Submitted:
31 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Rice cultivation and sampling
2.2. Measurement of nitrogenase activity
2.3. Measurements of sucrose and glucose concentrations
2.4. Evaluation of endophytic bacterial microbiota
2.5. Quantification of bacteria read/chloroplast read and nifH/bacterial 16S rRNA gene
3. Results
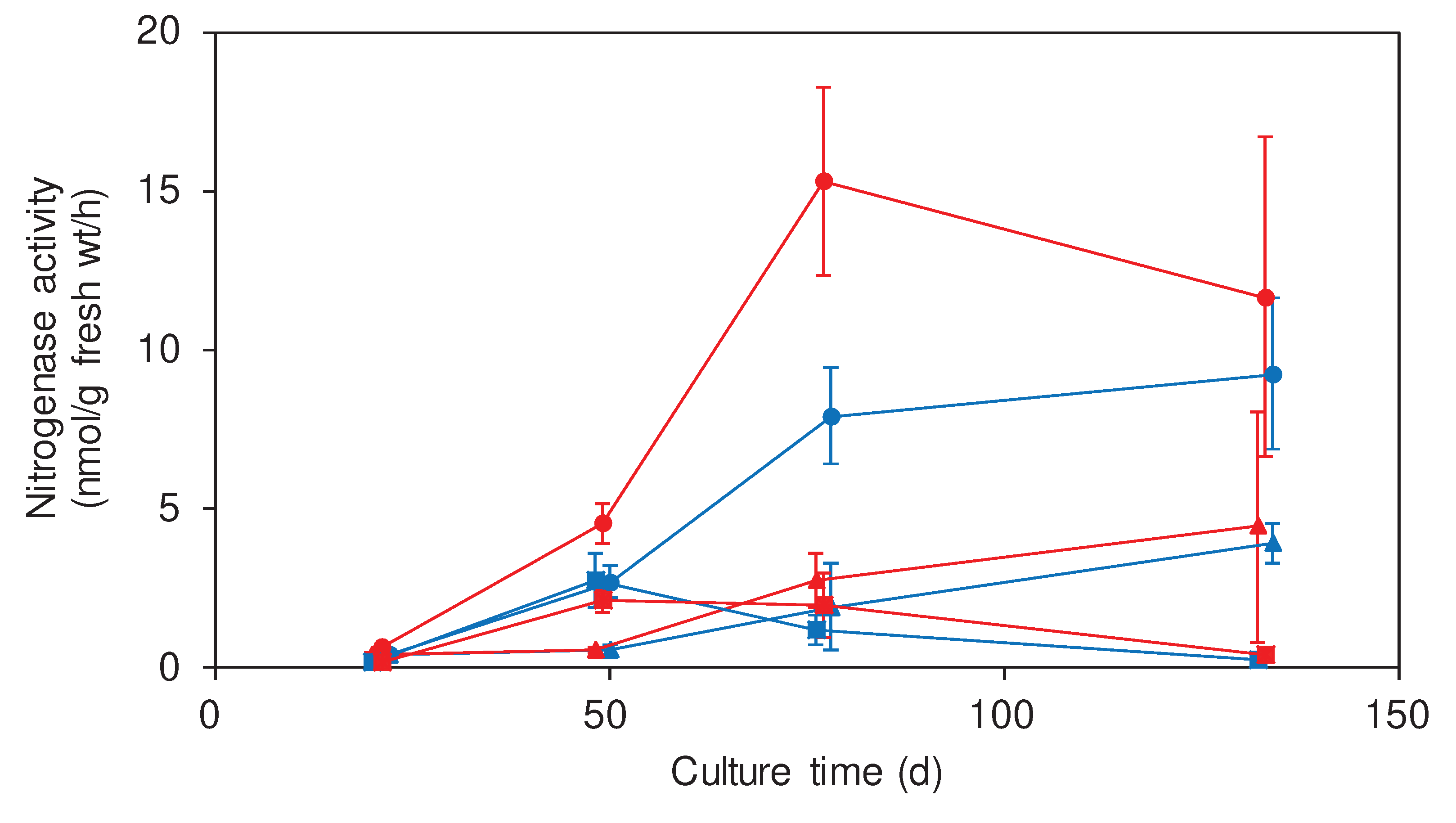
3.1. Seasonal changes of bacterial nitrogenase activity in the rice parts
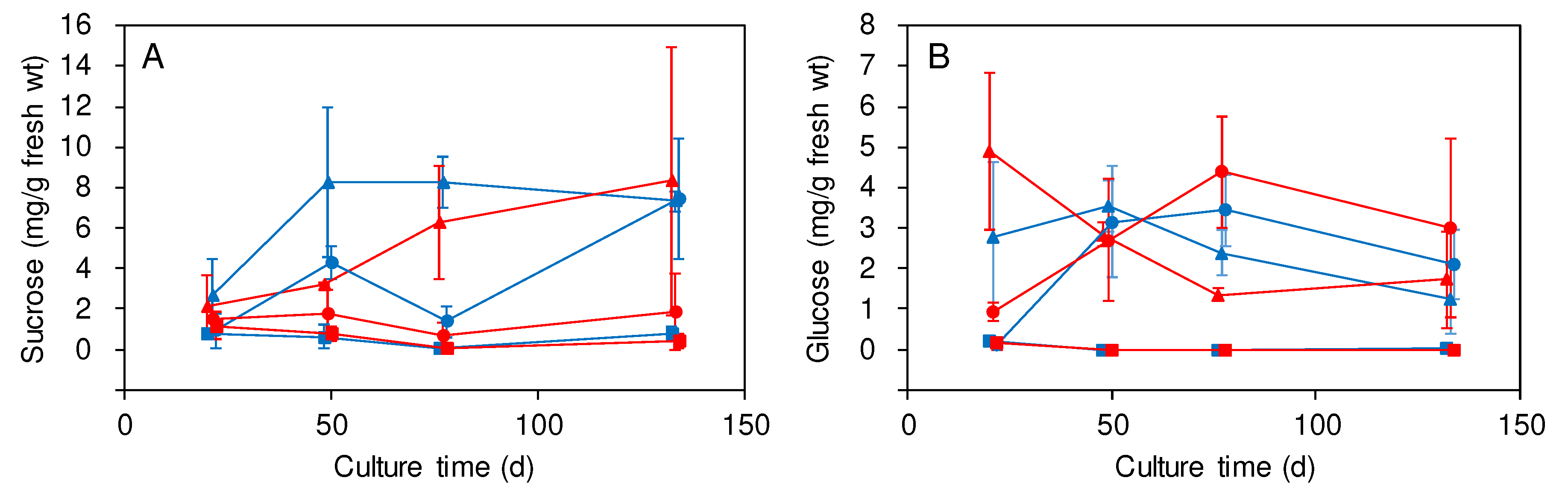
3.2. Seasonal changes of sucrose and glucose contents in the rice parts
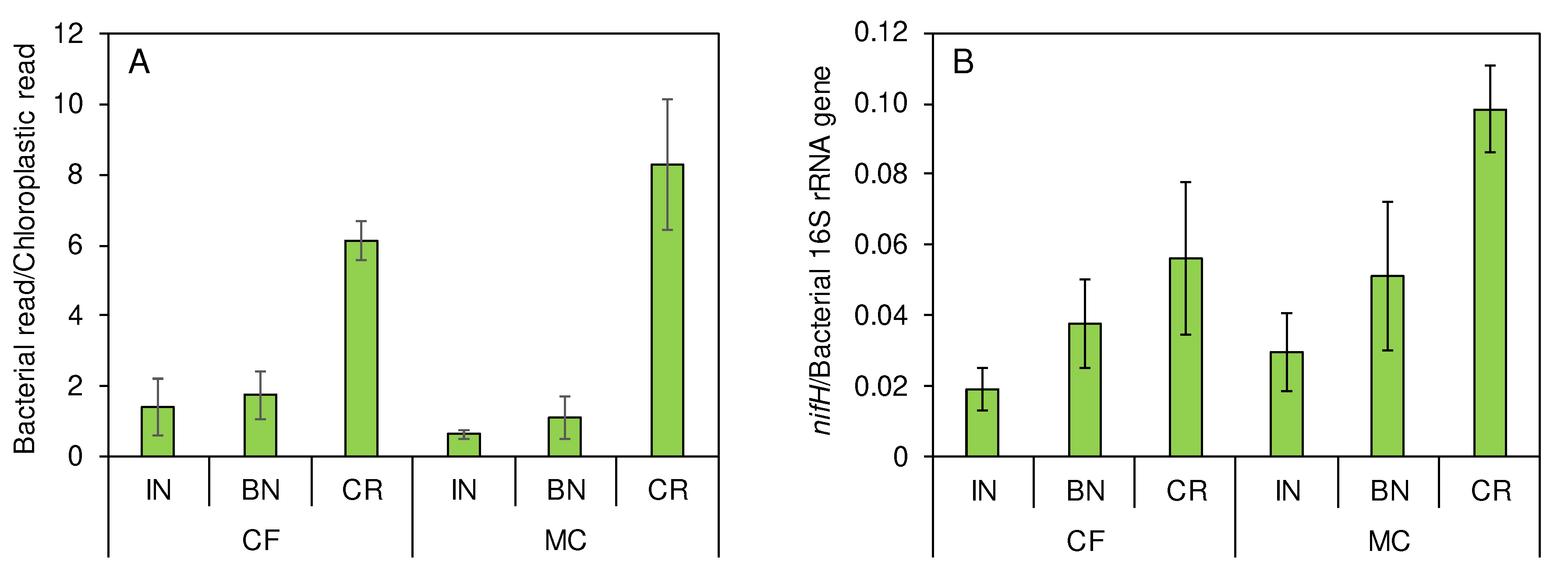
3.3. Bacterial and nifH abundances in the rice parts at panicle initiation
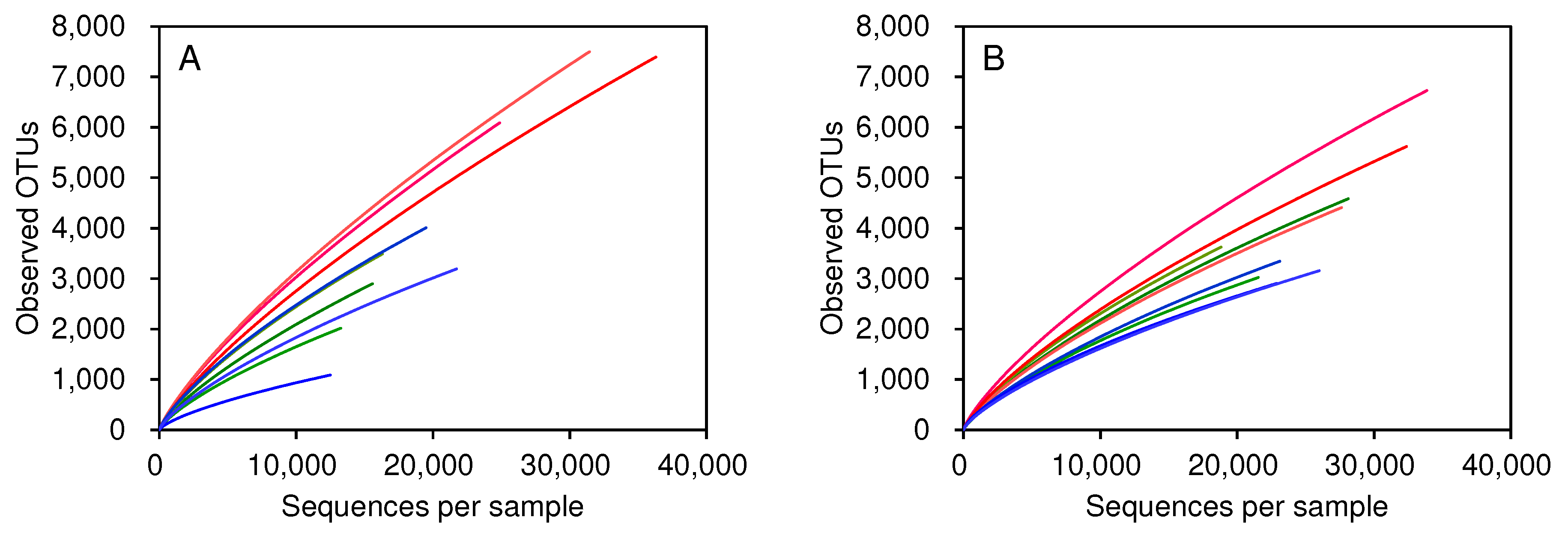
3.4. Evaluation of bacterial microbiota in the rice parts at panicle initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodwig, E.M.; Hosie, A.H.; Bourdes, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Downie, J.; Poole, P.S. Amino-acid cycling drives nitrogen fixation in the legume–Rhizobium symbiosis. Nature 2003, 422, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Terakado-Tonooka, J.; Bao, Z.; Minamisawa, K. Molecular analyses of the distribution and function of diazotrophic rhizobia and methanotrophs in the tissues and rhizosphere of non-leguminous plants. Plants 2019, 8, 408. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS biology 2018, 16, e2006352. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Terakado-Tonooka, J.; Minamisawa, K. Exploration of bacterial N2-fixation systems in association with soil-grown sugarcane, sweet potato, and paddy rice: a review and synthesis. Soil Science and Plant Nutrition 2017, 63, 578–590. [Google Scholar] [CrossRef]
- Ikeda, S.; Sasaki, K.; Okubo, T.; Yamashita, A.; Terasawa, K.; Bao, Z.; Liu, D.; Watanabe, T.; Murase, J.; Asakawa, S. Low nitrogen fertilization adapts rice root microbiome to low nutrient environment by changing biogeochemical functions. Microbes and environments 2014, 29, 50–59. [Google Scholar] [CrossRef]
- Bao, Z.; Watanabe, A.; Sasaki, K.; Okubo, T.; Tokida, T.; Liu, D.; Ikeda, S.; Imaizumi-Anraku, H.; Asakawa, S.; Sato, T. A rice gene for microbial symbiosis, Oryza sativa CCaMK, reduces CH4 flux in a paddy field with low nitrogen input. Applied and environmental microbiology 2014, 80, 1995–2003. [Google Scholar] [CrossRef]
- Bidyarani, N.; Prasanna, R.; Chawla, G.; Babu, S.; Singh, R. Deciphering the factors associated with the colonization of rice plants by cyanobacteria. Journal of Basic Microbiology 2015, 55, 407–419. [Google Scholar] [CrossRef]
- Maeda, I. Potential of phototrophic purple nonsulfur bacteria to fix nitrogen in rice fields. Microorganisms 2021, 10, 28. [Google Scholar] [CrossRef]
- Yamaji, N.; Sasaki, A.; Xia, J.X.; Yokosho, K.; Ma, J.F. A node-based switch for preferential distribution of manganese in rice. Nature Communications 2013, 4, 2442. [Google Scholar] [CrossRef]
- Yamamuro, C.; Ihara, Y.; Wu, X.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. The Plant Cell 2000, 12, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Gyaneshwar, P.; James, E.K.; Mathan, N.; Reddy, P.M.; Reinhold-Hurek, B.; Ladha, J.K. Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. Journal of bacteriology 2001, 183, 2634–2645. [Google Scholar] [CrossRef] [PubMed]
- James, E.K.; Gyaneshwar, P.; Mathan, N.; Barraquio, W.L.; Reddy, P.M.; Iannetta, P.P.; Olivares, F.L.; Ladha, J.K. Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Molecular Plant-Microbe Interactions 2002, 15, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Morisaki, H. Endophytic bacteria in the rice plant. Microbes and environments 2008, 23, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Xia, J.; Seino, H.; Inaba, K.; Takahashi, Y.; Hayakawa, C.; Hirai, H.; Maeda, I. Adaptations of Potential Nitrogenase Activity and Microbiota with Long-Term Application of Manure Compost to Paddy Soil. Environments 2023, 10, 103. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree physiology 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and environmental microbiology 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Research in microbiology 2001, 152, 95–103. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and environmental microbiology 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Teske, A.; Wawer, C.; Muyzer, G.; Ramsing, N.B. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Applied and Environmental Microbiology 1996, 62, 1405–1415. [Google Scholar] [CrossRef]
- Chen, T.; Li, G.; Islam, M.R.; Fu, W.; Feng, B.; Tao, L.; Fu, G. Abscisic acid synergizes with sucrose to enhance grain yield and quality of rice by improving the source-sink relationship. BMC plant biology 2019, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Wang, S.-J. Molecular regulation of sink–source transition in rice leaf sheaths during the heading period. Acta Physiologiae Plantarum 2008, 30, 639–649. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, K.; Li, G.; Pan, J.; Huang, J.; Peng, S. Stem small vascular bundles have greater accumulation and translocation of non-structural carbohydrates than large vascular bundles in rice. Physiologia Plantarum 2022, 174, e13695. [Google Scholar] [CrossRef] [PubMed]
- Slewinski, T.L. Non-structural carbohydrate partitioning in grass stems: a target to increase yield stability, stress tolerance, and biofuel production. Journal of Experimental Botany 2012, 63, 4647–4670. [Google Scholar] [CrossRef]
- Okamoto, T.; Shinjo, R.; Nishihara, A.; Uesaka, K.; Tanaka, A.; Sugiura, D.; Kondo, M. Genotypic variation of endophytic nitrogen-fixing activity and bacterial flora in rice stem based on sugar content. Frontiers in Plant Science 2021, 1610. [Google Scholar] [CrossRef]
- Che, J.; Yamaji, N.; Ma, J.F. Role of a vacuolar iron transporter OsVIT2 in the distribution of iron to rice grains. New Phytologist 2021, 230, 1049–1062. [Google Scholar] [CrossRef]
- Mu, S.; Yamaji, N.; Sasaki, A.; Luo, L.; Du, B.; Che, J.; Shi, H.; Zhao, H.; Huang, S.; Deng, F. A transporter for delivering zinc to the developing tiller bud and panicle in rice. The Plant Journal 2021, 105, 786–799. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, Y.; Cao, L.; Tan, H.; Zhang, R. Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L.). Microbiological Research 2016, 188, 1–8. [Google Scholar] [CrossRef]
- Knelman, J.E.; Legg, T.M.; O’Neill, S.P.; Washenberger, C.L.; González, A.; Cleveland, C.C.; Nemergut, D.R. Bacterial community structure and function change in association with colonizer plants during early primary succession in a glacier forefield. Soil Biology and Biochemistry 2012, 46, 172–180. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, M.; Chen, A.; Zhang, W.; Wei, W.; Sheng, R. Impact of fertilization regimes on diazotroph community compositions and N2-fixation activity in paddy soil. Agriculture, Ecosystems & Environment 2017, 247, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Li, Y.; Chen, D.; Ao, J.; Zhou, W.; Shen, D.; Li, Q.; Huang, Z.; Jiang, Y. Influence of nitrogen and phosphorus additions on N2-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation. Science of the Total Environment 2018, 619, 1530–1537. [Google Scholar] [CrossRef]
- Ramšak, A.; Peterka, M.; Tajima, K.; Martin, J.C.; Wood, J.; Johnston, M.E.; Aminov, R.I.; Flint, H.J.; Avguštin, G. Unravelling the genetic diversity of ruminal bacteria belonging to the CFB phylum. FEMS Microbiology Ecology 2000, 33, 69–79. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, F.; Zhang, X.; Dai, X.; Dong, X.; Song, W. Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microbial ecology 2008, 55, 415–424. [Google Scholar] [CrossRef]
- Yasuda, M.; Dastogeer, K.M.; Sarkodee-Addo, E.; Tokiwa, C.; Isawa, T.; Shinozaki, S.; Okazaki, S. Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions. Agronomy 2022, 12, 1367. [Google Scholar] [CrossRef]
- Choudhury, A.; Kennedy, I. Prospects and potentials for systems of biological nitrogen fixation in sustainable rice production. Biology and Fertility of Soils 2004, 39, 219–227. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Cao, Y.; Sheirdil, R.; Wang, X.; Zhang, L. Isolation and proposal novel rice promoting endophytic bacteria, Rhizobium oryzicola sp. nov. Int J Syst Evol Microbiol 2015, 65, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, L.; Ma, X.; Sui, X.H.; Jiang, R. Rhizobium pseudoryzae sp. nov., isolated from the rhizosphere of rice. International journal of systematic and evolutionary microbiology 2011, 61, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Tang, X.; Sheirdil, R.A.; Sun, L.; Ma, X.-T. Rhizobium rhizoryzae sp. nov., isolated from rice roots. International Journal of Systematic and Evolutionary Microbiology 2014, 64, 1373–1377. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Wang, J.; Gong, Q.; Zhang, X.; Zhang, G. Isolation, identification and characterization of endophytic bacterium Rhizobium oryzihabitans sp. nov., from rice root with biotechnological potential in agriculture. Microorganisms 2020, 8, 608. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Zhang, J.; Sun, L.; Zhang, R.-J.; Zhang, C.-W.; Yin, H.-Q.; Zhang, X.-X. Rhizobium oryziradicis sp. nov., isolated from rice roots. International journal of systematic and evolutionary microbiology 2017, 67, 963–968. [Google Scholar] [CrossRef]
- Wegner, C.E.; Liesack, W. Microbial community dynamics during the early stages of plant polymer breakdown in paddy soil. Environmental microbiology 2016, 18, 2825–2842. [Google Scholar] [CrossRef]
- Bao, Z.; Sasaki, K.; Okubo, T.; Ikeda, S.; Anda, M.; Hanzawa, E.; Kakizaki, K.; Sato, T.; Mitsui, H.; Minamisawa, K. Impact of Azospirillum sp. B510 inoculation on rice-associated bacterial communities in a paddy field. Microbes and environments 2013, 28, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lianfeng, W.; Zhongjun, J. Heterotrophy-coordinated diazotrophy is associated with significant changes of rare taxa in soil microbiome. Pedosphere 2022, 32, 402–413. [Google Scholar] [CrossRef]
- Li, G.; Hu, Q.; Shi, Y.; Cui, K.; Nie, L.; Huang, J.; Peng, S. Low nitrogen application enhances starch-metabolizing enzyme activity and improves accumulation and translocation of non-structural carbohydrates in rice stems. Frontiers in plant science 2018, 9, 1128. [Google Scholar] [CrossRef] [PubMed]

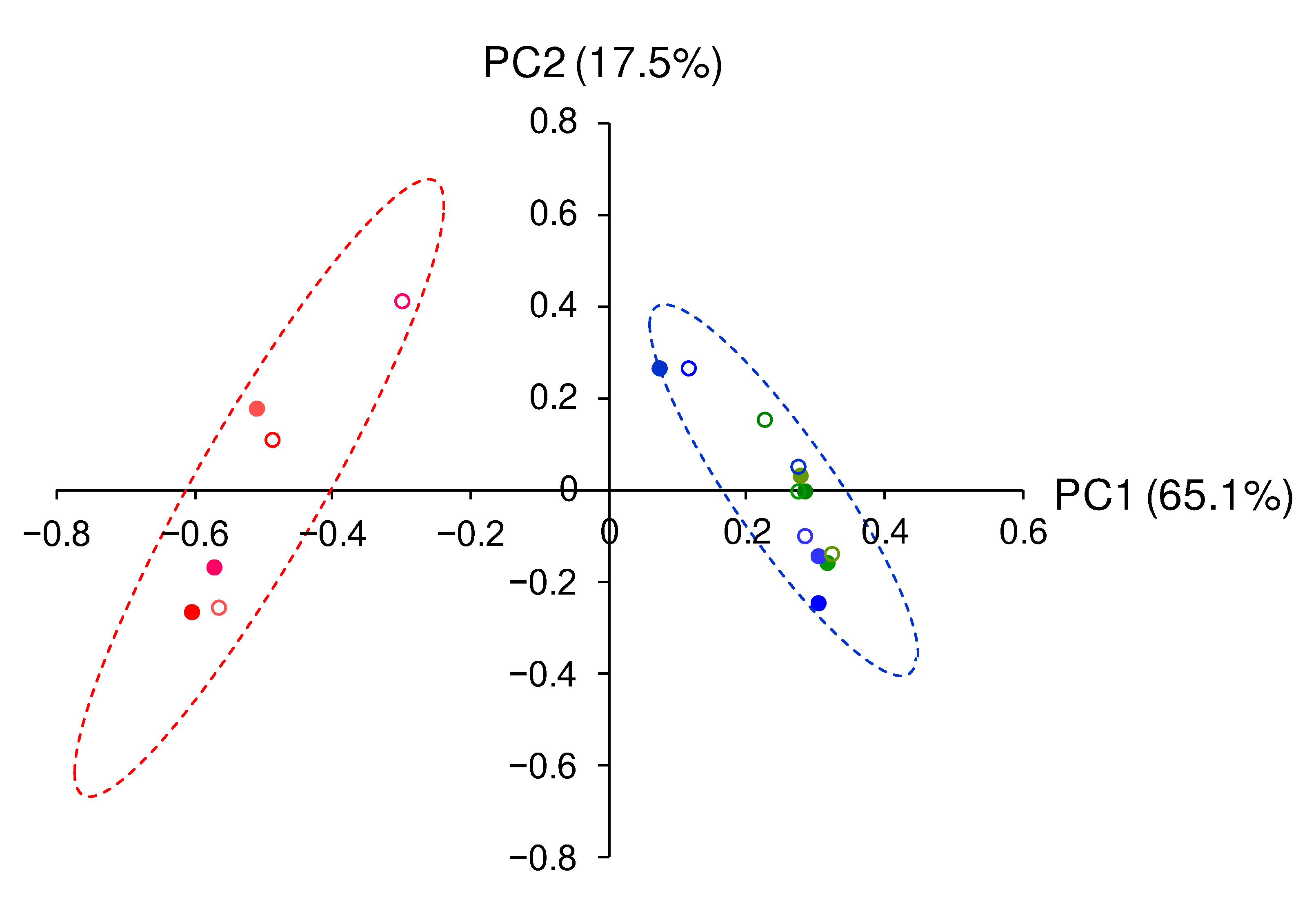
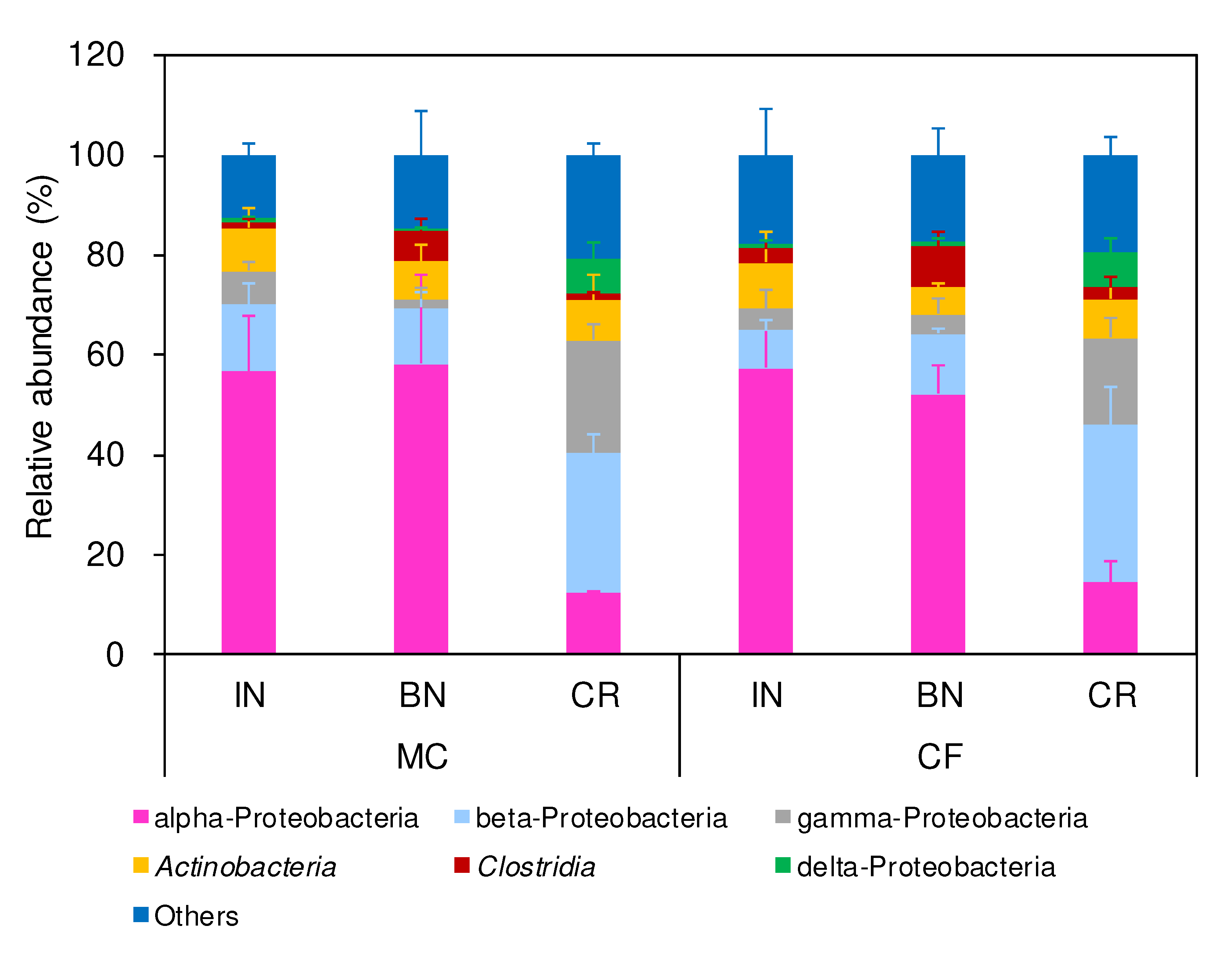
| Material applied | Rice part | Shannon | Inverse Simpson |
|---|---|---|---|
| MC | IN BN CR |
5.39 ± 0.63 4.87 ± 1.34 6.46 ± 0.28 |
14.5 ± 6.2 15.6 ± 15.6 33.1 ± 12.1 |
| CF | IN BN CR |
5.45 ± 0.42 5.35 ± 0.33 6.07 ± 0.55 |
18.1 ± 10.9 23.7 ± 14.0 43.4 ± 23.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





