Submitted:
01 August 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material
2.2. Extraction of Essential Oil from P. tortuousus
2.3. GC-MS analysis of the EO
2.4. Process development and formulation of the nanoemulsion (NE)
2.5. Characterization of the formulation
2.5.1. Particle size distribution and polydispersity index determinations
2.5.2. ZETA potential of optimal nanoemulsion (NE)
2.5.3. Thermodynamic stability study of the optimal NE
2.5.4. Transmission electron microscopy study
2.6. Gelification process of the nanoemulsion
2.7. Characterization of the Nanoemulsion-Gel
2.7.1. pH measurement
2.7.2. Rheological study
2.7.3. In-vivo wound healing study
3. Discussion
3.1. Essential oil yield
3.2. Chemical composition of the essential oils
3.3. NE formulation.
3.4. Selection and characterization of the optimal NE.
3.4.1. ZETA potential
3.4.2. Thermodynamic stability of the optimal NE
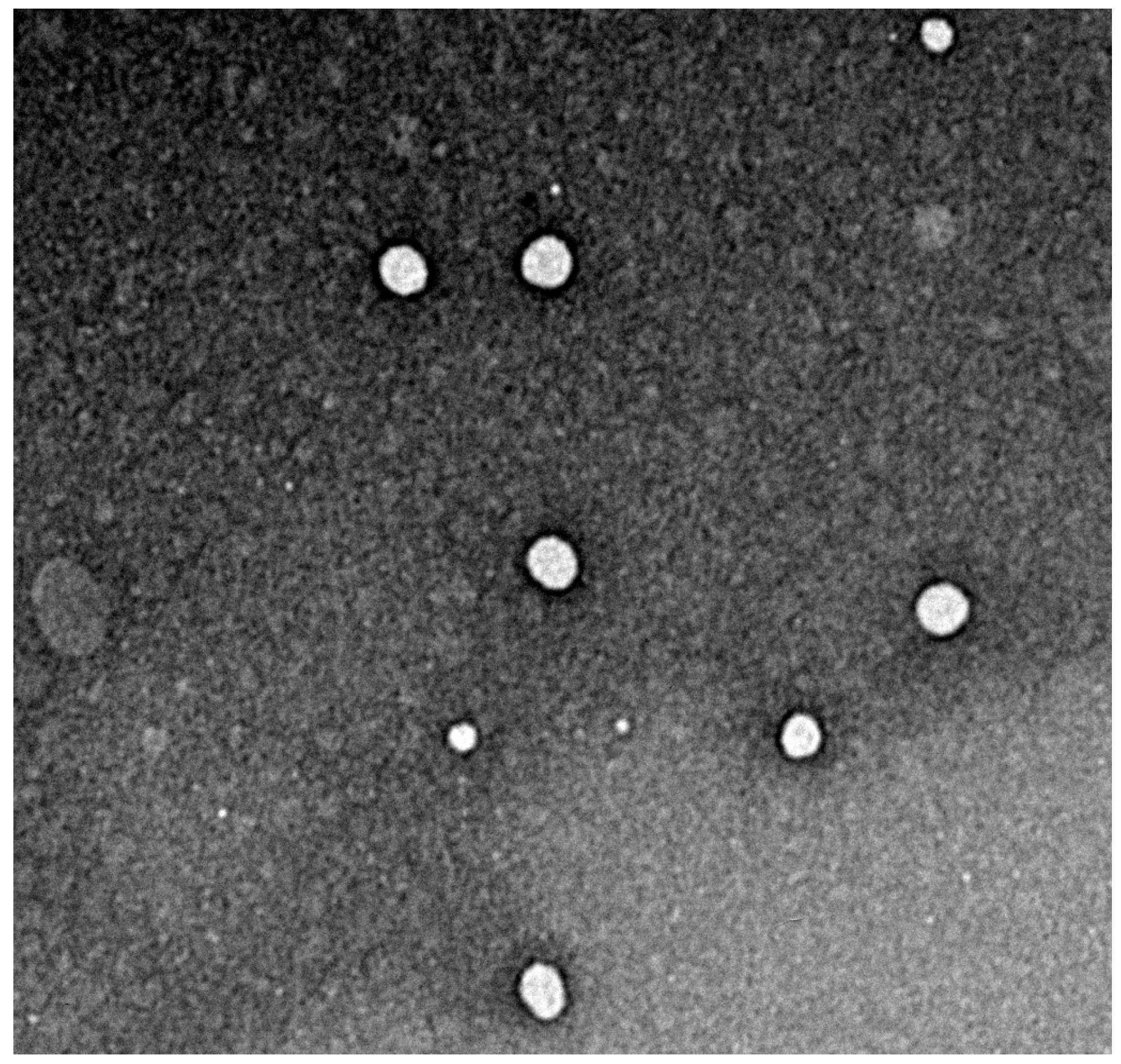
3.4.3. Characterization by Transmission Electron Microscopy
3.5. Gelification and Characterization of the Optimal Nanoemulsion Gel (NE/Gel).
3.5.1. pH measurement
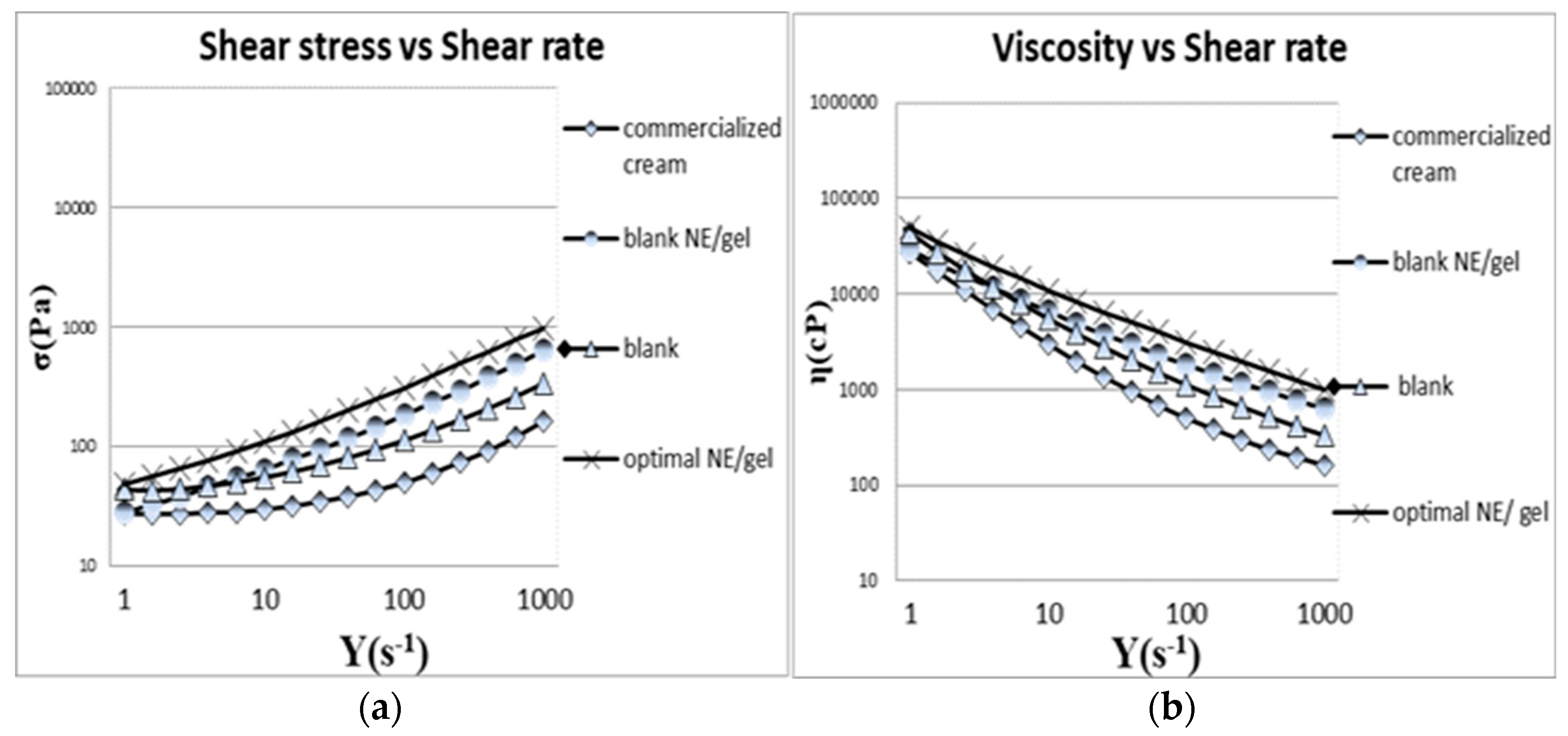
3.5.2. Rheology study
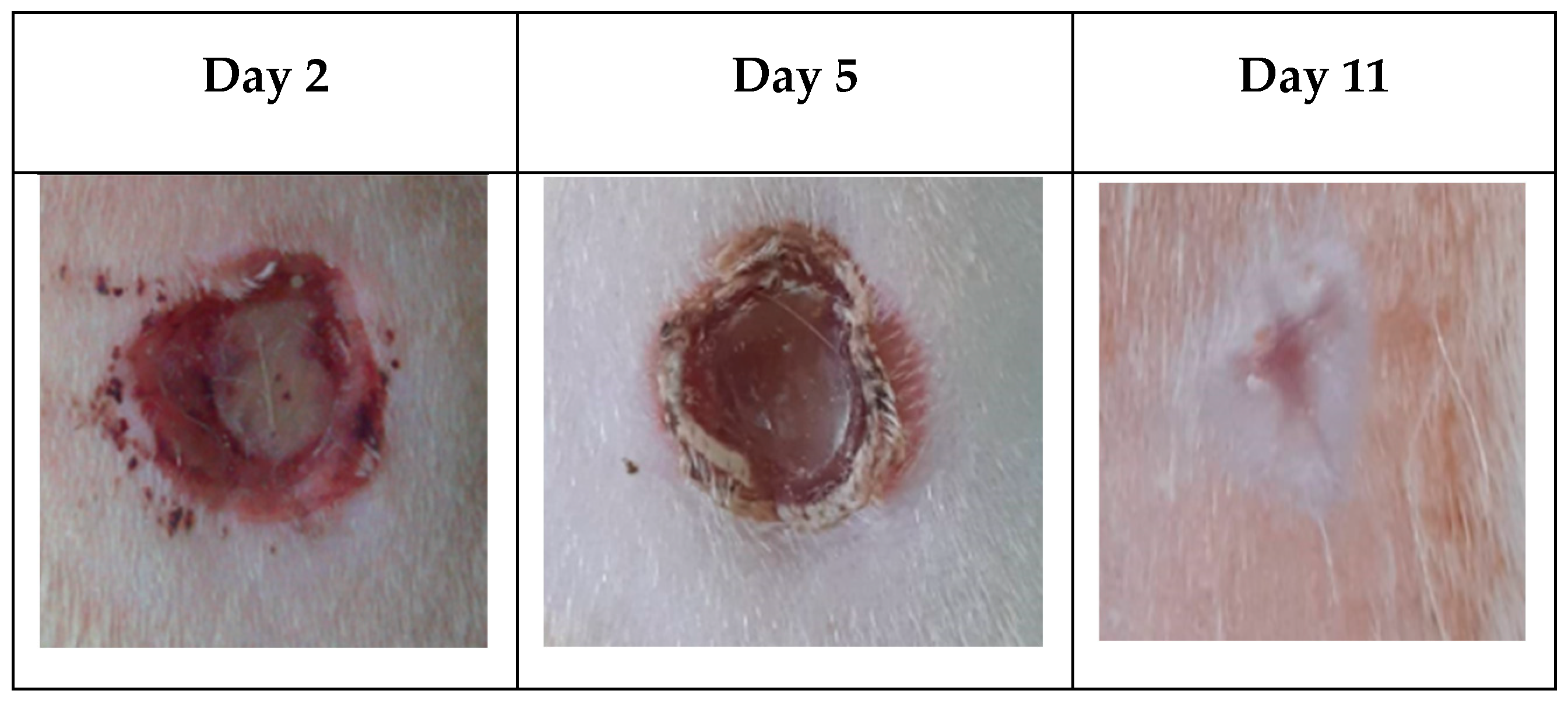
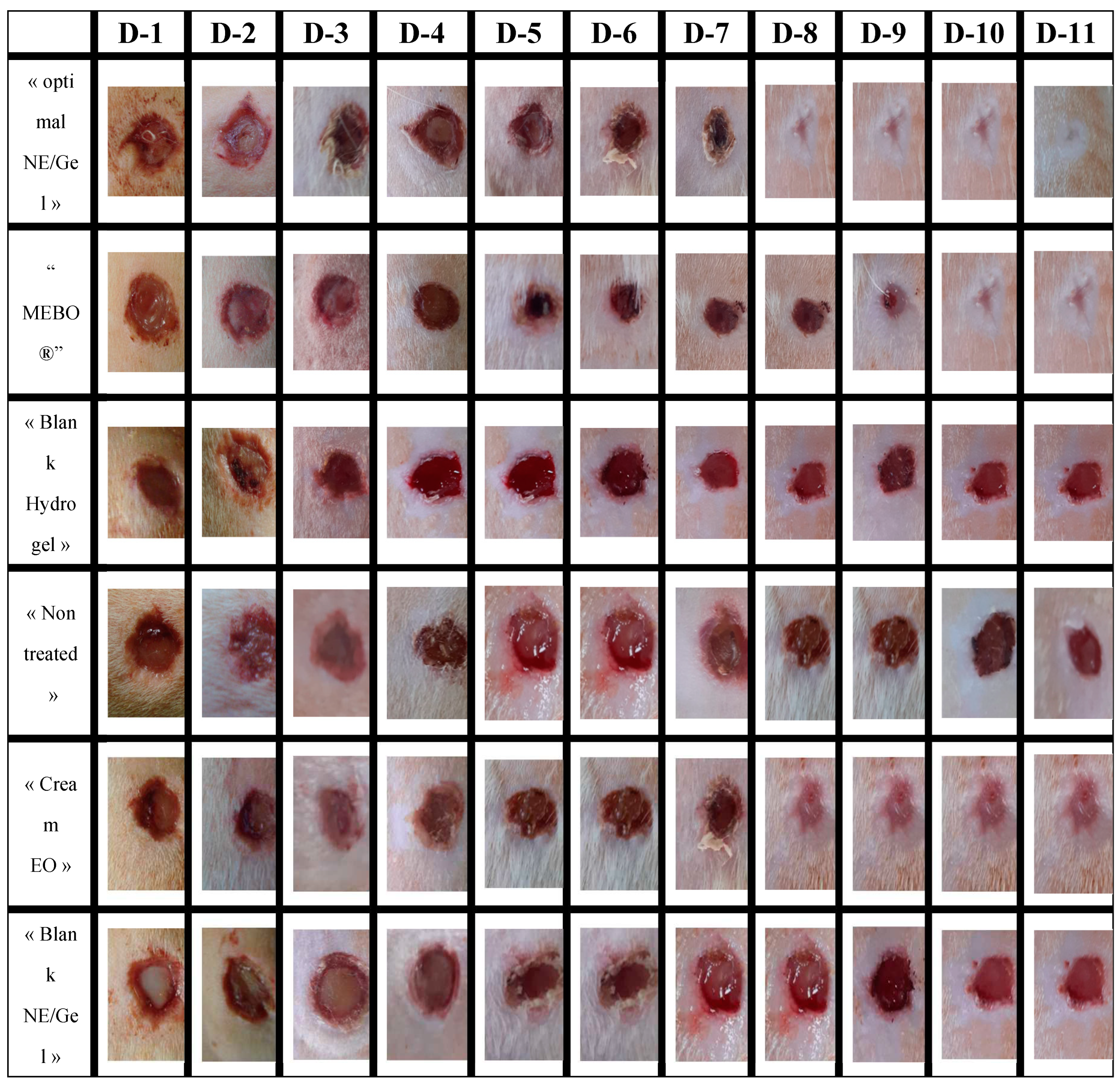
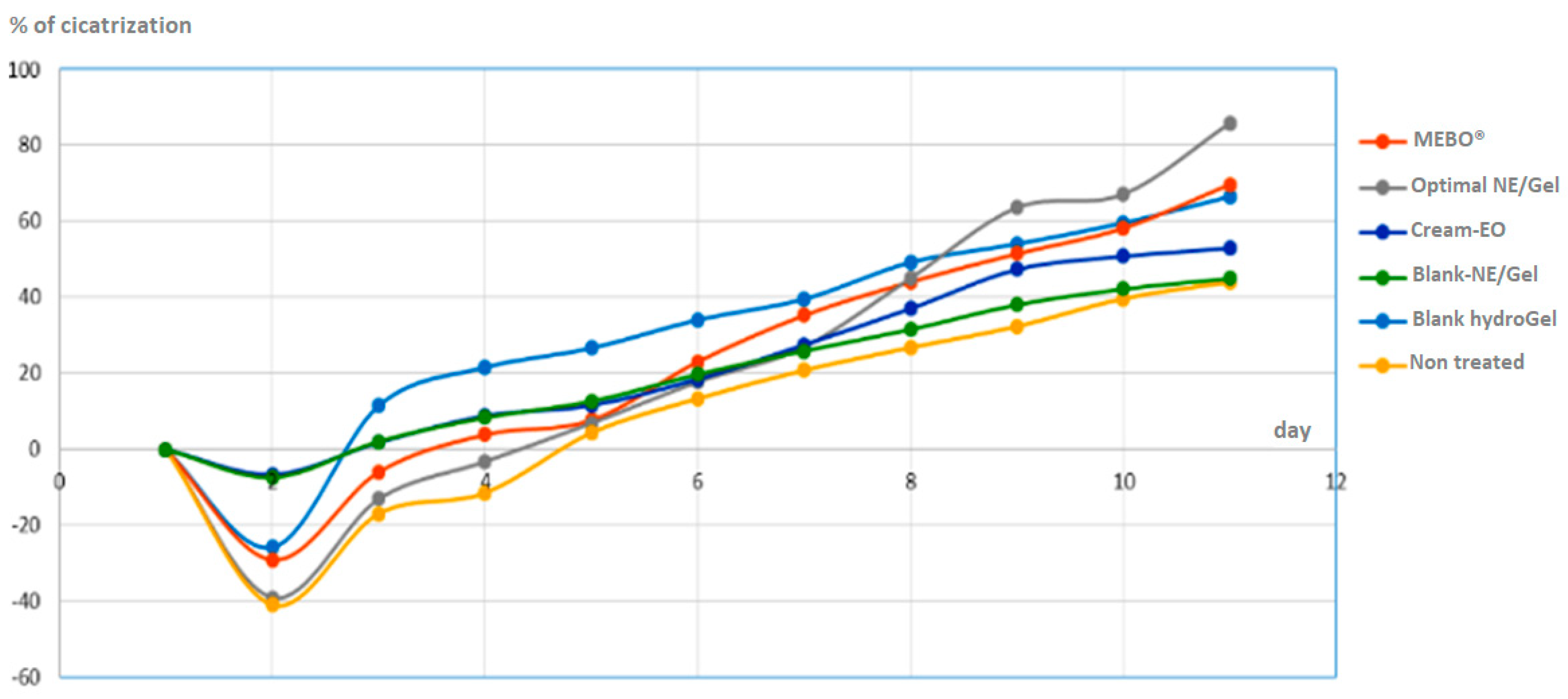
3.6. In-vivo wound healing study.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sabale, P.; Bhimani, B.; Prajapati, C.; & Sabale, V. An overview of medicinal plants as wound healers. J Appl Pharm Sci 2012, 2, 11, 143-150. [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The Relationship between Skin Function, Barrier Properties, and Body-Dependent Factors. Skin Res Technol 2018, 24, 165–174. [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J Int Med Res 2009, 37, 1528–1542. [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen 2009, 17, 763–771. [CrossRef]
- Rice, J.B.; Desai, U.; Cummings, A.K.G.; Birnbaum, H.G.; Skornicki, M.; Parsons, N.B. Burden of Diabetic Foot Ulcers for Medicare and Private Insurers. Diabetes Care 2014, 37, 651–658. [CrossRef]
- Purohit, S.K.; Solanki, R.; Soni, M.K.; Mathur, V. Experimental Evaluation of Indian Aloe (Aloe Vera) Leaves Pulp as Topical Medicament on Wound Healing. Int J Pharm Res 2012, 2. [CrossRef]
- Sikka, M.P.; Midha, V.K. 16 - The Role of Biopolymers and Biodegradable Polymeric Dressings in Managing Chronic Wounds. In Advanced Textiles for Wound Care (Second Edition); Rajendran, S., Ed.; The Textile Institute Book Series; Woodhead Publishing, 2019; pp. 463–488 ISBN 978-0-08-102192-7. [CrossRef]
- Modarresi, M.; Farahpour, M.-R.; Baradaran, B. Topical Application of Mentha Piperita Essential Oil Accelerates Wound Healing in Infected Mice Model. Inflammopharmacology 2019, 27, 531–537. [CrossRef]
- Ashtikar, M.; Wacker, M.G. Nanopharmaceuticals for Wound Healing - Lost in Translation?. Adv Drug Deliv Rev 2018, 129, 194–218. [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv Wound Care (New Rochelle) 2014, 3, 511–529. [CrossRef]
- Farahpour, M.R.; Vahid, M.; Oryan, A. Effectiveness of Topical Application of Ostrich Oil on the Healing of Staphylococcus Aureus- and Pseudomonas Aeruginosa-Infected Wounds. Connect Tissue Res 2018, 59, 212–222. [CrossRef]
- Pereira, M.; Vilela, G.; Costa, L.; Silva, R.; Fernandes, A.; Fonseca, E.; Piccoli, R. Inibição Do Desenvolvimento Fúngico Através Da Utilização de Óleos Essenciais de Condimentos. Ciencia E Agrotecnologia - CIENC AGROTEC 2006, 30. [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv Drug Deliv Rev 2018, 127, 138–166. [CrossRef]
- Orlowski, P.; Zmigrodzka, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Czupryn, M.; Antos-Bielska, M.; Szemraj, J.; Celichowski, G.; Grobelny, J.; Krzyzowska, M. Tannic Acid-Modified Silver Nanoparticles for Wound Healing: The Importance of Size. Int J Nanomed 2018, 13, 991–1007. [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid Based Complement Alternat Med 2019, 2684108. [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent Advances in Inorganic Nanomaterials for Wound-Healing Applications. Biomater Sci 2019, 7, 2652–2674. [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin Wound Healing and Phytomedicine: A Review. Skin Pharmacol Physiol 2014, 27, 303–310. [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers-A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl Mater Interfaces 2017, 9, 5916–5926. [CrossRef]
- Cerchiara, T.; Abruzzo, A.; Ñahui Palomino, R.A.; Vitali, B.; De Rose, R.; Chidichimo, G.; Ceseracciu, L.; Athanassiou, A.; Saladini, B.; Dalena, F.; et al. Spanish Broom (Spartium Junceum L.) Fibers Impregnated with Vancomycin-Loaded Chitosan Nanoparticles as New Antibacterial Wound Dressing: Preparation, Characterization and Antibacterial Activity. Eur J Pharm Sci 2017, 99, 105–112. [CrossRef]
- Manca, M.L.; Manconi, M.; Nacher, A.; Carbone, C.; Valenti, D.; Maccioni, A.M.; Sinico, C.; Fadda, A.M. Development of Novel Diolein–Niosomes for Cutaneous Delivery of Tretinoin: Influence of Formulation and in Vitro Assessment. Int J Pharm 2014, 477, 176–186. [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of Gellan-Cholesterol Nanohydrogels Embedding Baicalin and Evaluation of Their Wound Healing Activity. Eur J Pharm Biopharm 2018, 127, 244–249. [CrossRef]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [CrossRef]
- And Alternative Medicine, E.-B.C. Retracted: Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid Based Complement Alternat Med 2021, 2021, 7259208. [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of Chitosan Nanoparticles Loaded with Summer Savory (Satureja Hortensis L.) Essential Oil for Antimicrobial and Antioxidant Delivery Applications. LWT 2016, 70, 104–110. [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ Health Perspect 2005, 113, 823–839. [CrossRef]
- Brahimi, s; Dahia, M.; Blel, A.; Laouer, H. Composition Chimique et Activité Antimicrobienne de l’huile Essentielle de Deverra Reboudii (Coss. & Durieu). Phytothérapie 2018, 18. [CrossRef]
- Yangui, T.; Bouaziz, M.; Dhouib, A.; Sayadi, S. Potential Use of Tunisian Pituranthos Chloranthus Essential Oils as a Natural Disinfectant. Lett Appl Microbiol 2009, 48, 112–117. [CrossRef]
- Krifa, M.; Gharad, T.; Haouala, R. Biological Activities of Essential Oil, Aqueous and Organic Extracts of Pituranthos Tortuosus (Coss.) Maire. Scientia Horticulturae 2011, 128, 61–67. [CrossRef]
- Lograda, T.; Ramdani, M.; Kiram, A.; Chalard, P. Variation of essential oils composition of Pituranthos scoparius in Algeria. Global J Res Med Plants Indigen Med 2013, 2, 1, 1-11.
- Vérité, P.; Nacer, A.; Kabouche, Z.; Seguin, E. Composition of Seeds and Stems Essential Oils of Pituranthos Scoparius (Coss. & Dur.) Schinz. Flavour Fragr J 2004, 19, 562–564. [CrossRef]
- Louhaichi, M.; A.K., S.; H.E., E.; S., B. Initial Assessment of Medicinal Plants Across the Libyan Mediterranean Coast. Adv Env Biol 2011, 5, 359–370.
- Hedi, M.; Sabri, K.; Eljeni, H.; Neffati, M.; Akrout, A. Chemical Composition and Antimicrobial Activity of Pituranthos Chloranthus (Benth.) Hook and Pituranthos Tortuosus (Coss.) Maire Essential Oils from Southern Tunisia. Adv Biol Chem 2016, 5, 273–278. [CrossRef]
- Chaieb, M.; Boukhris, M. Flore Succinte et Illustrée Des Zones Arides et Sahariennes de Tunisie.; Association pour la protection de la nature et de l'environnement ; L'Or du temps Pub., 1998; ISBN 9973757491.
- Boulos, L. Flora of Egypt; Cairo, Egypt : Al Hadara Pub., 1999; ISBN 978-977-5429-14-8.
- Guetat, A.; Boulila, A.; Boussaid, M. Phytochemical Profile and Biological Activities of Deverra Tortuosa (Desf.)DC.: A Desert Aromatic Shrub Widespread in Northern Region of Saudi Arabia. Nat Prod Res 2019, 33, 2708–2713. [CrossRef]
- Abdelwahed, A.; Hayder, N.; Kilani, S.; Mahmoud, A.; Chibani, J.; Hammami, M.; Chekir-Ghedira, L.; Ghedira, K. Chemical Composition and Antimicrobial Activity of Essential Oils from Tunisian Pituranthos Tortuosus (Coss.) Maire. Flavour Fragr J 2006, 21, 129–133. [CrossRef]
- Abdallah, H.M.; Ezzat, S.M. Effect of the Method of Preparation on the Composition and Cytotoxic Activity of the Essential Oil of Pituranthos Tortuosus. Z Naturforsch C J Biosci 2011, 66, 143–148. [CrossRef]
- Elshibani, F.; Alshalmani, S.; Mohammed, H.A. Pituranthos Tortuosus Essential Oil from Libya: Season Effect on the Composition and Antioxidant Activity. J Essent Oil Bear Plants 2020, 23, 1095–1104. [CrossRef]
- Aloui, L.; Kossentini, M.; Zouari, S. Characterization of Aromatic Compounds and Antioxidant Activity of Essential Oils from Tunisian Pituranthos Tortuosus (Coss.) Maire (Apiaceae). J Essent Oil Bear Plants 2018, 21, 769–778. [CrossRef]
- Abdel-Mogib, M.; S.N.Ayyad, M.A.M.; Dawidar, A. Lactones from Pituranthos Tortusus. Pak J Sci Ind Res 1992, 35, 39.
- Abdel-Kader, M. New Ester and Furocoumarins from the Roots of Pituranthos Tortuosus. J Braz Chem Soc 2003, 14. [CrossRef]
- Oueslati, M.H.; Guetat, A.; Bouajila, J.; Alzahrani, A.K.; Basha, J. Deverra Tortuosa (Desf.) DC from Saudi Arabia as a New Source of Marmin and Furanocoumarins Derivatives with α-Glucosidase, Antibacterial and Cytotoxic Activities. Heliyon 2021, 7, e06656. [CrossRef]
- Guesmi, F.; Ben Hadj, A.S.; Landoulsi, A. Investigation of Extracts from Tunisian Ethnomedicinal Plants as Antioxidants, Cytotoxins, and Antimicrobials. Biomed Environ Sci 2017, 30, 811–824. [CrossRef]
- Abdelgaleil, S.; Badawy, M.; Shawir, M.S.; Mohamed, M.I.E. Chemical Composition, Fumigant and Contact Toxicities of Essential Oils Isolated from Egyptian Plants against the Stored Grain Insects; Sitophilus Oryzae L. and Tribolium Castaneum (Herbst). Egypt J Biol Pest Control 2015.
- Chang, A.C.; Dearman, B.; Greenwood, J.E. A Comparison of Wound Area Measurement Techniques: Visitrak versus Photography. Eplasty 2011, 11, e18.
- Trapp, M. Is There Room for Improvement in the Emollients for Adjuvant Therapy? J Eur Acad Dermatol Venereol 2007, 21 Suppl 2, 14–18. [CrossRef]
- Yoon, W.H.; Lee, K.H. Rheological Properties and Efficacy of the Formulation of Hyaluronic Acid with Tamarind Seed Polysaccharide for Arthritis. Biorheology 2019, 56, 31–38. [CrossRef]
- Qwist, P.K.; Sander, C.; Okkels, F.; Jessen, V.; Baldursdottir, S.; Rantanen, J. On-Line Rheological Characterization of Semi-Solid Formulations. Eur J Pharm Sci 2019, 128, 36–42. [CrossRef]
- Shanley, A. Topical Formulation: Moving From Art to Science. Pharm Technol 2016, 2016 Supplement, s26–s29.
- Devaux, S.; Castela, A.; Archier, E.; Gallini, A.; Joly, P.; Misery, L.; Aractingi, S.; Aubin, F.; Bachelez, H.; Cribier, B.; et al. Adherence to Topical Treatment in Psoriasis: A Systematic Literature Review. J Eur Acad Dermatol Venereol 2012, 26 Suppl 3, 61–67. [CrossRef]
- Guest, S.; Ma, A.; Mehrabyan, A.; Essick, G.; Hopkinson, A.; McGlone, F. Perception of Fluids with Diverse Rheology Applied to the Underarm versus Forearm Skin. Somatosens Mot Res 2012, 29, 89–102. [CrossRef]
- J Mastropietro, D. Rheology in Pharmaceutical Formulations-A Perspective. J Dev Drugs 2013, 02. [CrossRef]
- Al-Gaby, A.M.; Allam, R.F. Chemical Analysis, Antimicrobial Activity, and the Essential Oils from Some Wild Herbs in Egypt. J Herbs Spices Med Plants 2000, 7, 15–23. [CrossRef]
- São Pedro, A.; Santo, I.; Detoni, C.; Silva, C.; Cabral-Albuquerque, E. The Use of Nanotechnology as an Approach for Essential Oil-Based Formulations with Antimicrobial Activity. In; 2013; pp. 1364–1374 ISBN 978-84-942134-0-3.
- Kazemi, M.; Pierson, R.A.; McBreairty, L.E.; Chilibeck, P.D.; Zello, G.A.; Chizen, D.R. A Randomized Controlled Trial of a Lifestyle Intervention with Longitudinal Follow-up on Ovarian Dysmorphology in Women with Polycystic Ovary Syndrome. Clin Endocrinol (Oxf) 2020, 92, 525–535. [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Micelles and Nanoemulsions. In Nanocosmetics: From Ideas to Products; Cornier, J., Keck, C.M., Van de Voorde, M., Eds.; Springer International Publishing: Cham, 2019; pp. 47–72 ISBN 978-3-030-16573-4.
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could Essential Oils Enhance Biopolymers Performance for Wound Healing? A Systematic Review. Phytomedicine 2018, 38, 57–65. [CrossRef]
- Ben Djemaa, F.G.; Bellassoued, K.; Zouari, S.; El Feki, A.; Ammar, E. Antioxidant and Wound Healing Activity of Lavandula Aspic L. Ointment. J Tissue Viability 2016, 25, 193–200. [CrossRef]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the Antioxidant, Wound Healing, and Anti-Inflammatory Activities and the Phytochemical Composition of Maritime Pine (Pinus Pinaster Ait). J Ethnopharmacol 2018, 211, 235–246. [CrossRef]
- Gutiérrez-Del-Río, I.; Fernández, J.; Lombó, F. Plant Nutraceuticals as Antimicrobial Agents in Food Preservation: Terpenoids, Polyphenols and Thiols. Int J Antimicrob Agents 2018, 52, 309–315. [CrossRef]
- Hortelano, S.; González-Cofrade, L.; Cuadrado, I.; de Las Heras, B. Current Status of Terpenoids as Inflammasome Inhibitors. Biochem Pharmacol 2020, 172, 113739. [CrossRef]
- Ludwiczuk, A.; Asakawa, Y. Bryophytes as a Source of Bioactive Volatile Terpenoids - A Review. Food Chem Toxicol 2019, 132, 110649. [CrossRef]
- Ma, Y.-R.; Wang, K.-F.; Wang, W.-J.; Ding, Y.; Shi, T.-Q.; Huang, H.; Ji, X.-J. Advances in the Metabolic Engineering of Yarrowia Lipolytica for the Production of Terpenoids. Bioresour Technol 2019, 281, 449–456. [CrossRef]
- Sharma, S.H.; Thulasingam, S.; Nagarajan, S. Terpenoids as Anti-Colon Cancer Agents - A Comprehensive Review on Its Mechanistic Perspectives. Eur J Pharmacol 2017, 795, 169–178. [CrossRef]
- Valente, J.; Zuzarte, M.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, Antioxidant and Anti-Inflammatory Activities of Oenanthe Crocata L. Essential Oil. Food Chem Toxicol 2013, 62, 349–354. [CrossRef]
- Shareef, S.; AL-Medhtiy, M.; Abdel, I.; Ibrahim, A.; Alzahrani, A.; Abduljabbar, A.; Galali, Y.; Shakir Agha, N.; Aziz, P.; Thabit, M.; et al. Gastroprophylactic Effects of P-Cymene in Ethanol-Induced Gastric Ulcer in Rats. Processes 2022, 10, 1314. [CrossRef]
- Periasamy, M.; Schafleitner, R.; Muthukalingan, K.; Ramasamy, S. Phylogeographical Structure in Mitochondrial DNA of Legume Pod Borer (Maruca Vitrata) Population in Tropical Asia and Sub-Saharan Africa. PLoS One 2015, 10, e0124057. [CrossRef]
- Wu, T.; Mazhar, Z.; Alsayrafi, D.; Garelnabi, M. P-Cymene Modulate Oxidative Stress and Inflammation in Murine Macrophages: Potential Implication in Atherosclerosis. Cardiovasc Hematol Agents Med Chem 2020, 18, 151–157. [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Chun, H.S. P-Cymene and Its Derivatives Exhibit Antiaflatoxigenic Activities against Aspergillus Flavus through Multiple Modes of Action. Appl Biol Chem 2018, 61, 489–497. [CrossRef]
- Shang, X.; Wang, Y.; Zhou, X.; Guo, X.; Dong, S.; Wang, D.; Zhang, J.; Pan, H.; Zhang, Y.; Miao, X. Acaricidal Activity of Oregano Oil and Its Major Component, Carvacrol, Thymol and p-Cymene against Psoroptes Cuniculi in Vitro and in Vivo. Vet Parasitol 2016, 226, 93–96. [CrossRef]
- Abbasi, S.; Gharaghani, S.; Benvidi, A.; Rezaeinasab, M. New Insights into the Efficiency of Thymol Synergistic Effect with P-Cymene in Inhibiting Advanced Glycation End Products: A Multi-Way Analysis Based on Spectroscopic and Electrochemical Methods in Combination with Molecular Docking Study. J Pharm Biomed Anal 2018, 150, 436–451. [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of Herpes Simplex Virus Type 1 to Monoterpenes Thymol, Carvacrol, p-Cymene and Essential Oils of Sinapis Arvensis L., Lallemantia Royleana Benth. and Pulicaria Vulgaris Gaertn. Cell Mol Biol (Noisy-le-grand) 2017, 63, 42–47. [CrossRef]
- Sikalov, A.A.; Pavlovskiy, V.V.; Kirilchuk, A.A. Ruthenium(II) p-Cymene Complexes Bearing 2-(1,2,4-triazol-3-yl)Pyridines: Linkage Isomerism and Related NMR/DFT Studies. Inorg Chem Commun 2019, 99, 156–159. [CrossRef]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective Effect of Limonene in Rats: Influence on Oxidative Stress, Inflammation and Gene Expression. Phytomedicine 2019, 53, 37–42. [CrossRef]
- Santana, H.S.R.; de Carvalho, F.O.; Silva, E.R.; Santos, N.G.L.; Shanmugam, S.; Santos, D.N.; Wisniewski, J.O.; Junior, J.S.C.; Nunes, P.S.; Araujo, A.A.S.; et al. Anti-Inflammatory Activity of Limonene in the Prevention and Control of Injuries in the Respiratory System: A Systematic Review. Curr Pharm Des 2020, 26, 2182–2191. [CrossRef]
- Araújo-Filho, H.G. de; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer Activity of Limonene: A Systematic Review of Target Signaling Pathways. Phytother Res 2021, 35, 4957–4970. [CrossRef]
- Parise-Filho, R.; Pastrello, M.; Pereira Camerlingo, C.E.; Silva, G.J.; Agostinho, L.A.; de Souza, T.; Motter Magri, F.M.; Ribeiro, R.R.; Brandt, C.A.; Polli, M.C. The Anti-Inflammatory Activity of Dillapiole and Some Semisynthetic Analogues. Pharm Biol 2011, 49, 1173–1179. [CrossRef]
- Brazão, M.A.B.; Brazão, F.V.; Maia, J.G.S.; Monteiro, M.C. Antibacterial Activity of the Piper Aduncum Oil and Dillapiole, Its Main Constituent, against Multidrug-Resistant Strains. Bol Latinoam Caribe Plantas Med Aromat 2014, 517–526.
- Monteiro, M. Antifungal Action of the Dillapiole-Rich Oil of Piper Aduncum against Dermatomycoses Caused by Filamentous Fungi. Br J Med Med Res 2016, 15, 1–10. [CrossRef]



| Formula | EO(%) | Oily Vehicle | Surf. + CoS. (%) | Cosolvent (%) | Water (QSP100%) | Process |
|---|---|---|---|---|---|---|
| F1 | 1 | P.O. 5% | 25 | - | QSP 100% | P1 : Sonication 15 min + vortex stir 3 min |
| F2 | 1 | P.O. 10% | 20 | - | QSP 100% | P1: Sonication 15 min + vortex stir 3 min |
| F1’ | 1 | P.O. 5% | 25 | - | QSP 100% | P2: vortex stir 3 min + sonication 20 min |
| F2’ | 1 | P.O. 10% | 20 | - | QSP 100% | P2: vortex stir 3 min + sonication 20 min |
| F3 | 1 | Triacetin 5% | 25 | - | QSP 100% | P2: vortex stir 3 min + sonication 20 min |
| F4 | 0.5 | Triacetin 5% | 30 | - | QSP 100% | P2: vortex stir 3 min + sonication 20 min |
| F4’ | 0.5 | Triacetin 5% | 30 | - | QSP 100% | P3: 13.000 rpm homogenization 2 min |
| F5 | 0.5 | Triacetin 5% | 25 | 1 | QSP 100% | P3: 13.000 rpm homogenization 2 min |
| F6 | 1 | Triacetin 10% | 20 | - | QSP 100% | P3: 13.000 rpm homogenization 2 min |
| F7 | 1 | Triacetin 10% | 20 | - | QSP 100% | P3: 13.000 rpm homogenization 2 min |
| Group | Titre |
|---|---|
| G1 | Optimal NE/Gel preparation « Optimal NE/Gel » |
| G2 | Blank NE/Gel (without EO) « Blank NE/Gel » |
| G3 | Conventional EO cream « Cream EO » |
| G4 | Blank gel « Blank Hydrogel » |
| G5 | Non treated « Non-treated » |
| G6 | Commercialized medicinal cream (API: ß-sitostérol ) « MEBO ®» |
| N° | Compounds | *LRI | **P. tortuos EO ±STDEVA |
N° | Compounds | *LRI | **P. tortuosus EO ±STDEVA |
|---|---|---|---|---|---|---|---|
| 1 | α-Thujene | 933 | 0.8 ±0.06abcd | 24 | Bornyl acetate | 1287 | 0.8±0.10abcd |
| 2 | α-Pinene | 941 | 2.1±0,15fg | 25 | p-Cymen-7-ol (syn. cumin alcohol) | 1290 | 0.9±0.21abcd |
| 3 | Sabinene | 977 | 8.7±0.81k | 26 | Carvacrol | 1299 | 1.7±0.10ef |
| 4 | β-Pinene | 982 | 0.6±0.06abc | 27 | Pinanediol | 1317 | 0.4±0.12abc |
| 5 | Myrcene | 992 | 0,6±0,06abc | 28 | p-Mentha-1,4-dien-7-ol | 1331 | 0.4±0.06ab |
| 6 | α-Phellandrene | 1006 | 0.3±0.06a | 29 | α-Longipinene | 1338 | 0.9±0.15abcd |
| 7 | α-Terpinene | 1020 | 0.9±0.06abcd | 30 | Methyl eugenol | 1403 | 0.4±0.06ab |
| 8 | p-Cymene | 1028 | 6.0±0.26j | 31 | β-Bisabolene | 1508 | 0.5±0.06abc |
| 9 | Limonene | 1032 | 5.2±0.38i | 32 | Spathulenol | 1576 | 0.7±0.06abc |
| 10 | γ-Terpinene | 1063 | 2.5±0.15g | 33 | Dillapiole | 1623 | 13.0±0.62l |
| 11 | Terpinolene | 1090 | 1.1±0.06bcd | 34 | β-Eudesmol | 1650 | 1.0±0.12abcd |
| 12 | cis-p-Menth-2-en-1-ol | 1123 | 1.8±0.10ef | 35 | (Z)-3-Butylidenephthalide | 1677 | 8.5±0.64k |
| 13 | α-Campholenal | 1125 | 0.4±0.06abc | 36 | (E)-3-Butylidenephthalide | 1716 | 3.9±0.47h |
| 14 | trans-p-Menth-2-en-1-ol | 1142 | 0.9±0.06abcd | 37 | (Z)-Ligustilide | 1737 | 6.4±0.62j |
| 15 | Camphor | 1145 | 0.4±0.06ab | 38 | Hexahydrofarnesylacetone | 1845 | 0.7±0.10abcd |
| 16 | Sabinaketone | 1159 | 0.8±0.06abcd | Monoterpene hydrocarbons | 28.9±1.97 | ||
| 17 | 4-Terpineol | 1179 | 16.2±1.46m | Oxygenated monoterpenes | 28.9±1.37 | ||
| 18 | p-Cymen-8-ol | 1185 | 1.4±0.06de | Sesquiterpene hydrocarbons | 1.4±0.20 | ||
| 19 | α-Terpineol | 1191 | 1.1±0.10cd | Oxygenated sesquiterpenes | 1.6±0.15 | ||
| 20 | p-Mentha-1,5-dien-7-ol | 1193 | 0.3±0.00a | Phenylpropanoids | 13.4±0.68 | ||
| 21 | Myrtenal | 1194 | 0.5±0.06abc | Apocarotenes | 0.7±0.10 | ||
| 22 | Carvone | 1244 | 0.5±0.00abc | Non-terpene derivatives | 18.9±1.74 | ||
| 23 | trans-Ascaridol glicol | 1271 | 0.4±0.06abc | Total identified | 93.8±0.51 |
| Process | Formulation | EO (%) | Oily Vehicle ( %) |
Co-solvent (%) | Particle Size (nm) |
Polydispersity Index |
Visual Aspect |
|---|---|---|---|---|---|---|---|
| (P1): sonication 15min + vortex 3min | Formula 1 | 1.0 | 5 Paraffin oil |
107±0.68 | 0.6 | Milky yellowish |
|
| (P1) | Formula 2 | 1.0 | 10 Paraffin oil |
438±1.00 | 1.00 | Milky yellowish |
|
| (P2): Vortex 3min + sonication 20min |
Formula 1 | 1.0 | 5 Paraffin oil |
111±0.70 | 0.7 | Milky | |
| (P2) | Formula 2 | 1.0 | 10 Paraffin oil |
450±0.90 | 0.9 | Milky | |
| (P2) | Formula 3 | 1.0 | 5 Triacetin | 76±0.50 | 0.5 | Milky | |
| (P2) | Formula 4 | 0.5 | 5 Triacetin | 37±0.53 | 0.5 | Milky | |
| (P3): Homogenization at high-speed 13,000rpm | Formula 4 | 0.5 | 5 Triacetin | 47±0.42 | 0.4 | Bluish | |
| (P3) | Formula 5 | 0.5 | 5 Triacetin | 1 | 29±0.44 | 0.4 | Bluish |
| (P3) | Formula 6 | 1.0 | 10 Triacetin | 45±0.40 | 0.4 | Bluish | |
| (P3) | Formula 7 | 1.0 | 10 Triacetin | 1 | 27±0.39 | 0.3 | Bluish |
| Group | Formulation | EO content (%) |
|---|---|---|
| G1 | Optimal preparation « optimal NE/Gel » |
1 |
| G2 | NEs gelified without EO « Blank NE/Gel » |
0 |
| G3 | Conventional EO cream « Cream EO » |
1 |
| G4 | Blank gel « Blank Hydrogel » |
0 |
| G5 | Non treated « Non treated » |
0 |
| G6 | Commercialized medicinal cream (API : ß-sitostérol ) “ MEBO ®” |
0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





