Submitted:
26 July 2023
Posted:
27 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
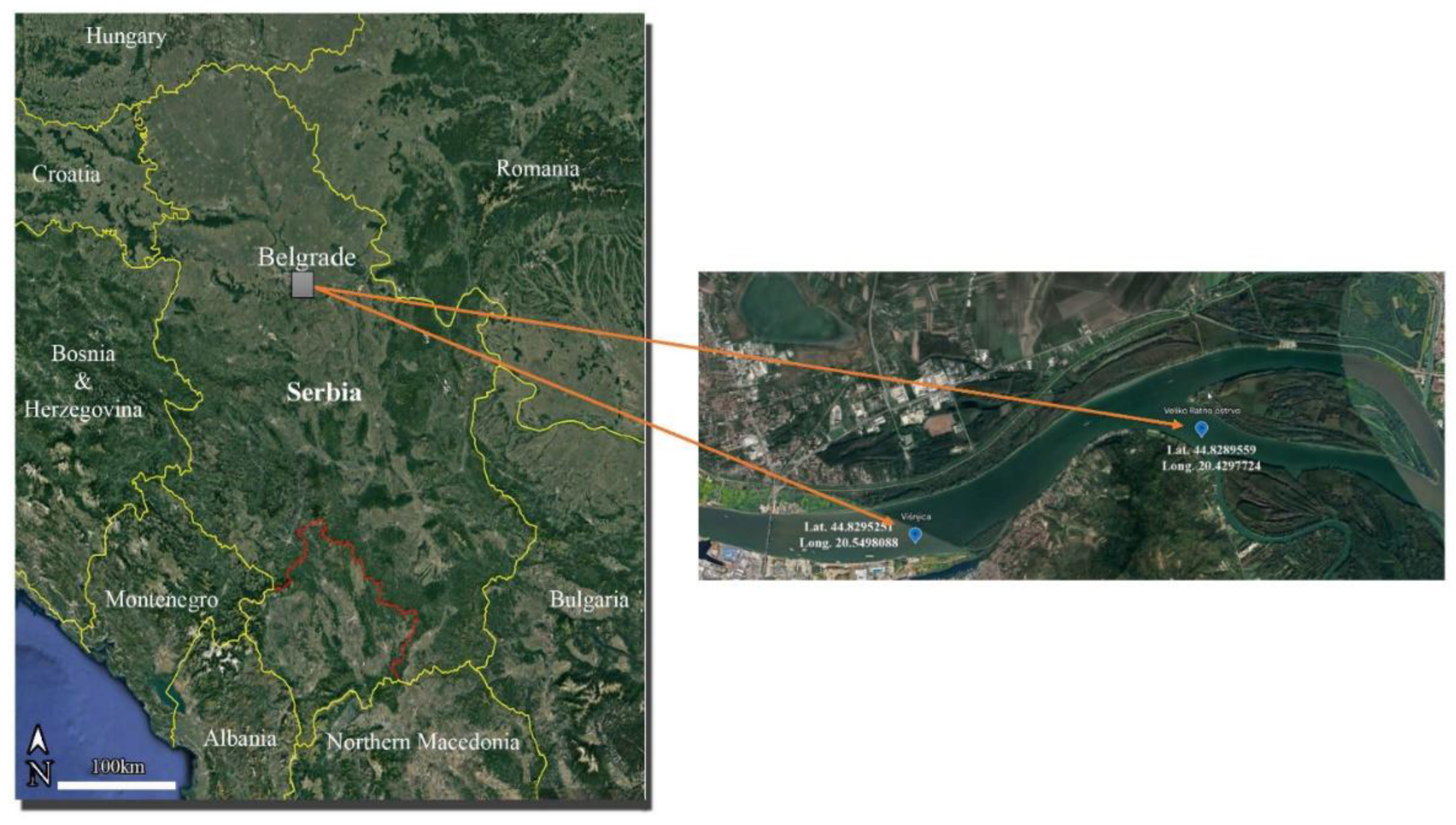
2.1. Study site and sample collection
2.2. Elemental accumulation analysis
2.3. Fatty acid profile analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Metal concentrations in two fish species
3.2. Fatty acid composition
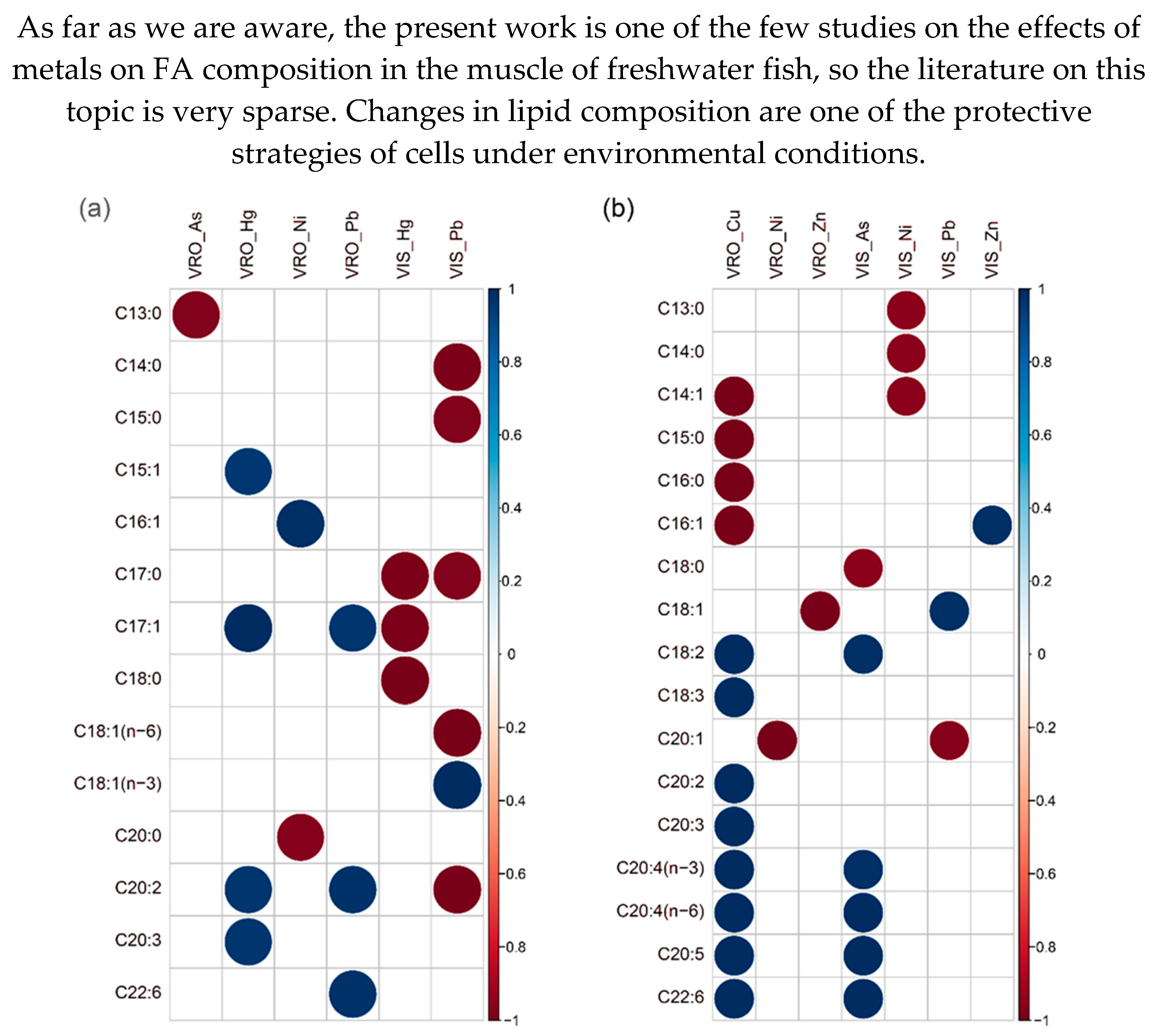
3.3. Correlation of metals concentrations with fatty acids content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khoshnoud, M.J.; Mobini, K.; Javidnia, K.; Hosseinkhezri, P.; Aeen Jamshid, K. Heavy metals (Zn, Cu, Pb, Cd and Hg) contents and fatty acids ratios in two fish species (Scomberomorus commerson and Otolithes ruber) of the Persian Gulf. Iran. J. Pharm. Sci. 2011, 7, 191–196. [Google Scholar]
- Zhao, X.M.; Yao, L.A.; Ma, Q.L.; Zhou, G.J.; Wang, L.; Fang, Q.L.; Xu, Z.C. Distribution and ecological risk assessment of cadmium in water and sediment in Longjiang River, China: Implication on water quality management after pollution accident. Chemosphere 2018, 194, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, D.S.; Loyau, A.; Bao, K.; Brack, W.; Chatzinotas, A.; De Vleeschouwer, F.; Friesen, J.; Gandois, L.; Hansson, S.V.; Haver, M.; Le Roux, G.; Shen, J.; Teisserenc, R.; Vredenburg, V.T. People, pollution and pathogens - Global change impacts in mountain freshwater ecosystems. Sci. Total Environ. 2018, 622-623, 756–763. [Google Scholar] [CrossRef]
- Erdoğrul, Ö.; Erbilir, F. Heavy metal and trace elements in various fish samples from Sir Dam Lake, Kahramanmaraş, Turkey. Environ. Monit. Assess. 2007, 130, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Fozia, F.; Ahmad, I.; Ullah, H. , Iqbal, A.; Ullah, R.; Moharram, B.A., Kowalczyk, A. Ecotoxicological assessment of heavy metal and its biochemical effect in fishes. Biomed. Res. Int. 2022, 3787838. [Google Scholar]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Senthamilselvan, D.; Chezhian, A.; Suresh, E. Synergistic effect of nickel and mercury on fatty acid composition in the muscle of fish Lates calcarifer. J. Fish Aquat. Sci. 2016, 11, 77–84. [Google Scholar]
- Strandberg, U.; Palviainen, M.; Eronen, A.; Piirainen, S.; Laurén, A.; Akkanen, J.; Kankaala, P. Spatial variability of mercury and polyunsaturated fatty acids in the European perch (Perca fluviatilis)–Implications for risk-benefit analyses of fish consumption. Environ. Pollut. 2016, 219, 305–314. [Google Scholar] [CrossRef]
- Li, D.; Hu, X. Fish and its multiple human health effects in times of threat to sustainability and affordability: are there alternatives? Asia Pac. J. Clin. Nutr. 2009, 218, 553–563. [Google Scholar]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef]
- Kaur, N.; Brraich, O.S. Impact of industrial effluents on physico-chemical parameters of water and fatty acid profile of fish, Labeo rohita (Hamilton), collected from the Ramsar sites of Punjab, India. Environ. Sci. Pollut. Res. 2022, 29, 11534–11552. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, A.; Aliakbarlu, J.; Tahmasebi, R. Antioxidant potential and fatty acid profile of fish fillet: effects of season and fish species. Vet. Res. Forum. 2022, 13, 91–99. [Google Scholar]
- Duarte, B.; Carreiras, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Matos, A.R.; Marques, J.C.; Caçador, I. Halophyte fatty acids as biomarkers of anthropogenic-driven contamination in Mediterranean marshes: sentinel species survey and development of an integrated biomarker response (IBR) index. Ecol. Indic. 2018, 87, 86–96. [Google Scholar] [CrossRef]
- Silva, C.O.; Simões, T.; Novais, S.C.; Pimparel, I.; Granada, L.; Soares, A.M.V.M.; Barata, C.; Lemos, M.F.L. Fatty acid profile of the sea snail Gibbula umbilicalis as a biomarker for coastal metal pollution. Sci. Total Environ. 2017, 586, 542–550. [Google Scholar] [CrossRef]
- Meng, H.; Lin, Y.; Zhong, W.; Zhao, Z.; Shen, L.; Ling, Z.; Zhao, K.; Xu, S. Fish biomonitoring and ecological assessment in the Dianchi Lake basin based on environmental DNA. Water 2023, 15, 399. [Google Scholar] [CrossRef]
- Nordov, A.; Macholi, R.; Arnesen, H.; Videbaek, J. N-3 polyunsaturated fatty acids and cardiovascular diseases. Lipids 2001, 36, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Van Dael, P. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Kottelat, M.; Freyhof, J. Handbook of European freshwater fishes. Publications Kottelat, Cornol and Freyhof, Berlin, 2007, pp 646.
- Milanović, A. , Kovačević-Majkić, J.; Milivojević, M. Water quality analysis of Danube River in Serbia: pollution and protection problems. Bull. Serb. Geogr. Soc. 2010, 90, 47–68. [Google Scholar]
- Filimonova, V.; Goncalves, F.; Marques, J.C.; De Troch, M.; Goncalves, A.M. Fatty acid profiling as bioindicator of chemical stress in marine organisms: a review. Ecol. Indic. 2016, 67, 657–672. [Google Scholar] [CrossRef]
- Fonseca, V.F.; Duarte, I.A.; Feijão, E.; Matos, A.R.; Duarte, B. Fatty acid-based index development in estuarine organisms to pinpoint environmental contamination. Mar. Pollut. Bull. 2022, 180, 113805. [Google Scholar] [CrossRef]
- Signa, G.; Di Leonardo, R.; Vaccaro, A.; Tramati, C.D.; Mazzola, A.; Vizzini, S. Lipid and fatty acid biomarkers as proxies for environmental contamination in caged mussels Mytilus galloprovincialis. Ecol. Indic. 2015, 57, 384–394. [Google Scholar] [CrossRef]
- Fokina, N.N.; Ruokolainen, T.R.; Nemova, N.N.; Bakhmet, I.N. Changes of blue mussels Mytilus edulis L. lipid composition under cadmium and copper toxic effect. Biol. Trace Elem. Res. 2013, 154, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Łuczyńska, J.; Paszczyk, B. Health risk assessment of heavy metals and lipid quality indexes in freshwater fish from lakes of Warmia and Mazury region, Poland. Int. J. Environ. Res. Public Health. 2019, 16, 3780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ning, X.; He, X.; Sun, X.; Yu, X.; Cheng, Y.; Yu, R.Q; Wu, Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS One. 2020, 15, e0228276. [Google Scholar] [CrossRef]
- Linhartová, Z.; Krejsa, J.; Zajíc, T.; Másílko, J.; Sampels, S.; Mráz, J. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquacult. Int. 2018, 26, 695–711. [Google Scholar] [CrossRef]
- Jovičić, K.; Janković, S.; Nikolić, D.; Đikanović, V.; Skorić, S.; Krpo-Ćetković, J.; Jarić, I. Prospects of fish scale and fin samples usage for nonlethal monitoring of metal contamination: a study on five fish species from the Danube River. Knowl. Manag. Aquat. Ecosyst. 2023, 424, 4. [Google Scholar] [CrossRef]
- Nędzarek, A.; Formicki, K.; Kowalska-Góralska, M.; Dobrzański, Z. Concentration and risk of contamination with trace elements in acipenserid and salmonid roe. J. Food Compos. Anal. 2022, 110, 104525. [Google Scholar] [CrossRef]
- Harrell, F.E. Hmisc: a package of miscellaneous R functions. 2020, http://biostat.mc.vanderbilt.
- Tepe,Y. Metal concentrations in eight fish species from Aegean and Mediterranean Seas, Environ. Monit. Assess. 2009, 159, 501–509.
- Ali, M.M.; Ali, M.L.; Proshad, R. ; Islam,S; Rahman, Z; Kormoker, T. Assessment of trace elements in the demersal fishes of a Coastal River in Bangladesh: a public health concern Thalassas. Int. J. Mar. Sci 2020, 36, 641–655. [Google Scholar]
- Töre, Y.; Ustaoğlu, F. ; Tepe,Y., Kalipci,E. Levels of toxic metals in edible fish species of the Tigris River (Turkey); Threat to public health. Ecol. Indic. 2021, 123, 107361. [Google Scholar] [CrossRef]
- EU, 2006. Commission Regulation (EC) No. 1881/2006 of 19 december 2006 setting maximum levels for certain contaminants in foodstuffs (text with EEA relevance). Off. J. Eur. Union 364, 5 - 24. No. 1881/2006.
- Official Gazette of the Republic of Serbia Nos 22/2018 & 90/2018, 2018. Regulation on the Maximum Permitted Residue Levels of Pesticides in Food and Animal Feed and Feed and Animal Feed for Which Maximum Quantities of Residues of Pesticides Are Permitted.
- Subotić, S.; Višnjić-Jeftić, Ž.; Spasić, S.; Hegediš, A. ; Krpo-Ćetković, J; Lenhardt, M. Concentrations of 18 Elements in Muscle, Liver, Gills, and Gonads of Sichel (Pelecus cultratus), Ruffe (Gymnocephalus cernua), and European Perch (Perca fluviatilis) in the Danube River near Belgrade (Serbia). Water Air Soil Pollut. 2015, 226, 287. [Google Scholar] [CrossRef]
- Subotić, S.; Višnjić-Jeftić, Ž.; Đikanović, V.; Spasić, S.; Krpo-Ćetković, J; Lenhardt, M. Metal Accumulation in Muscle and Liver of the Common Nase (Chondrostoma nasus) and Vimba Bream (Vimbavimba) from the Danube River, Serbia: Bioindicative Aspects. Bull. Environ. Contam. Toxicol. 2019, 103, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kostić-Vuković, J.; Kolarević, S.; Kračun-Kolarević, M.; Višnjić-Jeftić, Ž.; Rašković, B.; Poleksić, V.; Gačić, Z.; Lenhardt, M.; Vuković-Gačić, B. Temporal variation of biomarkers in common bream Abramis brama (L., 1758) exposed to untreated municipal wastewater in the Danube River in Belgrade, Serbia. Environ. Monit. Assess. 2021, 193, 465. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Özogul, F.; Alagoz, S. Fatty acid profiles and fat contents of commercially important seawater and freshwater fish species of Turkey: A comparative study. Food Chem. 2007, 103, 217–223. [Google Scholar] [CrossRef]
- Parzanini, C.; Colombo, S.M.; Kainz, M.J.; Wacker, A.; Parrish, C.C.; Arts, M.T. Discrimination between freshwater and marine fish using fatty acids: ecological implications and future perspectives. Environmental Reviews 2020, 28, 546–546. [Google Scholar] [CrossRef]
- Jardine, T.D.; Galloway, A.W.E.; Kainz, M.J. Unlocking the power of fatty acids as dietary tracers and metabolic signals in fishes and aquatic invertebrates. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2020, 375, 20190639. [Google Scholar] [CrossRef]
- Bazarsadueva, S.V.; Radnaeva, L.D.; Shiretorova, V.G.; Dylenova, E.P. The comparison of fatty acid composition and lipid quality indices of roach, perch, and pike of Lake Gusinoe (Western Transbaikalia). Int. J. Environ. Res. Public Health. 2021, 18, 9032. [Google Scholar] [CrossRef]
- Zheng, S.; Qiu, M.; Wu, J.H.Y.; Pan, X.F.; Liu, X.; Sun, L.; Zhu, H.; Wu, J.; Huang, Y. Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure. Ther. Adv. Chronic Dis. 2022, 13, 20406223221081616. [Google Scholar] [CrossRef]
- Liu, F.; Xie, Q.; Yu, R.Q.; Xie, Z.; Wu, J.; Zhang, X.; Wu, Y. Fatty acids as bioindicators of organohalogen exposure in marine fish from a highly polluted estuary: First insight into small-scale regional differences, J. Hazard. Mater. 2023, 452, 131337. [Google Scholar] [CrossRef]
- Ferain, A.; Delbecque, E.; Neefs, I.; Dailly, H.; Saeyer, N.D.; Larebeke, M.V.; Cornet, V.; Larondelle, Y.; Rees, J.F.; Kestemont, P.; De Schamphelaere, K.A.C.; Debier, C. Interplay between dietary lipids and cadmium exposure in rainbow trout liver: Influence on fatty acid metabolism, metal accumulation and stress response, Aquat. Toxicol. 2021, 231, 105676. [Google Scholar] [CrossRef]
- Gabryelak, T.; Filipiak, A.; Brichon, G. Effects of zinc on lipids of erythrocytes from carp (Cyprinus carpio L.) acclimated to different temperatures. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2000, 127, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Imran, A.; Nosheen, F.; Fatima, M.; Arshad, M.U.; Afzaal, M.; Ijaz, N.; Noreen, R.; Mehta, S.; Biswas, S.; Rasool, I.F.U.; Aslam, M.A.; Usman, I.; Zahra, S.M.; Segueni, N.; Amer Ali, Y. Functional roles and novel tools for improving-oxidative stability of polyunsaturated fatty acids: A comprehensive review. Food Sci Nutr. 2023, 11, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Carminato, A.; Pascoli, F.; Trocino, A.; Locatello, L.; Maccatrozzo, L.; Palazzi, R.; Radaelli, G.; Ballarin, C.; Bortoletti, M.; Bertotto, D. Productive results, oxidative stress and contaminant markers in european sea bass: Conventional vs. organic feeding. Animals 2020, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Vlahogianni, T.H.; Valavanidis, A. Heavy-metal effects on lipid peroxidation and antioxidant defence enzymes in mussels Mytilus galloprovincialis. Chemistry and Ecology 2007, 23, 361–371. [Google Scholar] [CrossRef]
- Maria, B.; Maria, M.C.; Antonio, B.; Simona, M.; Rosaria, A.; Andrea, S.; Giulia, M.; Mario, S. Chemical and biochemical responses to sub−lethal doses of mercury and cadmium in gilthead seabream (Sparus aurata). Chemosphere 2022, 307, 135822. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogiannia, T.; Dassenakisb, M.; Scoullosb, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Fokina, N. Copper and Nickel Induce Changes in the Lipid and Fatty Acid Composition of Anodonta cygnea. J. Xenobiot. 2023, 13, 132–147. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef]
| Roach(Rutilus rutilus) | White bream(Blicca bjoerkna) | |||
|---|---|---|---|---|
| Metal (µg g -1) | VRO | VIS | VRO | VIS |
| As | 0.019 ± 0.003 | 0.072 ± 0.056* | 0.115 ± 0.067 | 0.048 ± 0.059 |
| Cd | bdl | bdl | bdl | bdl |
| Co | bdl | bdl | bdl | bdl |
| Cr | 0.019 ± 0.033 | 0.020 ± 0.034 | 0.110 ± 0.217 | 0.051 ± 0.033 |
| Cu | 0.264 ± 0.255 | 0.566 ± 0.107 | 0.202 ± 0.235 | 0.269 ± 0.186 |
| Hg | 0.080 ± 0.067 | 0.081 ± 0.031 | 0.109 ± 0.043 | 0.050 ± 0.029 |
| Ni | 0.480 ± 0.224 | 0.158 ± 0.085 | 0.317 ± 0.510 | 0.591 ± 0.542 |
| Pb | 0.023 ± 0.003 | 0.029 ± 0.002* | 0.028 ± 0.007 | 0.024 ± 0.007 |
| Zn | 9.002 ± 8.040 | 27.641 ± 7.890* | 12.935 ± 7.606 | 12.228 ± 7.730 |
| Fatty acid | Roach (Rutilus rutilus) | White bream (Blicca bjoerkna) | ||
|---|---|---|---|---|
| VRO | VIS | VRO | VIS | |
| C11:0 | 0.009±0.008 | 0.008±0.006 | / | / |
| C12:0 | 0.592±0.145 | 0.707±0.327 | 0.400±0.060 | 0.653±0.271 |
| C13:0 | 0.099±0.027 | 0.156±0.133 | 0.082±0.029 | 0.189±0.047 |
| C14:0 | 6.517±0.963 | 7.545±2.456 | 5.035±0.530 | 6.477±1.471 |
| C14:1 | 0.099±0.049 | 0.144±0.093 | 0.076±0.034 | 0.133±0.036 |
| C15:0 | 1.381±0.242 | 1.767±0.841 | 0.825±0.153 | 1.320±0.328 |
| C15:1 | 0.876±0.172 | 1.328±0.928 | / | / |
| C16:0 | 19.255±13.279 | 25.456±10.740 | 38.399±2.296 | 35.152±3.605 |
| C16:1 | 14.210±2.222 | 10.063±7.105 | 11.379±0.648 | 11.973±1.705 |
| C17:0 | 0.988±0.435 | 1.346±0.699 | 0.528±0.185 | 0.949±0.325 |
| C17:1 | 0.692±0.208 | 0.928±0.469 | / | / |
| C18:0 | 7.123±2.120 | 7.711±3.395 | 4.975±0.461 | 6.887±1.292 |
| C18:1 | 0.157±0.051 | 0.185±0.090 | 27.772±1.551 | 24.905±1.381 |
| C18:1 | 19.360±10.579 | 19.512±12.102 | 4.056±0.123 | 5.090±0.832 |
| C18:2 | 11.770±2.241 | 10.551±1.807 | 3.556±2.464 | 3.256±2.010 |
| C18:2 | / | / | 0.090±0.057 | 0.076±0.012 |
| C18:3 | 0.289±0.054 | 0.299±0.109 | 0.231±0.033 | 0.378±0.360 |
| C18:3 | 1.635±0.469 | 1.284±0.512 | / | / |
| C20:0 | 0.347±0.144 | 0.419±0.271 | 0.181±0.039 | 0.167±0.038 |
| C20:1 | 1.106±0.459 | 1.402±0.795 | 0.419±0.063 | 0.788±0.264 |
| C20:2 | 2.095±0.602 | 1.446±0.474 | 0.299±0.219 | 0.394±0.196 |
| C20:3 | 0.593±0.117 | 0.547±0.148 | 0.097±0.054 | 0.057±0.046 |
| C20:4 | 2.543±0.885 | 1.857±0.558 | 0.313±0.057 | 0.241±0.041 |
| C20:4 | / | / | 0.048±0.040 | 0.051±0.019 |
| C20:5 | 3.719±2.439 | 2.563±1.871 | 0.603±0.030 | 0.508±0.297 |
| C22:6 | 4.546±2.799 | 2.774±1.713 | 0.635±0.087 | 0.354±0.169 |
| ƩSFA | 36.311 | 45.116 | 50.424 | 51.794 |
| ƩMUFA | 36.499 | 33.563 | 43.703 | 42.890 |
| Ʃn-3 PUFA | 12.443 | 8.478 | 1.782 | 1.481 |
| Ʃn-6 PUFA | 14.746 | 12.843 | 4.091 | 3.836 |
| n-3/n-6 | 0.844 | 0.660 | 0.436 | 0.386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





