Submitted:
31 July 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Patient collection and study protocol
2.2. Echocardiographic measurements
2.3. Surgical procedures
2.4. Laboratory tests
2.5. Statistical analysis
3. Results
3.1. Laboratory blood tests
3.2. Echocardiographic findings within the groups
3.3. Echocardiographic findings between groups
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rana, J.S.; Khan, S.S.; Lloyd-Jones, D.M.; Sidney, S. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med 2021, 36, 2517–2518. [Google Scholar] [CrossRef]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J Clin 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Sangha, R.; Price, J.; Butts, C.A. Adjuvant therapy in non-small cell lung cancer: current and future directions. Oncologist 2010, 15, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameri, M.; Bergman, P.; Franco-Cereceda, A.; Sartipy, U. Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study. J Thorac Dis 2018, 10, 3499–3506. [Google Scholar] [CrossRef]
- Batihan, G.; Ceylan, K.C.; Usluer, O.; Kaya, Ş.Ö. Video-assisted thoracoscopic surgery vs thoracotomy for non-small cell lung cancer greater than 5 cm: is VATS a feasible approach for large tumors? J Cardiothorac Surg 2020, 15, 261–261. [Google Scholar] [CrossRef]
- Pinsky, M.R. Cardiopulmonary interactions: physiologic basis and clinical applications. Ann Am Thorac Soc 2018, 15, S45–S48. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.S. Heart-lung interactions in health and disease. Am J Med Sci 1987, 294, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Vainshelboim, B.; Fox, B.D.; Saute, M.; Sagie, A.; Yehoshua, L.; Fuks, L.; Schneer, S.; Kramer, M.R. Limitations in exercise and functional capacity in long-term postpneumonectomy patients. J Cardiopulm Rehabil Prev 2015, 35, 56–64. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Chu, W.; Kou, Y.; Zhang, X. Evaluation of left and right ventricular myocardial function after lung resection using speckle tracking echocardiography. Medicine (Baltimore) 2016, 95, e4290–e4290. [Google Scholar] [CrossRef]
- Pedoto, A.; Amar, D. Right heart function in thoracic surgery: role of echocardiography. Curr Opin Anaesthesiol 2009, 22, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef]
- Peyrou, J.; Parsaï, C.; Chauvel, C.; Simon, M.; Dehant, P.; Abergel, E. Echocardiographic assessment of right ventricular systolic function in a population of unselected patients before cardiac surgery: a multiparametric approach is necessary. Arch Cardiovasc Dis 2014, 107, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Urheim, S.; Edvardsen, T.; Torp, H.; Angelsen, B.; Smiseth, O.A. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000, 102, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Boe, E.; Skulstad, H.; Smiseth, O.A. Myocardial work by echocardiography: a novel method ready for clinical testing. Eur Heart J Cardiovasc Imaging 2019, 20, 18–20. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Singh A, Huang X, Dai L, et al. Right ventricular function is reduced during cardiac surgery independent of procedural characteristics, reoperative status, or pericardiotomy. J Thorac Cardiovasc Surg. 2020;159(4):1430-1438.e4. 1: Surg. 2020;159(4). [CrossRef]
- Steffen HJ, Kalverkamp S, Zayat R, et al. Is Systolic Right Ventricular Function Reduced after Thoracic Non-Cardiac Surgery? A Propensity Matched Echocardiographic Analysis. Ann Thorac Cardiovasc Surg. 2018;24(5):238-246. [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191. [CrossRef]
- Falcoz, P.E.; Conti, M.; Brouchet, L.; Chocron, S.; Puyraveau, M.; Mercier, M.; Etievent, J.P.; Dahan, M. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007, 133, 325–332. [Google Scholar] [CrossRef]
- Reed, C.E.; Spinale, F.G.; Crawford, F.A., Jr. Effect of pulmonary resection on right ventricular function. Ann Thorac Surg 1992, 53, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Shelley, B.; Glass, A.; Keast, T.; McErlane, J.; Hughes, C.; Lafferty, B.; Marczin, N.; McCall, P. Perioperative cardiovascular pathophysiology in patients undergoing lung resection surgery: a narrative review. Br J Anaesth 2023, 130, e66–e79. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, B.; Hofhuizen, C.; van Lavieren, M.; van Swieten, H.; Lemson, J.; van Putten, M.J.; van der Hoeven, J.G.; Pickkers, P. Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interactions*. Crit. Care Med. 2014, 42, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.F.; Ueda, K. Pulmonary hypertension in thoracic surgical patients. Curr Opin Anaesthesiol 2010, 23, 25–33. [Google Scholar] [CrossRef]
- Koshino, Y.; Villarraga, H.R.; Orban, M.; Bruce, C.J.; Pressman, G.S.; Leinveber, P.; Saleh, H.K.; Konecny, T.; Kara, T.; Somers, V.K.; et al. Changes in left and right ventricular mechanics during the Mueller maneuver in healthy adults. Circ Cardiovasc Imaging 2010, 3, 282–289. [Google Scholar] [CrossRef]
- Wei, X.; Yu, H.; Dai, W.; Mu, Y.; Wang, Y.; Liao, J.; Peng, L.; Han, Y.; Li, Q.; Shi, Q. Patient-reported outcomes of video-assisted thoracoscopic surgery versus thoracotomy for locally advanced lung cancer: a longitudinal cohort study. Ann Surg Oncol 2021, 28, 8358–8371. [Google Scholar] [CrossRef]
- Homma, T.; Shimada, Y.; Tanabe, K. Decreased postoperative complications, neuropathic pain and epidural anesthesia-free effect of uniportal video-assisted thoracoscopic anatomical lung resection: a single-center initial experience of 100 cases. J Thorac Dis 2022, 14, 3154–3166. [Google Scholar] [CrossRef]
- D'Andrea, A.; Sperlongano, S.; Formisano, T.; Tocci, G.; Cameli, M.; Tusa, M.; Novo, G.; Corrado, G.; Ciampi, Q.; Citro, R.; et al. Stress echocardiography and strain in aortic regurgitation (SESAR protocol): left ventricular contractile reserve and myocardial work in asymptomatic patients with severe aortic regurgitation. Echocardiography (Mount Kisco, N.Y.) 2020, 37, 1213–1221. [Google Scholar] [CrossRef]
- D'Andrea, A.; Radmilovic, J.; Carbone, A.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; D'Ascenzi, F.; Bossone, E.; Galderisi, M.; et al. Speckle tracking evaluation in endurance athletes: the "optimal" myocardial work. Int J Cardiovasc Imaging 2020, 36, 1679–1688. [Google Scholar] [CrossRef]
- Yerebakan, C.; Klopsch, C.; Niefeldt, S.; Zeisig, V.; Vollmar, B.; Liebold, A.; Sandica, E.; Steinhoff, G. Acute and chronic response of the right ventricle to surgically induced pressure and volume overload--an analysis of pressure-volume relations. Interact. Cardiovasc Thorac Surg 2010, 10, 519–525. [Google Scholar] [CrossRef]

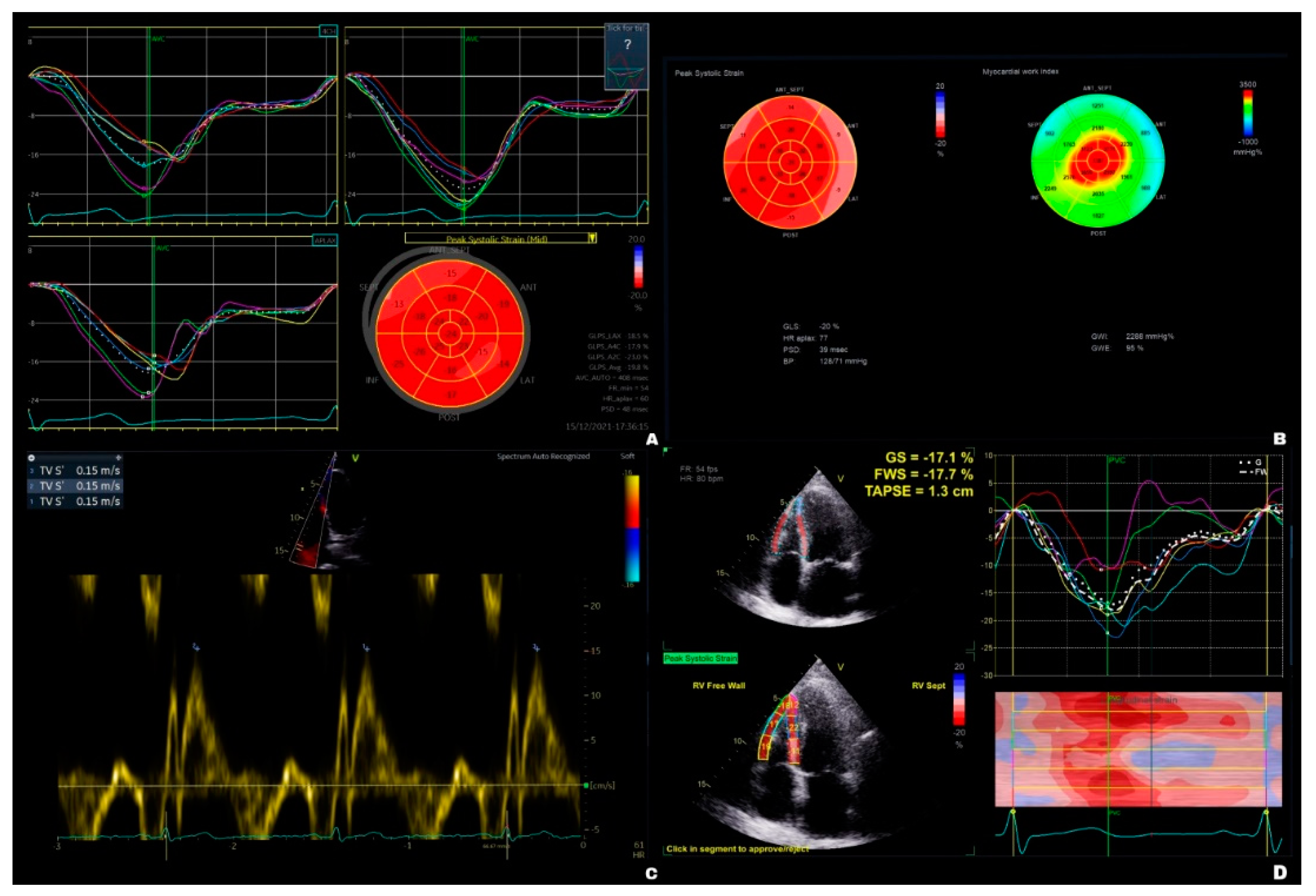
| Thoracotomy (n = 25) | VATS (n = 20) | p values | |
|---|---|---|---|
| Mean age, years | 63 ± 14 | 70 ± 11 | 0.10 |
| Female, n (%) | 10 (40) | 9 (45) | 0.74 |
| COPD ≥ GOLD III, n (%) | 2 (8) | 0 (0) | 0.06 |
| PAD, n (%) | 3 (12) | 1 (5) | 0.62 |
| Diabetes mellitus, n (%) | 3 (12) | 6 (20) | 0.16 |
| Hypertension, n (%) | 17 (68) | 15 (75) | 0.61 |
| Smoking, n (%) | 15 (60) | 8 (40) | 0.18 |
| KDIGO ≥ G3, n (%) | 3 (12) | 3 (15) | 0.37 |
| NYHA ≥ III, n (%) | 3 (12) | 1 (5) | 0.59 |
| Non-cardiopulmonary prior thoracic surgery, n (%) | 3 (12) | 0 (0) | 0.49 |
| BMI (kg/m2) | 26.9 ± 4.12 | 25.2 ± 4.36 | 0.13 |
| Thoracoscore PPD, (%) | 2.67 ± 2.75 | 2.67 ± 2.52 | 0.87 |
| Creatinine (mg/dL) | 0.96 ± 0.41 | 0.88 ± 0.2 | 0.85 |
| Hemoglobin (mg/dL) | 13.00 ± 1.91 | 13.70 ± 1.36 | 0.59 |
| Leukocytes (103/µL) | 7.30 ± 2.82 | 8.05 ± 3.28 | 0.27 |
| Thrombocytes (103/µL) | 246 ± 47.2 | 276 ± 92.1 | 0.13 |
| LDH (U/L) | 198 ± 30.9 | 210 ± 64.7 | 0.74 |
| AST (U/L) | 23 ± 14.7 | 24 ± 5.39 | 0.88 |
| ALT (U/L) | 19 ± 16.4 | 18 ± 7.29 | 0.57 |
| Thoracotomy (n = 25) | VATS (n = 20) | VATS vs. thoracotomy postoperative |
|||||
| Preoperative | Postoperative | p values | Preoperative | Postoperative | p values | p values | |
| Creatinine (mg/dL) | 1.00 ± 0.41 | 1.02 ± 0.76 | 0.989 | 0.92 ± 0.20 | 0.84 ± 0.23 | 0.076 | 0.328 |
| Hemoglobin (mg/dL) | 13.20 ± 1.91 | 11.30 ± 1.82 | < 0.001 | 13.40 ± 1.36 | 12.00 ± 1.74 | < 0.001 | 0.631 |
| Leukocytes (103/µL) | 8.04 ± 2.82 | 9.98 ± 2.74 | 0.007 | 8.89 ± 3.28 | 9.51 ± 4.42 | 0.766 | 0.668 |
| Thrombocytes (103/µL) | 249.00 ± 47.20 | 224.00 ± 82.10 | 0.236 | 297.00 ± 92.00 | 224.00 ± 98.90 | 0.087 | 0.175 |
| LDH (U/L) | 206.00 ± 30.90 | 209.00 ± 50.60 | 0.992 | 220.00 ± 64.70 | 210.00 ± 41.80 | 0.871 | 0.944 |
| AST (U/L) | 26.40 ± 14.70 | 26.30 ± 13.00 | 1.000 | 23.50 ± 5.39 | 31.90 ± 8.40 | 0.663 | 0.576 |
| ALT (U/L) | 24.90 ± 16.40 | 24.30 ± 13.60 | 0.992 | 20.10 ± 7.29 | 19.50 ± 11.20 | 0.994 | 0.214 |
| Thoracotomy (n = 25) | VATS (n = 20) | |||||
| Preoperative | Postoperative | p values | Preoperative | Postoperative | p values | |
| LVIDd (cm) | 4.44 ± 0.55 | 4.84 ± 0.74 | 0.101 | 4.43 ± 0.82 | 4.53 ± 0.90 | 0.961 |
| LVIDs (cm) | 3.20 ± 0.74 | 3.50 ± 0.84 | 0.343 | 3.18 ± 0.92 | 3.31 ± 0.93 | 0.883 |
| LVEDV (mL) | 103.00 ± 17.90 | 106.00 ± 15.20 | 0.991 | 101.00 ± 20.30 | 100.00 ± 22.20 | 0.992 |
| LVESV (mL) | 41.80 ± 11.10 | 45.20 ± 11.30 | 0.832 | 45.00 ± 13.70 | 45.10 ± 12.90 | 1.000 |
| LVEF (%) | 59.40 ± 6.26 | 59.2 ± 7.43 | 0.997 | 56.3 ± 7.04 | 56.50 ± 7.70 | 0.994 |
| CO (L/min) | 5.31 ± 1.19 | 5.66 ± 1.30 | 0.606 | 4.90 ± 1.23 | 4.97 ± 1.09 | 0.963 |
| GLSLV avg. (%) | -17.40 ± 4.14 | -16.7 ± 4.13 | 0.813 | -17.5 ± 2.78 | -16.10 ± 4.46 | 0.532 |
| GWILV | 1617 ± 529 | 1637 ± 462 | 0.997 | 1778 ± 580 | 1505 ± 636 | 0.074 |
| GWELV (%) | 90.90 ± 9.05 | 90.60 ± 7.56 | 0.991 | 93.20 ± 5.72 | 89.30 ± 10.70 | 0.068 |
| RVIDd (cm) | 3.13 ± 0.63 | 3.02 ± 0.67 | 0.674 | 2.90 ± 0.45 | 2.81 ± 0.65 | 0.926 |
| RVIDs (cm) | 1.85 ± 0.46 | 1.90 ± 0.51 | 0.968 | 1.64 ± 0.40 | 1.71 ± 0.41 | 0.441 |
| RVD1basal (cm) | 3.89 ± 0.79 | 3.69 ± 0.73 | 0.714 | 3.79 ± 0.63 | 3.81 ± 0.72 | 0.992 |
| RVD3long (cm) | 6.92 ± 0.79 | 7.20 ± 0.59 | 0.356 | 6.87 ± 0.97 | 6.81 ± 0.86 | 0.923 |
| RVFAC (%) | 51.2 ± 9.62 | 51.40 ± 7.55 | 0.997 | 47.90 ± 10.60 | 48.20 ± 7.33 | 0.994 |
| PASP (mm Hg) | 21.00 ± 8.42 | 21.00 ± 9.01 | 0.992 | 16.90 ± 6.59 | 19.70 ± 9.38 | 0.482 |
| TAPSE (mm) | 20.80 ± 3.25 | 17.90 ± 3.83 | 0.005 | 20.60 ± 3.79 | 20.60 ± 3.51 | 1.000 |
| TASV (cm/s) | 13.90 ± 3.45 | 12.40 ± 2.86 | 0.184 | 14.10 ± 2.25 | 14.60 ± 2.54 | 0.874 |
| RV4CHGLS (%) | -16.80 ± 3.28 | -16.00 ± 4.46 | 0.911 | -16.70 ± 6.46 | -16.40 ± 4.91 | 0.992 |
| RVFWGLS (%) | -17.20 ± 9.07 | -11.50 ± 8.50 | 0.133 | -18.60 ± 10.30 | -17.50 ± 9.71 | 0.983 |
| Thoracotomy | VATS | p values | |
|---|---|---|---|
| preoperative | |||
| LVIDd (cm) | 4.40 ± 0.60 | 4.40 ± 0.80 | 0.975 |
| LVIDs (cm) | 3.20 ± 0.70 | 3.20 ± 0.90 | 0.921 |
| LVEDV (mL) | 102.70 ± 17.90 | 100.60 ± 20.30 | 0.712 |
| LVESV (mL) | 41.80 ± 11.10 | 45.00 ± 13.70 | 0.391 |
| LVEF (%) | 59.40 ± 6.30 | 56.30 ± 7.00 | 0.130 |
| CO (L/min) | 5.30 ± 1.20 | 4.90 ± 1.20 | 0.264 |
| LVGLS (%) | -17.40 ± 4.10 | -17.50 ± 2.80 | 0.922 |
| LVGWI | 1616.80 ± 529.50 | 1778.00 ± 580.00 | 0.347 |
| LVGWE (%) | 90.90 ± 9.10 | 93.20 ± 5.70 | 0.344 |
| RVIDd (cm) | 3.10 ± 0.60 | 2.90 ± 0.40 | 0.178 |
| RVIDs (cm) | 1.80 ± 0.50 | 1.60 ± 0.40 | 0.135 |
| RVD1basal (cm) | 3.90 ± 0.80 | 3.80 ± 0.60 | 0.625 |
| RVD3basal (cm) | 6.90 ± 0.80 | 6.90 ± 1.00 | 0.823 |
| RVFAC (%) | 51.20 ± 9.60 | 47.90 ± 10.60 | 0.283 |
| PASP (mm Hg) | 21.00 ± 8.40 | 16.90 ± 6.60 | 0.095 |
| TAPSE (mm) | 20.80 ± 3.20 | 20.60 ± 3.80 | 0.857 |
| TASV (cm/s) | 13.90 ± 3.50 | 14.10 ± 2.20 | 0.835 |
| RV4CHGLS (%) | -16.80 ± 3.30 | -16.70 ± 6.50 | 0.957 |
| RVFWGLS (%) | -17.20 ± 9.10 | -18.60 ± 10.30 | 0.647 |
| postoperative | |||
| LVIDd (cm) | 4.80 ± 0.70 | 4.50 ± 0.90 | 0.228 |
| LVIDs (cm) | 3.50 ± 0.80 | 3.30 ± 0.90 | 0.481 |
| LVEDV (mL) | 105.90 ± 15.20 | 100.00 ± 22.20 | 0.313 |
| LVESV (mL) | 45.20 ± 11.30 | 45.10 ± 12.90 | 0.993 |
| LVEF (%) | 59.20 ± 7.40 | 56.50 ± 7.70 | 0.249 |
| CO (L/min) | 5.70 ± 1.30 | 5.00± 1.10 | 0.072 |
| LVGLS (%) | -16.70 ± 4.10 | -16.10 ± 4.50 | 0.670 |
| LVGWI | 1636.80 ± 462.00 | 1504.90 ± 636.40 | 0.448 |
| LVGWE (%) | 90.60 ± 7.60 | 86.30 ± 10.60 | 0.141 |
| RVIDd (cm) | 3.00 ± 0.70 | 2.80 ± 0.60 | 0.329 |
| RVIDs (cm) | 1.90 ± 0.50 | 1.70 ± 0.40 | 0.212 |
| RVD1basal (cm) | 3.70 ± 0.70 | 3.80 ± 0.70 | 0.597 |
| RVD3basal (cm) | 7.20 ± 0.60 | 6.80 ± 0.90 | 0.093 |
| RVFAC (%) | 51.40 ± 7.50 | 48.20 ± 7.30 | 0.174 |
| PASP (mm Hg) | 21.00 ± 9.00 | 19.70 ± 9.40 | 0.664 |
| TAPSE (mm) | 17.90 ± 3.80 | 20.60 ± 3.50 | 0.018 |
| TASV (cm/s) | 12.40 ± 2.90 | 14.60 ± 2.50 | 0.010 |
| RV4CHGLS (%) | -16.00 ± 4.50 | -16.40 ± 4.90 | 0.787 |
| RVFWGLS (%) | -11.50 ± 8.50 | -17.50 ± 9.70 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




