Introduction
The most powerful available mRNA vaccines against Covid-19, reach seroconversion and efficacy rates of about 95% in the general population. In Immunocompromised patients, as kidney transplant recipients (KTR), successful seroconversion varies between 30% and 50%, and SARS-CoV-2 infection is associated with a high rate of mortality [
1]. The current vaccine strategy for KTRs appears not to provide effective protection against coronavirus disease 2019 (COVID-19) [
2] and the occurrence of severe COVID-19 in some vaccinated KTRs may suggest a lack of immunity. The first vaccine, accepted by European Medicines Agency (EMA) was the BNT162b2 mRNA COVID-19 vaccine (Comirnaty) and the active substance in Pfizer-BioNtech Comirnaty vaccine is the mRNA encoding the S protein of SARS-Cov 2 (1). There are four major structural proteins in SARS-CoV-2: spike (S), envelope (E), membrane (M), and nucleocapsid (NCP) encoded by the S, E, M, and N genes, respectively. The S protein is a critical target for inducing antibodies, particularly neutralising antibodies (Nabs): it contains the S1 and S2 subunits (1). Antibodies against all major viral antigens are detectable both during and after COVID-19, as well as after vaccination (2). Even in successfully vaccinated persons, breakthrough COVID-19 infections and disease are possible and apparently play a substantial role in the pandemia. Moreover, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with a high rate of mortality in kidney transplant recipients (KTRs) [
1,
3]. There is a wide phenotypic variation in human antibody response against SARS COV-2. The adaptive immunity involves the immunological memory and the capacity of the immune system to “learn” from many encounters with the same infections, thereby allowing the immune response to become more responsive and effective over time. When all three immunoglobulin classes (i.e. IgG, IgM, and IgA) are detectable, the maximum neutralisation activity against SARS-CoV-2 is achieved. This is a measure of the ability of the antibodies to work together in a synergistic manner. The neutralizing antibodies (Nabs) are crucial for virus clearance and to achieve protection against the virus. They may achieve this in several ways, including interfering with virion binding to receptors, blocking virus uptake into host cells, and preventing uncoating of viral genomes in endosome or causing aggregation of virus particles. In the case of COVID-19, however, their roles remain less defined, e.g. the predictive value of neutralisation with regard to disease outcome. Neutralizing antibodies are currently the most generally recognized and accepted protective correlation against a wide range of human respiratory infections. There is hitherto, no evidence of a link between in vitro neutralization titers and in vivo protection against SARS-COV-2 (4). The aim of this study was to evaluate the prevalence and levels of anti-SARS-COV 2 IgG, IgA and IgM against S1, S2 and NCP structural proteins before and after vaccination, in kidney transplant recipients, with previous o no Covid-19 infection .
Materials and methods
We assessed the humoral immune response in a group of 32 renal transplanted patients (KTR) (24 males (age 38-78); 8 females (age 46-83) (enrolled at the Hospital Pio XI of Desio, ASST Brianza, in November 2021, before and after the administration of the first booster (3° dose) of vaccine doses of the mRNA vaccine BNT162b2 (Comirnaty, Pfizer-BioNTech) to prevent coronavirus disease 2019 (COVID-19). The time interval between two blood drawn was of 17 days. Patients were divided in two subgroups: previously infected individuals (Covid positive) and no infected patients (Covid negative) to compare their antibody response directed against the antigenic nucleocapsid protein (NCP) , confirmed by the nasopharyngeal swab and the molecular testing (RT-PCR). Their humoral response was analysed by typing the Immunoglobulin classes, and compared with a group of 23 healthy workers classified as Late responders (HCW-LR) (10 males age 27-62) and 13 females (age 46-64) which, in a previous work (5), showed a slow but steady increase in antibody concentration in the first 4 months, after 2 doses of vaccine; this distinguishes this group from the others in whom the value at the same time, shows a vaning trend. Antibodies against the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) and the nucleocapsid (N) protein were evaluated using two methods: ElecsysR anti SARS-CoV-2, (ECLIA) Roche Diagnostics, chosen as reference, and with the heterogeneous competitive immunoenzymatic method ACE2-RBD neutralization assay (Diagnostics bioprobes srl DIA.PRO, (Via G. Carducci, 27, 20099 Sesto San Giovanni, MI) for the semiquantitative determination of inhibition activity of RBD-ACE2 binding induced by antibodies to SARS CoV-2. The Roche method is an immunoassay for quantitative determination of antibodies (including IgG) to the SARS-CoV-2 spike (S) protein receptor binding domain (RBD) in human serum and plasma. The assay uses a recombinant protein representing the RBD of the S antigen in a double-antigen sandwich assay format, which favors detection of high affinity antibodies against SARS-CoV-2. The test is intended as an aid to assess the adaptive humoral immune response to the SARS-CoV-2 S protein, based on ECLIA methodology and used on the Cobas e602 module 8000. Samples with result > 0.80 BAU /mL (binding arbitrary units) were classified as positive for anti-SARS CoV-2 antibodies according to the manufacturer's directions. The 1:10 dilutions were performed on serum samples with values greater than 250 BAU/mL, while 1: 100 on serum samples with values greater than 2500 BAU /mL. The heterogeneous competitive immunoenzymatic method of Diagnostics bioprobes srl (DIA.PRO), allows to determine the neutralizing activity of anti-SARS-CoV-2 antibodies in serum samples, by incubating the sample to be analyzed with the spike / RBD protein. After washing, free RBD / spikes are determined by adding the recombinant ACE2 biotinylated protein and then streptavidin conjugated with horseradish peroxidase (SAV-HRP). The color is generated by tetramethylbenzidine / hydrogen peroxide (TMB /H2O2) if no antibody is bound, while a strong inhibition of color development is observed if antibodies to RBD have blocked the binding of ACE2 labeled with biotin. The presence of this antigen on the solid phase is determined by the addition of SAV-HRP which binds to ACE2 if no neutralizing antibody is present, or does not bind, if the antibodies have blocked the RBD adhering to the well. The serum samples examined were analyzed in the typing test of the immunoglobulin classes IgA, IgG and IgM anti-S1, S2 and NCP for sera collected at several time point. The immunoenzymatic method allows to carry out both the qualitative screening and the quantitative titration test. Furthermore it is possible to carry out a typing of Ig classes produced before and after vaccination; all serum samples were analyzed on the basis of the type of Ig classes produced IgG, IgM, IgA, against the main antigens of Sars-CoV-2 (S1, S2 and NCP) after natural infection or vaccination.
Results
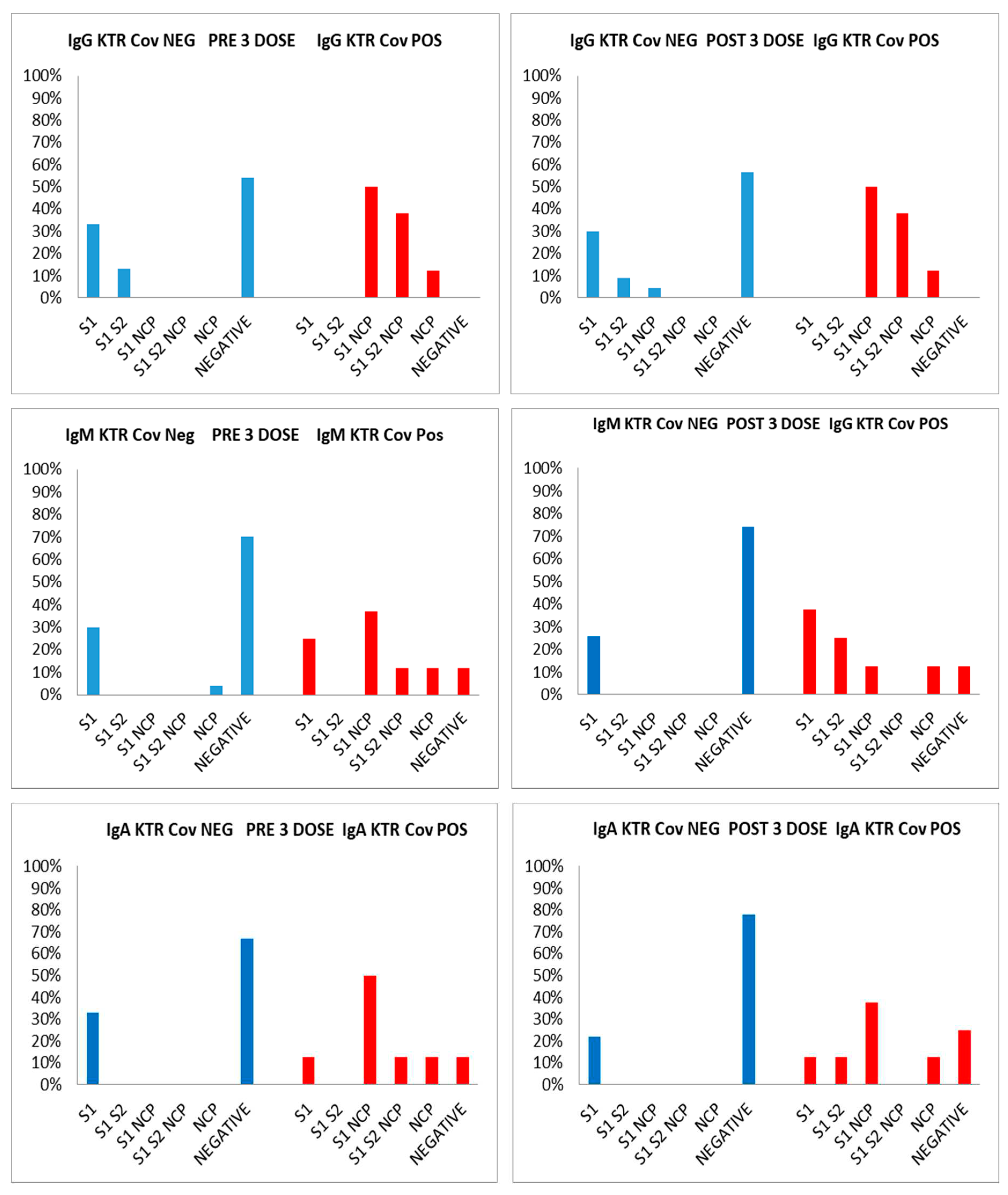
Serum samples (CAT serum sep clot activator 3.5 mL Greiner) were taken from the patients and from operators and gap time between two bloodrawn for this group was comprised between 62 and 128 days (median 60 days). Both groups received the first cycle of vaccine (2 doses) 10 months before. Regarding antibody response of the 24 kidney transplant recipients KTR Covid negative and the 8 positive subjects, the time lapse between two bloodrawn and third dose was 17 days. The analysis of the kinetics of the antibody response before the third dose, have shown the following results for Covid negative patients : 33% (8 pts) were IgG positive to S1 protein, 13% (3 pts) to S1/S2; 54% (13 pts) showed no response at all; for IgM only one patient (4%) was positive to NCP, 7 patients (30%) to S1 and 66% show no response at all; for IgA 6 patients were positive to S1, 18 patients show no response at all. As regards Covid positive patients 50% (4 out of 8 subjects) had IgG vs S1/NCP, 38% (3 pts) develop Ig anti S1/S2/NCP, and only 12.5% (1 subject) with anti-NCP Ab. For IgM: 25% (2 pts) with anti S1, 37% with anti S1/NCP, 12% with anti S1/S2/NCP, and 12% with anti NCP, and 12% negative for all antigens. For IgA only one patient (12.5%) had anti S1 Ab, 50% with anti S1/NCP Ab. After the third dose in Covid negative patients, 9 patients (30%) showed IgG to S1, 9% (2 patients) to S1/S2, and 1 to S2/NCP (4.5%). The 56.5 % individuals of this group (13) were negative to all proteins. An interesting data concerns the IgM and IgA type response with 26% (6) and 22% (4) of subjects who responded only to S1 and 74% (17) and 78% (18) without any Ig response (see Table 2). After the booster the following results were observed for patients Covid positive: 50% had anti S1/NCP IgG (4 pts), 38% had anti S1/S2/NCP (3 pts), 12% i.e. only one patient with anti NCP IgG. For the IgM response, 37.5% (3 pts) had anti S1 ab, 25% anti S1/S2, 12.5% anti S1/NCP ab, and only 1 pt positive for anti-NCP. For IgA we observe 12.5% (1 pt only) positive for anti S1, and also for anti S1/S2; 37.5% (3 subjects) have anti S1/NCP IgA, 1 only with anti-NCP ab, while 25% (2 pts) did not produce antibodies. In both groups, Covid and negative, we observed that patients that show no response at all when on major immunosoppressive therapy (see
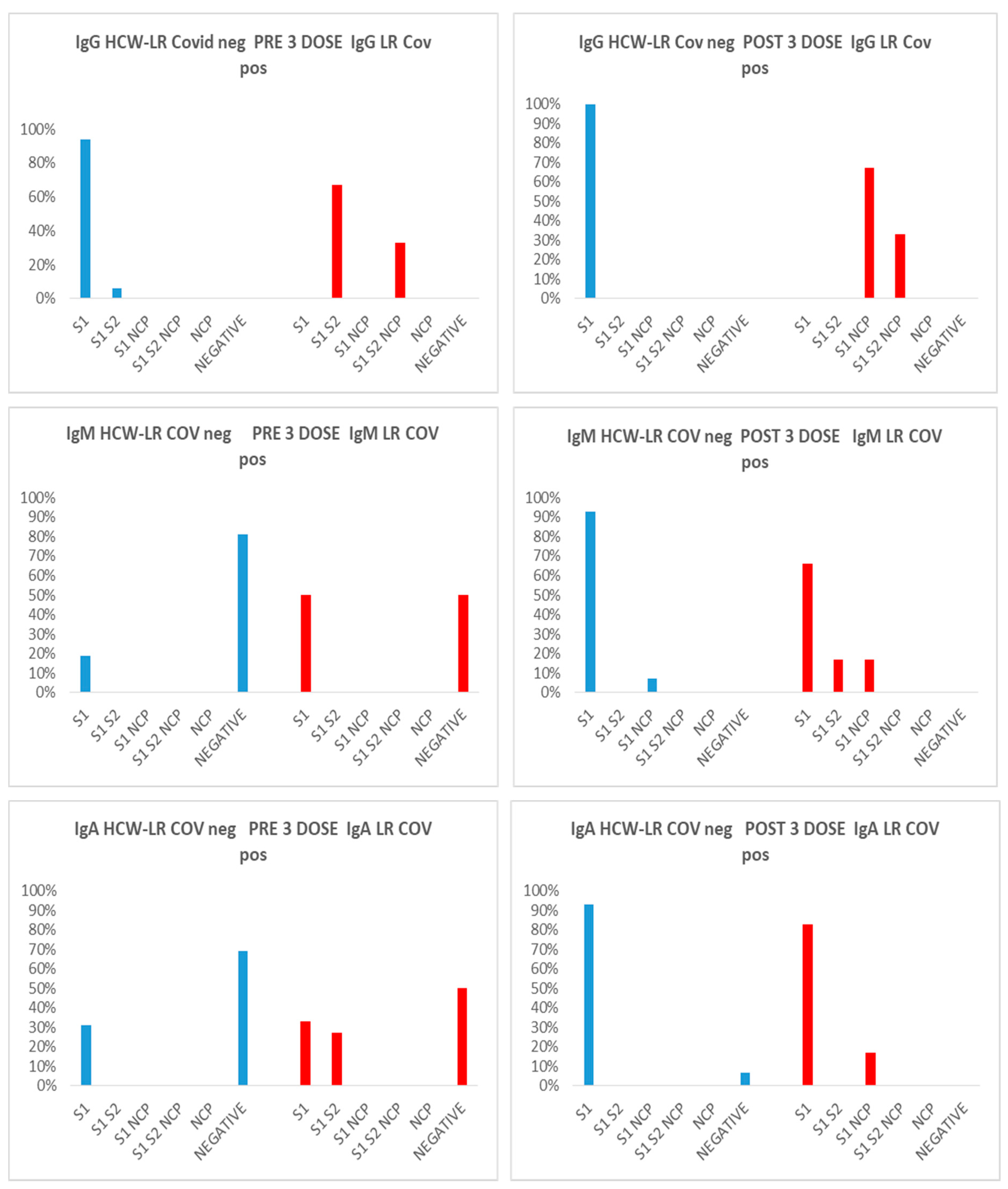
Figure 1). The prevalence of antibodies was also analyzed according to gender; the prevalence of males was higher than females in most groups, but, interestingly, in Covid positive patients, the prevalence of IgG anti S1/S2/NCP was higher in women than in men , either before or after the third dose, 67% vs 33%, and 67% vs 33% respectively. In the same way, in Covid positive patients, before the third dose, also prevalence of IgM anti S1/NCP was higher in women than in men, 67% vs 33%, while, after the third dose, we have the same prevalence in the groups. The same analysis was performed on the group of healthy workers HCW-LR with the following results: the negative Covid group responds positively to the vaccine stimulus received 9-10 months after the 1 cycle of two doses producing Ig of all three classes with an increase in the anti-S1 IgG response. Regarding the group of Covid positive subjects, an increase in the anti-S1 IgG response was observed after the booster, an index of vaccine efficacy, and the appearance in 43.5% of cases (10 subjects/23) of a direct IgM and IgA response also against the NCP protein, expression of a reinfection of these subjects after the 3rd dose. Of these, 70% of the positive are females (see
Figure 2).
Discussion
We compared the prevalence of all types of antibodies before and after the booster vaccine in a group of kidney transplant recipients with or without previous Covid infection. The group of Covid positive patients, either before or after vaccination, have IgG antibodies against S1S2NCP, while patients with no previous Covid infection don’t develop this class of antibodies. Blaszczuk et al. (6) showed, similarly, that NCP and S2 IgG antibodies were more frequently present in people with COVID infection. Currently approved COVID-19 mRNA vaccines generates antibodies to S1 protein, while antibodies to NCP are detectable only after natural infection. More than two years passed by, after the pandemic declaration for Coronavirus disease 2019 (COVID-19), but severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) still continues to cause a significant number of infections despite many individuals are immunized or naturally infected with the virus. The presence of neutralizing antibodies is generally correlated with being immune protected from SARS-CoV-2 infection. These antibodies bind the receptor-binding domain (RBD) of the spike protein and prevent the entry of the virus inside human cells. Several studies indicated that COVID-19 vaccination and SARS-CoV-2 infection form neutralizing anti-spike antibodies and robust T-cell responses against several viral epitopes; such responses were detectable up to one-year post-immunization, but a significant decrease was observed within the first few months. This can explain why several immunized individuals were re-infected with the virus (7,8). In a previous paper, we demonstrated that specific anti-S antibodies are significantly decreased 4 months post priming dose of Comirnaty vaccine although prior COVID-19 infection seems to escalate humoral response (5); further evaluation concerning antibody persistence beyond this point, and the proportion of neutralizing antibodies with higher affinity towards SARS-CoV-2 is needed, especially in naїve and immunosuppressed subjects. The investigation of humoral response to SARS-CoV-2 represents a key aspect in facing the COVID-19 pandemic. Although neutralizing antibodies are considered to have an important protective role, the association between seropositivity and immunity, as well as the duration of protective humoral response represent key questions of current research [
1,
2,
3,
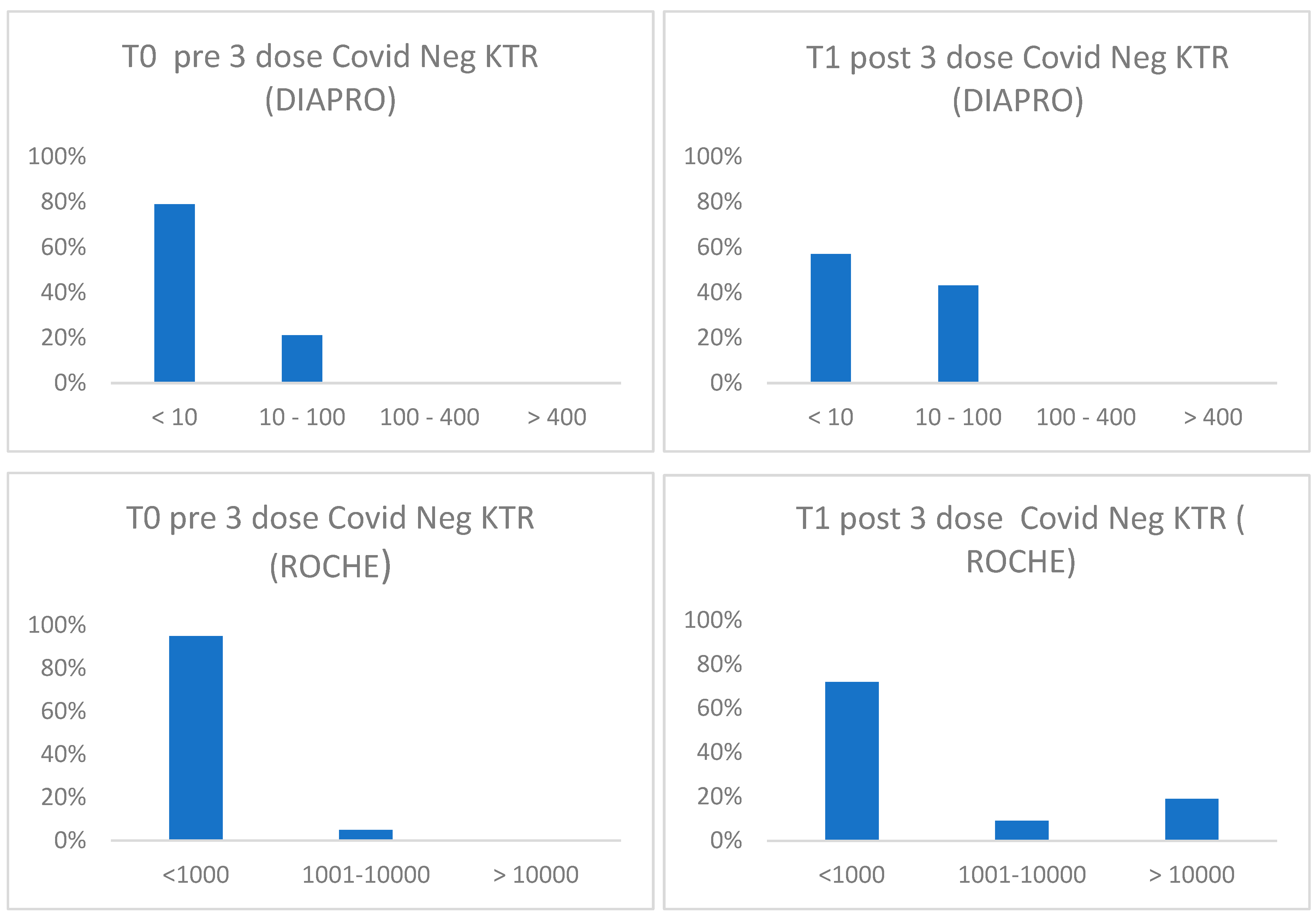
9]. The FDA declared a neutralizing titer ≥ 1:160 as sufficient for plasma donations. However, the definition of an antibody titer conferring protection is still missing. Recently a group of researcher determined the levels of protective antibodies after vaccination against COVID-19 in nearly 9,000 healthcare workers, establishing for certain levels of antibody concentration, measured by two laboratory methods, increasing levels of protection. We therefore used a similar approach to verify the distribution of our group of individuals based on the results obtained with the two methods to analyse their antibody response (
Figure 3). Consistent with literature data, the humoral response of vaccinated KTR patients without previous infection is not optimal compared to vaccinated people with a previous natural infection. Among all the subjects, a lower drug-related immunosuppression was associated with a better antibody response. In all transplanted patients (covid positive and negative) the titrated concentration of neutralizing antibodies (nAb) increases after the booster but does not go beyond 64 BAU/mL indicating the need for further vaccine boosters in this group of immunocompromised patient (10,11). In fact, after the 3rd dose 9 out of 32 subjects (28,1%) reinfected themselves compared to only 2 subjects (6,25%) before the booster. Given the high number of people who have survived at least one SARS-CoV-2 infection and the high vaccination coverage in the population, it is important to estimate the protective role of immunity associated with both the vaccine and the previous infection in preventing infection and severe COVID-19 disease. Maximum protection against the diagnosis of SARS-CoV-2 infection and severe disease may be achieved through hybrid immunity (the combined effect of vaccination and previous infection) while higher risk levels are always found among unvaccinated people and without a previous diagnosis of infection.
Figure 3.
Distribution of antibodies against SARS-CoV-2 according to total binding antibody concentration BAU/mL (Roche) or neutralizing antibody titer (Diapro) in covid negative KTR before and after 1° booster.
Figure 3.
Distribution of antibodies against SARS-CoV-2 according to total binding antibody concentration BAU/mL (Roche) or neutralizing antibody titer (Diapro) in covid negative KTR before and after 1° booster.
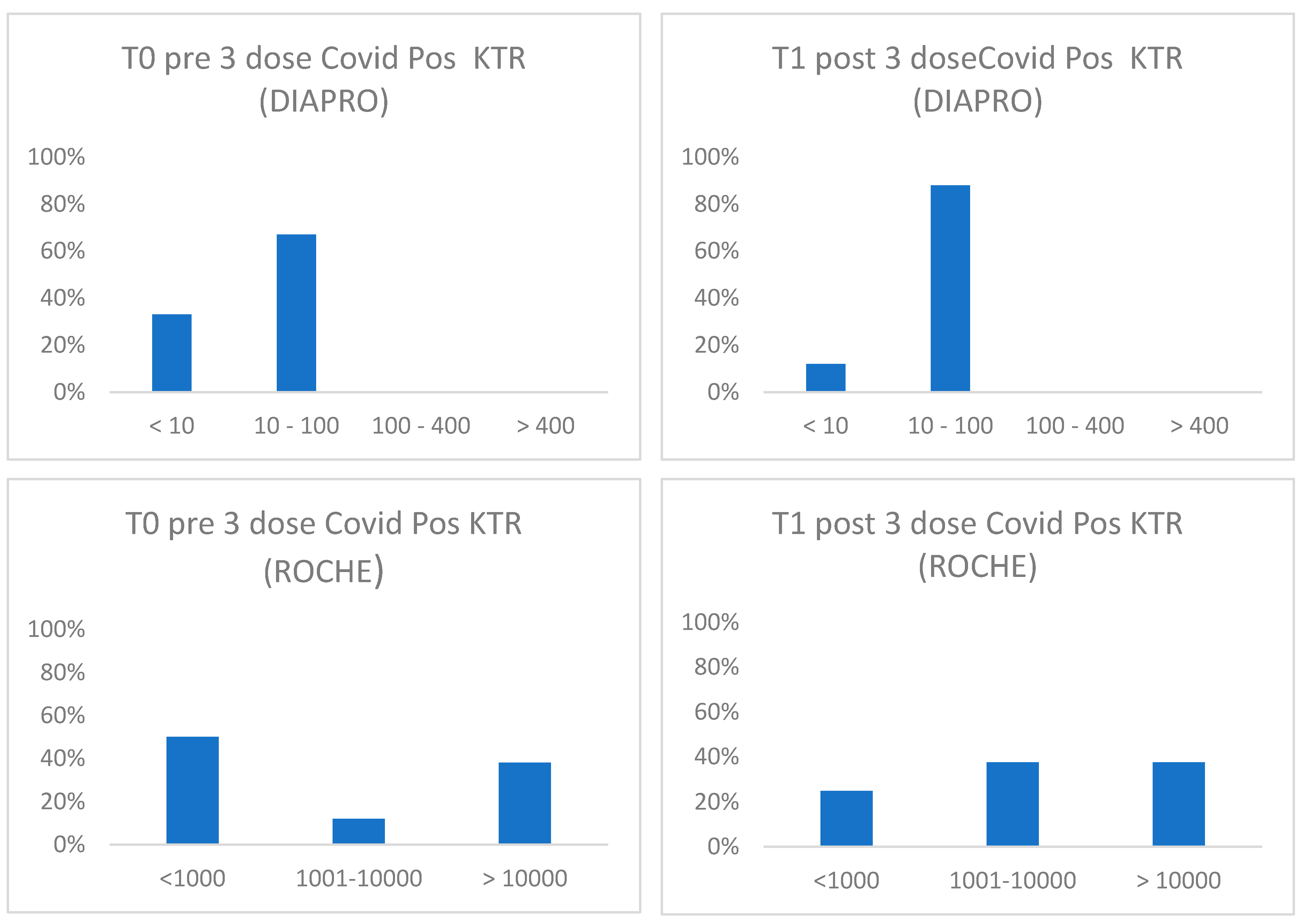
Figure 4.
Distribution of antibodies against SARS-CoV-2 according to binding (Roche) or neutralizing antibody classes (Diapro) in covid positive KTR before and after 1° booster.
Figure 4.
Distribution of antibodies against SARS-CoV-2 according to binding (Roche) or neutralizing antibody classes (Diapro) in covid positive KTR before and after 1° booster.
An immune response capable of determining protection and statistically correlated to it, is defined as correlate of protection (CoP), which needed identification of immunological markers and a relative threshold of protection against infection. To date these correlates have not been unequivocally defined although neutralizing antibodies are thought to be an essential component.
Limitations of our study include is the relatively small participants group, the observational, non-randomized study character, the selection bias towards personnel and patients interested in SARS-CoV-2 vaccination and lack of demographic matching between different groups. Kidney transplant recipients demonstrate an impaired humoral response, which correlated with the type and number of immunosuppressive agents too. Our study did not assess cell-mediated immunity. However, the data suggest that monitoring the neutralizing antibody response and total antibody concentrations, practically more feasible can be used to optimize vaccination strategies evaluating the duration and degree of protection provided by vaccines. The thresholds of protection found in our study should be compared to those obtained in further studies on other populations and with of a larger number of patients. It is also essential to estimate the influence of an antibody’s reduced neutralizing capacity against new emerging viruses variants.
References
- Risk of strong antibody decline in dialysis and transplant patients after SARS-CoV-2mRNA vaccination: Six months data from the observational Dia-Vacc study. Julian Stumpf, Jorg Schwobel, Tom Lindner, Leona Anders, Torsten Siepmann, Claudia Karger, Jan Huther, Heike Martin, Petra Muller, Robert Faulhaber-Walter, Torsten Langer, Holger Schirutschke, Thomas Stehr, Frank Meistring, Annegret Pietzonka, Kirsten Anding-Rost, Katja Escher, Frank Pistrosch, Jens Schewe, Harald Seidel, Kerstin Barnett, Thilo Pluntke, Simon Cerny, Alexander Paliege, Ingolf Bast, Anne Steglich, Florian Gembardt, Friederike Kessel, Hannah Kroger, Patrick Arndt, Jan Sradnick, Kerstin Frank, Anna Klimova, Ren Mauer, Xina Gr€ahlert, Torsten Tonn, ad and Christian Hugo. www.thelancet.com Vol. 17 Month June, 2022, 1-18. [CrossRef]
- Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. Boyarsky BJ, Werbel WA, RK Avery et al. JAMA 2021; 325: 2204–2206. [CrossRef]
- Occurrence of severe COVID-19 in vaccinated transplant patients. Caillard S, Chavarot N, Bertrand D et al. French Society of Transplantation: Kidney Int 2021; 100: 477–479. [CrossRef]
- Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice Natalie Yan-Lin Pang, Alexander Shao-Rong Pang, Vincent T. Chow, and De-Yun Wang. Military Med Res (2021) 8: 47. [CrossRef]
- Antibody response after two doses of the SARS-CoV-2 Comirnaty vaccine in a Covid-19 positive and Covid-19 negative Italian healthcare workers cohort. Sulejmani A, Giacobone C, Spiti S, et al. Scand J Clin Lab Invest 2022; 82:90-5. [CrossRef]
- Antibodies to NCP, RBD and S2 SARS-CoV-2 in Vaccinated and Unvaccinated Healthcare Workers. Agata Błaszczuk , Aleksander Michalski, Maria Malm, Bartłomiej Drop and Małgorzata Polz-Dacewicz. Vaccines 2022, 10, 1169. [CrossRef]
- Humoral and T-cell response to SARS-CoV-2 mRNA BNT162b2 vaccination in a cohort of kidney transplant recipients and their cohabitant living kidney donor partners. Vincenzo La Milia, Silvia Tonolo, Francesco Luzzaro, Claudio Bonato, Donatella Casartelli, Monica Limardo, Selena Longhi, Chiara Ravasi, Sara Viganò and Andrea Cavalli. Clinical Kidney Journal, 2022, vol. 15, no. 4, 820–821. [CrossRef]
- Antibody titers and protection against a SARS-CoV-2 infection. Chloé Dimeglio, Fabrice Herin, Guillaume Martin-Blondel, Marcel Miedougé, Jacques Izopet. Letters to the Editor / Journal of Infection 84 (2022) 248–288. [CrossRef]
- Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Werbel WA, Boyarsky BJ, Ou MT et al. Ann Intern Med 2021; 174: 1330–1332. [CrossRef]
- Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Julian Stumpfi, Torsten Siepmann, Tom Lindner, Claudia Karger, Jörg Schwöbel, Leona Anders, Robert Faulhaber-Walterg, Jens Scheweh, Heike Martini, Holger Schirutschk, Kerstin Barnett, Jan Hüther, Petra Müllerm, Torsten Langern, Thilo Pluntk, Kirsten Anding-Rost, Frank Meistring, Thomas Stehrr, Annegret Pietzonkas, Katja Eschert, Simon Cernyu, Hansjörg Rothev, Frank Pistroschw, Harald Seidelx, Alexander Paliegea, Joachim Beiged,, Ingolf Bast, Anne Steglich, Florian Gembardt, Friederike Kessel, Hannah Kröger, Patrick Arndt, Jan Sradnick, Kerstin Frank, Anna Klimova, René Mauer, Xina Grählert, Moritz Anft, Arturo Blazquez-Navarro, Timm H Westhoff, Ulrik Stervboad, Torsten Tonn, Nina Babel, Christian Hugo. The Lancet Regional Health Europe 9 (2021) 100178. [CrossRef]
- Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. Dominique Bertrand,1 Mouad Hamzaoui, Veronique Lemee, Julie Lamulle, Melanie Hanoy, Charlotte Laurent, Ludivine Lebourg, Isabelle Etienne, Mathilde Lemoine Frank Le Roy, Dorian Nezam, Jean-Christophe Plantier,2 Olivier Boyer , Dominique Guerrot , and Sophie Candon. JASN 32: 2147–2152, 2021. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).