1. Introduction
One of the priority tasks set by the United Nations (UN) and the world community is the creation of systems that help reduce the negative impact of emissions from industrial facilities on the environment. The recovery of clean water from enterprises wastewater in the industrial and energy sector is becoming an integral part of the wastewater treatment process due to the depletion of water resources on the planet.
From an environmental and economic point of view, an important criterion is the ratio of extracted water to wastewater, known as the recovery factor. The low recovery factor provides the following advantages: a reduction in the size of consumption of the desalination plant, and a reduction in the volume of produced brine that requires disposal. Conversely, a high recovery factor results in the formation of a highly concentrated brine, which, depending on the degree of dispersion and/or dilution, will have an adverse effect on the flora and fauna in the place of its storage.
The establishment of "zero liquid discharge" (ZLD) technology with 95-98% recovery of feed water can mainly be achieved using membrane and thermal technologies or a combination of both [
1,
2,
3].
The thermal technologies commonly used to treat reverse osmosis (RO) concentrate are evaporative (flash method, multi-stage evaporation), mechanical (vapor compression technology, thermocompression technology, concentrators, and brine crystallizers) or natural (evaporation ponds) [
4].
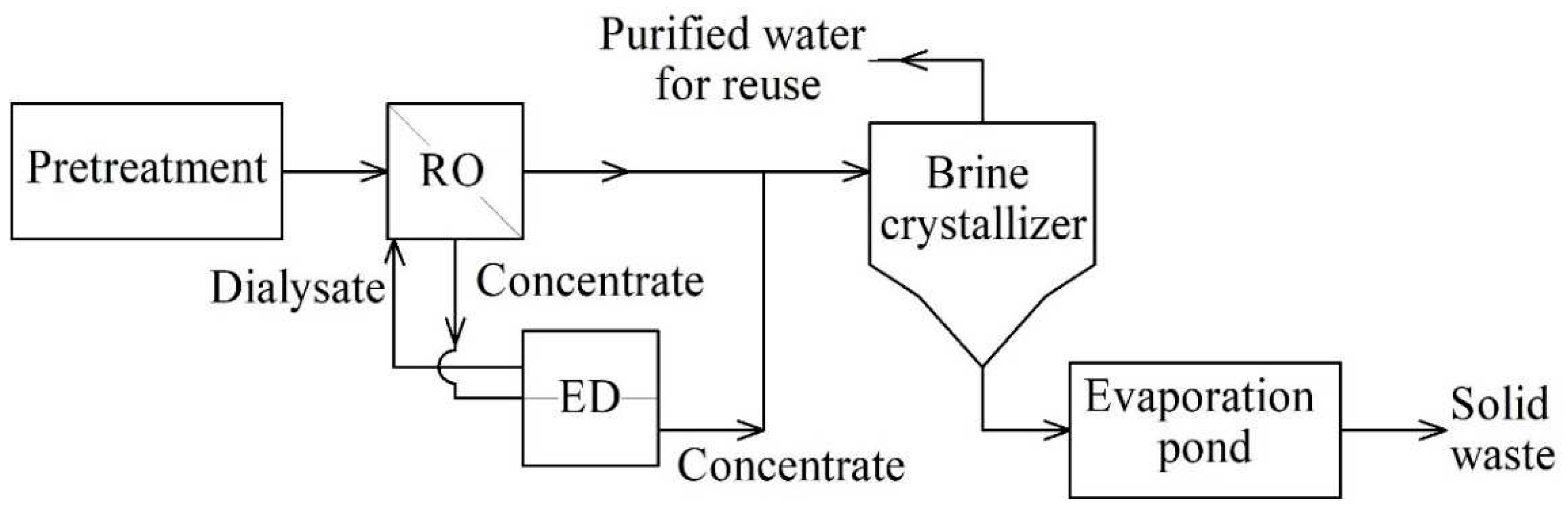
The main known disadvantage of thermal systems is their energy consumption. In addition, powerful thermal power plants (TPPs) discharge more than 500 tons of wastewater every hour. Such streams can be evaporated only by high-performance evaporators, which makes the process as uneconomical and energy-intensive as possible. To level such costs, hybrid systems of water treatment and water purification have been developed. Thus, the combination of membrane and thermal evaporative systems leads to the extraction of feed water up to 99% with more attractive economic indicators than single evaporators (
Figure 1). The use of reverse osmosis as the first stage of wastewater treatment with subsequent evaporation minimizes capital and operating costs (about 58-75% of energy and 48-67% of wastewater treatment costs compared to an evaporation plant) [
5,
6,
7].
Hybridization of mechanical thermal systems with reverse osmosis allows to extract of about 75-85% of water, and more than 85% of water in combination with electrodialysis [
8,
9,
10].
Electromembrane technologies include simple electrodialysis and its varieties (reverse electrodialysis, electrometathesis, electromembrane extraction, etc.) [
11]. Electrodialysis shows a high-water recovery ratio. This is due, firstly, to the preferential movement of salt ions through membranes, i.e., electrodialysis is a salt removal process, not a water removal technology, and therefore most feed water is easily recovered as a product. In a stand-alone electrodialysis system, salt is removed in the range of 3000 ppm (feed water concentration) to 350 ppm (product concentration). The maximum experimental salt concentration achieved by electrodialysis ranges from 104.2 to 267.6 g/kg at a consumption of 7–15 kWh/m
3 [
12]. This is in contrast to reverse osmosis where high recovery rates require multiple steps in a continuous process or longer exposure times. Secondly, electrodialysis is capable of producing a concentration of solids in the brine above 10% of the total, which is impossible to achieve with modern reverse osmosis systems. Thirdly, the requirements for pre-treatment of feed water due to possible deposits are in some cases lower for electrodialysis in comparison with reverse osmosis. Thus, electrodialysis may offer the advantage of a higher recovery factor compared to reverse osmosis systems [
13,
14].
However, the costs of electrodialysis are higher and increase at a low concentration of nutrient solutions, when the rate of salt removal, which scales with electric current, is limited by the rate of ion diffusion to the membrane surface [
15]. This phenomenon, known as limiting current density, as well as the high electrical resistance of solutions at low concentrations, increases the cost of electrodialysis at low salinity. Although the cost of water from a reverse osmosis system operating at a lower recovery is initially less, if the costs of the subsequent disposal of the reverse osmosis concentrate are taken into account, electrodialysis may be more cost-effective [
16].
Baromembrane technologies for liquid waste desalination are used both as an independent method and in combination with thermal desalination and other technologies [
17].
The use of reverse osmosis as an independent technology for wastewater treatment is connected with significant difficulties associated with restrictions on the salinity of treated water and membrane fouling [
18,
19]. Therefore, it is necessary to use pre-treatment, for example, reagent softening, ion-exchange softening, pH adjustment and other methods that help reduce deposits on reverse osmosis membranes [
20].
In connection with the above-mentioned features of electro- and baromembrane technologies, it was the synergy of electrodialysis, which provides high recovery, and reverse osmosis systems, with the final high purity of the product, that gave rise to the analysis of hybrid electrodialysis-reverse osmosis systems. In practice, the recovery factor of a reverse osmosis plant in a hybrid configuration is a design variable. Increasing the recovery factor results in enhanced reverse osmosis costs associated with a multiply risk of scale formation due to higher brine concentrations; higher energy costs due to increased osmotic pressure; the need for a second reverse osmosis stage if recovery above 50% is required [
21].
The hybrid system has two advantages compared to a stand-alone electrodialysis system: it is possible to reduce the number of membrane pairs of the electrodialyzer since the reverse osmosis concentrate is supplied to it as a nutrient solution, therefore, the salt removal rates (current density) become higher; the requirements for the electrodialysis product are relaxed since the final product consists of a high purity mixture reverse osmosis permeate and the electrodialysis product.
Comparing electrodialysis systems operating in stand-alone and hybrid configurations, it can be concluded that the cost of desalination of the electrodialysis product is always higher for a stand-alone system since the degree of product purification is completely dependent on the operation of the electrodialyzer. The study [
22] compared various configurations of membrane systems for desalination and concentration of effluents: a standard single-stage reverse osmosis (RO) system, a hybrid reverse osmosis - electrodialysis (RO-ED) system, a nanofiltration - reverse osmosis (NF-RO), and nanofiltration - reverse osmosis - electrodialysis (NF-RO-ED) system. Indicators such as the percentage of diluate and concentrate yield, the total salt content of the concentrate (TSC), the energy expended, the risk of scale formation, and the possibility of reusing products were evaluated (
Table 1).
The most effective was the NF-OO-ED system, which is capable of producing a highly concentrated brine close to saturation with respect to sodium chloride and the largest volume of diluate. The brine can be further used in the production of table salt or in the chlorine industry. In the case of one-stage RO, for every 1 m
3 of demineralized water, 0.574 m
3 of concentrate (OSC 60 g/l) is produced, which must be disposed of, which creates additional costs. The integration of NF and RO does not significantly change the salinity and volume of the resulting brine compared to the RO configuration. The introduction of ED helps to solve the problem of a large volume of low-mineralized waste. The NF-OO-ED system produces less clean water than the OO-ED configuration but produces a more concentrated brine. In the NF-OO-ED NF system remove most of the divalent hardness ions from the RO-ED circuit. Due to the reduced risk of scale formation in the ED module, recirculation occurs on the concentrate side, resulting in a concentrate that is practically saturated with sodium chloride [
23].
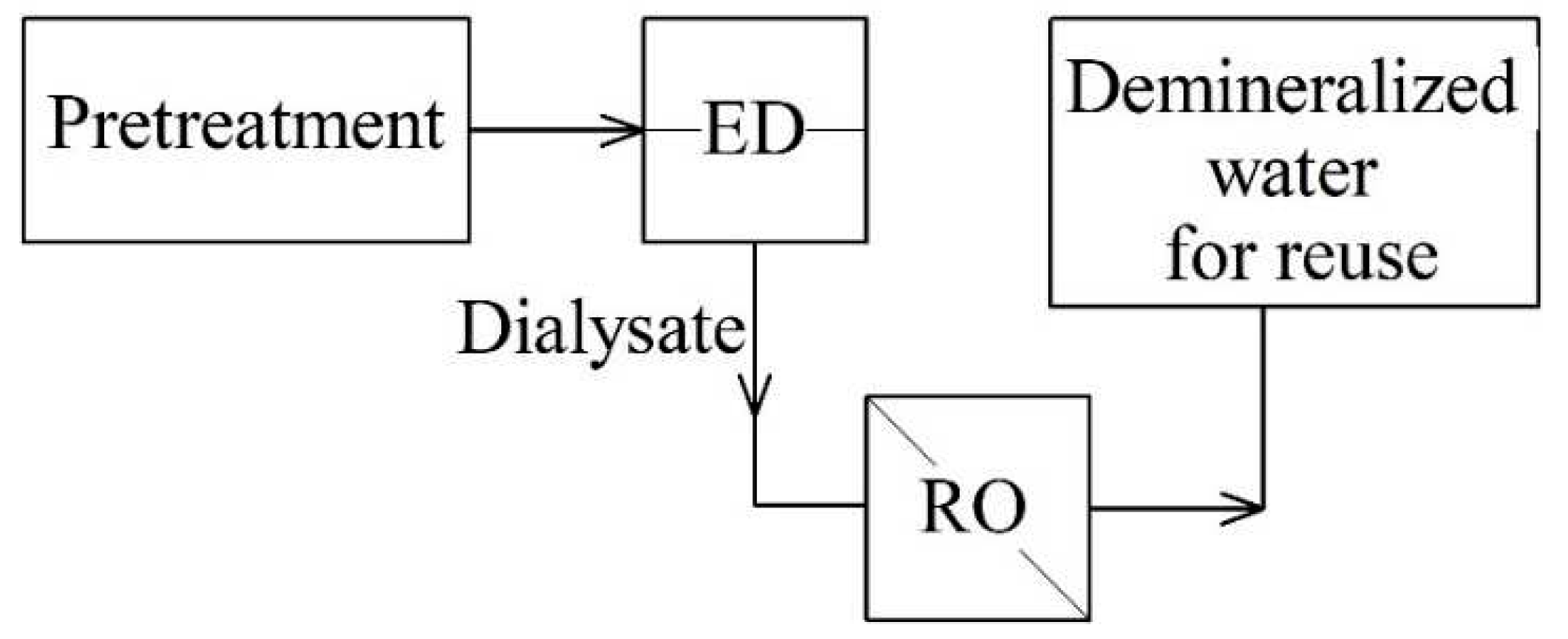
In 2018, industrial plants were tested in Brazil, consisting of series-connected reversible ED-RO (version 1,
Figure 2) or RO-reversible ED (version 2,
Figure 3) devices for petrochemical wastewater treatment in order to obtain process water for reuse.
The hybrid system of reverse ED-RO (
Figure 2) showed the degree of removal of more than 98% of impurities from wastewater. The results obtained meet the quality requirements for reuse in cooling towers. Through reverse ED, 75% water recovery was achieved, while 50% was recovered by RO, so that the hybrid process provided an average 41% of the total water recovery [
24].
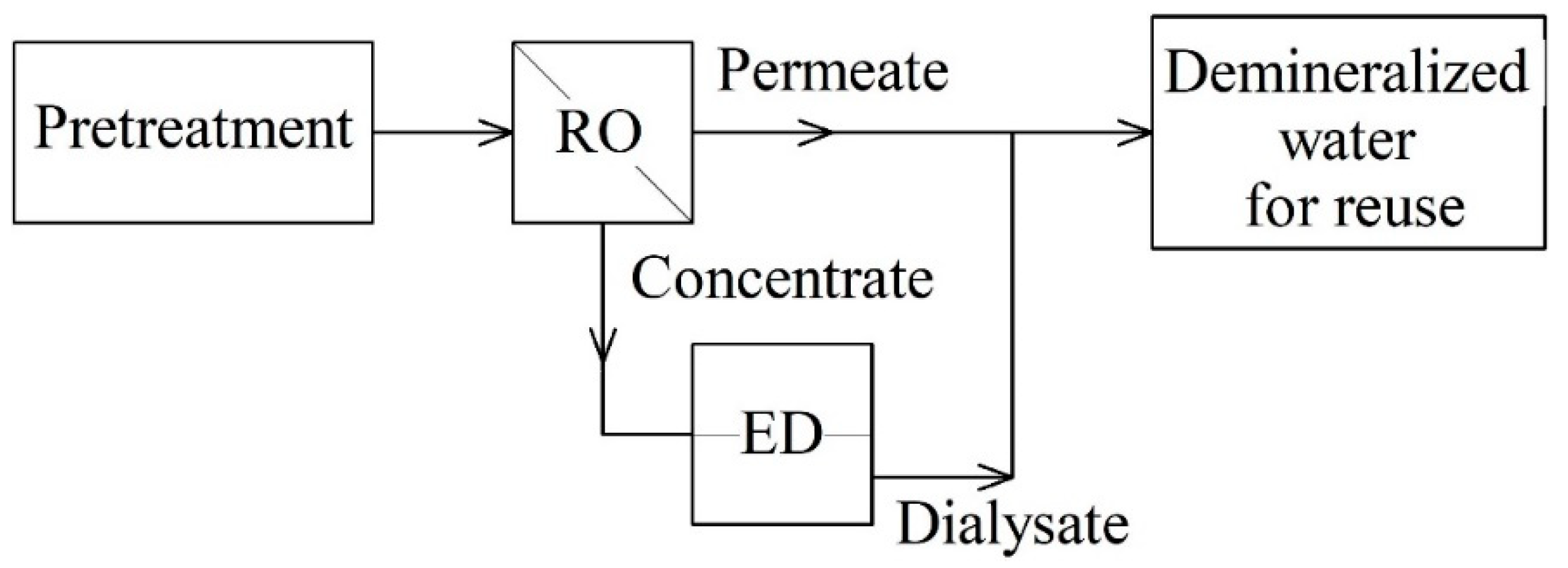
In the case of changing the order of conducting ED and RO according to the second option [
25] (
Figure 3), the volume of recovered diluate significantly increased (87.3%). The degree of impurities removal was more than 75%. This percentage turned out to be less than in the first version of the ED-RO, but nevertheless met the standards for using diluate as cooling circulating water.
An example of boiler make-up water treatment and wastewater disposal at TPPs with "zero" discharge is the installation of the company "Doswell Limited Partnership". The source water is preliminarily desalinated at a six-stage reverse osmosis unit, degassed in a deaerator and additionally desalted at a chemical desalination unit. Reverse osmosis brine, chemical desalination plant concentrate, and boiler blowdown water after a mechanical filter enter the electrodialysis unit, and then into the reverse osmosis unit. The concentrate from the installations is evaporated in the evaporator-crystallizer and then dehydrated in a filter press [
26].
Despite the large number of proposed theoretical computational and modeling studies of hybrid systems, wastewater treatment technologies for real industrial facilities and energy enterprises are presented in insufficient quantities [
27]. There is also insufficient information on the economic and environmental efficiency of hybrid technologies for the industrial and energy sector. Thus, one study compares a hybrid electrodialysis-reverse osmosis system with a reverse osmosis-mechanical vapor compression system, concluding that hybrid membrane technologies have lower initial capital costs and lower operating costs [
28]. In another study, it was shown that the use of hybrid electro-biomembrane systems for wastewater treatment is justified only if the cost of reverse osmosis is less than 40% of an electrodialysis system cost [
29].
Thus, combined methods can provide not only a synergistic effect for a particular industrial separation but also an optimization of the materials use, energy, and space. In this sense, integration is a practical strategy for sustainable industrial growth that the energy industry and industry, in general, are facing today. However, additional experimental and pilot-industrial studies of the hybrid membrane plant operation using both model solutions and industrial wastewater are required, followed by a calculation of economic efficiency and an analysis of the environmental feasibility of their use.
2. Materials and Methods
Experimental studies on utilization at a hybrid baro-electro membrane system were carried out on acidic (ACWR) and alkaline (ALWR) waste regeneration solutions of an ion-exchange chemical-desalting water treatment plant (WTP) of a thermal power plant (
Table 2). Technical characteristics of the electromembrane and baromembrane installations are given in
Table 3 and
Table 4.
The electromembrane apparatus used commercially available industrial heterogeneous ion-selective cation- and anion-exchange membranes IONSEP, which are a highly filled polymer composite consisting of finely ground polymer particles with ion-exchange functional groups fixed in the internal polymer matrix and a reinforcing fabric that improves the mechanical properties of the membrane. Passport characteristics of the used ion-exchange membranes are presented in
Table 5.
The analysis of solutions was carried out for hydrate alkalinity and carbonate alkalinity, total hardness, sulfates, color by spectrophotometric method, pH, and electrical conductivity according to known methods of analysis. The total salt content is calculated from the calibration curve from the electrical conductivity data.
According to the initial conditions of the experiment, in the line of dialysate and concentrate of the electrodialysis apparatus, there were 10 dm3 of solution with a temperature of 25°С each. The volumetric circulation rate of working solutions was 300 dm3/h. The voltage supplied to the device was 75 V.
3. Results
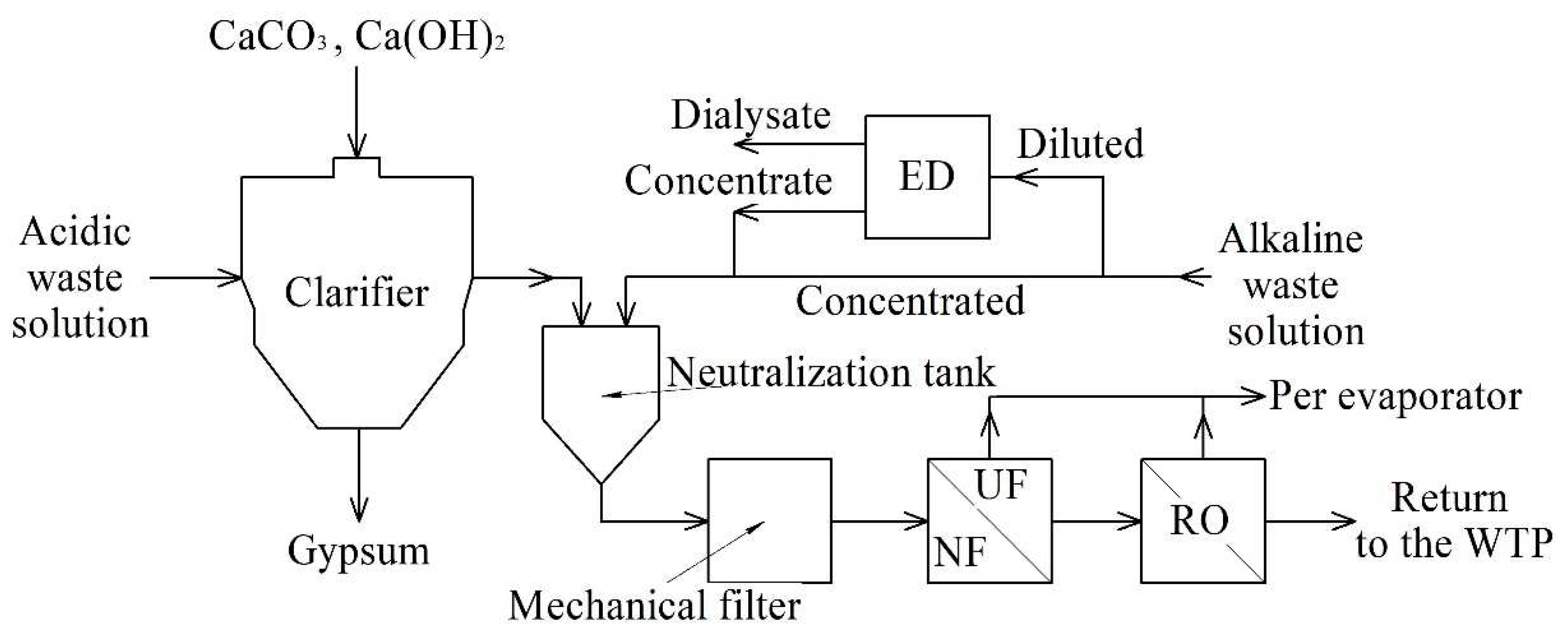
To develop the technology of wastewater treatment by combined membrane methods, a technological scheme was created, including reverse osmosis, electrodialysis, filter press, and an evaporator unit (
Figure 4).
Acidic and alkaline waste regeneration solutions are treated separately in the hybrid plant before being discharged. A well-known problem of effluents from an ion-exchange water treatment plant is the large amount of acidic, highly mineralized effluents with high hardness and exceeding the allowable concentration limit for sulfates, for which TPPs pay heavy fines. According to the proposed technological scheme, the waste of an ion-exchange water treatment plant is proposed to be processed as follows:
- ACWR with a high content of hardness ions and sulfates is subjected to preliminary liming and dehydration in a filter press to precipitate gypsum;
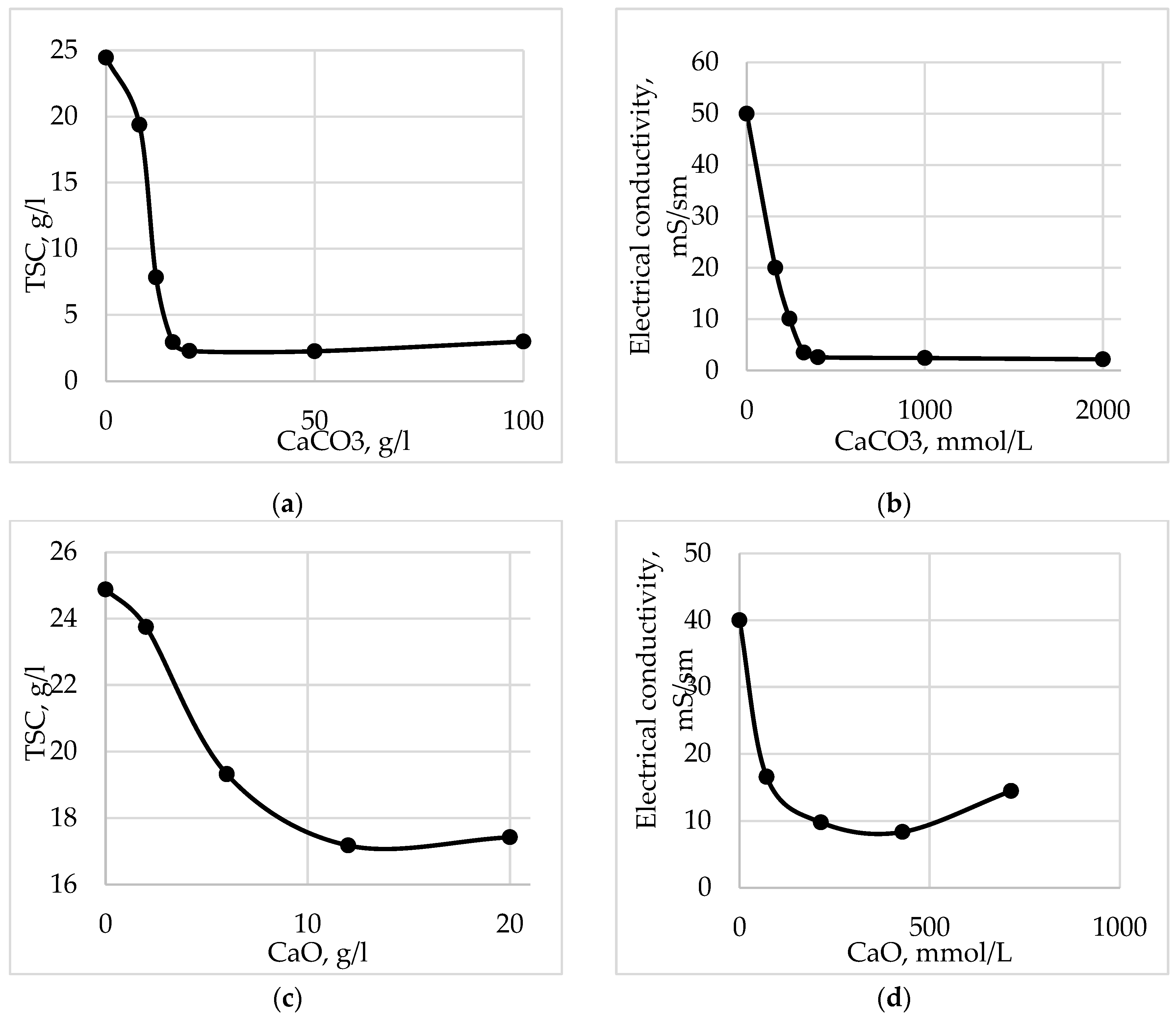
- ALWR is sent for electrodialysis if the electrical conductivity is less than 40 mS/cm for separation into a contaminated concentrate and a purified dialysate;
- if the electrical conductivity of ALWR is more than 40 mS/cm, then it is mixed in a neutralizer tank with limed ACWR;
- the concentrate after electrodialysis is sent to the neutralization tank for mixing with limed ACWR, and the purified dialysate is sent as the source water of the ion-exchange water treatment plant;
- after the neutralizer tank, the neutralized concentrated acidic and alkaline WRs pass sequentially through a mechanical and carbon filter and then baromembrane system - nanofiltration and reverse osmosis;
-concentrate of the baromembrane plant, which is mainly sodium salts, can be reused for the regeneration of Na-cation exchange filters, or evaporated to obtain a distillate and a dry residue, and the reverse osmosis permeate is sent to the water treatment plant as source water.
CaO, CaCO
3, and alkaline WR were used to treat acidic WR.
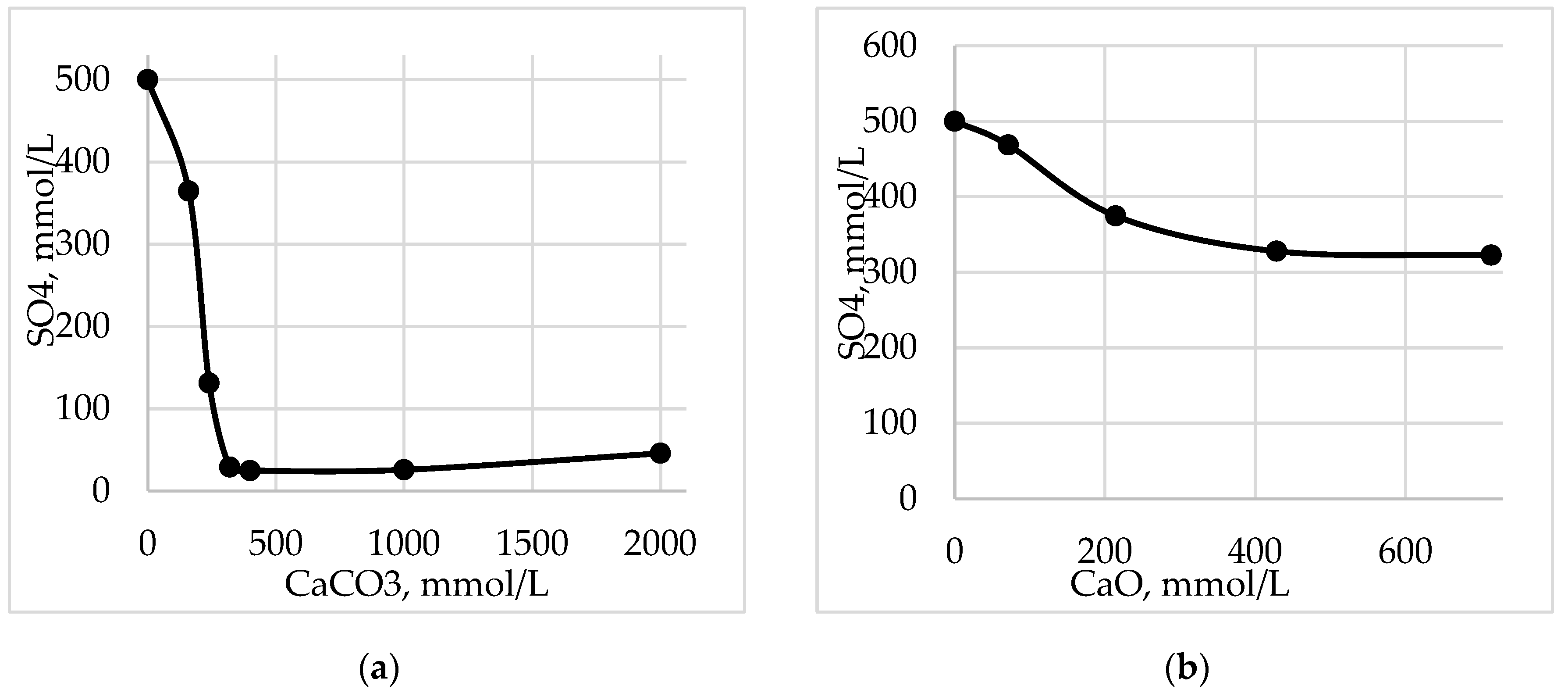
Figure 5 shows that when calcium carbonate is used, the content of sulfates in acid waste is reduced by a factor of 20 or more then 90%. When dosing quicklime, the decrease in sulfates occurs much less by 1.5 times.
When dosing calcium carbonate, the electrical conductivity and total salt content are also significantly reduced in comparison with the dosing of quicklime (
Figure 6). Thus the total salt content is reduced by 91% and the specific electrical conductivity by 95%.
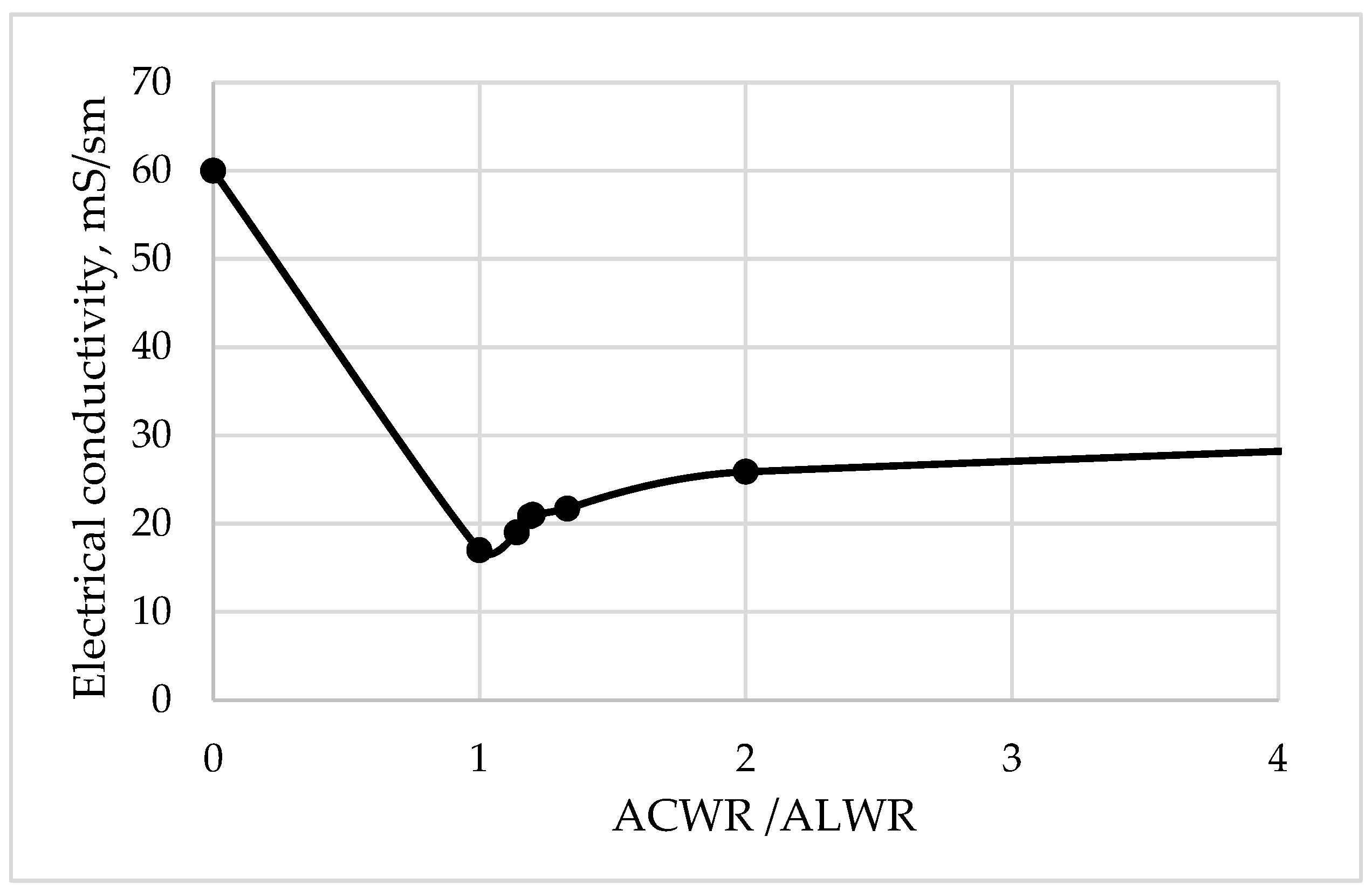
When ACWR is neutralized with ALWR, a small amount of precipitate is formed (
Figure 7). This is characterized by a dip in the curve on the graph, then the electrical conductivity of the solution begins to increase.
The obtained results of the experimental data indicate the need for reagent treatment of the acidic regeneration solution before mixing it with an alkaline regeneration solution, and not after, as is traditionally done at thermal power plants. After mixing, the neutralized acidic and alkaline WR are sent to the baromembrane unit according to the proposed technological scheme (
Table 6).
After liming of acidic regeneration solutions, mutual neutralization and a baromembrane purification, sulfates, hardness, electrical conductivity, color, and organic molecules in the permeate are reduced. To reduce sedimentation on the membranes, the reverse osmosis unit must be periodically flushed with acid solutions. The concentrate is sent to the evaporation plant to obtain a distillate in the amount of 6 liters and a dry residue. Another variation allows to abandon of using an evaporative unit. According to this variation concentrate of the baromembrane plant, which consists mainly sodium salts, can be reused for the regeneration of Na-cation exchange filters. The reverse osmosis permeate is reused in the water treatment plant as source water. Thus, a complete processing of highly concentrated effluents from the ion-exchange water treatment plant of TPP with "zero discharge" of liquid waste is carried out.
In case of discharge of low-concentrated alkaline waste regeneration solutions (electrical conductivity < 40 mS/cm), they must first be concentrated in an electrodialysis unit (
Table 7).
The electrodialysis concentrate is then sent for mixing with the limed acidic regeneration solutions, and the dialysate is returned to the water treatment plant as raw water.
Calculations were made of the returned water cost for various recycling schemes.
Based on the calculations of the membranes characteristics from different manufacturers, the average power consumption for transferring 1 kg is 1.4 kWh/kg of salt. The total power consumption for electromembrane separation of 300 l/h and the production of 20.15 g of salt per 1 dm3, taking into account the costs of preliminary pumping of solutions through the apparatus, is 915.2 Wh. Thus, the cost of operating the electrodialysis part of the plant will be 1.3 euro cents per 100 dm3 of wastewater.
Energy costs for reverse osmosis are on average 55.5 kWh/m3. Thus, the cost of processing 100 dm3 of wastewater will be 22 euro cents.
According to the experimental data obtained, calcium carbonate showed better results in reducing the content of sulfates in acidic effluents than quicklime. The cost of calcium carbonate is 2 euro cents per kilogram. The cost of sulphate reduction is 3.2 euro cents per 100 dm3 of waste.
The proposed technological scheme allows to return all the water from wastewater for reuse. The cost of returned water is 10 euro cents per 1 m3.
Thus, the total cost of wastewater treatment of the ion-exchange chemical desalination plant at the TPP will be 2.6 euros per 1 m3.
4. Conclusions
A technological scheme of a hybrid membrane system was developed for the disposal of highly mineralized waste from an ion-exchange chemical-desalting water treatment plant to implement the principle of resource-saving when creating thermal power plants with "zero discharge". The hybrid system uses baromembrane and electromembrane technologies. If necessary, a reagent treatment, a filter press, and an evaporator unit are added. Waste from the water treatment plant is proposed to be treated separately, using data on the composition of the treated waste, prior to pre-mixing and neutralization according to the principle of separate waste collection. The result of membrane treatment is the production of partially or completely demineralized water, which can be reused in the technological cycle of an energy facility, as well as a dehydrated residue. The total cost of working on the proposed technological scheme with a hybrid membrane system will be 2.6 euros per 1 m3.
Author Contributions
A.A.C. and A.P. worked on all the tasks, A.A.F. and N.C. worked on the literature review, A.P. and A.A.F. conducted experimental studies, I.I. and I.B. performed the supervision, all authors analyzed the results. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by a grant Russian Science Foundation № 22-29-01300,
https://rscf.ru/en/project/22-29-01300/. This work has been accomplished with financial support by Grant No BG05M2OP001-1.002-0011-C02 financed by the Science and Education for Smart Growth Operational Program (2014-2020) and co-financed by the European Union through the European Structural and Investment funds.
Data Availability Statement
All data can be used on request.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ED |
electrodialysis; |
| RO |
reverse osmosis; |
| ZLD |
zero liquid discharge; |
| NF |
nanofiltration; |
| TPPs |
thermal power plants; |
| ACWR |
acidic waste regeneration solutions; |
| ALWR |
alkaline waste regeneration solutions; |
| WTP |
water treatment plant; |
| TSC |
total salt content. |
References
- McGovern, R.K.; Zubair, S.M.; Lienhard, J.H. Hybrid electrodialysis reverse osmosis system design and its optimization for treatment of highly saline brines. IDA J. Desalination Water Reuse. 2014, 6, 15-23. https://doi.org/10.1179/2051645214Y.0000000016. [CrossRef]
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Appl. Energy. 2013, 104 592–602. https://doi.org/10.1016/j.apenergy.2012.11.064. [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard V, J.H. The benefits of hybridising electrodialysis with reverse osmosis. J. Membrane Sci. 2014, 469, 326–335. https://doi.org/10.1016/j.memsci.2014.06.040. [CrossRef]
- Al-Anzi, B.S.; Al-Rashidi, A.; Litty, A.; Fernandes, J.; Al-Sheikh, A.; Alhazza, A. Brine management from desalination plants for salt production utilizing high current density electrodialysis-evaporator hybrid system: A case study in Kuwait. Desalin. 2021, 498,114760. https://doi.org/10.1016/j.desal.2020.114760. [CrossRef]
- Johannsen, P.; Karlapudi, R.; Reinhold, G. High pressure reverse osmosis for wastewater minimization and zero liquid discharge applications. Desalin. 2006, 199, 84–85. https://doi.org/10.1016/j.desal.2006.03.021. [CrossRef]
- Neilly, A.; Jegatheesan, V.; Shu, L. Evaluating the potential for zero discharge from reverse osmosis desalination using integrated processes - A review. Desalin. Water Treat. 2009, 11, 58−65. https://doi.org/10.5004/dwt.2009.843. [CrossRef]
- M. Yaqub, W. Lee, Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review, Sci. Total Environt. 681 (2019) 551–563. https://doi.org/10.1016/j.scitotenv.2019.05.062. [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part I: sensitivity to superficial velocity. Desalin. 2014, 344, 152–162. https://doi.org/10.1016/j.desal.2014.03.035. [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis — Part II: Sensitivity to voltage application and membranes. Desalin. 2014, 345, 128–135. https://doi.org/10.1016/j.desal.2014.04.026. [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis — Part III: Sensitivity to composition and hydraulic recovery. Desalin. 2014, 347, 158–164. https://doi.org/10.1016/j.desal.2014.05.034. [CrossRef]
- Filimonova, A.A. Electro-membrane technologies in energy and industry. Membr. Membr. Technol. 2020, 2, 221-229. https://doi.org/10.1134/S2517751620040046. [CrossRef]
- Havelka, J.; Fárová, H.; Jiříček, T.; Kotala, T.; Kroupa, J. Electrodialysis-based zero liquid discharge in industrial wastewater treatment. Water Sci. Technol. 2019, 79, 1580–1586. https://doi.org/10.2166/wst.2019.161. [CrossRef]
- Medina, V.F.; Johnson, J.L.; Waisner, S.A.; Wade, R.; Mattei-Sosa, J. Development of a Treatment Process for Electrodialysis Reversal Concentrate with Intermediate Softening and Secondary Reverse Osmosis to Approach 98-Percent Water Recovery. J. Environ. Eng. 2015, 147. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000929. [CrossRef]
- Generous, M.M.; Qasem, N.A.A.; Zubair, S.M. An innovative hybridization of electrodialysis with reverse osmosis for brackish water desalination. Energy Convers. Manag. 2021, 245, 114589. https://doi.org/10.1016/j.enconman.2021.114589. [CrossRef]
- Gurreri, L.; La Cerva, M.; Moreno, J.; Goossens, B.; Trunz, A.; Tamburini, A. Coupling of electromembrane processes with reverse osmosis for seawater desalination: Pilot plant demonstration and testing. Desalin. 2022, 526, 115541. https://doi.org/10.1016/j.desal.2021.115541. [CrossRef]
- Generous, M.M.; Qasem, N.A.A.; Akbar, U.A.; Zubair, S.M. Techno-economic assessment of electrodialysis and reverse osmosis desalination plants. Sep. Purif. 2021, 272, 118875. https://doi.org/10.1016/j.seppur.2021.118875. [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317−2348. https://doi.org/10.1016/j.watres.2009.03.010. [CrossRef]
- Pandey, S.R.; Jegatheesan, V.; Baskaran, K.; Shu, L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: a review, Rev. Environ. Sci. Biotechnol. 2012, 11, 125-145. https://doi.org/10.1007/s11157-012-9272-0. [CrossRef]
- Xie, R.J.; Gomez, M.J.; Xing, Y.J.; Klose, P.S. Fouling assessment in a municipal water reclamation reverse osmosis system as related to concentration factor, J. Environ. Eng. Sci. 2004, 3, 61–72. https://doi.org/10.1139/s03-054. [CrossRef]
- Feria-Díaz, J.J.; Correa-Mahecha, F.; López-Méndez, M.C.; Rodríguez-Miranda, J.P.; Barrera-Rojas, J. Recent Desalination Technologies by Hybridization and Integration with Reverse Osmosis: A Review. Water 2021, 13, 1369. https://doi.org/10.3390/w13101369. [CrossRef]
- Patel, S.K.; Biesheuvel, P.M.; Elimelech, M. Energy Consumption of Brackish Water Desalination: Identifying the Sweet Spots for Electrodialysis and Reverse Osmosis. ACS EST Engg. 2021, 1, 851–864. https://doi.org/10.1021/acsestengg.0c00192. [CrossRef]
- Schäfer, A.I.; Andritsot, N.; Karabelas, A.A.; Hoek, E.MV; Schneider, R.; Nyström, M. Nano-filtration − principle and applications. Elsev. Adv Tech. 2005, 1-543.
- Turek, М.; Mitko, K.; Laskowska, E.; Chorążewska, M.; Piotrowski, K.; Jakóbik-Kolon, A.; Dydo, P. Energy consumption and gypsum scaling assessment in a hybrid nanofiltration-reverse osmosis-electrodialysis system. Chem. Eng. Technol. 2018, 2, 392-400. https://doi.org/10.1002/ceat.201700371. [CrossRef]
- Venzke, C.D.; Giacobbo, A.; Klauck, C.R.; Viegas, C.; Hansen, E.; de Aquim, P.M.; Rodrigues, M.A.S.; Bernardes, A.M. Integrated membrane processes (EDR-RO) for water reuse in the petrochemical industry. J. Membr. Sci. Res. 2018, 4, 218-226. https://doi.org/10.22079/jmsr.2018.82055.1180. [CrossRef]
- Venzke, C.D.; Giacobbo, A.; Ferreira, J.Z. Increasing water recovery rate of membrane hybrid process on the petrochemical wastewater treatment. Process. Saf. Environ. Prot. 2018, 117, 152-158. https://doi.org/10.1016/j.psep.2018.04.023. [CrossRef]
- Seigworth, A.; Ludlum, R.; Reahl, E. Case study: Integrating membrane processes with evaporation to achieve economical zero liquid discharge at the Doswell Combined Cycle Facility. Desalin. 1995, 102, 81-86. https://doi.org/10.1016/0011-9164(95)00044-3. [CrossRef]
- Chichirov, A.A.; Chichirova, N.D.; Filimonova, A.A.; Minibaev, A.I.; Tolmachev, L.I. Electrodialysis concentration of highly mineralized wastes of water treatment plants modeling. IOP Conf. Ser.: Earth Environ. Sci. 2019, 288, 012006. https://doi.org/10.1088/1755-1315/288/1/012006. [CrossRef]
- Bordbar, B.; Khosravi, A.; Azin, R. A Review on Sustainable Hybrid Water Treatment Processes, 3rd International Biennial Conference on Oil, Gas, and Petrochemical Engineering. Persian Gulf University Bushehr, Iran 2020.
- Valdés, H.; Saavedra, A.; Flores, M.; Vera-Puerto, I.; Aviña, H.; Belmonte, M. Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membr. 2021, 11, 753. https://doi.org/10.3390/membranes11100753. [CrossRef]
- Hangzhou Lanran Technologe Co. Ltd., Membrane manufacturer. Available online: http://lanran.com.cn/, 2023 (accessed 20 July 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).