Submitted:
27 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Evolution of the CD4+ Helper T Cell
2. Discovery of the CD4+ Helper T Cell
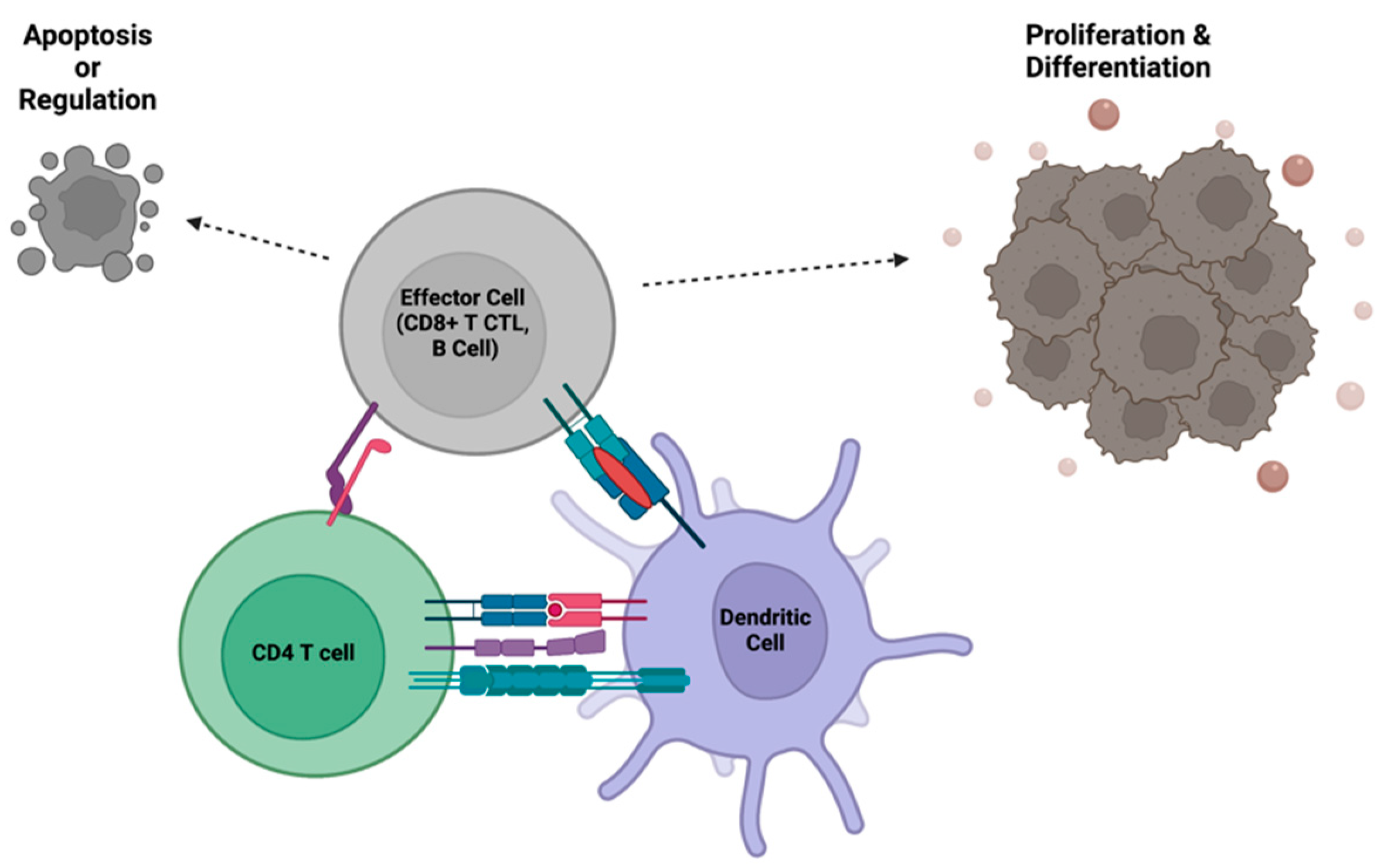
3. The Importance of CD4+ T Cell Help in Orchestrating and Balancing the Power of Immunity
4. The Loss of Efficient CD4+ T Cell Help Through Pathophysiological Migration and Activation
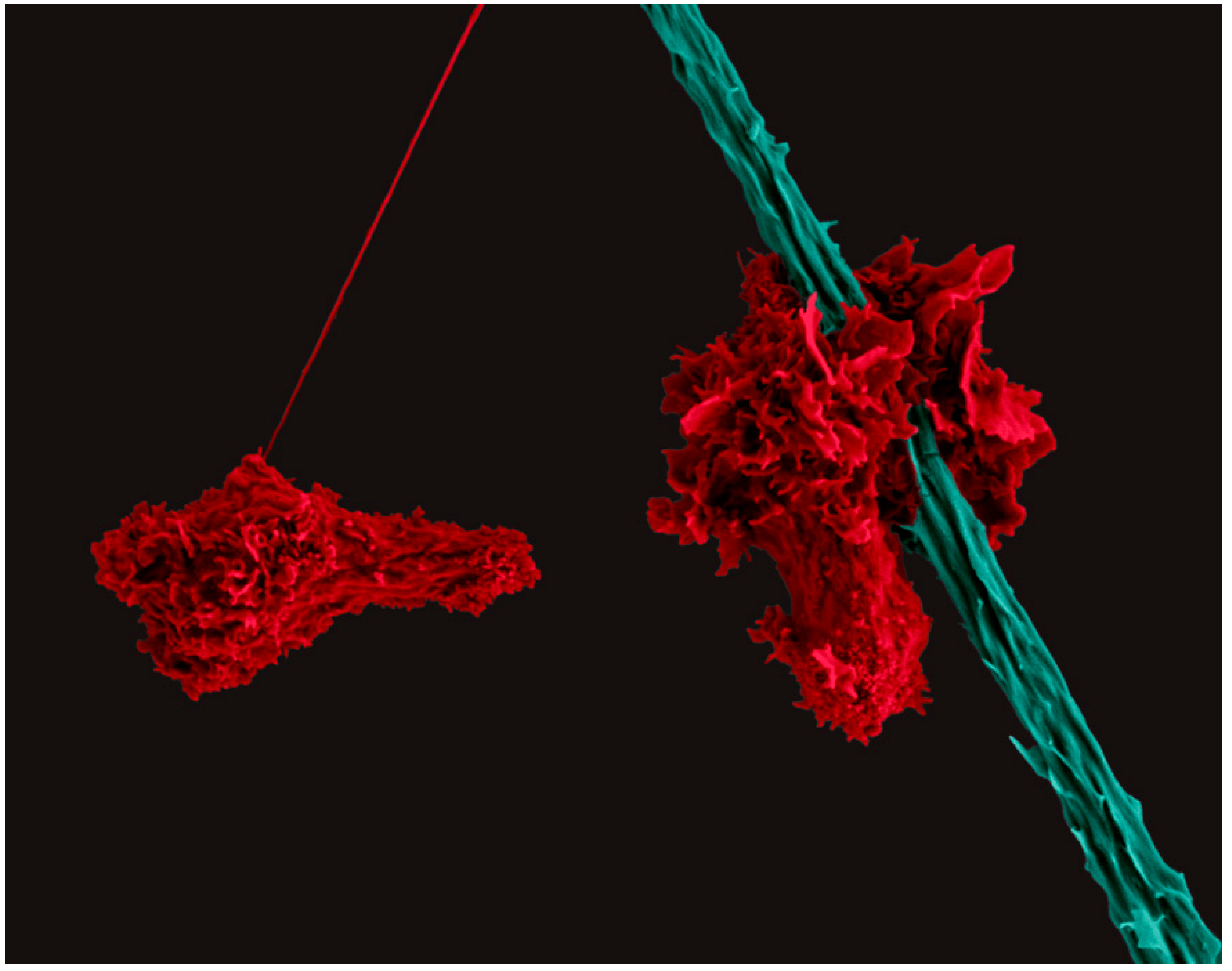
5. The Nomadic Life of a CD4+ Helper T Cell
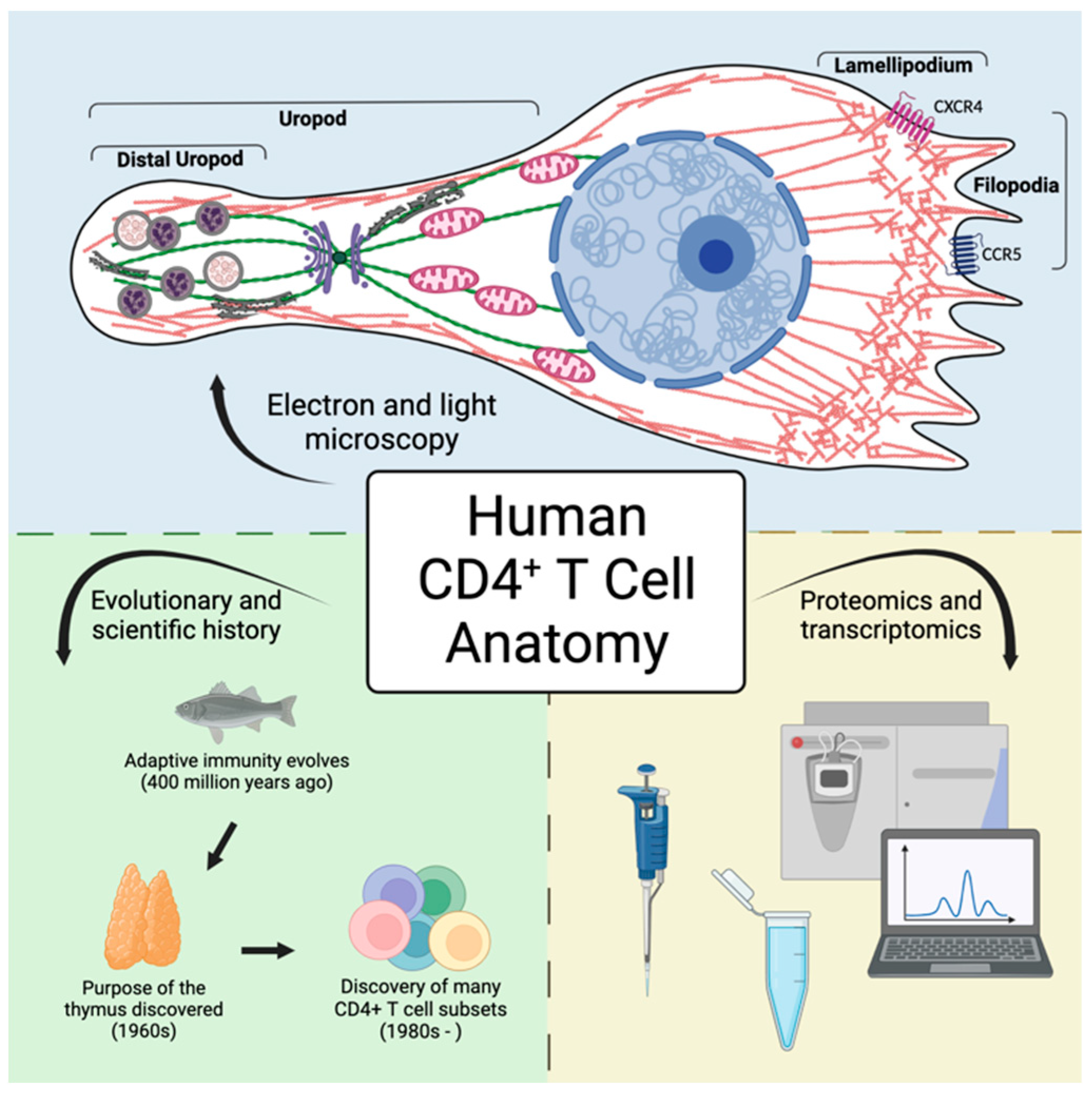
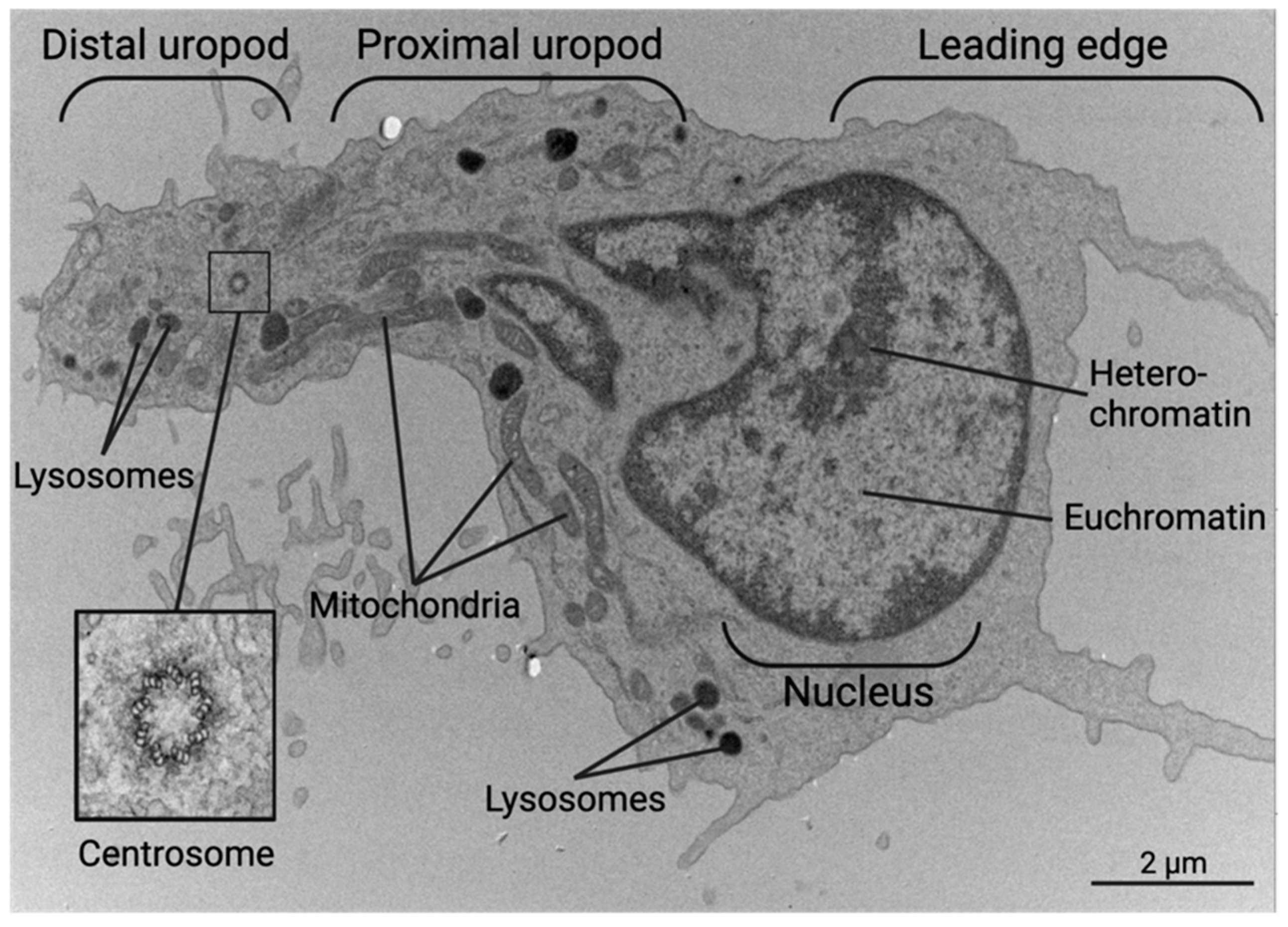
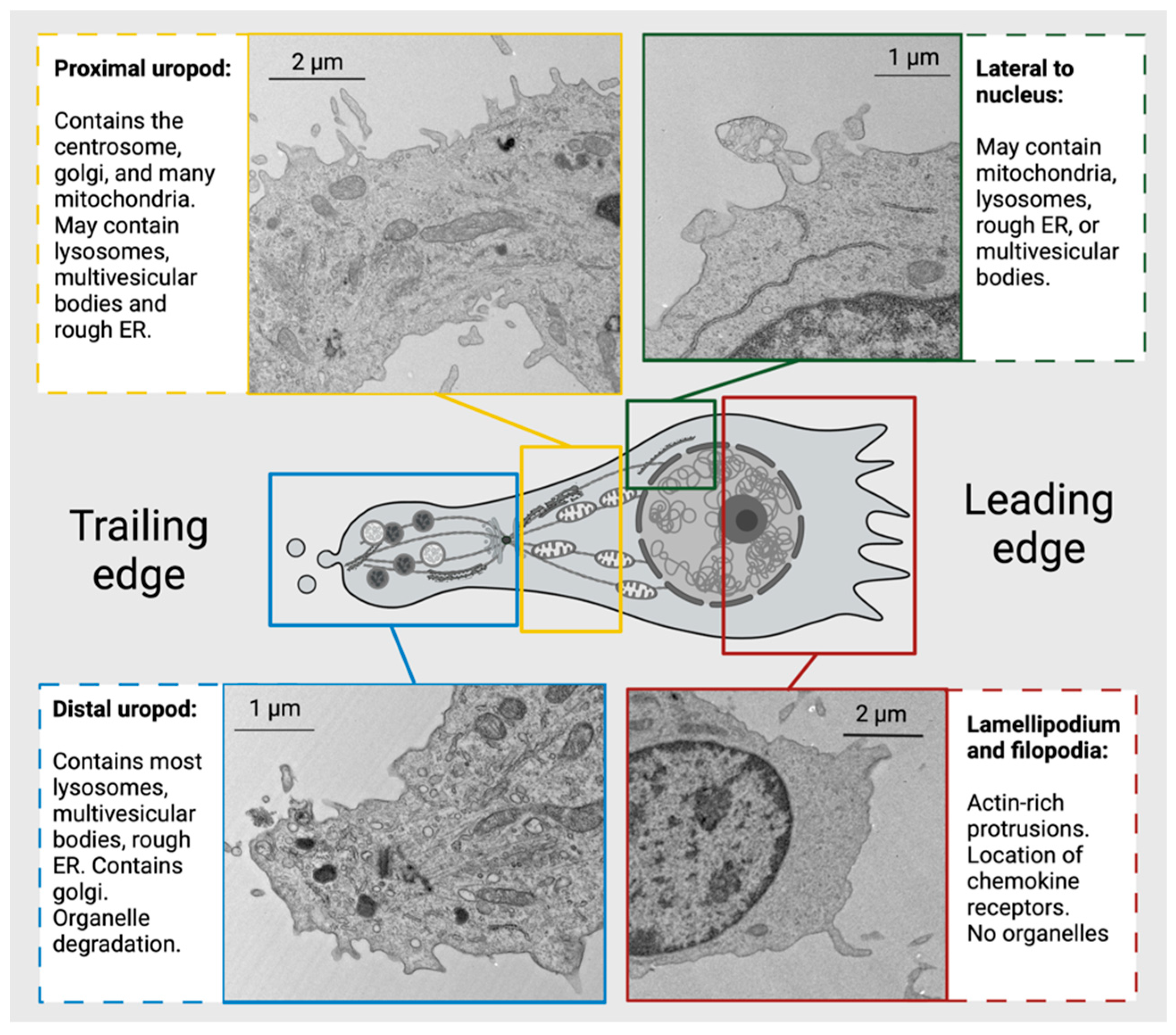
6. Anatomy of a CD4+ Helper T Cell
7. Transcriptomic and Proteomic Atlas of the Human CD4+ Helper T Cell
8. Conclusions
9. Materials and Methods
CD4+ T cell isolation and activation
Mass Spectrometry
Transmission Electron Microscopy
Scanning Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flajnik, M.F.; Kasahara, M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet 2010, 11, 47–59. [Google Scholar] [CrossRef]
- Hirano, M.; Das, S.; Guo, P.; Cooper, M.D. The evolution of adaptive immunity in vertebrates. Adv Immunol 2011, 109, 125–157. [Google Scholar] [CrossRef]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems--not homogeneous, not simple, not well understood. Immunol Rev 2004, 198, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kubick, N.; Klimovich, P.; Flournoy, P.H.; Bienkowska, I.; Lazarczyk, M.; Sacharczuk, M.; Bhaumik, S.; Mickael, M.E.; Basu, R. Interleukins and Interleukin Receptors Evolutionary History and Origin in Relation to CD4+ T Cell Evolution. Genes (Basel) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M. Evolution of vertebrate adaptive immunity: immune cells and tissues, and AID/APOBEC cytidine deaminases. Bioessays 2015, 37, 877–887. [Google Scholar] [CrossRef]
- Hsu, E. V(D)J recombination: of mice and sharks. Adv Exp Med Biol 2009, 650, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Hirano, M.; Herrin, B.R.; Li, J.; Yu, C.; Sadlonova, A.; Cooper, M.D. Dual nature of the adaptive immune system in lampreys. Nature 2009, 459, 796–801. [Google Scholar] [CrossRef]
- Cooper, M.D.; Alder, M.N. The evolution of adaptive immune systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef]
- Brazeau, M.D.; Friedman, M. The origin and early phylogenetic history of jawed vertebrates. Nature 2015, 520, 490–497. [Google Scholar] [CrossRef]
- Litman, G.W.; Anderson, M.K.; Rast, J.P. Evolution of antigen binding receptors. Annu Rev Immunol 1999, 17, 109–147. [Google Scholar] [CrossRef]
- van Niekerk, G.; Davis, T.; Engelbrecht, A.M. Was the evolutionary road towards adaptive immunity paved with endothelium? Biol Direct 2015, 10, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, T.C.; Huang, G.; Lu, Q.; Surleac, M.D.; Mandell, J.D.; Pontarotti, P.; Petrescu, A.J.; Xu, A.; Xiong, Y.; et al. Transposon molecular domestication and the evolution of the RAG recombinase. Nature 2019, 569, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F. Immunological function of the thymus. Lancet 1961, 2, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Fagraeus, A. The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol 1948, 58, 1–13. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Miller, J.F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med 1968, 128, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Cerottini, J.C.; Nordin, A.A.; Brunner, K.T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature 1970, 228, 1308–1309. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Sosman, J.; Irigoyen, O.; Friedman, S.M.; Kung, P.C.; Goldstein, G.; Chess, L. Functional analysis of human T cell subsets defined by monoclonal antibodies. I. Collaborative T-T interactions in the immunoregulation of B cell differentiation. J Immunol 1980, 125, 2402–2408. [Google Scholar] [CrossRef]
- Reinherz, E.L.; Kung, P.C.; Goldstein, G.; Schlossman, S.F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A 1979, 76, 4061–4065. [Google Scholar] [CrossRef] [PubMed]
- Phan-Dinh-Tuy, F.; Niaudet, P.; Bach, J.F. Molecular identification of human T-lymphocyte antigens defined by the OKT5 and OKT8 monoclonal antibodies. Mol Immunol 1982, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Kung, P.; Goldstein, G.; Reinherz, E.L.; Schlossman, S.F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science 1979, 206, 347–349. [Google Scholar] [CrossRef]
- Del Prete, G.F.; De Carli, M.; Ricci, M.; Romagnani, S. Helper activity for immunoglobulin synthesis of T helper type 1 (Th1) and Th2 human T cell clones: the help of Th1 clones is limited by their cytolytic capacity. J Exp Med 1991, 174, 809–813. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995, 155, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008, 9, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009, 119, 3573–3585. [Google Scholar] [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis. Front Immunol 2019, 10, 353. [Google Scholar] [CrossRef]
- Wei, X.; Niu, X. T follicular helper cells in autoimmune diseases. J Autoimmun 2023, 134, 102976. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Goswami, T.K.; Singh, M.; Dhawan, M.; Mitra, S.; Emran, T.B.; Rabaan, A.A.; Mutair, A.A.; Alawi, Z.A.; Alhumaid, S.; Dhama, K. Regulatory T cells (Tregs) and their therapeutic potential against autoimmune disorders - Advances and challenges. Hum Vaccin Immunother 2022, 18, 2035117. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hong, Y.K.; Yun, H.J.; Kim, Y.M.; Kim, J.R.; Yoo, W.H. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford) 2008, 47, 789–794. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Speiser, D.E.; Chijioke, O.; Schaeuble, K.; Munz, C. CD4(+) T cells in cancer. Nat Cancer 2023, 4, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.; Lacouture, C.; Fusaro, M.; Dupre, L. Molecular Tuning of Actin Dynamics in Leukocyte Migration as Revealed by Immune-Related Actinopathies. Frontiers in immunology 2021, 12, 750537. [Google Scholar] [CrossRef] [PubMed]
- Dupre, L.; Boztug, K.; Pfajfer, L. Actin Dynamics at the T Cell Synapse as Revealed by Immune-Related Actinopathies. Frontiers in cell and developmental biology 2021, 9, 665519. [Google Scholar] [CrossRef] [PubMed]
- Kreins, A.Y.; Maio, S.; Dhalla, F. Inborn errors of thymic stromal cell development and function. Semin Immunopathol 2021, 43, 85–100. [Google Scholar] [CrossRef]
- Markert, M.L.; Boeck, A.; Hale, L.P.; Kloster, A.L.; McLaughlin, T.M.; Batchvarova, M.N.; Douek, D.C.; Koup, R.A.; Kostyu, D.D.; Ward, F.E.; et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med 1999, 341, 1180–1189. [Google Scholar] [CrossRef]
- Castiello, M.C.; Brandas, C.; Capo, V.; Villa, A. HyperIgE in hypomorphic recombination-activating gene defects. Curr Opin Immunol 2023, 80, 102279. [Google Scholar] [CrossRef]
- de Saint-Basile, G.; Le Deist, F.; de Villartay, J.P.; Cerf-Bensussan, N.; Journet, O.; Brousse, N.; Griscelli, C.; Fischer, A. Restricted heterogeneity of T lymphocytes in combined immunodeficiency with hypereosinophilia (Omenn’s syndrome). J Clin Invest 1991, 87, 1352–1359. [Google Scholar] [CrossRef]
- Flinn, A.M.; Gennery, A.R. Adenosine deaminase deficiency: a review. Orphanet J Rare Dis 2018, 13, 65. [Google Scholar] [CrossRef]
- Vakkilainen, S.; Taskinen, M.; Mäkitie, O. Immunodeficiency in cartilage-hair hypoplasia: Pathogenesis, clinical course and management. Scand J Immunol 2020, 92, e12913. [Google Scholar] [CrossRef] [PubMed]
- Caudy, A.A.; Reddy, S.T.; Chatila, T.; Atkinson, J.P.; Verbsky, J.W. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol 2007, 119, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sharfe, N.; Dadi, H.K.; Shahar, M.; Roifman, C.M. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A 1997, 94, 3168–3171. [Google Scholar] [CrossRef]
- Jiang, Y.; Firan, M.; Nandiwada, S.L.; Reyes, A.; Marsh, R.A.; Vogel, T.P.; Hajjar, J. The Natural History of X-Linked Lymphoproliferative Disease (XLP1): Lessons from a Long-Term Survivor. Case Reports Immunol 2020, 2020, 8841571. [Google Scholar] [CrossRef]
- Lum, S.H.; Neven, B.; Slatter, M.A.; Gennery, A.R. Hematopoietic Cell Transplantation for MHC Class II Deficiency. Front Pediatr 2019, 7, 516. [Google Scholar] [CrossRef]
- Yazdani, R.; Fekrvand, S.; Shahkarami, S.; Azizi, G.; Moazzami, B.; Abolhassani, H.; Aghamohammadi, A. The hyper IgM syndromes: Epidemiology, pathogenesis, clinical manifestations, diagnosis and management. Clin Immunol 2019, 198, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Puel, A.; Casanova, J.L.; Kobayashi, M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin Transl Immunology 2016, 5, e114. [Google Scholar] [CrossRef] [PubMed]
- Cavannaugh, C.; Ochs, H.D.; Buchbinder, D. Diagnosis and clinical management of Wiskott-Aldrich syndrome: current and emerging techniques. Expert Rev Clin Immunol 2022, 18, 609–623. [Google Scholar] [CrossRef]
- Niggli, V. Insights into the mechanism for dictating polarity in migrating T-cells. Int Rev Cell Mol Biol 2014, 312, 201–270. [Google Scholar] [CrossRef]
- Lanzi, G.; Moratto, D.; Vairo, D.; Masneri, S.; Delmonte, O.; Paganini, T.; Parolini, S.; Tabellini, G.; Mazza, C.; Savoldi, G.; et al. A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP. The Journal of experimental medicine 2012, 209, 29–34. [Google Scholar] [CrossRef]
- Dobbs, K.; Domínguez Conde, C.; Zhang, S.Y.; Parolini, S.; Audry, M.; Chou, J.; Haapaniemi, E.; Keles, S.; Bilic, I.; Okada, S.; et al. Inherited DOCK2 Deficiency in Patients with Early-Onset Invasive Infections. N Engl J Med 2015, 372, 2409–2422. [Google Scholar] [CrossRef]
- Su, H.C. Insights into the pathogenesis of allergic disease from dedicator of cytokinesis 8 deficiency. Curr Opin Immunol 2023, 80, 102277. [Google Scholar] [CrossRef]
- Castro, C.N.; Rosenzwajg, M.; Carapito, R.; Shahrooei, M.; Konantz, M.; Khan, A.; Miao, Z.; Gross, M.; Tranchant, T.; Radosavljevic, M.; et al. NCKAP1L defects lead to a novel syndrome combining immunodeficiency, lymphoproliferation, and hyperinflammation. The Journal of experimental medicine 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Lenardo, M.J.; Freeman, A.F. HEM1 Actin Immunodysregulatory Disorder: Genotypes, Phenotypes, and Future Directions. J Clin Immunol 2022, 42, 1583–1592. [Google Scholar] [CrossRef]
- Brigida, I.; Zoccolillo, M.; Cicalese, M.P.; Pfajfer, L.; Barzaghi, F.; Scala, S.; Oleaga-Quintas, C.; Alvarez-Alvarez, J.A.; Sereni, L.; Giannelli, S.; et al. T-cell defects in patients with ARPC1B germline mutations account for combined immunodeficiency. Blood 2018, 132, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Somech, R.; Lev, A.; Lee, Y.N.; Simon, A.J.; Barel, O.; Schiby, G.; Avivi, C.; Barshack, I.; Rhodes, M.; Yin, J.; et al. Disruption of Thrombocyte and T Lymphocyte Development by a Mutation in ARPC1B. J Immunol 2017, 199, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.; Massaad, M.J.; Bainter, W.; Ohsumi, T.K.; Föger, N.; Chan, A.C.; Akarsu, N.A.; Aytekin, C.; Ayvaz, D.; Tezcan, I.; et al. Recurrent viral infections associated with a homozygous CORO1A mutation that disrupts oligomerization and cytoskeletal association. J Allergy Clin Immunol 2016, 137, 879–888. [Google Scholar] [CrossRef]
- Kashani, P.; Marwaha, A.; Feanny, S.; Kim, V.H.; Atkinson, A.R.; Leon-Ponte, M.; Mendoza-Londono, R.; Grunebaum, E. Progressive decline of T and B cell numbers and function in a patient with CDC42 deficiency. Immunol Res 2021, 69, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lougaris, V.; Baronio, M.; Gazzurelli, L.; Benvenuto, A.; Plebani, A. RAC2 and primary human immune deficiencies. J Leukoc Biol 2020, 108, 687–696. [Google Scholar] [CrossRef]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front Immunol 2017, 8, 580. [Google Scholar] [CrossRef]
- Mocroft, A.; Furrer, H.J.; Miro, J.M.; Reiss, P.; Mussini, C.; Kirk, O.; Abgrall, S.; Ayayi, S.; Bartmeyer, B.; Braun, D.; et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥ 200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis 2013, 57, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Nalwoga, A.; Whitby, D. Adaptive immune responses to Kaposi’s sarcoma-associated herpesvirus. Curr Opin Immunol 2022, 77, 102230. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Mariano, L.C.; Singh, S.; Gupta, S. Highly active antiretroviral therapy (HAART) and outcome of cervical lesions and high-risk HPV in women living with HIV (WLHIV): A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2022, 278, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Calvi, L.M.; Adams, G.B.; Weibrecht, K.W.; Weber, J.M.; Olson, D.P.; Knight, M.C.; Martin, R.P.; Schipani, E.; Divieti, P.; Bringhurst, F.R.; et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003, 425, 841–846. [Google Scholar] [CrossRef]
- Schwarz, B.A.; Bhandoola, A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol Rev 2006, 209, 47–57. [Google Scholar] [CrossRef]
- Takahama, Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 2006, 6, 127–135. [Google Scholar] [CrossRef]
- Lind, E.F.; Prockop, S.E.; Porritt, H.E.; Petrie, H.T. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. The Journal of experimental medicine 2001, 194, 127–134. [Google Scholar] [CrossRef]
- Melichar, H.J.; Ross, J.O.; Herzmark, P.; Hogquist, K.A.; Robey, E.A. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Sci Signal 2013, 6, ra92. [Google Scholar] [CrossRef]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 2014, 14, 377–391. [Google Scholar] [CrossRef]
- Fink, P.J. The biology of recent thymic emigrants. Annu Rev Immunol 2013, 31, 31–50. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Nordenfelt, P.; Elliott, H.L.; Springer, T.A. Coordinated integrin activation by actin-dependent force during T-cell migration. Nat Commun 2016, 7, 13119. [Google Scholar] [CrossRef] [PubMed]
- Rothballer, A.; Kutay, U. The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma 2013, 122, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.; Rudnicka, D.; Hasan, M.; Aulner, N.; Porrot, F.; Machu, C.; Renaud, O.; Prevost, M.C.; Hivroz, C.; Schwartz, O.; et al. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. Journal of virology 2010, 84, 2282–2293. [Google Scholar] [CrossRef]
- Usmani, S.M.; Murooka, T.T.; Deruaz, M.; Koh, W.H.; Sharaf, R.R.; Di Pilato, M.; Power, K.A.; Lopez, P.; Hnatiuk, R.; Vrbanac, V.D.; et al. HIV-1 Balances the Fitness Costs and Benefits of Disrupting the Host Cell Actin Cytoskeleton Early after Mucosal Transmission. Cell host & microbe 2019, 25, 73–86. [Google Scholar] [CrossRef]
- Furler, R.L.; Nixon, D.F. The Intimate Relationship Between CD4+ T Cell Morphology and HIV-1 Infection. AIDS Res Hum Retroviruses 2019, 35, 509–510. [Google Scholar] [CrossRef]
- Barski, A.; Cuddapah, S.; Kartashov, A.V.; Liu, C.; Imamichi, H.; Yang, W.; Peng, W.; Lane, H.C.; Zhao, K. Rapid Recall Ability of Memory T cells is Encoded in their Epigenome. Sci Rep 2017, 7, 39785. [Google Scholar] [CrossRef]
- Ikegame, S.; Carmichael, J.C.; Wells, H.; Furler O’Brien, R.L.; Acklin, J.A.; Chiu, H.P.; Oguntuyo, K.Y.; Cox, R.M.; Patel, A.R.; Kowdle, S.; et al. Metagenomics-enabled reverse-genetics assembly and characterization of myotis bat morbillivirus. Nat Microbiol 2023. [Google Scholar] [CrossRef]
| Mutation |
Cell Type(s) Affected |
Mechanism of immunodeficiency | Clinical implications and treatments | ||
| Developmental deficiencies | |||||
| DiGeorge Syndrome | 22q11.2 deletion | T cells | Small or no thymus, low T cell counts. | Hematopoietic stem cell transplant or thymus transplant (in infancy) may be necessary | [36,37] |
| Omenn Syndrome | Mutation in RAG1 or RAG2 | T and B cells | Diminished lymphocyte activation receptor variability, low B cell counts, defective negative selection in thymus (Normal-high T cell counts) | Elevated IgE levels, predisposition to autoimmunity | [38,39] |
| Adenosine Deaminase (ADA) deficiency | Limited to no ADA expression (ADA is normally expressed in the thymus at high levels) | All lymphocytes, but mainly T cells | Depletion of developing lymphocytes via toxic accumulation of 2′deoxyadenosine and 2′dioxyinoside | Can result in severe combined immunodeficiency (SCID), and increased susceptibility to viral infections. | [40] |
| Cartilage Hair Hypoplasia | RMRP gene mutation, important in RNA processing | All lymphocytes, but mainly T cells | Limited T cell maturation and differentiation, increased T cell apoptosis | Can result in SCID and a form of dwarfism, may have decreased antibody levels. | [41] |
| Receptor deficiencies leading to pathophysiological activation: | |||||
| CD25 deficiency | Mutation in IL2RA gene (α chain of IL-2 receptor) | T cells | Limited T cell development, proliferation, and activation, diminished IL-10 production (normal B cells) | Can result in SCID | [42,43] |
| X-linked lymphoproliferative syndrome-1 (XLP1) | Mutation in signaling lymphocyte associated molecule (SLAM)- associated protein (SAP) | Lymphocytes | Limited T cell help and cytotoxicity, limited NK cell function | Increased incidence of lymphoma. HSCT is needed to cure | [44] |
| MHCII Deficiency (Bare lymphocyte syndrome) | MHCII gene intact, mutations in genes regulating MHC transcription | CD4+ T cells and APCs | Reduced CD4+ T cell counts due to incomplete maturation from perturbed positive and negative selection in thymus | Persistent viral infections. HSCT is needed to cure | [45] |
| Hyper IgM Syndrome | Mutation of CD40 on CD4+ T cells, or CD40L on B cells | CD4+ T cells and B cells | B cells cannot class switch out of IgM due to no CD40/CD40L interactions with CD4+ T cells | Increased bacterial infections, increased serum IgM levels. HSCT may be used to treat. | [46] |
| Chronic Mucocutaneous Candidiasis | Many causes, some include RORγT or IL-17 receptor deficiency | Th17 cells | Limited to no differentiation into Th17 cells and limited anti-fungal immunity | Chronic candida fungus infection, treatments may include antifungals. HSCT may be used to cure | [47] |
| Cytoskeletal defects that lead to pathophysiological migration and activation: | |||||
| Wiskott-Aldrich Syndrome | Dysfunctional Wiskott-Aldrich syndrome protein (WASp) | All lymphocytes, but mainly T cells | Inability of lymphocytes to create branched actin filaments, critical for immune cell migration and TCR activation. | Limited CD8+ T cell and B cell function, both from intrinsic defects and restricted CD4+ T cell help | [48,49] |
| Wiskott-Aldrich Syndrome-2 | WIPF1 gene mutation- WIP protein mutation (WASP-interacting protein) | Mainly T cells | Defective F-actin polymerization, leading to limited T cell migration and TCR activation. Low B and CD8+ T cell counts | Similar presentation to Wiskott-Aldrich Syndrome | [50] |
| DOCK2 deficiency | DOCK2 | Hematopoietic cells, but mainly T cells | Limited Rac1 activation in T cells, reduced F actin polymerization | Possible decreased antibody production, decreased antiviral response. HSCT is needed to cure | [51] |
| DOCK8 Deficiency | Deficient DOCK8 protein (Normally, DOCK8 interacts with Cdc42, leading to branched actin creation) | All lymphocytes, but mainly T cells | Limited T cell migration, activation, and proliferation. | Severe allergic responses, elevated IgE levels, high risk for skin infections. HSCT is necessary to cure | [52] |
| NCKAP1 gene mutation | HEM1 protein (part of WAVE complex) | All immune cells, but mainly T cells and NK cells | Limited leading edge actin polymerization and migration, diminished immune synapse formation | Hyperinflammation, autoimmunity, recurring infections. May be treated with corticosteroids | [53,54] |
| ARPC1B Deficiency | ARPC1B (assists ARP2/3 complex) | Hematopoietic cells | No immune synapse formation in T cells, limited migration | Autoimmunity, combined immunodeficiency | [55,56] |
| CORO1A mutation | CORO1A C-terminal domain truncation | Hematopoietic cells, but mainly T cells | Inability for CORO1A to depolymerize actin cytoskeleton, leading to increased F-actin accumulation. Decreased T cell help | Limited CD4+ T cells, chronic viral infections, similar presentation to Wiskott-Aldrich syndrome | [57] |
| CDC42 Deficiency | CDC42 | T cells and B cells | Impaired antibody production and T cell effectors function | Decline in T cell numbers and function, can treat some opportunistic infections with antibiotic prophylaxis | [58] |
| RAC2 Deficiency | RAC2 | Hematopoietic cells | Decreased naïve CD4+ T cells, decreased neutrophil chemotaxis | Recurrent infections. HSCT can be used to cure | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





