1. Introduction
Quantum dots (QDs) are fluorescent semiconductor nanocrystals with a size from 2 to 10 nm [
1]. Their optical characteristics, such as broad excitation and narrow emission spectra, high photostability, and size-depending spectral position of the fluorescence peak, make them a promising tool in the biomedical field [
2]. The possible biomedical applications of QDs include their use in bioanalytical systems, cell and tissue imaging, and drug delivery and tracking
in vivo [
3,
4,
5]. However, the potential toxicity of QDs is a serious limitation to their use in living organisms. In particular, the immunotoxicity of QDs is one of major issues because the immune system plays an important role in protecting the body from foreign substances and preventing diseases.
A number of studies on QD toxicity have clearly demonstrated that QDs can accumulate in various organs and tissues and cause pathological effects. The liver has been shown to be one of the main organs where QDs accumulate [
6]. A study on the hepatotoxicity of CdSe/ZnS QDs carrying surface ligands with carboxyl groups showed that they caused hepatocyte damage and liver inflammation involving inflammosome activation and increased secretion of IL-1β cytokine [
7]. A study on Cd/Se/Te-based QDs with a ZnS shell showed an elevation of the IL-6 and TNF-α levels, although no histopathological damage was observed [
8]. A study on
in vivo biodistribution of water-soluble CdTe QDs demonstrated initial accumulation of QDs in the liver and their subsequent absorption in the kidneys during long-term blood circulation [
9]. In addition, it was shown that Cd-containing QDs upset the redox balance of primary kidney cells, thus inducing their damage [
10]. In conclusion, there is much evidence that QDs can accumulate in body organs and cause pathological damage [
11].
It was shown that, after intravenous administration, QDs accumulated in the spleen and thymus and retained their capacity for fluorescence for a long time after the injection [
12]. The results of immunotoxicity study of CdSe/ZnS QDs showed that the majority of the administered QDs were taken up by immune organs (the spleen and thymus). Lymphocytes from QD-treated mice exhibited a lower viability and an increased release of TNF-α and IL-6 [
13]. A study on the acute toxicity of CdSe/CdS-MPA QDs after repeated intraperitoneal injections demonstrated the accumulation of QDs in the liver and spleen and elevated levels of IL-6 in these organs and the blood plasma [
14]. A study on intraperitoneal injection of PEG-InP/ZnS QDs to mice showed an increase in the percentage of neutrophils and in IL-6 levels in the peritoneal lavage fluid (PLF) and plasma leading to acute-phase inflammation in mice [
15]. Intravenous administration of CdTe QDs resulted in the alteration of the levels of several proinflammatory cytokines. Specifically, the IL-6 level was significantly increased at QD doses of 0.4 mg/kg and higher, while the IL-12 (p70) and TNF-α levels were elevated at doses of 5 mg/kg and higher but were significantly reduced at the highest doses studied [
16]. In contrast, an immunotoxicity study
in vivo demonstrated only a weak effect of CdInS
2/ZnS QDs on the immune organs and the level of proinflammatory cytokines [
17]. Another study revealed the dependence of QD immunotoxicity on the substances used for surface coating of QDs [
18]. In summary, the immunotoxicity of QDs can be affected by the QD type, surface modifications, and dose in a non-linear manner. Moreover, a detailed assessment of the effects of QDs
in vivo requires systematic studies of series of QDs with different properties.
One of the most informative methods for assessing immunotoxicity is to measure the concentrations of cytokines [
19], both proinflammatory ones, such as IL-6 [
20], TNF [
21], IL-12 (p70) [
22], and IFN-γ [
23]. Monocyte chemoattractant protein 1 (MCP-1) is one of the key chemokines; it regulates migration and infiltration of monocytes/macrophages during the inflammatory response [
24]. Thus, alterations in the cytokine and chemokine concentrations can reflect the response of the immune system to the administration of potentially toxic substances.
The goal of our study was to assess the acute in vivo toxicities of QDs with different core compositions and surface charges and to estimate LD50 values for all these types of QDs. Then, we used the most toxic CdSe/ZnS-PEG-OH QDs to assess their immunotoxicity after intravenous administration of a relatively low non-lethal dose to mice. For this purpose, we examined the spleen, thymus and bone marrow, and measured the concentration of proinflammatory cytokines (IL-12p70, TNF, IFN-γ, MCP-1, and IL-6 ) to evaluate the possible harmful effects of QDs on the immune system.
2. Materials and Methods
2.1. Quantum dot synthesis and solubilization
CdSe/ZnS (core/shell) quantum dots with a fluorescence maximum at 592 nm were synthesized as described earlier [
25]. CuInS
2/ZnS QDs were prepared by colloidal synthesis in organic medium using a two-step shell growth procedure as described in [
26]. PbS/CdS/ZnS QDs were obtained by sequential synthesis procedure starting with the fabrication of PbS cores by hot injection method and their treatment by ion exchange as described in [
27] and ending with the coating of intermediate thin CdS shell atop PbS/CdS QDs and an outermost thick two-component CdS/ZnS shells. After the synthesis, the QDs were transferred from the organic phase to a water solution by replacing hydrophobic surface ligands with polyethylene glycol derivatives as described earlier [
25]. Briefly, the QDs were dissolved in chloroform (Sigma-Aldrich, Saint-Quentin-Fallavier, France) and precipitated with 10 mg/ml DL-cysteine (Sigma-Aldrich, Saint-Quentin-Fallavier, France.) solution in methanol (Sigma-Aldrich, Saint-Quentin-Fallavier, France). Excess DL-cysteine was washed off with methanol. The precipitate was dried and then dissolved in a weakly alkaline solution.
At the next stage, DL-cysteine ligands were replaced with PEG derivatives containing a hydroxyl group. We used three types of PEG derivatives: HS-(CH2)11-EG6-OH, HS-(CH2)11-EG6-OCH2-COOH, and HS-(CH2)11-EG6-NH2. (ProChimia Surfaces, Gdynia, Poland). For the modification of CdSe/ZnS QDs, we used mixtures of PEG derivatives at the following ratios: 70% of HS-(CH2)11-EG6-OH / 30% of HS-(CH2)11-EG6-OCH2-COOH, 70% of HS-(CH2)11-EG6-OH / 30% of HS-(CH2)11-EG6-NH2, and 100% of HS-(CH2)11-EG6-OH. The specified amounts of ligands were added to working mixtures with the corresponding pH values: 0.1 M sodium phosphate buffer solution (pH 7.2) if the HS-(CH2)11-EG6-OH ligand was used, 0.1 M sodium phosphate buffer solution (pH 8.0) in the case of the mixture of 70% of HS-(CH2)11-EG6-OH / 30% of HS-(CH2)11-EG6-OCH2-COOH, and 0.1 M sodium phosphate buffer solution (pH 6.6) in the case of 70% of HS-(CH2)11-EG6-OH/30% of HS-(CH2)11-EG6-NH2. After that, the mixtures were incubated at a temperature of 4°C overnight.
After the incubation, the samples were purified from unbound PEG derivatives using Amicon Ultra-15 centrifugal filter units with a 10 kDa cut-off (Millipore SAS, Molsheim, France) by centrifugation, after which 15 mL of 0.1 M sodium phosphate buffer solution (pH 7.2) was added in the case of HS-(CH2)11-EG6-OH; 0.1 M sodium phosphate buffer solution (pH 8.0), in the case of the mixture of 70% of HS-(CH2)11-EG6-OH / 30% of HS-(CH2)11-EG6-OCH2-COOH; and 0.1 M sodium phosphate buffer solution (pH 6.6), in the case of 70% of HS-(CH2)11-EG6-OH / 30% of HS-(CH2)11-EG6-NH2. The centrifugation was performed three times at room temperature at 4000 rpm for 10 min. Then, the obtained QD samples were additionally purified by gel exclusion chromatography on PD MiniTrap 25G columns (GE Healthcare, Chicago, Illinois, USA) according to the manufacturer's protocol. For this purpose, 500 μL of a QD solution was applied onto a column preliminarily equilibrated with a 0.1 M sodium phosphate buffer solution, pH 7.2. After that, 1 mL of the same buffer solution was used for elution. The fractions containing QDs were collected into separate test tubes. The purification procedure was performed twice. The resultant solution was sequentially filtered through sterile 0.22 μm Millex-GV (Sigma-Aldrich, Saint-Quentin-Fallavier, France) and sterile 0.1 μm Whatman Anotop filter units (Sigma-Aldrich, Saint-Quentin-Fallavier, France). The solutions of PEG derivatives with different functional groups (PEG filtrate solutions) were prepared by centrifugation of QD solutions using Amicon Ultra-4 centrifugal filter units with a 10 kDa cut-off (Millipore SAS, Molsheim, France) and filtered through sterile 0.1 μm Whatman Anotop filter units (Sigma-Aldrich, Saint-Quentin-Fallavier, France).
2.2. Quantum dot characterization
For the determination of the QD concentration in the final solution, we used the weight method. The aliquot of 35 μL of the final solution of the QD preparation was placed into a preliminarily weighted 0.5-mL low-bind test tube (Eppendorf) and then dried in a Concentrator Plus vacuum concentrator (Eppendorf France SAS, Montesson, France) for 3 h at a temperature of 30°C. After that, the test tube was weighted again. The quantity of QDs contained in 35 μL of the original QD solution was calculated by subtracting the initial weight of the empty test tube from the final weight of the test tube containing the QD preparation after drying. The QD quantity per milliliter of solution was calculated to obtain the mass concentration. The hydrodynamic diameter and zeta potential of solubilized QDs were measured by dynamic laser scattering and laser Doppler electrophoresis, respectively, by using a Zetasizer Nano-ZS device (Malvern Instrument Ltd., Malvern, UK).
2.3. Animals
Adult BALB/c and CBA×C57BL/6 female mice were obtained from the N.N. Blokhin National Medical Research Center of Oncology of the Russian Ministry of Health. Standard animals weighing 18–22 g were used in the study. All the animals were healthy; they were kept in special roomy cages at room temperature (20–23°C), a relative humidity of 60–65%, natural illumination, and forced air supply, on a litter of wood shavings sterilized in a hot-air oven. The mice were fed on the standard commercial certified pelletized feed for rodents with a known expiry date. The mice had free round-the-clock access to pure drinking water. All manipulations with animals were performed in accordance with the European Convention for the Protection of Vertebrates Used for Experimental and Other Scientific Purposes and were approved by the Animal Ethics Committee of the N.N. Blokhin National Medical Research Center of Oncology of the Russian Ministry of Health.
2.4. Estimation of the acute toxicity of quantum dots in vivo
For the
in vivo analysis of the acute toxicity of QDs, including the estimation of their median lethal doses (LD50), the BALB/c mice were divided into five experimental groups treated with different types of QDs (24 mice per group, 4 mice for each QD concentration). CuInS
2/ZnS, PbS/CdS/ZnS, and CdSe/ZnS QDs modified with the thiol-containing ligand HS-(CH
2)
11-EG
6-OH, as well as CdSe/ZnS QDs modified with the mixture of 70% of HS-(CH
2)
11-EG
6-OH / 30% of HS-(CH
2)
11-EG
6-OCH
2-COOH, the mixture of 70% of HS-(CH
2)
11-EG
6-OH / 30% of HS-(CH
2)
11-EG
6-NH
2, or 100% HS-(CH
2)
11-EG
6-OH, were administered at doses from 100 to 300 mg/kg as a single injection into the caudal vein. The QD samples were prepared in sterile 0.1 M sodium phosphate buffer solution (pH 7.2) under sterile conditions. Sterile 0.1 M sodium phosphate buffer solution (pH 7.2) and sterile filtrates of the QDs preparations were administered to mice of control groups. The day when the QD samples were injected was taken to be day 0. In that day, the mice were visually monitored for 6 h after the injection. After that, they were examined twice a day for 15 days. The number of the animals that died was the criterion for estimating the acute toxicity of the QDs. The toxicity was measured as the lethal dose (LD), i.e., the amount of QDs causing death of a specified percentage of the animals. LD50 values were estimated using Karber's arithmetic method [
28,
29].
2.5. Quantum dot immunotoxicity
2.5.1. Assessment of alterations in the spleen, thymus, and bone marrow
CdSe/ZnS QDs modified with the thiol-containing ligand HS-(CH2)11-EG6-OH were administered at the 0.2 LD100 dose as a single injection into the caudal vein of the CBA×C57BL/6 mice. A sterile solvent (0.1 M sodium phosphate buffer, pH 7.2) was injected to the mice of the negative control group. On the 7th or 21st day after administration, the animals were euthanized and immediately dissected. The thymus, spleen, and tubular bones were isolated. The spleen and thymus were weighed, and the corresponding cell suspensions were prepared in Hanks balanced salt solution (HBSS, Thermo Fisher Scientific, Waltham, MA, USA) with the use of a glass homogenizer. The resulting suspensions were filtered and washed twice by centrifugation. The bone marrow was extracted from the bones with Medium 199 (Thermo Fisher Scientific, Waltham, MA, USA) and then homogenized. The concentration of nucleus-containing cells (NCCs) was then determined. The results were expressed as the absolute numbers of NSCs in the organ.
2.5.2. Assessment of changes in the serum cytokine concentrations
BALB/c mice were divided into three experimental and six control groups, four mice each. CdSe/ZnS QDs modified with the thiol-containing ligand HS-(CH2)11-EG6-OH were administered at the 0.2 LD100 dose as a single injection into the caudal vein of experimental mice. A sterile solvent solution (0.1 M sodium phosphate buffer, pH 7.2) was injected to the mice of the negative control group. All preparations were injected into the tail vein. Serum samples were collected immediately, 6 h after, or 24 h after the injection of the QD preparation or the control solution. Serum samples from intact animals at the time points of 0, 6, and 24 h were used as an additional negative control. Serum samples from all mice in each group were pooled to obtain the necessary amount of material and stored at –20°С until analysis.
The concentrations of proinflammatory cytokines were measured using a cytometric bead array kit (BD™ CBA Mouse Inflammation Kit, BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. This technique employs bead populations with distinct fluorescence intensities coated with antibodies against IL-12p70, TNF, IFN-γ, MCP-1, and IL-6. The cytokine capture beads and phycoerythrin-conjugated detection antibodies were incubated with serum samples or standard solutions to form sandwich complexes. Then the samples were analyzed using a BD FACS Canto™ II cytometer (BD Biosciences, San Jose, CA, USA). The FCAP Array™ 3.0 software (BD Biosciences, San Jose, CA, USA) was used to calculate the cytokine concentrations from the data on fluorescence intensity.
3. Results
3.1. Quantum dot synthesis, solubilization, and characterization
We used QDs with different chemical compositions and surface charges to investigate their toxic effects in mouse models. After the synthesis of QDs in the organic phase and the transfer of water-insoluble QDs to the aqueous phase using DL-cysteine, we modified their surface with thiol-containing PEG derivatives with different end groups (-OH, -COOH, or -NH
2), obtaining stable and homogeneous aqueous QD preparations. Mixtures of PEG derivatives with different functional groups for the modification of CdSe/ZnS QDs were used to obtain QD samples with different surface charges. The surface charge and hydrodynamic diameter of QDs were determined by the electrophoretic mobility method employing the Doppler effect and by the dynamic light scattering method, respectively, using a Zetasizer Nano ZS instrument.
Table 1 shows the sizes and charges of the QDs used in the study.
3.2. In vivo acute toxicity of quantum dots
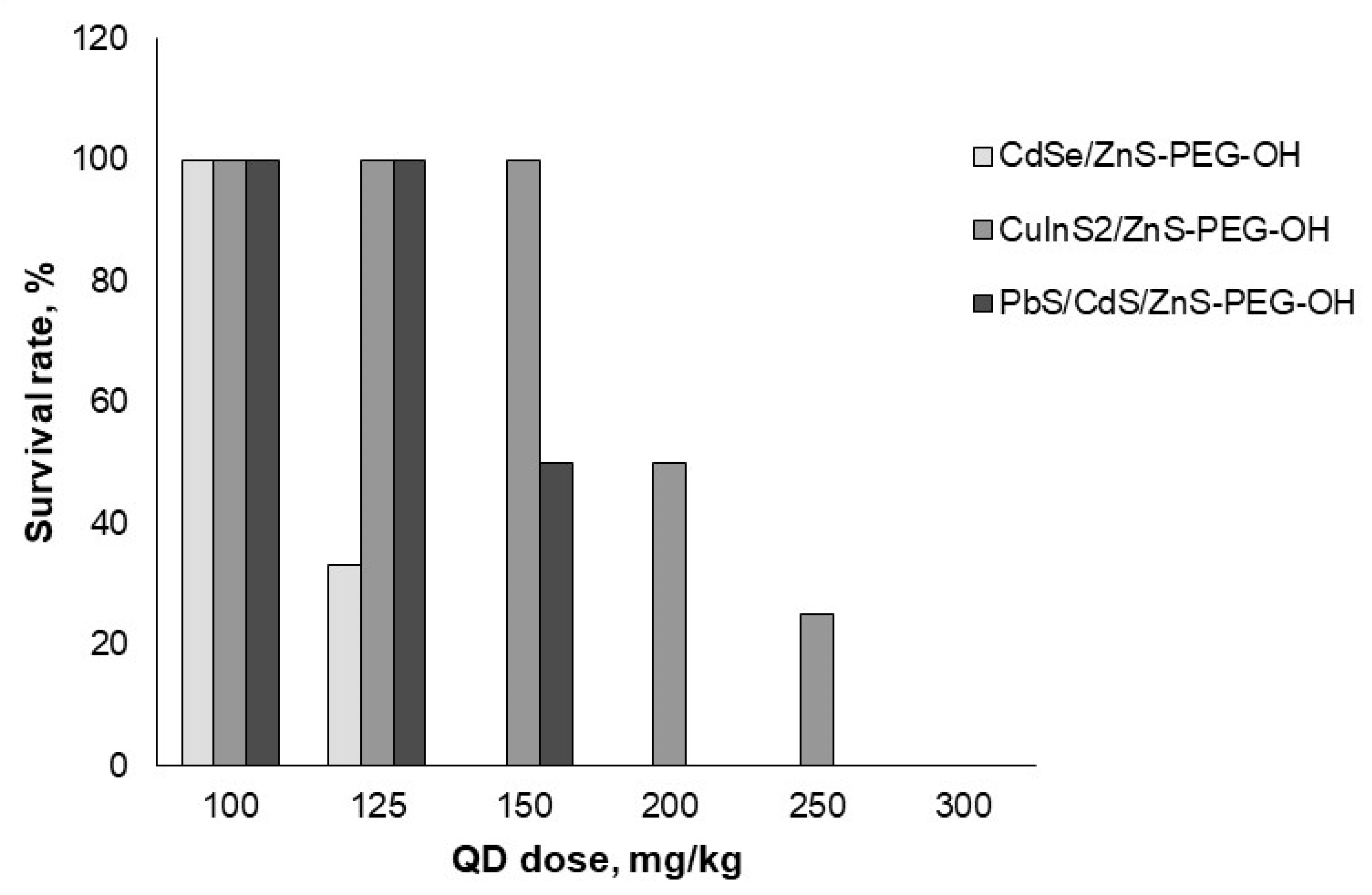
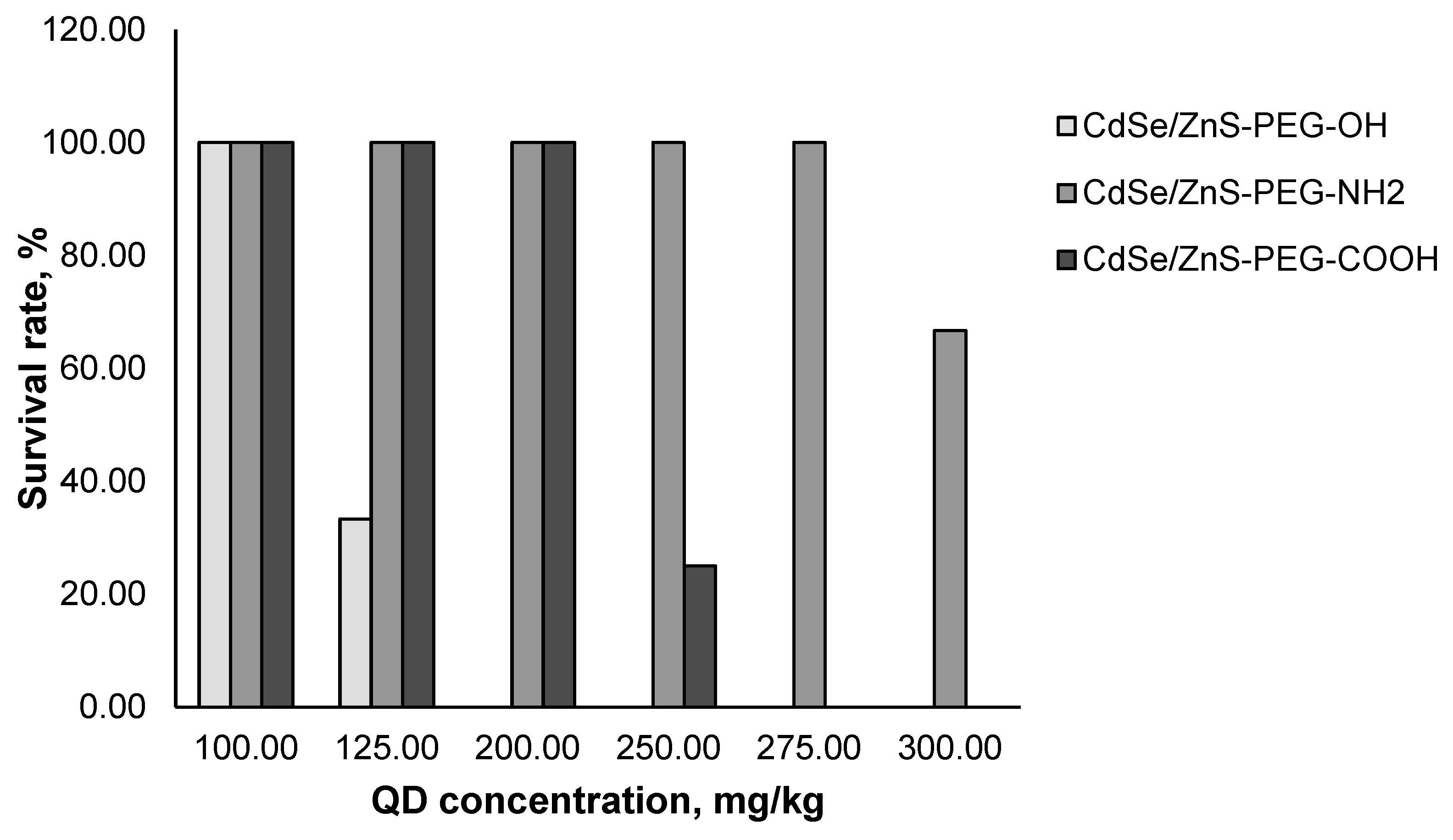
The mice were injected with the QD preparations into the caudal vein, after which we visually monitored the animals carefully for 24 h. After that, the number of surviving animals was recorded.
We estimated the
in vivo acute toxicity of QDs with different chemical compositions of the core (
Figure 1) and with different surface charges (
Figure 2). The data obtained in this experiment were used to calculate the LD50 values for all types of QDs studied (
Table 2). Our results showed that CuInS
2/ZnS QDs had a weaker toxic effect on mice than PbS/CdS/ZnS and CdSe/ZnS QDs, although their surface charges were similar, because the same PEG derivative was used for their modification (
Figure 1). Furthermore, the obtained mean values of LD50 for QDs with the same chemical composition of the core (CdSe/ZnS) but different surface charges differed considerably. CdSe/ZnS-PEG-OH QDs with a low negative charge had the highest toxic effect on mice, whereas the LD50 of CdSe/ZnS-PEG-NH
2 QDs with a low positive charge turned out to be approximately two times higher. CdSe/ZnS-PEG-COOH QDs were shown to cause the weakest toxic effect on mice.
3.3. Effects of quantum dots on the mouse spleen, thymus, and bone marrow
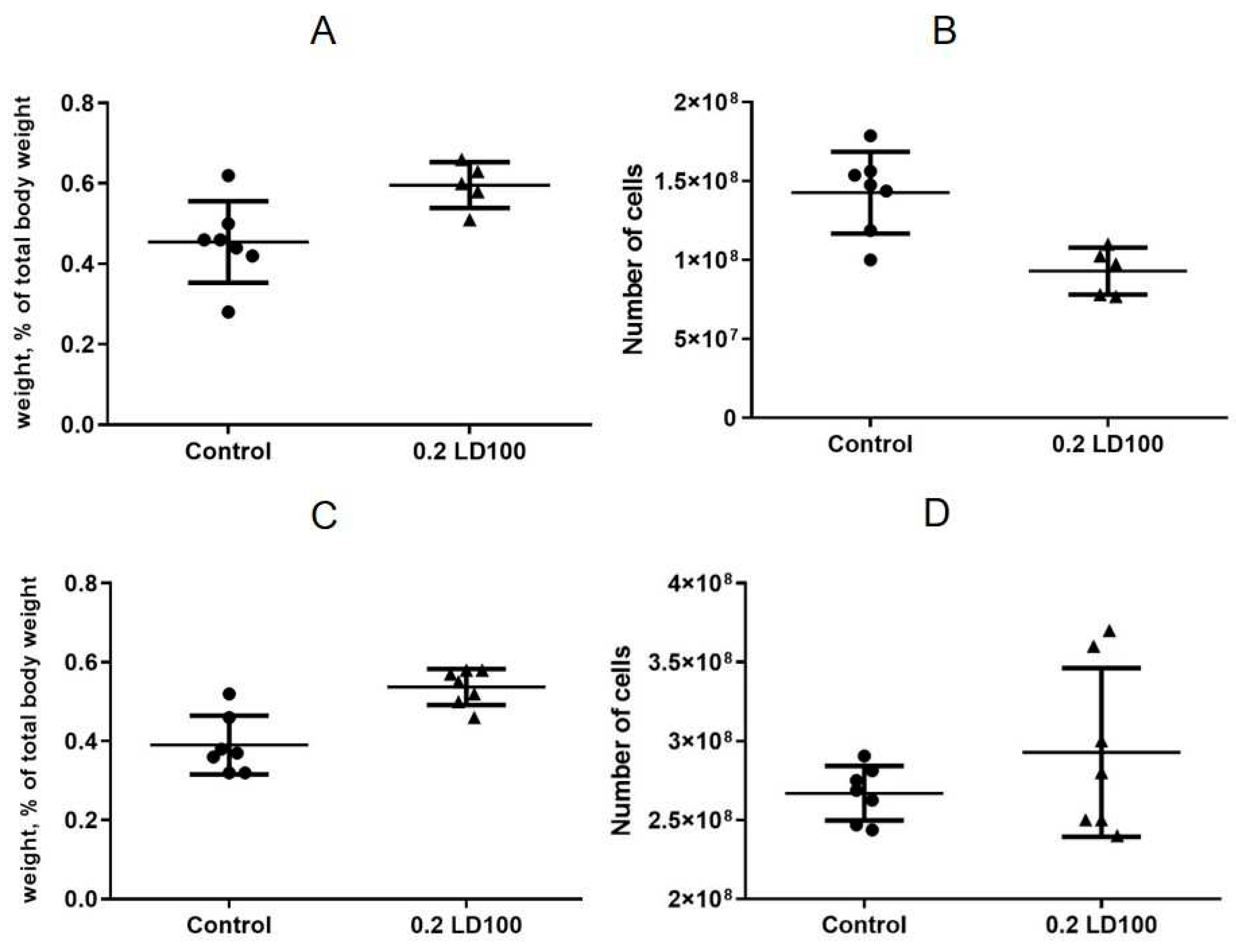
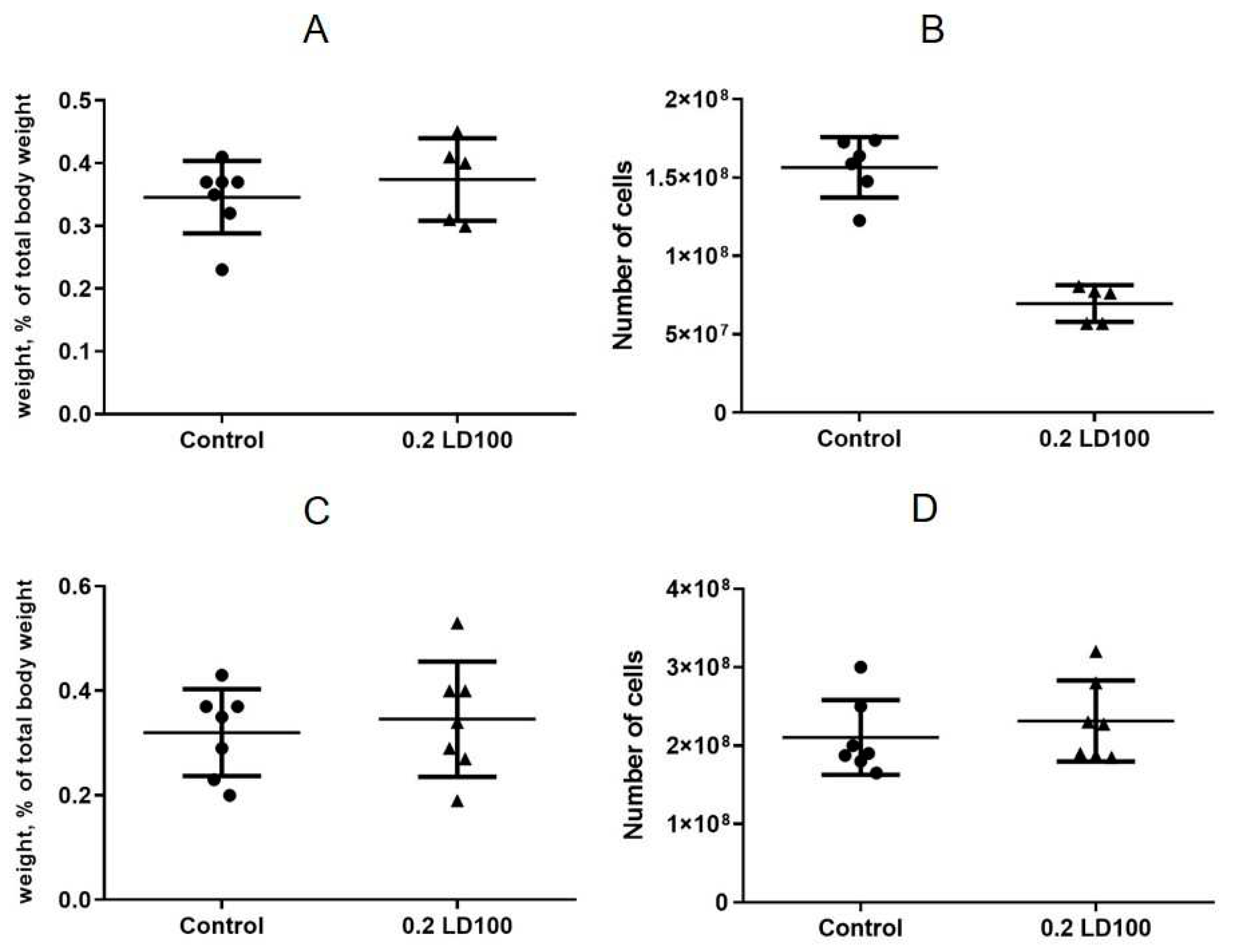
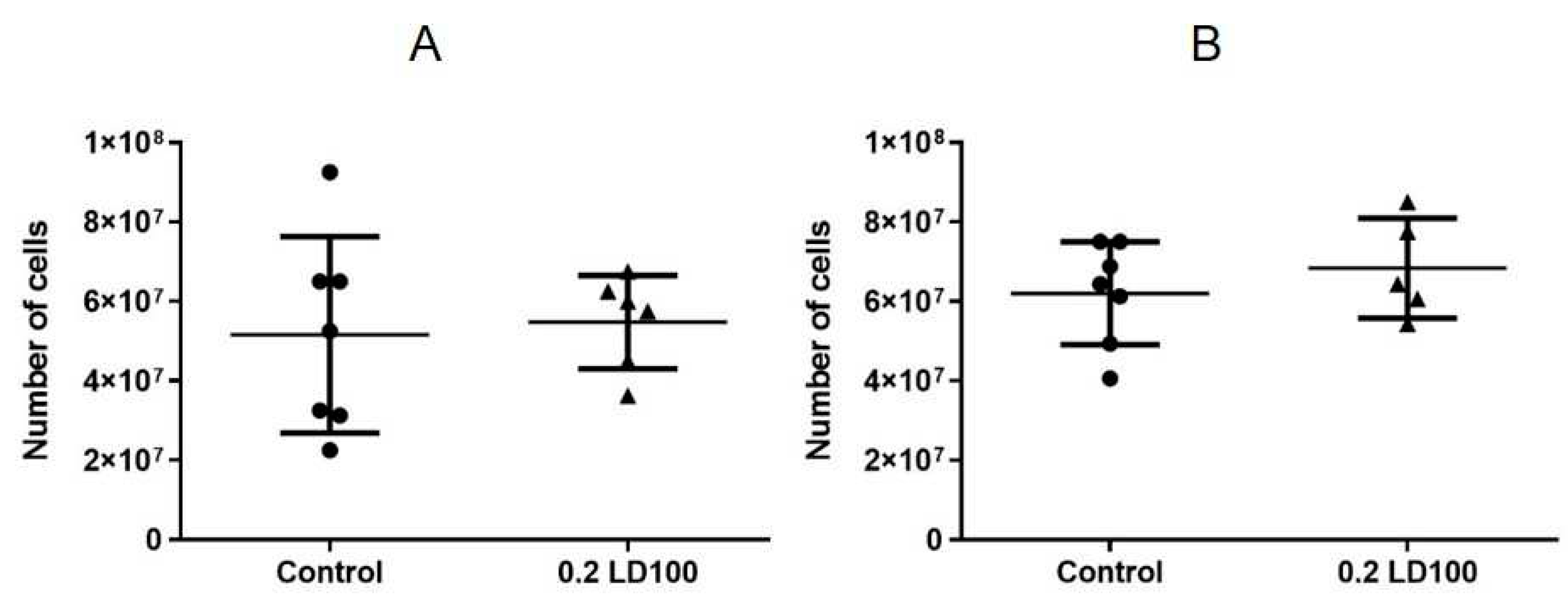
The results showed that injection of QDs with the chemical composition of CdSe/ZnS-PEG-OH at a low dose (0.2 LD100) caused cell depletion and a slight increase in the spleen weight on the seventh day after the QD injection (
Figure 3AB). At the same time, on the 21st day after injection, the spleen was still slightly enlarged but completely repopulated (
Figure 3C,D).
The thymus weight after the QD injection was practically unchanged, but there was also a significant cell depletion on day 7 after the QD administration, which may have been caused by the toxic effect of QDs on T cells, thymus macrophages, and/or stromal cells (
Figure 4A,B). After 21 days, the thymus cell content was also restored (
Figure 4C,D).
The cell content of the bone marrow did not change compared to the control group seven days after QD administration (
Figure 5A), which indicated that QDs did not affect the hematopoietic function of the bone marrow within a short period of time. However, after 21 days (
Figure 5B), a 20% decrease in the bone marrow cell content was observed; hence, the bone marrow hematopoietic function was gradually suppressed.
3.4. Effects of quantum dots on the cytokine profile
Three experimental groups of mice were treated with the control 0.1 M sodium phosphate buffer or the CdSe/ZnS-PEG-OH QD solution at the 0.2 LD100 dose. The concentrations of five cytokines (IL-12p70, TNF, IFN-γ, MCP-1, and IL-6) in mouse blood serum determined by the CBA assay are shown in
Table 3. The data show a slight decrease in the IL-12 (p70) and TNF production 6 h after the QD administration, whereas the concentration of IFN-γ remained unchanged. On the contrary, the serum levels of MCP-1 and IL-6 were significantly elevated 6 h after the QD administration but were close to the initial values 24 h after the treatment.
4. Discussion
Fluorescent semiconductor nanocrystals have a great potential in the field of biomedicine, but their use is restricted by the potential toxic effects. The
in vivo toxicity of CdSe/ZnS QDs has been reported in several studies [
30,
31,
32,
33]. However, a more systematic evaluation of the harmful effects of QDs on living organisms is needed for their safe use. One of the important aspects is immunotoxicity, because the immune system functions include protection from foreign substances.
We have estimated the LD50 values of different types of QDs, acute toxic effects of QDs on different organs of the immune system, and the cytokine profile after a single intravenous injection of QD preparations to mice. Such experiments could help to improve the safety testing standards for nanotechnological products based on nanocrystals.
The QD preparations were injected into the caudal vein of BALB/c mice, after which we carefully monitored the condition of the mice for 24 h and then examined them twice a day for 15 days. We estimated the
in vivo toxicity of QDs with different chemical compositions of the core (
Figure 1) and those with different surface charges (
Figure 2). The results were used to calculate the LD50 values for all types of QDs studied (
Table 2). Our data showed that the most toxic QDs are CdSe/ZnS-PEG-OH with a weakly negatively charged surface. In addition, QDs with a CuInS
2 core also exhibited a low toxicity compared with the QDs whose core contained heavy metals, which suggested degradation of the protective shell of QDs in a living body and manifestation of an additional toxic effect of heavy metals.
Our results clearly demonstrate that the QD surface charge also influences their toxicity. Indeed, the QDs with a low negative surface charge have proved to be the most toxic, and those with a higher negative charge, the least toxic. This effect is most likely to be determined by the differences in the distribution and accumulation of QDs in the mouse body and the differences in the interactions with the components of biological fluids. For example, negatively charged QDs may rapidly accumulate in lymph nodes [
34], and electrically neutral QDs are less prone to electrostatic interaction with proteins, which interferes with the formation of the "protein corona" and promotes QD degradation [
35].
At the same time, the lowest toxicity of the QDs with a negative surface charge agrees with our earlier data on
in vitro cytotoxicity [
36]. However, in contrast to our previously obtained results on the cytotoxicity of QDs
in vitro [
36], the acute toxicity of the same QDs
in vivo depends on the chemical composition of the nucleus, which may be due a faster degradation of the protective shell in the living organism, and the overall toxic effect is also caused by the toxicity of heavy metals.
Cadmium-containing nanomaterials are widely used in modern industrial composites. At the same time, they are highly toxic, which has been further confirmed by our study on their acute in vivo toxicity. Therefore, we have performed additional experiments to evaluate the effects of this type of QD on the immune system. We have monitored the effect of a relatively low dose (0.2 LD100) of CdSe/ZnS-PEG-OH QDs on the mouse spleen, thymus, and bone marrow for 21 days after a single intravenous injection.
The spleen was used as a model organ because it is the site of the transformation of monocytes into macrophages, cells intended for inactivation of toxic substances. The thymus was used because it is one of the organs most sensitive to immunotoxins, as well as the site of maturation, differentiation, and "immunological sorting" of T cells. The alterations in the red bone marrow were analyzed because it is an organ of hematopoiesis and immunopoiesis and consists of lymphocytic, monocytic, and megakaryocytic progenitor cells.
The results showed that a single administration of a low dose of CdSe/ZnS-PEG-OH QDs insignificantly increased the spleen weight, had almost no effect on the thymus weight, but caused strong cell depletion of both the spleen and the thymus on the seventh day after QD injection. However, the cellular contents of the spleen and thymus were completely restored on the 21st day after injection (
Figure 3 and
Figure 4), which indicated that the effect of a single dose of QDs was reversible.
The effect of CdSe/ZnS-PEG-OH QDs on the bone marrow was somewhat different. Cell depletion of the bone marrow exhibited a time delay: it was not observed until 21 days after a single administration of QDs, which indicates a remote adverse effect of QDs on the hematopoietic function of the bone marrow. This could be because QDs are known to mainly accumulate in other target organs immediately after administration [
37], reaching the bone marrow considerably later.
In addition, we estimated the acute effect of a low dose of CdSe/ZnS-PEG-OH QDs on the profile of proinflammatory cytokines in mice. The concentrations of IL-12p70, TNF, IFN-γ, MCP-1, and IL-6 were determined using a set of cytometric beads (
Table 3) in mouse serum samples collected immediately, 6 h after, and 24 h after the single administration of QDs and compared with those in control serum samples. The results demonstrated a rapid increase in the serum levels of MCP-1 and IL-6 6 h after administration, which may have been a response to acute inflammation in the target organs where the QDs accumulated immediately after administration. These data, together with the observed reversion of these concentrations to the control values 24 h after the QD administration, suggest that a single low dose of CdSe/ZnS-PEG-OH QDs may induce a short-term inflammatory response in mice. Thus, our results suggest that the acute immune response to CdSe/ZnS-PEG-OH QDs at low doses is reversible, but it should be taken in account for predicting short-term harmful effects and for safety assessment of nanomaterials.
5. Conclusions
We propose a new systematic approach to investigate the acute toxicity of QDs
in vivo. For this purpose, a series of water-soluble QDs with a core/shell structure, differing from each other in either the core chemical composition or the surface charge, have been obtained and characterized. The obtained PEGylated QDs have an organic shell that provides a high colloidal stability of the ODs, making them suitable for
in vivo studies. Experimental data have shown that the cadmium-containing CdSe/ZnS-PEG-OH and PbS/CdS/ZnS-PEG-OH QDs with a weakly negatively charged surface are considerably more toxic than the QDs with the same surface properties but with a CuInS
2 core. In addition, the results demonstrate that not only the chemical composition, but also the surface charge of QDs determine their acute toxicity
in vivo. Our data indicate that QDs with a low negative surface charge tend to be more toxic than those with a high negative charge. The immunotoxic effect of QDs is mainly directed to the organs producing immune cells, which may reduce the immunity of experimental animals in the long term. A short-term increase in the levels of proinflammatory cytokines, in particular, MCP1 and IL-6, suggests an acute but reversible inflammatory response to a single administration of a low dose of QDs. Systemic analysis of the
in vivo toxicity of QDs with different chemical compositions, sizes, and surface charges has allowed us to identify correlations and differences with our previously obtained results on the
in vitro toxicity of these QDs [
36]. Obviously,
in vitro models cannot provide fully relevant data on toxicity. Studies on the behavior of nanomaterials
in vivo can yield new knowledge about the mechanisms and pathways involved in the toxic effects of nanomaterials. This will undoubtedly help improving the quality control of the starting materials for the engineering of safe hybrid nano-biomaterials and extend the use of these materials in biotechnological developments.
Author Contributions
Conceptualization, A.S. and I.N.; methodology, E.G., M.B., Z.S., P.S.; formal analysis, A.K. and A.S.; investigation, E.G., S.B., P.S., M.B. and Z.S.; data curation, A.S., I.N. and A.K.; writing—original draft preparation, E.G., S.B. and A.S.; writing—review and editing, E.G., A.K., and I.N.; supervision, I.N. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The part of this study related to study of the effects of quantum dots on the cytokine profile was supported by the Russian Science Foundation under the grant no. 23-75-30016 (to A.K.). The part related to the development of new surface chemistry approaches was supported by the French National Research Agency (ANR-20-CE19-009-02).
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, A.S. or I.N., upon reasonable request.
Acknowledgments
A.S. and I.N. acknowledge the support of the French Ministry of Higher Education, Research and Innovation and the University of Reims Champagne-Ardenne. We thank Vladimir Ushakov for proofreading the manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomedicine 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Bilan, R.; Nabiev, I.; Sukhanova, A. Quantum Dot-Based Nanotools for Bioimaging, Diagnostics, and Drug Delivery. ChemBioChem 2016, 17, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Brazhnik, K.; Sokolova, Z.; Baryshnikova, M.; Bilan, R.; Efimov, A.; Nabiev, I.; Sukhanova, A. Quantum Dot-Based Lab-on-a-Bead System for Multiplexed Detection of Free and Total Prostate-Specific Antigens in Clinical Human Serum Samples. Nanomedicine Nanotechnology, Biol. Med. 2015, 11, 1065–1075. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Bioomaterialia 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Lu, J.; Tang, M.; Zhang, T. Review of Toxicological Effect of Quantum Dots on the Liver. J. Appl. Toxicol. 2019, 39, 72–86. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, S.; Chen, H.; He, M.; Deng, Y.; Cao, Z.; Pi, H.; Chen, C.; Li, M.; Ma, Q.; et al. CdSe/ZnS Quantum Dots Induce Hepatocyte Pyroptosis and Liver Inflammation via NLRP3 Inflammasome Activation. Biomaterials 2016, 90, 27–39. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, M.H.; Chang, L.W.; Yang, C.S.; Chang, H.; Chang, W.H.; Tsai, M.H.; Wang, C.J.; Lin, P. Cd/Se/Te-Based Quantum Dot 705 Modulated Redox Homeostasis with Hepatotoxicity in Mice. Nanotoxicology 2011, 5, 650–663. [Google Scholar] [CrossRef]
- Su, Y.; Peng, F.; Jiang, Z.; Zhong, Y.; Lu, Y.; Jiang, X.; Huang, Q.; Fan, C.; Lee, S.T.; He, Y. In Vivo Distribution, Pharmacokinetics, and Toxicity of Aqueous Synthesized Cadmium-Containing Quantum Dots. Biomaterials 2011, 32, 5855–5862. [Google Scholar] [CrossRef]
- Zhao, L.; Zong, W.; Zhang, H.; Liu, R. Kidney Toxicity and Response of Selenium Containing Protein-Glutathione Peroxidase (Gpx3) to CdTe QDs on Different Levels. Toxicol. Sci. 2019, 168, 201–208. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, T.; Tang, M. Toxicity of Quantum Dots on Target Organs and Immune System. J. Appl. Toxicol. 2021, 1–24. [Google Scholar] [CrossRef]
- Fitzpatrick, J.A.J.; Andreko, S.K.; Ernst, L.A.; Waggoner, A.S.; Ballou, B.; Bruchez, M.P. Long Term Persistence and Spectral Blue Shifting of Quantum Dots in Vivo. Nano Lett. 2009, 9, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, J.; Yong, K.T.; Zhu, X.; Lin, M.C.M.; Jiang, W.; Li, J.; Huang, Q.; Lin, G. Immunotoxicity Assessment of CdSe/ZnS Quantum Dots in Macrophages, Lymphocytes and BALB/c Mice. J. Nanobiotechnology 2016, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Im, H.Y.; Seo, J.E.; Hasan, M.; Woo, K.; Kwon, O.S. Acute Toxicity and Tissue Distribution of CdSe/CdS-MPA Quantum Dots after Repeated Intraperitoneal Injection to Mice. J. Appl. Toxicol. 2013, 33, 940–950. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Chen, Y.; Yao, Z. InP/ZnS Quantum Dots Cause Inflammatory Response in Macrophages through Endoplasmic Reticulum Stress and Oxidative Stress. Int. J. Nanomedicine 2019, 14, 9577–9586. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Zhang, Y.; Todd, J.; Kittle, K.; Patry, D.; Caldwell, D.; Lalande, M.; Smith, S.; Parks, D.; Navarro, M.; et al. Biodistribution and Systemic Effects in Mice Following Intravenous Administration of Cadmium Telluride Quantum Dot Nanoparticles. Chem. Res. Toxicol. 2019, 32, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, L.; Lin, X.; Yang, Z.; Zou, W.; Chen, Y.; Xu, J.; Liu, D.; Wang, X.; Lin, G. In Vitro and in Vivo Immunotoxicity of PEGylated Cd-Free CuInS2/ZnS Quantum Dots. Nanotoxicology 2020, 14, 372–387. [Google Scholar] [CrossRef]
- Dai, T.; Li, N.; Liu, L.; Liu, Q.; Zhang, Y. AMP-Conjugated Quantum Dots: Low Immunotoxicity Both In Vitro and In Vivo. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Corsini, E.; House, R. V. Evaluating Cytokines in Immunotoxicity Testing. Methods Mol. Biol. 2018, 1803, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A. .; Kuchroo, V.K. IL-12 Family Cytokines: Immunological Playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Nabiev, I.; Sukhanova, A.; Even-Desrumeaux, K.; Chames, P.; Baty, D.; Artemyev, M.; Oleinikov, V.; Nabiev, I. Engineering of Ultra-Small Diagnostic Nanoprobes through Oriented Conjugation of Single-Domain Antibodies and Quantum Dots. Protoc. Exch. 2012. [Google Scholar] [CrossRef]
- Vokhmintcev, K. V; Linkov, P.A.; Samokhvalov, P.S.; Nabiev, I.R. Two-Stage ZnS Shell Coating on the CuInS2 Quantum Dots for Their Effective Solubilization. KnE Energy 2018, 3, 535. [Google Scholar] [CrossRef]
- Ren, F.; del Rosal, B.; An, S.Y.; Yang, F.; Carrasco, E.; Benayas, A.; Kwon Oh, J.; Jaque, D.; de la Fuente, Á.J.; Vetrone, F.; Ma, D. Development and Investigation of Ultrastable PbS/CdS/ZnS Quantum Dots for Near-Infrared Tumor Imaging. Part. Part. Syst. Charact. 2017, 34, 1600242. [Google Scholar] [CrossRef]
- Lee, H.; Park, K. Acute Toxicity of Benzalkonium Chloride in BALB/c Mice Following Intratracheal Instillation and Oral Administration. Environ. Anal. Health Toxicol. 2019, 34, e2019009. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Z.S.; Cao, S.J.; Chang, Y.F.; Cao, Y.Q.; Li, J.B.; Yao, Z.X.; Wen, Y.P.; Huang, X.B.; Wu, R.; Yan, Q.G. Acute Oral Toxicity Test and Assessment of Combined Toxicity of Cadmium and Aflatoxin B1 in Kunming Mice. Food Chem. Toxicol. 2019, 131, 110577. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Li, L.; Chen, Y.; Chen, T.; Yang, Z.; Wang, J.; Liu, D.; Lin, G.; Wang, X. In Vivo Toxicity Evaluation of PEGylated CuInS2/ZnS Quantum Dots in BALB/c Mice. Front. Pharmacol. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chang, T.; Marcq, G.; Liu, C.; Kiss, B.; Rouse, R.; Mach, K.E.; Cheng, Z.; Liao, J.C. In Vivo Biodistribution and Toxicity of Intravesical Administration of Quantum Dots for Optical Molecular Imaging of Bladder Cancer. Sci. Reps. 2017, 7, 9309. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ouyang, Q.; Hu, R.; Ding, Z.; Tian, J.; Yin, F.; Xu, G.; Chen, Q.; Wang, X.; Yong, K.T. In Vivo Toxicity Assessment of Non-Cadmium Quantum Dots in BALB/c Mice. Nanomedicine: NBM. 2015, 11, 341–350. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Robe, A.; Pic, E.; Lassalle, H.P.; Bezdetnaya, L.; Guillemin, F.; Marchal, F. Quantum Dots in Axillary Lymph Node Mapping: Biodistribution Study in Healthy Mice. BMC Cancer 2008, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, W.; Choi, H.S.; Zimmer, J.P.; Tanaka, E.; Frangioni, J. V; Bawendi, M.G. Compact Cystein-Coated CdSe(ZnCdS) QDs for in Vivo Applications. J. Am. Chem. Soc. 2008, 129, 14530–14531. [Google Scholar] [CrossRef] [PubMed]
- Bozrova, S.; Gerasimovich, E.; Baryshnikova, M.; Sokolova, Z.; Samokhvalov, P.; Guhrenz, C.; Gaponik, N.; Karaulov, A.; Nabiev, I. Dependence of Quantum Dot Toxicity in Vitro on Their Size, Chemical Composition, and Surface Charge. Nanomaterials 2022, 12, 2734. [Google Scholar] [CrossRef]
- Su, Y.; Peng, F.; Jiang, Z.; Zhong, Y.; Lu, Y.; Jiang, X.; Huang, Q.; Fan, C.; Lee, S.T.; He, Y. In Vivo Distribution, Pharmacokinetics, and Toxicity of Aqueous Synthesized Cadmium-Containing Quantum Dots. Biomaterials 2011, 32, 5855–5862. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).