1. Introduction
Uncorrected refractive errors in young children can cause substantial visual impairment [
1,
2]. Spectacles or contact lenses are sufficient typically to correct refractive errors, but a subset of children with ASD and ASD-like neurodevelopmental disorders (NDD) refuse to wear glasses and are unsuited to contact lens wear. Their vision may be degraded bilaterally – by isoametropia – to the level of legal blindness [
3], or their vision may be degraded in one eye – by anisometropia – causing amblyopia. [
4] Uncorrected refractive errors may also promote strabismus [
5] and aggravate the visual degradations caused by nystagmus [
6]. We have performed refractive surgery on ASD children with these disorders to improve visual function. The working hypothesis is that refractive surgery improves both visual behavior and quality of life.
Here we analyze a large cohort of children with refractive errors and ASD alone, or ASD-like behaviors and additional NDD. The aim of this study was to determine: a) their refractive outcomes using ophthalmic measures and b) their behavioral and school performance alterations – employing parent-proxy reports – after refractive surgery.
2. Materials and Methods
Data were collated retrospectively for 267 children, adolescents, and young adults (444 eyes, for brevity hereinafter referred to as children) in the pediatric refractive surgery database at St. Louis Children’s Hospital from 1/1/2000-1/1/2015. Of the 267 patients in the treated cohort, 256/267 (96%) were < 18 yrs. All of the patients had a diagnosis of ASD or NDD with ASD-like associated behaviors: Down syndrome, Angelman syndrome, Cornelia De Lange syndrome, fetal alcohol syndrome, Rhett syndrome, or self-injurious behavior. The ASD diagnosis was established in the majority of cases by use of the Social Responsiveness Scale, and in a minority by alternative ASD measures [
7,
8].
Methods
The pre-operative and post-operative examination techniques, and refractive surgery methods used have been recounted in detail in our prior reports [
9,
10,
11,
12,
13,
14]. Briefly, all of the children had at least 2 office examinations performed before surgery, as well as examination-under-anesthesia (EUA) at the primary procedure. The examinations included age-appropriate testing of uncorrected (UDVA) and corrected (CDVA) distance visual acuity in each eye (ETDRS; HOTV; Allen figure acuity; Cardiff figure acuity; or Teller grating acuity), pupillary examination for afferent defects, diameter and dyscoria/corectopia, sensorimotor examination for strabismus, nystagmus, gaze apraxia, binocular fusion and stereopsis, at least 2 manual and, when feasible, automated cycloplegic refractions, slit-lamp biomicroscope evaluation of the anterior segment for corneal clarity, assessment of tear film, crystalline lens clarity and absence of inflammation, indirect ophthalmoscopy, and measurement of intraocular pressure with a Tonopen (Minter O&O, Norwell, MA) or iCare (iCare USA) tonometer. Spatial sweep visually evoked potential (VEP) measurements of acuity also were obtained in children who would not cooperate for optotype or acuity card testing. Additional measurements obtained under anesthesia, before the surgical procedure, included central corneal thickness ultrasound pachymetry, Righton Retinomax auto keratometry, digital caliper corneal diameter, portable slit lamp biomicroscopy with gonioscopy, A-scan ultrasonographic axial length (Nidek Echoscan or Ellex Eye Cubed), anterior chamber depth, lens thickness measurement, repeat indirect ophthalmoscopy using scleral depression and, when indicated, Retcam II digital imaging of the fundi.
Children were treated using the least invasive surgical method. The first choice was photorefractive keratectomy (PRK); if not suitable for PRK then phakic intraocular lens (pIOL) implantation; if not suitable for either PRK or pIOL implantation then refractive lensectomy/refractive lens exchange (RLE). PRK was performed for spherical-equivalent refractive error (SEQRE) +5 to -5 D, with astigmatism < 3 D, pachymetry exceeding 450 microns, a normal tear film and absence of blepharitis. pIOL implantation was employed for children whose SEQRE exceeded the PRK range, with anterior chamber depth ≥ 3.2 mm, corneal diameter > 10.8 mm, and normal crystalline lens clarity. Refractive lens exchange (RLE) was performed on children who exceeded the PRK range of refractive error and who failed to meet the anterior chamber depth, corneal diameter, or crystalline lens clarity criteria for safe pIOL implantation.
The Carl Zeiss IOL Master or Nidek IOL Biometer was utilized to record corneal curvature radius (mm/diopters/meridian degrees), axial length (mm), anterior chamber depth (mm), central corneal thickness (µm), white-to-white diameter (mm), and pupil size (mm). Children unable to cooperate for awake testing had these measures obtained at a pre-operative examination-under-anesthesia (EUA). Power of the pIOL (Artisan-Ophtec or Visian) was calculated using manufacturer-designated software. For RLE, after removal of the crystalline lens a standard posterior chamber IOL (Alcon SA 60AT or MA 60AC) was implanted into the capsular bag or sulcus to achieve target refraction. PRK, pIOL, and RLE correction was planned to achieve a target refraction of emmetropia, with some adjustment for patient age and anticipated refractive regression and/or axial length elongation, i.e., 1 D under (hyperopia) or over (myopia) correction.
Written informed consent was obtained from the parent(s) and when possible, also the child. The consent document itemized the rationale for the procedure and the potential risks, the possible benefits, and alternatives to pediatric refractive surgery. The consent also explained the need for continuing monitoring, and the possible need for additional eye surgery.
Inclusion and Exclusion Criteria
A diagnosis of ASD or an ASD-like disorder was the leading inclusion criteria for the study. Additional indications were: 1) an SEQRE > 3 D myopia, hyperopia or astigmatism; 2) noncompliance, aversion to or chronic difficulties with the wearing of spectacles in children also ill-suited to use of contact lens correction; and 3) good rapport with the child’s parent(s), who acknowledged the risks and alternatives to the procedure and the importance of follow-up ophthalmic examinations. Exclusion criteria were: corneal dystrophy, endothelial dysfunction, corneal scarring, keratitis, or systemic inflammatory disease; glaucoma, uveitis, recurrent conjunctivitis, tear film insufficiency, untreated lattice degeneration or a family history of retinal detachment.
Outcome Measures
The major outcome measures were: a) uncorrected distance and near visual acuity and b) post-operative refractive error. The post-operative acuities reported are uncorrected and converted to logMAR for calculation of geometric means [
15]. The visual acuity measures include potentially improvements during the follow-up interval attributable to increased performance with advancing maturity. But the measures also incorporate any visual decline occurring as the result of refractive regression or other confound.
The secondary outcome measure was parent proxy or caregiver reports of behavioral changes during the entire follow-up interval. A series of questions was posed to the parent that addressed any changes, for better or worse, in the child’s overall visual and behavioral function, in particular (a) detection, recognition, visual awareness, attentiveness, or social interactions and (b) observations or reports of these behaviors from those outside the child’s home, including teachers, therapists, or other caregivers.
Statistical Analysis
Fractional (e.g. Snellen) visual acuities were converted to logMAR notation for calculation of geometric means. Outcome measures were collected throughout the follow-up interval. Visual acuity outcomes reported are those obtained at the most recent follow-up examination. Means for parametric measures were compared with 1-tailed or 2-tailed, paired t tests, with significance defined as P < 0.05. Categorial variables were compared with Chi-square goodness-of-fit, with significance defined as P < 0.05.
3. Results
For the 267 children median age at surgery was 10.9 yrs. (range 1.3 – 25.8 yrs.) and median follow-up 3.1 yrs. (0.04 – 15.1). Sixty percent (161/267) were male. These patient characteristics are listed in
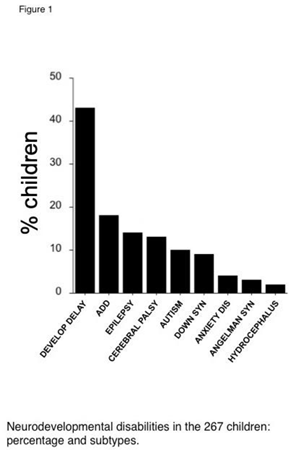
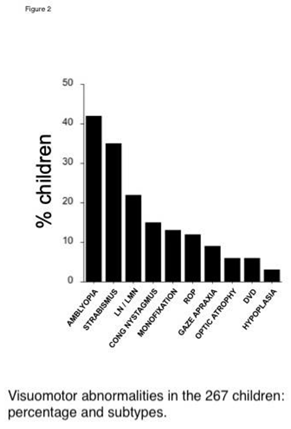
Table 1. The percent of children with ASD alone, or NDD subtypes with associated ASD behaviors are shown in Figure 1. ASD alone accounted for 12% of the cohort. Among other NDD, the most prevalent was idiopathic developmental delay (43%), followed by ADD/ADHD (18%), epilepsy (14%), and cerebral palsy (13%). The percent of children affected by an ocular co-morbidity are shown in Figure 2. The most prevalent was amblyopia (42%, strabismic and/or anisometropic), followed by strabismus (35%), fusion maldevelopment/latent nystagmus (23%), idiopathic infantile (“congenital”) nystagmus (15%), microtropia/monofixation syndrome (13%), retinopathy of prematurity (12%, ROP) and gaze apraxia (8%). Children could have more than one subtype of NDD or ocular disorder.
Myopic PRK was performed on 68 children (116 eyes); hyperopic PRK on 63 children (107 eyes); myopic pIOL implantation on 101 children (161 eyes), hyperopic pIOL implantation on 14 children (26 eyes), and RLE on 21 children (34 eyes). Treatment was bilateral in 68% (181/267 children) for isoametropia and unilateral in 32% (86/267 children) for anisometropia (
Table 1).
Refractive and Visual Acuity Outcomes
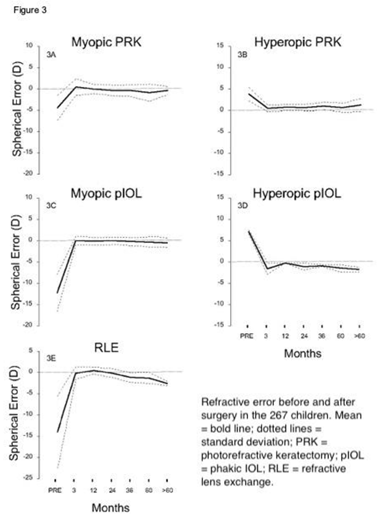
SEQRE in the myopic PRK-treated children (Figure 3A) improved from - 4.4 ± 2.9 D to 0 ± 1.2 D; in the hyperopic PRK group (Figure 3B) from +4.0 ± 1.5 D to +0.75± 1.0 D; in the myopic pIOL children (Figure 3C) from -12.0 ± 6.1 D to 0 ± 1.0 D, in the hyperoic pIOL group from +7.5 ±.09 D to -1.1 ±1.0D, and in the RLE-treated (Figure 3D) from -14.3. ± 4.8 D to -2.8 ± 0.7D. At median 3 yr. follow-up 87% were corrected to within ± 1 D emmetropia, and 95% to within ± 2 D.
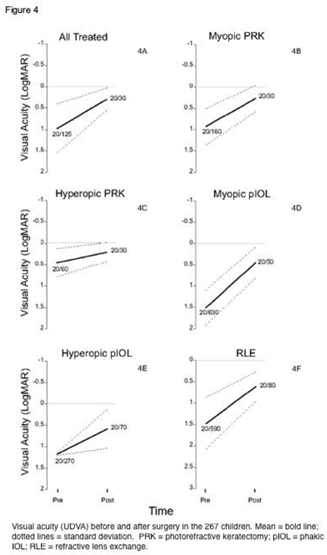
For the entire cohort of 267 children (444 eyes; Figure 4A), UDVA improved an average 0.6 logMAR: from 0.9 ± 0.6 (20/150) to 0.3 ± 0.3 (20/40). For myopic PRK patients (Figure 4B), UDVA improved from logMAR 0.92 ± 0.4 (20/160) to 0.27±1.0 (20/30); for hyperopic PRK (Figure 4C) from 0.45±0.33 (20/60) to 0.21±0.22 (20/30); for myopic pIOL (Figure 4D) from 1.50 ± 0.41 (20/630) to 0.45±0.36 (20/50); for hyperopic pIOL (Figure 4E) from 1.1 ±0.5 (20/270) to 0.5 ±0.5 (20/70); and for RLE patients (Figure 4F) from 1.48±0.61 (20/590) to 0.61±0.35 (20/80). The changes in both SEQRE and in UDVA were significant (p < .01) for each of these subgroups.
Behavioral Outcomes
Parents and caregivers reported improvement in visual and/or social behaviors in 72% (192/267) of the treated children. The gains recorded were for one or more of the following: visual recognition; eye contact and tracking; motor skills; and school performance. Ten percent of the total cohort of NDD children (27/267) had a specific diagnosis of ASD. Improvements were reported in 69% (19/27) of the ASD children. There were no reports of decreased quality of life or exacerbations of NDD behavior.
Complications
Complications encountered through the last follow-up examination were as follows, ranked from most to least common. Each of these complications entailed a return to the operating room for a secondary procedure. Among all eyes treated by RLE, 17.6% (6/34 eyes) required either YAG laser capsulotomy or vitrector membranectomy for posterior capsule phimosis and/or opacification. For all eyes implanted with a pIOL, 3% (5/187 eyes) required re-enclavation to repair traumatic de-enclavation of one haptic. Two eyes (1%; 2/187 eyes) implanted with a pIOL developed a cataract, requiring pIOL explantation and pediatric cataract/IOL surgery an average 4.2 years after pIOL implantation. Two eyes (1%; 2/187 eyes) implanted with a pIOL required a return to the operating room to restore patency to the peripheral iridotomy after an episode of pupillary block ocular hypertension. Two eyes treated by PRK (0.9%; 2/223 eyes) had a persistent epithelial defect in the first 30 days after surgery, requiring lateral tarsorrhaphy (released 60 – 90 days later).
4. Discussion
Our group has performed refractive surgeries for NDD children over the past 20 years. The majority of treated children have substantially improved visual acuity [
9,
10,
11]. The benefit of refractive surgery is, however, not limited to correction of ametropia. Children with ASD and ASD-like NDD behaviors treated by these procedures may achieve improved visual attention, school performance, and quality of life (QoL).
Refractive Surgery for Boosting ASD QoL
Improvement in QoL assumes reasonably that children with uncorrected high myopia or hyperopia who are aversive to spectacle or contact lens wear have decreased QoL. Pediatric patients with impaired visual acuity have reported difficulty with activities of daily living, dependency, psychosocial interaction and school [
16,
17]. Congenitally blind children have an increased likelihood of ASD or “autistic like” features [
18,
19,
20]. It is reasonable therefore to posit that ASD phenotypes are exacerbated by ametropic blur.
Enhanced QoL is common after refractive surgery in adult patients [
21]. After Lasik surgery [
22] or ICL surgery to correct myopia [
23], 95% of patients and 88% of adults respectively, reported improved QoL. In previous work our group has reported improved QoL after pediatric refractive surgery in smaller cohorts of ASD and NDD children. Among NDD children who underwent PRK, 15/17 (88%) experienced improved visual behaviors [
12]. Similar gains were observed for NDD children treated by Artisan-Ophtec pIOL surgery [
14], Visian pIOL surgery [
9] or RLE [
13]. In each of these smaller case series, children were noted to have enhanced visual awareness, attentiveness, or social interactions by observers other than parents. Lesueur and Arne also reported a boost in QoL in 4 children treated by pIOL implantation for anisometropic myopic amblyopia [
1].
In the current study, two NDD patients with ASD behaviors who had not previously walked started walking after RLE. One patient first walked at the age of 8 and the other at the age of 22. Another patient went from reading braille to reading books and watching movies. Determining which patients pre-operatively could benefit from these dramatic responses would be helpful. Further research is needed to unravel how decreased visual acuity contributes to a child’s overall ASD and NDD disabilities.
Refractive Surgery Adaptations for ASD Children
Adaptations are required when employing refractive surgery methods to treat any child, but especially ASD children. Children with ASD may be less cooperative, and require more time, energy, and expertise than a neurotypical child during peri-operative care. For this reason, it is useful to review helpful adaptations that can optimize the efficacy and safety of PRK, pIOL implantation and RLE procedures in this population of children.
For PRK, at the conclusion of laser ablation, a plano bandage contact lens is applied to the operated eye(s), and disposable goggles are fitted. As in adults, the corneal epithelium heals in 3-6 days [
10,
24]. Topical drops are instilled beginning the first post-operative day. Children with ASD tend to tolerate post-PRK discomfort and photophobia better than adults, resuming normal play. About 10% may require per oral narcotic analgesia for the first 48 hours. For post-operative awake exams in the office, an adequate view of the healing cornea(s) often requires restraint and use of a portable slit lamp. Despite the best efforts of parents, ~ 20% of children can be expected to dislodge the goggles, rub, and poke their eyes, and lose the contact lens. These behaviors are distressing to the child’s parents but do not impede – with rare exception – timely healing. The lost contact lens is replaced and reinserted in the office by the surgeon’s staff. Less than 1% of the children we have treated by PRK have had a persistent corneal epithelial defect. To avoid a sight-threatening subepithelial scar it is advisable to return to the operating room for a surgical tarsorrhaphy.
For pIOLs, the implant we have preferred to use for the last 7 years is the foldable, sulcus-positioned intraocular collamer lens (Visian ICL, Staar Surgical, Monrovia CA) [
9]. In the 10 years preceding the introduction of the Visian ICL, we used the PMMA iris-enclaved myopic (Verisyse) lens supplied in the U.S.A. by Ophtec Inc. (Boca Raton FL) [
14]. Precise sizing of the ICL requires accurate white-to-white measurement of corneal diameter. In cooperative children this can be achieved using the Nidek IOL Biometer or IOL Master. Alternatively, the measurement is performed using digital calipers during a planning EUA. Proper vaulting of the ICL in the posterior chamber – between the anterior surface of the crystalline lens and posterior surface of the iris – and lower long-term risk for corneal endothelial cell loss, requires a corneal diameter 10.8 mm or more and an anterior chamber depth ≥ 3.2 mm. The previous version of the ICL required an iridotomy, created during the procedure, to circumvent pupillary block ocular hypertension. The current EVO model of the ICL has an aperture in the optic allowing aqueous flow, obviating the need for the iridotomy or concern of pupillary block. In cases of high bilateral ametropia, the eyes are implanted sequentially, with several days to a week elapsing before operation on the second eye. Because children’s eyes heal rapidly, a superior 3.0 mm clear cornea incision can be employed, which achieves the therapeutic effect of a relaxing limbal incision. Common with-the-rule, pre-operative astigmatism is reduced by ~ 40%. If pre-operative astigmatism exceeds 2.5 D, a myopic toric ICL is implanted. Absorbable 9-0 polyglactin sutures are used to close the corneal incision, avoiding re-anesthetizing the child for suture removal. It is inadvisable in children to leave the incision un-sutured. In some children, to prevent eye rubbing, arm restraints will need to be fitted for the first several post-operative days or weeks.
RLE is useful for children with myopia exceeding 16 to 24 D (the upper limit for ICL or Ophtec phakic IOL power, respectively), corneal diameter less than 10.8 mm, or anterior chamber depth less than 3.2 mm [
13,
25]. Standard pediatric lensectomy, posterior capsulectomy and pars plana vitrectomy techniques are employed, using a high-speed 25-gauge vitrector and separate anterior chamber infusion. If needed to achieve emmetropia, a foldable, acrylic IOL (Alcon SA60AT or MA60AC) is injected into the capsular bag – depending on axial length and lens power calculations. A primary capsulectomy/anterior vitrectomy is advisable because of the high rate of formation of dense, posterior capsule fibrosis in pediatric eyes when the capsule is preserved. We perform an EUA a few weeks to a month before the planned lensectomy. The peripheral retina is examined in detail by depression. Accurate, immersion axial length measurements are obtained. If the axial length exceeds ~ 27 mm, barrier diode laser therapy may be applied to reduce the risk (estimated ~ 2 %) of future, aphakic/ pseudophakic retinal detachment [
26,
27].
We acknowledge that our results should be considered preliminary. The principal limitations of the study are its retrospective design and lack of a QoL questionnaire to measure behavioral improvements more rigorously. We are currently engaged in a prospective study employing ASD-specific QoL parent-proxy questionnaires.
5. Conclusions
Refractive error and visual acuity improved substantially in this cohort of difficult-to-manage, ASD and NDD children. PRK, pIOL and RLE procedures appear to be effective and reasonably safe methods for improving behaviors in many ametropic children with ASD and ASD-like neurodevelopmental disorders.
Author Contributions
Conceptualization, M.R. and L.T.; methodology, M.R., J.H., and L.T.; software, N.F. and M.R.; validation, M.R., N.F. and L.T.; formal analysis, M.R. and N.F.; investigation, M.R..; resources, J.H. and M.R.; data curation, N.F. and M.R; writing—original draft preparation, M.R.; writing—review and editing, L.T.; visualization, N.F. and M.R.; supervision, M.R. and L.T.; project administration, M.R.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Washington University in St. Louis (protocol number 202001151, date of approval March 5, 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank all of the family members of autistic people who helped in the dissemination of the study findings.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lesueur, L.; Arne, J. Phakic posterior chamber lens implantation in children with high myopia. J Cataract Refract Surg 1999, 25, 1571-1575. [CrossRef]
- Holden, B. Uncorrected refractive error: the major and most easily avoidable cause of visual loss. Community Eye Health 2007, 20, 37-29.
- Tychsen, L. Refractive surgery for special needs children. Arch Ophthalmol 2009, 127, 810-813, doi:127/6/810 [pii]. https://doi.org/10.1001/archophthalmol.2009.72. [CrossRef]
- Holmes, J.M.; Levi, D.M. Treatment of amblyopia as a function of age. Vis Neurosci 2018, 35, E015. https://doi.org/10.1017/S0952523817000220. [CrossRef]
- Bonafede, L.; Bender, L.; Shaffer, J.; Ying, G.S.; Binenbaum, G. Refractive change in children with accommodative esotropia. Br J Ophthalmol 2020, 104, 1283-1287. https://doi.org/10.1136/bjophthalmol-2019-314891. [CrossRef]
- Tychsen, L. Refractive surgery for children: laser, implants, current results and future directions. Expert Rev Ophthalmol 2008, 3, 635-643. [CrossRef]
- Constantino, J.; Gruber, J. Social Responsiveness Scale (SRS) Manual; Western Psychological Services: Los Angeles, 2005.
- Constantino, J.N.; Charman, T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol 2016, 15, 279-291. https://doi.org/10.1016/S1474-4422(15)00151-9. [CrossRef]
- Tychsen, L.; Faron, N.; Hoekel, J. Phakic intraocular collamer lens (Visian ICL) implantation for correction of myopia in spectacle-aversive special needs children. Am J Ophthalmol 2017, 175, 77-86. https://doi.org/10.1016/j.ajo.2016.11.016. [CrossRef]
- Tychsen, L.; Packwood, E.; Berdy, G. Correction of large amblyopiogenic refractive errors in children using the excimer laser. J AAPOS 2005, 9, 224-233. https://doi.org/10.1016/j.jaapos.2005.01.006. [CrossRef]
- Tychsen, L.; Hoekel, J. Outcomes of phakic IOL implantation in children and adolescents with high ametropia. J AAPOS 2011, 15, 31. [CrossRef]
- Tychsen, L.; Hoekel, J. Refractive surgery for high bilateral myopia in children with neurobehavioral disorders: 2. Laser-assisted subepithelial keratectomy (LASEK). J AAPOS 2006, 10, 364-370. https://doi.org/10.1016/j.jaapos.2006.04.004. [CrossRef]
- Tychsen, L.; Packwood, E.; Hoekel, J.; Lueder, G. Refractive surgery for high bilateral myopia in children with neurobehavioral disorders: 1. Clear lens extraction and refractive lens exchange. J AAPOS 2006, 10, 357-363. https://doi.org/10.1016/j.jaapos.2006.04.003. [CrossRef]
- Tychsen, L.; Hoekel, J.; Ghasia, F.; Yoon-Huang, G. Phakic intraocular lens correction of high ametropia in children with neurobehavioral disorders. J AAPOS 2008, 12, 282-289, doi:S1091-8531(07)00579-4 [pii]10.1016/j.jaapos.2007.12.001. [CrossRef]
- Holladay, J.; Prager, T. Mean visual acuity. Am J Ophthalmol 1991, 111(3), 372-374.
- Decarlo, D.; McGwin, G., Jr.; Bixler, M.; Wallander, J.; Owsley, C. Impact of pediatric vision impairment on daily life: results of focus groups. Optom Vis Sci 2012, 89, 1409-1416. https://doi.org/10.1097/OPX.0b013e318264f1dc. [CrossRef]
- Salt, A. How should an ophthalmologist tell if a child’s development is normal? . In Pediatric Ophthalmology and Strabismus, Hoyt, C., Taylor, D., Eds.; Elsevier: New York, 2013; pp. 1055-1059.
- Hobson, R.; Bishop, M. The pathogenesis of autism: insights from congenital blindness. Philos Trans R Soc Lond B Biol Sci 2003, 358, 335-344. https://doi.org/10.1098/rstb.2002.1201. [CrossRef]
- Perez-Pereira, M.; Conti-Ramsden, G. Language Development and Social Interaction in Blind Children; University Press: Hove and New York, 1999.
- McAlpine, L.; Moore, C. The development of social understanding in children with visual impairment. J Vis Impair Blind 1995, 89, 349-358, doi:doi.org/10.1177/0145482X9508900408. [CrossRef]
- Chuck, R.S.; Jacobs, D.S.; Lee, J.K.; Afshari, N.A.; Vitale, S.; Shen, T.T.; Keenan, J.D.; American Academy of Ophthalmology Preferred Practice Pattern Refractive Management/Intervention, P. Refractive Errors & Refractive Surgery Preferred Practice Pattern(R). Ophthalmology 2018, 125, P1-P104. https://doi.org/10.1016/j.ophtha.2017.10.003. [CrossRef]
- Garamendi, E.; Pesudovs, K.; Elliott, D. Changes in quality of life after laser in situ keratomileusis for myopia. J Cataract Refract Surg 2005, 31, 1537-1543. https://doi.org/10.1016/j.jcrs.2004.12.059. [CrossRef]
- Ieong, A.; Hau, S.; Rubin, G.; Allan, B. Quality of life in high myopia before and after implantable Collamer lens implantation. Ophthalmology 2010, 117, 2295-2300. https://doi.org/10.1016/j.ophtha.2010.03.055. [CrossRef]
- Paysse, E.; Hamill, M.; Koch, D.; Hussein, M.; Brady McCreery, K.; Coats, D. Epithelial healing and ocular discomfort after photorefractive keratectomy in children. J Cataract Refract Surg 2003, 29, 478-481. [CrossRef]
- Ali, A.; Packwood, E.; Lueder, G.; Tychsen, L. Unilateral lens extraction for high anisometropic myopia in children and adolescents. J AAPOS 2007, 11, 153-158. https://doi.org/10.1016/j.jaapos.2006.09.004. [CrossRef]
- Lee, K.; Lee, J. Long-term results of clear lens extraction for severe myopia. J Cataract Refract Surg 1996, 22, 1411-1415. [CrossRef]
- Colin, J.; Robinet, A. Clear lensectomy and implantation of a low-power posterior chamber intraocular lens for correction of high myopia. Ophthalmology 1997, 104, 73-78. [CrossRef]
Table 1.
Characteristics of 267 children (444 eyes) with ASD or ASD-like neurodevelopmental disorders treated by refractive surgery.
Table 1.
Characteristics of 267 children (444 eyes) with ASD or ASD-like neurodevelopmental disorders treated by refractive surgery.
| Age at Surgery |
10.9 ± 4.8 yrs. (range 1.3 –25.8) |
| Length of Follow-up |
3.1 ± 1.9 yrs. (range 0.04 – 15.1) |
| Sex |
60% male (161/267) |
| Method of Refractive Surgery |
|
| PRK myopic |
68 children (116 eyes) |
| PRK hyperopic |
63 children (107 eyes) |
| pIOL myopic |
101 children (161 eyes) |
| pIOL hyperopic |
14 children (26 eyes) |
| RLE myopic |
21 children (34 eyes) |
| Laterality Surgery |
|
| Bilateral: isoametropia |
68%, 179 children (358 eyes) |
| Unilateral: anisometropia |
32%, 86 children (86 eyes) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).