1. Introduction
COVID-19 is an acute, sometimes severe, respiratory illness caused by a novel SARS-CoV-2 (Severe Acute Respiratory Syndrome-related Coronavirus - 2). The presence of COVID-19 was first reported in late 2019 in Wuhan, China, and since then the infection has spread widely around the world. The most common method for nucleic acid diagnosis is based on real time RT-PCR [
1,
2,
3]. Although real-time RT-PCR is sensitive and reliable, it is time-consuming (~2 to 4 h) and requires a specific detection device or instrument, which limits its broad application to current huge demand for the global pandemic of COVID-19.
Loop-mediated isothermal amplification (LAMP) is a rapid technology of DNA amplification [
4,
5,
6], which has been applied to pathogen detection such as virus, bacteria and parasite such as malaria [
4,
7,
8,
9,
10].The LAMP reaction generally takes place in a constant temperature, and the target DNA can be amplified in 30 min [
5]. The LAMP method employs 4 or 6 primers to bind six regions of a target DNA, and the specificity is extremely high[
5,
6]. Initially, the LAMP reactions used 4 primers, later, it was found that the use of two additional loop primers can shorten by a half the time required for the original LAMP reaction [
11]. Warm Start RTx Reverse Transcriptase (New England Biolabs, UK) makes possible to combine both reverse transcription and LAMP in one reaction. Since SARS-CoV-2 has a RNA length of approximately 30 kb [
12,
13,
14], a single step of reverse transcription (RT)- LAMP reactions can be carried out together significantly shortening the time needed. Additionally, the use of a direct sample without need of any RNA extraction can significantly shorten even more the process thus a rapid detection of SARS-CoV-2 can be achieved.
We developed, in this work, a simple COVID-19 diagnosis kit for the rapid detection of SARS-CoV-2, using one-step reverse transcription and loop-mediated isothermal amplification (RT-LAMP) from direct nasopharyngeal swabs. The whole process can be as short as 60 min at a constant temperature of 64°C and can be carried out without the need of any sophisticated instrument. The detection limit is between 50 and 100 copies of viral RNA per ml. The indication of a simple color change can be visualized by the naked eye to confirm the result of specific viral RNA sequence detection.
Multiple isolates of SARS-CoV-2 variants were described. In the United Kingdom (UK), a new variant of SARS-CoV-2 (known as 20I/501Y.V1, VOC 202012/01, or B.1.1.7)[
15]emerged with a large number of mutations. This variant has been detected in several countries around the world. In January 2021, scientists from the UK reported evidence suggesting that the B.1.1.7 variant may be associated with an increased risk of death compared with other variants [
16]. In South Africa, another variant of SARS-CoV-2 (known as 20H/501Y.V2 or B.1.351) emerged independently of B.1.1.7. This variant shares some mutations with B.1.1.7. Cases attributed to this variant have been detected in multiple countries outside of South Africa [
17]. In Brazil, a variant of SARS-CoV-2 (known as P.1) was first identified in four travelers from Brazil who were tested during routine screening at Haneda airport outside Tokyo, Japan. This variant has 17 unique mutations, including three in the receptor binding domain of the spike protein [
18]. Recent studies indicate that B.1.427 and B.1.429lineages (California or West Coast variants) likely emerged in late spring or early summer of 2020. These two variants carry similar, though slightly different, genetic mutations [
19].The emergence of the Omicron variant of the COVID virus has raised significant concerns worldwide. This new variant, first identified in November 2021, has demonstrated an unprecedented number of mutations in its spike protein, which plays a crucial role in viral entry and infectivity. The Omicron variant exhibits over 50 mutations in the spike protein, including several deletions and insertions, resulting in potential changes in its antigenic properties [
20] and increase in transmissibility of compared to previous variants [
21]. Moreover, studies indicate a potential reduction in neutralizing antibodies generated by previous infection or vaccination against the Omicron variant [
22]. Our kit was validated by clinical COVID-19 samples and in silico analysis confirmed the detection of all circulating variants including the Omicron variant.
2. Methods
2.1. Design of RT-LAMP primers for SARS CoV-2genome detection
Primer sets used for LAMP were designed based on the Wuhan-Hu-1 SARS-CoV-2 complete genome sequence (GenBank: MN908947.3)[
23].
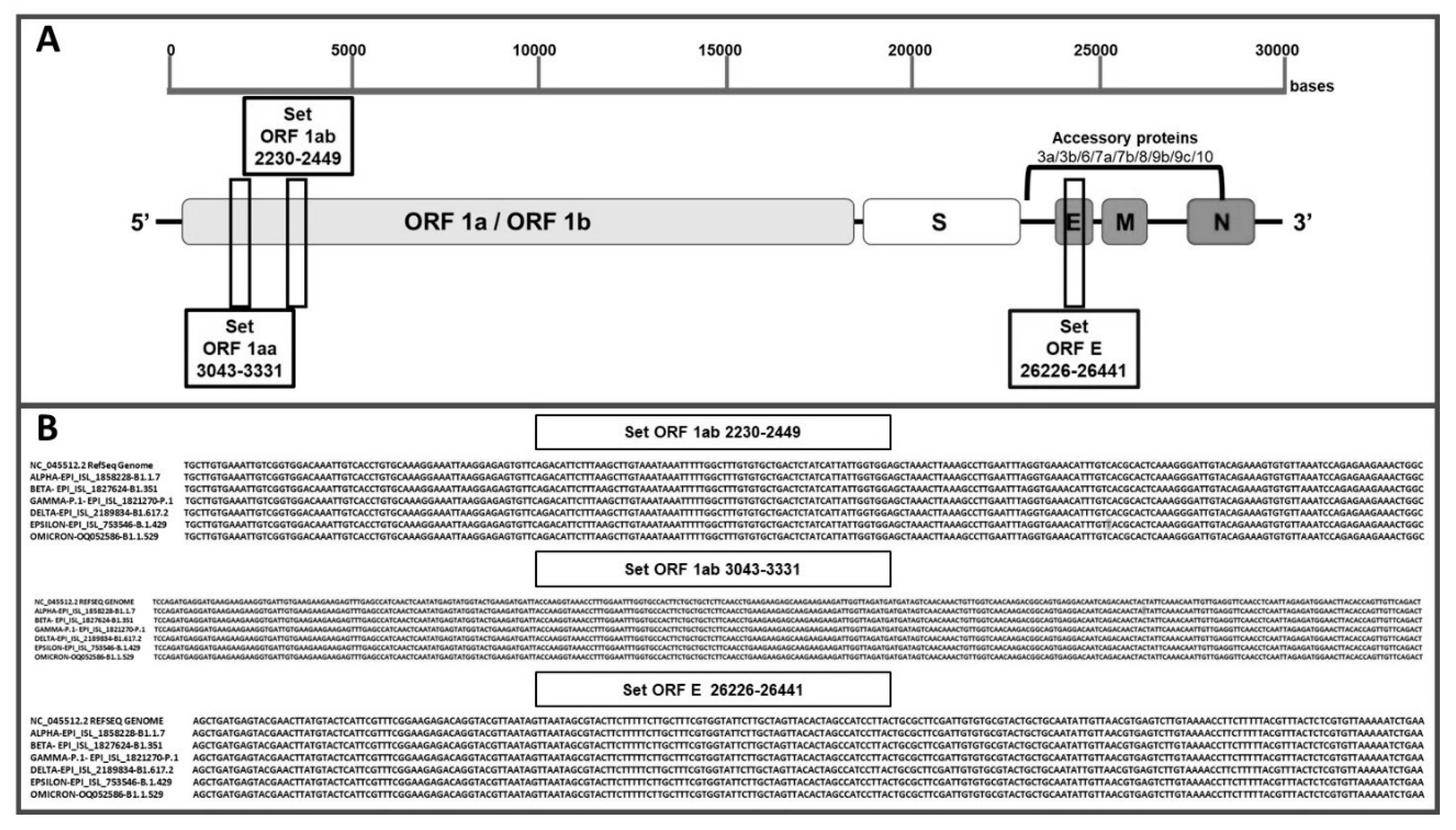
Three sets of LAMP primers were designed to target the open reading frame ORF1aa, open reading frameORF1ab and envelopment (E) genes, respectively (
Table 1). The primer design was performed using Primer Explorer V5 software tool (EIKEN CHEMICAL Co., Ltd., Tokyo, Japan). The loop primers (LF and LB) were manually designed, ensuring appropriate melting temperatures using Vector NTi Advance software (Invitrogen).
The amplicon sizes of
ORF1AA,
ORF1AB and E were 220 bp, 289 bp and 215bp, respectively. All the primers were validated using Quantitative Synthetic SARS-CoV-2 RNA (ATCC® VR-3276SD™), and oligos were ordered from Macrogen Inc, Koreaand resuspended in UltraPure water at a 100 μM concentration. The localization of LAMP target regions on the genome of SARS-CoV-2 is illustrated in
Figure 1A.
2.2. Sample Inactivation and treatment for direct assay
Nasopharyngeal swab samples were obtained from the Virology Unit at Infectious Diseases Hospital “Francisco Javier Muñiz” and analyzed by RT-PCR (GeneFinder) after automatized RNA purification (Perkin Elmer). The same samples were assayed by direct RT-LAMP, without RNA extraction. For RT-LAMP, 10 µl of the swab samples were pre-treated at 95°C for 8 minutes with 40 µl of Lysis buffer A (Tris-HCl pH 8.8, 20 mM; EDTA, 0.1 mM and lysis (buffer B Tris(2-carboxyethyl) phosphine hydrochloride 2.5 mM) in 180:1 (v/v) ratio. The samples were then briefly centrifuged and 10 µl were used for the subsequent reaction.
2.3. RT-LAMP assay
The RT-LAMP assay was performed using a dry thermal block with a 0.5-mL PCR tube holder. The final LAMP conditions for LAMP included 40 pmol of each FIP and BIP primers, 5 pmol of each of F3 and B3 primers, 20 pmol of each LF and LB loop primers, 8 U of Bst DNA polymerase Large Fragment (New England Biolabs), 7.5 U of WarmStartRTx Reverse Transcriptase (New England Biolabs), 120 µM hydroxy naphthol blue (Sigma-Aldrich), 8 mM MgSO4, 1.4 mM of dNTP mix, 20 mM Tris–HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 0.1% tween-20 and 1 M betaine, in a final volume of 40 μL including the template(30 µl reaction buffer and 10 µl of sample template). The reaction mixture was incubated at 64°C for 60 minutes. The amplification products were visualized by the color change, from violet to blue-sky blue, indicating the presence of the target viral RNA sequence. In some cases, the products were also analyzed by electrophoresis on a 2% agarose gel.
2.4. Primers cross-reactivity (in silico sequences analysis)
To evaluate the analytical specificity and cross-reactivity, BLASTN analysis of the 3 sets of 6 primers was performed against a high-priority pathogens database that included other closely related coronaviruses from the public domain NCBI Nucleotides database. Current GenBank Release 240.0, (219055207 sequences, 698688094046 bases, for traditional GenBank records 1947019989 sequences, 9627627030647 bases, for set-based (WGS/TSA/TLS) records). Software used for the search: BLASTN 2.9.0+. Latest inclusivity data were collected from whole genome sequences of SARS-CoV-2 published via GISAID (
www.gisaid.org) and whole genome sequences published via National Center for Biotechnology Information (
www.ncbi.nlm.nih.gov) (supplementary material).
2.5. Analytical Specificity of the RT-LAMP Assay
Cross-specificity tests for the multiplex RT-LAMP assay were carried out using viral and bacterial pathogens associated with respiratory infections, comprising influenza A, influenza B, Canine coronavirus, Dengue 1-4, Chikungunya, Yellow Fever, Zika, Mayaro, Mpox, Hela RNA, Trypanosoma Cruzi, M tuberculosis, E. coli, Saccharomyces cerevisiae, Saccharomyces pombe, Pichia Pastoris.
2.6. Repeatability, Reproducibility and Detection limit of the RT-LAMP assay
Purified RNA SARS-CoV-2 S (AMPLIRUN SARS-CoV-2 RNA CONTROL, 12000-20000 copies/ μL once reconstituted), were used as template in RT-LAMP reactions, with different operators and 8 replicates at each template and a no-target control included. The detection limit was determined by conducting serial dilutions ofviral genomes copies, including 1,000, 500, 100, 50, 25, 12, 6 and 0 copy per reaction.
2.7. Clinical evaluation of the RT-LAMP assay for SARS-CoV-2 detection
To assess the performance of RT-LAMP assay using the final combined primer sets,192 samples obtained from nasopharyngeal swabs collected from patients in theVirology Section of Francisco Javier Muñiz Infectious Diseases Hospital, Buenos Aires, Argentina, were utilized. The samples were processed with lysis buffer and incubated at 98°C for 8 minutes for inactivation. These samples included individuals who tested positive (n=139) and negative (n=53) for SARS-CoV-2 RNA based on RT-qPCR, which served as gold standard.
3. Results and discussion
3.1. Design and specificity of ORF1aa, ORF1ab and gene E primers for SARS-CoV-2 target sequence identification
To identify the most specific target region for LAMP primers design, we used VECTOR NTi SOFTWARE ALIGN CLUSTAL MULTIALIGNto identify conserved genomic areas SARS-CoV- 2. Three conserved genomic regions were selected: two sequences from ORF1a (ORF1aa and ORF1ab) that encode the replicase polyprotein [
24], and one sequence from gene E, that encodes envelope protein[
25] (
Figure 1A). For each selected region 6 primers were designed, targeting regions of approximately 240–260 bp length, suitable for rapid amplification (Fig. 1 and
Table 1). Alignments of the primers against SARS-CoV-2 reference genome (MN908947) showed 100 % identity, indicating the absence of mismatch (
Table S1). Additionally,
in silico specificity analysis was performed using genome sequences from other coronavirus and respiratory pathogens, demonstrating no more than 80% homology between the designed primers and those genomes (
Table S2). Thus, cross-reactivity is not expected in this SARS-CoV-2 detection methods.
3.2. Evaluation of primer annealing with SARS-CoV-2 variants and mutations
To investigate the impact of mutations on primer annealing, Reactivity
in silico was performed using our designed primers with variants and mutations: several new variants emerged in the fall of 2020, 2021 and 2022, including a UK variant known as 501Y.V1, VOC 202012/01, or B.1.1.7 lineage, a South African variant known as 501Y.V2 or B.1.351 lineage, a Brazilian variant known as 501Y.V3 or P.1 lineage, California variants known as B.1.427/ B.1.429 and the most recent lineages of variant Omicron (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4 and BA.5). The analysis, based on the latest inclusivity data from GISAID and NCBI, confirmed no mismatches between the primers and the target regions of the variants (Fig. 1B and
Table S3).
3.3. Development of an RT-LAMP assay and its Analytical Sensitivity Testing
The detection of SARS-CoV-2 RNA was assayed with the Quantitative SARS-CoV-2 RNA standard (AMPLIRUN SARS-CoV-2 RNA CONTROL, VIRCELL).
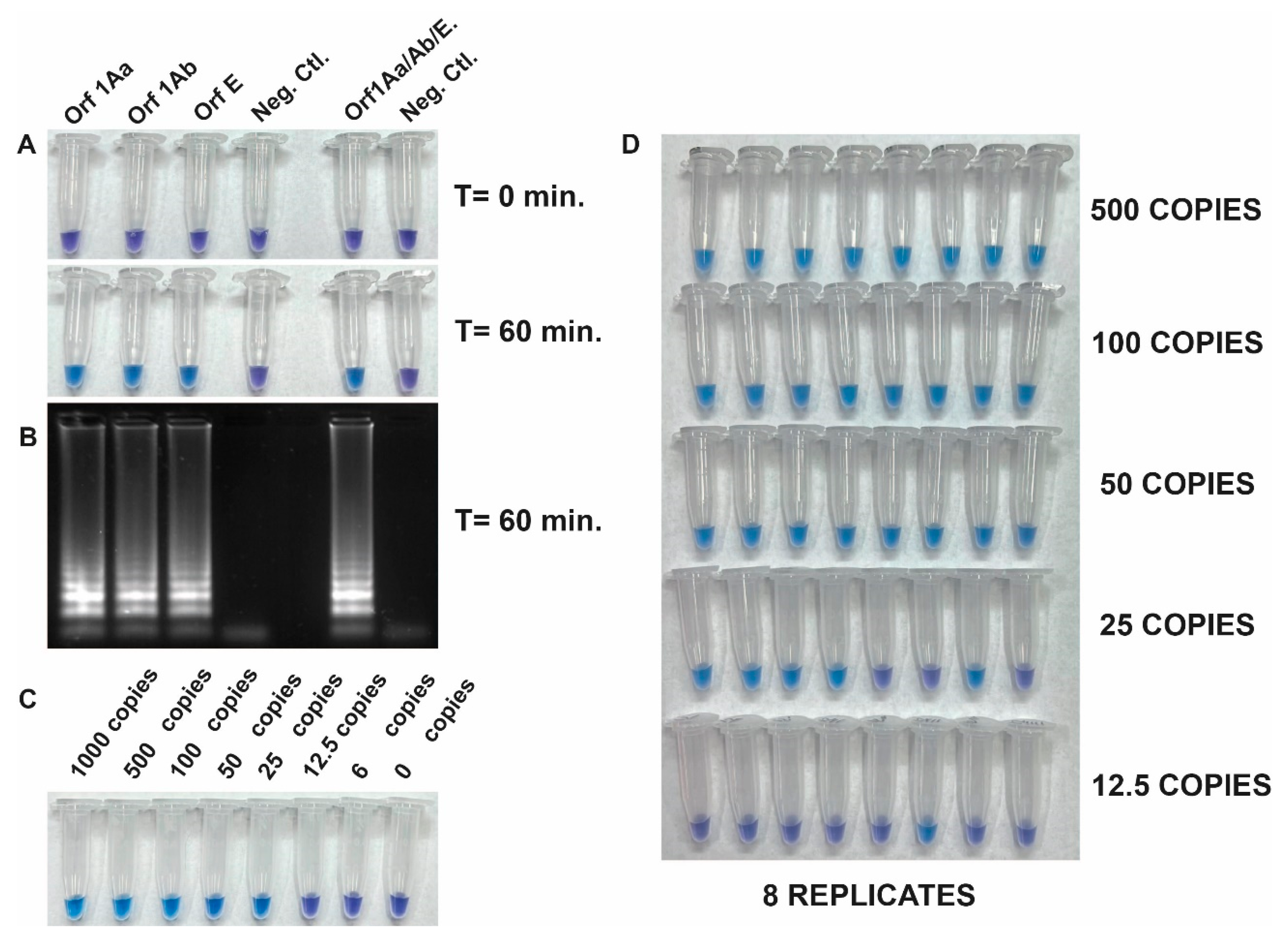
Figure 2 shows the specificity and the integration of reverse transcription and LAMP into a single RT-LAMP reaction to specifically amplify viral RNA fragments. This is achieved by using the set of three primers separately, and all together with the Quantitative SARS-CoV-2 RNA standard. Positive reactions resulted in a color change from violet to blue-sky-blue indicating amplification of the target sequence by Bst DNA polymerase activity, while negative reactions retained the purple color (
Figure 2A). The visible read-outs are consistent with the results obtained by electrophoresis gel (
Figure 2B).
To determine the limit of detection (LOD) of the RT-LAMP assay we evaluated the sensitivity using the Quantitative synthetic SARS-CoV-2 RNA standard. The copy number of RNA targets was diluted from 1000 to 6 copies per reaction to assess the assay's sensitivity. The efficiency of the assay was determined by sampling the reaction mixture at 60 minutes using a pool of the 3 primer sets. It was found that 25 copies of the target RNA could be successfully amplified. However, a repeatability assay conducted by different operators demonstrated that 100% amplification efficiency was achieved in samples with more than 50 copies (
Figure 2C and 2D). This indicates that the tested RT-LAMP assay is sensitive enough to detect viral RNA down to 50 copies per reaction.
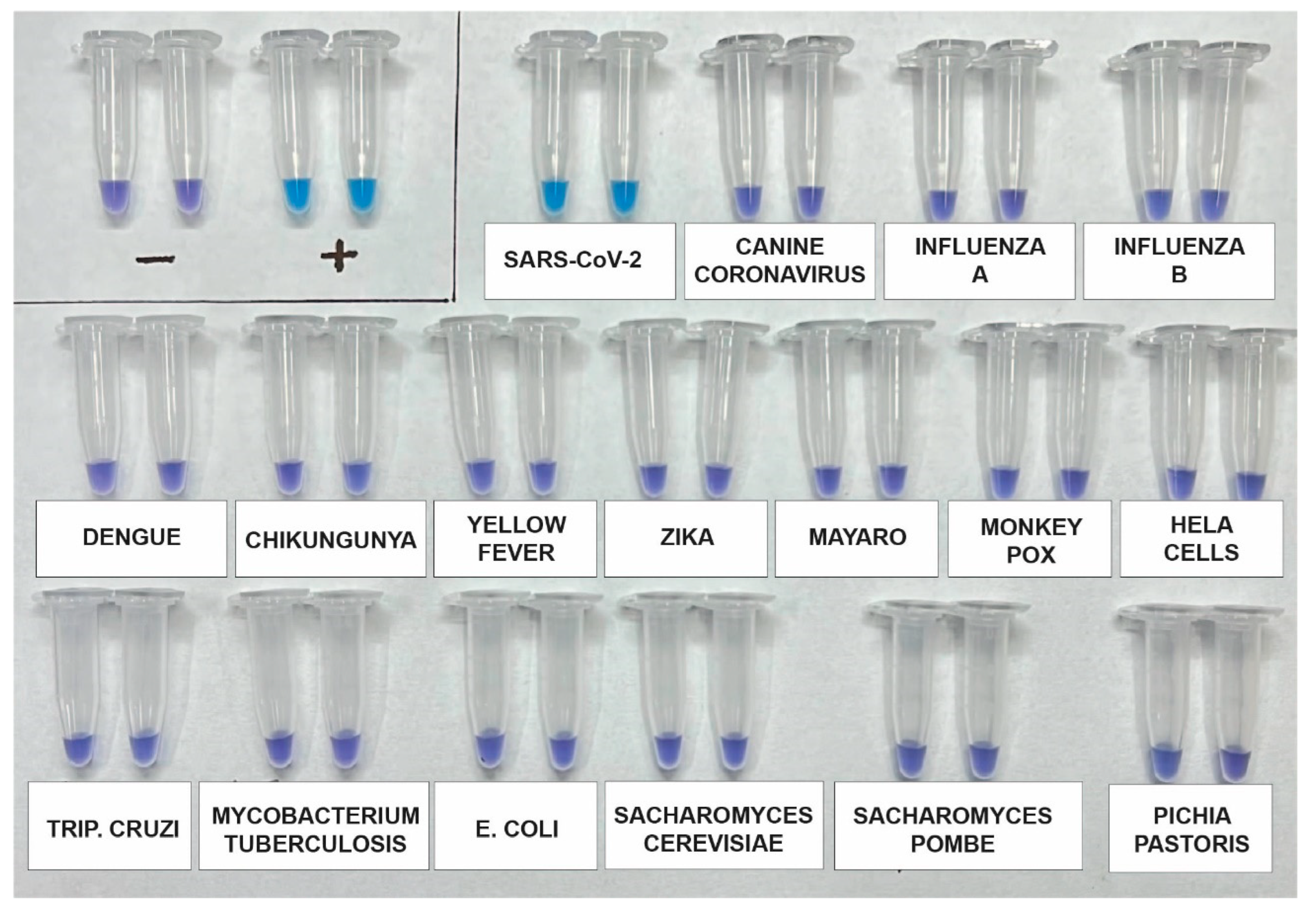
3.4. Analytical Specificity Testing
The analytical specificity of the assay was evaluated again using a synthetic SARS-CoV-2 RNA standard, as well as RNA genomes from other viruses such as Influenza A (H1N1), Influenza B, Canine coronavirus, Dengue 1-4, Zika, Chikungunya, and Mayaro. Additionally, potential contaminants including Saccharomyces cerevisiae, Saccharomyces pombe, Escherichia coli, and genomic human DNA were included for testing using DNA from HeLa Cells. After incubating the samples for 60 minutes, the results indicated that the primers used in the kit specifically and accurately detected SARS-CoV-2 without any cross-reactivity (Fig. 3).
3.5. Clinical Validation
The clinical validation of the developed RT-LAMP assay to detect SARS-CoV-2 RNA was performed from direct nasopharyngeal swab samples. The samples were pre-treated with lysis buffer and heat for 8 minutes to 98 C.A test kit comprising tubes with pools of primers (ORF1Aa, ORF1Ab, and E primers) was used. Swab samples (139 positive and 53 negative samples) collected from patients following standard procedures were initially tested using conventional RT-qPCR. Subsequently, the same samples were subjected to RT-LAMP after the treatment described in the Materials and Methods section. The contingency table of the results, presented in
Table 2, indicates that out of 139 samples identified as positive by RT-qPCR, 126 were also detected as positive by RT-LAMP. In other words, there were only 13 false negative results. In addition, the negative samples that were previously identified as negative using RT-PCR were also found to be negative when tested with RT-LAMP. This confirms that the RT-LAMP test has a specificity of 100%, meaning it accurately identifies true negatives and avoids false positive results.
The obtained results demonstrated a clinical specificity of 100% and a clinical sensitivity of 90.6% (
Table 2), indicating a strong agreement between the RT-LAMP assay and the conventional RT-qPCR.
4. Conclusions
In this study, we present the development of a simple diagnostic test for the rapid detection of SARS-CoV-2, the virus responsible for |COVID-19, using one-step reverse transcription and loop-mediated isothermal amplification (RT-LAMP) directly from nasopharyngeal swab samples without RNA purification. The primer designs in our study showed 100% identity, and no discrepancies were detected against the target sequence, as confirmed by alignments with the SARS-CoV-2019 reference (MN908947). We also performed in silico analysis of complete genome sequences from different variants to evaluate reactivity with the E gene and two regions of the ORF1ab target-specific oligonucleotide sequences contained in our test kit. The absence of discrepancies and compatibility with different viral variants further supported the robustness of our primer design.
Importantly, our RT-LAMP kit showed comparable specificity and sensitivity to conventional methods such as real-time reverse transcription polymerase chain reaction (RT-qPCR). This demonstrated the effectiveness of our test in accurately identifying SARS-CoV-2 infections. Additionally, the simplicity and speed of our development allowed for its use in clinical and resource-limited settings, addressing the need for rapid and accessible diagnostic tools.
By leveraging the advantages of RT-LAMP, our developed diagnostic test offered a reliable and efficient solution for COVID-19 diagnosis. It exhibited excellent primer specificity, compatibility with viral variants, and comparable performance to established methods. This positioned our kit as a valuable tool in the fight against the pandemic, contributing to timely and accurate detection of SARS-CoV-2.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
SW: LL, CC, FS, LO were responsible for conducting the research and performing the experiments. SP, SC took charge of managing and coordinating the research activity planning and execution. LM, IZ, MBB contributed by providing laboratory samples, with MBB critically reviewing the initial draft. AV oversaw and led the research activity planning and execution, in the preparation, creation, and presentation of the published work and handling the writing. Additionally, AV was responsible for acquiring the financial support for the project leading to this publication. All authors read and approved the final manuscript.
Funding
This work was supported by the Agencia de Promoción Científica y Tecnológica (ANPCyT) and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. PICT-2017-1544- Extension to COVID-19.
Ethical approval
Pre-existing samples were used and de-identified. This work was therefore determined to be research not involving human subjects.
Acknowledgments
SW are research of Pablo Cassara Laboratory. LL, FS, CC, AV are members of CONICET. LO is supported by a ANPCyT fellowship. We thank Dr Gustavo Leiros for the critical reading of the manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Drosten, C.; Göttig, S.; Schilling, S.; Asper, M.; Panning, M.; Schmitz, H.; Günther, S. Rapid Detection and Quantification of RNA of Ebola and Marburg Viruses, Lassa Virus, Crimean-Congo Hemorrhagic Fever Virus, Rift Valley Fever Virus, Dengue Virus, and Yellow Fever Virus by Real-Time Reverse Transcription-PCR. J. Clin. Microbiol. 2002, 40, 2323–2330. [Google Scholar] [CrossRef]
- Espy, M.J.; Uhl, J.R.; Sloan, L.M.; Buckwalter, S.P.; Jones, M.F.; Vetter, E.A.; Yao, J.D.C.; Wengenack, N.L.; Rosenblatt, J.E.; Cockerill, F.R.; et al. Real-Time PCR in Clinical Microbiology: Applications for Routine Laboratory Testing. Clin. Microbiol. Rev. 2006, 19, 165–256. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E.; Nitsche, A. SURVEY AND SUMMARY SURVEY AND SUMMARY Real-Time PCR in Virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef]
- Boehme, C.C.; Nabeta, P.; Henostroza, G.; Raqib, R.; Rahim, Z.; Gerhardt, M.; Sanga, E.; Hoelscher, M.; Notomi, T.; Hase, T.; et al. Operational Feasibility of Using Loop-Mediated Isothermal Amplification for Diagnosis of Pulmonary Tuberculosis in Microscopy Centers of Developing Countries. J. Clin. Microbiol. 2007, 45, 1936–1940. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP) of Gene Sequences and Simple Visual Detection of Products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28. [Google Scholar] [CrossRef]
- Mori, Y.; Notomi, T. Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Accurate, and Cost-Effective Diagnostic Method for Infectious Diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef]
- Law, J.W.F.; Mutalib, N.S.A.; Chan, K.G.; Lee, L.H. Rapid Metho Ds for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Thai, H.T.C.; Le, M.Q.; Vuong, C.D.; Parida, M.; Minekawa, H.; Notomi, T.; Hasebe, F.; Morita, K. Development and Evaluation of a Novel Loop-Mediated Isothermal Amplification Method for Rapid Detection of Severe Acute Respiratory Syndrome Coronavirus. J. Clin. Microbiol. 2004, 42, 1956–1961. [Google Scholar] [CrossRef]
- Reboud, J.; Xu, G.; Garrett, A.; Adriko, M.; Yang, Z.; Tukahebwa, E.M.; Rowell, C.; Cooper, J.M. Vector Control Division, Ministry of Health. 2019. [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated Reaction by Loop-Mediated Isothermal Amplification Using Loop Primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A Novel Coronavirus Outbreak of Global Health Concern Published. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Lu, Y.; Meng, Y.; Ye, Z.; Wang, L.; Wang, R.; Zheng, Q.; Wu, H.; Wu, J. Field Detection of Citrus Huanglongbing Associated with ’ Candidatus Liberibacter Asiaticus’ by Recombinese Polymerase Amplification within 15 Min. J. Agric. Food Chem. 2018, 66, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, H.; Thai, S.; Li, X.; Hui, J.; Tang, H.; Zhai, S.; Sun, L.; Wang, T. Development and Validation of an Algorithm to Identify Drug-Induced Anaphylaxis in the Beijing Pharmacovigilance Database. Int. J. Clin. Pharm. 2018, 40, 862–869. [Google Scholar] [CrossRef]
- Yao, M.; Jiang, F.; Zhang, Y.; Li, Y.; Pang, B.; Liang, H.; Kou, Z.; Jiang, X.; Wen, H.; Xu, Y. SARS-CoV-2 Variant of Concern 202 012/01 (B.1.1.7) in a Traveller from the UK to China. J Travel Med 2021, 28. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G. Covid-19: New UK Variant May Be Linked to Increased Death Rate, Early Data Indicate. BMJ 2021, 372, 1–2. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and Rapid Spread of a New Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Lineage with Multiple Spike Mutations in South Africa. medRxiv 2020, 2. [Google Scholar] [CrossRef]
- Voloch, C.M.; Ronaldo da Silva Francisco, J.; Almeida, L.G.P. de; Cardoso, C.C.; Brustolini, O.J.; Gerber, A.L.; Guimarães, A.P. de C.; Mariani, D.; Costa, R.M. da; Orlando C. Ferreira, J.; et al. Genomic Characterization of a Novel SARS-CoV-2 Lineage from Rio de Janeiro, Brazil. J. Virol. 2021, 95, 19–21. [Google Scholar] [CrossRef]
- Long, S.W.; Olsen, R.J.; Christensen, P.A.; Subedi, S.; Olson, R.; Davis, J.J. SHORT COMMUNICATION Sequence Analysis of 20 , 453 Severe Acute Respiratory Syndrome Coronavirus 2 Genomes from the Houston Metropolitan Area Identi Fi Es the Emergence and Widespread Distribution of Multiple Isolates of All Major Variants of Concern. 2021, 191, 983–992.
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The Emergence and Epidemic Characteristics of the Highly Mutated SARS-CoV-2 Omicron Variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Ahmed, W.; Bivins, A.; Smith, W.J.M.; Metcalfe, S.; Stephens, M.; Jennison, A. V.; Moore, F.A.J.; Bourke, J.; Schlebusch, S.; McMahon, J.; et al. Detection of the Omicron (B.1.1.529) Variant of SARS-CoV-2 in Aircraft Wastewater. Sci. Total Environ. 2022, 820. [Google Scholar] [CrossRef]
- Lyke, K.E.; Atmar, R.L.; Dominguez Islas, C.; Posavad, C.M.; Szydlo, D.; PaulChourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid Decline in Vaccine-Boosted Neutralizing Antibodies Against SARS-CoV-2 Omicron Variant. Cell reports Med. 2022, 3, 1–7. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Huang, Y.; Lau, S.K.P.; Yuen, K.Y. Coronavirus Genomics and Bioinformatics Analysis. Viruses 2010, 2, 1805–1820. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus Envelope Protein: Current Knowledge. Virol. J. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).