Submitted:
03 May 2024
Posted:
07 May 2024
You are already at the latest version
Abstract
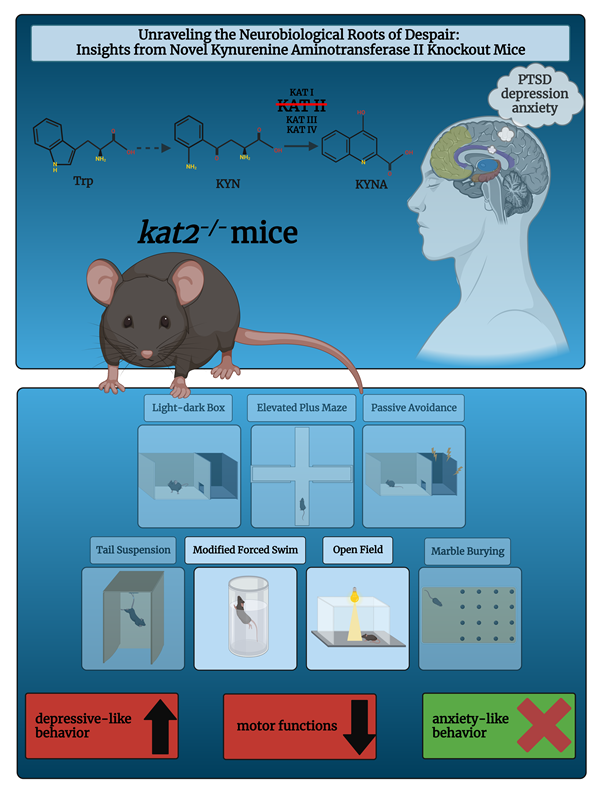
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
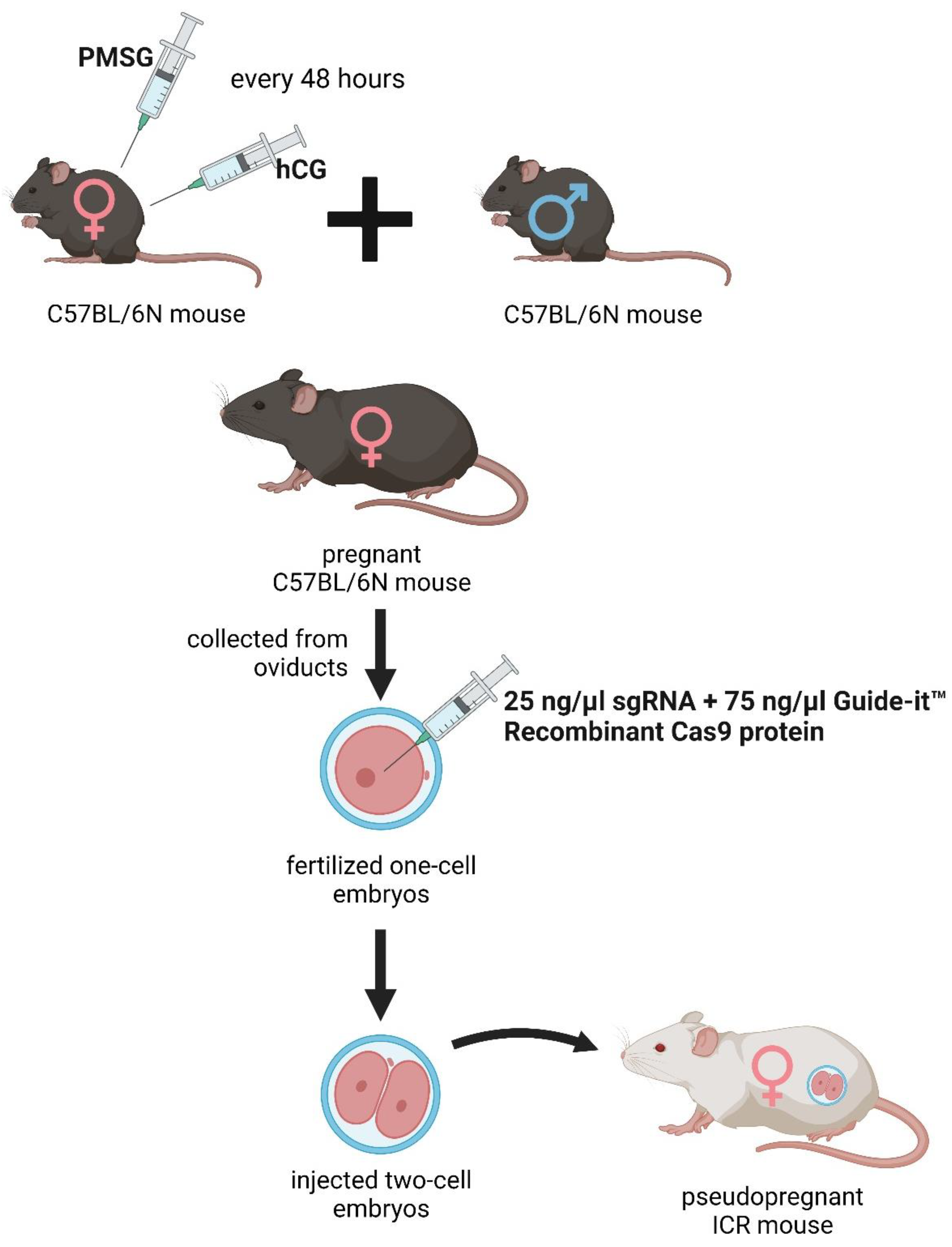
2.2. Animals
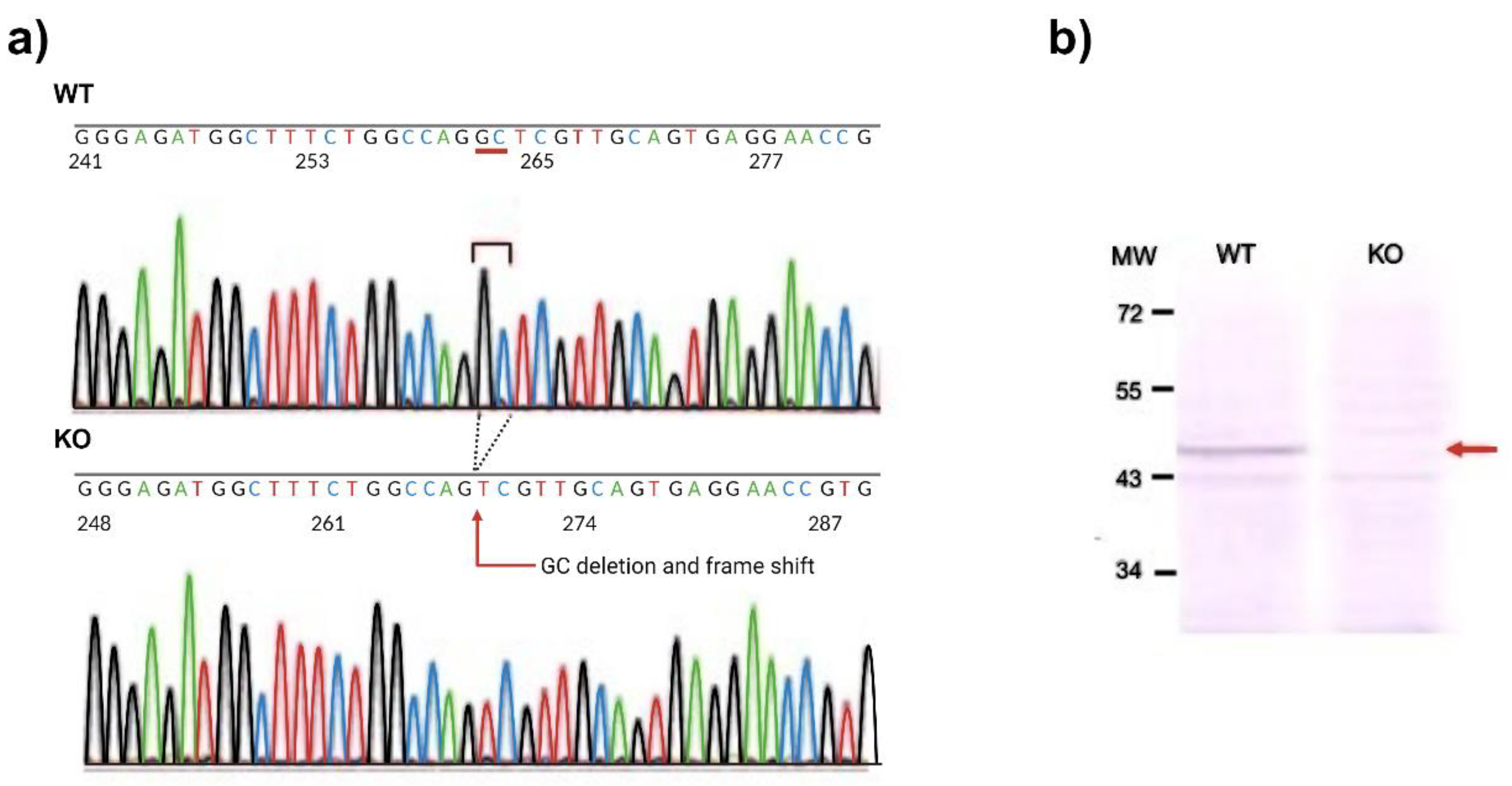
2.3. DNA Extraction and Sequencing
2.4. Western Blotting
2.5. Phenotype Analysis with Modified SHIRPA Test
2.6. Behavioral Tests
2.6.1. Modified Forced Swim Test (FST)
2.6.2. Tail Suspension Test (TST)
2.6.3. Elevated Plus Maze (EPM) Test
2.6.4. Light Dark Box (LDB) Test
2.6.5. Passive Avoidance Test (PAT)
2.6.6. Open Field (OF) Test
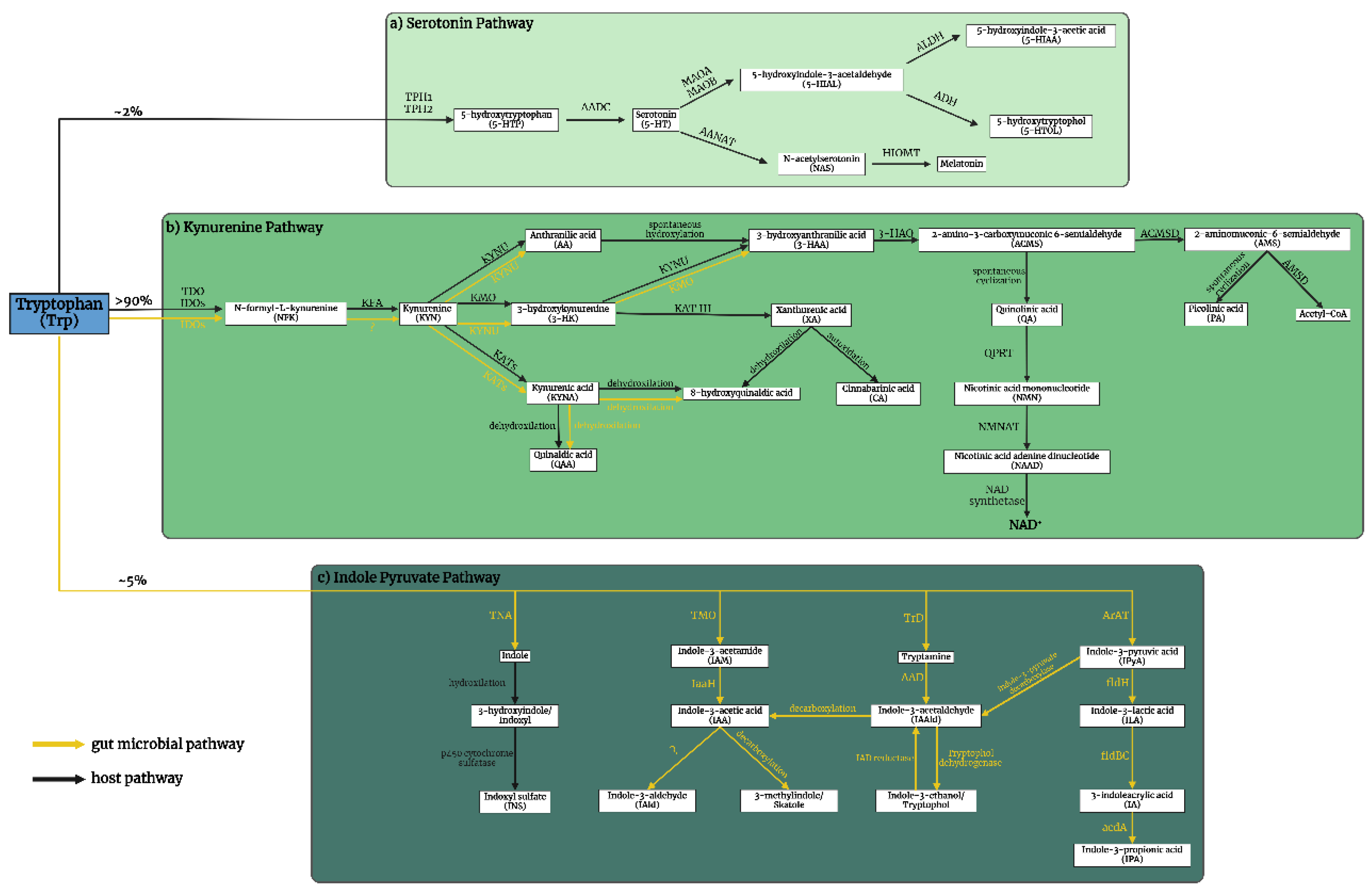
2.7. Ultra-High-Performance Liquid Chromatography with Tandem Mass Spectrometry
2.8. The Enzyme Activities of Trptophan (Trp) Metabolism
2.9. Oxidative Stress and Excitotoxicity Indices
3.0. Statistical Analysis
3. Results
3.1. DNA Sequence Analysis and Western Blot
3.2. Phenotype Analysis with SHIRPA Protocol
3.3. Behavioral Tests
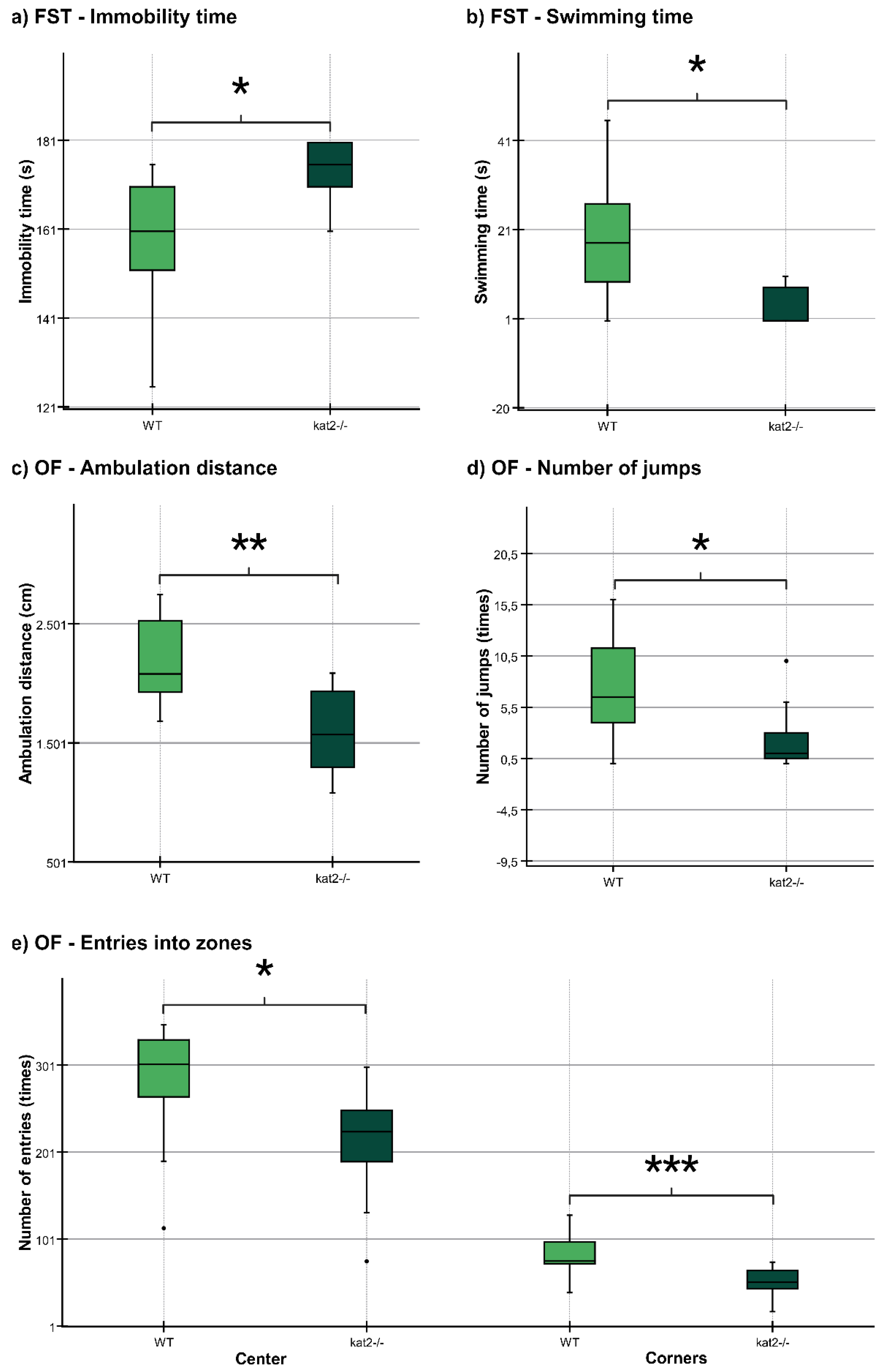
3.3.1. Forced Swim Test (FST)
3.3.2. Open Field (OF) Test
3.3.3. Other Behavioral Tests
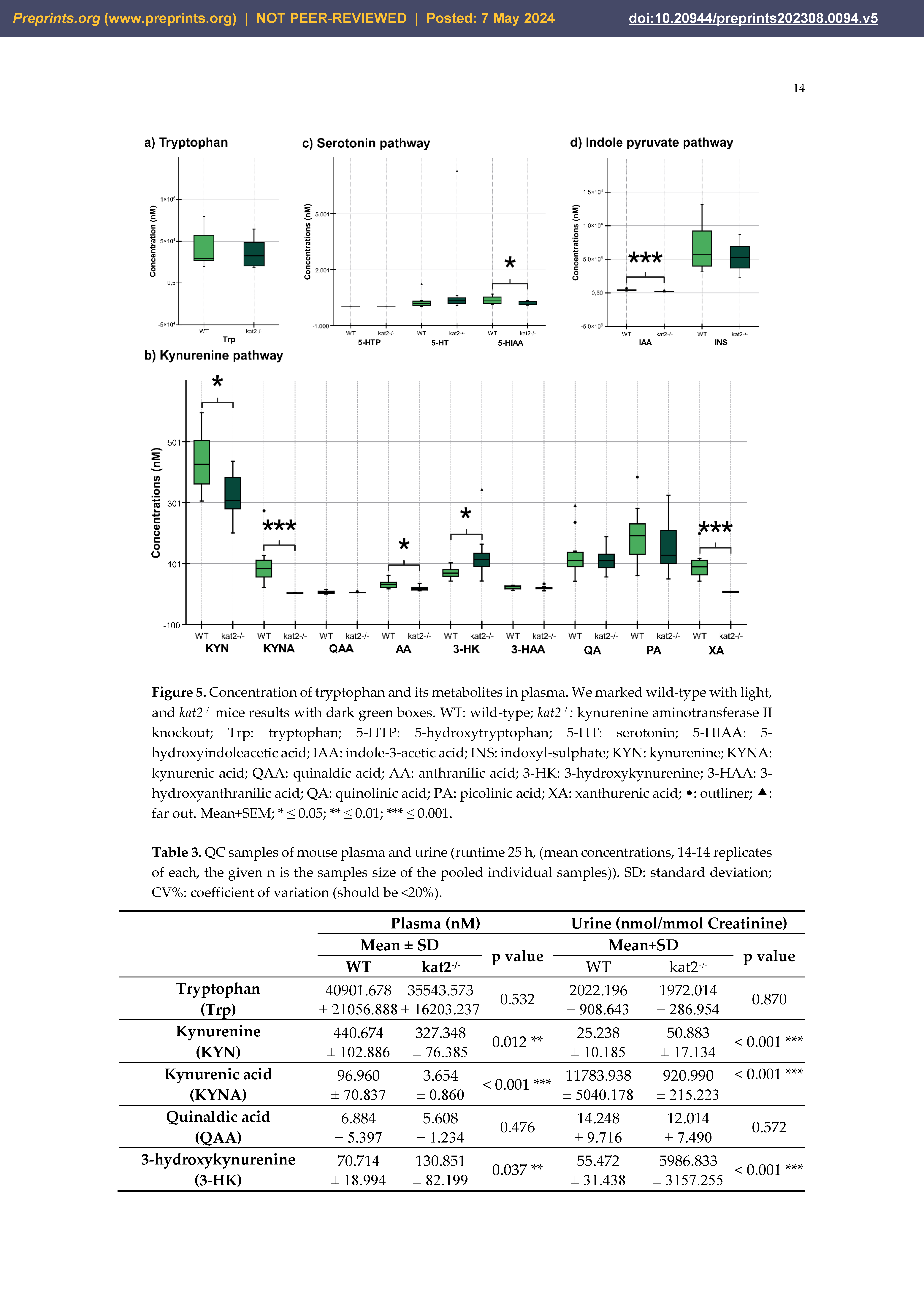
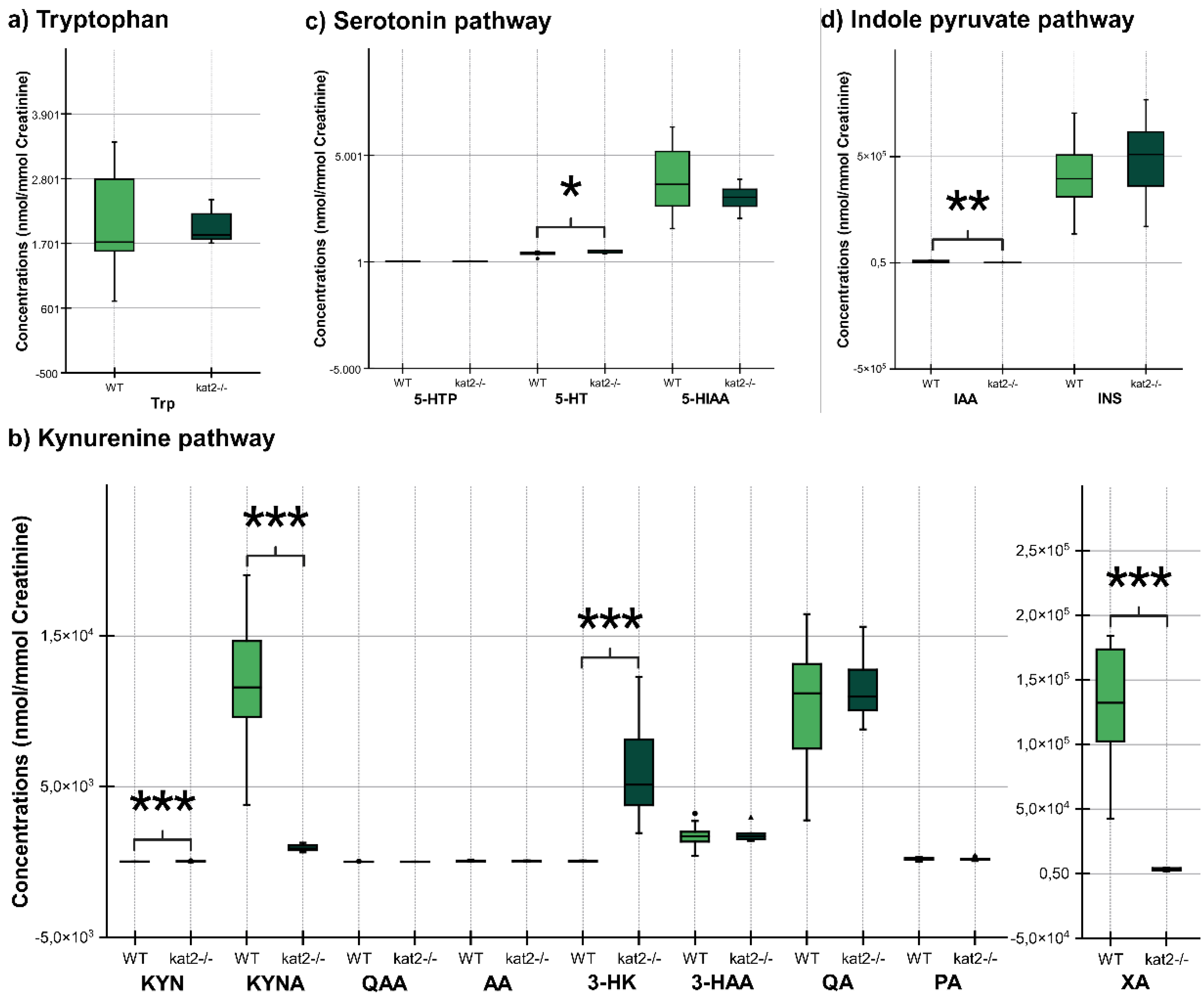
3.4. Ultra-High-Performance Liquid Chromatography with Tandem Mass Spectrometry
3.5. Enzyme Activities in Trptophan (Trp) Metabolism
2.9. Oxidative Stress and Excitotoxicity Indices
3. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT: serotonin |
| AD: Alzheimer’s disease |
| CBIR: cannabinoid 1 receptor |
| CNS: central nervous system |
| COMT: catechol-O-methyltransferase |
| EPM: elevated plus maze |
| FST: forced swim test |
| GABA: gamma-aminobutyric acid |
| GAD: glutamic acid decarboxylase |
| hCG: human chorionic gonadotropin |
| IAA: indole-3-acetic acid |
| ISN: indoxyl sulfate |
| KAT II: α-aminoadipate aminotransferase/kynurenine aminotransferase II |
| KATs: kynurenine aminotransferases |
| KYN: kynurenine |
| KYNA: kynurenic acid |
| LDB: light dark box |
| MDD: major depressive disorder |
| MRM: multiple reaction monitoring |
| OF: open field |
| PAT: passive avoidance test |
| PCR: polymerase chain reaction |
| PMSG: pregnant mare serum gonadotropin |
| PTSD: posttraumatic stress disorder |
| SCZ: schizophrenia |
| sgRNA: single guide RNA |
| SSRI: selective serotonin reuptake inhibitors |
| TNA tryptophanase |
| Trp: tryptophan |
| TST: tail suspension test |
| UHPLC-MS: ultra-high performance liquid chromatography-tandem mass spectrometry |
| WT: wild-type |
References
- Tyng CM, Amin HU, Saad MN, Malik AS (2017): The influences of emotion on learning and memory. Frontiers in psychology.1454.
- Battaglia S, Garofalo S, di Pellegrino G, Starita F (2020): Revaluing the role of vmPFC in the acquisition of Pavlovian threat conditioning in humans. Journal of Neuroscience. 40:8491-8500.
- Battaglia S, Harrison BJ, Fullana MA (2022): Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Molecular Psychiatry. 27:784-786.
- Sumsuzzman DM, Choi J, Jin Y, Hong Y (2021): Neurocognitive effects of melatonin treatment in healthy adults and individuals with Alzheimer’s disease and insomnia: a systematic review and meta-analysis of randomized controlled trials. Neuroscience & Biobehavioral Reviews. 127:459-473.
- Borgomaneri S, Battaglia S, Sciamanna G, Tortora F, Laricchiuta D (2021): Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neuroscience & Biobehavioral Reviews. 127:334-352.
- Hasson U, Chen J, Honey CJ (2015): Hierarchical process memory: memory as an integral component of information processing. Trends in cognitive sciences. 19:304-313.
- Clewett D, Sakaki M, Nielsen S, Petzinger G, Mather M (2017): Noradrenergic mechanisms of arousal’s bidirectional effects on episodic memory. Neurobiology of learning and memory. 137:1-14.
- Battaglia S, Nazzi C, Thayer J (2023): Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neuroscience & Biobehavioral Reviews.105163.
- Battaglia S, Di Fazio C, Vicario CM, Avenanti A (2023): Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: Synaptic transmission and plasticity. International Journal of Molecular Sciences. 24:5926. [CrossRef]
- Hayes JP, VanElzakker MB, Shin LM (2012): Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Frontiers in integrative neuroscience. 6:89.
- Dillon DG, Pizzagalli DA (2018): Mechanisms of memory disruption in depression. Trends in neurosciences. 41:137-149.
- Mathews A, MacLeod C (2005): Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 1:167-195.
- Hasebe K, Kendig MD, Morris MJ (2021): Mechanisms underlying the cognitive and behavioural effects of maternal obesity. Nutrients. 13:240.
- Junges VM, Closs VE, Nogueira GM, Gottlieb MGV (2018): Crosstalk between Gut Microbiota and Central Nervous System: A Focus on Alzheimer's Disease. Curr Alzheimer Res. 15:1179-1190.
- Guzmán-Vélez E, Feinstein JS, Tranel D (2014): Feelings without memory in Alzheimer disease. Cognitive and behavioral neurology. 27:117.
- Mack J, Marsh L (2017): Parkinson’s disease: cognitive impairment. Focus. 15:42-54.
- Mckee AC, Daneshvar DH (2015): The neuropathology of traumatic brain injury. Handbook of clinical neurology. 127:45-66.
- Li M, Feng L, Liu X, Zhang M, Fu B, Wang G, et al. (2018): Emotional working memory in patients with major depressive disorder. Journal of International Medical Research. 46:1734-1746. [CrossRef]
- Samuelson KW (2011): Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues in clinical neuroscience. 13:346-351.
- Dere E, Pause BM, Pietrowsky R (2010): Emotion and episodic memory in neuropsychiatric disorders. Behavioural brain research. 215:162-171.
- Berger M, Gray JA, Roth BL (2009): The expanded biology of serotonin. Annual review of medicine. 60:355-366.
- Ressler KJ, Nemeroff CB (2000): Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 12 Suppl 1:2-19.
- Bacqué-Cazenave J, Bharatiya R, Barrière G, Delbecque JP, Bouguiyoud N, Di Giovanni G, et al. (2020): Serotonin in Animal Cognition and Behavior. Int J Mol Sci. 21. [CrossRef]
- Meneses A, Liy-Salmeron G (2012): Serotonin and emotion, learning and memory. Rev Neurosci. 23:543-553.
- Švob Štrac D, Pivac N, Mück-Šeler D (2016): The serotonergic system and cognitive function. Translational neuroscience. 7:35-49.
- Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ (2006): Serotonin and human cognitive performance. Curr Pharm Des. 12:2473-2486.
- Buhot MC, Martin S, Segu L (2000): Role of serotonin in memory impairment. Ann Med. 32:210-221.
- Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S (2022): Stopping in (e) motion: Reactive action inhibition when facing valence-independent emotional stimuli. Frontiers in Behavioral Neuroscience. 16:998714.
- Cowen P, Sherwood AC (2013): The role of serotonin in cognitive function: evidence from recent studies and implications for understanding depression. Journal of psychopharmacology. 27:575-583.
- Battaglia S, Cardellicchio P, Di Fazio C, Nazzi C, Fracasso A, Borgomaneri S (2022): The influence of vicarious fear-learning in “infecting” reactive action inhibition. Frontiers in Behavioral Neuroscience. 16:946263.
- Battaglia S, Thayer JF (2022): Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends in Neurosciences.
- Battaglia S, Orsolini S, Borgomaneri S, Barbieri R, Diciotti S, di Pellegrino G (2022): Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology. 59:e14122.
- Di Gregorio F, La Porta F, Petrone V, Battaglia S, Orlandi S, Ippolito G, et al. (2022): Accuracy of EEG biomarkers in the detection of clinical outcome in disorders of consciousness after severe acquired brain injury: preliminary results of a pilot study using a machine learning approach. Biomedicines. 10:1897. [CrossRef]
- Borgomaneri S, Battaglia S, Avenanti A, di Pellegrino G (2021): Don't hurt me no more: State-dependent transcranial magnetic stimulation for the treatment of specific phobia. Journal of affective disorders. 286:78-79.
- Khalil R, Godde B, Karim AA (2019): The link between creativity, cognition, and creative drives and underlying neural mechanisms. Frontiers in neural circuits. 13:18. [CrossRef]
- Borgomaneri S, Battaglia S, Garofalo S, Tortora F, Avenanti A, di Pellegrino G (2020): State-dependent TMS over prefrontal cortex disrupts fear-memory reconsolidation and prevents the return of fear. Current Biology. 30:3672-3679. e3674.
- Battaglia S, Garofalo S, di Pellegrino G (2018): Context-dependent extinction of threat memories: Influences of healthy aging. Scientific reports. 8:12592.
- Albert PR, Vahid-Ansari F, Luckhart C (2014): Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre-and post-synaptic 5-HT1A receptor expression. Frontiers in behavioral neuroscience. 8:199.
- Tortora F, Hadipour AL, Battaglia S, Falzone A, Avenanti A, Vicario CM (2023): The role of Serotonin in fear learning and memory: a systematic review of human studies. Brain Sciences. 13:1197.
- Brewerton TD (1995): Toward a unified theory of serotonin dysregulation in eating and related disorders. Psychoneuroendocrinology. 20:561-590.
- Savitz J, Lucki I, Drevets WC (2009): 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 88:17-31. [CrossRef]
- Nutt DJ (2001): Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry. 62 Suppl 11:22-27; discussion 28.
- Steiger H (2004): Eating disorders and the serotonin connection: state, trait and developmental effects. J Psychiatry Neurosci. 29:20-29.
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J (2003): Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 27:1159-1172.
- Kelmendi B, Adams TG, Yarnell S, Southwick S, Abdallah CG, Krystal JH (2016): PTSD: from neurobiology to pharmacological treatments. Eur J Psychotraumatol. 7:31858.
- Chu A, Wadhwa R (2022): Selective Serotonin Reuptake Inhibitors.[Updated 2022 May 8]. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing.
- Alvares GA, Quintana DS, Hickie IB, Guastella AJ (2016): Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 41:89-104.
- Stahl SM, Lee-Zimmerman C, Cartwright S, Morrissette DA (2013): Serotonergic drugs for depression and beyond. Curr Drug Targets. 14:578-585.
- Teleanu RI, Niculescu A-G, Roza E, Vladâcenco O, Grumezescu AM, Teleanu DM (2022): Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. International Journal of Molecular Sciences. 23:5954.
- Liu Y, Zhao J, Guo W (2018): Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front Psychol. 9:2201.
- Nutt DJ (2006): The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 67 Suppl 6:3-8.
- Muneer A (2020): Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clinical Psychopharmacology and Neuroscience. 18:507.
- Tanaka M, Török N, Tóth F, Szabó Á, Vécsei L (2021): Co-players in chronic pain: neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines. 9:897.
- Huang Y, Zhao M, Chen X, Zhang R, Le A, Hong M, et al. (2023): Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging and Disease. 14:858. [CrossRef]
- Polyák H, Galla Z, Nánási N, Cseh EK, Rajda C, Veres G, et al. (2023): The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines. 11:945. [CrossRef]
- Hubková B, Valko-Rokytovská M, Čižmárová B, Zábavníková M, Mareková M, Birková A (2022): Tryptophan: Its metabolism along the kynurenine, serotonin, and indole pathway in malignant melanoma. International Journal of Molecular Sciences. 23:9160.
- Mor A, Tankiewicz-Kwedlo A, Krupa A, Pawlak D (2021): Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells. 10:1603.
- Bosi A, Banfi D, Bistoletti M, Giaroni C, Baj A (2020): Tryptophan metabolites along the microbiota-gut-brain axis: an interkingdom communication system influencing the gut in health and disease. International Journal of Tryptophan Research. 13:1178646920928984.
- Spekker E, Tanaka M, Szabó Á, Vécsei L (2021): Neurogenic inflammation: The participant in migraine and recent advancements in translational research. Biomedicines. 10:76.
- Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J (2015): Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. Journal of psychiatric research. 68:316-328.
- Jones AW (2023): Brief history of the alcohol biomarkers CDT, EtG, EtS, 5-HTOL, and PEth. Drug Testing and Analysis.
- Höglund E, Øverli Ø, Winberg S (2019): Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Frontiers in endocrinology.158.
- Jayamohananan H, Kumar MKM, Aneesh T (2019): 5-HIAA as a potential biological marker for neurological and psychiatric disorders. Advanced pharmaceutical bulletin. 9:374.
- Hardeland R (2010): Melatonin metabolism in the central nervous system. Current neuropharmacology. 8:168-181.
- Ishidoh K, Kamemura N, Imagawa T, Oda M, Sakurai J, Katunuma N (2010): Quinolinate phosphoribosyl transferase, a key enzyme in de novo NAD+ synthesis, suppresses spontaneous cell death by inhibiting overproduction of active-caspase-3. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1803:527-533. [CrossRef]
- Raffaelli N, Sorci L, Amici A, Emanuelli M, Mazzola F, Magni G (2002): Identification of a novel human nicotinamide mononucleotide adenylyltransferase. Biochemical and biophysical research communications. 297:835-840.
- Jauch R, Humm A, Huber R, Wahl MC (2005): Structures of Escherichia coli NAD synthetase with substrates and products reveal mechanistic rearrangements. Journal of Biological Chemistry. 280:15131-15140.
- Tanaka M, Tóth F, Polyák H, Szabó Á, Mándi Y, Vécsei L (2021): Immune influencers in action: metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines. 9:734.
- Tanaka M, Szabó Á, Spekker E, Polyák H, Tóth F, Vécsei L (2022): Mitochondrial impairment: A common motif in neuropsychiatric presentation? The link to the tryptophan–kynurenine metabolic system. Cells. 11:2607.
- Fila M, Chojnacki J, Pawlowska E, Szczepanska J, Chojnacki C, Blasiak J (2021): Kynurenine pathway of tryptophan metabolism in migraine and functional gastrointestinal disorders. International Journal of Molecular Sciences. 22:10134.
- Wirthgen E, Hoeflich A, Rebl A, Günther J (2018): Kynurenic acid: the Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Frontiers in immunology. 8:1957.
- Takahashi H, Price J (1958): Dehydroxylation of xanthurenic acid to 8-hydroxyquinaldic acid. Journal of Biological Chemistry. 233:150-153.
- Walczak K, Langner E, Szalast K, Makuch-Kocka A, Pożarowski P, Plech T (2020): A tryptophan metabolite, 8-hydroxyquinaldic acid, exerts antiproliferative and anti-migratory effects on colorectal cancer cells. Molecules. 25:1655. [CrossRef]
- Espi M, Koppe L, Fouque D, Thaunat O (2020): Chronic kidney disease-associated immune dysfunctions: impact of protein-bound uremic retention solutes on immune cells. Toxins. 12:300.
- Mishra P, Kaur S, Sharma AN, Jolly RS (2016): Characterization of an indole-3-acetamide hydrolase from Alcaligenes faecalis subsp. parafaecalis and its application in efficient preparation of both enantiomers of chiral building block 2, 3-dihydro-1, 4-benzodioxin-2-carboxylic acid. PloS one. 11:e0159009.
- Ye X, Li H, Anjum K, Zhong X, Miao S, Zheng G, et al. (2022): Dual role of indoles derived from intestinal microbiota on human health. Frontiers in Immunology. 13:903526. [CrossRef]
- Mousseau DD (1993): Tryptamine: a metabolite of tryptophan implicated in various neuropsychiatric disorders. Metabolic brain disease. 8:1-44.
- Brydges CR, Fiehn O, Mayberg HS, Schreiber H, Dehkordi SM, Bhattacharyya S, et al. (2021): Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature. Scientific reports. 11:21011. [CrossRef]
- Hou Y, Li J, Ying S (2023): Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites. 13:1166.
- Li X, Zhang B, Hu Y, Zhao Y (2021): New insights into gut-bacteria-derived indole and its derivatives in intestinal and liver diseases. Frontiers in Pharmacology. 12:769501.
- Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. (2018): Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Frontiers in cellular and infection microbiology. 8:13. [CrossRef]
- Ranhotra HS (2023): Discrete interplay of gut microbiota L-tryptophan metabolites in host biology and disease. Molecular and Cellular Biochemistry.1-18.
- Hyland NP, Cavanaugh CR, Hornby PJ (2022): Emerging effects of tryptophan pathway metabolites and intestinal microbiota on metabolism and intestinal function. Amino Acids. 54:57-70.
- Kumar P, Lee JH, Lee J (2021): Diverse roles of microbial indole compounds in eukaryotic systems. Biological Reviews. 96:2522-2545.
- Su X, Gao Y, Yang R (2022): Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells. 11:2296.
- Gasaly N, De Vos P, Hermoso MA (2021): Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Frontiers in immunology. 12:658354.
- Hubbard TD, Murray IA, Perdew GH (2015): Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metabolism and Disposition. 43:1522-1535.
- Fu Y, Lyu J, Wang S (2023): The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Frontiers in Immunology. 14.
- Madella AM, Van Bergenhenegouwen J, Garssen J, Masereeuw R, Overbeek SA (2022): Microbial-derived tryptophan catabolites, kidney disease and gut inflammation. Toxins. 14:645.
- Sun L-J, Li J-N, Nie Y-Z (2020): Gut hormones in microbiota-gut-brain cross-talk. Chinese medical journal. 133:826-833.
- Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O'Connor G, et al. (2017): Neurotransmitters: The critical modulators regulating gut–brain axis. Journal of cellular physiology. 232:2359-2372.
- Tran SM-S, Mohajeri MH (2021): The role of gut bacterial metabolites in brain development, aging and disease. Nutrients. 13:732.
- Han Y, Wang B, Gao H, He C, Hua R, Liang C, et al. (2022): Vagus nerve and underlying impact on the gut microbiota-brain Axis in behavior and neurodegenerative diseases. Journal of inflammation research.6213-6230. [CrossRef]
- Gershon MD, Margolis KG (2021): The gut, its microbiome, and the brain: connections and communications. The Journal of clinical investigation. 131. [CrossRef]
- Caspani G, Swann J (2019): Small talk: microbial metabolites involved in the signaling from microbiota to brain. Current opinion in pharmacology. 48:99-106.
- Zhou Y, Chen Y, He H, Peng M, Zeng M, Sun H (2023): The role of the indoles in microbiota-gut-brain axis and potential therapeutic targets: A focus on human neurological and neuropsychiatric diseases. Neuropharmacology.109690.
- Caspani G, Kennedy S, Foster JA, Swann J (2019): Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microbial Cell. 6:454.
- Chernikova MA, Flores GD, Kilroy E, Labus JS, Mayer EA, Aziz-Zadeh L (2021): The brain-gut-microbiome system: pathways and implications for autism spectrum disorder. Nutrients. 13:4497.
- Ju S, Shin Y, Han S, Kwon J, Choi TG, Kang I, Kim SS (2023): The Gut–Brain Axis in Schizophrenia: The Implications of the Gut Microbiome and SCFA Production. Nutrients. 15:4391.
- Pappolla MA, Perry G, Fang X, Zagorski M, Sambamurti K, Poeggeler B (2021): Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer's disease. Neurobiology of disease. 156:105403.
- Tanaka M, Bohár Z, Martos D, Telegdy G, Vécsei L (2020): Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacological Reports. 72:449-455.
- Martos D, Tuka B, Tanaka M, Vécsei L, Telegdy G (2022): Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines. 10:849.
- Goh DL, Patel A, Thomas GH, Salomons GS, Schor DS, Jakobs C, Geraghty MT (2002): Characterization of the human gene encoding α-aminoadipate aminotransferase (AADAT). Molecular genetics and metabolism. 76:172-180.
- Modoux M, Rolhion N, Mani S, Sokol H (2021): Tryptophan metabolism as a pharmacological target. Trends in Pharmacological Sciences. 42:60-73.
- Palotai M, Telegdy G, Tanaka M, Bagosi Z, Jászberényi M (2014): Neuropeptide AF induces anxiety-like and antidepressant-like behavior in mice. Behavioural brain research. 274:264-269.
- Tanaka M, Telegdy G (2008): Involvement of adrenergic and serotonergic receptors in antidepressant-like effect of urocortin 3 in a modified forced swimming test in mice. Brain research bulletin. 77:301-305.
- Tanaka M, Schally A, Telegdy G (2012): Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behavioural brain research. 228:388-391.
- Telegdy G, Tanaka M, Schally AV (2011): Effects of the growth hormone-releasing hormone (GH-RH) antagonist on brain functions in mice. Behavioural brain research. 224:155-158.
- Tanaka M, Csabafi K, Telegdy G (2013): Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regulatory peptides. 180:1-4.
- Rákosi K, Masaru T, Zarándi M, Telegdy G, Tóth GK (2014): Short analogs and mimetics of human urocortin 3 display antidepressant effects in vivo. Peptides. 62:59-66.
- Tanaka M, Vécsei L (2022): Editorial of special issue ‘dissecting neurological and neuropsychiatric diseases: Neurodegeneration and neuroprotection’. MDPI, pp 6991.
- Tran KN, Nguyen NPK, Nguyen LTH, Shin H-M, Yang I-J (2023): Screening for Neuroprotective and Rapid Antidepressant-like Effects of 20 Essential Oils. Biomedicines. 11:1248.
- Tanaka M, Szabó Á, Vécsei L (2022): Integrating armchair, bench, and bedside research for behavioral neurology and neuropsychiatry. MDPI, pp 2999. [CrossRef]
- Tanaka M, Kádár K, Tóth G, Telegdy G (2011): Antidepressant-like effects of urocortin 3 fragments. Brain Research Bulletin. 84:414-418.
- Telegdy G, Adamik A, Tanaka M, Schally A (2010): Effects of the LHRH antagonist Cetrorelix on affective and cognitive functions in rats. Regulatory peptides. 159:142-147.
- Baliellas DE, Barros MP, Vardaris CV, Guariroba M, Poppe SC, Martins MF, et al. (2023): Propentofylline Improves Thiol-Based Antioxidant Defenses and Limits Lipid Peroxidation following Gliotoxic Injury in the Rat Brainstem. Biomedicines. 11:1652. [CrossRef]
- Montanari M, Imbriani P, Bonsi P, Martella G, Peppe A (2023): Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson’s Disease from Mice to Human. Biomedicines. 11:1560.
- Garifulin R, Davleeva M, Izmailov A, Fadeev F, Markosyan V, Shevchenko R, et al. (2023): Evaluation of the Autologous Genetically Enriched Leucoconcentrate on the Lumbar Spinal Cord Morpho-Functional Recovery in a Mini Pig with Thoracic Spine Contusion Injury. Biomedicines. 11:1331. [CrossRef]
- Bueno CRdS, Tonin MCC, Buchaim DV, Barraviera B, Junior RSF, Santos PSdS, et al. (2023): Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals. 16:653.
- Scalise S, Zannino C, Lucchino V, Lo Conte M, Scaramuzzino L, Cifelli P, et al. (2022): Human iPSC modeling of genetic febrile seizure reveals aberrant molecular and physiological features underlying an impaired neuronal activity. Biomedicines. 10:1075. [CrossRef]
- Tanaka M, Szabó Á, Vécsei L, Giménez-Llort L (2023): Emerging translational research in neurological and psychiatric diseases: from in vitro to in vivo models. MDPI, pp 15739.
- Datki Z, Sinka R (2022): Translational biomedicine-oriented exploratory research on bioactive rotifer-specific biopolymers. Advances in Clinical and Experimental Medicine.1-5.
- Kwon K-M, Lee M-J, Chung H-S, Pak J-H, Jeon C-J (2022): The Organization of Somatostatin-Immunoreactive Cells in the Visual Cortex of the Gerbil. Biomedicines. 10:92.
- Chen B, Hasan MM, Zhang H, Zhai Q, Waliullah A, Ping Y, et al. (2023): UBL3 Interacts with Alpha-synuclein in Cells and the Interaction is Downregulated by the EGFR Pathway Inhibitor Osimertinib. Biomedicines. 11:1685. [CrossRef]
- Song A, Cho G-W, Vijayakumar KA, Moon C, Ang MJ, Kim J, et al. (2021): Neuroprotective effect of valproic acid on salicylate-induced tinnitus. International Journal of Molecular Sciences. 23:23. [CrossRef]
- Ibos KE, Bodnár É, Bagosi Z, Bozsó Z, Tóth G, Szabó G, Csabafi K (2021): Kisspeptin-8 induces anxiety-like behavior and hypolocomotion by activating the HPA axis and increasing GABA release in the nucleus accumbens in rats. Biomedicines. 9:112.
- Puri S, Kenyon BM, Hamrah P (2022): Immunomodulatory Role of Neuropeptides in the Cornea. Biomedicines. 10.
- Mirchandani-Duque M, Barbancho MA, López-Salas A, Alvarez-Contino JE, García-Casares N, Fuxe K, et al. (2022): Galanin and Neuropeptide Y Interaction Enhances Proliferation of Granule Precursor Cells and Expression of Neuroprotective Factors in the Rat Hippocampus with Consequent Augmented Spatial Memory. Biomedicines. 10. [CrossRef]
- Taschereau-Dumouchel V, Michel M, Lau H, Hofmann SG, LeDoux JE (2022): Putting the "mental" back in "mental disorders": a perspective from research on fear and anxiety. Mol Psychiatry. 27:1322-1330.
- Li J, Li C, Subedi P, Tian X, Lu X, Miriyala S, et al. (2023): Light Alcohol Consumption Promotes Early Neurogenesis Following Ischemic Stroke in Adult C57BL/6J Mice. Biomedicines. 11. [CrossRef]
- Petković A, Chaudhury D (2022): Encore: Behavioural animal models of stress, depression and mood disorders. Front Behav Neurosci. 16:931964.
- Bahor Z, Nunes-Fonseca C, Thomson LD, Sena ES, Macleod MR (2016): Improving our understanding of the in vivo modelling of psychotic disorders: A protocol for a systematic review and meta-analysis. Evid Based Preclin Med. 3:e00022.
- Tanaka M, Spekker E, Szabó Á, Polyák H, Vécsei L (2022): Modelling the neurodevelopmental pathogenesis in neuropsychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents-in celebration of 80th birthday of Professor Peter Riederer. J Neural Transm (Vienna). 129:627-642.
- Sobolewska-Nowak J, Wachowska K, Nowak A, Orzechowska A, Szulc A, Płaza O, Gałecki P (2023): Exploring the Heart-Mind Connection: Unraveling the Shared Pathways between Depression and Cardiovascular Diseases. Biomedicines. 11.
- Tug E, Fidan I, Bozdayi G, Yildirim F, Tunccan OG, Lale Z, Akdogan D (2024): The relationship between the clinical course of SARS-CoV-2 infections and ACE2 and TMPRSS2 expression and polymorphisms. Adv Clin Exp Med. 33:39-51.
- Fan P, Miranda O, Qi X, Kofler J, Sweet RA, Wang L (2023): Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer's Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals (Basel). 16.
- Festa F, Medori S, Macrì M (2023): Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines. 11. [CrossRef]
- Alhaddad A, Radwan A, Mohamed NA, Mehanna ET, Mostafa YM, El-Sayed NM, Fattah SA (2023): Rosiglitazone Mitigates Dexamethasone-Induced Depression in Mice via Modulating Brain Glucose Metabolism and AMPK/mTOR Signaling Pathway. Biomedicines. 11.
- Statsenko Y, Habuza T, Smetanina D, Simiyu GL, Meribout S, King FC, et al. (2023): Unraveling Lifelong Brain Morphometric Dynamics: A Protocol for Systematic Review and Meta-Analysis in Healthy Neurodevelopment and Ageing. Biomedicines. 11. [CrossRef]
- Dang J, Tao Q, Niu X, Zhang M, Gao X, Yang Z, et al. (2022): Meta-Analysis of Structural and Functional Brain Abnormalities in Cocaine Addiction. Front Psychiatry. 13:927075. [CrossRef]
- Okanda Nyatega C, Qiang L, Jajere Adamu M, Bello Kawuwa H (2022): Altered striatal functional connectivity and structural dysconnectivity in individuals with bipolar disorder: A resting state magnetic resonance imaging study. Front Psychiatry. 13:1054380.
- Du H, Yang B, Wang H, Zeng Y, Xin J, Li X (2023): The non-linear correlation between the volume of cerebral white matter lesions and incidence of bipolar disorder: A secondary analysis of data from a cross-sectional study. Front Psychiatry. 14:1149663.
- Chen Y, Yu R, DeSouza JFX, Shen Y, Zhang H, Zhu C, et al. (2023): Differential responses from the left postcentral gyrus, right middle frontal gyrus, and precuneus to meal ingestion in patients with functional dyspepsia. Front Psychiatry. 14:1184797. [CrossRef]
- Adamu MJ, Qiang L, Nyatega CO, Younis A, Kawuwa HB, Jabire AH, Saminu S (2023): Unraveling the pathophysiology of schizophrenia: insights from structural magnetic resonance imaging studies. Front Psychiatry. 14:1188603.
- Chang CH, Wang WL, Shieh YH, Peng HY, Ho CS, Tsai HC (2022): Case Report: Low-Frequency Repetitive Transcranial Magnetic Stimulation to Dorsolateral Prefrontal Cortex and Auditory Cortex in a Patient With Tinnitus and Depression. Front Psychiatry. 13:847618.
- Zakia H, Iskandar S (2022): Case report: Depressive disorder with peripartum onset camouflages suspected intracranial tuberculoma. Front Psychiatry. 13:932635.
- Nyatega CO, Qiang L, Adamu MJ, Kawuwa HB (2022): Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson's disease: A voxel-based morphometry study. Front Psychiatry. 13:1027907. [CrossRef]
- Rymaszewska J, Wieczorek T, Fila-Witecka K, Smarzewska K, Weiser A, Piotrowski P, Tabakow P (2022): Various neuromodulation methods including Deep Brain Stimulation of the medial forebrain bundle combined with psychopharmacotherapy of treatment-resistant depression-Case report. Front Psychiatry. 13:1068054.
- Kim BH, Kim SH, Han C, Jeong HG, Lee MS, Kim J (2023): Antidepressant-induced mania in panic disorder: a single-case study of clinical and functional connectivity characteristics. Front Psychiatry. 14:1205126.
- Zhou J, Cao Y, Deng G, Fang J, Qiu C (2023): Transient splenial lesion syndrome in bipolar-II disorder: a case report highlighting reversible brain changes during hypomanic episodes. Front Psychiatry. 14:1219592.
- Veldema J (2023): Non-Invasive Brain Stimulation and Sex/Polypeptide Hormones in Reciprocal Interactions: A Systematic Review. Biomedicines. 11.
- Manuello J, Costa T, Cauda F, Liloia D (2022): Six actions to improve detection of critical features for neuroimaging coordinate-based meta-analysis preparation. Neurosci Biobehav Rev. 137:104659.
- Nani A, Manuello J, Mancuso L, Liloia D, Costa T, Vercelli A, et al. (2021): The pathoconnectivity network analysis of the insular cortex: A morphometric fingerprinting. Neuroimage. 225:117481. [CrossRef]
- Liloia D, Crocetta A, Cauda F, Duca S, Costa T, Manuello J (2022): Seeking Overlapping Neuroanatomical Alterations between Dyslexia and Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Replication Study. Brain Sci. 12.
- Liloia D, Cauda F, Uddin LQ, Manuello J, Mancuso L, Keller R, et al. (2023): Revealing the Selectivity of Neuroanatomical Alteration in Autism Spectrum Disorder via Reverse Inference. Biol Psychiatry Cogn Neurosci Neuroimaging. 8:1075-1083. [CrossRef]
- Tanaka M, Chen C (2023): Editorial: Towards a mechanistic understanding of depression, anxiety, and their comorbidity: perspectives from cognitive neuroscience. Front Behav Neurosci. 17:1268156.
- Battaglia S, Schmidt A, Hassel S, Tanaka M (2023): Case reports in neuroimaging and stimulation. Frontiers in Psychiatry. 14:1264669.
- Cauda F, Nani A, Liloia D, Manuello J, Premi E, Duca S, et al. (2020): Finding specificity in structural brain alterations through Bayesian reverse inference. Human brain mapping. 41:4155-4172. [CrossRef]
- Balogh L, Tanaka M, Török N, Vécsei L, Taguchi S (2021): Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines. 9:340.
- Gregorio F, Battaglia S (2023): Advances in EEG-based functional connectivity approaches to the study of the central nervous system in health and disease. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University.
- Hakamata Y, Hori H, Mizukami S, Izawa S, Yoshida F, Moriguchi Y, et al. (2023): Blunted diurnal interleukin-6 rhythm is associated with amygdala emotional hyporeactivity and depression: a modulating role of gene-stressor interactions. Frontiers in Psychiatry. 14:1196235. [CrossRef]
- Rassler B, Blinowska K, Kaminski M, Pfurtscheller G (2023): Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines. 11:1028.
- Vasiliu O (2023): Efficacy, Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder—A Narrative Review. Pharmaceuticals. 16:411.
- Tanaka M, Szabó Á, Körtési T, Szok D, Tajti J, Vécsei L (2023): From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells. 12:2649. [CrossRef]
- Masuya H, Inoue M, Wada Y, Shimizu A, Nagano J, Kawai A, et al. (2005): Implementation of the modified-SHIRPA protocol for screening of dominant phenotypes in a large-scale ENU mutagenesis program. Mamm Genome. 16:829-837. [CrossRef]
- Mandillo S, Tucci V, Hölter SM, Meziane H, Banchaabouchi MA, Kallnik M, et al. (2008): Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics. 34:243-255. [CrossRef]
- Detke MJ, Rickels M, Lucki I (1995): Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl). 121:66-72.
- Khisti RT, Chopde CT, Jain SP (2000): Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 67:137-143.
- Steru L, Chermat R, Thierry B, Simon P (1985): The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 85:367-370.
- Cryan JF, Mombereau C, Vassout A (2005): The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 29:571-625.
- Lister RG (1987): The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl). 92:180-185.
- Pellow S, Chopin P, File SE, Briley M (1985): Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 14:149-167.
- Costall B, Coughlan J, Horovitz ZP, Kelly ME, Naylor RJ, Tomkins DM (1989): The effects of ACE inhibitors captopril and SQ29,852 in rodent tests of cognition. Pharmacol Biochem Behav. 33:573-579.
- Onaivi ES, Martin BR (1989): Neuropharmacological and physiological validation of a computer-controlled two-compartment black and white box for the assessment of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 13:963-976.
- van der Poel AM (1967): Ethological study of the behaviour of the albino rat in a passive-avoidance test. Acta Physiol Pharmacol Neerl. 14:503-505.
- Stanford SC (2007): The Open Field Test: reinventing the wheel. J Psychopharmacol. 21:134-135.
- Walsh RN, Cummins RA (1976): The Open-Field Test: a critical review. Psychol Bull. 83:482-504.
- Galla Z, Rajda C, Rácz G, Grecsó N, Baráth Á, Vécsei L, et al. (2021): Simultaneous determination of 30 neurologically and metabolically important molecules: A sensitive and selective way to measure tyrosine and tryptophan pathway metabolites and other biomarkers in human serum and cerebrospinal fluid. J Chromatogr A. 1635:461775. [CrossRef]
- Galla Z, Rácz G, Grecsó N, Baráth Á, Kósa M, Bereczki C, Monostori P (2021): Improved LC-MS/MS method for the determination of 42 neurologically and metabolically important molecules in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 1179:122846.
- Sahin EK, Caykoylu A, Senat A, Erel O (2019): A comprehensive study of oxidative stress in patients with somatic symptom disorder. Acta Neuropsychiatrica. 31:100-105.
- Polat N, Beyaztas H, Aktas S, Maden O, Guler EM (2023): Comparison of oxidative stress parameters, thiol-disulfide homeostasis, and pro-inflammatory cytokines levels in patients with bipolar disorder and their first-degree relatives. Journal of Psychiatric Research. 162:103-112. [CrossRef]
- Juchnowicz D, Dzikowski M, Rog J, Waszkiewicz N, Zalewska A, Maciejczyk M, Karakuła-Juchnowicz H (2021): Oxidative stress biomarkers as a predictor of stage illness and clinical course of schizophrenia. Frontiers in Psychiatry. 12:728986.
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, et al. (2015): Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 40:463-471. [CrossRef]
- Barone P (2019): The ‘Yin’and the ‘Yang’of the kynurenine pathway: excitotoxicity and neuroprotection imbalance in stress-induced disorders. Behavioural pharmacology. 30:163-186.
- Globus MY-T, Ginsberg MD, Busto R (1991): Excitotoxic index—a biochemical marker of selective vulnerability. Neuroscience letters. 127:39-42.
- Schwarz MJ, Ackenheil M (2002): The role of substance P in depression: therapeutic implications. Dialogues Clin Neurosci. 4:21-29.
- Tanaka M, Török N, Vécsei L (2021): Are 5-HT(1) receptor agonists effective anti-migraine drugs? Expert Opin Pharmacother. 22:1221-1225.
- Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, et al. (2020): Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 25:2860-2872.
- Van Praag HM, Kahn RS, Asnis GM, Wetzler S, Brown SL, Bleich A, Korn ML (1987): Denosologization of biological psychiatry or the specificity of 5-HT disturbances in psychiatric disorders. Journal of Affective Disorders. 13:1-8.
- Li D, Yu S, Long Y, Shi A, Deng J, Ma Y, et al. (2022): Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol. 13:985378. [CrossRef]
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T (2008): A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 11:198-209.
- Correia AS, Vale N (2022): Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. International journal of molecular sciences. 23:8493.
- Han Q, Cai T, Tagle DA, Li J (2010): Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci. 67:353-368. [CrossRef]
- Okada K, Angkawidjaja C, Koga Y, Kanaya S (2014): Structural and mechanistic insights into the kynurenine aminotransferase-mediated excretion of kynurenic acid. J Struct Biol. 185:257-266.
- Kucukkarapinar M, Yay-Pence A, Yildiz Y, Buyukkoruk M, Yaz-Aydin G, Deveci-Bulut TS, et al. (2022): Psychological outcomes of COVID-19 survivors at sixth months after diagnose: the role of kynurenine pathway metabolites in depression, anxiety, and stress. J Neural Transm (Vienna). 129:1077-1089. [CrossRef]
- Ford JD, Courtois CA (2021): Complex PTSD and borderline personality disorder. Borderline Personal Disord Emot Dysregul. 8:16.
- Horowitz MJ (2011): Stress response syndromes: PTSD, grief, adjustment, and dissociative disorders. Jason Aronson, Incorporated.
- Dorfman WI, Walker LE (2007): The Anxiety, Somatoform and Dissociative Disorders. First Responder’s Guide to Abnormal Psychology: Applications for Police, Firefighters and Rescue Personnel.87-107.
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A (2009): Amygdala inhibitory circuits and the control of fear memory. Neuron. 62:757-771.
- Hartley CA, Phelps EA (2010): Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 35:136-146.
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M (2014): Direct reactivation of a coherent neocortical memory of context. Neuron. 84:432-441.
- Barsy B, Kocsis K, Magyar A, Babiczky Á, Szabó M, Veres JM, et al. (2020): Associative and plastic thalamic signaling to the lateral amygdala controls fear behavior. Nat Neurosci. 23:625-637. [CrossRef]
- Tsigos C, Chrousos GP (2002): Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 53:865-871.
- Rodrigues SM, LeDoux JE, Sapolsky RM (2009): The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 32:289-313.
- Battaglia S, Di Fazio C, Mazzà M, Tamietto M, Avenanti A (2024): Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. International Journal of Molecular Sciences. 25:864.
- Spielberg JM, Stewart JL, Levin RL, Miller GA, Heller W (2008): Prefrontal Cortex, Emotion, and Approach/Withdrawal Motivation. Soc Personal Psychol Compass. 2:135-153.
- Frankland PW, Josselyn SA, Köhler S (2019): The neurobiological foundation of memory retrieval. Nat Neurosci. 22:1576-1585.
- Grace AA (2016): Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 17:524-532.
- Westerhof GJ, Bohlmeijer ET, McAdams DP (2017): The Relation of Ego Integrity and Despair to Personality Traits and Mental Health. J Gerontol B Psychol Sci Soc Sci. 72:400-407.
- Jing L, Duan TT, Tian M, Yuan Q, Tan JW, Zhu YY, et al. (2015): Despair-associated memory requires a slow-onset CA1 long-term potentiation with unique underlying mechanisms. Sci Rep. 5:15000. [CrossRef]
- van der Kolk BA (1994): The body keeps the score: memory and the evolving psychobiology of posttraumatic stress. Harv Rev Psychiatry. 1:253-265.
- van Meerkerk-Aanen PJ, de Vroege L, Khasho D, Foruz A, van Asseldonk JT, van der Feltz-Cornelis CM (2017): La belle indifférence revisited: a case report on progressive supranuclear palsy misdiagnosed as conversion disorder. Neuropsychiatr Dis Treat. 13:2057-2067.
- Reid S, Barbui C (2010): Long term treatment of depression with selective serotonin reuptake inhibitors and newer antidepressants. Bmj. 340:c1468.
- Raison S, Weissmann D, Rousset C, Pujol JF, Descarries L (1995): Changes in steady-state levels of tryptophan hydroxylase protein in adult rat brain after neonatal 6-hydroxydopamine lesion. Neuroscience. 67:463-475.
- Jacobsen JP, Medvedev IO, Caron MG (2012): The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 367:2444-2459.
- Xu CJ, Wang JL, Jing P, Min L (2019): Tph2 Genetic Ablation Contributes to Senile Plaque Load and Astrogliosis in APP/PS1 Mice. Curr Alzheimer Res. 16:219-232.
- Angoa-Pérez M, Kane MJ, Briggs DI, Herrera-Mundo N, Sykes CE, Francescutti DM, Kuhn DM (2014): Mice genetically depleted of brain serotonin do not display a depression-like behavioral phenotype. ACS Chem Neurosci. 5:908-919.
- Sbrini G, Hanswijk SI, Brivio P, Middelman A, Bader M, Fumagalli F, et al. (2022): Peripheral Serotonin Deficiency Affects Anxiety-like Behavior and the Molecular Response to an Acute Challenge in Rats. Int J Mol Sci. 23. [CrossRef]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT (2006): Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 31:101-111.
- Bouchekioua Y, Nebuka M, Sasamori H, Nishitani N, Sugiura C, Sato M, et al. (2022): Serotonin 5-HT(2C) receptor knockout in mice attenuates fear responses in contextual or cued but not compound context-cue fear conditioning. Transl Psychiatry. 12:58. [CrossRef]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. (2007): Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 27:684-691. [CrossRef]
- Sorgdrager FJH, Naudé PJW, Kema IP, Nollen EA, Deyn PP (2019): Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front Immunol. 10:2565.
- Myint AM (2012): Kynurenines: from the perspective of major psychiatric disorders. Febs j. 279:1375-1385.
- Battaglia MR, Di Fazio C, Battaglia S (2023): Activated Tryptophan-Kynurenine metabolic system in the human brain is associated with learned fear. Front Mol Neurosci. 16:1217090.
- Skorobogatov K, Autier V, Foiselle M, Richard JR, Boukouaci W, Wu CL, et al. (2023): Kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Brain Behav Immun Health. 27:100584. [CrossRef]
- Liang Y, Xie S, He Y, Xu M, Qiao X, Zhu Y, Wu W (2022): Kynurenine Pathway Metabolites as Biomarkers in Alzheimer's Disease. Dis Markers. 2022:9484217.
- Marszalek-Grabska M, Walczak K, Gawel K, Wicha-Komsta K, Wnorowska S, Wnorowski A, Turski WA (2021): Kynurenine emerges from the shadows - Current knowledge on its fate and function. Pharmacol Ther. 225:107845.
- Yu P, Di Prospero NA, Sapko MT, Cai T, Chen A, Melendez-Ferro M, et al. (2004): Biochemical and phenotypic abnormalities in kynurenine aminotransferase II-deficient mice. Mol Cell Biol. 24:6919-6930. [CrossRef]
- Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R (2010): Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 35:1734-1742.
- Desbonnet L, Tighe O, Karayiorgou M, Gogos JA, Waddington JL, O'Tuathaigh CM (2012): Physiological and behavioural responsivity to stress and anxiogenic stimuli in COMT-deficient mice. Behav Brain Res. 228:351-358.
- Kash SF, Tecott LH, Hodge C, Baekkeskov S (1999): Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 96:1698-1703.
- Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC (2009): Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J Neurosci. 29:15713-15720.
- Zhang WH, Zhou J, Pan HQ, Wang XY, Liu WZ, Zhang JY, et al. (2017): δ Subunit-containing GABA(A) receptor prevents overgeneralization of fear in adult mice. Learn Mem. 24:381-384.
- Sideris A, Piskoun B, Russo L, Norcini M, Blanck T, Recio-Pinto E (2016): Cannabinoid 1 receptor knockout mice display cold allodynia, but enhanced recovery from spared-nerve injury-induced mechanical hypersensitivity. Mol Pain. 12. [CrossRef]
- de Kloet ER, Molendijk ML (2016): Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural Plast. 2016:6503162.
- Lee DJ, Bovin MJ, Weathers FW, Palmieri PA, Schnurr PP, Sloan DM, et al. (2019): Latent factor structure of DSM–5 posttraumatic stress disorder: Evaluation of method variance and construct validity of novel symptom clusters. Psychological Assessment. 31:46. [CrossRef]
- McSweeney LB, Koch EI, Saules KK, Jefferson S (2016): Exploratory factor analysis of Diagnostic and Statistical Manual, criteria for posttraumatic stress disorder. The Journal of Nervous and Mental Disease. 204:9-14.
- Seligowski AV, Rogers AP, Orcutt HK (2016): Relations among emotion regulation and DSM-5 symptom clusters of PTSD. Personality and Individual Differences. 92:104-108.
- Di Gregorio F, Steinhauser M, Maier ME, Thayer JF, Battaglia S (2024): Error-related cardiac deceleration: functional interplay between error-related brain activity and autonomic nervous system in performance monitoring. Neuroscience & Biobehavioral Reviews.105542.
- Battaglia S, Nazzi C, Thayer JF (2024): Genetic differences associated with dopamine and serotonin release mediate fear-induced bradycardia in the human brain. Translational Psychiatry. 14:24.
| Name of sgRNA | Sequence | ||
|---|---|---|---|
| M-KAT II-2 | GTTCCTCACTGCAACGAGCCguuuuagagcuagaaauagcaaguuaaaaaaggcuaguccguuaucaacuugaaaaaguggcacggacucggugcuuuu |
||
| Name of primer | Sequence | ||
| M-KAT II_1st_F | CCCTCTGTGGATGGACTTTG | ||
| M-KAT II_1st _R | TTGAAAGATGTGCCTCATGC | ||
| M-KAT II_2nd_F | GGATGGACTTTGTCCCTTCT | ||
| M-KAT II_2nd_R | ATGTGCCTCATGCTTGGCCC | ||
| Name of KAT gene | Transcript ID | CCDS | CCDS Nucleotide Sequence |
| Aadat-201 | ENSMUST00000079472.4 | CCDS22320 | 32-33 (2 nucleotide deletion) |
| Test type | Number of animals | Perspectives | Mean ± SEM of wild-type |
Mean ± SEM of kat2-/- |
p-value |
| Modified forced swim test (FST) | WT: n = 12 kat2-/-: n = 11 |
Immobility time (s) | 157,73 ± 4,97 | 174,09 ± 2,00 | 0,022 * |
| Swimming time (s) | 18,18 ± 4,44 | 3,18 ± 1,39 | 0,014 * | ||
| Climbing time (s) | 4,09 ± 1,26 | 1,82 ± 1,82 | 0,681 | ||
| Tail suspension test (TST) | WT: n = 10 kat2-/-: n = 13 |
Immobility time (s) | 194,50 ± 21,11 | 209,58 ± 18,65 | 0,625 |
| Passive avoidance (PA) test | WT: n = 12 kat2-/-: n = 12 |
Time spent in the lit box on the training day (s) | 48,33 ± 8,44 | 64,67 ± 16,10 | 0,979 |
| Time spent in the lit box on the test day (s) | 256,0 ± 22,2 | 283,8 ± 11,4 | 0,822 | ||
| Elevated plus maze (EPM) test | WT: n = 10 kat2-/-: n = 11 |
Time spent in the open arms (s) | 42,90 ± 19,48 | 30,64 ± 13,17 | 0,500 |
| Light-dark box (LDB) test | WT: n = 12 kat2-/-: n = 11 |
Time spent in the lit box (s) | 119,00 ± 9,06 | 113,91 ± 7,36 | 0,957 |
| Open-field test (OFT) | WT: n = 12 kat2-/-: n = 11 |
Number of entries to the center zones (times) | 281,67 ± 19,96 | 210,73 ± 19,66 | 0,011 * |
| Number of entries to the corner zones (times) | 83,08 ± 7,78 | 51,27 ± 5,39 | 0,001 *** | ||
| Ambulation distance (cm) | 2191,75 ± 105,21 | 1609,27 ± 115,17 | 0,002 ** | ||
| Number of jumps (times) | 7,33 ± 1,43 | 2,45 ± 0,93 | 0,034 * |
| Plasma (nM) | Urine (nmol/mmol Creatinine) | |||||
| Mean ± SD | p value | Mean+SD | p value | |||
| WT | kat2-/- | WT | kat2-/- | |||
|
Tryptophan (Trp) |
40901.678 ± 21056.888 |
35543.573 ± 16203.237 |
0.532 | 2022.196 ± 908.643 |
1972.014 ± 286.954 |
0.870 |
|
Kynurenine (KYN) |
440.674 ± 102.886 |
327.348 ± 76.385 |
0.012 ** | 25.238 ± 10.185 |
50.883 ± 17.134 |
< 0.001 *** |
|
Kynurenic acid (KYNA) |
96.960 ± 70.837 |
3.654 ± 0.860 |
< 0.001 *** | 11783.938 ± 5040.178 |
920.990 ± 215.223 |
< 0.001 *** |
|
Quinaldic acid (QAA) |
6.884 ± 5.397 |
5.608 ± 1.234 |
0.476 | 14.248 ± 9.716 |
12.014 ± 7.490 |
0.572 |
|
3-hydroxykynurenine (3-HK) |
70.714 ± 18.994 |
130.851 ± 82.199 |
0.037 ** | 55.472 ± 31.438 |
5986.833 ± 3157.255 |
< 0.001 *** |
|
Xanthurenic acid (XA) |
93.624 ± 45.637 |
7.406 ± 1.452 |
< 0.001 *** | 127228.662 ± 52582.223 |
3273.334 ±1021.511 |
< 0.001 *** |
|
Anthranilic acid (AA) |
32.655 ± 13.114 |
19.335 ± 7.280 |
0.012 ** | 69.112 ± 45.347 |
60.862 ± 22.368 |
0.612 |
|
3-Hydroxyanthranilic acid (3-HAA) |
22.992 ± 6.140 |
20.920 ± 5.921 |
0.452 | 1741.538 ± 824.887 |
1789.475 ± 454.422 |
0.874 |
|
Quinolinic acid (QA) |
132.185 ± 75.409 |
112.000 ± 41.600 |
0.468 | 10059.485 ± 4601.597 |
11718.491 ± 2401.051 |
0.326 |
|
Picolinic acid (PA) |
193.797 ± 93.230 |
154.895 ± 88.753 |
0.352 | 190.435 ± 91.394 |
193.898 ± 113.072 |
0.941 |
|
5-Hydroxytryptophan (5-HTP) |
2.790 ± 1.577 |
2.708 ± 1.297 |
0.901 | 21.742 ± 8.520 |
19.297 ± 3.833 |
0.419 |
|
Serotonin (5-HT) |
277.309 ± 353.179 |
1010.379 ± 2219.355 |
0.316 | 371.974 ± 125.489 |
479.383 ± 63.304 |
0.027 * |
|
5-hydroxyindoleacetic acid (5-HIAA) |
362.241 ± 199.450 |
201.217 ± 99.184 |
0.035 ** | 3774.968 ± 1666.005 |
2969.725 ± 598.373 |
0.167 |
|
Indole-3-acetic acid (IAA) |
457.329 ± 153.046 |
229.142 ± 68.266 |
< 0.001 *** | 6030.306 ± 4737.901 |
1513.400 ± 1097.122 |
0.009 ** |
|
Indoxyl-sulphate (INS) |
6738.111 ± 3559.896 |
5404.257 ± 2292.535 |
0.332 | 400636.750 ± 185880.105 |
497063.585 ± 190235.646 |
0.267 |
| Enzyme | Product/Substrate | Plasma | Urine | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | p value | Mean ± SD | p value | ||||
| WT | kat2-/- | WT | kat2-/- | ||||
| TDO/IDOs (KFA) | KYN/Trp | 0.013 ± 0.007 |
0.011 ± 0.006 |
0.532 | 0.013 ± 0.002 |
0.026 ± 0.006 |
< 0.001 *** |
| KATs | KYNA/KYN | 0.205 ± 0.107 |
0.011 ± 0.002 |
< 0.001 *** | 476.464 ± 164.156 |
18.937 ± 5.057 |
< 0.001 *** |
| KMO | 3-HK/KYN | 0.168 ± 0.062 |
0.386 ± 0.180 |
0.002 ** | 2.219 ± 0.827 |
122.983 ± 75.543 |
< 0.001 *** |
| KYNU | AA/KYN | 0.075 ± 0.028 |
0.059 ± 0.016 |
0.120 | 2.593 ± 0.862 |
1.253 ± 0.529 |
< 0.001 *** |
| KYNU | 3-HAA/3-HK | 0.330 ± 0.070 |
0.194 ± 0.080 |
< 0.001 *** | 35.177 ± 16.776 |
0.372 ± 0.182 |
< 0.001 *** |
| KAT III | XA/3-HK | 1.374 ± 0.714 |
0.070 ± 0.033 |
< 0.001 *** | 2702.990 ± 1524.430 |
0.629 ± 0.229 |
< 0.001 *** |
| 3-HAO | QA/3-HAA | 5.l771 ± 2.978 |
5.486 ± 1.994 |
0.804 | 6.240 ± 2.487 |
6.856 ± 1.779 |
0.532 |
| 3-HA | PA/3-HAA | 8.797 ± 4.263 |
7.681 ± 4.872 |
0.592 | 1.286 ± 0.594 |
0.119 ± 0.085 |
< 0.001 *** |
| TPH | 5-HTP/Trp | < 0.001 ± <0.001 |
< 0.001 ± < 0.001 |
0.128 | 15.653 ± 6.024 |
0.010 ± 0.003 |
< 0.001 *** |
| AADC | 5-HT/5-HTP | 97.585 ± 87.384 |
307.233 ± 509.276 |
0.216 | 10.209 ± 2.530 |
25.997 ± 7.185 |
< 0.001 *** |
| MAO &ALDH | 5-HIAA/5-HT | 4.217 ± 4.818 |
0.905 ± 0.712 |
0.045 * | < 0.001 ± < 0.001 |
6.181 ± 0.859 |
< 0.001 *** |
| TMO(TrD, ArAT) | IAA/Trp | 0.013 ± 0.005 |
0.007 ± 0.002 |
0.005 ** | 5.243 ± 1.794 |
0.786 ± 0.636 |
< 0.001 *** |
| TNA | INS/Trp | 0.208 ± 0.178 |
0.170 ± 0.089 |
0.555 | 203.069 ± 108.000 |
248.916 ± 81.413 |
0.298 |
| Oxidative stress index | ||||||
|---|---|---|---|---|---|---|
| Oxidant/antioxidant metabolites |
Plasma (nM) | Urine (nmol/mmol Creatinine) | ||||
| Mean ± SD | p value | Mean ± SD | p value | |||
| WT | kat2-/- | WT | kat2-/- | |||
| 3-HK/KYNA+AA+XA | 0.378 ± 0.163 |
4.090 ± 1.478 |
< 0.001 *** | 0.085 ± 0.011 |
1.352 ± 0.473 |
< 0.001 *** |
| Excitotoxicity index | ||||||
|
NMDA agonist/antagonist metabolites |
Plasma (nM) |
Urine (nmol/mmol Creatinine) |
||||
| QA/KYNA | 1.648 ± 0.810 |
30.514 ± 8.618 |
< 0.001 *** | 0.884 ± 0.320 |
13.092 ± 2.833 |
< 0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





