Submitted:
01 August 2023
Posted:
02 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
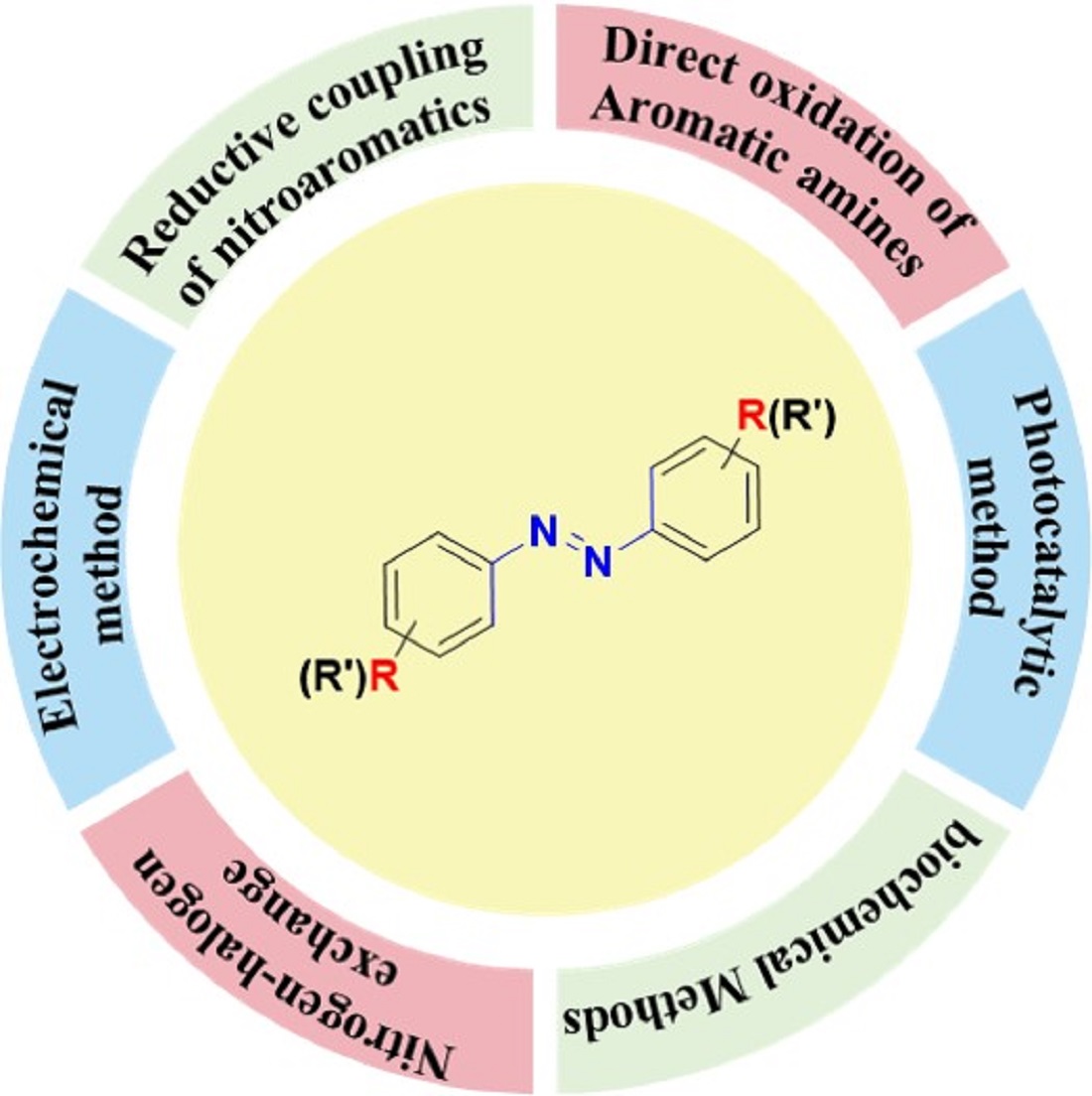
2. Advances in the Synthesis of Aromatic Azo Compounds
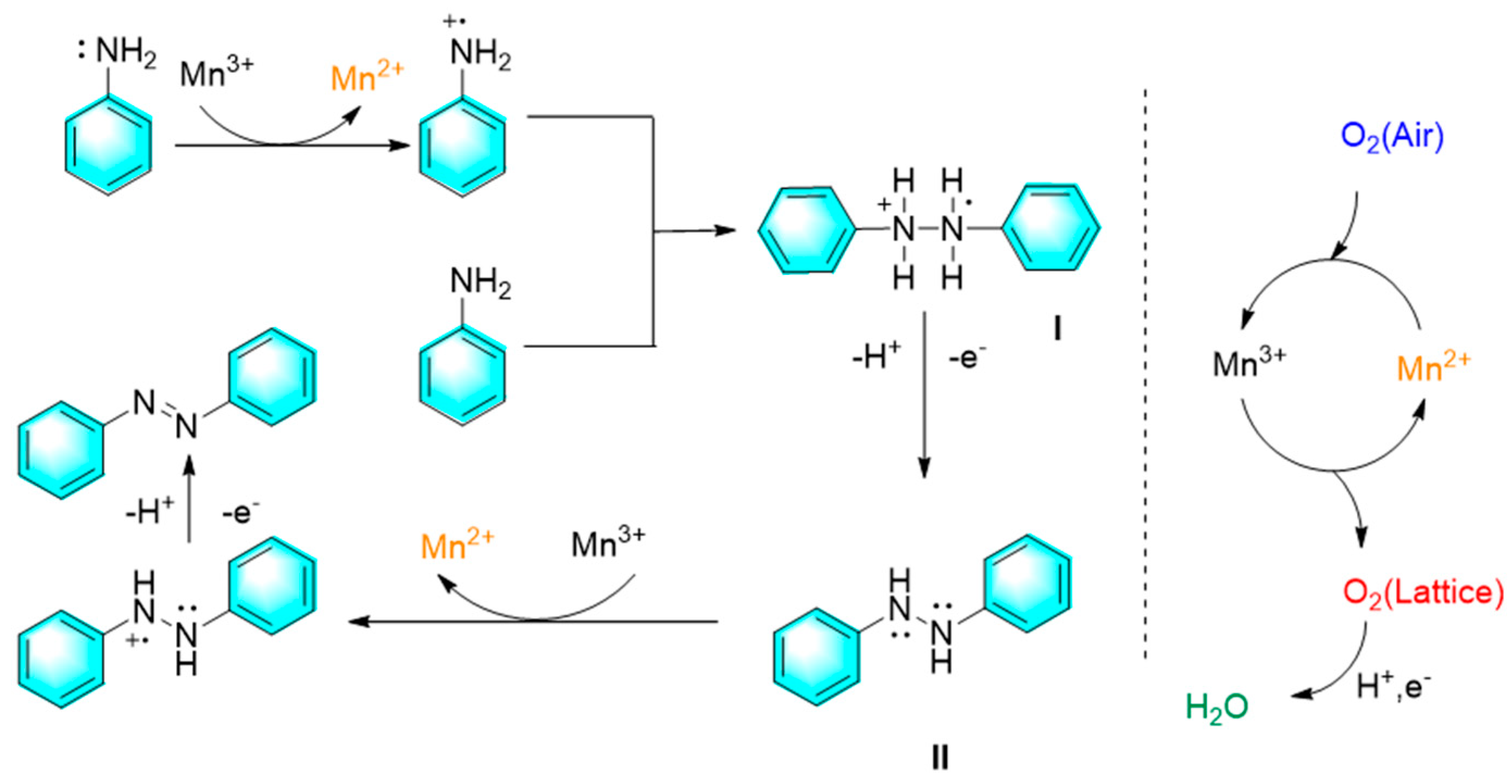
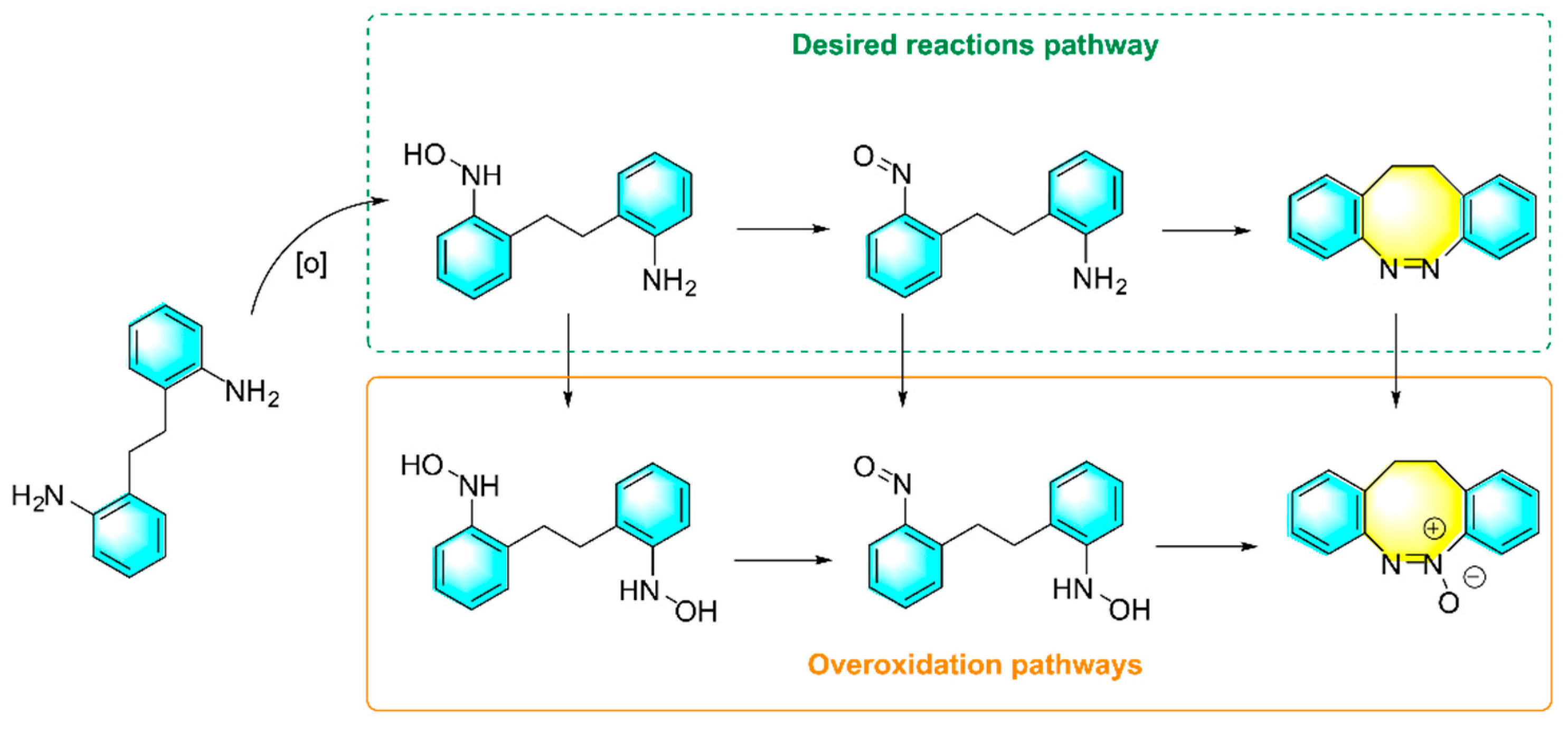
2.1. Direct Oxidation of Aromatic Amines
2.2. Reductive Coupling of Aromatic Nitro Compound
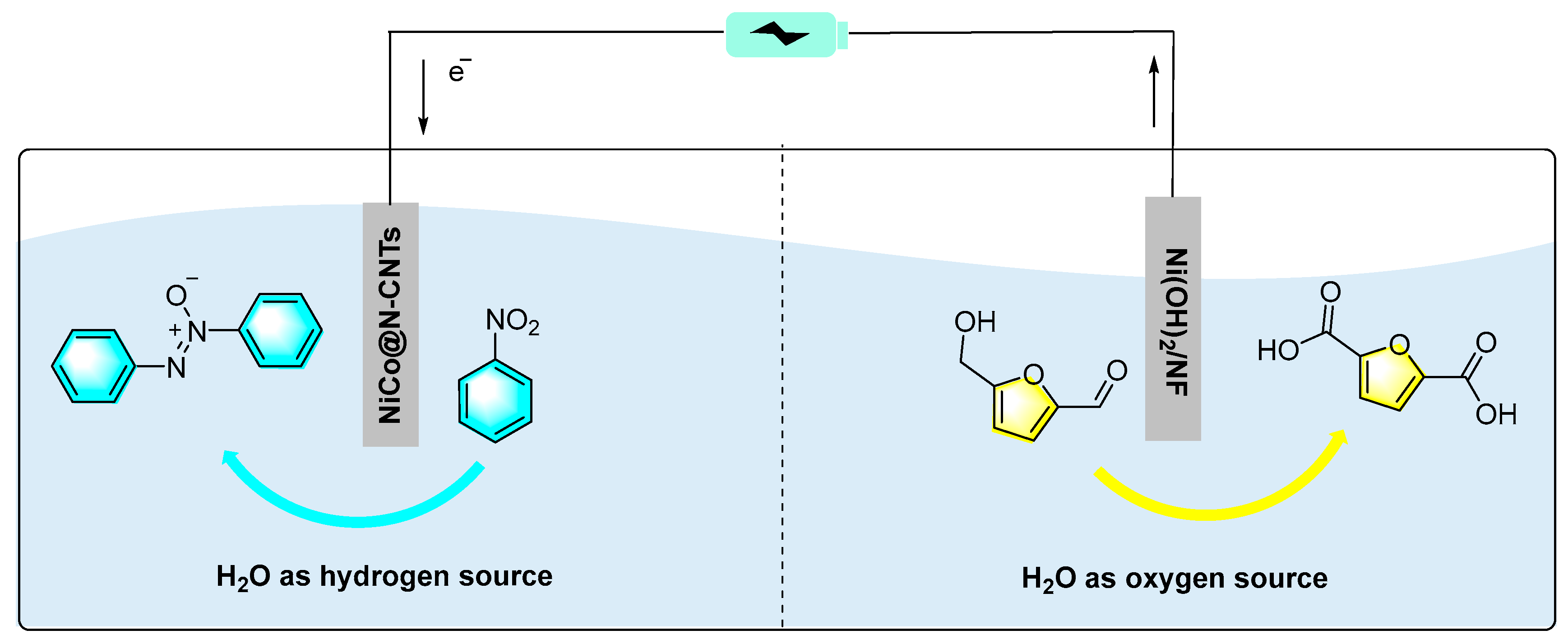
2.3. Electrochemical Method
2.4. Photocatalytic Method
2.5. Biochemical Methods
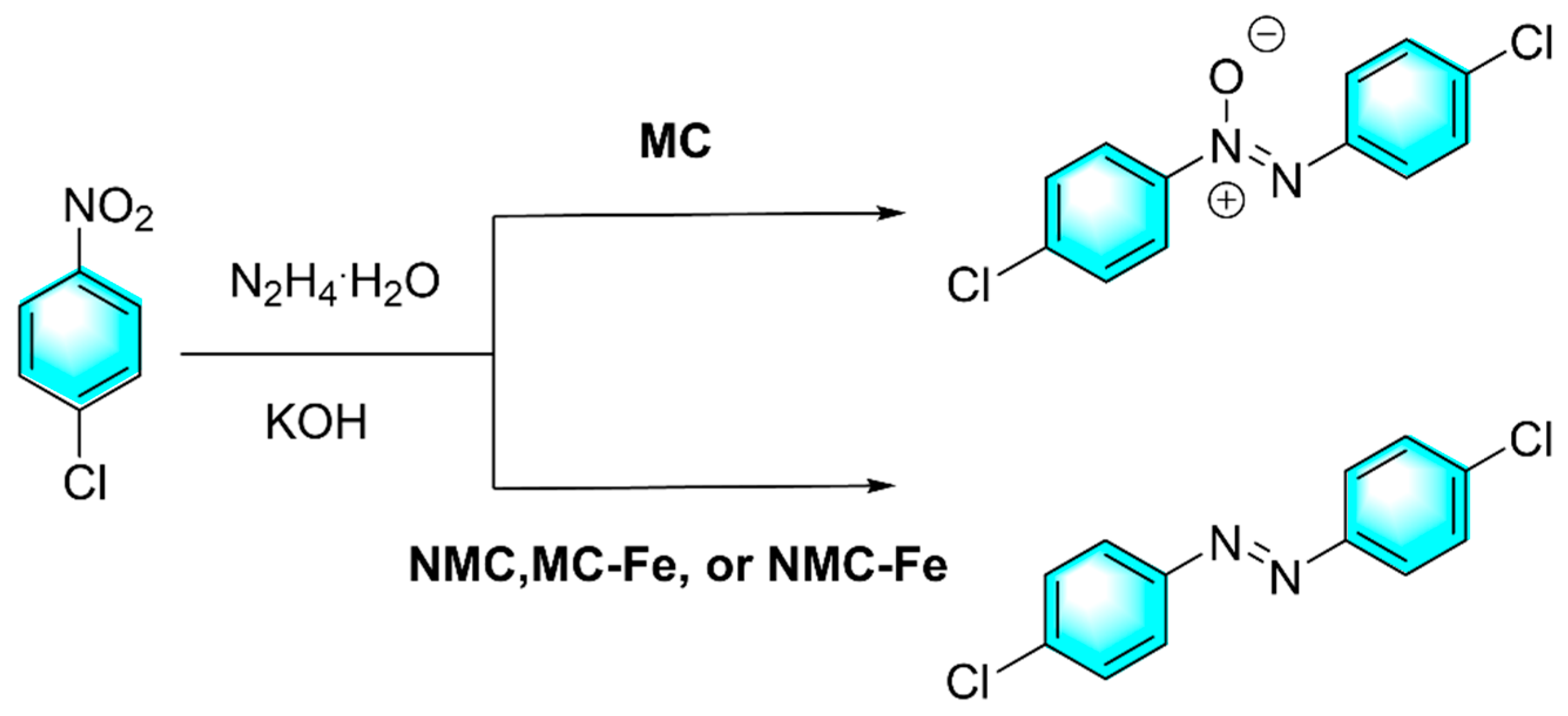
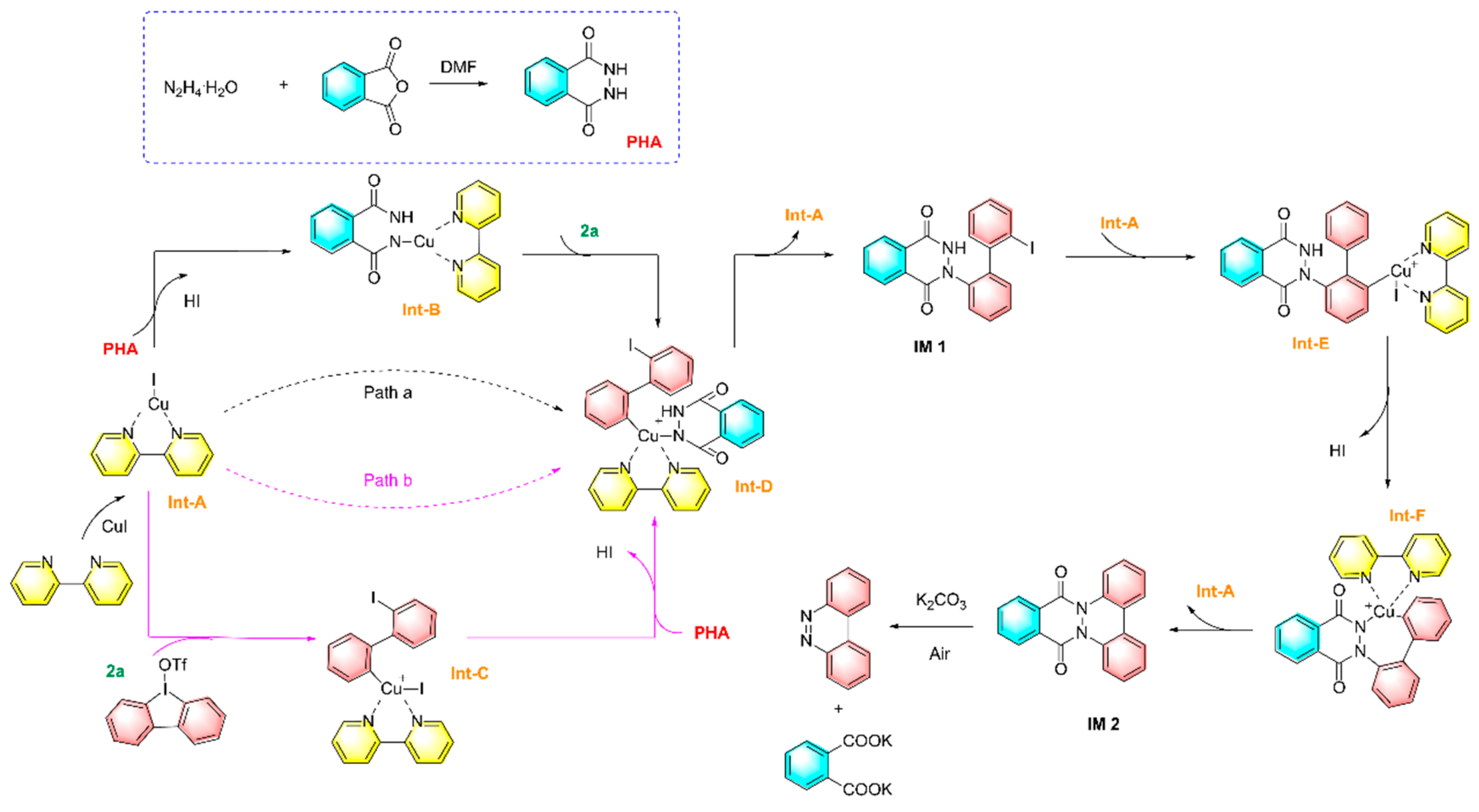
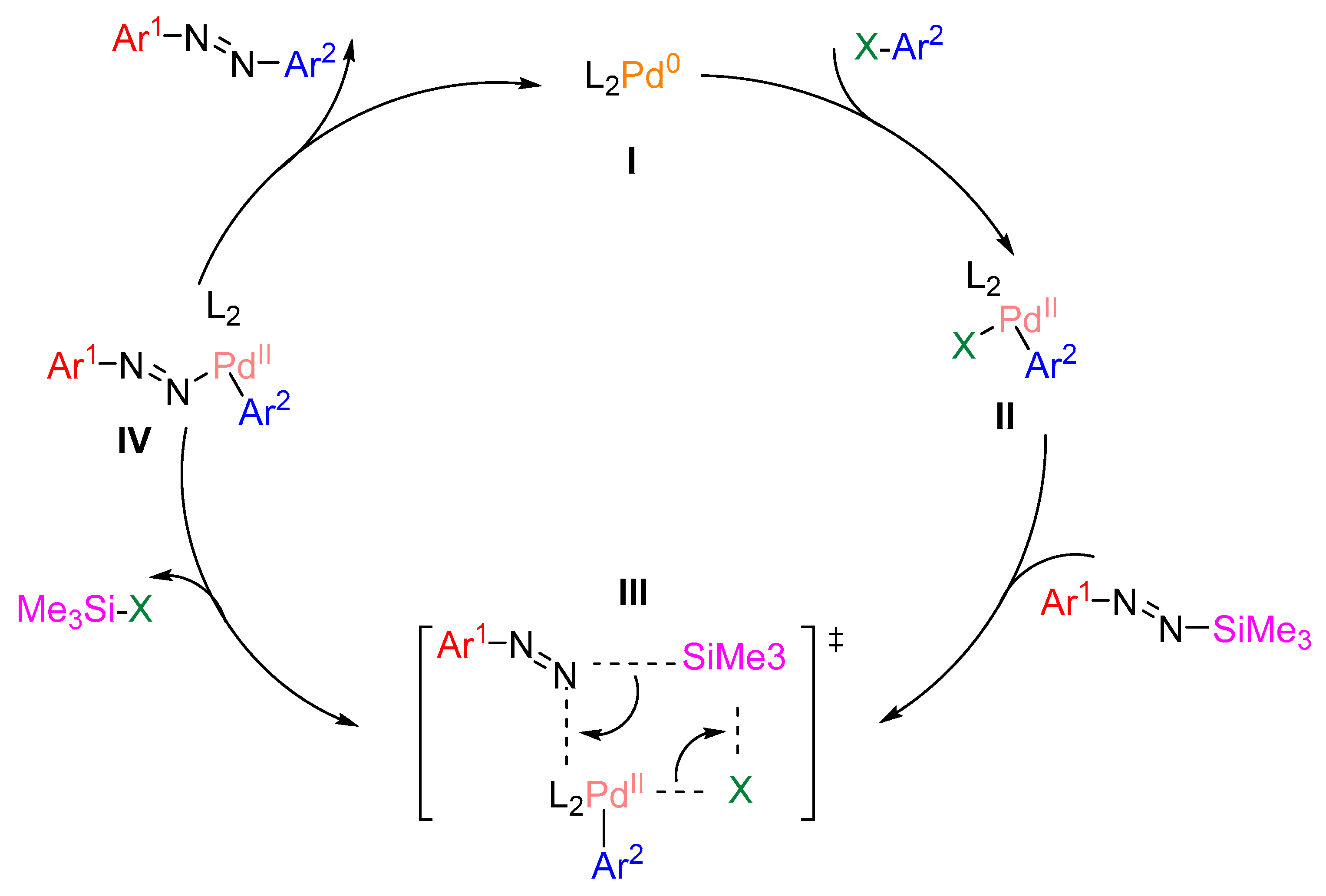
2.6. Nitrogen-Halogen Exchange
3. Conclusions
References
- Hu, D.; Wang, Y.; Liu, J.; Mao, Y.; Chang, X.; Zhu, Y. Light-Driven Sequential Shape Transformation of Block Copolymer Particles through Three-Dimensional Confined Self-Assembly. Nanoscale 2022, 14, 6291–6298. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Tanifuji, N.; Yoshikawa, H. Azo Compounds as Active Materials of Energy Storage Systems. Angew Chem Int Ed 2022, 61. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Wu, Y.; Li, W.; Yang, Y. A 3D Adenine-based Cd-MOF: Synthesis, Structure and Photoluminescent Sensing for an Aromatic Azo Compound. Z. Anorg. Allg. Chem. 2020, 646, 1911–1915. [Google Scholar] [CrossRef]
- Wibowo, M.; Ding, L. Chemistry and Biology of Natural Azoxy Compounds. J. Nat. Prod. 2020, 83, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Pharmacological and Predicted Activities of Natural Azo Compounds. Nat. Prod. Bioprospect. 2017, 7, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pi, J.; Chen, S.; Liu, P.; Sun, P. Construction of a 4 H -Pyrido[4,3,2- Gh ]Phenanthridin-5(6 H )-One Skeleton via a Catalyst-Free Radical Cascade Addition/Cyclization Using Azo Compounds as Radical Sources. Org. Chem. Front. 2018, 5, 793–796. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Fuentes-Lemus, E.; Figueroa, J.D.; Dorta, E.; Schöneich, C.; Davies, M.J. Azocompounds as Generators of Defined Radical Species: Contributions and Challenges for Free Radical Research. Free Radical Biology and Medicine 2020, 160, 78–91. [Google Scholar] [CrossRef]
- Udoikono, A.D.; Louis, H.; Eno, E.A.; Agwamba, E.C.; Unimuke, T.O.; Igbalagh, A.T.; Edet, H.O.; Odey, J.O.; Adeyinka, A.S. Reactive Azo Compounds as a Potential Chemotherapy Drugs in the Treatment of Malignant Glioblastoma (GBM): Experimental and Theoretical Studies. Journal of Photochemistry and Photobiology 2022, 10, 100116. [Google Scholar] [CrossRef]
- Na Joo, H.; Huy Le, B.; Jun Seo, Y. Azo-Pyrene–Based Fluorescent Sensor of Reductive Cleavage of Isomeric Azo Functional Group. Tetrahedron Letters 2017, 58, 679–681. [Google Scholar] [CrossRef]
- Kohei, M.; Takizawa, N.; Tsutsumi, R.; Xu, W.; Kumagai, N. Azo-Tagged C4N4 Fluorophores: Unusual Overcrowded Structures and Their Application to Fluorescent Imaging. Org. Biomol. Chem. 2023, 21, 2889–2893. [Google Scholar] [CrossRef]
- Concilio, S.; Iannelli, P.; Sessa, L.; Olivieri, R.; Porta, A.; De Santis, F.; Pantani, R.; Piotto, S. Biodegradable Antimicrobial Films Based on Poly(Lactic Acid) Matrices and Active Azo Compounds. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Luo, C.; Xu, G.-L.; Ji, X.; Hou, S.; Chen, L.; Wang, F.; Jiang, J.; Chen, Z.; Ren, Y.; Amine, K.; et al. Reversible Redox Chemistry of Azo Compounds for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 2879–2883. [Google Scholar] [CrossRef]
- Li, J.; Huo, F.; Chen, T.; Yan, H.; Yang, Y.; Zhang, S.; Chen, S. In-Situ Construction of Stable Cathode/Li Interfaces Simultaneously via Different Electron Density Azo Compounds for Solid-State Lithium Metal Batteries. Energy Storage Materials 2021, 40, 394–401. [Google Scholar] [CrossRef]
- Adu, J.K.; Amengor, C.D.K.; Mohammed Ibrahim, N.; Amaning-Danquah, C.; Owusu Ansah, C.; Gbadago, D.D.; Sarpong-Agyapong, J. Synthesis and In Vitro Antimicrobial and Anthelminthic Evaluation of Naphtholic and Phenolic Azo Dyes. Journal of Tropical Medicine 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Luo, C.; Borodin, O.; Ji, X.; Hou, S.; Gaskell, K.J.; Fan, X.; Chen, J.; Deng, T.; Wang, R.; Jiang, J.; et al. Azo Compounds as a Family of Organic Electrode Materials for Alkali-Ion Batteries. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 2004–2009. [Google Scholar] [CrossRef]
- Fan, F.; Wang, C. Preparation and Photochromic Properties of Nanocapsules Containing Azo Compound with Polyurethane as Wall Material Using in Situ Polymerization. Polymer-Plastics Technology and Engineering 2014, 53, 1062–1069. [Google Scholar] [CrossRef]
- Nehls, E.M.; Rosales, A.M.; Anseth, K.S. Enhanced User-Control of Small Molecule Drug Release from a Poly(Ethylene Glycol) Hydrogel via Azobenzene/Cyclodextrin Complex Tethers. J. Mater. Chem. B 2016, 4, 1035–1039. [Google Scholar] [CrossRef]
- Xu, G.; Li, S.; Liu, C.; Wu, S. Photoswitchable Adhesives Using Azobenzene-Containing Materials. Chem. Asian J. 2020, 15, 547–554. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Zhang, H.; Liu, K. Azobenzene-Based Photomechanical Biomaterials. Adv NanoBio Res 2021, 1, 2100020. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Chen, J.; Gaskell, K.J.; He, X.; Liang, Y.; Jiang, J.; Wang, C. Solid-State Electrolyte Anchored with a Carboxylated Azo Compound for All-Solid-State Lithium Batteries. Angew. Chem. Int. Ed. 2018, 57, 8567–8571. [Google Scholar] [CrossRef]
- Smaniotto, A.; Mezalira, D.Z.; Zapp, E.; Gallardo, H.; Vieira, I.C. Electrochemical Immunosensor Based on an Azo Compound for Thyroid-Stimulating Hormone Detection. Microchemical Journal 2017, 133, 510–517. [Google Scholar] [CrossRef]
- Peiris, S.; Sarina, S.; Han, C.; Xiao, Q.; Zhu, H.-Y. Silver and Palladium Alloy Nanoparticle Catalysts: Reductive Coupling of Nitrobenzene through Light Irradiation. Dalton Trans. 2017, 46, 10665–10672. [Google Scholar] [CrossRef] [PubMed]
- Chong, X.; Liu, C.; Huang, Y.; Huang, C.; Zhang, B. Potential-Tuned Selective Electrosynthesis of Azoxy-, Azo- and Amino-Aromatics over a CoP Nanosheet Cathode. National Science Review 2020, 7, 285–295. [Google Scholar] [CrossRef]
- Chaiseeda, K.; Nishimura, S.; Ebitani, K. Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene. ACS Omega 2017, 2, 7066–7070. [Google Scholar] [CrossRef]
- Yadav, G.D.; Mewada, R.K. Novelties of Azobenzene Synthesis via Selective Hydrogenation of Nitrobenzene over Nano-Fibrous Ag-OMS-2 – Mechanism and Kinetics. Chemical Engineering Journal 2013, 221, 500–511. [Google Scholar] [CrossRef]
- Arora, A.; Oswal, P.; Kumar Rao, G.; Kumar, S.; Kumar, A. Organoselenium Ligands for Heterogeneous and Nanocatalytic Systems: Development and Applications. Dalton Trans. 2021, 50, 8628–8656. [Google Scholar] [CrossRef] [PubMed]
- Pothula, K.; Tang, L.; Zha, Z.; Wang, Z. Bismuth Nanoparticles: An Efficient Catalyst for Reductive Coupling of Nitroarenes to Azo-Compounds. RSC Adv. 2015, 5, 83144–83148. [Google Scholar] [CrossRef]
- Agnoli, S. Interfacial Chemistry of Low-Dimensional Systems for Applications in Nanocatalysis. Eur. J. Inorg. Chem. 2018, 2018, 4311–4321. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, M.; Cao, F.; Li, J.; Zhang, S.; Qu, Y. Single Crystal MnOOH Nanotubes for Selective Oxidative Coupling of Anilines to Aromatic Azo Compounds. J. Mater. Chem. A 2021, 9, 19692–19697. [Google Scholar] [CrossRef]
- Liu, X.; Ye, S.; Li, H.-Q.; Liu, Y.-M.; Cao, Y.; Fan, K.-N. Mild, selective and switchable transfer reduction of nitroarenes catalyzed by supported gold nanoparticles. Catal. Sci. Technol. 2013, 3, 3200. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, X.; Wang, M.; Liu, Q.; Shao, Y.; Li, H.; Liang, C.; Chen, X.; Wang, H. A Schiff-Base Modified Pt Nano-Catalyst for Highly Efficient Synthesis of Aromatic Azo Compounds. Catalysts 2019, 9, 339. [Google Scholar] [CrossRef]
- Gao, S.; Han, Y.; Fan, M.; Li, Z.; Ge, K.; Liang, X.-J.; Zhang, J. Metal-Organic Framework-Based Nanocatalytic Medicine for Chemodynamic Therapy. Sci. China Mater. 2020, 63, 2429–2434. [Google Scholar] [CrossRef]
- Ingale, G.; Seo, Y.J. Azo Compounds with Different Sized Fluorophores and Characterization of Their Photophysical Properties Based on Trans to Cis Conformational Change. Tetrahedron Letters 2014, 55, 5247–5250. [Google Scholar] [CrossRef]
- Grebenkin, S.Yu.; Syutkin, V.M.; Baranov, D.S. Mutual Orientation of the n → π * and π → π * Transition Dipole Moments in Azo Compounds: Determination by Light-Induced Optical Anisotropy. Journal of Photochemistry and Photobiology A: Chemistry 2017, 344, 1–7. [Google Scholar] [CrossRef]
- Rezaei-Seresht, E.; Mireskandari, E.; Kheirabadi, M.; Cheshomi, H.; Rezaei-Seresht, H.; Aldaghi, L.S. Synthesis and Anticancer Activity of New Azo Compounds Containing Extended π-Conjugated Systems. Chem. Pap. 2017, 71, 1463–1469. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, P.; Ghosh, P. Selective Single-Step Oxidation of Amine to Cross-Azo Compounds with an Unhampered Primary Benzyl Alcohol Functionality. Org. Lett. 2018, 20, 6725–6729. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Xie, C.; Liu, H.; Wang, Y.; Mei, Q.; Liu, H.; Han, B. Ethylenediamine Promoted the Hydrogenative Coupling of Nitroarenes over Ni/C Catalyst. Chinese Chemical Letters 2019, 30, 203–206. [Google Scholar] [CrossRef]
- Dana, S.; Sahoo, H.; Bhattacharyya, A.; Mandal, A.; Prasad, E.; Baidya, M. Copper-Catalyzed Chelation-Assisted Synthesis of Unsymmetrical Aliphatic Azo Compounds. ChemistrySelect 2017, 2, 2029–2033. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Y.; Wang, J.; Ren, W.; Liu, S.; Zheng, C.; Gao, X. Enhancing Nitrobenzene Reduction to Azoxybenzene by Regulating the O-Vacancy Defects over Rationally Tailored CeO2 Nanocrystals. Applied Surface Science 2022, 572, 151343. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.; Lin, X.; Hu, Q.; Hu, B.; Zhou, Y.; Zhu, G. Modular Synthesis of Alkylarylazo Compounds via Iron(III)-Catalyzed Olefin Hydroamination. Org. Lett. 2019, 21, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, B.; Guo, A.; Dong, Z.; Jin, S.; Lu, Y. Reduction of Nitroarenes to Azoxybenzenes by Potassium Borohydride in Water. Molecules 2011, 16, 3563–3568. [Google Scholar] [CrossRef]
- Da Silva, M.A.R.; Rocha, G.F.S.R.; Diab, G.A.A.; Cunha, C.S.; Pastana, V.G.S.; Teixeira, I.F. Simple and Straightforward Method to Prepare Highly Dispersed Ni Sites for Selective Nitrobenzene Coupling to Azo/Azoxy Compounds. Chemical Engineering Journal 2023, 460, 141068. [Google Scholar] [CrossRef]
- Han, S.; Cheng, Y.; Liu, S.; Tao, C.; Wang, A.; Wei, W.; Yu, H.; Wei, Y. Selective Oxidation of Anilines to Azobenzenes and Azoxybenzenes by a Molecular Mo Oxide Catalyst. Angew. Chem. Int. Ed. 2021, 60, 6382–6385. [Google Scholar] [CrossRef] [PubMed]
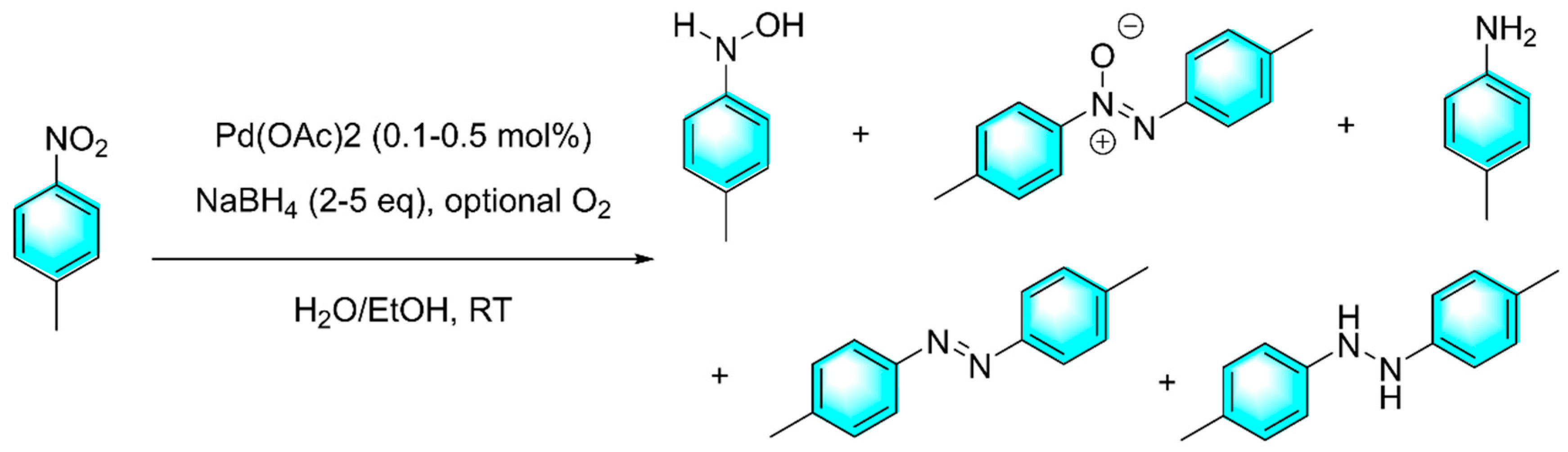
- Doherty, S.; Knight, J.G.; Backhouse, T.; Summers, R.J.; Abood, E.; Simpson, W.; Paget, W.; Bourne, R.A.; Chamberlain, T.W.; Stones, R.; et al. Highly Selective and Solvent-Dependent Reduction of Nitrobenzene to N -Phenylhydroxylamine, Azoxybenzene, and Aniline Catalyzed by Phosphino-Modified Polymer Immobilized Ionic Liquid-Stabilized AuNPs. ACS Catal. 2019, 9, 4777–4791. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Jin, Y.; Wu, C.; Yuan, Z.; Ma, P.; Wang, J.; Niu, J. An Intriguing Tetranuclear Rh-Based Polyoxometalate for the Reduction of Nitroarene and Oxidation of Aniline. Chem. Commun. 2022, 58, 9902–9905. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.-Q.; Ye, S.; Liu, Y.-M.; He, H.-Y.; Cao, Y. Gold-Catalyzed Direct Hydrogenative Coupling of Nitroarenes To Synthesize Aromatic Azo Compounds. Angew. Chem. Int. Ed. 2014, 53, 7624–7628. [Google Scholar] [CrossRef]
- Shen, J.; Xu, J.; Zhu, Q.; Zhang, P. Hypervalent Iodine(III)-Promoted Rapid Cascade Reaction for the Synthesis of Unsymmetric Azo Compounds. Org. Biomol. Chem. 2021, 19, 3119–3123. [Google Scholar] [CrossRef]
- Shen, J.; Xu, J.; He, L.; Ouyang, Y.; Huang, L.; Li, W.; Zhu, Q.; Zhang, P. Photoinduced Rapid Multicomponent Cascade Reaction of Aryldiazonium Salts with Unactivated Alkenes and TMSN 3. Org. Lett. 2021, 23, 1204–1208. [Google Scholar] [CrossRef]
- Khaligh, N.G.; Hamid, S.B.A.; Johari, S. Telescopic Synthesis of Azo Compounds via Stable Arenediazonium Tosylates by Using n -Butyl Nitrite as Diazotization Reagent. Polycyclic Aromatic Compounds 2019, 39, 346–352. [Google Scholar] [CrossRef]
- Wang, Y.; Yihuo, A.; Wang, L.; Dong, S.; Feng, X. Catalytic Asymmetric Synthesis of Chiral Azo Compounds via Interrupted Japp-Klingemann Reaction with Aryldiazonium Salts. Sci. China Chem. 2022, 65, 546–553. [Google Scholar] [CrossRef]
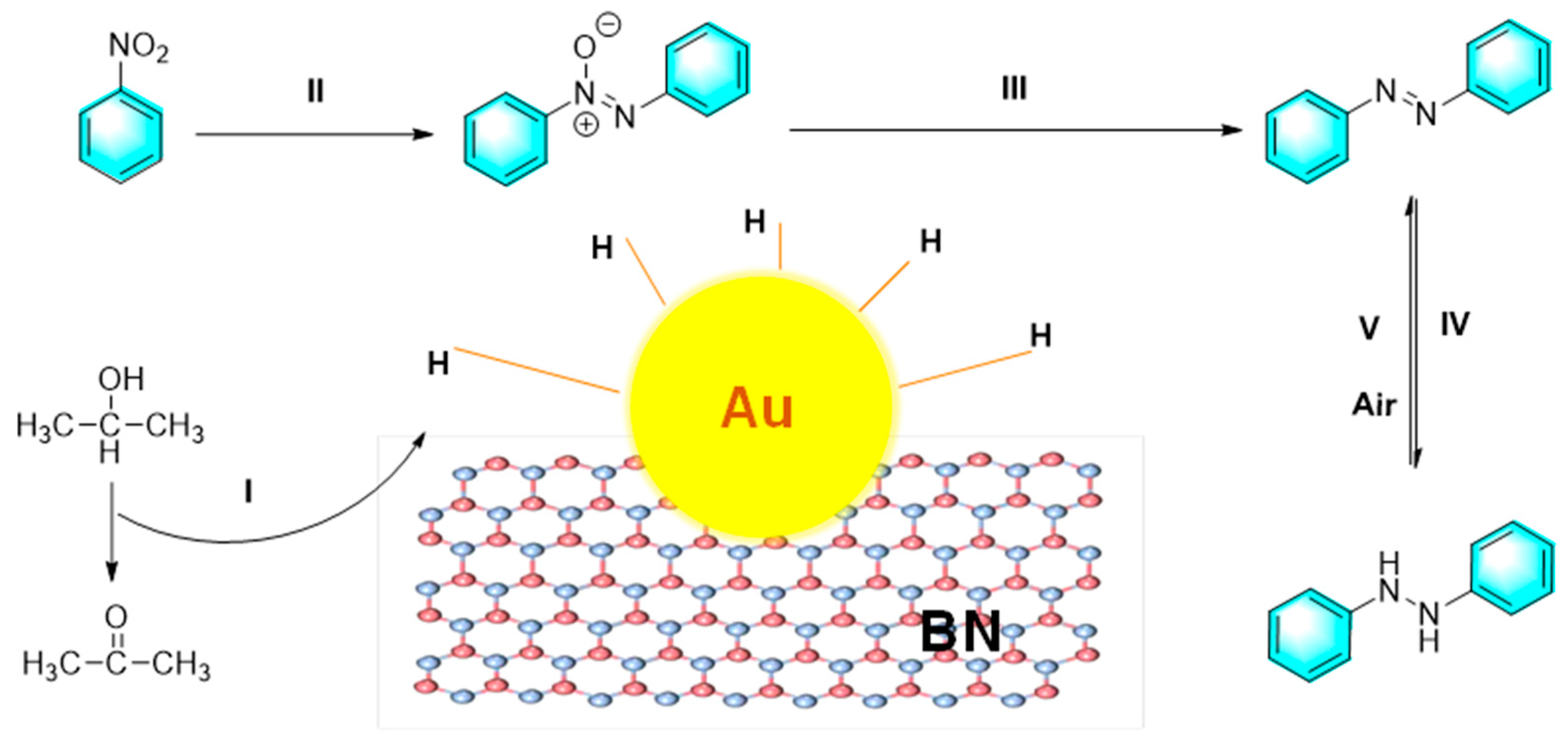
- Liu, Q.; Xu, Y.; Qiu, X.; Huang, C.; Liu, M. Chemoselective Hydrogenation of Nitrobenzenes Activated with Tuned Au/h-BN. Journal of Catalysis 2019, 370, 55–60. [Google Scholar] [CrossRef]
- An, Y.; Tan, H.; Zhao, S. Ag 2 CO 3 Mediated Oxidative Dehydrogenative Coupling of Anilines Giving Aromatic Azo Compounds. Chin. J. Org. Chem. 2017, 37, 226. [Google Scholar] [CrossRef]
- Monir, K.; Ghosh, M.; Mishra, S.; Majee, A.; Hajra, A. Phenyliodine(III) Diacetate (PIDA) Mediated Synthesis of Aromatic Azo Compounds through Oxidative Dehydrogenative Coupling of Anilines: Scope and Mechanism: PIDA-Mediated Synthesis of Aromatic Azo Compounds. Eur. J. Org. Chem. 2014, 2014, 1096–1102. [Google Scholar] [CrossRef]
- Antoine John, A.; Lin, Q. Synthesis of Azobenzenes Using N -Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]Undec-7-Ene (DBU). J. Org. Chem. 2017, 82, 9873–9876. [Google Scholar] [CrossRef]
- Sarkar, P.; Mukhopadhyay, C. First Use of P-Tert-Butylcalix[4]Arene-Tetra-O-Acetate as a Nanoreactor Having Tunable Selectivity towards Cross Azo-Compounds by Trapping Silver Ions. Green Chem. 2016, 18, 442–451. [Google Scholar] [CrossRef]
- Amtawong, J.; Balcells, D.; Wilcoxen, J.; Handford, R.C.; Biggins, N.; Nguyen, A.I.; Britt, R.D.; Tilley, T.D. Isolation and Study of Ruthenium–Cobalt Oxo Cubanes Bearing a High-Valent, Terminal Ru V –Oxo with Significant Oxyl Radical Character. J. Am. Chem. Soc. 2019, 141, 19859–19869. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Liu, C.; Lv, N.; He, W.; Meng, J.; Fang, Z.; Guo, K. Continuous and Green Microflow Synthesis of Azobenzene Compounds Catalyzed by Consecutively Prepared Tetrahedron CuBr. Dyes and Pigments 2020, 174, 108071. [Google Scholar] [CrossRef]
- Hu, L.; Cao, X.; Chen, L.; Zheng, J.; Lu, J.; Sun, X.; Gu, H. Highly Efficient Synthesis of Aromatic Azos Catalyzed by Unsupported Ultra-Thin Pt Nanowires. Chem. Commun. 2012, 48, 3445. [Google Scholar] [CrossRef] [PubMed]
- Ötvös, S.B.; Georgiádes, Á.; Mészáros, R.; Kis, K.; Pálinkó, I.; Fülöp, F. Continuous-Flow Oxidative Homocouplings without Auxiliary Substances: Exploiting a Solid Base Catalyst. Journal of Catalysis 2017, 348, 90–99. [Google Scholar] [CrossRef]
- Oseghale, C.O.; Fapojuwo, D.P.; Alimi, O.A.; Akinnawo, C.A.; Mogudi, B.M.; Onisuru, O.R.; Meijboom, R. Bifunctional Cs−Au/Co 3 O 4 (Basic and Redox)-Catalyzed Oxidative Synthesis of Aromatic Azo Compounds from Anilines. European J Organic Chem 2021, 2021, 5063–5073. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Zhi, C.; Luo, B.; Li, X.; Pan, Y.; Cao, X.; Gu, H. Highly Efficient Synthesis of Azos Catalyzed by the Common Metal Copper (0) through Oxidative Coupling Reactions. RSC Adv. 2014, 4, 16607. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Ke, X. Tuning Catalytic Selectivity in Cascade Reactions by Light Irradiation. Catal Lett 2018, 148, 1124–1129. [Google Scholar] [CrossRef]
- Saha, A.; Payra, S.; Selvaratnam, B.; Bhattacharya, S.; Pal, S.; Koodali, R.T.; Banerjee, S. Hierarchical Mesoporous RuO 2 /Cu 2 O Nanoparticle-Catalyzed Oxidative Homo/Hetero Azo-Coupling of Anilines. ACS Sustainable Chem. Eng. 2018, 6, 11345–11352. [Google Scholar] [CrossRef]
- Cai, S.; Rong, H.; Yu, X.; Liu, X.; Wang, D.; He, W.; Li, Y. Room Temperature Activation of Oxygen by Monodispersed Metal Nanoparticles: Oxidative Dehydrogenative Coupling of Anilines for Azobenzene Syntheses. ACS Catal. 2013, 3, 478–486. [Google Scholar] [CrossRef]
- Yaghoubian, A.; Hodgson, G.K.; Adler, M.J.; Impellizzeri, S. Direct Photochemical Route to Azoxybenzenes via Nitroarene Homocoupling. Org. Biomol. Chem. 2022, 20, 7332–7337. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wu, S.; Jiang, S.; Xiao, F.; Deng, G. Electrosynthesis of Azobenzenes Directly from Nitrobenzenes. Chin. J. Chem. 2021, 39, 3334–3338. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, X.; Zhao, J.; Sarina, S.; Barry, J.; Zhu, H. Selective Reductions Using Visible Light Photocatalysts of Supported Gold Nanoparticles. Green Chem. 2013, 15, 236–244. [Google Scholar] [CrossRef]
- Tran, V.H.; Kim, H.-K. Visible-Light-Driven SAQS-Catalyzed Aerobic Oxidative Dehydrogenation of Alkyl 2-Phenylhydrazinecarboxylates. RSC Adv. 2022, 12, 30304–30309. [Google Scholar] [CrossRef]
- Du, K.-S.; Huang, J.-M. Electrochemical Dehydrogenation of Hydrazines to Azo Compounds. Green Chem. 2019, 21, 1680–1685. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Hou, S.; Eidson, N.; Fan, X.; Liang, Y.; Deng, T.; Jiang, J.; Wang, C. Azo Compounds Derived from Electrochemical Reduction of Nitro Compounds for High Performance Li-Ion Batteries. Adv. Mater. 2018, 30, 1706498. [Google Scholar] [CrossRef]
- Dutta, B.; Biswas, S.; Sharma, V.; Savage, N.O.; Alpay, S.P.; Suib, S.L. Mesoporous Manganese Oxide Catalyzed Aerobic Oxidative Coupling of Anilines To Aromatic Azo Compounds. Angew. Chem. Int. Ed. 2016, 55, 2171–2175. [Google Scholar] [CrossRef]
- Jiang, B.; Du, Y.-Y.; Han, G.-Z. Palladium-Mediated Base-Free and Solvent-Free Synthesis of Aromatic Azo Compounds from Anilines Catalyzed by Copper Acetate. Green Processing and Synthesis 2022, 11, 823–829. [Google Scholar] [CrossRef]
- Maier, M.S.; Hüll, K.; Reynders, M.; Matsuura, B.S.; Leippe, P.; Ko, T.; Schäffer, L.; Trauner, D. Oxidative Approach Enables Efficient Access to Cyclic Azobenzenes. J. Am. Chem. Soc. 2019, 141, 17295–17304. [Google Scholar] [CrossRef]
- Shukla, A.; Singha, R.K.; Konathala, L.N.S.; Sasaki, T.; Bal, R. Catalytic Oxidation of Aromatic Amines to Azoxy Compounds over a Cu–CeO 2 Catalyst Using H 2 O 2 as an Oxidant. RSC Adv. 2016, 6, 22812–22820. [Google Scholar] [CrossRef]
- Mondal, B.; Mukherjee, P.S. Cage Encapsulated Gold Nanoparticles as Heterogeneous Photocatalyst for Facile and Selective Reduction of Nitroarenes to Azo Compounds. J. Am. Chem. Soc. 2018, 140, 12592–12601. [Google Scholar] [CrossRef]
- Yan, Z.; Xie, X.; Song, Q.; Ma, F.; Sui, X.; Huo, Z.; Ma, M. Tandem Selective Reduction of Nitroarenes Catalyzed by Palladium Nanoclusters. Green Chem. 2020, 22, 1301–1307. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.; Qiu, X.; Huang, C.; Liu, M. Chemoselective Hydrogenation of Nitrobenzenes Activated with Tuned Au/h-BN. Journal of Catalysis 2019, 370, 55–60. [Google Scholar] [CrossRef]
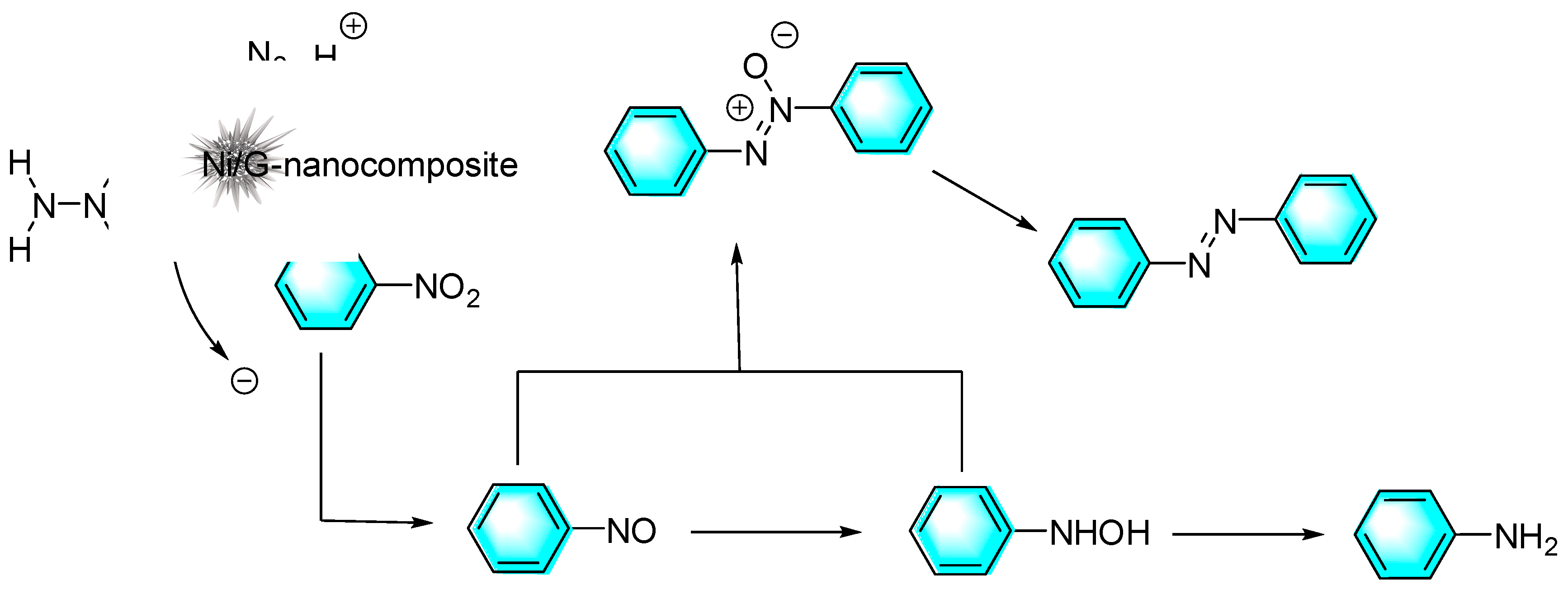
- Pahalagedara, M.N.; Pahalagedara, L.R.; He, J.; Miao, R.; Gottlieb, B.; Rathnayake, D.; Suib, S.L. Room Temperature Selective Reduction of Nitrobenzene to Azoxybenzene over Magnetically Separable Urchin-like Ni/Graphene Nanocomposites. Journal of Catalysis 2016, 336, 41–48. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Shi, C.; Lin, D.; Li, J.; Jin, H.; Chen, X.; Wang, S. Iron and Nitrogen Co-Doped Mesoporous Carbon-Based Heterogeneous Catalysts for Selective Reduction of Nitroarenes. Adv. Synth. Catal. 2019, 361, 3525–3531. [Google Scholar] [CrossRef]
- Moran, M.J.; Martina, K.; Baricco, F.; Tagliapietra, S.; Manzoli, M.; Cravotto, G. Tuneable Copper Catalysed Transfer Hydrogenation of Nitrobenzenes to Aniline or Azo Derivatives. Adv. Synth. Catal. 2020, 362, 2689–2700. [Google Scholar] [CrossRef]
- Hu, L.; Cao, X.; Shi, L.; Qi, F.; Guo, Z.; Lu, J.; Gu, H. A Highly Active Nano-Palladium Catalyst for the Preparation of Aromatic Azos under Mild Conditions. Org. Lett. 2011, 13, 5640–5643. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Liu, X.; Zhou, P.; Jin, S.; Zhang, Z. Co–N x Catalyst: An Effective Catalyst for the Transformation of Nitro Compounds into Azo Compounds. React. Chem. Eng. 2021, 6, 112–118. [Google Scholar] [CrossRef]
- Qiao, W.; Waseem, I.; Shang, G.; Wang, D.; Li, Y.; Besenbacher, F.; Niemantsverdriet, H.; Yan, C.; Su, R. Paired Electrochemical N–N Coupling Employing a Surface-Hydroxylated Ni 3 Fe-MOF-OH Bifunctional Electrocatalyst with Enhanced Adsorption of Nitroarenes and Anilines. ACS Catal. 2021, 11, 13510–13518. [Google Scholar] [CrossRef]
- Gong, W.; Mao, X.; Zhang, J.; Lin, Y.; Zhang, H.; Du, A.; Xiong, Y.; Zhao, H. Ni–Co Alloy Nanoparticles Catalyze Selective Electrochemical Coupling of Nitroarenes into Azoxybenzene Compounds in Aqueous Electrolyte. ACS Nano 2023, 17, 3984–3995. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Mellah, M. Convenient Electrocatalytic Synthesis of Azobenzenes from Nitroaromatic Derivatives Using SmI2. ACS Catal. 2017, 7, 8480–8486. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Wu, T.; Liu, H.; Xie, C.; Yang, G.; Han, B. Simultaneous and Selective Transformation of Glucose to Arabinose and Nitrosobenzene to Azoxybenzene Driven by Visible-Light. Green Chem. 2016, 18, 3852–3857. [Google Scholar] [CrossRef]
- Wang, B.; Deng, Z.; Li, Z. Efficient Chemoselective Hydrogenation of Nitrobenzene to Aniline, Azoxybenzene and Azobenzene over CQDs/ZnIn2S4 Nanocomposites under Visible Light. Journal of Catalysis 2020, 389, 241–246. [Google Scholar] [CrossRef]
- Sousa, A.C.; Baptista, S.R.; Martins, L.O.; Robalo, M.P. Synthesis of Azobenzene Dyes Mediated by CotA Laccase. Chem. Asian J. 2019, 14, 187–193. [Google Scholar] [CrossRef]
- Pariyar, G.C.; Kundu, T.; Mitra, B.; Mukherjee, S.; Ghosh, P. Ethyl Lactate: An Efficient Green Mediator for Transition Metal Free Synthesis of Symmetric and Unsymmetric Azobenzenes. ChemistrySelect 2020, 5, 9781–9786. [Google Scholar] [CrossRef]
- Xie, R.; Xiao, Y.; Wang, Y.; Xu, Z.-W.; Tian, N.; Li, S.; Zeng, M.-H. Hydrazine–Halogen Exchange Strategy Toward N═N-Containing Compounds and Process Tracking for Mechanistic Insight. Org. Lett. 2023, 25, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Finck, L.; Oestreich, M. Synthesis of Non-Symmetric Azoarenes by Palladium-Catalyzed Cross-Coupling of Silicon-Masked Diazenyl Anions and (Hetero)Aryl Halides. Angew Chem Int Ed 2022, 61. [Google Scholar] [CrossRef] [PubMed]

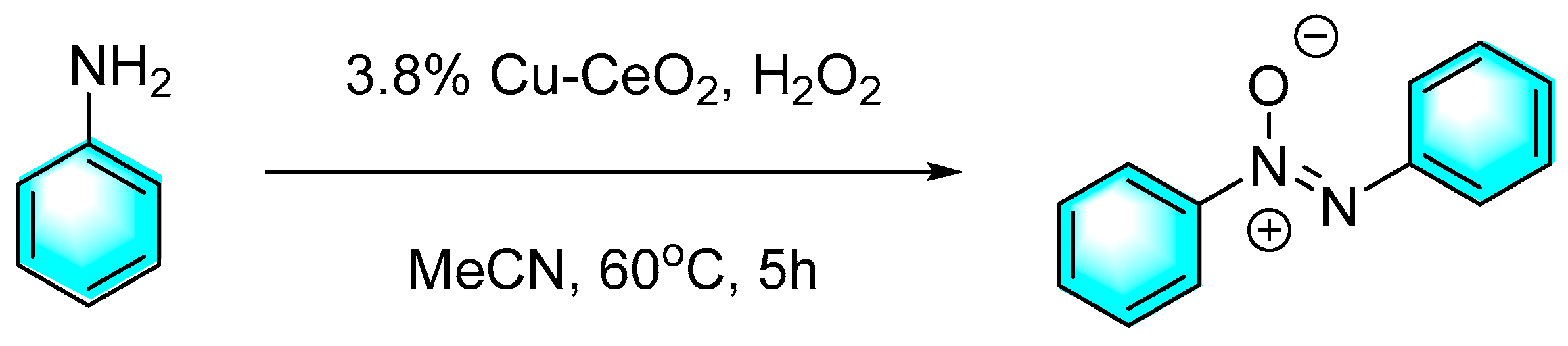










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




