Submitted:
31 July 2023
Posted:
01 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dopamine, Reward, and Behavioral Regulation
2.1. Impulsive Behavior
2.2. Craving and Bingeing
2.3. Negative Affect
2.4. Sleep
3. Dopamine and Post-Pandemic Mental Health
- ➢
- significant rise in addictions and related mental illnesses
- ➢
- significant rise in corresponding anti-depressant prescription uptake
- ➢
- increased risk of suicidal ideation or suicide
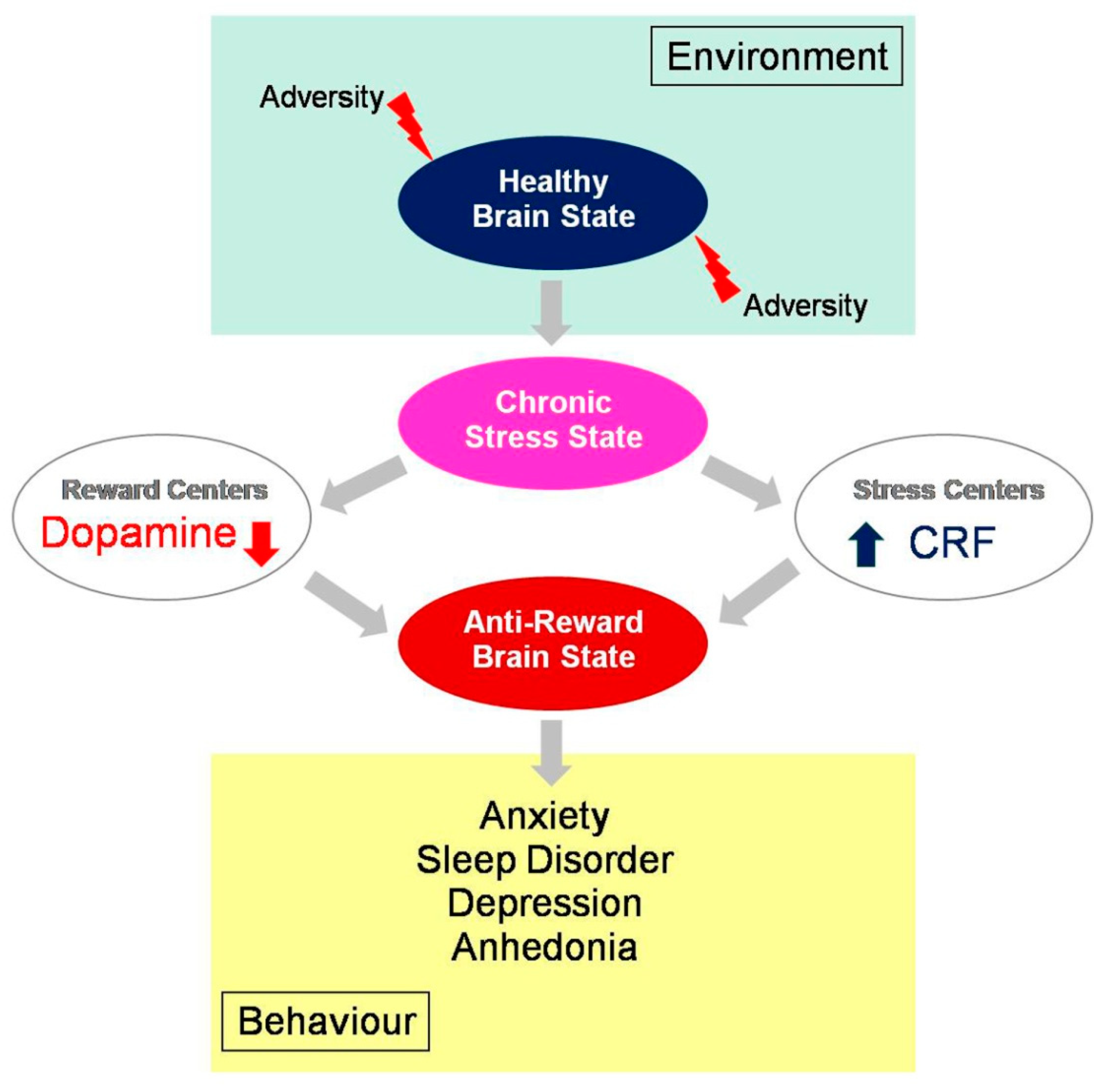
3.1. Adversity and Vulnerability
3.2. From Reward to Anhedonia
3.3. The New «Digital Drug»
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewis RG, Florio E, Punzo D, Borrelli E. The Brain's Reward System in Health and Disease. Adv Exp Med Biol 2021, 1344, 57–69. [Google Scholar] [CrossRef]
- Kringelbach ML, Berridge KC. The Functional Neuroanatomy of Pleasure and Happiness. Discov Med 2010, 9, 579–587. [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 1996, 6, 228–36. [Google Scholar] [CrossRef] [PubMed]
- De Decker A, Verbeken S, Sioen I, Van Lippevelde W, Braet C, Eiben G, Pala V, Reisch LA, De Henauw S; I. Family Consortium. Palatable food consumption in children: interplay between (food) reward motivation and the home food environment. Eur J Pediatr. 2017, 176, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Everitt, BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990, 14, 217–32. [Google Scholar] [CrossRef]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res 2000, 126, 325–41. [Google Scholar] [CrossRef]
- Johnston, JD. Physiological responses to food intake throughout the day. Nutr Res Rev 2014, 27, 107–18. [Google Scholar] [CrossRef]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiological Psychology 1954, 47, 419–27. [Google Scholar] [CrossRef]
- Kapsimali M, Dumond H, Le Crom S, Coudouel S, Vincent JD, Vernier P. Evolution et développement des systèmes neuromodulateurs dopaminergiques chez les Vertébrés [Evolution and development of dopaminergic neurotransmitter systems in vertebrates]. J Soc Biol 2000, 194, 87–93. [Google Scholar] [CrossRef]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Schultz, W. Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev 2015, 95, 853–951. [Google Scholar] [CrossRef] [PubMed]
- Peters KZ, Cheer JF, Tonini R. Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System. Trends Neurosci 2021, 44, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol 1989, 40, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration US Office of the Surgeon General (US). Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health Washington (DC) US Department of Health and Human Services, 2016; CHAPTER 2, THE NEUROBIOLOGY OF SUBSTANCE USE, MISUSE, AND ADDICTION. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK424849/. 4248.
- 15. Jahan AR, Burgess DM. Substance Use Disorder. [Updated 2023 Apr 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023; Available online at: https://www.ncbi.nlm.nih.gov/books/NBK570642/. /.
- Volkow ND, Michaelides M, Baler R. The Neuroscience of Drug Reward and Addiction. Physiol Rev 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Kato A, Shimomura K, Ognibene D, Parvaz MA, Berner LA, Morita K, Fiore VG. Computational models of behavioral addictions: State of the art and future directions. Addict Behav 2023, 140, 107595. [Google Scholar] [CrossRef]
- Michaelsen MM, Esch T. Understanding health behavior change by motivation and reward mechanisms: a review of the literature. Front Behav Neurosci. 2023, 17, 1151918. [Google Scholar] [CrossRef]
- Koob GF, & Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 2016, 3, 760–773.
- Keramati M, Gutkin B. Imbalanced decision hierarchy in addicts emerging from drug-hijacked dopamine spiraling circuit. PLoS One. 2013, 8, e61489. [Google Scholar] [CrossRef]
- Sussman S, Sinclair DL. Substance and Behavioral Addictions, and Their Consequences among Vulnerable Populations. Int J Environ Res Public Health 2022, 19, 6163. [Google Scholar] [CrossRef]
- Maldonado R, Calvé P, García-Blanco A, Domingo-Rodriguez L, Senabre E, Martín-García E. Vulnerability to addiction. Neuropharmacology. 2021, 186, 108466. [Google Scholar] [CrossRef]
- Velazquez-Sanchez C, Muresan L, Marti-Prats L, Belin D. The development of compulsive coping behaviour is associated with a downregulation of Arc in a Locus Coeruleus neuronal ensemble. Neuropsychopharmacology 2023, 48, 653–663. [Google Scholar] [CrossRef]
- Moreno M, Flores P. Schedule-induced polydipsia as a model of compulsive behavior: neuropharmacological and neuroendocrine bases. Psychopharmacology 2012, 219, 647–59. [Google Scholar] [CrossRef] [PubMed]
- Robbins TW, Koob GF. Selective disruption of displacement behaviour by lesions of the mesolimbic dopamine system. Nature 1980, 285, 409–12. [Google Scholar] [CrossRef] [PubMed]
- Mora S, Merchan A, Vilchez O, Aznar S, Klein AB, Ultved L, et al. Reduced cortical serotonin 5-HT2A receptor binding and glutamate activity in high compulsive drinker rats. Neuropharmacology 2018, 143, 10–9. [Google Scholar] [CrossRef] [PubMed]
- Ansquer S, Belin-Rauscent A, Dugast E, Duran T, Benatru I, Mar AC, et al. Atomoxetine decreases vulnerability to develop compulsivity in high impulsive rats. Biol Psychiatry. 2014, 75, 825–32. [Google Scholar] [CrossRef] [PubMed]
- Higgins GA, Brown M, St John J, MacMillan C, Silenieks LB, Thevarkunnel S. Effects of 5-HT2C receptor modulation and the NA reuptake inhibitor atomoxetine in tests of compulsive and impulsive behaviour. Neuropharmacology 2020, 170, 108064. [Google Scholar] [CrossRef]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol 1999, 11, 361–9. [Google Scholar] [CrossRef]
- Thrivikraman KV, Kinkead B, Owens MJ, Rapaport MH, Plotsky PM. Locus coeruleus noradrenergic modulation of diurnal corticosterone, stress reactivity and cardiovascular homeostasis in male rats. Neuroendocrinology. 2021, 112, 763–76. [Google Scholar]
- Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacology 2020, 237, 1973–87. [Google Scholar] [CrossRef]
- Bari A, Robbins TW. Noradrenergic versus dopaminergic modulation of impulsivity, attention and monitoring behaviour in rats performing the stop-signal task: possible relevance to ADHD. Psychopharmacology 2013, 230, 89–111. [Google Scholar] [CrossRef]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 2008, 29, 192–9. [Google Scholar] [CrossRef]
- Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A. Neurobiological links between stress and anxiety. Neurobiol Stress 2019, 11, 100191. [Google Scholar] [CrossRef] [PubMed]
- Tanaka M, Yoshida M, Emoto H, Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol 2000, 405, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Coban DA, Tan O. The relationship between childhood trauma and obsessive-compulsive disorder, comorbid attention deficit hyperactivity disorder, and impulsivity. Arch Neuropsychiatry 2019, 57, 37–43. [Google Scholar]
- Saxena S, Maidment KM, Vapnik T, Golden G, Rishwain T, Rosen RM, et al. Obsessive-compulsive hoarding: symptom severity and response to multimodal treatment. J Clin Psychiatry 2002, 63, 21–7. [Google Scholar] [CrossRef]
- Rosa-Alcázar Á, García-Hernández MD, Parada-Navas JL, Olivares-Olivares PJ, Martínez-Murillo S, Rosa-Alcázar AI. Coping strategies in obsessive-compulsive patients during Covid-19 lockdown. Int J Clin Health Psychol 2021, 21, 100223. [Google Scholar] [CrossRef]
- Mathis V, Kenny PJ. From controlled to compulsive drug-taking: The role of the habenula in addiction. Neurosci Biobehav Rev 2019, 106, 102–111. [Google Scholar] [CrossRef]
- Asensio S, Hernández-Rabaza V, Orón Semper JV. What Is the "Trigger" of Addiction? Front Behav Neurosci 2020, 14, 54. [Google Scholar] [CrossRef]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010, 68, 815–34. [Google Scholar] [CrossRef]
- 42. Schaefer LM, Forester G, Burr EK, Laam L, Crosby RD, Peterson CB, Crow SJ, Engel SG, Dvorak RD, Wonderlich SA. Examining the role of craving in affect regulation models of binge eating: Evidence from an ecological momentary assessment study. J Psychopathol Clin Sci 2023. [CrossRef]
- 43. Moore CF, Panciera JI, Sabino V, Cottone P. Neuropharmacology of compulsive eating. Philos Trans R Soc Lond B Biol Sci. 2018, 373, 20170024. [CrossRef]
- Parylak SL, Koob GF, Zorrilla EP. The dark side of food addiction. Physiol. Behav 2011, 104, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001, 937, 1–26. [Google Scholar]
- Koob, GF. Anhedonia, Hyperkatifeia, and Negative Reinforcement in Substance Use Disorders. Curr Top Behav Neurosci 2022, 58, 147–165. [Google Scholar] [CrossRef]
- Piper, ME. Withdrawal: Expanding a Key Addiction Construct. Nicotine Tob Res 2015, 17, 1405–15. [Google Scholar] [CrossRef] [PubMed]
- Backström T, Winberg S. Central corticotropin releasing factor and social stress. Front. Neurosci, 2013, 7, 117. [Google Scholar] [CrossRef]
- Han KS, Kim L, Shim I. Stress and sleep disorder. Exp Neurobiol 2012, 21, 141–50. [Google Scholar] [CrossRef]
- Grabowska K, Ziemichód W, Biała G. Recent Studies on the Development of Nicotine Abuse and Behavioral Changes Induced by Chronic Stress Depending on Gender. Brain Sci 2023, 13, 121. [Google Scholar] [CrossRef]
- Hales CA, Stuart SA, Griffiths J, Bartlett J, Arban R, Hengerer B, Robinson ES. Investigating neuropsychological and reward-related deficits in a chronic corticosterone-induced model of depression. Psychoneuroendocrinology, 2023, 147, 105953. [CrossRef]
- Baik, JH. Stress and the dopaminergic reward system. Exp Mol Med 2020, 52, 1879–1890. [Google Scholar] [CrossRef]
- Sochal M, Ditmer M, Gabryelska A, Białasiewicz P. The Role of Brain-Derived Neurotrophic Factor in Immune-Related Diseases: A Narrative Review. Journal of Clinical Medicine 2022, 11, 6023. [Google Scholar] [CrossRef]
- Correia AS, Cardoso A, Vale N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants (Basel) 2023, 12, 470. [Google Scholar] [CrossRef]
- Smevik H, Habli S, Saksvik SB, Kliem E, Evensmoen HR, Conde V, Petroni A, Asarnow RF, Dennis EL, Eikenes L, Kallestad H, Sand T, Thompson PM, Saksvik-Lehouillier I, Håberg AK, Olsen A. Poorer sleep health is associated with altered brain activation during cognitive control processing in healthy adults. Cereb Cortex 2023, 33, 7100–7119. [Google Scholar] [CrossRef]
- Uccella S, Cordani R, Salfi F, Gorgoni M, Scarpelli S, Gemignani A, Geoffroy PA, De Gennaro L, Palagini L, Ferrara M, Nobili L. Sleep Deprivation and Insomnia in Adolescence: Implications for Mental Health. Brain Sci, 2023, 13, 569. [CrossRef]
- Sollenberger NA, Sequeira S, Forbes EE, Siegle GJ, Silk JS, Ladouceur CD, Ryan ND, Dahl RE, Mattfeld AT, McMakin DL. More time awake after sleep onset is linked to reduced ventral striatum response to rewards in youth with anxiety. J Child Psychol Psychiatry. 2023, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alloy LB, Chat IK, Grehl MM, Stephenson AR, Adogli ZV, Olino TM, Ellman LM, Miller GE, Nusslock R. Reward and Immune Systems in Emotion (RISE) prospective longitudinal study: Protocol overview of an integrative reward-inflammation model of first onset of major depression in adolescence. Brain Behav Immun Health, 2023, 30, 100643. [CrossRef]
- Scaplen KM, Kaun KR. Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J Neurogenet. 2016, 30, 133–48. [Google Scholar] [CrossRef]
- Blum K, Gondré-Lewis M, Steinberg B, et al. Our evolved unique pleasure circuit makes humans different from apes: Reconsideration of data derived from animal studies. J Syst Integr Neurosci. 2018, 4, 10. [Google Scholar]
- Wise, RA. Dopamine and Reward: The Anhedonia Hypothesis 30 years on. Neurotox Res. 2008, 14, 169–183. [Google Scholar] [CrossRef]
- Volkow ND, Michaelides M, Baler R. The Neuroscience of Drug Reward and Addiction. Physiol Rev, 2019, 99, 2115–2140. [CrossRef]
- Gong L, Liao T, Liu D, Luo Q, Xu R, Huang Q, Zhang B, Feng F, Zhang C. Amygdala Changes in Chronic Insomnia and Their Association with Sleep and Anxiety Symptoms: Insight from Shape Analysis. Neural Plast, 2019, 2019, 8549237. [CrossRef]
- Cirrincione L, Plescia F, Malta G, Campagna M, Lecca LI, Skerjanc A, Carena E, Baylon V, Theodoridou K, Fruscione S, Cannizzaro E. Evaluation of Correlation between Sleep and Psychiatric Disorders in a Population of Night Shift Workers: A Pilot Study. Int J Environ Res Public Health. 2023, 20, 3756. [Google Scholar] [CrossRef]
- World Health Organization. Mental health and COVID-19: Early evidence of the pandemics impact. Geneva: World Health Organization, https://apps.who.int/iris/bitstream/handle/10665/352189/WHO-2019-nCoV-Sci-Brief-Mental-health-2022.1-eng.pdf. 2022.
- Oakes, MB. Ontological insecurity in the post-covid-19 fallout: using existentialism as a method to develop a psychosocial understanding to a mental health crisis. Med Health Care Philos. 2023 Jun 30. [CrossRef]
- Tomlinson M, Marlow M. COVID-19 and mental health: Building back better or reimagining a new way forward? PLoS Med 2023, 20, e1004216. [Google Scholar] [CrossRef]
- Winkler P, Mohrova Z, Mlada K, Kuklova M, Kagstrom A, Mohr P, et al. Prevalence of current mental disorders before and during the second wave of COVID-19 pandemic: An analysis of repeated nationwide cross-sectional surveys. J Psychiatr Res 2021, 139, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Pierce M, McManus S, Hope H, Hotopf M, Ford T, Hatch SL, et al. Mental health responses to the COVID-19 pandemic: a latent class trajectory analysis using longitudinal UK data. Lancet Psychiatry 2021, 8, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Knudsen AKS, Stene-Larsen K, Gustavson K, Hotopf M, Kessler RC, Krokstad S, et al. Prevalence of mental disorders, suicidal ideation and suicides in the general population before and during the COVID-19 pandemic in Norway: A population-based repeated cross-sectional analysis. Lancet Reg Health Eur 2021, 4, 100071. [Google Scholar] [CrossRef]
- Davies HL, Hübel C, Herle M, Kakar S, Mundy J, Peel AJ, Ter Kuile AR, Zvrskovec J, Monssen D, Lim KX, Davies MR, Palmos AB, Lin Y, Kalsi G, Rogers HC, Bristow S, Glen K, Malouf CM, Kelly EJ, Purves KL, Young KS, Hotopf M, Armour C, McIntosh AM, Eley TC, Treasure J, Breen G. Risk and protective factors for new-onset binge eating, low weight, and self-harm symptoms in >35,000 individuals in the UK during the COVID-19 pandemic. Int J Eat Disord 2023, 56, 91–107. [Google Scholar] [CrossRef]
- Güzel Â, Mutlu NL, Molendijk M. COVID-19-related changes in eating disorder pathology, emotional and binge eating and need for care: a systematic review with frequentist and Bayesian meta-analyses. Eat Weight Disord 2023, 28, 19. [Google Scholar] [CrossRef]
- Martinelli TF, Nagelhout GE, Best D, Vanderplasschen W, van de Mheen D. Factors associated with problematic substance use before and during the COVID-19 pandemic among a drug addiction recovery cohort: A prospective study in the Netherlands, Belgium, and UK. J Subst Use Addict Treat. 2023, 148, 209025. [Google Scholar] [CrossRef]
- Zhao L, Li X, Yang Q, Peng Y, Jiang L, Jia P, Shi W. The longitudinal association between internet addiction and depressive and anxiety symptoms among Chinese adolescents before and during the COVID-19 pandemic. Front Public Health. 2023, 10, 1096660. [Google Scholar] [CrossRef]
- Stanley IH, Flarity KM, April MD. Suicide Ideation, Plans, and Attempts Attributed to the COVID-19 Pandemic Among US Veterans. JAMA Netw Open. 2023, 6, e2320193. [Google Scholar] [CrossRef] [PubMed]
- Tardeh S, Adibi A, Mozafari AA. Prevalence of Suicide Ideation and Attempt during COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Int J Prev Med. 2023, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Egerton A, Valmaggia LR, Howes OD, Day F, Chaddock CA, Allen P, Winton-Brown TT, Bloomfield MAP, Bhattacharyya S, Chilcott J, Lappin JM, Murray RM, McGuire P. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr Res 2016, 176, 171–176. [Google Scholar] [CrossRef]
- Chuang SP, Wu JYW, Wang CS. Resilience and Quality of Life in People with Mental Illness: A Systematic Review and Meta-Analysis. Neuropsychiatr Dis Treat 2023, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Østergaard MLD, Aponte-Canencio DM, Barajas Ortiz Y, Velez Botero HJ, Simon Modvig J, Brasholt M. Vulnerability factors in conflict-related mental health. Med Confl Surviv 2023, 39, 63–80. [Google Scholar] [CrossRef]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 2005, 30, 821–32. [Google Scholar] [CrossRef]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology 2007, 32, 2310–20. [Google Scholar] [CrossRef]
- Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: A review. EXCLI J 2017, 16, 1057–1072. [Google Scholar] [CrossRef]
- Fleischer E, Landaeta-Díaz L, González-Medina G, Horovitz O. Anxiety, anhedonia, and related food consumption in Israelis populations: An online cross-sectional study two years since the outbreak of COVID-19. Heliyon 2023, 9, e17211. [Google Scholar] [CrossRef]
- Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef]
- Costa R, Pinto TM, Conde A, Mesquita A, Motrico E, Figueiredo B. Women's perinatal depression: Anhedonia-related symptoms have increased in the COVID-19 pandemic. Gen Hosp Psychiatry 2023, 84, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne SJ, Melendez SI, Olmstead MC. Investigating dopamine and glucocorticoid systems as underlying mechanisms of anhedonia. Psychopharmacology (Berl). 2018, 235, 3103–3113. [Google Scholar] [CrossRef]
- Kim HJJ, Zagzoog A, Ceni C, Ferrisi R, Janz N, Laprairie RB. Dual Cannabinoid and Orexin Regulation of Anhedonic Behaviour Caused by Prolonged Restraint Stress. Brain Sci 2023, 13, 314. [Google Scholar] [CrossRef]
- Gold MS, Blum K, Febo M, Baron D, Modestino EJ, Elman I, Badgaiyan RD. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front Biosci (Schol Ed) 2018, 10, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Phillips R, Walsh E, Jensen T, Nagy G, Kinard J, Cernasov P, Smoski M, Dichter G. Longitudinal associations between perceived stress and anhedonia during psychotherapy. J Affect Disord 2023, 330, 206–213. [Google Scholar] [CrossRef]
- Zhang C, Liu B, Pawluski J, Steinbusch HWM, Kirthana Kunikullaya U, Song C. The effect of chronic stress on behaviors, inflammation and lymphocyte subtypes in male and female rats. Behav Brain Res 2023, 439, 114220. [Google Scholar] [CrossRef] [PubMed]
- Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: A web-based cross-sectional survey. Psychiatry Res 2020, 288, 112954. [Google Scholar] [CrossRef]
- Lin M-P. Prevalence of Internet Addiction during the COVID-19 Outbreak and Its Risk Factors among Junior High School Students in Taiwan. International Journal of Environmental Research and Public Health 2020, 17, 8547. [Google Scholar] [CrossRef]
- Dresp-Langley B, Hutt A. Digital Addiction and Sleep. Int J Environ Res Public Health. 2022, 19, 6910. [Google Scholar] [CrossRef]
- Kuss DJ, Griffiths MD, Karila L, Billieux, J. Internet addiction: a systematic review of epidemiological research for the last decade. Curr Pharm Des 2014, 20, 4026–52. [Google Scholar] [CrossRef]
- Griffiths MD, Pontes HM. Internet Addiction Disorder and Internet Gaming Disorder are not the same. J Addict Res Ther, 2014, 5, e1. [Google Scholar]
- Cheng C, Li AY. Internet addiction prevalence and quality of (real) life: a meta-analysis of 31 nations across seven world regions. Cyberpsychol Behav Soc Netw. 2014, 17, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Liu M, Luo J. Relationship between peripheral blood dopamine level and internet addiction disorder in adolescents: A pilot study. Int J Clin Exp Med, 2015, 8, 9943–9948. [Google Scholar]
- Nalwa K, Anand AP. Internet addiction in students: a cause of concern. Cyberpsychol Behav, 2003, 6, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Cao F, Su L. Internet addiction among Chinese adolescents: prevalence and psychological features. Child Care and Health Development, 2006, 33, 275–281. [Google Scholar]
- Fu KW, Chan WS, Wong PW, Yip PS. Internet addiction: prevalence, discriminant validity and correlates among adolescents in Hong Kong. Br J Psychiatry, 2010, 196, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Saikia AM, Das J, Barman P, Bharali MD. Internet Addiction and its Relationships with Depression, Anxiety, and Stress in Urban Adolescents of Kamrup District, Assam. J Family Community Med, 2019, 26, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Dalbudak E, et al. Relationship of internet addiction severity with depression, anxiety and alexithymia, temperament, and character in university students. Cyberpsychol Behav Soc Netw, 2013, 16, 272–278. [Google Scholar] [CrossRef]
- Shakya HB, Christakis NA. Association of ‘Facebook’ use with compromised well-being: A longitudinal study. American Journal of Epidemiology 2017, 185, 203–211. [Google Scholar]
- Lau JTF, Walden DL, Wu AMS, Cheng KM, Lau MCM, Mo PKH. Bidirectional predictions between Internet addiction and probable depression among Chinese adolescents. J Behav Addict, 2018, 7, 633–643. [Google Scholar] [CrossRef]
- Hinojo-Lucena FJ, Aznar-Díaz I, Cáceres-Reche MP, Trujillo-Torres JM, Romero-Rodríguez JM. Problematic Internet Use as a Predictor of Eating Disorders in Students: A Systematic Review and Meta-Analysis Study. Nutrients, 2019, 11, e2151. [Google Scholar] [CrossRef] [PubMed]
- Moreno MA, Eickhoff J, Zhao Q, Suris, JC. College Students and Problematic Internet Use: A Pilot Study Assessing Self-Appraisal and Independent Behavior Change. J Adolesc Health, 2019, 64, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy S, Chetlapalli SK. Internet addiction: Prevalence and risk factors: A cross-sectional study among college students in Bengaluru, the Silicon Valley of India. Indian J Public Health, 2015, 59, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li JB, Lau JTF, Mo PKH, Su XF, Tang J, Qin ZG, Gross DL. Insomnia partially mediates the association between problematic Internet use and depression among secondary school students in China. J Behav Addict 2017, 6, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Chen YL, Gau SS. Sleep problems and internet addiction among children and adolescents: a longitudinal study. J Sleep Res 2016, 25, 458–65. [Google Scholar] [CrossRef]
- Kim K, et al. Internet addiction in Korean adolescents and its relation to depression and suicidal ideation: a questionnaire survey. Int J Nurs Stud 2006, 43, 185–192. [Google Scholar] [CrossRef]
- Keles B, McCrae N, Grealish A. A systematic review: the influence of social media on depression, anxiety and psychological distress in adolescents, International Journal of Adolescence and Youth. 2020, 25, 79–93.
- Yau YH, Potenza MN. Gambling disorder and other behavioral addictions: recognition and treatment. Harv Rev Psychiatry 2015, 23, 134–146. [Google Scholar] [CrossRef]
- Greenfield, D. Treatment Considerations in Internet and Video Game Addiction: A Qualitative Discussion. Child and Adolescent Clinics of North America Reviews, Youth Internet Habits and Mental Health 2018. [Google Scholar]
- Twenge, JM. Have Smartphones Destroyed a Generation? The Atlantic, 2017. [Google Scholar]
- Shaw M, Black DW. Internet addiction: definition, assessment, epidemiology and clinical management. CNS Drugs 2008, 22, 353–365. [Google Scholar] [CrossRef]
- Kuss DJ, Griffiths MD, Karila L, Billieux, J. Internet addiction: a systematic review of epidemiological research for the last decade. Curr Pharm Des 2014, 20, 4026–52. [Google Scholar] [CrossRef] [PubMed]
- Rumpf H, et al. Including gaming disorder in the ICD-11: The need to do so from a clinical and public health perspective. Journal of Behavioral Addictions 2018, 7, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Onukwuli VO, Onyinye EN, Udigwe IB, Umeh UM, Enebe JT, Umerah AT. Internet addiction during the COVID-19 pandemic among adolescents in southeast Nigeria and implications for adolescent care in the post-pandemic era: A cross-sectional study. SAGE Open Med 2023, 11, 20503121231152763. [Google Scholar] [CrossRef]
- Kumar G, Dash P, Jnaneswar A, Suresan V, Jha K, Ghosal S. Impact of internet addiction during COVID-19 on anxiety and sleep quality among college students of Bhubaneswar city. J Educ Health Promot 2022, 11, 156. [Google Scholar] [CrossRef]
- Melca IA, Teixeira EK, Nardi AE, Spear AL. Association of Internet Addiction and Mental Disorders in Medical Students: A Systematic Review. Prim Care Companion CNS Disord 2023, 25, 22r03384. [Google Scholar] [CrossRef]
- Dresp-Langley, B. Children's Health in the Digital Age. Int J Environ Res Public Health 2020, 17, 3240. [Google Scholar] [CrossRef]
- Dresp-Langley, B. Dresp-Langley B. Consciousness Beyond Neural Fields: Expanding the Possibilities of What Has Not Yet Happened. Front Psychol 2022, 12, 762349. [Google Scholar] [CrossRef]
- Oster, H. The interplay between stress, circadian clocks, and energy metabolism. J Endocrinol 2020, 247, R13–R25. [Google Scholar] [CrossRef]
- Arias D, Saxena S, Verguet, S. Quantifying the global burden of mental disorders and their economic value. eClinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




