Submitted:
28 July 2023
Posted:
31 July 2023
You are already at the latest version
Abstract
Keywords:
1. Background
2. Search Methodology
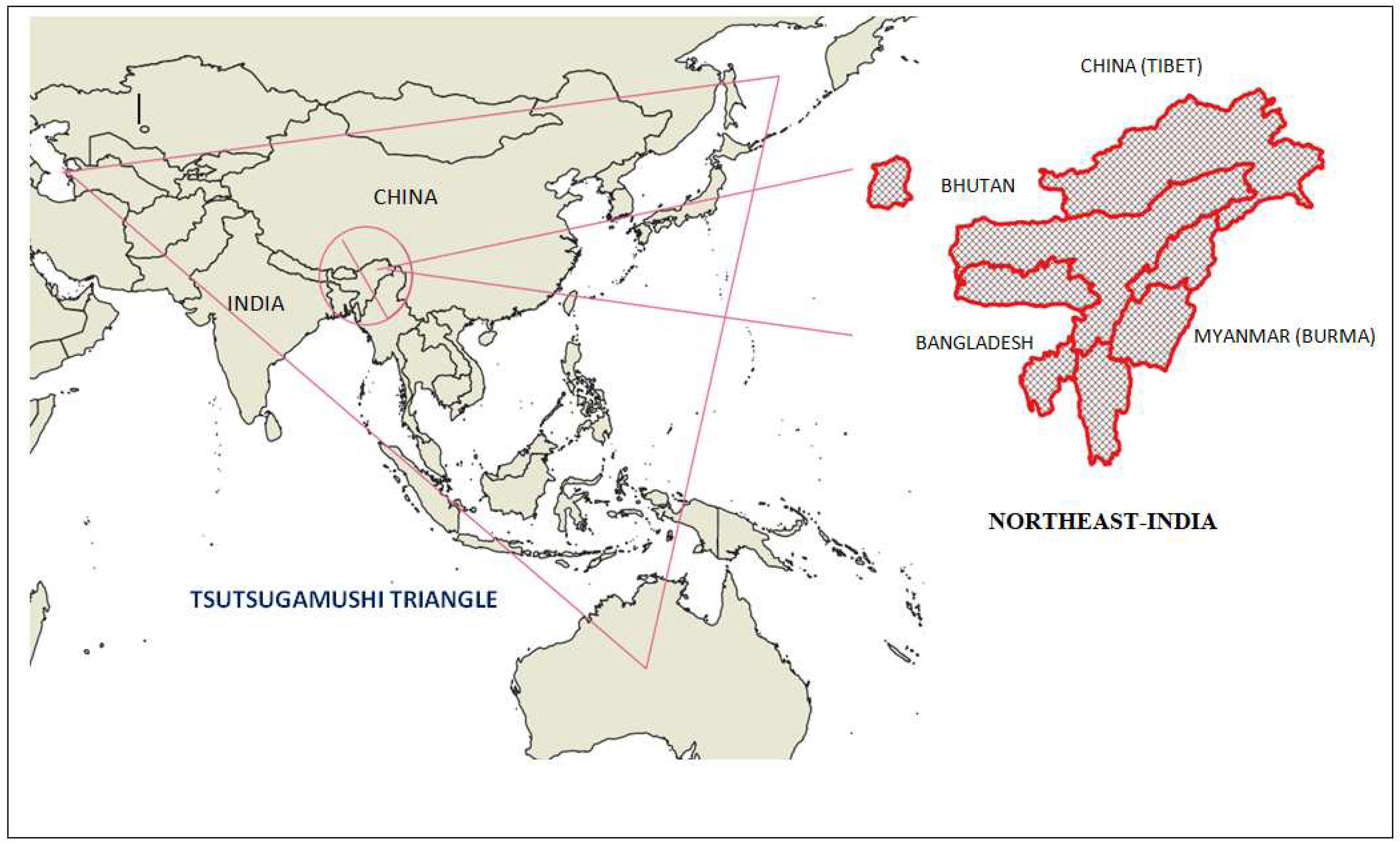
3. Geography of North-East India
4. Scrub Typhus in Northeast India
4.1. Descriptive Epidemiology:
4.2. Age and Gender Cases
4.3. Diagnostic Conformation
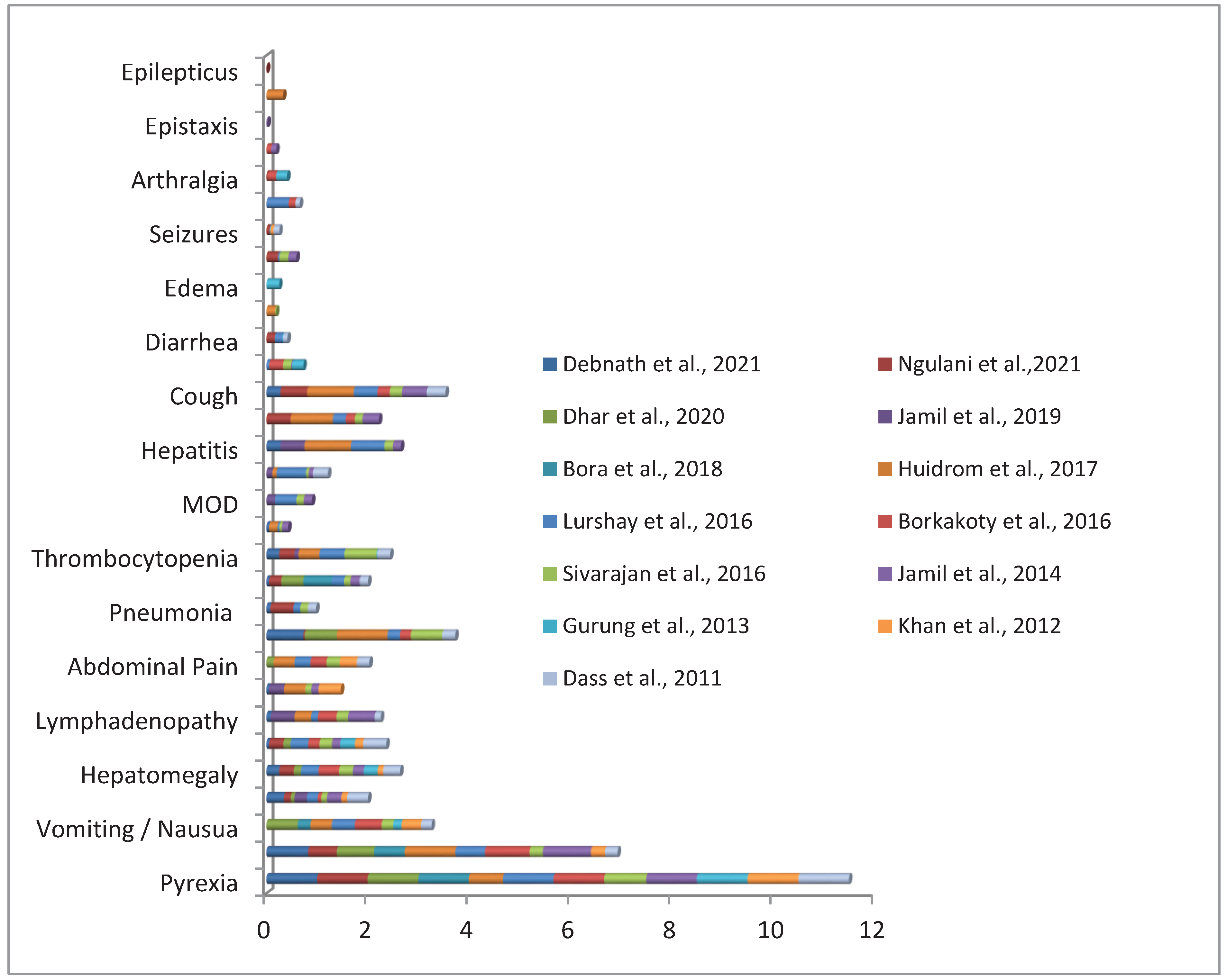
4.4. Clinical Presentations
4.5. Co-Infection
4.6. Genetic Diversity
4.7. Treatment and Outcomes
4.8. Entomological Investigations
5. Discussion
6. Conclusions and Future Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trent, B.; Fisher, J.; Soong, L. Scrub Typhus Pathogenesis: Innate Immune Response and Lung Injury During Orientia tsutsugamushi Infection. Front. Microbiol. 2019, 10, 2065. [CrossRef]
- Santos, H.A.; Massard, C.L. The Family Rickettsiaceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Heidelberg, 2014; pp. 619–635 ISBN 978-3-642-30197-1.
- Bonell, A.; Lubell, Y.; Newton, P. N.; Crump, J. A.; Paris, D. H. Estimating the burden of scrub typhus: A systematic review. PLoS Neglected Trop. Dis. 2017, 11, e0005838. [CrossRef]
- Walker; David H. Scrub Typhus - Scientific Neglect, Ever-Widening Impact. New Engl. J. Med. 2016, 375, 913–15. https://pubmed.ncbi.nlm.nih.gov/27602663/.
- Jiang, J.; Richards, A.L. Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle. Trop. Med. Infect. Dis. 2018, 3, 11. https://doi.org/10.3390/tropicalmed3010011. [CrossRef]
- Paris, D.H.; Shelite, T.R.; Day, N.P.; Walker, D.H. Unresolved Problems Related to Scrub Typhus: A Seriously Neglected Life-Threatening Disease. Am. J. Trop. Med. Hyg. 2013, 89, 301–307. https://doi.org/10.4269/ajtmh.13-0064. [CrossRef]
- World Health Organization. WHO Recommended Surveillance Standards; World Health Organization: Geneva, Switzerland, 1999.
- Chakraborty, S.; Sarma, N. Scrub typhus: an emerging threat. Indian. J. Dermatol. 2017, 62 478–485.
- Saraswati, K.; Day, N. P. J.; Mukaka, M.; Blacksell, S. D. Scrub typhus point-of-care testing: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12. https://doi.org/10.1371/journal.pntd.0006330. [CrossRef]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A Review of the Global Epidemiology of Scrub Typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. https://doi.org/10.1371/journal.pntd.0006062. [CrossRef]
- Biswal, M.; Zaman, K.; Suri, V.; Rao, H.; Kumar, A.; Kapur, G.; Sharma, N.; Bhalla, A.; Jayashree, M. Use of Eschar for the Molecular Diagnosis and Genotypic Characterisation of Orientia Tsutsugamushi Causing Scrub Typhus. Indian. J. Med. Microbiol. 2018, 36, 422–425. https://doi.org/10.4103/ijmm.IJMM_18_8. [CrossRef]
- Phetsouvanh, R.; Thojaikong, T.; Phoumin, P.; Sibounheuang, B.; Phommasone, K.; Chansamouth, V.; Lee, S.J.; Newton, P.N.; Blacksell, S.D. Inter- and Intra-Operator Variability in the Reading of Indirect Immunofluorescence Assays for the Serological Diagnosis of Scrub Typhus and Murine Typhus. Am. J. Trop. Med. Hyg. 2013, 88, 932–936. https://doi.org/10.4269/ajtmh.12-0325. [CrossRef]
- Nadjm, B.; Thuy , P.T.; Trang, V.D.; Ha, L.D.; Kinh, N.V.; Wertheim, H.F. Scrub typhus in the northern provinces of Vietnam: An observational study of admissions to a national referral hospital. Trans. R. Soc. Trop. Med. Hyg, 2014, 108, 739–40. https://doi.org/10.1093/trstmh/tru145. [CrossRef]
- Tran, H.T.D.; Hattendorf, J.; Do, H.M.; Hoang, T.T.; Hoang, H.T.H; Lam, H.N.; Huynh, M.K.; Vu, L.T.H.; Zinsstag, J.; Paris, D.H.; Schelling, E. Ecological and behavioural risk factors of scrub typhus in central Vietnam: a case-control study. Infect. Dis. Poverty 2021, 10, 1–14. https://doi.org/10.1186/s40249-021-00893-6. [CrossRef]
- Wangrangsimakul, T.; Elliott, I.; Nedsuwan, S.; Kumlert, R.; Hinjoy, S.; Chaisiri, K.; Day, N.P.J.; Morand, S. The Estimated Burden of Scrub Typhus in Thailand from National Surveillance Data (2003-2018). PLoS Negl. Trop. Dis. 2020, 14, e0008233. https://doi.org/10.1371/journal.pntd.0008233. [CrossRef]
- Roberts, T.; Parker, D.M.; Bulterys, P.L.; Rattanavong, S.; Elliott, I.; Phommasone, K.; Mayxay, M.; Chansamouth, V.; Robinson, M.T.; Blacksell, S.D.; et al. A Spatio-Temporal Analysis of Scrub Typhus and Murine Typhus in Laos; Implications from Changing Landscapes and Climate. PLoS Negl. Trop. Dis. 2021, 15, e0009685. https://doi.org/10.1371/journal.pntd.0009685. [CrossRef]
- Trung, N.V.; Hoi, L.T.; Thuong, N.T.H.; Toan, T.K.; Huong, T.T.K.; Hoa, T.M.; Fox, A.; Kinh, N. van; van Doorn, H.R.; Wertheim, H.F.L.; et al. Seroprevalence of Scrub Typhus, Typhus, and Spotted Fever Among Rural and Urban Populations of Northern Vietnam. Am. J. Trop. Med. Hyg. 2017, 96, 1084–1087. https://doi.org/10.4269/ajtmh.16-0399. [CrossRef]
- Le Viet, N.; Laroche, M.; Thi Pham, H.L.; Viet, N.L.; Mediannikov, O.; Raoult, D.; Parola, P. Use of Eschar Swabbing for the Molecular Diagnosis and Genotyping of Orientia Tsutsugamushi Causing Scrub Typhus in Quang Nam Province, Vietnam. PLoS Negl. Trop. Dis. 2017, 11, e0005397. https://doi.org/10.1371/journal.pntd.0005397. [CrossRef]
- Costa, C.; Ferrari, A.; Binazzi, R.; Beltrame, A.; Tacconi, D.; Moro, L.; Edouard, S.; Parola, P.; Buonfrate, D.; Gobbi, F. Imported Scrub Typhus in Europe: Report of Three Cases and a Literature Review. Travel. Med. Infect. Dis. 2021, 42, 102062. https://doi.org/10.1016/j.tmaid.2021.102062. [CrossRef]
- Singh, P. Scrub typhus, a case report: Military and regional significance. Med. J. Armed Forces India 2004, 60, 89–90. [CrossRef]
- Northeast India. Available online: https://en.wikipedia.org/wiki/Northeast_India (accessed on 28 July 2023).
- Ravindranath, N.H.; Rao, S.; Sharma, N.; Nair, M.; Gopalakrishnan, R.; Rao, A.S.; Malaviya, S.; Tiwari, R.; Sagadevan, A.; Munsi, M.; et al. Climate Change Vulnerability Profiles for North East India. Curr. Sci. 2011, 101.
- Dikshit, K.R.; Dikshit, J.K. Relief Features of North-East India. In North-East India: Land, People and Economy, Dikshit, K.R.; Dikshit, J.K. Eds. Springer Netherlands: Dordrecht, 2014; pp. 91–125.
- Chakravarty, S.; Suresh, C.P.; Puri, A.; Shukla, G. North-East India, the Geographical Gateway of India. Indian. For. 2012, 138, 702–709.
- Dikshit, K.R.; Dikshit, J.K. Weather and Climate of North-East India. In North-East India: Land, People and Economy, Springer Netherlands: Dordrecht, 2014; pp. 149–173.
- Dikshit, K.R.; Dikshit, J.K. Agriculture in North-East India: Past and Present. In: North-East India: Land, People and Economy. Advances in Asian Human-Environmental Research. Springer, Dordrecht. 2013, pp 587–637 https://doi.org/10.1007/978-94-007-7055-3_16. [CrossRef]
- Richards, A.L.; Jiang, J. Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species. Trop. Med. Infect. Dis. 2020, 5, 49. https://doi.org/10.3390/tropicalmed5020049. [CrossRef]
- Chandna, A.; Chew, R.; Shwe Nwe Htun, N.; Peto, T. J.; Zhang, M.; Liverani, M.; Brummaier, T.; Phommasone, K.; Perrone, C.; Pyae Phyo, A.; Sattabongkot, J.; Roobsoong, W.; Nguitragool, W.; Sen, A.; Ibna Zaman, S.; Sandar Zaw, A.; Batty, E.; Waithira, N.; Abdad, M. Y.; Blacksell, S. D.; Lubell, Y. Defining the burden of febrile illness in rural South and Southeast Asia: an open letter to announce the launch of the Rural Febrile Illness project. Wellcome Open Res. 2022, 6, 64. [CrossRef]
- Aung, A.K.; Spelman, D.W.; Murray, R.J.; Graves, S. Rickettsial Infections in Southeast Asia: Implications for Local Populace and Febrile Returned Travelers. Am. J. Trop. Med. Hyg. 2014, 91, 451–460. https://doi.org/10.4269/ajtmh.14-0191. [CrossRef]
- Mittal, G.; Ahmad, S.; Agarwal, R.K.; Dhar, M.; Mittal, M.; Sharma, S. Aetiologies of Acute Undifferentiated Febrile Illness in Adult Patients – an Experience from a Tertiary Care Hospital in Northern India. J. Clin. Diagn. Res. 2015, 9, DC22–DC24. https://doi.org/10.7860/JCDR/2015/11168.6990. [CrossRef]
- Mørch, K.; Manoharan, A.; Chandy, S.; Chacko, N.; Alvarez-Uria, G.; Patil, S.; Henry, A.; Nesaraj, J.; Kuriakose, C.; Singh, A.; Kurian, S.; Haanshuus, C.G.;, Langeland, N.; Blomberg, B.; Antony, G.V.; & Mathai, D. Acute undifferentiated fever in India: a multicentre study of aetiology and diagnostic accuracy. BMC Infect. Dis. 2017, 17, 665. [CrossRef]
- Khan, S. A.; Bora, T.; Chattopadhyay, S.; Jiang, J.; Richards, A. L.; Dutta, P. Seroepidemiology of rickettsial infections in Northeast India. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 487–494. [CrossRef]
- Khan, S. A.; Bora, T.; Laskar, B.; Khan, A. M.; Dutta, P. Scrub Typhus Leading to Acute Encephalitis Syndrome, Assam, India. Emerg. Infect. Dis. 2017, 23, 148–150.
- Richards, A.L.; Jiang, J. Scrub Typhus: Historic Perspective and Current Status of the Worldwide Presence of Orientia Species. Trop. Med. Infect. Dis. 2020, 5, 49. [CrossRef]
- Jamil, M.; Lyngrah, K. G.; Lyngdoh, M.; Hussain, M. Clinical Manifestations and Complications of Scrub Typhus : A Hospital Based Study from North Eastern India. J. Assoc. Physicians India. 2014, 62, 19–23.
- Singh, S. I.; Devi, K. P.; Tilotama, R.; Ningombam, S.; Gopalkrishna, Y.; Singh, T. B.; Murhekar, M. V. An outbreak of scrub typhus in Bishnupur district of Manipur, India, 2007. Trop Doct. 2010, 40, 169–170. [CrossRef]
- Lalmalsawma, P.; Pautu, L.; and Lalremsiama, N. Scenario of scrub typhus disease in Mizoram, Northeast India. Int. J. Curr. Adv. Res. 2017, 6, 6341–6344.
- Meerburg, B. G.; Singleton, G. R.; Kijlstra, A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009, 35, 221–270. https://doi.org/10.1080/10408410902989837. [CrossRef]
- Davis, G.E,; Austrian, R.C.; Bell, E.J. Observations on tsutsugamushi disease (scrub typhus) in Assam and Burma: the recovery of strains of Rickettsia orientalis. Am. J. Epidemiol. 1947, 46, 268–286.
- Kochhar, R. K. "Observations on scrub typhus in NEFA and North Assam." Armed Forces Med. J. India 1972, 28, 130–46.
- Gurung, S.; Pradhan, J.; Bhutia, P. Y. Outbreak of scrub typhus in the North East Himalayan region-Sikkim: an emerging threat. Indian. J. Med. Microbiol. 2013, 31, 72–74. [CrossRef]
- Khamo, V.; Aiko, V.; Kapfo W.; Hiese M. A report on the present scenario of Scrub typhus in Nagaland, India. Int. J. Med. Sci. Public. Health 2016, 5, 160–161.
- Khan, S. A.; Dutta, P.; Khan, A. M.; Topno, R.; Borah, J.; Chowdhury, P.; Mahanta, J. Re-emergence of scrub typhus in northeast India. Int. J. Infect. Dis. 2012, 16, e889–e890. [CrossRef]
- The Hindu: 5 killed in Nagaland scrub typhus outbreak. Available online: https://www.thehindu.com/news/national/other-states/5-killed-in-nagaland-scrub-typhus-outbreak/article32647398.ece (accessed on 28 July 2023).
- Nagaland Post Scrub typhus kills 3 in Manipur village. Available online: https://nagalandpost.com/index.php/scrub-typhus-kills-3-in-manipur-village/ (accessed on 28 July 2023).
- Lalchhandama, K. "The saga of scrub typhus with a note on the outbreaks in Mizoram". Sci. Vis. 2018, 18, 50–57. https://doi.org/10.33493/scivis.18.02.01. [CrossRef]
- Min, K.-D.; Lee, J.-Y.; So, Y.; Cho, S.-i. Deforestation Increases the Risk of Scrub Typhus in Korea. Int. J. Environ. Res. Public. Health 2019, 16, 1518. https://doi.org/10.3390/ijerph16091518. [CrossRef]
- Alkathiry, H.; Al-Rofaai, A.; Ya’cob, Z.; Cutmore, T.S.; Mohd-Azami, S.N.I.; Husin, N.A.; Lim, F.S.; Koosakulnirand, S.; Mahfodz, N.H.; Ishak, S.N.; et al. Habitat and Season Drive Chigger Mite Diversity and Abundance on Small Mammals in Peninsular Malaysia. Pathogens 2022, 11, 1087. https://doi.org/10.3390/pathogens11101087. [CrossRef]
- Chen, Y.-L.; Guo, X.-G.; Ding, F.; Lv, Y.; Yin, P.-W.; Song, W.-Y.; Zhao, C.-F.; Zhang, Z.-W.; Fan, R.; Peng, P.-Y.; et al. Infestation of Oriental House Rat (Rattus tanezumi) with Chigger Mites Varies along Environmental Gradients across Five Provincial Regions of Southwest China. Int. J. Environ. Res. Public. Health 2023, 20, 2203. https://doi.org/10.3390/ijerph20032203. [CrossRef]
- Nallan, K.; Rajan, G.; Sivathanu, L.; Devaraju, P.; Thiruppathi, B.; Kumar, A.; Rajaiah, P. Molecular Detection of Multiple Genotypes of Orientia tsutsugamushi Causing Scrub Typhus in Febrile Patients from Theni District, South India. Trop. Med. Infect. Dis. 2023, 8, 174. https://doi.org/10.3390/tropicalmed8030174. [CrossRef]
- Pautu, L.; Lalmalsawma, P.; Vanramliana ; Rosangkima, G.; Malvi, Y. SCRUB TYPHUS DISEASE OUTBREAK AT CHEURAL VILLAGE, LAWNGTLAI DISTRICT, MIZORAM, NORTH-EAST INDIA. . J. Adv. Sci. Res. 2022, 13, 12–16. [CrossRef]
- Borkakoty, B.; Jakharia, A.; Biswas, D.; Mahanta, J. Co-infection of scrub typhus and leptospirosis in patients with pyrexia of unknown origin in Longding district of Arunachal Pradesh in 2013. Indian J Med Microbiol. 2016, 34, 88–91. [CrossRef]
- Dass, R.; Deka, N. M.; Duwarah, S. G.; Barman, H.; Hoque, R.; Mili, D.; Barthakur, D. Characteristics of pediatric scrub typhus during an outbreak in the North Eastern region of India: peculiarities in clinical presentation, laboratory findings and complications. Indian. J. Pediatr. 2011 , 78, 1365–1370. [CrossRef]
- Lurshay, R.M.; Gogoi, P.R.; Deb, S. Clinico-laboratory profile of severe pediatric scrub typhus. Sch. J. App Med. Sci. 2016, 4, 3714–3720.
- Gupta, N.; Mittal, V.; Gurung, B.; Sherpa, U. Pediatric Scrub typhus in South Sikkim. Indian. Pediatr. 2012, 49, 322–324.
- Jakharia, A.; Borkakoty, B.; Biswas, D.; Yadav, K.; Mahanta, J. Seroprevalence of Scrub Typhus Infection in Arunachal Pradesh, India. Vector Borne Zoonotic Dis. 2016, 16, 659–663. [CrossRef]
- Kala, D.; Gupta, S.; Nagraik, R.; Verma, V.; Thakur, A.; Kaushal, A. Diagnosis of scrub typhus: recent advancements and challenges. 3 Biotech. 2020 ,10, 396. [CrossRef]
- Huidrom S, Singh LK. Clinical and laboratory manifestations of scrub typhus: A study from a tertiary care hospital in Manipur Indian. J. Microbiol. Res. 2017, 4, 434–436.
- Yoo, J. S.; Kim, D.; Choi, H. Y.; Yoo, S.; Hwang, J. H.; Hwang, J. H.; Choi, S. H.; Achangwa, C.; Ryu, S.; Lee, C. S. Prevalence Rate and Distribution of Eschar in Patients with Scrub Typhus. Am. J. Trop. Med. Hyg. 2022 ,106, 1358–62. [CrossRef]
- Mahajan, S. K., Rolain, J. M., Kashyap, R., Bakshi, D., Sharma, V., Prasher, B. S., Pal, L. S., & Raoult, D. Scrub typhus in Himalayas. Emerg. Infect. Dis. 2006, 12, 1590–2.
- Varghese, G. M., Janardhanan, J., Trowbridge, P., Peter, J. V., Prakash, J. A., Sathyendra, S., Thomas, K., David, T. S., Kavitha, M. L., Abraham, O. C., & Mathai, D. Scrub typhus in South India: clinical and laboratory manifestations, genetic variability, and outcome. Int. J. Infect. Dis. 2013 , 17, e981-7. [CrossRef]
- Pokhrel, A.; Rayamajhee, B.; Khadka, S.; Thapa, S.; Kapali, S.; Pun, S.B.; Banjara, M.R.; Joshi, P.; Lekhak, B.; Rijal, K.R. Seroprevalence and Clinical Features of Scrub Typhus among Febrile Patients Attending a Referral Hospital in Kathmandu, Nepal. Trop. Med. Infect. Dis. 2021, 6, 78. [CrossRef]
- Dhar, D.; Choudhury, K. A Prospective Analysis of Seroprevalence and Demographic Profile of Scrub Typhus in a Tertiary Care Hospital of South Eastern Assam. Int. J. Res. Rev. 2020, 7, 179–182.
- Debnath, P.; Debbarma, R.K.; Debbarma, D. Clinical Profile of Scrub Typhus: A Cross Sectional Study in a Tertiary Care Centre of Tripura. Inter. J. Dent. Med. Sci. Res. 2021, 3, 302–305.
- Ngulani, K.S.; Kakati, S.; Hussain, S.; Singh, M.U.; Kaguilan, K. Outbreak of scrub typhus in Manipur - experience at a tertiary care hospital. IOSR J. Dent. Med. Sci. 2019, 18, 33–8.
- Bora, T.; Khan, S. A.; Jampa, L.; Laskar, B. Genetic diversity of Orientia tsutsugamushi strains circulating in Northeast India. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 2–30. [CrossRef]
- Jamil, M.; Bhattacharya, P.; Mishra, J., Akhtar, H.; Roy, A. Eschar in Scrub Typhus: A Study from North East India. J. Assoc. Physicians India 2019, 67, 38–40.
- Sivarajan, S.; Shivalli, S.; Bhuyan, D.; Mawlong, M.; Barman, R. Clinical and paraclinical profile, and predictors of outcome in 90 cases of scrub typhus, Meghalaya, India. Infect. Dis. Poverty 2016, 5, 91. [CrossRef]
- Jamil, M. D.; Hussain, M.; Lyngdoh, M.; Sharma, S.; Barman, B.; Bhattacharya, P. K. Scrub typhus meningoencephalitis, a diagnostic challenge for clinicians: A hospital based study from North-East India. J. Neurosci. Rural. Pract. 2015, 6, 488–493. [CrossRef]
- Sharma, S. R.; Masaraf, H.; Lynrah, K. G.; Lyngdoh, M. Tsutsugamushi Disease (Scrub Typhus) Meningoencephalitis in North Eastern India: A Prospective Study. Ann. Med. Health Sci. Res. 2015, 5, 163–7.
- Palani, M.T.; Mishra, M.N.; Odyuo, B.T.S.; Mishra, P.; Abraham, S. Infection pattern and Laboratory Profile of Scrub Typhus in A Secondary Care Centre from North-East India. J. Infect. Pathol, 2022, 5, 2.
- Vanlalruati, R.S.C.; Lalhmingmawii, S.R.; Lallawmkima, I.; Lalthantluanga, B.; Renthlei, L.; Pautu, L. Serological Evidence of Scrub Typhus in Mizoram, North Eastern Region of India. Infect. Dis. Clin. Microbiol. 2022, 4, 55–61. [CrossRef]
- Khan, S.A.; Saikia, J.; Bora, T.; Khamo, V.; Rahi, M. Molecular detection of Orientiatsutsugamushi strains circulating in Nagaland. Indian. J. Med. Microbiol. 2022, 40, 443–445. [CrossRef]
- Khan, S.A.; Murhekar, M.V.; Bora, T.; Kumar, S.; Saikia, J.; Kamaraj, P.; Sabarinanthan, R. Seroprevalence of Rickettsial Infections in Northeast India: A Population-Based Cross-Sectional Survey. Asia Pac. J. Public. Health. 2021, 33, 516–522. [CrossRef]
- Durairaj, E.; Lynrah, K.; Lyngdoh, C.J.; Barman, H.; Lyngdoh, W.V.; Paul, D.Performance Analysis and Evaluation of Quantitative Real Time PCR for Diagnosis of Scrub Typhus in North-East India. IJTDH.2021, 42, 24-33. [CrossRef]
- Singh, K.R.; Singh, C.G.; Khumukcham, S.; Marak, N.D.; Kumar, P.; Parasmani, H.; Kharshiing, G.A. Scrub typhus: An unusual cause of acute abdomen. J. Med. Soc. 2019, 33, 43–46.
- Lalrinkima, H.; Lalremruata, R.; Lalchhandama, C.; Khiangte, L.; Siamthara, F. H.; Lalnunpuia, C., Borthakur, S. K., & Patra, G. (2017). Scrub typhus in Mizoram, India. J. Vector Borne Dis. 2017, 54, 369–371.
- Mohan, D.G.; Teronpi,T.; Sharma, A.; Dey, S. “Scrub typhus”: One of the leading cause of febrile illness: A report from a tertiary care centre. IJMHR. 2018, 4, 76–78.
- Varghese, G. M.; Janardhanan, J.; Mahajan, S.K.; Tariang, D.; Trowbridge, P.; Prakash, J.A.; David, T.; Sathendra, S.; Abraham, O.C. Molecular epidemiology and genetic diversity of Orientia tsutsugamushi from patients with scrub typhus in 3 regions of India. Emerg. Infect. Dis. 2015, 21, 64–69.
- Singh, S.K.; Ram, R.; Marangmei, L.; Chakma, G. Case Series on Scrub Typhus from a tertiary care hospital of North East India. IOSR J. Dent. Med. Sci. 2014, 13, 62–64. [CrossRef]
- Basheer, A.; Iqbal, N.; Mookkappan, S.; Anitha, P.; Nair, S.; Kanungo, R.; Kandasamy, R. Clinical and Laboratory Characteristics of Dengue-Orientia tsutsugamushi co-Infection from a Tertiary Care Center in South India. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016028. https://doi.org/10.4084/MJHID.2016.028. PMID: 27413521; PMCID: PMC4928539. [CrossRef]
- Baruah, M.K.; Sharma, J. First report of scrub typhus cases in Dhemaji, Assam, India. South. Indian. J. Biol. Sci. 2015, 1, 156–157.
- Wilairatana, P.; Kuraeiad, S.; Rattaprasert, P.; Kotepui, M. Prevalence of malaria and scrub typhus co-infection in febrile patients: a systematic review and meta-analysis. Parasit. Vectors. 2021, 14, 471. [CrossRef]
- Md-Lasim, A.; Mohd-Taib, F.S.; Abdul-Halim, M.; Mohd-Ngesom, A.M.; Nathan, S.; Md-Nor, S. Leptospirosis and Coinfection: Should We Be Concerned? Int. J. Env. Res. Public. Health.2021, 18, 9411. [CrossRef]
- Gupta, N.; Mittal, V.; Gurung, B.; Sherpa, U. Pediatric Scrub typhus in South Sikkim. Indian. Pediatr. 2012, 49, 322–324.
- Lv. Y.; Guo, X.G.; Jin, D.C. Research Progress on Leptotrombidium deliense. Korean J. Parasitol. 2018, 56, 313–324. [CrossRef]
- Sharma, A.K. Entomological surveillance for rodent and their ectoparasites in Scrub typhus affected areas of Meghalaya, (India). J. Entomol. Zool.2013, 1, 27-29.
- Lalthazuali; Sharma, A.K.; Thomas, T.G.; Bhan, S.; Bhadauriya, A.S.; Ramteke, P.U.; Kumawat, R.; Singh, R. Entomological survey of vectors of Scrub typhus in Haulawng, Lunglei district, Mizoram, India. J. Commun. Dis. 2020, 52, 69–73.
- Devasagayam, E.; Dayanand, D.; Kundu, D.; Kamath, M.S.; Kirubakaran, R.; Varghese, G.M. The burden of scrub typhus in India: A systematic review. PLoS Negl. Trop. Dis. 2021,15, 9619. [CrossRef]
- Dey, R.K.; Imad, H.A.; Aung, P.L.; Faisham, M.; Moosa, M.; Hasna, M.; Afaa, A.; Ngamprasertchai, T.; Matsee, W.; Nguitragool, W.; Nakayama, E.E.; Shioda, T.Concurrent Infection with SARS-CoV-2 and Orientia tsutsugamushi during the COVID-19 Pandemic in the Maldives. Trop. Med. Infect. Dis. 2023, 8, 82. [CrossRef]
- Luce-Fedrow, A.; Lehman, M.L.; Kelly, D.J.; Mullins, K.; Maina, A.N.; Stewart, R.L.; Ge, H.; John, H.S.; Jiang, J.; Richards, A.L. A Review of Scrub Typhus (Orientia tsutsugamushi and Related Organisms): Then, Now, and Tomorrow. Trop. Med. Infect. Dis. 2018, 3, 8. [CrossRef]
- Subbalaxmi, M.V., Madisetty, M.K., Prasad, A.K., Teja, V.D., Swaroopa, K., Chandra, N., Upadhyaya, A.C., Shetty, M., Rao, M.N., Raju, Y.S.; Lakshmis, V. Outbreak of scrub typhus in Andhra Pradesh--experience at a tertiary care hospital. J. Assoc. Physicians India,2014, 62, 490-496.
- Blacksell, S.D.; Bryant, N.J.; Paris, D.H.; Doust, J.A.; Sakoda, Y.; Day, N.P.J. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin. Infect. Dis. 2007, 44, 391–401. [CrossRef]
- Lee, K.D.; Moon, C.; Oh, W.S.; Sohn, K.M.; Kim, B.N. Diagnosis of scrub typhus: introduction of the immunochromatographic test in Korea. Korean J. Intern. Med. 2014, 29, 253–255. [CrossRef]
- Blacksell, S.D.; Paris, D.H.; Chierakul, W.;Chierakul, W.;Wuthiekanun, V.; Teeratakul, A.; Kantipong, P.;Day, N.P.J.Prospective evaluation of commercial antibody-based rapid tests in combination with a loop-mediated isothermal amplification PCR assay for detection of Orientia tsutsugamushi during the acute phase of scrub typhus infection. Clin. Vaccine. Immunol.2012, 19, 391. [CrossRef]
- Blacksell, S.D.; Jenjaroen, K.; Phetsouvanh, R.; Tanganuchitcharnchai, A.; Phouminh, P.; Phongmany, S.; Day, N.P.;Newton, P.N.Accuracy of rapid IgM-based immunochromatographic and immunoblot assays for diagnosis of acute scrub typhus and murine typhus infections in Laos. Am. J. Trop. Med. Hyg. 2010, 83, 365–369. [CrossRef]
- Rahi, M.; Gupte, M.D.; Bhargava, A.; Varghese, G.M.; Arora, R. DHR-ICMR guidelines for diagnosis and management of rickettsial diseases in India. Indian. J. Med. Res. 2016, 417-422.
- Palani, M.T.; Mishra, M.N.; Odyuo, B.T.S.; Mishra, P.; Abraham, S. Infection pattern and Laboratory Profile of Scrub Typhus in A Secondary Care Centre from North-East India. J. Infect. Pathol.2022, 5, 149.
- Lallawmkima, I.; Vanlalruati, R.S.C.; Chongthu, J.L.; Renthlei, L. Scrub Typhus with Multi-Organ Dysfunction Syndrome and Immune Thrombocytopenia: A Case Report.IDCM. 2022, 4, 133–136. [CrossRef]
- Mohanty. A.; Gupta, P.; Singh, T.S; Gupta, P. Scrub typhus with a rare presentation in a child- a case report . J. Med. Sci. Clin. Res. 2017, 5, 19968–19970.
- Mohanty, A.; Kabi, A; Gupta, P.; Jha, M.K.; Rekha, U.S.; Raj, A.K. Scrub typhus - A case series from the state of Sikkim, India. Int. J. Crit. Illn. Inj. Sci. 2019, 9, 194–198.
- Mangaraj, J.; Sharma, A.; Alam, S.T. Report of sporadic cases of scrub typhus–A threat to re-emergence in Assam, India. Int. J. Curr. Microbiol. Appl. Sci.2017, 6, 1037-41. [CrossRef]
- Biradar, C.; Rama, S.K.; Nizami, A.I.; Handique, F.; Halemani, C. Soldiers from Indo Myanmar Border Presenting with Scrub Typhus.J. Dent. Med. Sci.2015, 14, 123-127.
- Tony, E.; Roy, A.; Barman, B.; Lynrah, K.; Komut, O.; Khonglah, Y. Hemophagocytic Syndrome Associated with Scrub Typhus: A Case Report from North East India. J. Clin. Case Rep.2015, 5, 10.
- Karim, H.M.R.; Bhattacharyya, P.; Yunus, M. A Case Report of an unusual presentation: Could the early use of interventional radiology have saved this patient of scrub typhus. Bangladesh Crit. Care J.2014, 2, 79-80. [CrossRef]
- Dhakal, M.; Dhakal, O.P.; Bhandari, D. Pancreatitis in scrub typhus: a rare complication. BMJ Case Rep.2014, bcr2013201849. [CrossRef]
- Sawale, V.; Upreti, S.; Singh, T.S.; Singh, N.; Singh, T.B. A rare case of Guillain-Barre syndrome following scrub typhus. Neurol. India.2014, 62, 82. [CrossRef]
- Goswami, D.; Hing, A.; Das, A.; Lyngdoh, M. Scrub typhus complicated by acute respiratory distress syndrome and acute liver failure: a case report from Northeast India. Int. J. Infect. Dis.2013, 17, e644-e645. [CrossRef]
- Dutt Y, Dhiman R, Singh T, Vibhuti A, Gupta A, Pandey RP, Raj VS, Chang CM, Priyadarshini A. The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches. Antibiotics (Basel). 2022 Jul 11;11(7):930. [CrossRef]
- Hardesh K. Maurya, H. K. Maurya, Ramendra Pratap, R. Pratap, Abhinav Kumar, A. Kumar, Brijesh Kumar, B. Kumar, Volker Huch, V. Huch, Vishnu K. Tandon, V. K. Tandon, & Vishnu Ji Ram, V. Ji Ram. (0000). A carbanion induced ring switching synthesis of spiranes: an unprecedented approach. RSC advances, 2, 9091-9099. [CrossRef]
- A carbanion induced ring switching synthesis of spiranes: an unprecedented approach HK Maurya, R Pratap, A Kumar, B Kumar, V Huch, VK Tandon, VJ Ram RSC advances 2 (24), 9091-9099.
- Physico-chemical properties of starch and protein and their relation to grain quality and nutritional value of rice, PK Mishra, R Pandey, Rice Breed. Pub. IRRI, Los Banos, Phillippines, 389-405.
- INFLUENCE OF FERTILITY LEVELS, VARIETIES AND TRANSPLANTING TIME ON RICE (ORYZA-SATIVA), R Pandey, MM Agarwal Indian Journal of Agronomy 36 (4), 459-463.
- Pandey RP, Mukherjee R, Priyadarshini A, Gupta A, Vibhuti A, Leal E, Sengupta U, Katoch VM, Sharma P, Moore CE, Raj VS, Lyu X. Potential of nanoparticles encapsulated drugs for possible inhibition of the antimicrobial resistance development. Biomed Pharmacother. 2021 Sep;141:111943. [CrossRef]
| Region | Outbreak/ study Duration | Age in months/ Years |
Diagnostic confirm cases | Method of diagnosis | Antibody/marker gene | Fatalities | Affected groups/Outcome/Remarks | References |
|---|---|---|---|---|---|---|---|---|
| Lawngtlai District, Mizoram | December , 2019 | Mean age was 40 years | 80 | WFT | OXK | NA | Affected group: adults (31-40 age group), farmers, female, rural areas. | (Pautu et al. 2022) [51] |
| Assam | April 2017 to March 2018 | 3 to 85 yrs (mean age 39.6 yrs) | 56 | ICT | IgG, IgM, IgA | NA | Liver function evaluation and proteinuria attained statistical significance. | (Palani et al. 2022)$ [71] |
| Mizoram | October 1, 2018 to September 31, 2019 | 7 months to 94 years | 36 | ICT, ELISA |
IgG, IgM, IgA IgM |
NA | The sensitivity of IgM ELISA and Rapid test were 44.19% and 46.40% respectively. 36 positive for both ICT and ELISA. |
(Vanlalruati et al. 2022) # [72] |
| Nagaland | December 2018 | NA | 2 (pcr) 87 (WFT)= 89 |
PCRWFT | 56 kDa OXK |
NA | Closest homology with the prototype strain TA763 (endemic in Thailand and Taiwan). | (Khan et al. 2022) [73] |
| Manipur | August 2017 to December 2018 Peak season : July to November |
≥12 years Mean age: 41 years (±16) |
176 | ICT | IgG, IgM, IgA | 8 | Affected group: rural areas, farmers, and adults. | (Ngulani et al. 2021) $ [65] |
| Assam, Meghalaya, and Tripura |
September 2017 to February 2018 | 3 to 45 years | 18 | ELISA | IgG | NA | Community based studies Documented low prevalence (0.76%) of scrub typhus. |
(Khan et al. 2021)# [74] |
| Meghalaya | June 2019 to May 2020 | NA | 37 | WFT ELISA PCR |
OXK IgM 56 kDa |
NA | 14% OXK positive 34 % ELISA positive 50% PCR positive (27/54) |
(Durairaj et al. 2021) # [75] |
| Tripura | January 2018 to December 2018 Peak season: September |
Mean age 28 to 50 years. | 40 | NA | NA | 1 | Deranged liver function and thrombocytopenia significant. | (Debnath et al. 2021)# [64] |
| Assam | January 2017 to September 2017 |
≥11 yearsMean age: 20-29 years | 14 | ELISA | IgM | NA | Prevalence rate of Scrub typhus: 20%. | (Dhar et al. 2020)# [63] |
| Manipur | August 2017 to November 2018 | ≥20 years | 8 | WFT ELISA |
OXK IgM |
1 | Scrub typhus Presenting as Acute Abdomen case. | (Singh et al. 2019) # [76] |
| Meghalaya | January 2013 to December 2015 | ≥ 18 years | 129 | ICT WFT ELISA |
IgM OXK IgM |
NA | Eschar was found in 24.8% of the scrub typhus patients. | (Jamil et al. 2019) $ [67] |
| Assam and Arunachal | December 2014 to December 2016. Peak season: May to August |
3 to 80 years | 278 | ELISA PCR |
IgM, IgG, 47 KDa, 56 KDa, 16S rRNA |
NA | Pre-dominance of Karp-like strains. | (Bora et al. 2018) [66] |
| Assam | NA | 1- >60years Mean age: 25-36 yrs |
33 | ELISA | IgM | NA | Prevalence rate of scrub typhus: 22.2% | (Mohan et al. 2018) [78] |
| Mizoram | January 2012 to July 2017 Peak season: July and November |
2 months -85 years Mean age:34 years |
907 | ICT | IgM, IgG and IgA | 34 | Age group 20-30 years were affected more. The youngest patient recorded was a 2 months old male. Male affected more. |
(Lalmalsawma, et al. 2017 ) [37] |
| Manipur | January 2016 to May 2017 | 6 to 67 years | 24 | ICT | IgM, IgG and IgA | NA | Affected group: Rural background. One patient was Dengue positive |
(Huidrom et al.2017) ^* [58] |
| Tezpur, Assam | April 2011–November 2012 | ≥5 years | 75 | IFA ELISA |
IgM | NA | Affected group: Rural areas Scrub typhus co-infection observed. |
(Mørch et al. 2017)* [31] |
| Dibrugarh, Assam | 2013–2015 Peak season: July–September |
3 to 80 years | 104 | ELISA PCR |
IgM 56-kDa |
26 | 13 (12.5%) co-infected with JEV IgM. Resemblances of Karp strain. Occupation= NA High case-fatality rate of 49% (26/53 follow-up) |
(S A Khan et al. 2017) $ * [33] |
| Aizawl, Mizoram | October 2014 to December 2016. Peak season: November to February and September to October (autumn) |
1 to ≥60 years | 283 | ICT | IgM | NA | 21–30 age groups were most affected. Prevalence of ST 6.9%. |
(Lalrinkima et al. 2017) # [77] |
| Nagaland | 2014 | - | 31 | ELISA | IgM | NA | Community based studies. 7.2% samples positive by IgM ELISA. |
(Khamo et al. 2016) [42] |
| Meghalaya | January 2014 to December 2014 | Below 18 years | 75 | ICT | IgM, IgG and IgA | 1 | Inclusion: children below 18 years. Antibiotics data not available. |
(Lurshay et al. 2016) [54] |
| Arunachal Pradesh | 2009 to 2013 | 2– 80 years | 121 | ELISA | IgG | NA | Community based studies. Farmers, adults, females were affected more. Seroprevalence was 40.3%. |
(Jakharia et al. 2016) $ [56] |
| Meghalaya | September 2011 to August 2012 Peak season: September to December |
≥ 18 years Mean age: 21–30 years |
90 | ICT | IgM, IgG and IgA | 5 | Farmer, 21–30 years were more affected. 13.6 % ST positive. Malaria co-infection observed in two patients. |
(Sivarajan et al. 2016) * + [68] |
| Arunachal Pradesh | NA | 0 - ≥ 15 years | 30 | ELISA | IgM | NA | 25% cases were co-infected with leptospira. | (Borkakoty et al. 2016)* [52] |
| Assam, Arunachal, Nagaland |
2013 to 2015 | - | 390 | ELISA PCR |
IgM 56-kDa |
NA | Community based studies 30.8% positive for scrub typhus. Sequence analysis revealed Karp-like strains from Thailand, Taiwan and Vietnam. |
( Khan et al. 2016)* [32] |
| Meghalaya | October 2009 to November 2011 | 19 to 68 years | 23 (meningitis) | WFT ELISA |
OX K IgM |
NA | Scrub meningitis cases. Majority of patients were either farmers or housewives. |
(Sharma et al. 2015) # [70] |
| Meghalaya | January 1, 2013 to December 31, 2014. Peak season: October to November |
Above 12 years Mean age: 34.84 ± 16.21 years |
113 | WFT ICT |
OXK IgM |
2 | 13.2% featured with meningitis/meningoencephalitis. 0.07% had multiple eschar. |
(Jamil et al. 2015)# [69] |
| Meghalaya | September 2010 to August 2012 | Mean age 40 ± 12 years | 96 | ELISA PCR |
IgM 56-kDa |
NA | Kato (Hualien-13) closest to Cambodia and Neimang-65 strains were observed. Karp and Gilliam like strains were also observed. |
(Varghese et al. 2015) [79] |
| Meghalaya | 7th January 2013 to 6th January 2014 Peak season: September to November |
≥ 18 years | 61 | WFT ICT |
OXK IgM, IgG and IgA |
5 | 18 to 30 year’s age group was affected. 4.9% scrub typhus patients’ pregnant women were treated with azithromycin. |
(Jamil et al. 2014)# +* [35] |
| Manipur | July to October | 15-65 years | 6 | ICT | IgM, IgG and IgA | NA | Affected group: rural areas. 2 scrub typhus patients had eschar |
(Singh et al. 2014) [80] |
| Sikkim | January 2011 to December 2011 Peak season: July and October |
Above 2 years | 63 | WFT ICT ELISA |
OXK IgA, IgM, and IgG IgM |
1 | Eschar was seen in 10 pediatric patients. 55.56% had pedal edema. 30.8% scrub typhus positive for IgM ELISA. |
(Gurung et al. 2013) + [41] |
| Assam, Arunachal Pradesh, and Nagaland | 2010 to 2011 | NA | 108 | ELISA PCR |
IgM 56-kDa |
NA | 34.39% scrub typhus positive for IgM ELISA. 15.38% were PCR-positive for ST. |
(Khan et al. 2012) [43] |
| Meghalaya | October 2009 to January 2010 | Average age: 9.4 years | 24 | WFT | OXK | NA | Pediatric scrub typhus cases. | (Dass et al. 2011) [53] |
| Manipur | 2007 | 0 to ≥ 45 years | 38 | WFT | OXK | 2 | 71% received treatment from traditional healers. 55% had eschar on the perineal area. |
(Singh et al. 2010) [36] |
| Region | Cases | Diagnosis | Treatment | Fatalities | References |
|---|---|---|---|---|---|
| Mizoram | 1 | IgM ELISA | Ceftriaxone-sulbactam Doxycycline Azithromycin |
- | (Lallawmkima et al. 2022) [99] |
| Sikkim | 3 | WFT IgM ELISA |
Ceftriaxone^, Amiodarone^ Doxycycline + |
- | (Mohanty et al. 2019) [100] |
| Sikkim | 1 | WFT, ELISA |
Ceftriaxone^ +, Doxycycline + |
- | (Mohanty et al. 2017) [101] |
| Assam | 3 | WFT | Cefotaxime, Doxycycline Derriphylline |
- | (Mangaraj et al, 2017) [102] |
| Nagaland | 2 | ICT | Doxycycline | - | (Biradar et al. 2015) [103] |
| Assam | 3 | ELISA | NA | - | ( Baruah et al.2015) * [81] |
| Meghalaya | 1 | ICT | Ceftriaxone, Doxycycline, Azithromycin |
- | (Ete et al. 2015) [104] |
| Meghalaya | 1 | WFT | Inj. Artesunate, Inj.Acyclovir and Inj. Ceftriaxone^ Tab. Doxycycline Pantoprazole Inj. Dobutamine Inj. Azithromycin |
1 | (Karim et al. 2014) [105,112] |
| Manipur | 1( Guillain-Barre syndrome | ICT | Doxycycline | - | (Sawale et al. 2014) [107] |
| Sikkim | 1 | WFT, ICT, ELISA |
Cefotaxime, Doxycycline |
- | (Dhakal et al. 2014) [106] |
| Meghalaya | 1 | WFT | Doxycycline, Ceftriaxone Chloramphenicol. | - | (Goswami et al. 2013) [108,109] |
| Sikkim | 5 | WFT ELISA |
Doxycycline+ Ceftriaxzone+ |
1 | (Gupta et al. 2012) [85,113,114] |
| Region | Host | Ectoparasites | Outcome | References |
|---|---|---|---|---|
| Mizoram | Rodent Species | Mites (chigger) | Reactive with any one or all of PROGEN OXK, OX19 or OX2. Chigger (mite) index =19.2 |
[52] |
| Mizoram | Rodent Species | Mites, Lice, Fleas | Chigger (mite) index =12.33 Chigger infestation rate=18.5 |
[88] |
| Assam, Arunachal Pradesh and Nagaland | Rodents, dogs, cats, cattle, and human | Ticks, mites, fleas and lice | NA for ST(scrub typhus) Positive for spotted fever group Rickettsia species. |
[32] |
| Meghalaya | Rodent Species | Ticks, mites, fleas and lice | Chigger (mite) index =1.8 Chigger infestation rate=4.8 |
[87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





