Submitted:
28 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Phytochemical analysis of prGSE
2.1.1. Total Phenolic Content
2.1.2. High Performance Liquid Chromatography (HPLC) Analysis
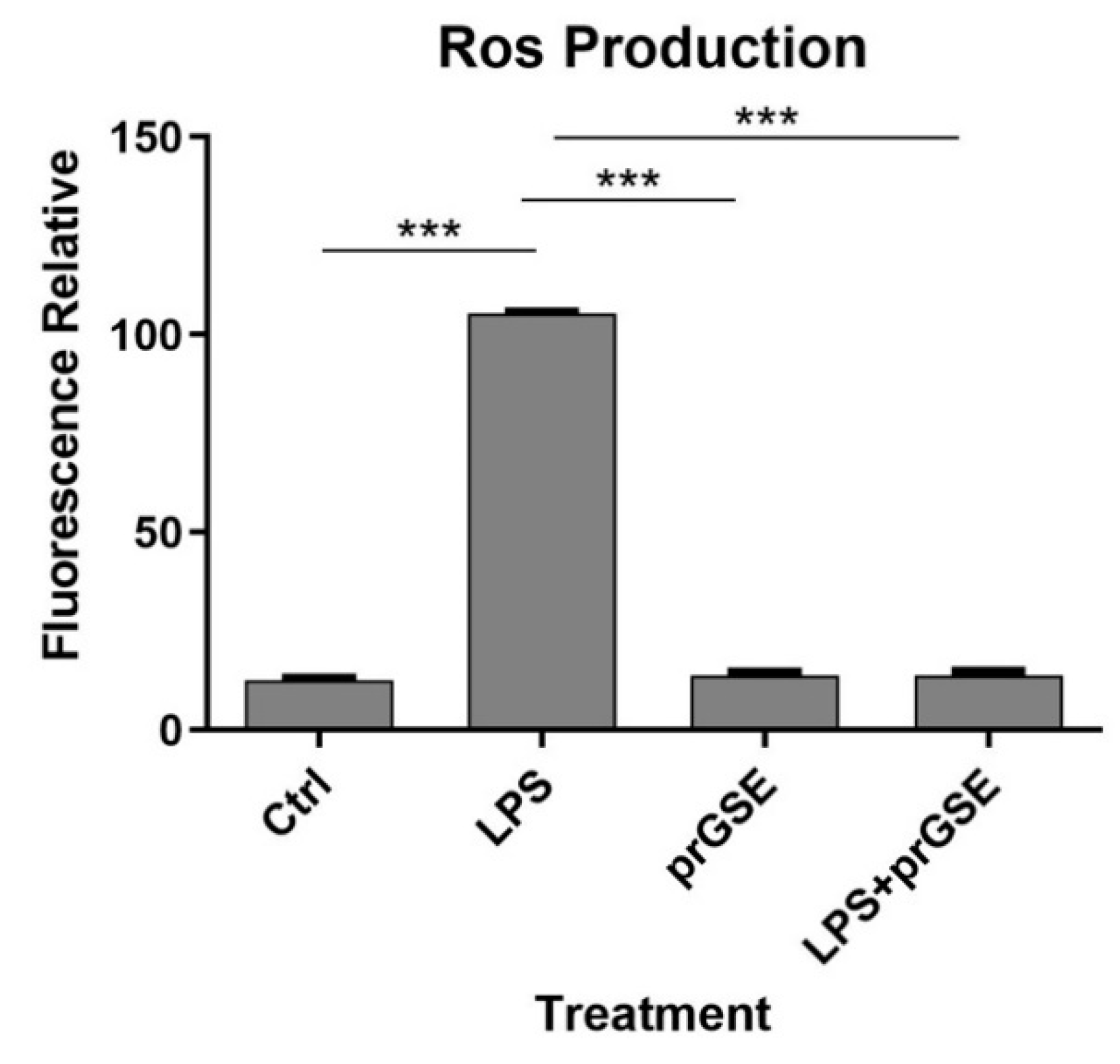
2.1.3. Nuclear Magnetic Resonance (NMR) Analysis
2.2. Caco-2 experiments
2.2.1. Effect of the Different Treatments with LPS, prGSE, and LPS-prGSE on the Caco-2 cell Monolayer Viability/Cytotoxicity
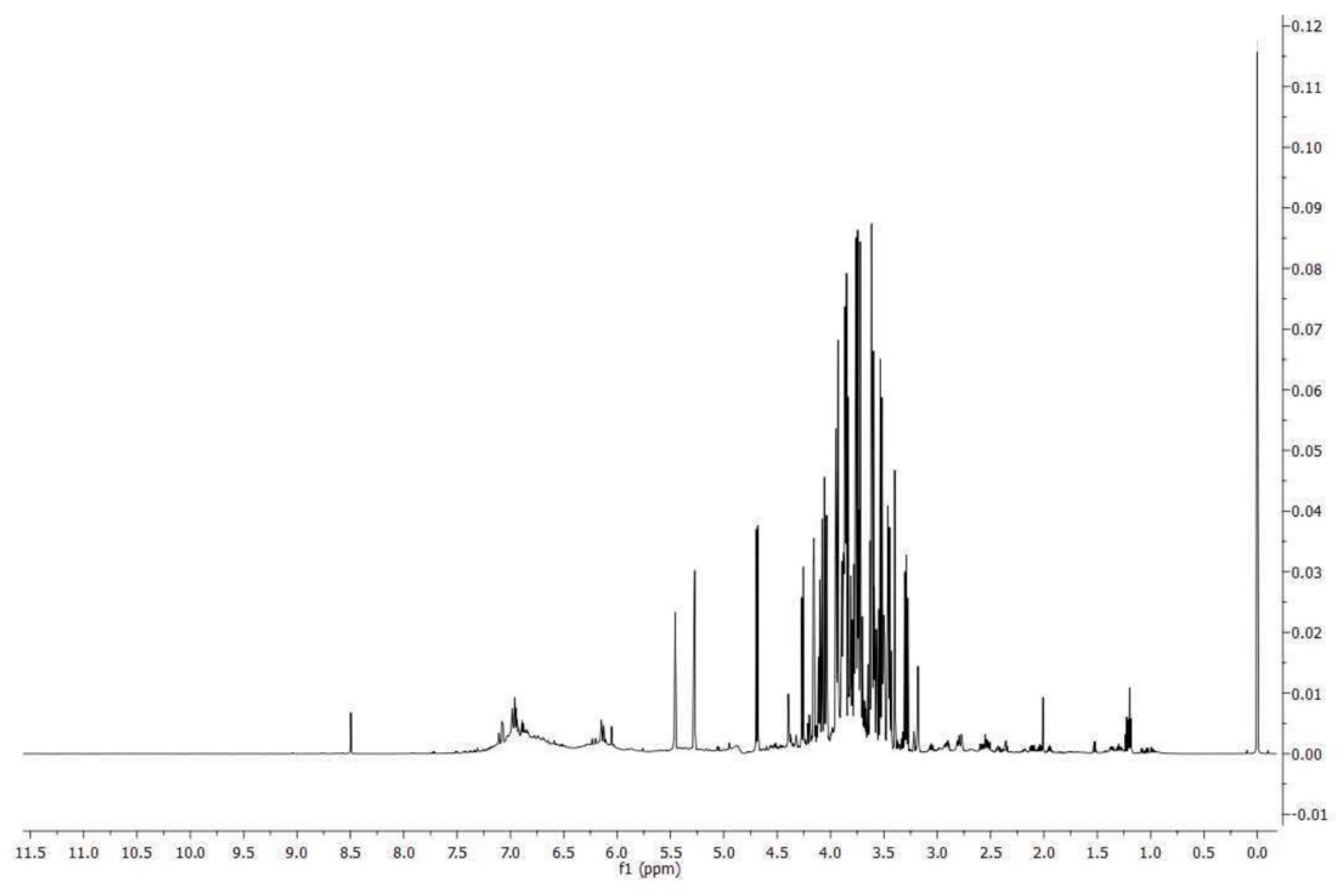
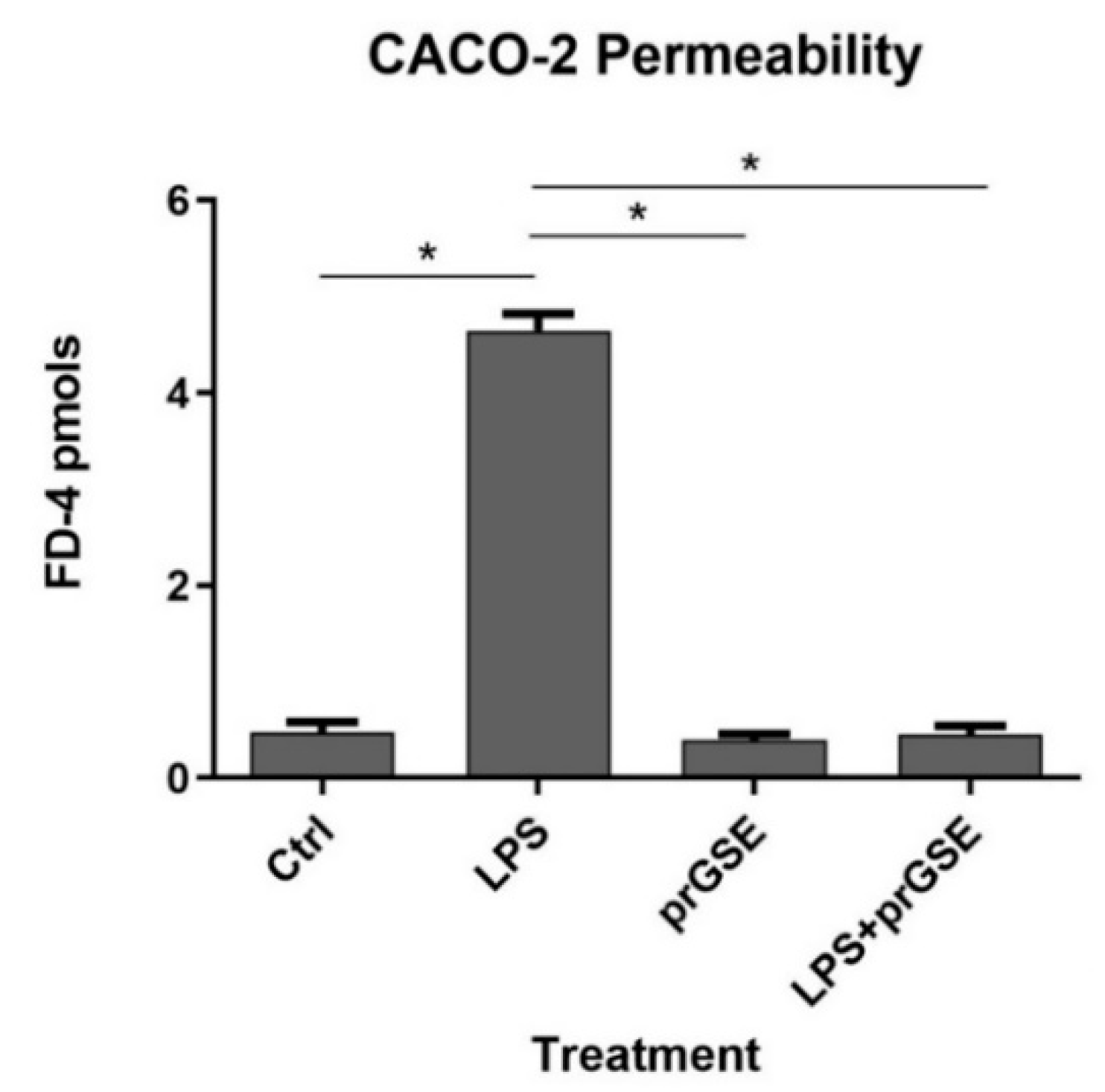
2.2.2. FD-4 Permeability Analysis after LPS, prGSE, and LPS-prGSE on the Caco-2 cells
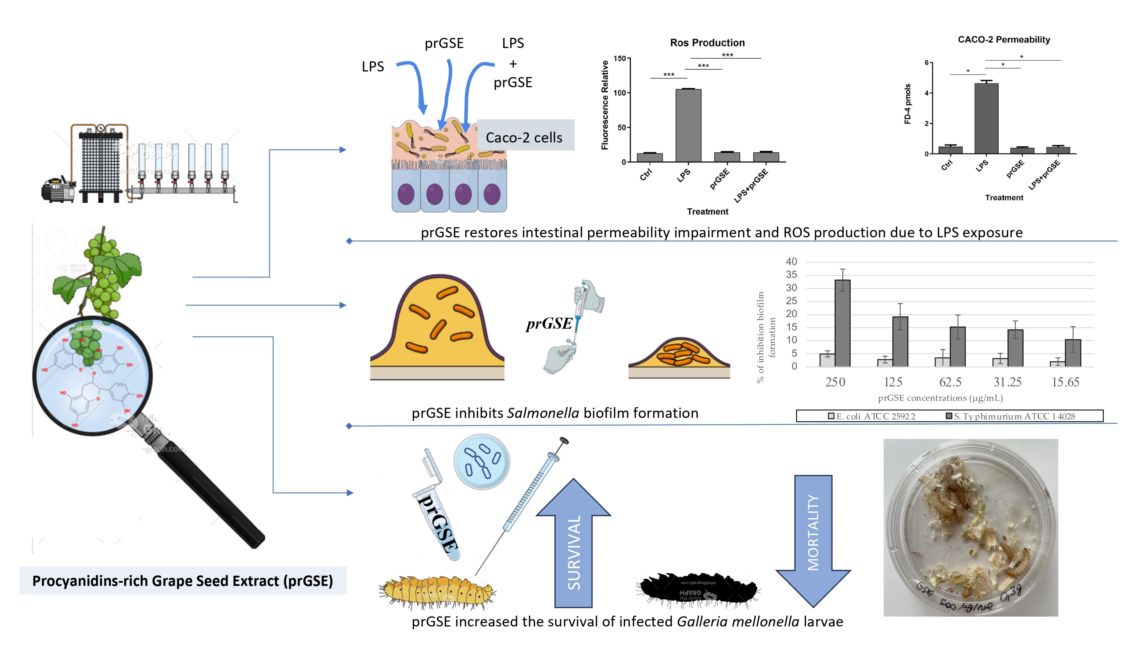
2.2.3. Analysis of Reactive Oxygen Species (ROS) production after LPS, prGSE, and LPS-prGSE treatment on the Caco-2 cells
2.3. In Vitro and In Vivo Activity of prGSE Against Salmonella Typhimurium and Escherichia coli Cells and Virulence Factors
2.3.1. In Vitro Antibacterial Activity Evaluation
| GM MIC50 (µg/mL) | GM MIC90 (µg/mL) | GM MIC100 (µg/mL) | |
|---|---|---|---|
| Salmonella Typhimurium ATCC 14028 | 44.17 | 88.39 | 222.73 |
| Escherichia coli ATCC 25922 | 55.68 | 99.21 | 396.85 |
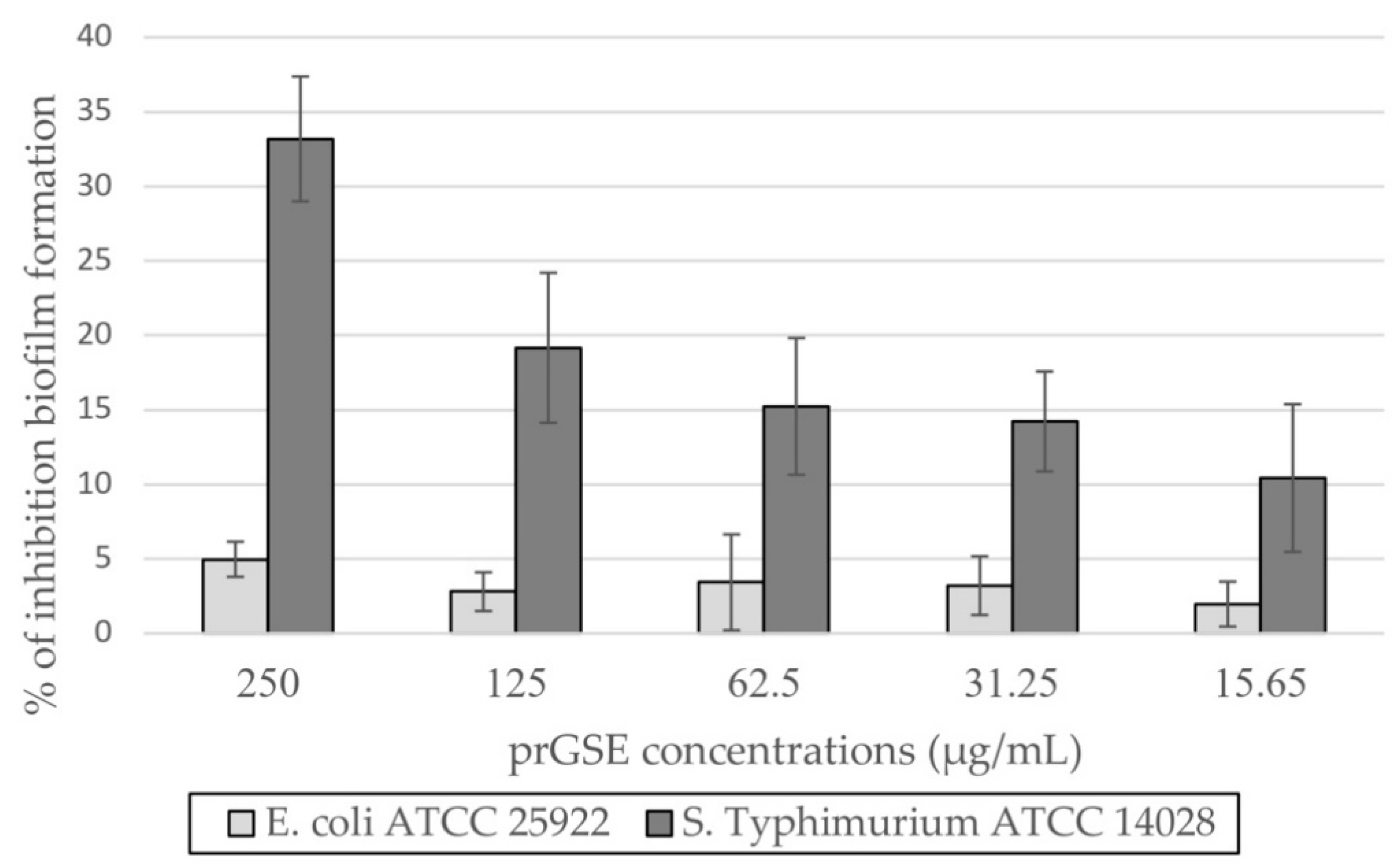
2.3.2. In Vitro Antibiofilm Activity
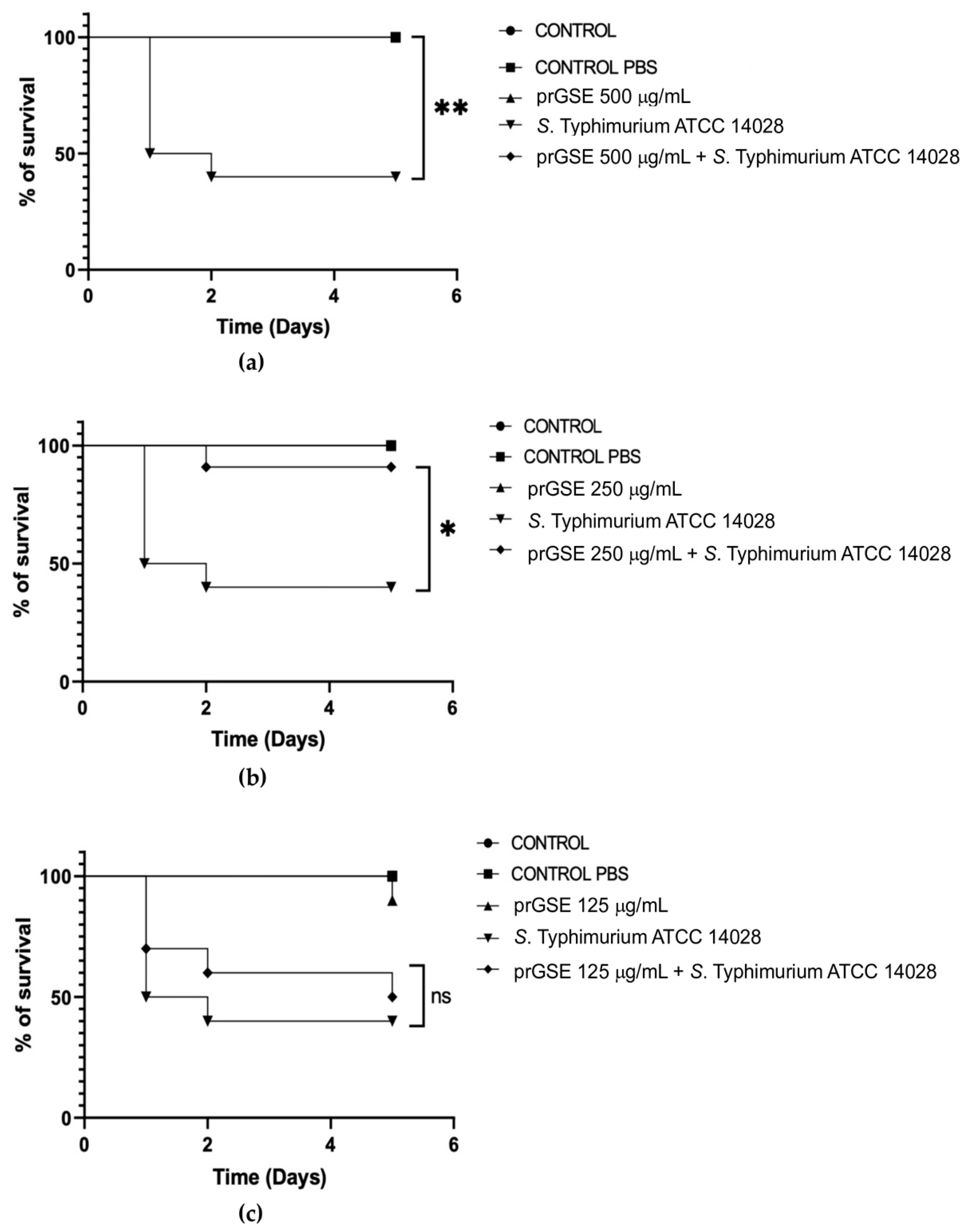
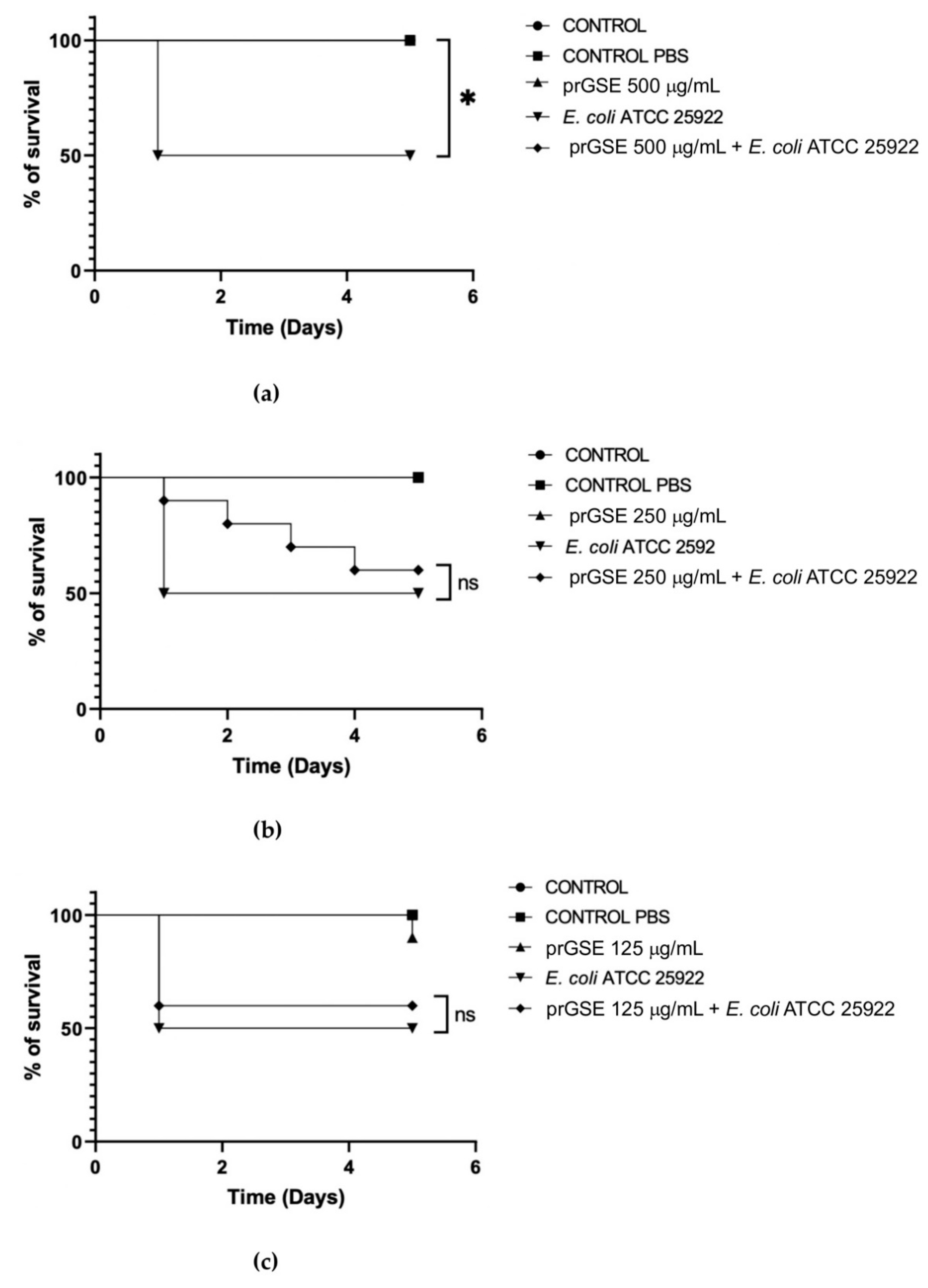
2.3.3. In Vivo Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Extraction and phytochemical analysis of prGSE
4.1.1. Plant Material
4.1.2. Sample Preparation
4.1.3. Total Phenolic Content
4.1.4. High Performance Liquid Chromatography (HPLC) Analysis
4.1.5. Nuclear Magnetic Resonance (NMR) Analysis
4.2. Caco-2 Culture experiments
4.2.1. Cell culture
4.2.2. Caco-2 cell Monolayer Viability/Cytotoxicity
4.2.3. Treatment of Caco-2 cells with LPS and prGSE
4.2.4. Cell permeability assay
4.2.5. Analysis of Oxidative Stress
4.3. In Vitro and In Vivo Activity of prGSE Against Salmonella enterica serovar Typhimurium and Escherichia coli Cells and Virulence Factors
4.3.1. Bacterial strains and growth conditions
4.3.2. Antimicrobial susceptibility tests
4.3.3. Anti-biofilm Activity
4.3.4. In vivo G. mellonella survival assay
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- C, P.; F, P.; D, C.; J, T.; Sh, I. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver 2018, 12. [Google Scholar] [CrossRef]
- Schultz, B.M.; Paduro, C.A.; Salazar, G.A.; Salazar-Echegarai, F.J.; Sebastián, V.P.; Riedel, C.A.; Kalergis, A.M.; Alvarez-Lobos, M.; Bueno, S.M. A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases. Front. Immunol. 2017, 8, 236225. [Google Scholar] [CrossRef]
- T, V.; J, T.; R, F. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Ij, M.; M, M.; J, W.; N, M.; D, W.; R, A.; J, B.-W.; E, M. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10. [Google Scholar] [CrossRef]
- Nayak, S.K. Biofilm-Mediated Gastrointestinal Diseases. In Biofilms in Human Diseases: Treatment and Control; Springer International Publishing AG, 2019; pp. 167–176. [Google Scholar]
- Martinez-Medina, M.; Garcia-Gil, L.J. Escherichia Coli in Chronic Inflammatory Bowel Diseases: An Update on Adherent Invasive Escherichia Coli Pathogenicity. World J. Gastrointest. Pathophysiol. 2014, 5, 213. [Google Scholar] [CrossRef] [PubMed]
- A, T.; A, M.; A, B.; J, F.; M, S. Preventing Bacterial Translocation in Patients with Leaky Gut Syndrome: Nutrition and Pharmacological Treatment Options. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- A, A.; A, de G; M, R. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72. [Google Scholar] [CrossRef]
- R, N.; H, Y. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann. Nutr. Metab 2017, 71 Suppl 1. [Google Scholar] [CrossRef]
- Gori, M.; Altomare, A.; Cocca, S.; Solida, E.; Ribolsi, M.; Carotti, S.; Rainer, A.; Francesconi, M.; Morini, S.; Cicala, M.; et al. Palmitic Acid Affects Intestinal Epithelial Barrier Integrity and Permeability In Vitro. Antioxidants 2020, 9. [Google Scholar] [CrossRef]
- Mp, G.; R, S.; A, A.; S, C.; M, D.P.; S, C.; G, S.; R, A.; S, E.; S, M.; et al. Human Colonic Myogenic Dysfunction Induced by Mucosal Lipopolysaccharide Translocation and Oxidative Stress. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2013, 45. [Google Scholar] [CrossRef]
- V, P.; A, A.; Mp, G.; V, L.; S, C.; S, C.; R, P.; R, A.; L, D.G.; M, C. Antioxidant Activity of Inulin and Its Role in the Prevention of Human Colonic Muscle Cell Impairment Induced by Lipopolysaccharide Mucosal Exposure. PloS One 2014, 9. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Barera, S.; Locato, V.; Cocca, S.; Franchin, C.; Arrigoni, G.; Vannini, C.; Grossi, S.; Campomenosi, P.; et al. Effect of Inulin on Proteome Changes Induced by Pathogenic Lipopolysaccharide in Human Colon. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Liu, K.; Deng, S.; Fan, Y.; Liu, Y. Sodium Humate Alleviates Enterotoxigenic Escherichia Coli-Induced Intestinal Dysfunction via Alteration of Intestinal Microbiota and Metabolites in Mice. Front. Microbiol. 2022, 13, 809086. [Google Scholar] [CrossRef]
- M, F.; M, A.-B.; M, A.-G.; Jp, G.M.; X, S.-R.; A, H.-P.; E, E.; Am, G.-C.; D, G.; B, L.; et al. Present and Future Therapeutic Approaches to Barrier Dysfunction. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Mp, G.; A, A.; E, S.; M, M.; C, S.; R, A.; G, D.; L, M.; R, C.; M, C. Effect of Acute Mucosal Exposure to Lactobacillus Rhamnosus GG on Human Colonic Smooth Muscle Cells. J. Clin. Gastroenterol 2008, 42 Pt 2. [Google Scholar] [CrossRef]
- F, A.; A, S.; A, A.; P, M.; C, P.; B, A.; R, C.; M, G.; M, M.; M, C.; et al. Lactobacillus Rhamnosus Protects Human Colonic Muscle from Pathogen Lipopolysaccharide-Induced Damage. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2013, 25. [Google Scholar] [CrossRef]
- P, L.; S, B.; J, G.; Hj, W.; G, F.; J, F.-G.; S, V. Beyond Heat Stress: Intestinal Integrity Disruption and Mechanism-Based Intervention Strategies. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- I, H.; W, H.; L, T.; A, M.; S, J.; X, D.; C, Y.; W, H.; G, Z.; Z, K.; et al. Phytochemicals and Inflammatory Bowel Disease: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60. [Google Scholar] [CrossRef]
- Pasqualetti, V.; Locato, V.; Fanali, C.; Mulinacci, N.; Cimini, S.; Morgia, A.M.; Pasqua, G.; De Gara, L. Comparison between In Vitro Chemical and Ex Vivo Biological Assays to Evaluate Antioxidant Capacity of Botanical Extracts. Antioxidants 2021, 10, 1136. [Google Scholar] [CrossRef]
- N, M.; A, V.; V, P.; M, I.; C, G.; M, B.; G, D.A.; A, C.; V, L.; C, D.V.; et al. Effects of Ionizing Radiation on Bio-Active Plant Extracts Useful for Preventing Oxidative Damages. Nat. Prod. Res. 2019, 33. [Google Scholar] [CrossRef]
- G, S.; Ar, S.; Fd, D.; N, M.; M, I.; F, C.; E, P.; E, G.; S, P.; D, A.; et al. Evaluation of Anti-Candida Activity of Vitis Vinifera L. Seed Extracts Obtained from Wine and Table Cultivars. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis Vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Kitsiou, M.; Purk, L.; Gutierrez-Merino, J.; Karatzas, K.A.; Klymenko, O.V.; Velliou, E. A Systematic Quantitative Determination of the Antimicrobial Efficacy of Grape Seed Extract against Foodborne Bacterial Pathogens. Foods 2023, 12, 929. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape Seed Extract: Having a Potential Health Benefits. J. Food Sci. Technol. 2020, 57, 1205. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.; Huang, W.; Zhou, H.; Zheng, Z.; Zhao, Y.; He, B.; Zhu, T.; Tang, S.; Zhu, Q. Kuwanon G Preserves LPS-Induced Disruption of Gut Epithelial Barrier In Vitro. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Y, H.; K, I.; R, K.; M, M.; T, U.; Y, I.; K, T. Protective Effects of Lactoferrin against Intestinal Mucosal Damage Induced by Lipopolysaccharide in Human Intestinal Caco-2 Cells. Yakugaku Zasshi 2008, 128. [Google Scholar] [CrossRef]
- A, B.; A, Z.; M, G.; D, D.; B, S. LPS Induces Hyper-Permeability of Intestinal Epithelial Cells. J. Cell. Physiol. 2017, 232. [Google Scholar] [CrossRef]
- Y, W.; J, T.; B, C.; B, W.; D, Z.; B, W. Effects of Alcohol on Intestinal Epithelial Barrier Permeability and Expression of Tight Junction-Associated Proteins. Mol. Med. Rep. 2014, 9. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Atzeri, A.; Lucas, R.; Incani, A.; Peñalver, P.; Zafra-Gómez, A.; Melis, M.P.; Pizzala, R.; Morales, J.C.; Deiana, M. Hydroxytyrosol and Tyrosol Sulfate Metabolites Protect against the Oxidized Cholesterol Pro-Oxidant Effect in Caco-2 Human Enterocyte-like Cells. Food Funct. 2016, 7, 337–346. [Google Scholar] [CrossRef]
- M07: Dilution AST for Aerobically Grown Bacteria - CLSI . Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 17 July 2023).
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria Mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef]
- Giannini, B.; Mulinacci, N.; Pasqua, G.; Innocenti, M.; Valletta, A.; Cecchini, F. Phenolics and Antioxidant Activity in Different Cultivars/Clones of Vitis Vinifera L. Seeds over Two Years. Plant Biosyst. - Int. J. Deal. Asp. Plant Biol. 2016. [Google Scholar]
- Abdel-Rhman, S.H. Role of Pseudomonas Aeruginosa Lipopolysaccharides in Modulation of Biofilm and Virulence Factors of Enterobacteriaceae. Ann. Microbiol. 2019, 69, 299–305. [Google Scholar] [CrossRef]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity Within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2021, 10, 624622. [Google Scholar] [CrossRef]
- A, D.; P, N.; M, D.; G, M.K.; J, N.; S, A.; G, L. Increased Serum Levels of Lipopolysaccharide and Antiflagellin Antibodies in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2015, 27. [Google Scholar] [CrossRef]
- O, P.R.; A, L.S.R.; E, A.A.; A, de la H, M.; E, R.S.; A, A.M. Serum Lipopolysaccharide-Binding Protein in Endotoxemic Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2007, 13. [Google Scholar] [CrossRef]
- S, G.; R, A.-S.; Hm, S.; Ty, M. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in Vitro and in Vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182. [Google Scholar] [CrossRef]
- S, B.; Pk, D.; Jk, T. Lipopolysaccharide Activates NF-KappaB by TLR4-Bcl10-Dependent and Independent Pathways in Colonic Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295. [Google Scholar] [CrossRef]
- Mt, A. Toll-like Receptor Signalling in the Intestinal Epithelium: How Bacterial Recognition Shapes Intestinal Function. Nat. Rev. Immunol. 2010, 10. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.O.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Ty, M.; D, N.; V, B.; H, N.; N, H. Ethanol Modulation of Intestinal Epithelial Tight Junction Barrier. Am. J. Physiol. 1999, 276. [Google Scholar] [CrossRef]
- Rm, C.; C, F.; A, T.; M, A.; Ca, M.; S, G. Differential Effect of Ethanol and Hydrogen Peroxide on Barrier Function and Prostaglandin E2 Release in Differentiated Caco-2 Cells: Selective Prevention by Growth Factors. J. Pharm. Sci. 2009, 98. [Google Scholar] [CrossRef]
- Ty, M.; D, H.; Lt, T.; D, N.; N, H.; D, B. Cytoskeletal Regulation of Caco-2 Intestinal Monolayer Paracellular Permeability. J. Cell. Physiol. 1995, 164. [Google Scholar] [CrossRef]
- Zt, B.; Sl, G.; Mr, D.; Km, G.; L, Y.; Sf, O.; Jd, L.; Ap, N. Cocoa Procyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of Colonic Inflammation. J. Nutr. Biochem. 2015, 26. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Chiu, M.; Taurino, G.; Brighenti, F.; Rio, D.D.; Mena, P.; Bussolati, O. Catechin and Procyanidin B2 Modulate the Expression of Tight Junction Proteins but Do Not Protect from Inflammation-Induced Changes in Permeability in Human Intestinal Cell Monolayers. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- K, G.-C.; R, C.; I, G.; A, A.; M, P.; F, V.; X, T.; M, B. Protective Effect of Proanthocyanidins in a Rat Model of Mild Intestinal Inflammation and Impaired Intestinal Permeability Induced by LPS. Mol. Nutr. Food Res. 2019, 63. [Google Scholar] [CrossRef]
- H, W.; T, L.; Ym, L.; Zp, G.; Kq, Z.; Jy, S.; Js, X.; Yp, C. Granny Smith Apple Procyanidin Extract Upregulates Tight Junction Protein Expression and Modulates Oxidative Stress and Inflammation in Lipopolysaccharide-Induced Caco-2 Cells. Food Funct. 2018, 9. [Google Scholar] [CrossRef]
- R, R.; S, M.; Sm, K.; B, O.; Jd, S.; S, A.; D, G.; M, F. Claudin-2, a Component of the Tight Junction, Forms a Paracellular Water Channel. J. Cell Sci. 2010, 123. [Google Scholar] [CrossRef]
- T, O.; H, M.; T, J. Changes in the Expression of Claudins in Active Ulcerative Colitis. J. Gastroenterol. Hepatol. 2008, 23 Suppl 2. [Google Scholar] [CrossRef]
- Das, P.; Goswami, P.; Das, T.; Nag, T.; Sreenivas, V.; Ahuja, V.; Panda, S.; Gupta, S.D.; Makharia, G. Comparative Tight Junction Protein Expressions in Colonic Crohn’s Disease, Ulcerative Colitis, and Tuberculosis: A New Perspective. Virchows Arch. 2012. [CrossRef]
- Talavera, M.M.; Nuthakki, S.; Cui, H.; Jin, Y.; Liu, Y.; Nelin, L.D. Immunostimulated Arginase II Expression in Intestinal Epithelial Cells Reduces Nitric Oxide Production and Apoptosis. Front. Cell Dev. Biol. 2017, 5, 227133. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, S.; Zhao, R.C. The Roles of Mesenchymal Stem Cells in Tumor Inflammatory Microenvironment. J. Hematol. Oncol.J Hematol Oncol 2014, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal Growth Factor, through Alleviating Oxidative Stress, Protect IPEC-J2 Cells from Lipopolysaccharides-Induced Apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef] [PubMed]
- C, G.-Q.; E, R.-G.; R, B.-D.; M, P.; A, A.; Mt, B.; X, T. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018 | EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6007 (accessed on 26 July 2023).
- Zha, L.; Garrett, S.; Sun, J. Salmonella Infection in Chronic Inflammation and Gastrointestinal Cancer. Diseases 2019, 7. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; He, Q.; Sun, K.; Wang, X.; Zhang, X.; Li, Y.; Zeng, J. Crosstalk between Microbial Biofilms in the Gastrointestinal Tract and Chronic Mucosa Diseases. Front. Microbiol. 2023, 14, 1151552. [Google Scholar] [CrossRef]
- G, M.; A, R.; V, C.; Py, D. Galleria Mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- N, R.; C, N.-L.; D, L. The Insect Galleria Mellonella as a Powerful Infection Model to Investigate Bacterial Pathogenesis. J. Vis. Exp. JoVE 2012. [Google Scholar] [CrossRef]
- Lf, G.; Pr, B.; S, B. The Structure and Function of the Smooth Septate Junction in a Transporting Epithelium: The Malpighian Tubules of the New Zealand Glow-Worm Arachnocampa Luminosa. Tissue Cell 1980, 12. [Google Scholar] [CrossRef]
- Pm, C.; At, C.; Er, H.; Pd, E.; Kh, G. Proteomic Analysis of the Peritrophic Matrix from the Gut of the Caterpillar, Helicoverpa Armigera. Insect Biochem. Mol. Biol. 2008, 38. [Google Scholar] [CrossRef]
- T, K.; O, B.; O, O.; N, B.; B, L. Genetic Evidence for a Protective Role of the Peritrophic Matrix against Intestinal Bacterial Infection in Drosophila Melanogaster. Proc. Natl. Acad. Sci. U. S. A. 2011, 108. [Google Scholar] [CrossRef]
- Mukherjee, K.; Hain, T.; Fischer, R.; Chakraborty, T.; Vilcinskas, A. Brain Infection and Activation of Neuronal Repair Mechanisms by the Human Pathogen Listeria Monocytogenes in the Lepidopteran Model Host Galleria Mellonella. Virulence 2013, 4, 324. [Google Scholar] [CrossRef] [PubMed]
- Emery, H.; Johnston, R.; Rowley, A.F.; Coates, C.J. Indomethacin-Induced Gut Damage in a Surrogate Insect Model, Galleria Mellonella. Arch. Toxicol. 2019, 93, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Grounta, A.; Harizanis, P.; Mylonakis, E.; Nychas, G.-J.E.; Panagou, E.Z. Investigating the Effect of Different Treatments with Lactic Acid Bacteria on the Fate of Listeria Monocytogenes and Staphylococcus Aureus Infection in Galleria Mellonella Larvae. PLOS ONE 2016, 11, e0161263. [Google Scholar] [CrossRef] [PubMed]
- A, U.; I, U.; S, M.; K, V. Eugenol in Combination with Lactic Acid Bacteria Attenuates Listeria Monocytogenes Virulence in Vitro and in Invertebrate Model Galleria Mellonella. J. Med. Microbiol. 2016, 65. [Google Scholar] [CrossRef]
- Scalfaro, C.; Iacobino, A.; Nardis, C.; Franciosa, G. Galleria Mellonella as an in Vivo Model for Assessing the Protective Activity of Probiotics against Gastrointestinal Bacterial Pathogens. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- K, M.; R, R.; R, F.; A, V. Galleria Mellonella as a Model Host to Study Gut Microbe Homeostasis and Brain Infection by the Human Pathogen Listeria Monocytogenes. Adv. Biochem. Eng. Biotechnol. 2013, 135. [Google Scholar] [CrossRef]
- Nj, S.; Mc, B.; Ol, C.; Se, R.; Rm, L.R.; Mj, W.; Fj, S.; Rw, T. Galleria Mellonella as an Infection Model for Campylobacter Jejuni Virulence. J. Med. Microbiol. 2011, 60. [Google Scholar] [CrossRef]
- Wagley, S.; Borne, R.; Harrison, J.; Baker-Austin, C.; Ottaviani, D.; Leoni, F.; Vuddhakul, V.; Titball, R.W. Galleria Mellonella as an Infection Model to Investigate Virulence of Vibrio Parahaemolyticus. Virulence 2018, 9, 197. [Google Scholar] [CrossRef]
- S, B.; H, G.; Y, Z.; Cl, H.; Dv, Z.; Mm, V. The Galleria Mellonella Larvae as an in Vivo Model for Evaluation of Shigella Virulence. Gut Microbes 2017, 8. [Google Scholar] [CrossRef]
- Card, R.; Vaughan, K.; Bagnall, M.; Spiropoulos, J.; Cooley, W.; Strickland, T.; Davies, R.; Anjum, M.F. Virulence Characterisation of Salmonella Enterica Isolates of Differing Antimicrobial Resistance Recovered from UK Livestock and Imported Meat Samples. Front. Microbiol. 2016, 7, 186885. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- V, S.; E, B.; F, S.; A, C.; O, G.; A, M.; G, P.; Am, P. Biostimulant Effects of Chaetomium Globosum and Minimedusa Polyspora Culture Filtrates on Cichorium Intybus Plant: Growth Performance and Metabolomic Traits. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- G, C.; F, S.; P, G.; S, M.; L, F.; G, G.; Ar, C.; G, C.; Me, D.C.; F, A. Mantonico and Pecorello Grape Seed Extracts: Chemical Characterization and Evaluation of In Vitro Wound-Healing and Anti-Inflammatory Activities. Pharm. Basel Switz. 2020, 13. [Google Scholar] [CrossRef]
- Dinicola, S.; Cucina, A.; Pasqualato, A.; D’Anselmi, F.; Proietti, S.; Lisi, E.; Pasqua, G.; Antonacci, D.; Bizzarri, M. Antiproliferative and Apoptotic Effects Triggered by Grape Seed Extract (GSE) versus Epigallocatechin and Procyanidins on Colon Cancer Cell Lines. Int. J. Mol. Sci. 2012, 13, 651. [Google Scholar] [CrossRef]
- Provenzani, R.; San-Martin-Galindo, P.; Hassan, G.; Legehar, A.; Kallio, A.; Xhaard, H.; Fallarero, A.; Yli-Kauhaluoma, J. Multisubstituted Pyrimidines Effectively Inhibit Bacterial Growth and Biofilm Formation of Staphylococcus Aureus. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Cui, Z.-H.; He, H.-L.; Zheng, Z.-J.; Yuan, Z.-Q.; Chen, Y.; Huang, X.-Y.; Ren, H.; Zhou, Y.-F.; Zhao, D.-H.; Fang, L.-X.; et al. Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram- Bacteria. Antibiotics 2023, 12, 760. [Google Scholar] [CrossRef]
- Efficacy and Safety of Phage Therapy against Salmonella Enterica Serovars Typhimurium and Enteritidis Estimated by Using a Battery of in Vitro Tests and the Galleria Mellonella Animal Model. Microbiol. Res. 2022, 261, 127052. [CrossRef]
| Compound | Retention Time (Min) | Area | mg/g |
|---|---|---|---|
| Catechin (1) | 11.65 | 408.94 | 10.92 |
| Procyanidin B2 (2) | 16.86 | 106.99 | 4.05 |
| Epicatechin (3) | 19.03 | 372 | 7.66 |
| Epicatechin gallate (4) | 30.54 | 261.12 | 2.80 |
| Procyanidin Pol 1 (5) | 48.59 | 9694.75 | 365.48 |
| Procyanidin Pol 2 (6) | 52.00 | 2078.38 | 78.36 |
| Molecule | Amount (mg/100 mg dry extract) | |
|---|---|---|
| Amino acids | Leucine | 0.01461 ± 0.00073 |
| Isoleucine | 0.01417 ± 0.00071 | |
| Valine | 0.0228 ± 0.0011 | |
| Threonine | 0.00199 ± 0.00010 | |
| Alanine | 0.0403 ± 0.0021 | |
| GABA | 0.1199 ± 0.0062 | |
| Glutamine | 0.1536 ± 0.0077 | |
| Aspartate | 0.372 ± 0.019 | |
| Asparagine | 0.027 ± 0.0014 | |
| Phenylalanine | 0.034 ± 0.0017 | |
| Tryptophan | 0.0838 ± 0.0042 | |
| Organic acids | Lactate | 0.00633 ± 0.00032 |
| Acetate | 0.056 ± 0.0028 | |
| Citrate | 0.1027 ± 0.0051 | |
| Malate | 0.445 ± 0.022 | |
| Ascorbate | 0.1343 ± 0.0067 | |
| Formate | 0.1587 ± 0.0079 | |
| Carbohydrates | Glucose | 13.55 ± 0.68 |
| Sucrose | 8.17 ± 0.41 | |
| Miscellaneous molecules | Ethanol | 0.1031 ± 0.0052 |
| Choline | 0.1146 ± 0.0057 | |
| Procyanidin B1 | 2.66 ± 0.13 | |
| Polymeric Procyanidin (eq. Procyanidin B1) | 25.3 ± 1.27 | |
| Trigonelline | 0.025 ± 0.0012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





