Submitted:
31 July 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Biosensor Working Principal and Measurement Setup
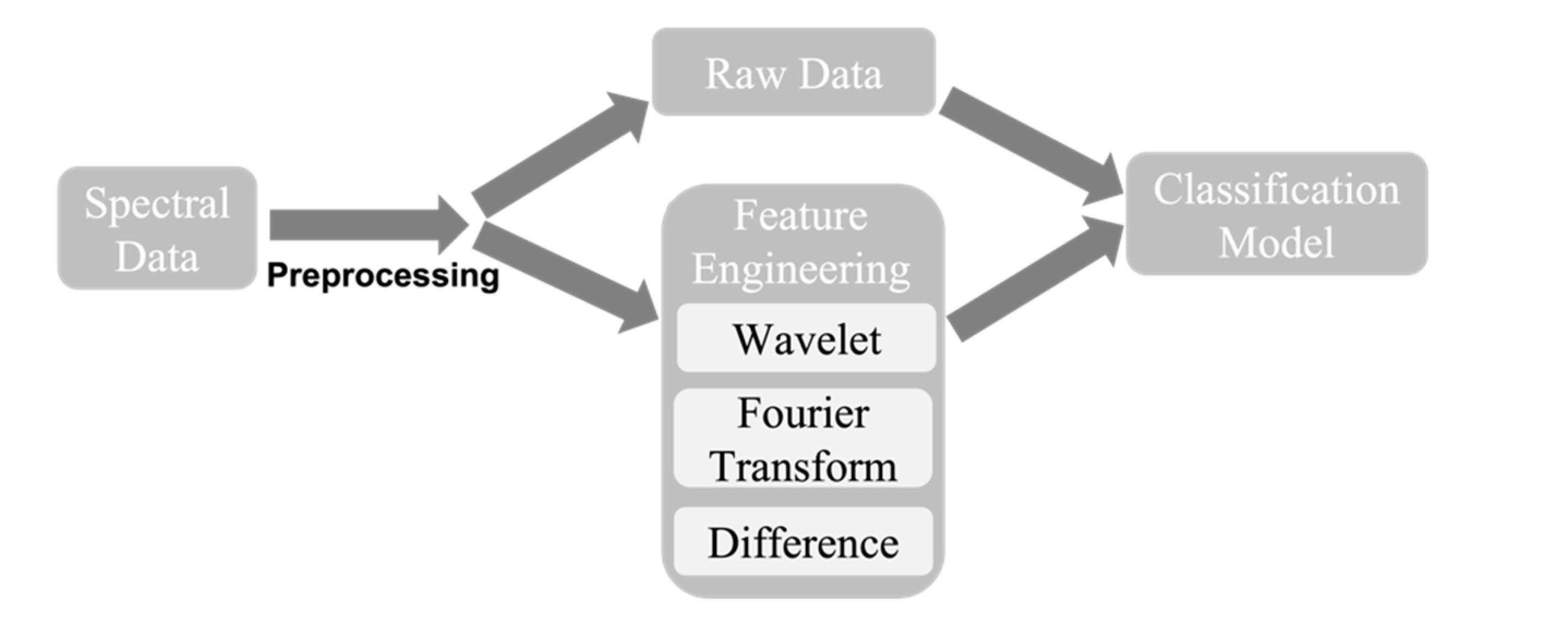
2.2. Data Preprocessing
2.3. Feature Engineering
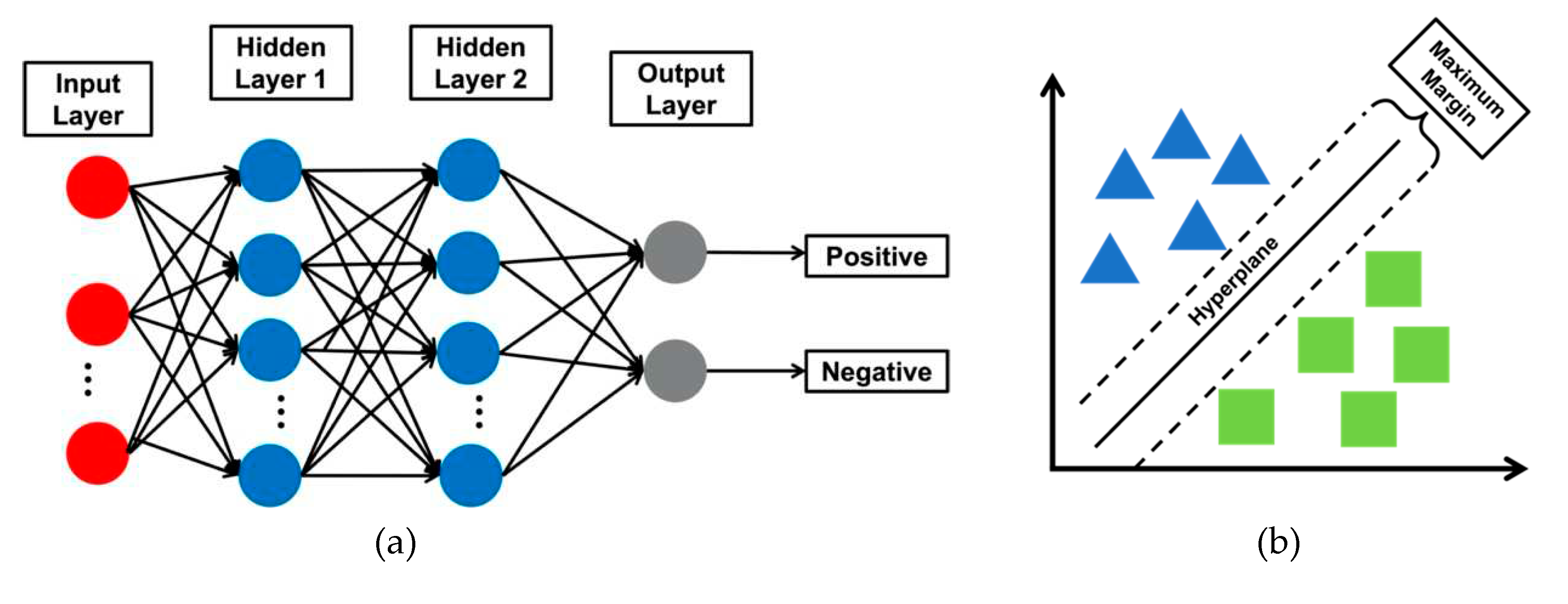
2.4. Classification Models
2.5. Control Experiments
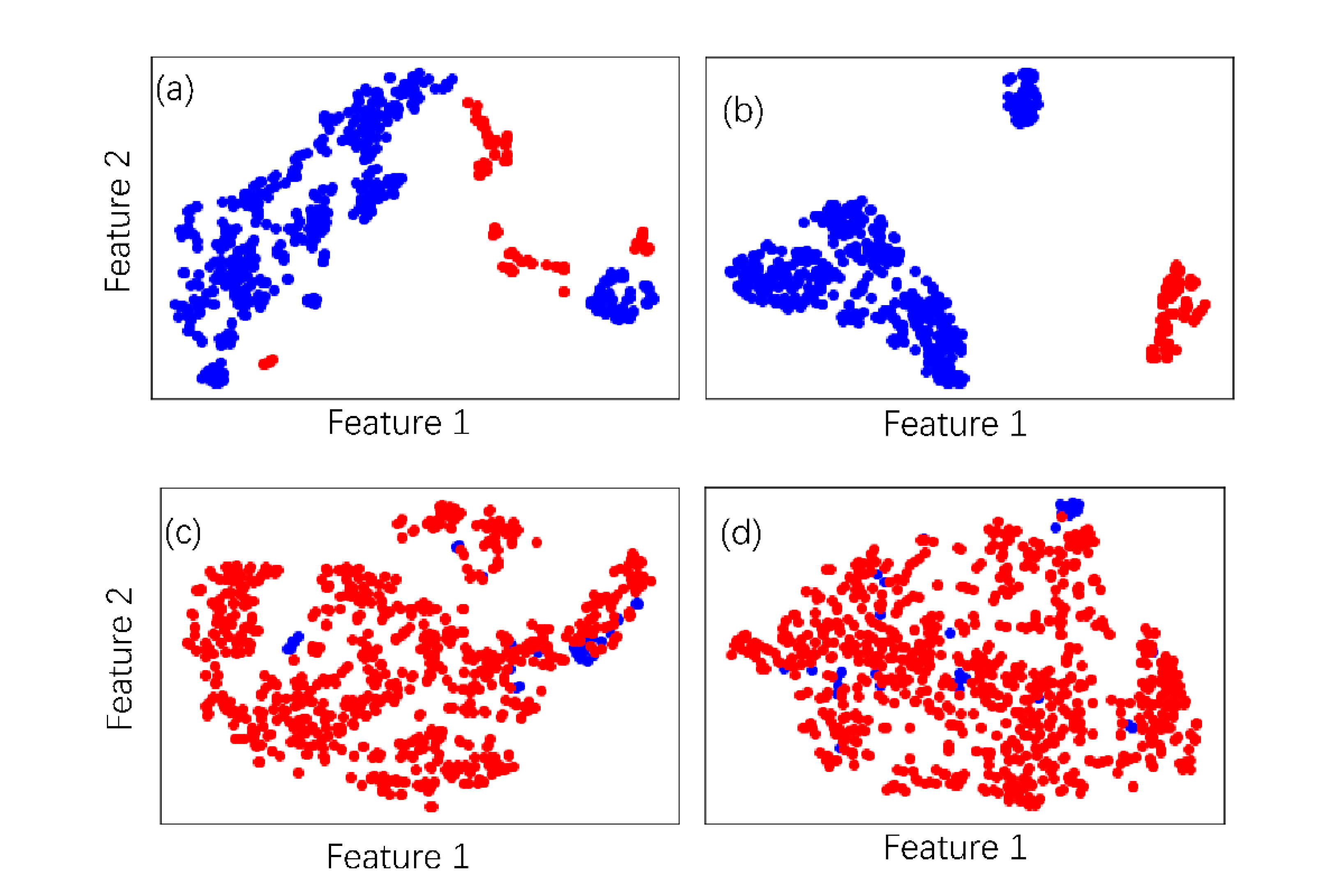
2.6. Dataset Distinguishability Analysis
3. Results and Discussion
4. Conclusion
Acknowledgements
References
- Tao, Y.D. , et al. , A Survey on Data-driven COVID-19 and Future Pandemic Management. Acm Computing Surveys, 2023, 55. [Google Scholar]
- Tenali, N. and G.R.M. Babu, A Systematic Literature Review and Future Perspectives for Handling Big Data Analytics in COVID-19 Diagnosis. New Generation Computing, 2023.
- Duncan, D.B. , et al., Performance of saliva compared with nasopharyngeal swab for diagnosis of COVID-19 by NAAT in cross-sectional studies: a systematic review and meta-analysis. Clinical Biochemistry, 2022, 109, 117–117. [Google Scholar]
- Tng, D.J.H. , et al., Amplified parallel antigen rapid test for point-of-care salivary detection of SARS-CoV-2 with improved sensitivity (vol 189, 14, 2021). Microchimica Acta, 2023, 190. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D. , et al., COVID-19 Diagnosis: A Comprehensive Review of the RT-qPCR Method for Detection of SARS-CoV-2. Diagnostics, 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Larremore, D.B. , et al., Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Science Advances, 2021; 7. [Google Scholar]
- He, J. , et al., Rapid detection of SARS-CoV-2: The gradual boom of lateral flow immunoassay. Frontiers in Bioengineering and Biotechnology, 2023, 10. [Google Scholar] [CrossRef]
- Al-Hashimi, O.T.M. , et al., The sensitivity and specificity of COVID-19 rapid anti-gene test in comparison to RT-PCR test as a gold standard test. Journal of Clinical Laboratory Analysis, 2023. [Google Scholar]
- Liu, K.S. , et al., Laboratory detection of SARS-CoV-2: A review of the current literature and future perspectives. Heliyon, 2022, 8. [Google Scholar]
- Chong, Y.P. , et al., SARS-CoV-2 Testing Strategies in the Diagnosis and Management of COVID-19 Patients in Low-Income Countries: A Scoping Review. Molecular Diagnosis & Therapy, 2023. [Google Scholar]
- El-Sherif, D.M. , et al. , New approach in SARS-CoV-2 surveillance using biosensor technology: a review. Environmental Science and Pollution Research, 2022, 29, 1677–1695. [Google Scholar]
- Abid, S.A. , et al., Biosensors as a future diagnostic approach for COVID-19. Life Sciences, 2021, 273. [Google Scholar] [CrossRef]
- Wei, H.S. , et al., Research progress of biosensors for detection of SARS-CoV-2 variants based on ACE2. Talanta, 2023, 251. [Google Scholar] [CrossRef]
- Rong, G.G. , et al., A high-throughput fully automatic biosensing platform for efficient COVID-19 detection. Biosensors & Bioelectronics, 2023, 220. [Google Scholar]
- Rong, G.G. , et al., A Closed-Loop Approach to Fight Coronavirus: Early Detection and Subsequent Treatment. Biosensors-Basel, 2022, 12. [Google Scholar]
- Takemura, K. , Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology. Biosensors-Basel, 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G., W. L. Hu, and Y.T. Fang, Direct excitation of the Tamm plasmon-polaritons on a dielectric Bragg reflector coated with a metal film. Opto-Electronics Review, 2013, 21, 338–343. [Google Scholar] [CrossRef]
- Liu, C.D., M. D. Kong, and B.C. Li, Tamm plasmon-polariton with negative group velocity induced by a negative index meta-material capping layer at metal-Bragg reflector interface. Optics Express, 2014, 22, 11376–11383. [Google Scholar] [CrossRef] [PubMed]
- Rong, G. , Sawan, M., Rapid Onsite Detection of SARS-CoV-2 with Novel Optical Biosensors and Detection Systems of Handheld and High Throughput Design. Talanta, 2023. submiited.
- Cao, Y.J. , et al., Efficient Optical Pattern Detection for Microcavity Sensors Based Lab-on-a-Chip. Ieee Sensors Journal, 2012, 12, 2121–2128. [Google Scholar] [CrossRef]
- Mariani, S. , et al., 10 000-Fold Improvement in Protein Detection Using Nanostructured Porous Silicon Interferometric Aptasensors. Acs Sensors, 2016, 1, 1471–1479. [Google Scholar] [CrossRef]
- Wu, C. , et al., Physical analysis of the response properties of porous silicon microcavity biosensor. Physica E-Low-Dimensional Systems & Nanostructures, 2012, 44, 1787–1791. [Google Scholar]
- Kotsiantis, S.B., I. D. Zaharakis, and P.E. Pintelas, Machine learning: a review of classification and combining techniques. Artificial Intelligence Review, 2006, 26, 159–190. [Google Scholar]
- Kliegr, T., S. Bahnik, and J. Furnkranz, A review of possible effects of cognitive biases on interpretation of rule-based machine learning models. Artificial Intelligence, 2021, 295. [Google Scholar]
- Metri-Ojeda, J. , et al., Rapid screening of mayonnaise quality using computer vision and machine learning. Journal of Food Measurement and Characterization, 2023. [Google Scholar]
- Mughaid, A. , et al., A novel machine learning and face recognition technique for fake accounts detection system on cyber social networks. Multimedia Tools and Applications, 2023. [Google Scholar]
- Xu, Y.S. , et al. , Machine Learning-Driven APPs Recommendation for Energy Optimization in Green Communication and Networking for Connected and Autonomous Vehicles. Ieee Transactions on Green Communications and Networking, 2022, 6, 1543–1552. [Google Scholar]
- Dai, Z.H., R. H. Wang, and J.H. Guan, Auxiliary Decision-Making System for Steel Plate Cold Straightening Based on Multi-Machine Learning Competition Strategies. Applied Sciences-Basel, 2022, 12. [Google Scholar]
- Fidencio, A.X., C. Klaes, and I. Iossifidis, Error-Related Potentials in Reinforcement Learning-Based Brain-Machine Interfaces. Frontiers in Human Neuroscience, 2022, 16. [Google Scholar]
- Brown, J.A. , et al., A Machine Learning System for Supporting Advanced Knowledge Discovery from Chess Game Data. 2017 16th Ieee International Conference on Machine Learning and Applications (Icmla), 2017, 649-654.
- Hu, J.L. , et al. , A hierarchical learning system incorporating with supervised, unsupervised and reinforcement learning. Advances in Neural Networks - Isnn 2007, Pt 1, Proceedings, 2007, 4491, 403. [Google Scholar]
- Kumar, B., O. P. Vyas, and R. Vyas, A comprehensive review on the variants of support vector machines. Modern Physics Letters B 2019, 33. [Google Scholar] [CrossRef]
- Champati, B.B. , et al., Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata. Molecules, 2022, 27. [Google Scholar] [CrossRef]
- Fang, X. and M. Ghosh, High-dimensional properties for empirical priors in linear regression with unknown error variance. Statistical Papers, 2023.
- Li, M. and B.Z. Yuan, 2D-LDA: A statistical linear discriminant analysis for image matrix. Pattern Recognition Letters, 2005, 26, 527–532. [Google Scholar] [CrossRef]
- Valero-Mas, J.J. , et al., Multilabel Prototype Generation for data reduction in K-Nearest Neighbour classification. Pattern Recognition, 2023; 135. [Google Scholar]
- Mohanty, S.K. , et al., Decision tree approach for fault detection in a TCSC compensated line during power swing. International Journal of Electrical Power & Energy Systems 2023, 146. [Google Scholar]
- Kim, T. and J.S. Lee, Maximizing AUC to learn weighted naive Bayes for imbalanced data classification. Expert Systems with Applications 2023, 217. [Google Scholar] [CrossRef]
- Meniailov, I., S. Krivtsov, and T. Chumachenko, Dimensionality Reduction of Diabetes Mellitus Patient Data Using the T-Distributed Stochastic Neighbor Embedding. Smart Technologies in Urban Engineering, Stue-2022 2023, 536, 86–95. [Google Scholar]
- Wu, B. , et al., A Nanoscale Porous Silicon Microcavity Biosensor for Novel Label-Free Tuberculosis Antigen-Antibody Detection. Nano 2012, 7. [Google Scholar] [CrossRef]
- Kaliteevski, M. , et al., Tamm plasmon-polaritons: Possible electromagnetic states at the interface of a metal and a dielectric Bragg mirror. Physical Review B 2007, 76. [Google Scholar] [CrossRef]
- van Erven, T. and P. Harremoes, Renyi Divergence and Kullback-Leibler Divergence. Ieee Transactions on Information Theory, 2014, 60, 3797–3820. [Google Scholar] [CrossRef]

| Method | Raw Data | Feature Engineering | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | SVM | MLP | SVM | MLP | ||||
| Parameter | SEN | SPE | SEN | SPE | SEN | SPE | SEN | SPE |
|
Performance on Control Detection Data |
100% | 0% | 100% | 0% | 0% | 83% | 0% | 90% |
| Performance on Valid Detection Data | 100% | |||||||
| Factor | Need data filtering and denoising | Need to take care of shift direction | Need stable light source and low noise spectroscopy system | Needed researcher work | |
|---|---|---|---|---|---|
| Technique | |||||
| Find peaks and calculate spectral shift | Yes | Yes | No | Algorithm design and test | |
| Interferogram average over wavelength | Yes | No | Yes | Algorithm design and test | |
| Intensity interrogation | Yes | No | Yes | Algorithm design and test | |
| Machine learning | Yes | No | No | Model training from data | |
| Specimen Collection Location | qPCR Result | Biosensor with ML Result | |
|---|---|---|---|
| Vaccination Site 1 | Operation Desktop | Weak positive | Positive |
| Vaccination Site 1 | Vaccination Station | Strong positive | Positive |
| Vaccination Site 2 | Operation Desktop | Weak positive | Positive |
| Vaccination Site 2 | Vaccination Station | Weak positive | Positive |
| Vaccination Site 2 | Ventilation Plate | Strong positive | Positive |
| Vaccination Site 2 | Innoculation Table Handle | Weak positive | Positive |
| Vaccination Site 4 | Keyboard and Mouse | Negative | Negative |
| Vaccination Site 5 | Pen and White Board | Strong positive | Positive |
| Vaccination Site 55 | Innoculation Table Handle | Negative | Negative |
| No. 4 ans No. 5 Innoculation Desk Room | Door Handle and Switch | Negative | Negative |
| Other | Hemostatic Swab | Weak positive | Positive |
| Other | Cleaner’s Hand | Negative | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





