1. Introduction
One of the main diseases of worldwide concern is non–insulin dependent Diabetes mellitus (NIDDM), caused by an insulin resistance or a decreased secretion by Langerhans β cells of this hypoglycemic hormone.
After ingestion, sugars and carbohydrates are digested and reduced to glucose, which is absorbed in the intestine. This increase of glycaemia (glucose levels in blood) promotes the release of hormone insulin in the pancreas that lowers glucose levels. The β–cells in pancreas secrete insulin so that glucose can enter into cells as a cell nutrient. In addition, insulin stimulates the formation of glycogen in the liver and thus blood glucose to normal values. The antagonistic hormone produced by the endocrine side of the pancreas, glucagon, stimulates glycogen degradation when there is a lack of glucose intake and glucose is needed. This is a simplification of endocrine feedback to control glycaemia and body energy balance. However, as occurs with the antioxidant reactionsin the body, these can also be altered giving rise a worldwide–extended disease: Diabetes. There are three main types of diabetes, depending on the cause of the problem [
1].
The first type of diabetes (
Type I diabetes), whose cause is autoimmune since the organism itself destroys the β–cells of the pancreas,concerns5–10% of all diabetic patients.[
2]. For being a non-curable disease, patients need to inject insulin daily as treatment because this function of the pancreas is partially or totally disabled. Although engineering and pharmaceuticals have played such a helpful role with diabetics (insulin pumps, different types of insulin depending on the speed of action, mobile applications with glycaemia predictions…), science continues to search for a cure in theories such as the microencapsulation of β–cells, transplantation or genetic theories.
On the other side, 90-95% of patients with diabetes suffers from
Type II diabetes, a metabolic diabetes whose prevalence is increasing in the world [
2]. Generally, these patients are adults whose risk factors (overweight, sedentary lifestyle, non–healthy diet or family history) lead their bodies to insulin resistance.This disease can be sufferedsilently and asymptomatically without medical diagnosis.Therefore, glycaemic monitoring is important to keep resistance under control with hygienic–dietary changes in lifestyle. As prevention, glycaemic can be periodically monitored in pharmacies or MD’s and takes theFindrisk test (questioning the risk of suffering type II diabetes in 10 years). A low score in this test does not mean a lack of pre–diabetes or diabetes. Treatment begins with daily oral medicines that affect different pathways (DPP–4, GLUT–1, α–glycosidase…). If glycaemia and HbA1c (glycosylated haemoglobin that reflects mean glycaemia within last 2–3 months) values remain under control, monotherapy is sufficient. However, when the patient presents glycaemic alterations –reflected by a high HbA1c value (>7%) – more than one type of hypoglycaemic treatment is needed and sometimes insulin is part of this treatment.
Last but not least, there is a third type of diabetes (Gestational diabetes) that can appear during pregnancy. Both mother and child may develop diabetes if the glycaemia is not controlled during pregnancy.
For all the three types of diabetes, patient education and selection of the optimal treatment for them are important. When the disease is not treatedwell, blood glucose is so high that it can cause tissue damage due to overproduction of superoxide in cells, leading to potentially fatal complications such as retinopathies, limb amputations, neuropathies, renal and cardiovascular (CVD) pathologies or even premature death.
Most of the current preclinical research with
in vivo model focus on the first type of diabetes, since the profile is a young normal-weighted patient and the cause is autoimmune and cannot be prevented. Nevertheless, could there be an effective natural alternative to prevent and treat Type II diabetes whose prevalence is 530 million adults worldwide[
3] and now also occurring increasingly frequently in children[
2]?
Among antidiabetic treatments, inhibition of the α–glucosidase pathway has been shown to be effective in delaying intestinal absorption of polysaccharides and disaccharides. This enzyme, located in the membrane–bound epithelium of the small intestine, is responsible for the cleavage of glucose from ingested disaccharides.
Despite the antidiabetic efficacy of acarbose (lowering HbA1c), digestive problems such as flatulence (probably affecting more than 1 in 10 people), stomach pain and diarrhoea (probably affecting fewer than 1 in 10 people) are the main side–effects that limit it success in prescribing[
4]. In addition, acarbose causes rare side effects (likely to affect less than 1 in 100 people): nausea, vomiting, increasing liver enzymes and indigestion [
5].
Given the current prevalence of diabetes type II in society [
1], research of natural compounds able to regulate glycaemia and insulin–resistance is nowadays covering importance in industry, as alternative or co–adjuvant treatment to existing hypoglycaemic medicines.Many plants with
α–glucosidaseinhibitory activity have been shown to generate less side effects [
6,
7,
8,
9,
10].
In pre–clinical steps of research,
C. elegans is considered a promising
in vivo model for the study of the molecular mechanism of glucose as part of anti–diabetic bioactivity [
11]. The strains are cheap to breed and can be frozen, allowing for long–term storage. Moreover, thanks to be the first multicellular organism with a complete genome sequence [
12], mutations and molecular identification of many key genes can be studied to identify behavioural defects. Indeed, at least 38 % of the protein–coding genes in
C. elegans have predicted orthologs in the human genome [
13], 60 – 80 % of human genes have predicted orthologs in the
C. elegans genome [
14], and 40 % of genes known to be associated with human diseases have clear orthologs in the
C. elegans genome [
15].
Most of the studies of insulin resistance with this
in vivo model are carried out by using mutant strains such as
daf-2, an insulin-like receptor gene, to understand the antidiabetic behaviour of a compound. However, the effects of the compounds are attributed
a posteriori [
11,
16,
17,
18], after a physiological response of the worms rather than directly measuring preservation of activity inside the worm. In this context, there is a need on finding a simple and reliable method with this
in vivo model in order to quantify directly pharmacological activity of the compound given as treatment for Type II diabetes.
In previous study, hydroalcoholic extract of
Origanumvulgareshowed significant antioxidant activity both
in vitro and
in vivo [
19]. According to literature, this medicinal plant count with many different pharmacological activities that might be significant in metabolic age-related pathologies of current interest in society such as Diabetes type II [
20,
21,
22]. Therefore, themain aim of this work isto find a new method able to easily determine hypoglycaemic activity
in vivo model and so, certainly attribute the response to the effect of the treatment.
In order to achieve that, the study starts evaluating the in vitro hypoglycemic activity of two oral formulations with Origanumvulgare extract before and after a simulated process of gastrointestinal digestion. After confirming the in vitro activity, the hypoglycemic activity is testedin vivo through the new method proposed. Results would determine the robustness of the method and if there is an in vitro-in vivo co-relation.
2. Results and Discussion
Diabetes mellitus is a chronic disease that, if left untreated, leads to serious and disabling complications that reduce the quality of life of those affected and increase the cost of care. It is the most common endocrine disease and its prevalence is steadily increasing worldwide. Of the different types of diabetes, type II is the most common. It is characterised by a gradual course in which changes in the mass and function of pancreatic beta cells precede a decrease in the responsiveness of peripheral tissues to insulin. These patients do not initially require insulin therapy, and glycaemic control can be achieved through weight loss, physical activity, or oral hypoglycemic agents. Oral hypoglycemic agents such as biguanides, thiazolidinediones, and sulfonylureas can be effective in controlling hyperglycemia. However, they have significant side effects, including hypoglycemia and gastrointestinal problems [
23].
There is an urgent need for effective substitutes to reduce diabetes complications with fewer side effects. In recent years, the search for alternative medicines for the treatment of diabetes has attracted considerable attention. Natural products play a key role in this effort. There are more than 400 traditional medicinal plants known to have antidiabetic properties from their bioactive compounds. However, only a few of them have been sufficiently studied scientifically and medically to prove their efficacy [
24].
Naturally, occurring polysaccharides are primary metabolites found in all plants and are therefore essential for their development. These compounds, usually derived from medicinal plants, grains, fruits, vegetables, edible fungi and foods, have been extensively studied in recent years due to their low toxicity and numerous pharmacological activities, including antidiabetic activity [
25,
26]. Several studies have shown that purified polysaccharides from pumpkin, sea cucumber, goji berry, mushrooms, green beans, tea, and oats have beneficial effects on glucose homeostasis, reduce diabetic complications through the defence mechanism against oxidative stress damage, and ultimately improve insulin sensitivity [
27,
28,
29]. Carbohydrates are ingested in the form of polysaccharides and are converted in the gastrointestinal tract by the digestive enzymes α-amylase (salivary or pancreatic) and α-glucosidase (small intestinal) into monosaccharides, which are readily absorbed by the small intestine and enter the bloodstream. Thus, inhibition of these enzymes prevents this conversion and is an effective step in controlling blood glucose levels in diabetics. Carbohydrates that are not digested in the small intestine are metabolised in the large intestine by intestinal bacteria, which explains the frequent side effects of these drugs (meteorism, flatulence, diarrhoea, etc.).
Therefore, the search for extracts/compounds that inhibit these enzymes could be an effective strategy to combat this pathology. In this work, the inhibitory activity of the enzyme α-glucosidase was tested two formulations proposed for a hydroalcoholic extract of
O. vulgare. Matsui
et al. [
30] describe a method to spectrophotometrically determine the inhibition of this enzyme by a substrate that decomposes into a yellow compound and glucose after exposure to α-glucosidase. Acarbose, one of the drugs marketed for oral treatment of noninsulin-dependent diabetes (type II diabetes), was used as a positive control in the analysis because it impairs intestinal absorption of carbohydrates and reduces postprandial hyperglycemia by competitively inhibiting the action of intestinal α-glucosidase, which hydrolyses oligosaccharides and polysaccharides. Rodriguez-Solana
et al. [
31] also used this compound (1 mg/ml) as a positive control and showed 78.34% inhibition of the enzyme α-glucosidase and 78.33% inhibition for α-amylase. As shown in
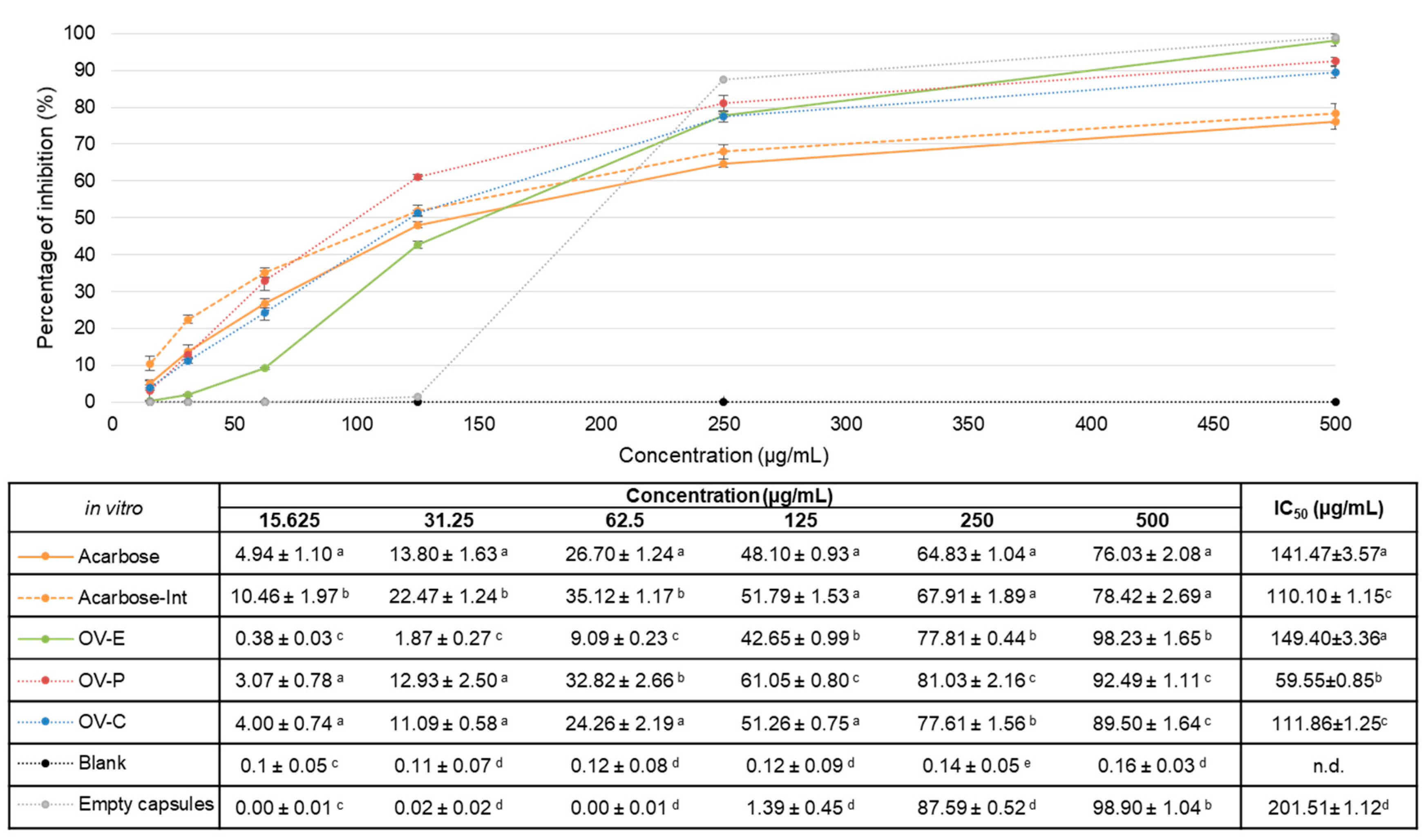
Figure 1, in this current study, the same positive control at 0.5 mg/mL showed a percentage of inhibition of 76.03±2.08% before digestion and 78.42±2.69% after the gastrointestinal digestion process.
In vitro was OV-E as effective as acarbose, a marketed oral anti-diabetic drug used as a positive control.
In general, the inhibition of the enzyme was enhanced with the intestinal fraction, what suggests that not only might the activity be maintained in the intestine – where the enzyme is physiological located -, but also improved. The crude extract (OV-E) before the gastrointestinal digestion process showed an IC
50 value similar to acarbose (positive control), 149.40±3.36 and 141.47±3.57 µg/mL, respectively with
p>0.05 (
Figure 1).According to the results, two formulations after the digestion step presented higher bioactivity than the extract before digestion (OV-E). Neither the activity of the excipients nor the digestion enzymes can be related to this enhance of activity since the
Blank did not present any inhibitory activity (being highest 0.16±0.03%, with
p<0.05 –
Figure 1). In the case of the capsules, even if the hard gelatine capsules were completely disaggregated, the intestinal fraction of the empty capsules, used as control, did not present any potentially inhibitory activity, being IC
50=201.51±1.12 µg/mL, with
p<0.001 for the rest of results.
Once dismissed possible secondary pharmacological activities from the pharmaceutical forms, the encapsulated extract (111.86±1.25 µg/mL) did not present statistical differences with the intestinal fraction of the positive control (110.10±1.15 µg/mL) with p=0.282. Thus, this dosage form did not show the same improvement in the pharmacological activity, but the efficacy was similar to acarbose after digestion. However, the extract in powder showed the highest hypoglycaemic because the IC50 value was the lowest (59.55±0.85 µg/mL) with p<0.05.
In a previous study with flavonoid fisetin from leaves of
Rhus succedanea L. [
32], which was more active than acarbose, encapsulation in nanoparticles did not improved this inhibitory activity against α–glucosidase. Using the computational molecular docking approach, the authors explained their results in relation to the structural determinants involved on the interaction between flavonoids and the enzyme, considering that the 3’,4’–dihydroxyl groups of B ring in flavonoids are crucial in engaging direct binding with the active–site residues. Since the flavonoid content was higher in the intestinal fraction of the capsule than in OV–P, our hypothesis relays on the fact that in OV–P, the binding site of flavonoids might be uncluttered and the chemical compounds are already completely released, comparing to the physical protection of the capsule as a formulation container. Thus, the extract given as powder clearly showed to enhance hypoglycaemic activity
in vitro.
Once the intestinal absorbable fractions have been shown to inhibit the enzyme in the site of action
in vitro, there is the doubt whether they will be maintaining the activity inside a living organism. Thus, the inhibitory activity of the pharmaceutical forms – and the positive control – after digestion was determined inside
C. elegans. The new method proposed was designed in order to quantify directly the activity of the samples inside this
in vivo model, instead of attributing physiological effects
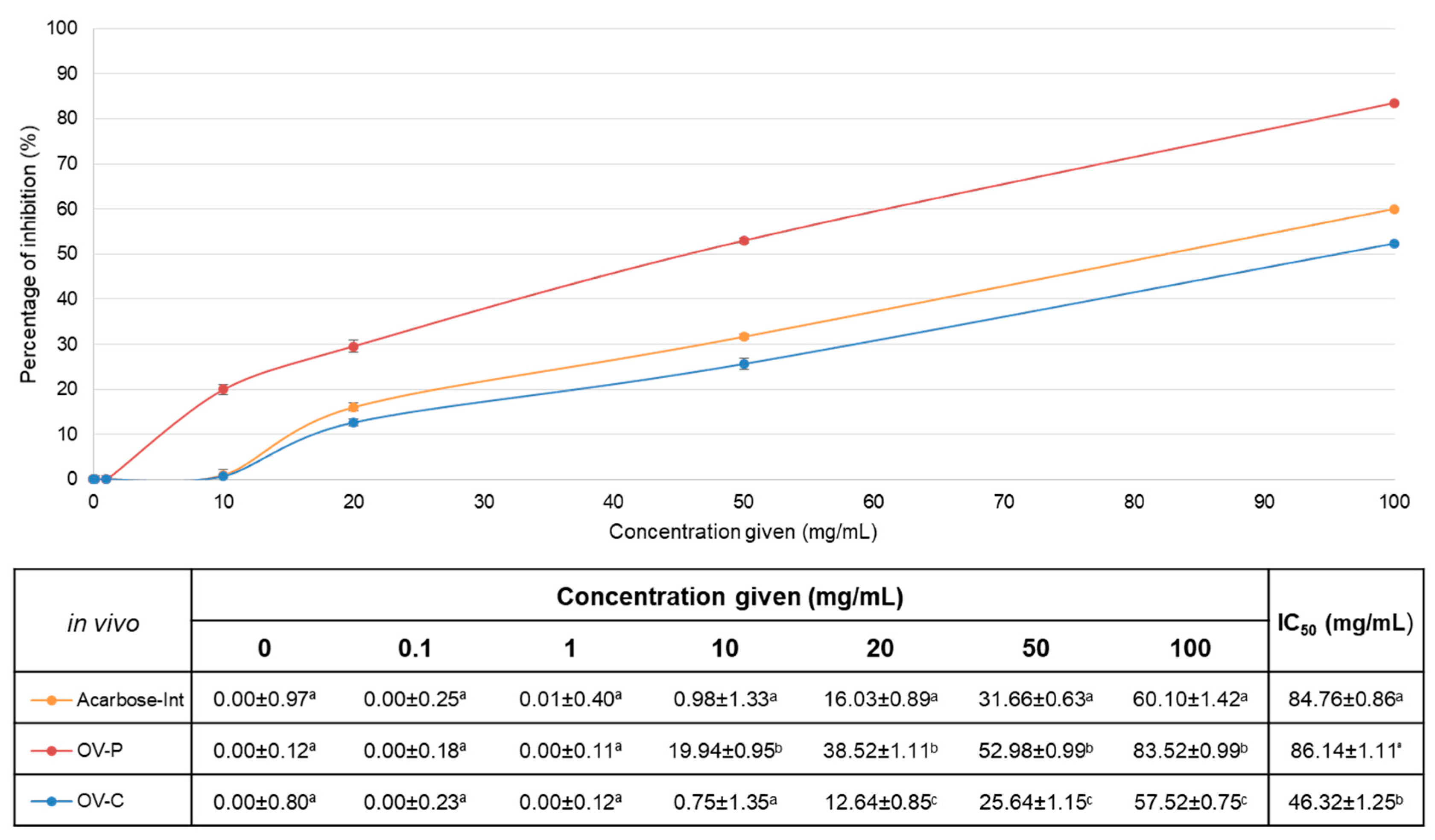
a posteriori. Figure 2 recompiles the inhibitory percentages of OV-C, OV-P and Acarbose-Int
in vivo as well as the calculated value of IC
50.
Results for in vivo pharmacological activity were again expressed as percentage of inhibition.At first glance, the extract encapsulated (OV-C) presented a similar behaviour as positive control (Acarbose-Int), as in vitro. Nevertheless, OVE given as powder showed to inhibit significantly higher the enzyme than the positive control at any of the concentrations, being at 50 mg/mL 52.98±0.99% against 31.66±0.63%, respectively with p<0.05. In this sense, the samples showed similar patron of activity among them than in the experiment performed in vitro.
Whereas
in vitro tests are used to demonstrate the intrinsic activity of compounds,
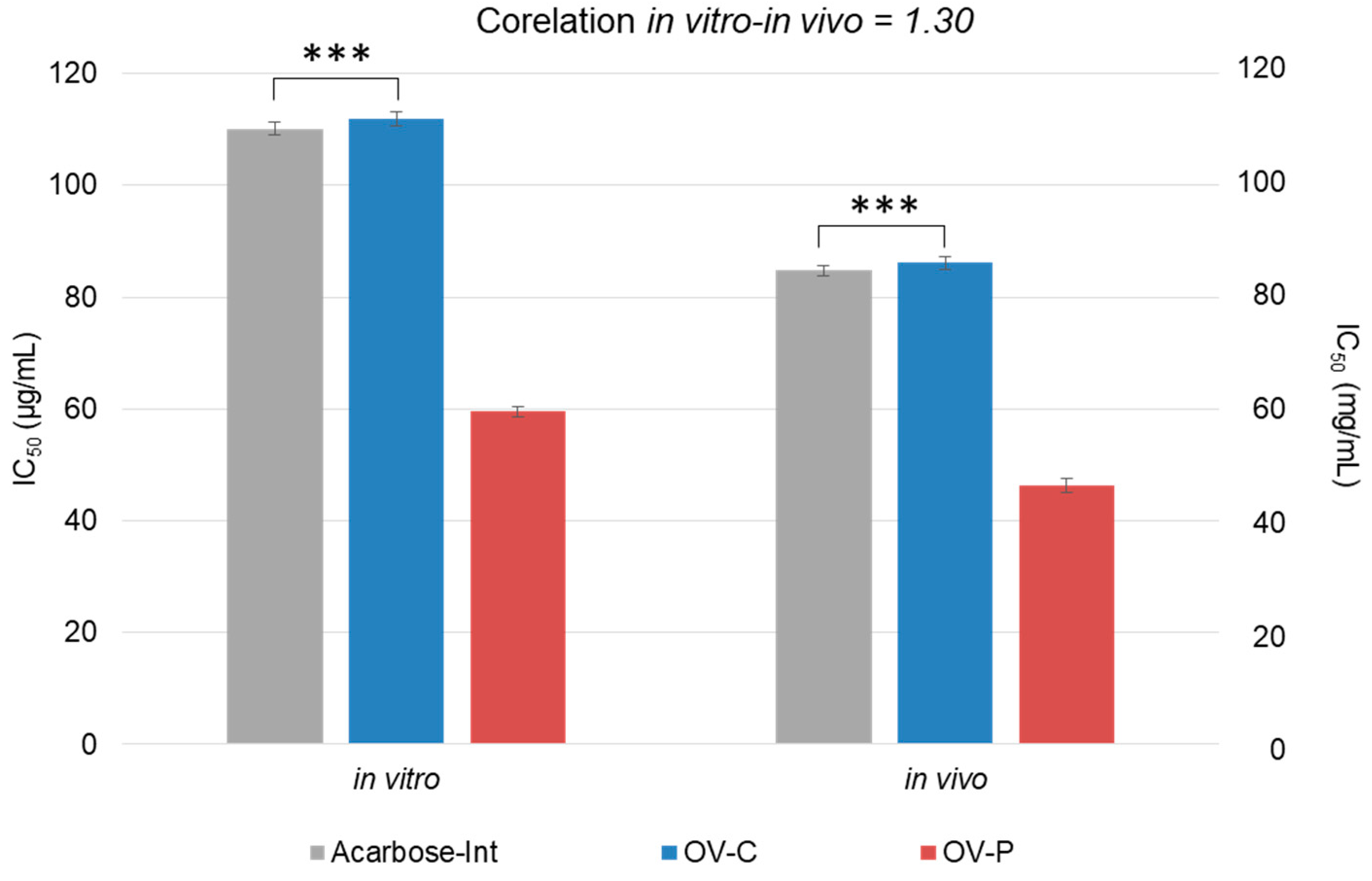
in vivo tests focus on the physiological effects and provide a second and essential line of evidence for antioxidant activity. Although sometimes is there no direct correlation between
in vitro and
in vivo results, our results showed a scale of 1.30 times on the values (
Figure 3).
This mathematical relation can still only be attributed to this case and should not be settled as universal until more data from different plants is recompiled. This new method set up provides a fast track to test treatments for diabetes in vivo without involving genes or any other costly material. Results confirm the robustness of the method. The assay was replicated three times from the start using independent worm stocks to validate the method. The reproducibility was tested by carrying out a minimum of 12 determinations for each compound concentration per repetition of the experiment under the same conditions. In contrast, our detectable results (more than 1% inhibition) had a C.V. of 1.87-6.72%, which is a good indication of the repeatability of the method, since it is less than 20%. For this reason, our method is accurate and valid for the quantification of the inhibition of in vivo, allowing the direct attribution of this in vivo hypoglycaemic activity to the treatment. In addition, it opens the door to further research into in vitro pharmacological activities that require an in vivo counterpart.
3. Material and Methods
3.1. Preparation of the extract and formulations
The two pharmaceutical forms used for this study were prepared as previously on de Torre
et al. [
33]. Briefly, the hydroalcoholic extract (OV-E) was prepared from
O. vulgare flowered aerial-dried parts by cold maceration with ethanol-water 50% v/v. Then, two simple solid oral pharmaceutical forms for this extract (500 mg with 30% of rosmarinic acid) were designed, divided powder and hard gelatine capsules.
3.2. In vitro gastrointestinal digestion process
The
in vitrogastrointestinal digestion process was performed according to Gayoso
et al. [
34] with some modifications previously described by de Torre
et al. [
33]. Each pharmaceutical form (equivalent a 500 mg of extract) weresubmitted into the gastrointestinal digestion process obtaining the lyophilised intestinal absorbable fraction, called for each sample: intestinal absorbable fraction of the powder (OV-P), intestinal absorbable fraction of the capsules (OV-C) and intestinal absorbable fraction of the positive control (Acarbose-Int). Finally, thefractions were lyophilised (Cryodos50, Telstar, Barcelona, Spain). A control with two empty capsules and a blank with the excipients and without plant extract were treated under the same conditions.
3.3. In vitro α–glucosidase activity assay
According to Matsui[
30]and Shuyuan
et al. [
10], the inhibitory activity of
α–glucosidase can be measured thanks to an analogous disaccharide (4–Nitrophenyl–α–
D–glucopyranoside –
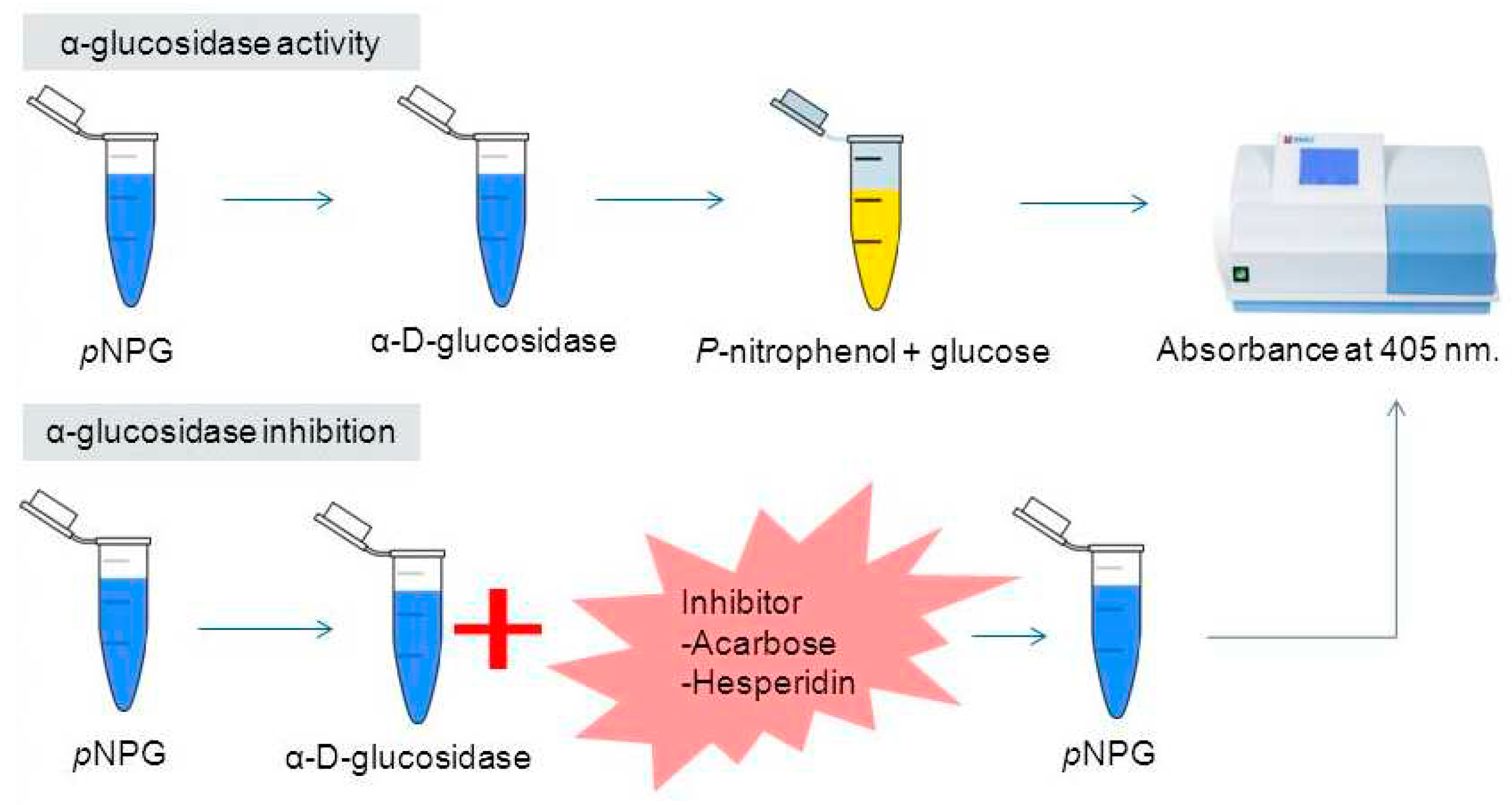
p–NPG–, used as substrate). The enzyme breaks down the α-chemical bond of the substrate, so that carbohydrate D–Glucopyranoside and the phenolic compound 4–Nitrophenylare released. 4–Nitrophenyl turns yellow at pH 6.8, making it quantifiable at 405 nm (
Figure 4).
The more inhibitory activity the extract has, the less yellow the solution will have (measurable, lower absorbance value at 405 nm). To enhance colour, a basic solution must be added (optimal efficient medium pH 6.8) [
35] so that there are enough electrons for the
p–nitrophenolate anion to make resonance and stabilize [
36]. This protocol was established in the laboratory.
The enzyme α–glucosidase needs to be stored at –20 ºC, and kept cold during handling. The enzyme was obtained from
Saccharomyces cerevisae (#G0660, Sigma–Aldrich Co., St. Louis, MO) and the optimal pH is 6.8, the same as the intestinal one [
37]. The enzyme was resuspended in 0.1 M PBS buffer, pH 6.8, at a concentration of 0.5 U/L –within the range suggested 0.2 U/L–1U/L [
38].
As the enzyme, substrate (4–Nitrophenyl–α–D–glucopyranoside– #N1377, Sigma–Aldrich Co., St. Louis, MO) must be stored at –20 ºC and kept on ice while handling. After several titration tests performed, the optimal substrate concentration was 1 mM in 0.1 M PBS buffer and pH 6.8.
It is necessary a solution to stop the reaction and measure the inhibitory activity avoiding biodegradation. 80 µL of 0.15 M Na2CO3 was added toeach well after the reaction occurred.
The different lyophilised sampleswere diluted in 0.1 M PBS buffer, pH 6.8 at six serial concentrations (1,000–0.03125 µg/mL). Acarbose (#A8980,Sigma–Aldrich Co., St. Louis, MO) was used as the positive control at the same concentrations and the sample solvent (PBS) was used for the negative control. Finally, the intestinal digestion fractions of OV1 were also analysed, as well as the intestinal fractions of the corresponding positive control (Acarbose–Int at the same concentrations).
Every plate needs a positive control (120 µL Acarbose) and a negative control (120 µL PBS) with the corresponding blanks. 20 µL PBS was used for blanks instead of enzyme (20 µL 0.5 U/L in PBS) and the volume of substrate was constant for each condition (20 µL).Sample, acarbose, PBS and enzyme were added to the 96–well plate and incubated for 15 min at 37 ºC with shaking.Then, substrate was added according to the design. It was incubated under the same circumstances for an additional 15 min. After the incubation time, 80 µL of the stop solution were added to each sample.
The absorbance measurement was made with the Power WaVe XS de BioTek
® spectrophotometer at 405nm.The results were processed with KC Junior BioTek data analysis software. Inhibition (%) of α-glucosidase was calculated by using the following equation:
where,
Inhibitory (%, percentage of inhibition) is obtained from the absorbance of the sample (
AbsS) without its blank (
AbsSB: Sample Blank Absorbance) against the absorbance of the negative control (
AbsC) without its blank (
AbsCB: Negative Control Blank Absorbance).
The inhibitory concentration (IC50) was calculated by GraphPad Prism v 4.00 analysis.
3.4. In vivo α–glucosidase activity assay
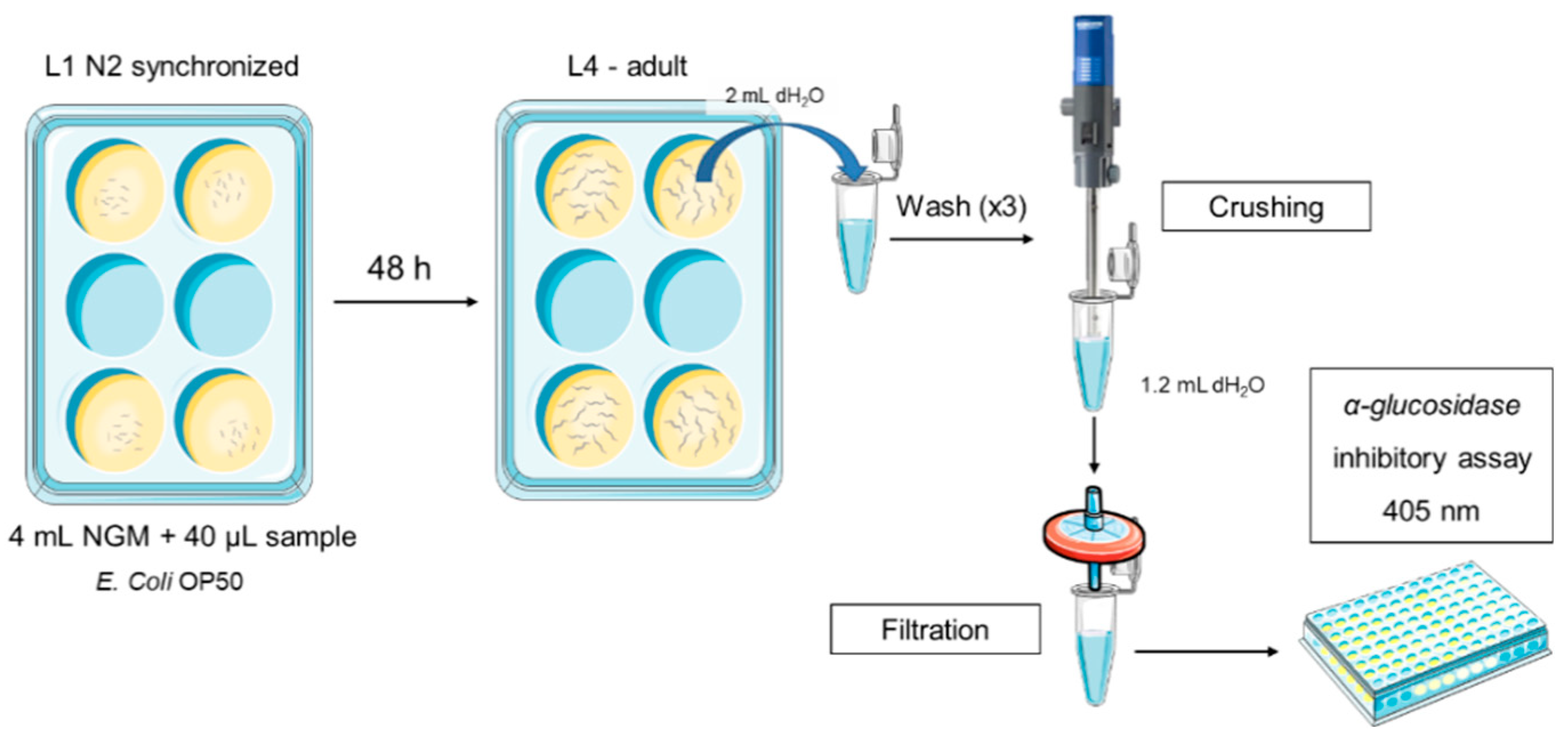
C. elegans was cultured as described previously and the strain used was N2 Bristol. All assays were performed in 6–well cell culture plates with 4 mL of Nematode Growth Medium (NGM) per well at 20 °C.
Intestinal samples of each formulation and acarbose as positive control were resuspended in distilled water at different concentrations: 0, 0.1, 1, 10, 20, 50 and 100 mg/mL.40 µL of each condition were added per well.
100 µL of
E. coli OP50 were seeded on each well as the worm food source and 2,000 L1 synchronized worms were placed onto each well. After 48 h, they were collected with 2 mL of sterile water per plate, washed three times and pelleted by centrifugation (314 g / 4 min / 20 ºC). Then, worms were suspended in 1.75 mL of sterile water and crushed for 20 s at maximum power using Ultraturrax T25. Final solution was filtered through a 0.45 µm filter (
Figure 5). Finally, hypoglycaemic
in vivo activity assay was performed in the same manner as previously described
in vitro.
3.5. Statistical analysis
All experiments were performed in triplicate. Means, standard deviations and graphs were obtained with Microsoft Excel 2013 (Microsoft Corp., Redmond, WA). Statistical analysis was performed using Stata v.12 (StataCorp LLC, College Station, TX). Normality was checked by Shapiro–Wilkinson test. Differences were estimated by ANOVA followed by pairwise comparison post hoc test using Tukey’s method (95 % CL) or post–estimation margins to check interaction among groups.