakao, F.; Hayashi, F. Influence of cataract surgery on automated perimetry in patients with glaucoma. Arch. Ophthalmol. 2001, 132, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Seol, B.R.; Jeoung, J.W.; Park, K.H. Changes of visual-field global indices after cataract surgery in primary open-angle glaucoma patients. Jpn. J. Ophthalmol. 2016, 60, 439–445. [Google Scholar] [CrossRef]
- Rao, H.L.; Jonnadula, G.B.; Addepalli, U.K.; Senthil, S.; Garudadri, C.S. Effect of Cataract Extraction on Visual Field Index in Glaucoma. Eur. J. Gastroenterol. Hepatol. 2013, 22, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Chak, G.; Garg, S.; Steinert, R.F. Reduction in Mean Deviation Values in Automated Perimetry in Eyes with Multifocal Compared to Monofocal Intraocular Lens Implants. Arch. Ophthalmol. 2014, 158, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Aychoua, N.; Montolio, F.G.J.; Jansonius, N.M. Influence of Multifocal Intraocular Lenses on Standard Automated Perimetry Test Results. JAMA Ophthalmol 2013, 131, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.J.; Samples, J.R.; Dustin, L. Relationship Between Foveal Threshold and Visual Acuity Using the Humphrey Visual Field Analyzer. Arch. Ophthalmol. 2007, 143, 875–877. [Google Scholar] [CrossRef]
| Variable | Enhanced monofoal IOL (n = 34) | Standard monofocal IOL (n = 38) | p-Value* |
|---|---|---|---|
| Age (years) | 68.76 ± 6.96 | 66.00 ± 7.59 | 0.113 |
| Sex (male/female) | 18/16 | 18/20 | 0.979 |
| Laterality (right/left) | 19/15 | 18/20 | 0.471 |
| SE (diopter) | 0.46 ± 1.67 | 0.75 ± 1.80 | 0.488 |
| Axial length (mm) | 24.74 ± 0.77 | 24.57 ± 0.84 | 0.370 |
| Mesopic pupil size (mm) | 3.76 ± 0.82 | 3.90 ± 0.83 | 0.478 |
| UDVA (logMAR) | 0.39 ± 0.17 | 0.36 ± 0.20 | 0.612 |
| CDVA (logMAR) | 0.22 ± 0.16 | 0.23 ± 0.14 | 0.698 |
| UIVA (logMAR) | 0.42 ± 0.14 | 0.43 ± 0.17 | 0.880 |
| UNVA (logMAR) | 0.56 ± 0.14 | 0.52 ± 0.14 | 0.183 |
| IOP (mmHg) | 12.76 ± 1.94 | 12.97 ± 1.81 | 0.638 |
| Global pRNFL thickness (μm) | 86.29 ± 10.67 | 84.53 ± 10.44 | 0.480 |
| Number of glaucoma medications | 1.47 ± 0.51 | 1.45 ± 0.50 | 0.846 |
| 30-2 VF parameters | |||
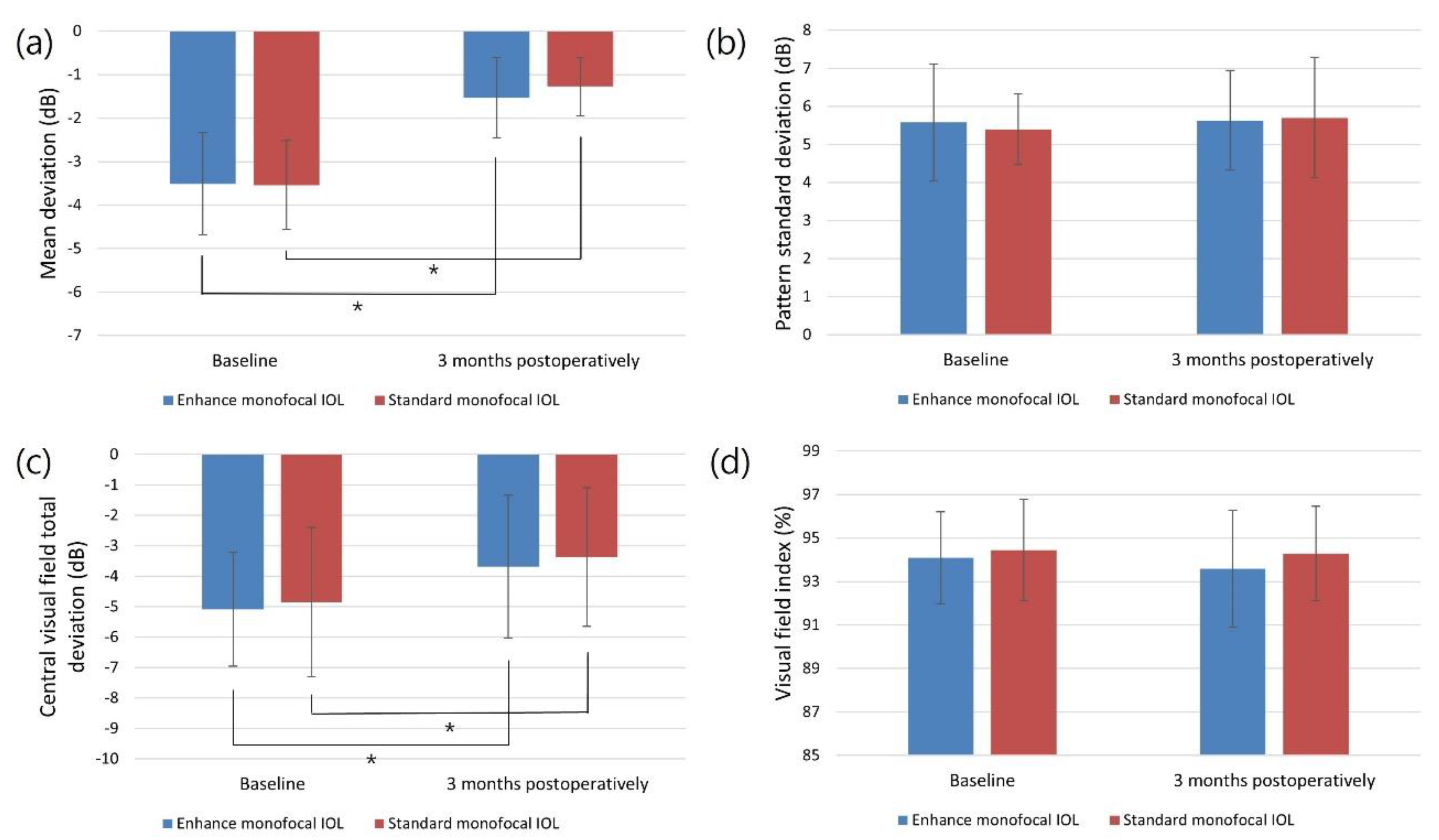
| MD (dB) | -3.51 ± 1.18 | -3.54 ± 1.02 | 0.903 |
| PSD (dB) | 5.58 ± 1.53 | 5.40 ± 0.93 | 0.562 |
| VFI (%) | 94.09 ± 2.11 | 94.45 ± 2.34 | 0.499 |
| Central VF total deviation (dB) | -5.09 ± 1.87 | -4.85 ± 2.45 | 0.645 |
| Variable | Enhanced monofoal IOL (n = 34) | Standard monofocal IOL (n = 38) | p-Value* |
|---|---|---|---|
| UDVA (logMAR) | 0.05 ± 0.07 | 0.05 ± 0.06 | 0.983 |
| CDVA (logMAR) | 0.01 ± 0.04 | 0.02 ± 0.04 | 0.678 |
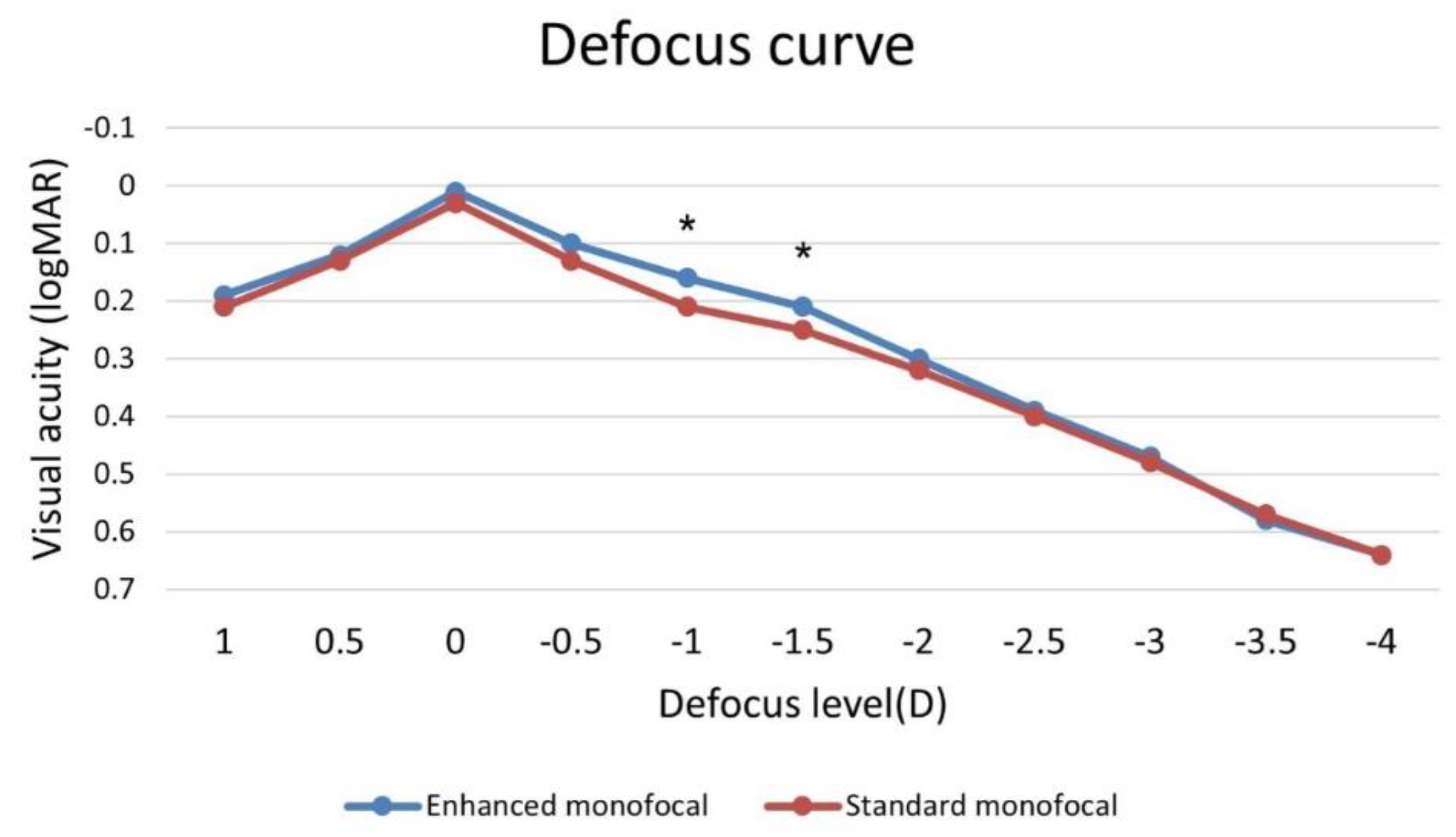
| UIVA (logMAR) | 0.34 ± 0.10 | 0.41 ± 0.10 | 0.003 |
| UNVA (logMAR) | 0.50 ± 0.10 | 0.53 ± 0.08 | 0.168 |
| SE (diopter) | -0.25 ± 0.22 | -0.21 ± 0.36 | 0.605 |
| Variable | Enhanced monofoal IOL (n = 34) | Standard monofocal IOL (n = 38) | p-Value* |
|---|---|---|---|
| Glare (yes/no) | 2/32 | 3/35 | >0.999 |
| Starbursts (yes/no) | 1/33 | 2/36 | >0.999 |
| Halos (yes/no) | 2/32 | 1/37 | 0.599 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





