1. Introduction
The ecological role played by carnivorous waterbirds in the productivity and functioning of inland wetlands ecosystems has recently gain attention [
1], and have contributed to wetlands conservation and management worldwide [
2]. Carnivorous waterbirds have been reported to enhance wetlands biodiversity by supporting and regulating ecosystem services that are provided by wetlands [
3,
4,
5]. Carnivorous waterbirds provides services such as mobile linking of ecosystems’ flora and fauna [
5,
6,
7], enhancement of primary production [
8] and nutrients accumulation and cycling within and between the wetlands [
9,
10] and regulate pest outbreaks (Wallensten et al., 2007; Ziegler et al., 2010; Otieno et al., 2015). They also provide important ecosystem services, such as nutrients cycling, pest control, provision of meat and indicators of environmental changes to indigenous communities residing along wetlands [
12].
The ecosystem services provided by species in an ecosystem are linked to the species traits (i.e. the qualities of each species) [
10,
13]. Species with similar traits normally perform similar functions in the ecosystem [
4,
14]. This trait-function relationship has led to grouping of species into functional groups [
15,
16] and forms the bases for niche differentiation among species. It is also believed that within a functional group, only few species are dominant (drivers) and many are minor (passengers) species similar to dominant species in an ecosystem at any time [
17,
18,
19]. Furthermore, a change in a species dominance should be counterbalanced by a previously similar minor species following environmental changes or disturbance [
18], and playing similar ecological roles in the ecosystem to previously more abundant species [
20]. The result being that the ecosystem maintains its functions and processes despite losses of previously dominant species. This shift in species abundances following environmental change and disturbances (functional redundancy/equivalence) [
21], implies that ecosystems with high functional redundancy are more sustainable in face of environmental changes and disturbances [
1,
22,
23] In addition, dominant species should be functionally dissimilar because they perform different functions and hence occupy different niches ([
21]).
The objective of this study was to assess and investigate the composition and diversity of carnivorous waterbirds in two freshwater tributaries of Zambezi River in Namibia, Sikunga and Lisikili tributaries. The two tributaries have different protection status. Part of the Sikunga tributary has been gazette as a Fish Protected Area (FPA) (thereafter referred to as FPA), while the Lisikili tributary is a Non-Fish Protected Area (NFPA) (thereafter referred to as NFPA). In the FPA, fishing is regulated through quota and is only done at certain portions of the tributary. In the NFPA, fishing is not regulated and there is high subsistence fishing using different fishing gears including traditional fishing systems. Specifically, we predicted that: 1) carnivorous waterbirds functional and taxonomical diversity were higher at the FPA sites than NFPA sites; 2) at both sites carnivorous waterbirds community abundance were dominated by few species and have many minor species; 3) changes in abundance of dominant carnivorous waterbirds species in FPA sites were counterbalanced by a functionally-similar dominant species in NFPA sites.
2. Materials and Methods
2.1. Study area
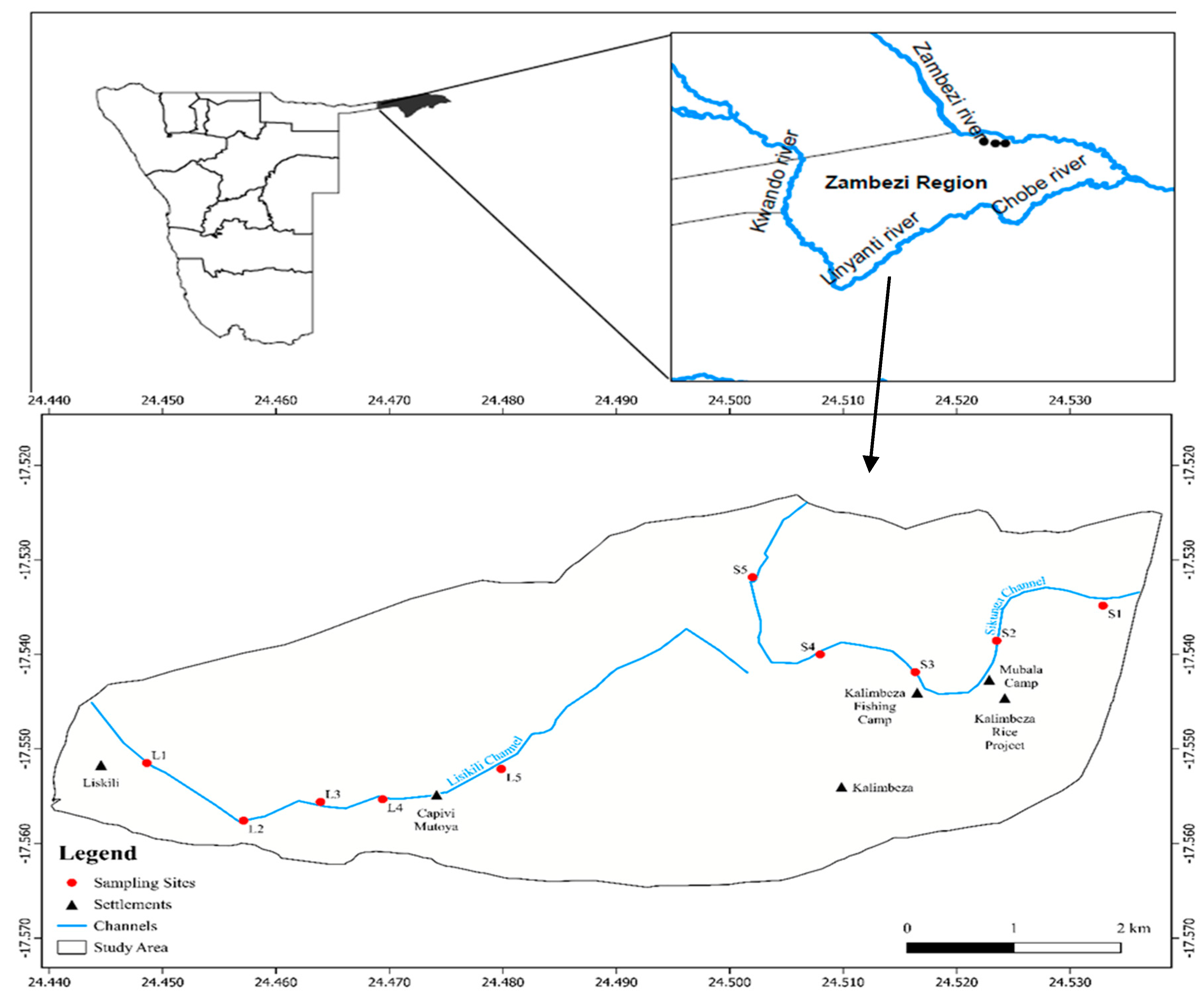
The study was conducted on the Zambezi River, located in the Zambezi Region of Namibia (
Figure 1). The region borders Botswana in the south, Angola and Zambia in the north, and Zimbabwe in the east. The Zambezi Region is home to two perennial rivers: the Kwando/Linyanti River to the west, and the Zambezi/Chobe River to the east. The Zambezi/Chobe is a highly pulsed and expansive river in terms of water volume during the flooding season. The topography of the Zambezi Region is flat terrain with an altitude ranging between 1100 m in the west and 930 m in the east. Seasonal floodwater transverses from the river catchments and spreads laterally by overflow, creating a single, large floodplain in the eastern Zambezi Region). The Zambezi/Chobe River usually reaches its peak flow between March and May, after which the water recedes until the end of September. During the dry months (November–April), the floodplains are dry and covered in terrestrial grasses.
2.2. Sampling sites
The waterbirds community was surveyed at two designated sampling stations: FPA and NFPA during the winter of 2020 (July to November) (see
Figure 1). A two (2) kilometer transect was laid out at each station. Within each transect, five (5) intra-sites were laid 500 meters apart and parallel to the water’s edge. At each site we enumerated and identified all the water-birds within a radius of 50 meters using binoculars and guides books by Reynolds and Tye, 2015.
2.3. Species composition, diversity and species richness
A comparison carnivorous water bird compositional structure between FPA and NFPA, was computed using the generalized Morisita’s similarity indices (Cm) based on the abundance data (Chao and others 2008; Jost 2008) while species richness was calculated by summing up the total number of species detected on any one the surveys trip per. Species diversity was calculated using the Shannon Diversity Index per site (Hill, 1973) using the following formula;
where: H - Shannon diversity index;
pi - proportion of individuals of i
th species in a whole community; ∑ - sum symbol; and log - the natural logarithm to base 10
where n – Number of individuals of a given species; and
N - Total number of individuals in a community,
2.4. Functional Diversity indices
A waterbirds species-trait matrix was created in order to assess functional diversity indices. We used traits that were previously suggested to situate waterbirds potential to effect and respond to wetlands changes due to disturbances (de Arruda Almeida, et al., 2016; Májek, et al., 2016). Traits included in this study were traits associated with resource use (feeding guild, major food items, feeding location and weight), breeding (breeding or non-breeding, nesting location, breeding season and clutch size) and movement (resident and migratory) (
Appendix A). We standardized each trait on a scale of 1 to 5 in order to account for equal treatment of variation with each trait (
Appendix B). We estimated functional diversity indices using FDiversity software [
25].
2.5. Species attributes
We used nine attributes that we considered important for assessing the effects and response of waterbirds to wetlands change due to anthropogenic activities. The traits used included (1) body weight (since it influence waterbirds metabolic rate, foraging behaviour, longevity and home-range size); (2) feeding guild and major food item (as it influence foraging behaviour and response to anthropogenic activities that change their main diet); (3) foraging location (since variability of anthropogenic activities may change the physical characteristics of the river); (4) breeding behaviour (nest type, location of nest, clutch size, seasonality of breeding) (as birds during breeding can transport nutrients from nearby terrestrial ecosystems to wetlands, unsustainable of wetland resources can negatively affect nesting sites of waterbirds, breeding of waterbirds are normally synchronised and clutch size influence survival rates and resilience to environmental condition; (5) and migratory status (resident or migratory) which can act as mobile linkers and influence nutrients cycling across different regions. Species attributes were obtained from Sinclair et. Al, (2000), Newman (2010) and Reynolds and Tye, (2015; Chittenden , Davies, Ingrid Weiersbye (2019). We used maximum recorded values for weight and clutch size (sensu Rutina and Moe 2014).
2.6. Functional groups and redundancy
To classify waterbirds species into different functional groups, a hierarchical classification in SPSS was performed in order to determine number of sampled groups using the elbow rule. Subsequently, the k-Classification was computed using the number of groups obtained from the hierarchical classification. The simplified Euclidean Distance (ED) was employed as a measure of variation among species in abstract space. The simplified version of ED has the formula
where ED
jk is the ecological distance between species j and k, and A
ij and A
ik are values of species
j and
k for attribute
i.
To predict the differences in species abundance between FPA and NFPA, an equation following [
18,
19] were applied
:
A significant high in abundance in FPA sites than NFPA sites requires the result of the equation to be > 1 while a significant low abundance should be < -1, with results of between -1 and 1 suggesting for equal abundance between the two sites.
2.7. Guild abundance
Community measurements of guild abundance and diversity were determined from the waterbirds surveys pooled over four months. Waterbirds were classified into three types; (predominantly feeding on Fish, predominantly feeding on Fish-insects and predominantly feeding on insects, based on their preferred food items [
23,
24]. Prior to analysis, species abundance for each site was standardized by the area of water so that abundance data were interpreted as usage per available habitat, or the relative abundance. Guild abundance was calculated as the sum of total individuals per guild averaged over the four visits and log-transformed to normalize the data.
3. Results
3.1. Carnivorous bird composition and their feeding modes
A total of 40 carnivorous waterbirds species belong to 9 orders were recorded during the survey (
Table 1). Of these 40 species, 10 (25%) predominantly feed on fish, 6 (15%) predominantly feed on insects and 24 (60%) species predominantly feed on both fish and insects. At the FPA site, a total of 35 species were recorded, comprising of 10 (29%) species that predominantly feed on fish, 4 (11%) species that predominantly feed on insects and 21 (60%) species that feed on both fish and insects. At the NFPA site, a total of 31 species were recorded comprising of 8 (26%) species that predominantly feed on fish, 4 (13%) species that predominantly feed on insects and 19 (61%) feed on both fish and insects (
Appendix A). Generally, Species composition among the three feeding guilds was averagely similar between the two sites (Morisita index: 63-71 for all pairwise combinations;
Table 2). The two sites had an overlap of 25 species, accounting for 62.5% of the total species on record (
Appendix B). Nine (9) species (Marabou Stork, Black Headed Heron, African Spoonbill, African Marsh Harrier, Water Thick-knee, Grey Heron, Whiskered Tern, African Wattled Plover, and Pink Backed Pelican) list those species herein) were exclusively recorded at the FPA while another five (5) species (Malachite Kingfisher, Long-toed Lapwing, Black Crowned Night Heron, Crowned Lapwing and Knob Billed Duck) were exclusively recorded at the NFPA.
3.2. Taxonomic diversity indices
Species diversity, species evenness and species dominance did not differ between FPA and NFA (
Table 2). However, species richness was higher at the FPA site than observed at the NFPA site (
Table 2
3.3. Functional diversity
Generally, when considering multi-trait functional diversity indices, carnivorous waterbirds ecological distance differed between FPA and Non-FPA sites (
Table 3). Carnivorous waterbirds ecological distance was higher in FPA site compared to NFPA sites. Similarity, functional evenness, function richness and functional divergence also differed between FPA and NFPA sites (
Table 3). Functional richness and divergence were higher in FPA sites compared to NFPA sites. Contrary these results, function evenness was higher at the NFPA sites compared to FPA sites.
When considering functional regulatory single traits indices, all the nine traits used in this study significantly differed between the FPA and NFPA sites, except for breeding type and clutch size. In terms of feeding guild, FPA sites were dominated by carnivorous waterbirds while NFPA sites were dominated by species that predominantly feed on both fish and insects. In terms of food items, FPA sites were dominated by piscivore birds and NFPA were dominated by water birds that feed both on fish and insects.
When considering community weighed single traits functional diversity, only four traits; food item, weight and movement type were significantly different between FPA and NFPA sites (
Table 4). Similarity to functional regulatory single trait diversity, FPA sites were dominated by carnivorous waterbirds while NFPA sites were dominated by waterbirds that feeds dominantly on insects. In terms of food items, FPA sites were dominated by piscivore birds and NFPA were dominated by water birds that feed both on fish and insects (
Table 4).
3.4. Shift in species dominance
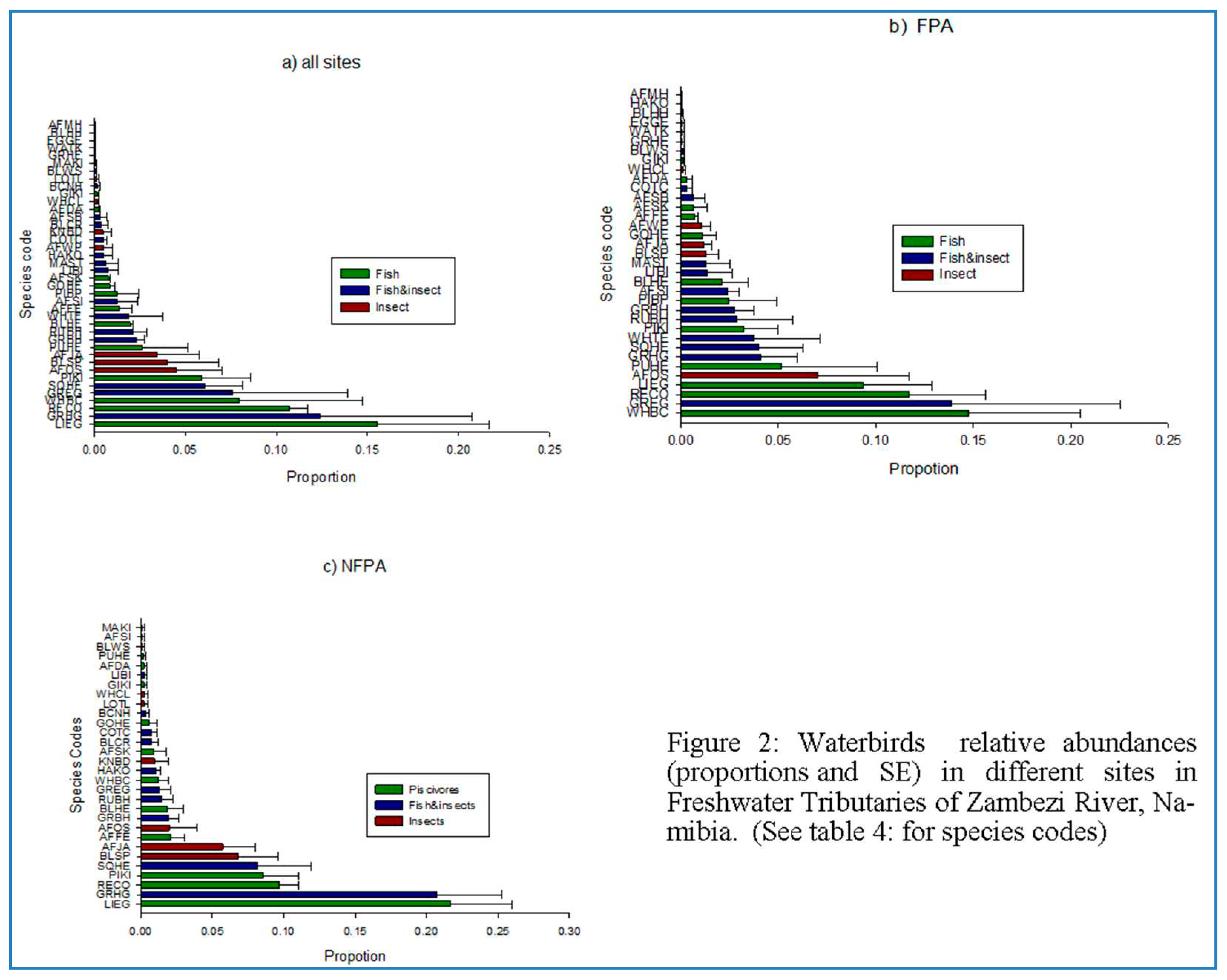
Carnivorous waterbirds at the study site were dominated by 9 (23%) species out of the possible 40 total number of sampled species. These dominant species were Little Egret (15%), Reed cormorant (15%), White Breasted Cormorant (14%), Great Egret (11%), Grey Headed Gull (10%), Squacco Heron (7%), African Open bill stock (5%), Pied Kingfisher (5%), and Blacksmith Plover (3%) (
Figure 2a). Of the nine dominant species, six (67%) feed predominantly on fish, two (22%) species feeds on both fish and insects, while only 1 (11%) feeds on insects. Further analysis showed that, at the FPA, carnivorous waterbirds were dominated by eight (23%) species out of the possible 40 total number of sampled species. These dominant species on FPA sites were White-breasted Cormorant (17%), Reed Cormorant (16%), Great Egret (14%), Little Egret (10%), Squacco, African Sacred Ibis (6%), White faced Duck (6%), Heron (5%), and Grey-headed Gull (%5). Of the eight species, three species (37.5%) feed predominantly on fish and five (62.5%) predominantly on Fish and insects. In contrast, at the NFPA sites carnivorous water birds were dominated by six (15%) species out of the possible 40 total number of sampled species. Of the, six species (67%) feed predominantly on fish, one species (17.5%) feeds on fish and insects and one species (17.5% predominantly feeds on insects. Three (3) (37.5%) out of the above 8 species (i.e. African Open bill Stork, Great Egret and White Breasted Cormorant) decreased in abundance at the NFPA sites. Of these 3 decreasing species, only the African Open bill Stork, was replaced by an increase in abundance of functionally similar but minor species (Black Smith Plover) (Fisher’s exact test, P > 0.05).
On NFPA site, the functional similarity (in terms of predominantly food items) of dominant species was similar to the functional similarity of dominant species of the whole study area (Fisher’s exact test = 1.277, P = 0.557). On FPA sites functional similarity of dominant species was dissimilar to the functional similarity of the dominant species of whole study area (Fisher’s exact test = 6.685, P= 0.034).
4. Discussion
4.1. Carnivorous waterbirds species composition and dominance
The current study aimed to explore the abundance and distribution of carnivorous waterbirds between fish protected and non-fish protected areas on the Zambezi River tributaries. A total of (40) different species, representing thirty five (35) species at the FPA and thirty (31) species at the NFPA were recorded from July to October 2021. Among these species, only a few dominant species were documented at both sampling sites, supporting our prediction that at both sites carnivorous waterbirds community abundance were dominated by few species and have many minor species. Throughout the study period, only a few dominant species were spotted at the two sampling sites, but dominancy was not equally distributed among the feeding guilds. Waterbirds species that feed on both fish and insects dominated the community assembles of waterbirds at both the FPA (60%) and NFPA (61%). This suggests that waterbirds feeding guild structure in the Zambezi tributaries is resilient to fishing pressure. It predicts that waterbirds recorded in the Zambezi tributaries can shift among available diet to sustain their food requirement. Similar distributions have been reported in other wetlands [
4,
27]), and terrestrial ecosystems [
18,
19]. Lorenzo et. Al: [
23] reported that wetlands birds were dominated by few species whenever water levels of wetlands were high or low, while Almeida
et. al; [
24] reported that waterbirds were domoinated by few species both at artificial and natural wetlands. Walker
et. al; [
25] and Rutina and Moe [
26] reported that graminoid and tree species were dominated by fewer species across grazing and browsing gradients, respectively. However, opposing results has been reported where no signs of redundancy in species dominance were observed [
22,
28]. Petchey
et. al; [
16] reported that in there were no redundancy in British avian assemblages.
Species composition between the two sampling sites was averagely similar (Morisita’s index: 0.63 to 0.71). The two sampling sites had an overlap of 25 species, accounting for 62.5% of the total species on record. These observations suggest that, the external effect of fishing did not influence the species richness and evenness distribution of carnivorous waterbirds between the two sampling sites. However, the influence of fishing pressure on other traits that are associated with growth forms and resources use strategies cannot be ruled out. Connectivity between the two sampling sites could equally explain similarity in catch composition as there is flow and exchange of similar species between two geographically connected sites in the absence of external factors such as fish pressure.
The distributions of minor species at the FPA and NFPA had functionally similar dominant species; except for waterbirds species that fed on insects (e.g. Black Smith Plover). It has been hypothesized that minor species have to be functionally similar to dominants species in order to stabilize the functions and process of an ecosystem (Brain Walker 1999; Walker, Kinzig, and Langridge 1999; Rutina and Moe 2014). However, a lack in the distributions of insectivorous species at both sites would suggest that, fish is the most predominant food item governing the distribution of carnivorous waterbirds at the two sampling sites and that competing activities such as fishing has not affected prey abundance for carnivorous waterbirds (Wenny et al. 2011; Anthal and Sahi 2017). Fishing on the NFPA is done mostly though traditional methods. In other wetlands fishing has been reported to promote fish diversity [
31,
32] and invertebrates diversity [
33] that increased abundance food resources to waterbirds [
34].
4.2. Species diversity, evenness and richness
Further analysis on species diversity and richness showed that, species richness was higher at the FPA compared to the NFPA sites. Similarly, the functional diversity indices was equally highest at the FPA (34 species) than at the NFPA sites (31 species) while the functional evenness was higher at the NFPA sites than FPA sites. Low disturbances has been associated with high diversities [
35] and species dominancy [
21]. If the concept of functional traits-ecosystem functions relationship holds, the FPA sites should act as an important refuge for a flock of species when water availability is low, enhance resource availability and accessibility to waterbirds [
36]. The current results showed that large body carnivorous waterbirds (e.g. Pink Backed Pelican) were dominantly cited at the FPA sites than at the NFPA sites. This could be linked to high abundances of prey food resources such as fish and insects at the FPA ( [
32,
33,
37]. It is expected that carnivorous waterbirds at the FPA would be expected to balance their energy intake with energy costs in order to maintain their body weight in response to prey choices.
4.3. Shift in species dominance between the two sites
Our third prediction that a decline in abundance of dominant waterbirds species at the FPA are counterbalanced by an increase in abundance of taxonomically and functionally-similar dominant species at the NFPA sites was partly supported by this study as only some species that declined in abundance under changes in fishing pressure were replaced by increasing functionally similar species at the FPA. The distributions of minor species at the FPA and NFPA had functionally similar dominant species; except for waterbirds species that fed on insects (e.g. Black Smith Plover). It has been hypothesized that minor species have to be functionally similar to dominants species in order to stabilize the functions and process of an ecosystem (Brain Walker 1999; Walker, Kinzig, and Langridge 1999; Rutina and Moe 2014). However, a lack in the distributions of insectivorous species at both sites would suggest that, fish is the most predominant food item governing the distribution of carnivorous waterbirds at the two sampling sites and that competing activities such as fishing has not affected prey abundance for carnivorous waterbirds (Wenny et al. 2011; Anthal and Sahi 2017). This has been observed in other freshwater systems like the Okavango Delta [
33] and lake Ngami [
31,
32] in Botswana. Species that predominantly feed on fish were generally reduced in their contributions to the overall abundance of species at the NFPA sites compared to FPA sites, suggesting that fishing pressure might have negatively affected the distribution and abundance of these species at the NFPA sites. On contrary insectivore waterbirds seemed to be favoured by fishing activities.
5. Conclusion
The results of this study provide an insight on the effects of fishing on population stability of the carnivorous waterbirds in the Zambezi tributaries. More studies are required to generate information on waterbirds species contributions to ecological functions and their responses to fishing at the two study sites, through intense monitoring surveys of all waterbirds food items and environmental and ecological conditions of the tributaries.
Author Contributions
Conceptualization, LPR and Y.Y.; methodology, LPR and JKM.; software, JKM.; validation, LPR., ES. and JMK.; formal analysis, JMK and LPR; investigation, LPR and ES.; resources, ES.; data curtain, JKM.; writing—original draft preparation, X.X.; writing—review and editing, X.X.; visualization, X.X.; supervision, LPR; project administration, LPR.; funding acquisition, ES.
Funding
This research was funded by University of Namibia under the office of the Pro-Vice Chancellor – Research, Innovation and Development.
Data Availability Statement
Data is available from the lead author on request.
Acknowledgments
We thank the Department of Inland Fisheries – Zambezi region, the Sikunga Conservancy management and members and the Lisikili community and fishermen for their support during data collection.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
| species name |
Species Code |
Food Item |
code itm |
weight |
weight code |
feedloc code |
Bredtype code |
Nest loca code |
maximum clutch size |
clush code |
move code |
IUCN code |
season code |
FPA |
NFPA |
| White Breasted Cormorant |
WHBC |
FISH |
1 |
3700 |
5 |
5 |
2 |
2 |
4 |
2 |
1 |
1 |
3 |
374 |
9 |
| Reed Cormorant |
RECO |
FISH |
1 |
580 |
5 |
5 |
1 |
1 |
4 |
2 |
1 |
1 |
1 |
335 |
79 |
| Little Egret |
LIEG |
FISH |
1 |
310 |
3 |
1 |
2 |
4 |
9 |
3 |
1 |
1 |
3 |
235 |
188 |
| Pink Backed Pelican |
PIBP |
FISH |
1 |
5400 |
5 |
5 |
1 |
1 |
6 |
2 |
1 |
1 |
5 |
57 |
0 |
| African Skimmer |
AFSK |
FISH |
1 |
200 |
3 |
1 |
2 |
1 |
4 |
2 |
1 |
5 |
1 |
27 |
5 |
| African Fish Eagle |
AFFE |
FISH |
1 |
3600 |
5 |
1 |
5 |
5 |
5 |
3 |
5 |
1 |
5 |
15 |
19 |
| African Darter |
AFDA |
FISH |
1 |
1400 |
5 |
5 |
5 |
5 |
4 |
2 |
5 |
1 |
1 |
7 |
2 |
| Goliath Heron |
GOHE |
FISH |
1 |
5000 |
5 |
5 |
1 |
2 |
5 |
3 |
1 |
5 |
3 |
7 |
5 |
| African Marsh Harrier |
AFMH |
FISH |
1 |
430 |
3 |
5 |
2 |
3 |
3 |
1 |
1 |
5 |
3 |
1 |
0 |
| Giant Kingfisher |
GIKI |
FISH |
1 |
425 |
3 |
1 |
1 |
2 |
12 |
4 |
1 |
1 |
3 |
1 |
2 |
| Great Egret |
GREG |
FISH-INSECTS |
3 |
1500 |
5 |
5 |
1 |
4 |
4 |
2 |
1 |
5 |
3 |
314 |
11 |
| Squacco Heron |
SQHE |
FISH-INSECTS |
3 |
290 |
3 |
1 |
1 |
2 |
11 |
4 |
5 |
1 |
5 |
131 |
70 |
| African Openbill Stork |
AFOS |
FISH-INSECTS |
3 |
1100 |
5 |
1 |
2 |
3 |
3 |
1 |
1 |
1 |
1 |
117 |
11 |
| Grey Headed Gull |
GRHG |
FISH-INSECTS |
3 |
370 |
3 |
5 |
2 |
4 |
4 |
2 |
1 |
1 |
1 |
102 |
178 |
| Pied Kingfisher |
PIKI |
FISH-INSECTS |
3 |
100 |
1 |
5 |
1 |
2 |
6 |
2 |
1 |
1 |
5 |
87 |
64 |
| African Sacred Ibis |
AFSI |
FISH-INSECTS |
3 |
1500 |
5 |
5 |
2 |
1 |
4 |
2 |
5 |
5 |
1 |
42 |
1 |
| Green Backed Heron |
GBHE |
FISH-INSECTS |
3 |
180 |
3 |
1 |
1 |
2 |
6 |
2 |
5 |
1 |
3 |
33 |
16 |
| Black Heron |
BLHE |
FISH-INSECTS |
3 |
390 |
3 |
5 |
1 |
2 |
12 |
4 |
1 |
1 |
5 |
14 |
17 |
| Whiskered Tern |
WHTE |
FISH-INSECTS |
3 |
88 |
1 |
1 |
2 |
2 |
3 |
1 |
5 |
1 |
1 |
7 |
0 |
| Coppery-tailed Coucal |
COTC |
FISH-INSECTS |
3 |
280 |
3 |
1 |
1 |
1 |
4 |
2 |
1 |
1 |
1 |
6 |
5 |
| Black Winged Stilt |
BLWS |
FISH-INSECTS |
3 |
160 |
3 |
1 |
1 |
1 |
4 |
2 |
1 |
1 |
5 |
3 |
1 |
| Purple Heron |
PUHE |
FISH-INSECTS |
3 |
1100 |
5 |
1 |
1 |
2 |
4 |
2 |
1 |
1 |
1 |
3 |
1 |
| Water Thick-knee |
WATK |
FISH-INSECTS |
3 |
440 |
3 |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
1 |
2 |
0 |
| White Crowned Lapwing |
WHCL |
FISH-INSECTS |
3 |
214 |
3 |
1 |
1 |
1 |
3 |
1 |
1 |
1 |
3 |
2 |
2 |
| Grey Heron |
GRHE |
FISH-INSECTS |
3 |
2100 |
5 |
1 |
2 |
2 |
4 |
2 |
5 |
1 |
1 |
2 |
0 |
| Black Crake |
BLCR |
FISH-INSECTS |
3 |
118 |
3 |
5 |
1 |
3 |
4 |
2 |
1 |
5 |
5 |
1 |
5 |
| Rufous Bellied Heron |
RUBH |
FISH-INSECTS |
3 |
300 |
3 |
1 |
1 |
4 |
5 |
2 |
1 |
1 |
3 |
1 |
13 |
| Hamerkop |
HAKO |
FISH-INSECTS |
3 |
470 |
3 |
1 |
1 |
3 |
5 |
2 |
1 |
1 |
1 |
1 |
8 |
| Little Bittern |
LIBI |
FISH-INSECTS |
3 |
150 |
3 |
5 |
1 |
2 |
15 |
4 |
1 |
1 |
3 |
1 |
2 |
| Marabou Stork |
MAST |
FISH-INSECTS |
3 |
8000 |
5 |
5 |
2 |
3 |
3 |
1 |
1 |
5 |
5 |
1 |
0 |
| African Spoonbill |
AFSB |
FISH-INSECTS |
3 |
1500 |
5 |
5 |
2 |
2 |
5 |
2 |
5 |
1 |
3 |
1 |
0 |
| Malachite Kingfisher |
MAKI |
FISH-INSECTS |
3 |
17 |
1 |
1 |
1 |
3 |
5 |
2 |
5 |
1 |
1 |
0 |
1 |
| Black Crowned Night Heron |
BCNH |
FISH-INSECTS |
3 |
800 |
5 |
1 |
1 |
3 |
4 |
2 |
1 |
1 |
5 |
0 |
3 |
| Knob Billed Duck |
KNBD |
FISH-INSECTS |
3 |
2900 |
5 |
1 |
1 |
2 |
5 |
3 |
1 |
1 |
1 |
0 |
9 |
| Black Smith Plover |
BLSP |
INSECTS |
5 |
160 |
3 |
5 |
1 |
1 |
4 |
2 |
1 |
1 |
3 |
44 |
48 |
| African Wattled Plover |
AFWP |
INSECTS |
5 |
250 |
3 |
5 |
1 |
2 |
2 |
1 |
1 |
1 |
1 |
29 |
0 |
| African Jacana |
AFJA |
INSECTS |
5 |
261 |
3 |
1 |
5 |
5 |
4 |
2 |
5 |
1 |
1 |
20 |
41 |
| Black Headed Heron |
BLHH |
INSECTS |
5 |
1100 |
5 |
5 |
1 |
1 |
12 |
4 |
1 |
1 |
5 |
1 |
0 |
| Crowned Lapwing |
CRLA |
INSECTS |
5 |
185 |
3 |
5 |
2 |
4 |
5 |
2 |
1 |
1 |
3 |
0 |
6 |
| Long-toed Lapwing |
LOTL |
INSECTS |
5 |
225 |
3 |
1 |
1 |
1 |
6 |
2 |
1 |
1 |
3 |
0 |
2 |
Appendix B
Table A1.
Functional attributes for the carnivorous waterbirds species recorded along the two Zambezi River tributaries, northern-eastern Namibia.
Table A1.
Functional attributes for the carnivorous waterbirds species recorded along the two Zambezi River tributaries, northern-eastern Namibia.
| Functional Attribute value |
Weight (g) |
Food item |
Feeding location |
Breeding type |
Nest location |
Maximum clutch size |
Breeding Season |
| 1 |
0-200 |
Predominantly Fish |
Ground/mud |
Monogamy |
Ground |
1-3 |
Dry |
| 2 |
|
|
|
|
Reed bed |
|
|
| 3 |
2001-1000 |
Fish and invertebrates |
|
polygamy |
Reed bed/tree |
4-10 |
Varies |
| 4 |
|
|
|
|
Tree |
|
|
| 5 |
> 1000 |
Predominantly invertebrates |
Water |
nonbreeding |
Nonbreeding |
>10 |
Wet |
References
- Green, A.J.; Elmberg, J. Ecosystem services provided by waterbirds. Biol. Rev. 2013, 89, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Bateman, H.L.; Franklin, J.; Allen, D. Waterbird community composition, abundance, and diversity along an urban gradient. Landsc. Urban Plan. 2018, 170, 103–111. [Google Scholar] [CrossRef]
- Green, A.J. The importance of waterbirds as an overlooked pathway of invasion for alien species. Divers. Distrib. 2015, 22, 239–247. [Google Scholar] [CrossRef]
- Almeida, B.d.A.; Green, A.J.; Sebastián-González, E.; dos Anjos, L. Comparing species richness, functional diversity and functional composition of waterbird communities along environmental gradients in the neotropics. PLOS ONE 2018, 13, e0200959. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Figuerola, J. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Divers. Distrib. 2005, 11, 149–156. [Google Scholar] [CrossRef]
- Frisch, D.; Green, A.J.; Figuerola, J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquat. Sci. 2007, 69, 568–574. [Google Scholar] [CrossRef]
- Green, A.J.; Jenkins, K.M.; Bell, D.; Morris, P.J.; Kingsford, R.T. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 2007, 53, 380–392. [Google Scholar] [CrossRef]
- Petrie, S. A. Influence of migrant tundra swans (Cygnus columbianus) and Canada geese (Branta canadensis) on aquatic vegetation at. ... Hydrobiologia 2006, 567, 195–211. [Google Scholar] [CrossRef]
- Hahn, S.; Bauer, S.; Klaassen, M. Estimating the contribution of carnivorous waterbirds to nutrient loading in freshwater habitats. Freshw. Biol. 2007, 52, 2421–2433. [Google Scholar] [CrossRef]
- Almeida, B.A.; Sebastián-González, E.; dos Anjos, L.; Green, A.J. Comparing the diversity and composition of waterbird functional traits between natural, restored, and artificial wetlands. Freshw. Biol. 2020, 65, 2196–2210. [Google Scholar] [CrossRef]
- Otieno, N.E.; Mutati, A.S.; Akoth, C.; Ogwanjg, D.; Mwinami, T.; Alaro, P.; Njoka, J. Role of Invertebrate Prey Abundance on Waterbird Distribution Across Rice Field Growth Stages in Western Kenya. Waterbirds 2015, 38, 47–57. [Google Scholar] [CrossRef]
- Campos-Silva, J.V.; Peres, C.A.; Hawes, J.E.; Abrahams, M.I.; Andrade, P.C.M.; Davenport, L. Community-based conservation with formal protection provides large collateral benefits to Amazonian migratory waterbirds. PLOS ONE 2021, 16, e0250022. [Google Scholar] [CrossRef] [PubMed]
- Tarakini, T.; Mabika, I.; Mwedzi, T.; Mundy, P.; Fritz, H. Diet and Life-History Traits of Savannah Dwelling Waterbirds in Southern Africa: Implications for Their Conservation Status. Birds 2021, 2, 173–184. [Google Scholar] [CrossRef]
- Májeková, M.; Paal, T.; Plowman, N.S.; Bryndová, M.; Kasari, L.; Norberg, A.; Weiss, M.; Bishop, T.R.; Luke, S.H.; Sam, K.; et al. Evaluating Functional Diversity: Missing Trait Data and the Importance of Species Abundance Structure and Data Transformation. PLOS ONE 2016, 11, e0149270. [Google Scholar] [CrossRef]
- Henry, D.A.W.; Cumming, G.S. Can waterbirds with different movement, dietary and foraging functional traits occupy similar ecological niches? . Landsc. Ecol. 2016, 32, 265–278. [Google Scholar] [CrossRef]
- Faucon, M.-P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Walker, B. The Ecosystem Approach to Conservation. pdf. Conserv. Biol. 1999, 13, 436–437. [Google Scholar]
- Walker, B.; Kinzig, A.; Langridge, J. Plant Attribute Diversity, Resilience, and Ecosystem Function: The Nature and Significance of Dominant and Minor Species. Ecosystems 1999, 2, 95–113. [Google Scholar] [CrossRef]
- Rutina, L.P.; Moe, S.R. Elephant (Loxodonta africana) Disturbance to Riparian Woodland: Effects on Tree-Species Richness, Diversity and Functional Redundancy. Ecosystems 2014, 17, 1384–1396. [Google Scholar] [CrossRef]
- Newbold, T.; Scharlemann, J.P.W.; Butchart, S.H.M. ; Şekercioğlu, H.; Joppa, L.; Alkemade, R.; Purves, D.W. Functional traits, land-use change and the structure of present and future bird communities in tropical forests. Glob. Ecol. Biogeogr. 2014, 23, 1073–1084. [Google Scholar] [CrossRef]
- Tsai, C.; Hsieh, C.; Nakazawa, T. Predator–prey mass ratio revisited: does preference of relative prey body size depend on individual predator size? . Funct. Ecol. 2016, 30, 1979–1987. [Google Scholar] [CrossRef]
- Petchey, O.L.; Evans, K.L.; Fishburn, I.S.; Gaston, K.J. Low functional diversity and no redundancy in British avian assemblages. J. Anim. Ecol. 2007, 76, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.A.W.; Cumming, G.S. Can waterbirds with different movement, dietary and foraging functional traits occupy similar ecological niches? . Landsc. Ecol. 2016, 32, 265–278. [Google Scholar] [CrossRef]
- Almeida, B.d.A.; Gimenes, M.R.; dos Anjos, L. Wading bird functional diversity in a floodplain: Influence of habitat type and hydrological cycle. Austral Ecol. 2016, 42, 84–93. [Google Scholar] [CrossRef]
- Casanoves, F.; Pla, L.; Di Rienzo, J.A.; Díaz, S. FDiversity: a software package for the integrated analysis of functional diversity. Methods Ecol. Evol. 2010, 2, 233–237. [Google Scholar] [CrossRef]
- R. P. G. Hockey P.A.R., Dean W.J.R., Roberts Birds of Southern Africa. 2005.
- Lorenzón, R.E.; Ronchi-Virgolini, A.L.; Blake, J.G. Wetland dependency drives temporal turnover of bird species between high- and low-water years in floodplain wetlands of the Paraná River. Ecohydrology 2019, 13. [Google Scholar] [CrossRef]
- Aarif, K.M.; Nefla, A.; Muzaffar, S.B.; Musammilu, K.K.; Prasadan, P.K. Traditional fishing activities enhance the abundance of selected waterbird species in a wetland in India. Avian Res. 2017, 8, 16. [Google Scholar] [CrossRef]
- Wenny, A.; Daniel, G.; Travis, L.; Matthew, D.; Cagan, H.; International, A. The Need to Quantify Ecosystem Services Provided By Birds THE NEED TO QUANTIFY ECOSYSTEM SERVICES PROVIDED BY BIRDS. 2011, 128, 1–14. [Google Scholar]
- A. Anthal and D. N. Sahi, “Feeding Guild Structure of Wetland Birds of Jammu ( J & K ), India,” pp. 1747–1753, 2017.
- D. K. Ketlhatlogile Mosepele, “FISHERY DYNAMICS IN A SHALLOW, FLUCTUATING TROPICAL LAKE: THE CASE OF LAKE NGAMI,” in Ecosystem Services and Human Well-being at Lake Ngami, Botswana: Implications for Sustainability, W. M. Donald Kgathi, Edwin Mosimanyana, Joseph mbaiwa, Ed., Environmetal Science, Engineering and Technology, 2018.
- M. M.-H. and L. P. R. Ketlhatlogile Mosepele, Kaelo Makati, Ineelo Mosie, “FISH BIODIVERSITY DYNAMICS IN A SHALLOW TROPICAL LAKE: THE CASE OF LAKE NGAMI,” in Ecosystem Services and Human Well-being at Lake Ngami, Botswana: Implications for Sustainability, wellington masamba Donald Kgathi, Edwin Mosimanyana, Joseph Mbaiwa, Ed., Environmetal Science, Engineering and Technology, 2018.
- K. Mosepele, Belda Quetina Mosepele, “Review of Aquatic Biodiversity Dynamics in the Okavango Delta: Resilience in a Highly Fluctuating Environment,” in inland waters - Dynamics and ecology, J. P. and M. M. S. Adam Devlin, Ed., 2021.
- Lucas, P. Lucas P. Rutina and Ketlhatlogile Mosepele, “POTENTIAL E COLOGICAL I MPORTANCE OF W ATERBIRDS AT L AKE N GAMI , B OTSWANA,” in Ecosystem Services and Human Well-being at Lake Ngami, Botswana: Implications for Sustainability, W. M. Donald Kgathi, Edwin Mosimanyana, Joseph Mosimanyana, Ed., Environmetal Science, Engineering and Technology, 2018.
- Costa, D.S.; Gerschlauer, F.; Pabst, H.; Kühnel, A.; Huwe, B.; Kiese, R.; Kuzyakov, Y.; Kleyer, M. Community-weighted means and functional dispersion of plant functional traits along environmental gradients on Mount Kilimanjaro. J. Veg. Sci. 2017, 28, 684–695. [Google Scholar] [CrossRef]
- Ma, Z.; Cai, Y.; Li, B.; Chen, J. Managing Wetland Habitats for Waterbirds: An International Perspective. Wetlands 2009, 30, 15–27. [Google Scholar] [CrossRef]
- Simasiku, E.; Hay, C. The significance of the Sikunga Fish Protected Area towards fisheries conservation in the Zambezi River, Namibia. Afr. J. Ecol. 2023, 61, 354–367. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).