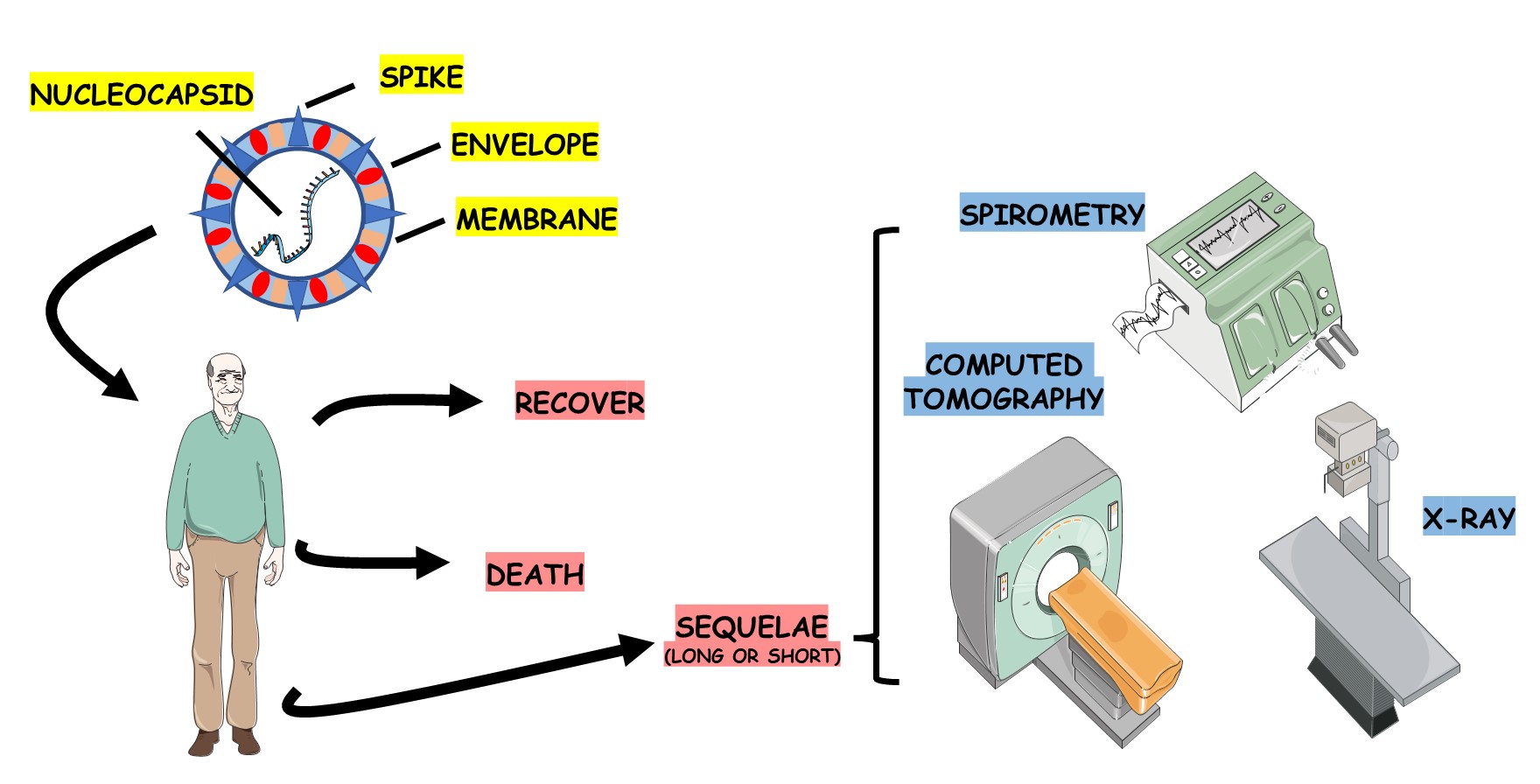
1. Introduction
In December 2019, the disease caused by the coronavirus called COVID19 was diagnosed in Wuhan, China, leading to a worldwide pandemic of greater relevance for all current humanity [
1,
2,
3]. The epidemic disease caused by SARS-CoV-2 has been called coronavirus disease-19 (COVID-19). By November 2022, Brazil had surpassed 35 million cases associated with more than 700,000 deaths. Manifestations ranged from asymptomatic patients with mild symptoms to severe illness and death. The viral infection has expanded internationally, and the WHO has announced a Public Health Emergency of International Concern [
1,
4,
5].
In approximately 80-90% of cases, the disease may manifest as mild or asymptomatic symptoms. However, in the remaining 10%, patients may develop a significant infection, with severe dyspnea, hypoxemia, and extensive pulmonary impairment [
6,
7]. COVID-19 is primarily a respiratory disease, which can manifest itself in the upper and lower respiratory tracts, with a progressive and gradual evolution, starting with oropharyngeal pain, rhinorrhea, nasal congestion, myalgia, arthralgia, fatigue (21-65%), fever (which becomes persistent) (81-94%), loss of taste and smell (chemosensory disturbances) [
8,
9,
10]. It can also evolve with cough (65-78%), progressive dyspnea, silent hypoxia, hemoptysis, chest pain, and acute respiratory failure [
9,
11].
Many patients may suffer from Acute Post-COVID-19 Syndrome, which manifests with persistent symptoms after the pathological condition. This syndrome was defined as a condition characterized by the persistence of symptoms or complications beyond four weeks after the onset of the disease and, in some cases, includes persistent symptoms and abnormalities beyond twelve weeks of the infectious condition [
12,
13].
Chest radiological imaging methods are fundamental, primordial, and valuable instruments for diagnosing, following up, and treating the disease [
14]. Radiological diagnostics constitute an essential component in assessing the extent and severity of the infection, and it is a pivotal element in guiding the treatment [
6]. In terms of imaging, plain chest radiography can be interesting and can be used as an initial test, but complementing it with Chest Computed Tomography (CT) may represent a better and more detailed test for analyzing the clinical picture of COVID-19, both in the assessment of the acute phase and in the Post-COVID-19 Syndrome [
11,
15,
16,
17,
18].
In addition to the characteristic clinical scenario that may occur and the various radiographic images that should be evaluated, checking pulmonary function (spirometry) is essential for the follow-up of these affected patients [
19,
20,
21]. The predictive values are evaluated according to the
Global Lung Function Initiative [
20,
22]. Values below 80% of the predicted value are considered pathological findings (calculated based on the variables: sex, age, height, and patient weight) [
19]. The Tiffeneau index is based on values below 70% to indicate a bronchoconstrictive disorder [
23].
Due to the complexity of COVID-19, the responses presented in the existing research have not been exhausted, leading to further investigations to assist in managing patients so that they have a better quality of life in the periods after the onset of the disease. For these reasons, this study aimed to investigate the relationship between the clinical evolution of lung function by spirometry and computed tomography with the need for therapeutic intervention and type of post-COVID treatment.
2. Materials and Methods
2.1. Study design
An observational study was performed. Data were obtained during an outpatient consultation and access to the medical record in a retroactive period of two years until the date of diagnosis of COVID-19.
This study included 302 patients affected by COVID-19 and treated at the Medical Specialties Clinic of the University of Marília – Sao Paulo - Brazil. All patients in the study were followed up from March 2020 to December 2022, with follow-up occurring every six months until the patient showed effective clinical, radiological, and functional improvement.
The criterion for inclusion in the study group was a positive PCR (poly-chain reaction) test. The exclusion criterion was negative PCR, even if the patient had compatible clinical signs and a positive antigen test.
Patient data were collected from records of general clinical, pulmonological, functional (spirometry), and radiological evaluations. These data were obtained at time zero (disease state), as well as up to six months, from six-twelve months, and after twelve months of evolution. Identification data such as race, sex, age, and profession were collected. This study was approved by the Research Ethics Committee of the University of Marilia – Sao Paulo - Brazil, under protocol 60359322.6.0000.5496.
2.2. Statistics
The absolute (f) and relative (%) frequency distribution described qualitative variables. Differences in the frequency distribution of response categories were analyzed using the univariate chi-square test. The association between qualitative variables was analyzed using Fisher's exact test. Wald's Backward logistic regression analysis was performed to analyze the effect of covariates on the probability of normalization of CT and spirometry exams. The effect of the logistic regression model was analyzed using the Omnibus test, and the model's goodness of fit using Cox's R2. The significance level adopted was 5%, and the data were analyzed using the SPSS software (version 24.0).
3. Results
Table 1 describes the general characteristics of the sample in relation to the qualitative variables of 302 patients. The sample's mean age ± standard deviation was 49±16, with a minimum age of 12 and a maximum age of 94 years. The difference in the sum of the absolute frequency for some variables is due to the absence of data (regarding post-COVID-19 pulmonary symptoms, dry cough, dyspnea, and chest pain were the most frequent (data not shown). Of the associated comorbidities, asthma was more frequent (23.5%) than COPD (6.6%).
Of the total of 302 patients evaluated, 191 underwent follow-up with CT and 57 with spirometry. The largest proportion of the sample presented CT and spirometry normalization in less than six months (< 6 months). However, 17.3% of the patients had no CT normalization, and 19.3% had no spirometry normalization (
Table 2). The relationship with the other comorbidities such as diabetes, hypertension, and obesity) was also performed. However, there was no significant difference.
Among patients with a greater need for time for CT normalization and who did not normalize, a higher proportion of patients required hospital, Intensive Care Unit (ICU) and Oro-tracheal intubation (OTI) treatment during the period of COVID treatment. Regarding Post-COVID treatment, the need for treatment with inhaled medication and the combination of medication with physiotherapy was greater among patients with a longer time for CT normalization and who did not normalize (
Table 3).
For the clinical evolution of spirometry, no association was observed between priority treatment and the need for OTI during the period of COVID treatment, as well as the need for post-COVID treatment. However, among patients who had a longer time to normalization, a higher proportion of patients who needed ICU during the COVID treatment period was observed. However, in patients who did not normalize spirometry, a higher proportion of patients who did not need ICU was observed. Although no significant association was observed, among patients who did not normalize spirometry, a higher proportion of patients with home treatment and who did not require OTI was observed (
Table 4).
A logistic regression model was constructed to analyze the effect of independent variables on the probability of normalization of CT and spirometry. For analysis, the dependent variables CT and spirometry were dichotomized into normalized and no normalized. The selection of independent variables considered the COVID treatment variables and the factors of age, sex, and morbidities, but only the morbidities of asthma and Chronic Obstructive Pulmonary Disease had a significant effect on the association analysis. A significant effect was observed in the initial model, but the variables explain only 18.9% of the variation in the probability of CT normalization. After excluding the independent variables that did not significantly affect the model for normalization of CT, a significant effect was observed for age and the need for ICU, which explains 17.0% of the variation in the probability of normalization of CT. Increasing age and the need for ICU reduce the likelihood of CT normalization. For the probability of spirometry normalization, the initial model did not show a significant effect. After excluding the independent variables that did not show a significant effect, the final model showed a significant effect only for COPD, which is responsible for 14.5% of the variation in the probability of spirometry normalization (
Table 5).
4. Discussion
The involvement of COVID-19 has substantial consequences for the body, especially the respiratory system. As our study, the literature shows that patients who have suffered from the disease exhibit patterns of lung function consistent with restrictive defects that normalize with time [
6,
14,
24].
Similar to our results, Péterfi et al. identified that COPD, hypertension, and diabetes are risk factors that caused a higher number of hospitalizations in older patients [
25]. Üçsular
et al [
26] carried out a retrospective study showing that hypertension, COPD, diabetes, and cardiovascular disease in the elderly were significantly higher compared to the non-elderly. In addition, most of the elderly underwent hospital treatment in the ward and ICU. Watanabe et al. [
27], when studying the probabilities of hospitalization according to age, they found that older adults suffered more from hospitalizations. The literature also shows that older patients present spirometry results of less than six months with significantly more alterations [
28].
As in our study, the literature shows that, in general, most patients recover completely in clinical terms after infection with COVID-19; however, an estimated 10-15% of patients maintain symptoms for a certain time post-infection [
29]. This phase is designated by clinicians as “Long-term COVID-19 effects”, which include those patients who do not recover normality within a period of more than 2-3 weeks after infection [
30,
31]. The post-COVID-19 syndrome is officially characterized by the persistent clinical picture about four weeks after onset in a sub-chronic phase of 12 or more weeks [
32,
33].
There are studies that compared the types of hospitalization between the different waves of the pandemic and showed that hospitalizations in the ward reduced from 22.41% of those admitted in the first wave (03/2020 to 10/2020) and to 17.16% in the second ( 11/2020 to 06/2021), as well as the need for an ICU, which reduced from 13.84% to 9.56%. A significant decrease in dyspnea was also observed (from 25.51% in the first wave to 13.13% in the second) [
34].
The study by Parashar et al. [
35] evaluated 255 COVID-19 survivors. Participants were classified as having mild, moderate, and severe disease and were followed for two months. Results indicated that pulmonary function test parameters were significantly associated with disease severity, with detected obstructive and restrictive changes suggesting a mixed pattern of long-term sequelae of COVID-19. However, no significant differences were found in peak expiratory flow and other parameters, indicating that COVID-19 is associated with a mixed pattern of spirometry results.
Diagnostic CT findings may be helpful to predict the prognosis of patients affected by COVID-19 [
14,
17]. The British Thoracic Society recommends radiographic evaluations for about 12 weeks after disease onset, during the follow-up of patients with Post-COVID-19 Acute Syndrome [
13]. In the follow-up of these patients, radiological alterations are often observed for an initially indeterminate period of time (the long-term evolution of these abnormalities remains an unresolved issue). After for months of infection, ground-glass opacities are usually observed (>40% of cases), and it is the most common abnormality of persistent disease
[13,22]. Studies have shown the presence of signs of reticulation, with fibrous bands with or without parenchymal distortion, bronchiectasis, and bronchiectasis in 67% of patients who survived three months after hospital discharge and who underwent orotracheal intubation and ventilatory assistance [
36].
Studies show that, with time, dyspnea improves, but a subgroup maintains radiological and physiological changes [
22]. Fibrotic lesions can be irreversible and lead to chronic interstitial lung disease, with a decline in lung function, worsening symptoms, and decreased quality of life, leading to early mortality [
36].
Pan
et al. evaluated the chest CT patterns of 209 participants, from diagnosis of the disease to 1 year of follow-up. Based on CT findings at 12 months, participants were categorized into complete resolution, residual linear opacities, and multifocal cystic or reticular lesions. Full resolution occurred mainly in the first three months after the onset of symptoms, and one year after the diagnosis of COVID-19, the three CT patterns could be observed, with complete resolution being the most common [
37].
In another study conducted by Corsi
et al. [
38], clinical status, pulmonary function tests, laboratory tests, and radiological findings were evaluated at three and twelve months after discharge in patients admitted between February 25 and May 2, 2020. At twelve months after discharge, most patients had laboratory tests and lung function tests significantly improved. All patients with negative CT findings at three months also had a negative CT at twelve months. Among the patients who presented CT changes in three months, 2% returned to normal, 82% improved, 14% remained stably abnormal, and 2% worsened. Thus, according to the above authors, pulmonary function tests are normal in most survivors 12 months after discharge, but structural CT abnormalities may persist. Watanabe
et al. [
39] found that the frequency of CT changes remained high 1 year after infection, especially among severe/critical patients for fibrotic changes. Lerum
et al. also found substantial improvement/normalization of CT over the twelve months [
40]. Bongiovanni
et al. found that, despite some abnormalities on chest CT after twelve months, lung function impairment persists only in a minority of individuals [
41]. In our results, most patients had a normal functional examination, and only a small percentage still had mild or moderate alterations.
5. Conclusions
The post-COVID-19 state incurs a constellation of symptoms involving various organs of the patient, including the pulmonary tract. As for the radiological image, it was observed that 4.2% had normal CT, 50.3% had normalized CT in less than 6 months, 23% normalized between 6 and 12 months and 5.2% normalized the images after 12 months. However, 17.3% of patients did not show CT normalization after 12 months, maintaining residual pulmonary sequelae. As for the functional status, it was found that 78% of the patients who underwent spirometry had a normal test, 15.4% had a mild change, 5.3% had a moderate change, and only 1.3% had a severe change.
Currently, due to vaccination schedules, patients have shown changes in the presentation of the disease, unlike the beginning of the pandemic, where the disease was very intense and compromised the body systemically with significant pulmonary involvement. Our results indicate that, in most cases, there was a satisfactory clinical, radiological, and functional evolution in the post-disease stage, with most patients returning to their normal organic status within one year after infection. However, a small percentage remained with clinical, radiological, and functional sequelae. Our results can contribute to the literature investigating the evolution of the consequences of COVID-19.
Author Contributions
Conceptualization, V.M.C.S., T.L.M.Z., H.F.G., and S.M.B.; methodology, E.F.B.C., L.F.L., E.L.G., A.C.A., and S.M.B.; validation, L.F.L., C.F.G.Z., and S.M.B.; formal analysis, E.F.B.C.; investigation, V.M.C.S., T.L.M.Z., H.F.G., V.C.S.C., and S.M.B.; writing—original draft preparation, V.M.C.S., T.L.M.Z., H.F.G., and S.M.B.; writing—review and editing, V.M.C.S., T.L.M.Z., and S.M.B.; visualization, V.M.C.S., V.C.S.C., T.L.M.Z., H.F.G., and S.M.B.; supervision, S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.-M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Alfredsson, E.; Psouni, E. Coparent exclusion, prenatal experiences, and mental health during COVID-19 in Sweden. J. Fam. Psychol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L.J.N.R.M. Characteristics of SARS-CoV-2 and COVID-19. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Rosa, R.G.; Cavalcanti, A.B.; Azevedo, L.C.P.; Veiga, V.C.; de Souza, D.; Santos, R.d.R.M.d.; Schardosim, R.F.d.C.; Rech, G.S.; Trott, G.; Schneider, D.; et al. Association between acute disease severity and one-year quality of life among post-hospitalisation COVID-19 patients: Coalition VII prospective cohort study. Intensiv. Care Med. 2023, 49, 166–177. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Minniti, G.; Miola, V.F.B.; Haber, J.F.d.S.; Bueno, P.C.d.S.; Haber, L.S.d.A.; Girio, R.S.J.; Detregiachi, C.R.P.; Dall’antonia, C.T.; Rodrigues, V.D.; et al. Organokines in COVID-19: A Systematic Review. Cells 2023, 12, 1349. [Google Scholar] [CrossRef] [PubMed]
- Churruca, M.; Martínez-Besteiro, E.; Couñago, F.; Landete, P. COVID-19 pneumonia: A review of typical radiological characteristics. World J. Radiol. 2021, 13, 327–343. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.J.T.l. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Kiener, M.; Roldan, N.; Machahua, C.; Sengupta, A.; Geiser, T.; Guenat, O.T.; Funke-Chambour, M.; Hobi, N.; Julio, M.K.-D. Human-Based Advanced in vitro Approaches to Investigate Lung Fibrosis and Pulmonary Effects of COVID-19. Front. Med. 2021, 8, 644678. [Google Scholar] [CrossRef]
- To, K.K.-W.; Sridhar, S.; Chiu, K.H.-Y.; Hung, D.L.-L.; Li, X.; Hung, I.F.-N.; Tam, A.R.; Chung, T.W.-H.; Chan, J.F.-W.; Zhang, A.J.-X.J.E.m.; et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. 2021, 10, 507–535. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Su, W.-L.; Lu, K.-C.; Chan, C.-Y.; Chao, Y.-C. COVID-19 and the lungs: A review. J. Infect. Public Heal. 2021, 14, 1708–1714. [Google Scholar] [CrossRef]
- Fugazzaro, S.; Contri, A.; Esseroukh, O.; Kaleci, S.; Croci, S.; Massari, M.; Facciolongo, N.C.; Besutti, G.; Iori, M.; Salvarani, C.; et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int. J. Environ. Res. Public Heal. 2022, 19, 5185. [Google Scholar] [CrossRef] [PubMed]
- Mogami, R.; Filho, R.C.A.; Chantong, C.G.C.; de Almeida, F.C.S.; Koifman, A.C.B.; Jauregui, G.F.; Mafort, T.T.; Costa, H.d.S.B.d.; dos Santos, G.A.P.; de Carvalho, B.Z.; et al. The Importance of Radiological Patterns and Small Airway Disease in Long-Term Follow-Up of Postacute COVID-19: A Preliminary Study. Radiol. Res. Pr. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanli, D.E.T.; Yildirim, D.; Sanli, A.N.; Turkmen, S.; Erozan, N.; Husmen, G.; Altundag, A.; Tuzuner, F. A practical approach to imaging characteristics and standardized reporting of COVID-19: a radiologic review. Mil. Med Res. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Awulachew, E.; Diriba, K.; Anja, A.; Getu, E.; Belayneh, F. Computed Tomography (CT) Imaging Features of Patients with COVID-19: Systematic Review and Meta-Analysis. Radiol. Res. Pr. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Islam, N.; Ebrahimzadeh, S.; Salameh, J.-P.; Kazi, S.; Fabiano, N.; Treanor, L.; Absi, M.; Hallgrimson, Z.; Leeflang, M.M.; Hooft, L.J.C.D.o.S.R. Thoracic imaging tests for the diagnosis of COVID-19. 2021.
- Sharif, P.M.; Nematizadeh, M.; Saghazadeh, M.; Saghazadeh, A.; Rezaei, N. Computed tomography scan in COVID-19: a systematic review and meta-analysis. Pol. J. Radiol. 2022, 87, 1–23. [Google Scholar] [CrossRef]
- Kotzé, P.B.; Manthey, R.; Griffith-Richards, S.; Ackermann, C.; Klusmann, K. Computed tomography chest findings in post-acute COVID-19 lung disease at a South African regional hospital - a descriptive study. The Pan African medical journal 2023, 44, 175. [Google Scholar] [PubMed]
- Lehmann, A.; Gysan, M.; Bernitzky, D.; Bal, C.; Prosch, H.; Zehetmayer, S.; Milos, R.-I.; Vonbank, K.; Pohl, W.; Idzko, M.; et al. Comparison of pulmonary function test, diffusion capacity, blood gas analysis and CT scan in patients with and without persistent respiratory symptoms following COVID-19. BMC Pulm. Med. 2022, 22, 1–8. [Google Scholar] [CrossRef]
- Niyatiwatchanchai, N.; Deesomchok, A.; Chaiwong, W.; Duangjit, P.; Pothirat, C.; Liwsrisakun, C.; Bumroongkit, C.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; et al. Comparative Study of Early Impacts of Post-COVID-19 Pneumonia on Clinical Manifestations, Pulmonary Function, and Chest Radiographs. Medicina 2022, 58, 216. [Google Scholar] [CrossRef]
- Sahin, M.E.; Gökçek, A.; Satar, S.; Ergün, P. Relation of impulse oscillometry and spirometry with quantitative thorax computed tomography after COVID-19 pneumonia. Revista da Associacao Medica Brasileira (1992) 2023, 69, e20221427. [Google Scholar] [CrossRef]
- Jutant, E.-M.; Meyrignac, O.; Beurnier, A.; Jaïs, X.; Pham, T.; Morin, L.; Boucly, A.; Bulifon, S.; Figueiredo, S.; Harrois, A.; et al. Respiratory symptoms and radiological findings in post-acute COVID-19 syndrome. ERJ Open Res. 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Munker, D.; Veit, T.; Barton, J.; Mertsch, P.; Mümmler, C.; Osterman, A.; Khatamzas, E.; Barnikel, M.; Hellmuth, J.C.; Münchhoff, M.; et al. Pulmonary function impairment of asymptomatic and persistently symptomatic patients 4 months after COVID-19 according to disease severity. Infection 2021, 50, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, V.T.; Vavougios, G.D.; Kalogiannis, P.; Tachoulas, K.; Touloudi, E.; Astara, K.; Mysiris, D.S.; Tsirimona, G.; Papayianni, E.; Boutlas, S.; et al. Breathlessness and exercise with virtual reality system in long-post-coronavirus disease 2019 patients. Front. Public Heal. 2023, 11, 1115393. [Google Scholar] [CrossRef] [PubMed]
- Péterfi, A.; Mészáros, Á.; Szarvas, Z.; Pénzes, M.; Fekete, M.; Fehér, Á.; Lehoczki, A.; Csípő, T.; Fazekas-Pongor, V. Comorbidities and increased mortality of COVID-19 among the elderly: A systematic review. Imaging 2022, 109, 163–176. [Google Scholar] [CrossRef]
- sular, F.D.; Karadeniz, G.; Polat, G.; Ayrancı, A.; Yalnız, E.; Kazankaya, F.; Güldaval, F.; Büyükşirin, M.; Anar, C.J.T.T.J. Clinical Differences Between Elderly and Non-elderly Patients with COVID-19. 2022, 23, 238. [Google Scholar]
- Watanabe, J.H.; Kwon, J.; Mehta, S.R. Association of Age and Hospitalization Amongst Those with Underlying High-risk Conditions at COVID-19 Diagnosis in a Large, State-wide Health System. J. Gen. Intern. Med. 2021, 36, 2906–2908. [Google Scholar] [CrossRef]
- Navarro, A.O.; Cervantes-Bojalil, J.; Quevedo, O.d.J.C.; Martínez, A.A.; Hernández-Jiménez, C.A.; Álvarez, E.P.; Gil, A.G.; Amaro, A.L.P.; Vera-Lastra, O.; Luis, B.A.L. Decreased quality of life and spirometric alterations even after mild-moderate COVID-19. Respir. Med. 2021, 181, 106391. [Google Scholar] [CrossRef]
- Nalbandian, A.; Desai, A.D.; Wan, E.Y.J.A.R.o.M. Post-COVID-19 condition. 2023, 74, 55–64. [Google Scholar] [CrossRef]
- Lam, I.C.H.; Wong, C.K.H.; Zhang, R.; Chui, C.S.L.; Lai, F.T.T.; Li, X.; Chan, E.W.Y.; Luo, H.; Zhang, Q.; Man, K.K.C.; et al. Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. EClinicalMedicine 2023, 60, 102000. [Google Scholar] [CrossRef]
- Salamanna, F.; Veronesi, F.; Martini, L.; Landini, M.P.; Fini, M. Post-COVID-19 Syndrome: The Persistent Symptoms at the Post-viral Stage of the Disease. A Systematic Review of the Current Data. Front. Med. 2021, 8, 653516. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.J.N.m. Post-acute COVID-19 syndrome. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Barros, D.; Moraes, T.; Hayashi, C.; Ralio, R.; Minenelli, F.; van Zon, K.; Ripardo, J.J.I.R. Clinical characteristics and outcomes of hospitalized COVID-19 patients in a Brazilian Hospital-A retrospective study comprising first and second waves. 2022.
- Parashar, R.; Joshi, A.; Raghuwanshi, P.; Joshi, R.; Hulke, S.; Sharma, J.P. Patterns and Trajectories of Pulmonary Function in Coronavirus Disease 2019 Survivors: An Exploratory Study Conducted in Central India. Cureus 2022, 14, e26955. [Google Scholar] [CrossRef]
- Zuo, H. Contribution of CT Features in the Diagnosis of COVID-19. Can. Respir. J. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Yang, L.; Liang, B.; Ye, T.; Li, L.; Li, L.; Liu, D.; Wang, J.; Hesketh, R.L.; Zheng, C. Chest CT Patterns from Diagnosis to 1 Year of Follow-up in Patients with COVID-19. Radiology 2022, 302, 709–719. [Google Scholar] [CrossRef]
- Corsi, A.; Caroli, A.; Bonaffini, P.A.; Conti, C.; Arrigoni, A.; Mercanzin, E.; Imeri, G.; Anelli, M.; Balbi, M.; Pace, M.; et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography 2022, 8, 2588–2603. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T.J.R. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. 2022, 27, 605–616. [Google Scholar] [PubMed]
- Lerum, T.V.; Meltzer, C.; Rodriguez, J.R.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.B.; Aarberg-Lund, K.M.; Durheim, M.T.; Ashraf, H.; Einvik, G.; et al. A prospective study of pulmonary outcomes and chest computed tomography in the first year after COVID-19. ERJ Open Res. 2023, 9. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Barilaro, G.; Bini, F.J.J.o.M.V. Twelve-month clinical, functional, and radiological outcomes in patients hospitalized for SARS-CoV-2 pneumonia. 2023, 95, e28524. [Google Scholar] [CrossRef]
Table 1.
Distribution of absolute (f) and relative (%) frequency of the participants' data, type of treatment, and the presence of comorbidities.
Table 1.
Distribution of absolute (f) and relative (%) frequency of the participants' data, type of treatment, and the presence of comorbidities.
| |
Category |
f |
% |
p-value |
| Year |
2020 |
41 |
13.6 |
0.006* |
| 2021 |
146 |
48.3 |
| 2022 |
115 |
38.1 |
| Sex |
Female |
184 |
60.9 |
<0.001* |
| Male |
118 |
39.1 |
| Age range |
<40 year |
86 |
28.5 |
0.006* |
| 40 - 59 years |
127 |
42.1 |
| >59 years |
89 |
29.5 |
| Priority treatment |
Home |
234 |
77.5 |
<0.001* |
| Hospital |
68 |
22.5 |
| Intensive Care Unity |
No |
273 |
90.4 |
<0.001* |
| Yes |
29 |
9.6 |
| Oro-tracheal intubation |
No |
283 |
93.7 |
<0.001* |
| Yes |
19 |
6.3 |
| Asthma |
No |
231 |
76.5 |
<0.001* |
| Yes |
71 |
23.5 |
| COPD |
No |
282 |
93.4 |
<0.001* |
| Yes |
20 |
6.6 |
| Hypertension |
No |
238 |
78.8 |
<0.001* |
| Yes |
64 |
21.2 |
| Diabetes |
No |
282 |
93.4 |
<0.001* |
| Yes |
20 |
6.6 |
| Smoke |
No |
255 |
84.4 |
<0.001* |
| Yes |
47 |
15.6 |
Table 2.
Distribution of absolute (f) and relative (%) frequency of normalization time for computed tomography (CT) and spirometry.
Table 2.
Distribution of absolute (f) and relative (%) frequency of normalization time for computed tomography (CT) and spirometry.
| |
Category |
f |
% |
p-value |
| Normalization of chest tomography |
Inicial |
8 |
4.2 |
<0.001* |
| <6 months |
96 |
50.3 |
| 6 a 12 months |
44 |
23.0 |
| > 12 months |
10 |
5.2 |
| No normalization |
33 |
17.3 |
| Total |
191 |
100.0 |
| Normalization of spirometry |
<6 months |
35 |
61.4 |
<0.001* |
| 6 a 12 months |
7 |
12.3 |
| >12 months |
4 |
7.0 |
| No normalization |
11 |
19.3 |
| Total |
57 |
100.0 |
Table 3.
Analysis of the absolute (f) and relative (%) distribution of priority treatment, need for Intensive Care Unit, need for orotracheal intubation, and post-covid treatment with clinical evolution of computed tomography (CT).
Table 3.
Analysis of the absolute (f) and relative (%) distribution of priority treatment, need for Intensive Care Unit, need for orotracheal intubation, and post-covid treatment with clinical evolution of computed tomography (CT).
| |
Category |
Normalization CT (n=191) |
p-value |
| Initial |
<6 months |
6 a 12 months |
>12 months |
No normalized |
| Priority treatment |
Home |
f |
8 |
82 |
21 |
4 |
12 |
<0.001* |
| % |
100.0 |
85.4 |
47.7 |
40.0 |
36.4 |
| Hospital |
f |
0 |
14 |
23 |
6 |
21 |
| % |
0.0 |
14.6 |
52.3 |
60.0 |
63.6 |
| Intensive Care Unity |
No |
f |
8 |
94 |
36 |
9 |
18 |
<0.001* |
| % |
100.0 |
97.9 |
81.8 |
90.0 |
54.5 |
| Yes |
f |
0 |
2 |
8 |
1 |
15 |
| % |
0.0 |
2.1 |
18.2 |
10.0 |
45.5 |
| Oro-tracheal intubation |
No |
f |
8 |
95 |
39 |
9 |
22 |
<0.001* |
| % |
100.0 |
99.0 |
88.6 |
90.0 |
66.7 |
| Yes |
f |
0 |
1 |
5 |
1 |
11 |
| % |
0.0 |
1.0 |
11.4 |
10.0 |
33.3 |
| Post-Covid 19 treatment |
No |
f |
2 |
3 |
1 |
0 |
3 |
<0.001* |
| % |
25.0 |
3.1 |
2.3 |
0.0 |
9.1 |
| Inhaled Medication |
f |
4 |
60 |
25 |
6 |
7 |
| % |
50.0 |
62.5 |
56.8 |
60.0 |
21.2 |
| Physiotherapy |
f |
2 |
19 |
6 |
1 |
3 |
| % |
25.0 |
19.8 |
13.6 |
10.0 |
9.1 |
| Both |
f |
0 |
14 |
12 |
3 |
20 |
| % |
0.0 |
14.6 |
27.3 |
30.0 |
60.6 |
Table 4.
Analysis of the absolute (f) and relative (%) distribution of priority treatment, need for Intensive Care Unity, need for Oro-tracheal intubation), and post-covid treatment with clinical evolution of spirometry.
Table 4.
Analysis of the absolute (f) and relative (%) distribution of priority treatment, need for Intensive Care Unity, need for Oro-tracheal intubation), and post-covid treatment with clinical evolution of spirometry.
| Variable |
Category |
Normalization of Espirometry (n=57) |
p-value |
| <6 months |
6 a 12 months |
>12 months |
No normalization |
| Priority treatment |
Home |
f |
20 |
3 |
1 |
7 |
0.561 |
| % |
57.1% |
42.9% |
25.0% |
63.6% |
| Hospital |
f |
15 |
4 |
3 |
4 |
| % |
42.9% |
57.1% |
75.0% |
36.4% |
| Intensive Care Unity |
No |
f |
31 |
5 |
1 |
9 |
0.025* |
| % |
88.6% |
71.4% |
25.0% |
81.8% |
| Yes |
f |
4 |
2 |
3 |
2 |
| % |
11.4% |
28.6% |
75.0% |
18.2% |
| Oro-tracheal intubation |
No |
f |
32 |
5 |
2 |
10 |
0.065 |
| % |
91.4% |
71.4% |
50.0% |
90.9% |
| Yes |
f |
3 |
2 |
2 |
1 |
| % |
8.6% |
28.6% |
50.0% |
9.1% |
| Post-Covid 19 treatment |
No |
f |
2 |
0 |
0 |
1 |
0.718 |
| % |
5.7% |
0.0% |
0.0% |
9.1% |
| Inhaled Medication |
f |
16 |
4 |
3 |
6 |
| % |
45.7% |
57.1% |
75.0% |
54.5% |
| Physiotherapy |
f |
5 |
0 |
1 |
0 |
| % |
14.3% |
0.0% |
25.0% |
0.0% |
| Both |
f |
12 |
3 |
0 |
4 |
| % |
34.3% |
42.9% |
0.0% |
36.4% |
Table 5.
Logistic regression analysis for the effect of independent variables on the probability of computed tomography (CT) and spirometry normalization.
Table 5.
Logistic regression analysis for the effect of independent variables on the probability of computed tomography (CT) and spirometry normalization.
| Variables |
B |
Wald |
Odds |
IC95% (Odds) |
Model |
| Dependent |
Independente |
p-value |
LL |
UL |
p-value |
R2 Cox |
| CT normalization (beginning) |
Age (years) |
-0.048 |
0.005* |
0.953 |
0.922 |
0.986 |
<0.001** |
0.189 |
| Sex |
-0.66 |
0.161 |
0.517 |
0.205 |
1.3 |
| Treatament |
-0.44 |
0.433 |
0.644 |
0.214 |
1.937 |
| ICU |
-1.734 |
0.055 |
0.177 |
0.03 |
1.038 |
| OTI |
-0.598 |
0.534 |
0.55 |
0.083 |
3.624 |
| COPD |
-0.057 |
0.931 |
0.945 |
0.263 |
3.395 |
| Constant |
1.306 |
0.204 |
3.692 |
0.491 |
27.757 |
| Age (years) |
5.695 |
<0.001* |
297.389 |
|
|
| CT normalization (in the end) |
Age (years) |
-0.043 |
0.004* |
0.958 |
0.93 |
0.986 |
<0.001** |
0.170 |
| ICU |
-2.413 |
<0.001* |
0.09 |
0.034 |
0.234 |
| Constant |
4.37 |
<0.001* |
79.015 |
|
|
| Spirometry normalization (beginning) |
Age (years) |
-0.031 |
0.284 |
0.97 |
0.917 |
1.026 |
0.066 |
0.188 |
| Sex |
0.103 |
0.899 |
1.108 |
0.226 |
5.442 |
| Treatament |
0.888 |
0.380 |
2.43 |
0.335 |
17.655 |
| ICU |
-1.867 |
0.235 |
0.155 |
0.007 |
3.374 |
| OTI |
1.915 |
0.279 |
6.788 |
0.212 |
217.78 |
| COPD |
-2.075 |
0.039* |
0.126 |
0.018 |
0.898 |
| Constant |
3.275 |
0.075 |
26.447 |
|
|
| Spirometry normalization (in the end) |
DPOC |
-2.48 |
0.004* |
0.084 |
0.016 |
0.443 |
0.003** |
0.145 |
| Constante |
1.969 |
<0.001* |
7.167 |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).