1. Introduction
Premenstrual syndrome (PMS) consists of multiple psychological and physical complaints and symptoms that develop during the luteal phase of the menstrual cycle (Gnanasambanthan et al., 2018). Dysmenorrhea is one of the most prevalent symptoms during PMS, although abdominal bloating, peripheral edema, mood swings, labile moods, lethargy, irritability, fatigue, breast-tenderness, anxiety/tension, headache, or migraine are frequently reported by women in the premenstrual period. These symptoms are of sufficient severity to interfere with interpersonal relationships and/or daily functioning. The prevalence of dysmenorrhea among women is consistently high and unrelated to the economic status of their country (Yonkers et al., 2018). In 2010, the prevalence rates of primary dysmenorrhea in young women were of 43%-91% (Armour et al., 2019). Literature has shown that an estimated 90% of females during the reproductive age are affected by premenstrual symptoms (Gao et al., 2002). Data from Mexico revealed that the prevalence of dysmenorrhea is around of 64% (Ortiz, 2010).
The aim of treatment to ameliorate or eliminate symptoms includes, reduce their impact on activities and interpersonal relationships, improving women´s quality of life. Initially, nonpharmacologic therapy should be considered for patients with PMS. Even though, women with persistent symptoms of PMS are eligible for pharmacological intervention (Dickerson et al., 2003). Many over the counter (OTC) medicines containing mild diuretics, analgesics, prostaglandin inhibitors, or antihistamines are frequently used by patients without medical prescription. Also, guidelines suggest the use of selective serotonin reuptake inhibitors (SSRIs), diuretics, androgens, and/or gonadotropin-releasing hormone drugs in patients with premenstrual dysphoric disorder, a severe type of premenstrual syndrome PMS (Hofmeister et al., 2016; Green Top Guideline, 2017). The fixed-dose combination (FDC) drugs have been extensively indicated considering the advantage of multimodal management of PMS (Jankzura et al., 2021). In Mexico, the oral FDC of naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg tablet (Analgen Fem®) is commercially available as an OTC medicine. The efficacy and safety of this FDC drug was previously evaluated in a randomized controlled clinical trial including 200 patients with primary dysmenorrhea. This study demonstrated that naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg reduced the pain intensity after the first eight hours of treatment (p<0.01). Furthermore, the proportion of patients who reported a pain reduction by at least 50% was 80.6 (70/98) in the test group. The treatment with naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg was safe and tolerated by patients, only 4 (4.0%) women experienced five adverse events: somnolence (1), headache (2), dizziness (1), increased thirst (1) and diarrhea (1). No serious adverse events were reported (Ortiz et al., 2016). On the other hand, literature shows different reports regarding the safety profile of non-steroidal anti-inflammatory drugs (NSAIDs), including single and fixed dose combinations. Women with primary dysmenorrhea often use OTC NSAIDs at variable doses, exposing to numerous side effects, including irritation gastric mucosa, stomach ulcers, nausea, vomiting, and nervous system disorders (Janczura et al., 2021). Therefore, the present study was aimed to evaluate the post-marketing safety and effectiveness of naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg tablets in Mexican women with a diagnosis of PMS.
2. Materials and Methods
2.1. Study design and patients
This prospective, open-label, non-interventional, multicenter, observational post-marketing study, conducted in the following clinical sites in Mexico: (1) American British Cowdray Medical Center (Mexico City), (2) Hospital San Angel Inn. (México City), (3) Clinical Trials México S.A. de C.V. (Hidalgo), and (4) ICARO Investigaciones en Medicina, S.A. de C.V. (Chihuahua), was conducted from December 2017 to December 2019.
Collected data from 270 female outpatients older than 18 years with clinical diagnosis of PMS and eligible for naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg treatment were enrolled. Patients were excluded if they were under therapy with non-steroidal anti-inflammatory drugs (NSAIDs) or hormonal contraceptives treatment. In addition, women with previous history of drug study hypersensitivity, cardiovascular diseases, active sex life without contraceptives, pregnant or nursing patients, or meet any other contraindication were excluded from the study. The study protocol (LIO-04-15) and unidentified subjects´ data for sharing was registered in the Mexican data base of clinical trials and in the data on file owned by Laboratorios Liomont, S.A. de C.V.
2.2. Ethics
The study was conducted in accordance with the Declaration of Helsinki, the Good Clinical Practices (ICH E6) and the health legislation of Mexico. The protocol (LIO-04-15) and the informed consent format were reviewed and approved by local ethics committee (LIO-04-15/28022018, CECYC Pharma, S.A. de C.V., México). Patients were included after signing the informed consent.
2.3. Study medication
Naproxen 220 mg + Paracetamol 300 mg + pamabrom 25 mg tablet (Analgen Fem®) was prescribed by the treating physicians under routine clinical practice, in compliance with the recommendations of the prescribing information (one or two tablets every 8 h for five days maximum). Study medication was allowed to discontinue once symptoms were controlled.
2.4. Safety and effectiveness variables
Primary safety measure was the patient proportion that was suspected or experienced an adverse event once the patients received the first dose of the product to the end of the observational period; all adverse events, associated or not with the study medication, were recorded. The effectiveness measures were:
- a)
Menstrual pain intensity reported by patients prior to the first dose-study medication, and the last dose administered. Pain intensity was determined by the Numerical Rating Scale (NRS), where 0 = “no pain at all”, 1 – 3 = mild; 4 – 6 = moderate; and 7 – 10 = severe.
- b)
The patient proportion with a baseline pain score reduction by at least 50%.
- c)
The premenstrual questionary (PMSQ) was applied by the investigator prior to the first dose of the study medication and the last dose administered aimed to evaluate the different dimensions of the scale, including: PMS-A (anxiety, irritability, and nervous tension); PMS-C (headaches, increased appetite, desire for sweets, fatigue, palpitations, and tremors); PMS-D (depression, insomnia, tearfulness, forgetfulness, and confusion); PMS-H (includes water retention, swelling, breast tenderness, bloating, and weight gain, this is the type most commonly related with edema). Patients assessed the presence of symptoms as follow (Barboza et al., 2014): “0” = symptom was absent; “1” = symptom was barely noticeable”; “2” = symptom inhibited activities; “3” = symptom altered the lifestyle.
2.5. Statistical analyses
The sample was calculated considering a single group and based on the article by Moore et al., 2015. A sample size of 187 patients was estimated considering a type I error of 5% (α = 0.05), a confidence interval 95%, statistical power of 80%, 14.5% as adverse events proportion, and assuming a 45% dropout rate, the final sample was 270 subjects. The analysis of safety and effectiveness was performed on the intention-to-treat population, which is defined as all the enrolled patients who fulfilled the inclusion criteria and received at least one dose of study medication. The significance level for the statistical tests was 5% (Type I error, α=0.05). Except for the exact binomial tests, since they are unilateral, the level of significance was established at 2.5% (Type I error, α=0.025). The Stata 15 (StataCorp LLC), NCSS 20 (NCSS, LLC., USA) and East 6 (Cytel Inc., USA) software were used for analysis.
3. Results
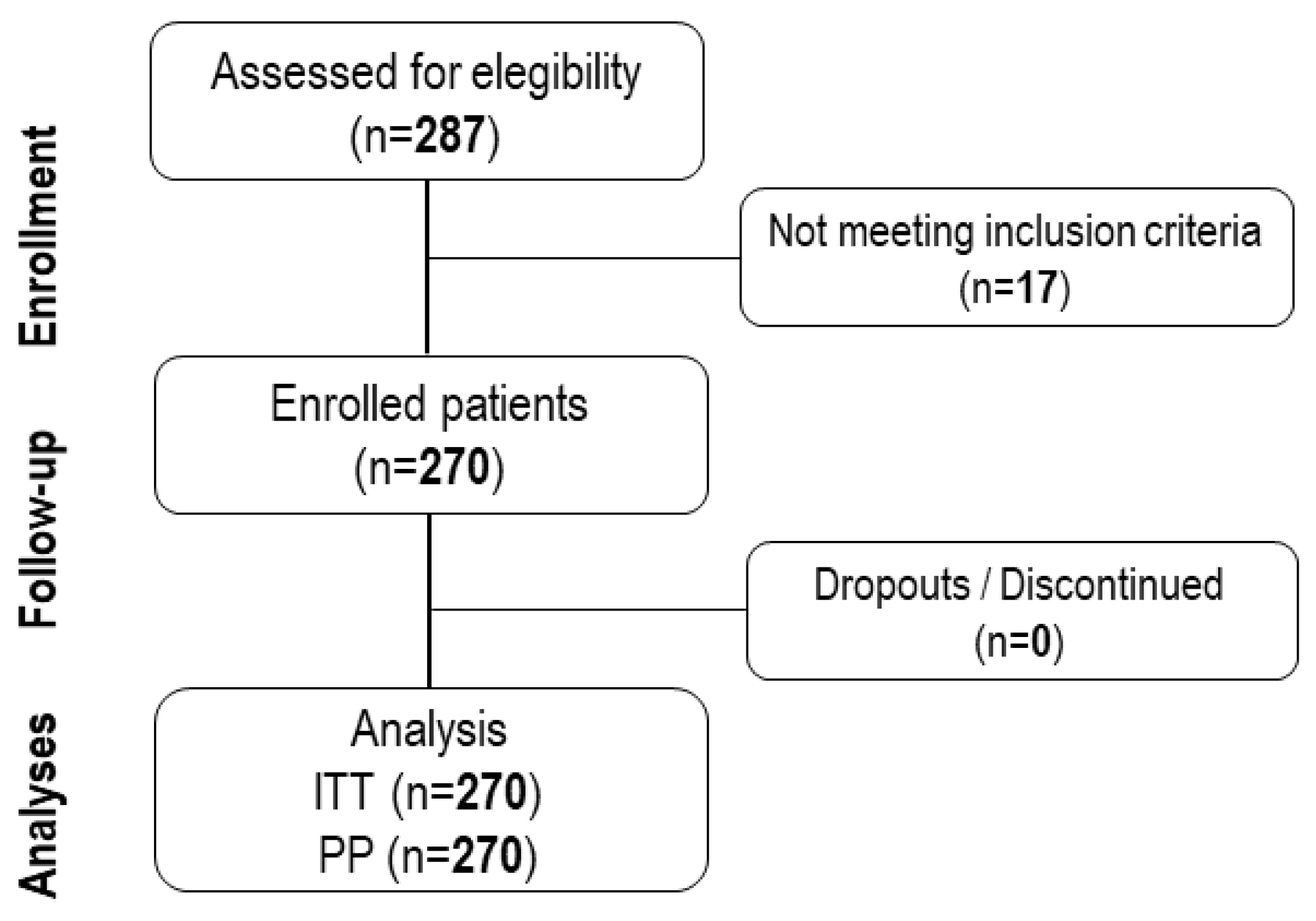
A total of two hundred and seventy patients with diagnosis of PMS aged from 18 to 57 years (28.9±8.8 years) were included in the present study to receive naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg tablet as prescribed by the investigator under the routine clinical practice. The study population was characterized by previous history of daily activities limitation (63.0%), school or work absence (51.5%), and hospitalization due to PMS symptoms (2.2%), among others showed in
Table 1. The CONSORT diagram represents the distribution of the study population during the recruitment, enrollment, and following periods (
Figure 1).
The safety and effectiveness analyses were performed in the population of intention-to-treat (ITT) and per-protocol (PP), respectively. Among the 270 patients analyzed for safety, 8 (3%) patients experienced 10 adverse events. The adverse events experienced were headache (5/8), gastritis (2/8) dyspepsia (1/8), diarrhea (1/8) and nausea (1/8). The investigator discontinued the study medication in 3/8 patients because of adverse events and classified 6/10 adverse events as “associated” with naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg tablet. All adverse events were coded with the preferred terms of MedDRA (version 22.0) and classified by System Organ Class (SOC) as shown in
Table 2.
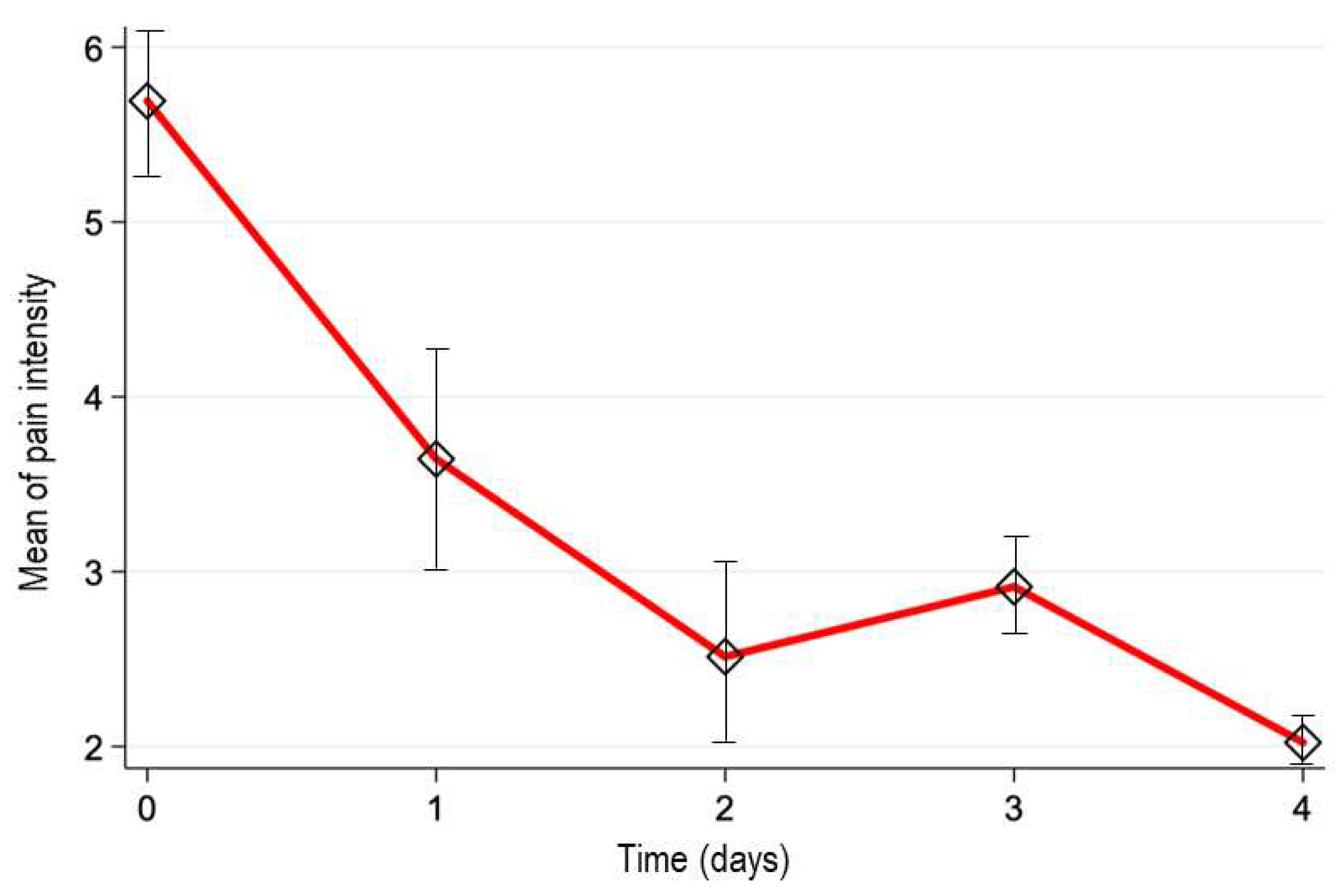
The pain intensity was measured by NRS scale at baseline resulting in 5.7±2.6 cm (mean ± SD, n=270), showing a reduction over the time: 3.6±2.6 cm (day 1; n=270), 2.5±2.4 cm (day 2; n=185), 2.9±2.1 (day 3; n=69), and 2.0±2.3 (day 4; n=44). As seen, the patients were discontinuing the treatment as the analgesic effect occurred. The estimated median difference of pain intensity scores between the baseline and the last observation (-4.5, 95%CI; -5, -4) demonstrated that naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg tablet significantly reduced the pain intensity over time (p<0.001). The
Figure 2 shows the mean of pain intensity vs. time profile. In addition, we determined the proportion of patients who reported a pain reduction by at least 50% at the end of the treatment. This proportion was 70.7% (95%CI; 64.9%, 76.1%).
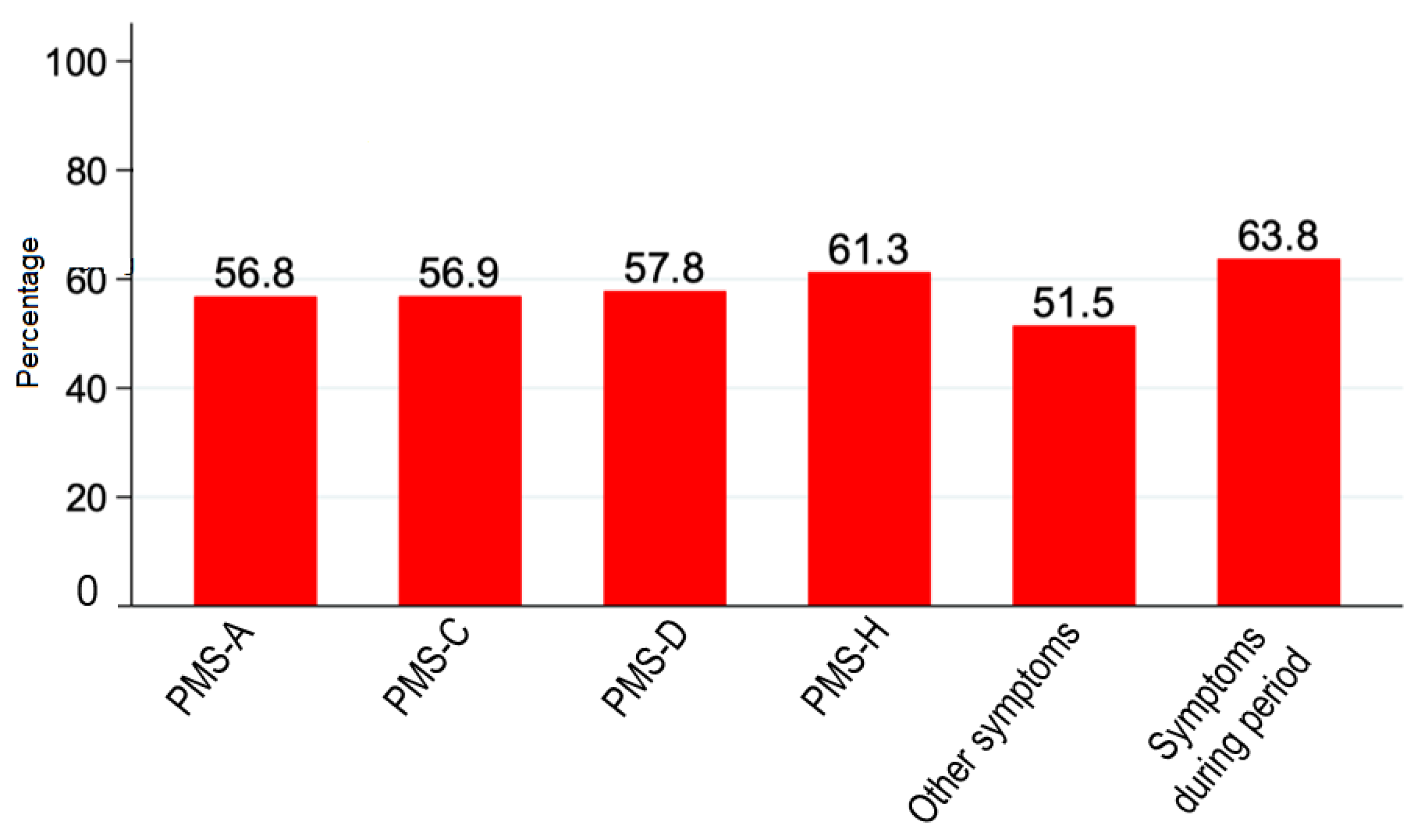
On the other hand, in the present study we evaluated the effect of naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg tablet on PMS symptoms. The mean scores of PMS questionnaire overtime are shown in the
Table 3; meanwhile the
Figure 3 showed the proportion of patients who reported a reduction of PMS symptoms score by at least 50%. These results indicate that naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg contributes to alleviate the PMS symptoms in more than 50% of women who received at least one dose of the oral FDC.
4. Discussion
The management of PMS is implemented in a stepwise manner, from non-pharmacological alternatives to analgesic, hormonal and/or with antidepressant therapy. NSAIDs have been used as first line of pharmacological treatment in patients with primary dysmenorrhea associated with PMS. The indication is supported by findings that have shown the key role of prostaglandins in the pathogenesis of primary dysmenorrhea and other premenstrual symptoms (Gnanasambanthan et al., 2018; Barcikowska et al., 2020). Although literature refers a great number of premenstrual symptoms, physicians categorize PMS into four groups: PMS-A, PMS-C, PMS-D, and PMS-H. Group A is predominant related with anxiety, irritability, and nervous tension; group C with headaches, and may be accompanied by increased appetite, desire for sweets, fatigue, palpitations, and tremors; group D with depression, insomnia, tearfulness, forgetfulness, and confusion, and finally, the group H includes water retention, swelling, breast tenderness, bloating, and weight gain, and this is the type most commonly related with peripheral edema (Tacani et al., 2015). Therefore, in many cases the multimodal therapies including analgesic, anti-inflammatory, and diuretics represent a plausible approach to improve the quality of life of women with PMS. The purpose of this approach is to combine drugs with different mechanisms of action to achieve a synergistic effect, yielding a measurable effectiveness at low doses with a better safety and tolerability profile.
The adverse events incidence in this observational study was 3.0% (8/270 patients). The characteristics of experienced adverse events were like the previously reported for these drugs in combination or individually. In addition, the incidence of these adverse events was essentially lower than those reported in previous studies (Moore et al., 2015; Ortiz et al., 2016).
On the other hand, previous studies have demonstrated the efficacy of fixed dose combination containing naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg in alleviating the symptoms caused by PMS (Ortiz et al., 2016). Naproxen and paracetamol are analgesic and anti-inflammatory drugs, respectively, that are commonly used for treating painful conditions such as primary dysmenorrhea (Ali et al., 2017; Nie et al., 2020). The anti-inflammatory and analgesic properties of naproxen have been attributed to the inhibition of cyclooxygenase and the consequent inhibition of prostaglandin synthesis (Angiolillo et al., 2017), meanwhile paracetamol exerts antinociceptive effect through the interference with serotonergic descending pain pathways, without omitting the potential inhibition of prostaglandin synthesis or through an active metabolite influencing cannabinoid receptors (Anderson et al., 2008). A meta-analysis demonstrated the efficacy of naproxen in primary dysmenorrhea (OR 3.99, 95%CI, 2.18 to 7.30) (Nie et al., 2020). In addition, Marjoribanks et al. 2015 published a meta-analysis to compare NSAIDs used in the treatment of primary dysmenorrhea. The authors found that naproxen was significantly more effective than placebo in producing moderate to excellent relief of dysmenorrhea (OR 3.67, 95%CI, 2.94 to 4.58). Fixed dose combinations of paracetamol with NSAIDs also showed a significant pain relief in women with primary dysmenorrhea (Eccles et al., 2010). Considering the water retention, swelling, and finally peripheral edema, the approach of a fixed dose combination including pamabrom, a mild diuretic, represent a plausible alternative. Previous studies demonstrated that NSAID plus pamabrom combination is effective in the control of pain and other symptoms in patients with primary dysmenorrhea (Ortiz et al., 2016; Guayasamín et al., 2020). These results demonstrated the validity of adding a mild diuretic to an NSAID to increase the effectiveness in the control of PMS symptoms. Studies reported a mild to moderate adverse events, mainly gastrointestinal effects, however the patients reported an acceptable level of tolerability. In line with literature reports, the present study showed the effectiveness of naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg since at day 3 more than 50% discontinued the treatment due to symptoms control; meanwhile, at day 4 the proportion of patients with symptom control was higher than 80%.
Diversity of the enrolled population was considered a main limitation of our study. Here, investigators enrolled women with PMS with similar demographic characteristics. However, it is well established that race, ethnicity, and other demographics can impact how different people respond to the same therapy. On the other hand, observational period was performed only during a single treatment period; thus, we consider the short observational period as the main limitation of our study. In future studies, the safety during two to three premenstrual period might be evaluated.
The present work contributes to the actual knowledge regarding the safety profile of the fixed-dose combination naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg (Analgen Fem®), including analgesic, anti-inflammatory and diuretic drugs for the management of PMS. The authors consider the safety information essential to the medical community and patient´s education programs since the drug is commercialized as OTC. The non-interventional methodology allowed to obtain safety and effectiveness data from women´s patterns of drug use under uncontrolled scenario. Finally, it is important to highlight that the present study contributed to generate new evidence on the safety and effectiveness of naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg combination in women with premenstrual syndrome. The monitoring of therapeutics in the post-marketing phase is of vital importance for health professionals, sponsors, and regulatory authorities. In this sense, the present study contributed with pharmacovigilance programs in Mexico for OTC drugs.
In summary, the present post-marketing study demonstrated that naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg (Analgen Fem®) treatment had a low frequency of adverse events. No serious nor unexpected adverse events were communicated during the study. On the other hand, the fixed dose combination drug improves the PMS symptoms, reducing the pain intensity and edema when it is administered under routine clinical practice. Therefore, naproxen 220 mg + paracetamol 300 mg + pamabrom 25 mg exert a favorable benefit-risk balance when is administered no longer than 5 days in women with PMS.
Author Contributions
LDR; MGP; AEPJ: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. AEPJ; OBA; MGP: Drafting the work or revising it critically for important intellectual content. JEV; EF; OBA; AEJP; MGP; LDR: provide approval for publication of the content. JEV; EF; OBA; AEJP; MGP; LDR: Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The present study did not receive funding.
Data Availability Statement
Acknowledgments
The authors want to acknowledge the administrative support of Infinite Clinical Research International CRO.
Conflicts of Interest
Dr. Livan Delgado-Roche is employee of Laboratorios Liomont, S.A. de C.V. The rest of the authors declare they have not conflict of interest.
References
- Gnanasambanthan, S., and Datta, S. (2019). Premenstrual syndrome. Obstet Gynaecol Reprod Med. 29:281–5.
- Yonkers, K.A., and Simoni, M.K. (2018). Premenstrual disorders. Am J Obstet Gynecol. 218:68–74.
- Armour, M., Parry, K., Manohar, N., Holmes, J., Ferfolja, T., Curry, C., et al. (2019). The Prevalence and Academic Impact of Dysmenorrhea in 21,573 Young Women: A Systematic Review and Meta-Analysis. J Women’s Heal. 28:1161–71. [CrossRef]
- Ortiz, M.I. (2010). Primary dysmenorrhea among Mexican university students: Prevalence, impact and treatment. Eur J Obstet Gynecol Reprod Biol. 152:73–7. [CrossRef]
- Dickerson, L.M., Mazyck, P.J., Hunter, M.H. (2003). Premenstrual Syndrome. Am Fam Physician. 67:1743–52.
- Green Top Guideline. (2017). Management of Premenstrual Syndrome: Green-top Guideline No. 48. BJOG An Int J Obstet Gynaecol. 124:73–105.
- Hofmeister, S., Bodden, S. (2016). Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 94:236–40.
- Ortiz, M.I., Murguía-Cánovas, G., Vargas-López, L.C., Silva, R., González-de la Parra, M. (2016). Naproxen, paracetamol and pamabrom versus paracetamol, pyrilamine and pamabrom in primary dysmenorrhea: a randomized, double-blind clinical trial. Medwave. 16:e6587–e6587. [CrossRef]
- Moore, N., Pollack, C., Butkerait, P. (2015). Adverse drug reactions and drug–drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 11:1061–75. [CrossRef]
- Barcikowska, Z., Rajkowska-Labon, E., Grzybowska, M.E., Hansdorfer-Korzon, R., Zorena, K. (2020). Inflammatory markers in dysmenorrhea and therapeutic options. Int J Environ Res Public Health. 17:1–14. [CrossRef]
- Tacani, P.M., de Oliveira Ribeiro, D., Guimarães, B.E.B., Perez-Machado, A.F., Tacani, RE. (2015). Characterization of symptoms and edema distribution in premenstrual syndrome. Int J Womens Health. 7:297–303. [CrossRef]
- Nie, W., Xu, P., Hao, C., Yingying, C., Yanling, Y., Lisheng, W. (2020). Efficacy and safety of over-the-counter analgesics for primary dysmenorrhea. J Med. 99:19:1–11.
- Ali, Z., Burnett, I., Eccles, R., North, M., Jawad, M., Jawad, S., et al. (2007). Efficacy of a paracetamol and caffeine combination in the treatment of the key symptoms of primary dysmenorrhoea. Curr Med Res Opin. 23(4):841–51. [CrossRef]
- Angiolillo, D.J., Weisman, S.M. (2017). Clinical Pharmacology and Cardiovascular Safety of Naproxen. Am J Cardiovasc Drugs. 17:97–107. [CrossRef]
- Anderson, B.J. (2008). Paracetamol (Acetaminophen): Mechanisms of action. Paediatr Anaesth. 18:915–21. [CrossRef]
- Marjoribanks, J., Ayeleke, R.O., Farquhar, C., Proctor, M. (2015). Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015: CD001751. [CrossRef]
- Eccles, R., Holbrook, A., Jawad, M. (2020). A double-blind, randomised, crossover study of two doses of a single-tablet combination of ibuprofen/paracetamol and placebo for primary dysmenorrhoea. Curr Med Res Opin. 26:2689–99. [CrossRef]
- Guayasamín, I., Rivera, V., González, Y., Campoverde, J., Contreras, S., Roque, R., et al. (2020). Dexketoprofen plus pamabrom versus acetaminophen in primary dysmenorrhea: a controlled, randomized, double-blind, multicenter study. Rev Med Electr 42:1-20.
- Janczura M, Kobus M, Sip S, Zarowski M, Warenczak A, Cielecka-Piontek J. (2021). Fixed-Dose Combination of NSAIDs and Spasmolytic Agents in the Treatment of Different Types of Pain—A Practical Review. J Clin Med; 10:3118. [CrossRef]
- Study information and results: http://siipris03.cofepris.gob.mx/Resoluciones/Consultas/ConWebRegEnsayosClinicosDetalle.asp?idsolicitud=3073.
- Barboza CR, Lasmar R, Gama GF, Oliveira L, de Oliveira EC, Gonçalves M, de Souza A, Geller M. Premenstrual syndrome: Clinical assessment of treatment outcomes following Borago officinalis extract therapy. Rev Bras Med 2014; 71:211-17.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).