Introduction
Streptococcus pneumoniae and influenza (H1N1) are common vaccinations given to the elderly general population. Infections by these organisms are associated with mortality and morbidity worldwide, and the regional incidence varies [
1]. Vaccinations against these organisms are associated with cardiovascular benefits and also a general reduction in mortality [
2,
3,
4]. Improvement in trained immunity has been postulated for non-specific protection of these vaccinations against Covid19 [
5]. The exact mechanisms of this protection. The study was performed to analyze the genome sequence overlap between these microorganisms and the human genome and to evaluate the possible modifications and influences of these vaccinations on human gene expression.
Methods
Blastn analysis [
6,
7] was performed by comparing the Streptococcus pneumoniae strain Hu17 and Haemophilus influenza (H1N1) 2009 California genome sequences against the human genome (Homo sapiens Hg38). The genome sequence of Influenza H1N1 2009 California (2009 California) was downloaded from NCBI (
https://www.ncbi.nlm.nih.gov/data-hub/taxonomy/641809/). The Influenza H1N1 2009 California genome sequences were compared against Homo sapiens hg38 downloaded from NCBI (
https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.26/) with blastn tools (2.13.0+) on a Linux server, respectively. The genome sequence of Streptococcus Pneumonia strain Hu17 (2009 California) was downloaded from NCBI (
https://www.ncbi.nlm.nih.gov/data-hub/taxonomy/641809/). The Streptococcus pneumoniae strain Hu17 genome sequences were compared against Homo sapiens hg38 downloaded from NCBI (
https://www.ncbi.nlm.nih.gov/assembly/GCF_00000 1405.26/) with blastn tools (2.13.0+) on a Linux server, respectively. The hits/alignments on the genome of Homo sapiens hg38 reported by the blast analysis were retrieved with customized Python scripts. The regions of hits were annotated with gene information, respectively.
The corresponding genes were studied for functional annotations to study background processes, which could influence physiological changes. The blast analysis results were further processed for functional analysis of the concerned genes through the database for annotation, visualization, and integrated discovery (DAVID) [
8] to understand the biological implications of the outcomes.
Results
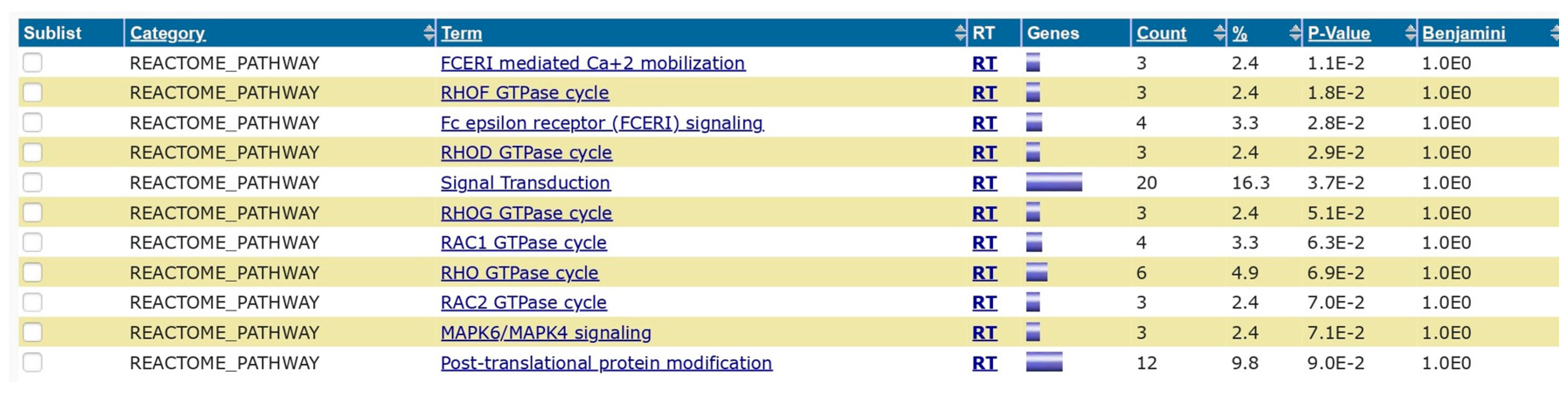
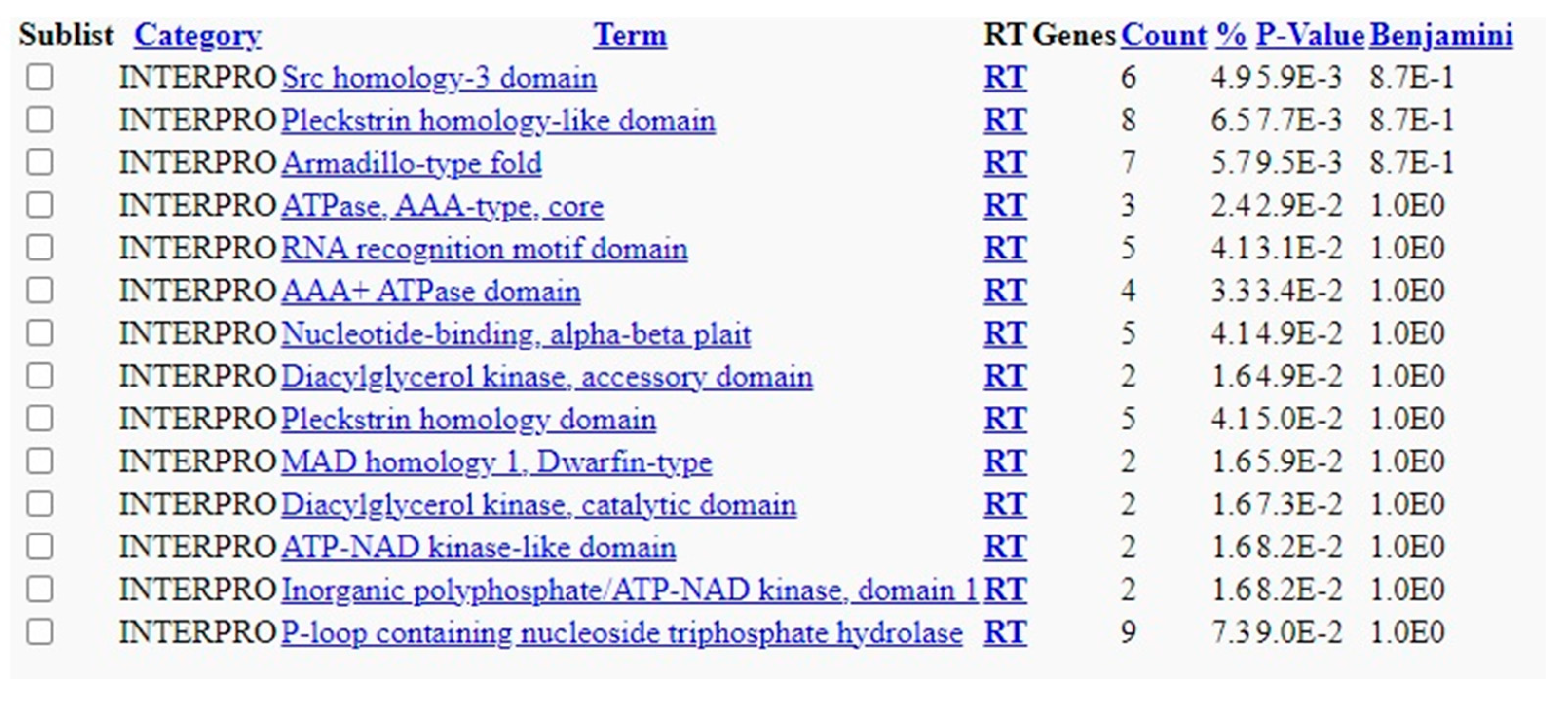
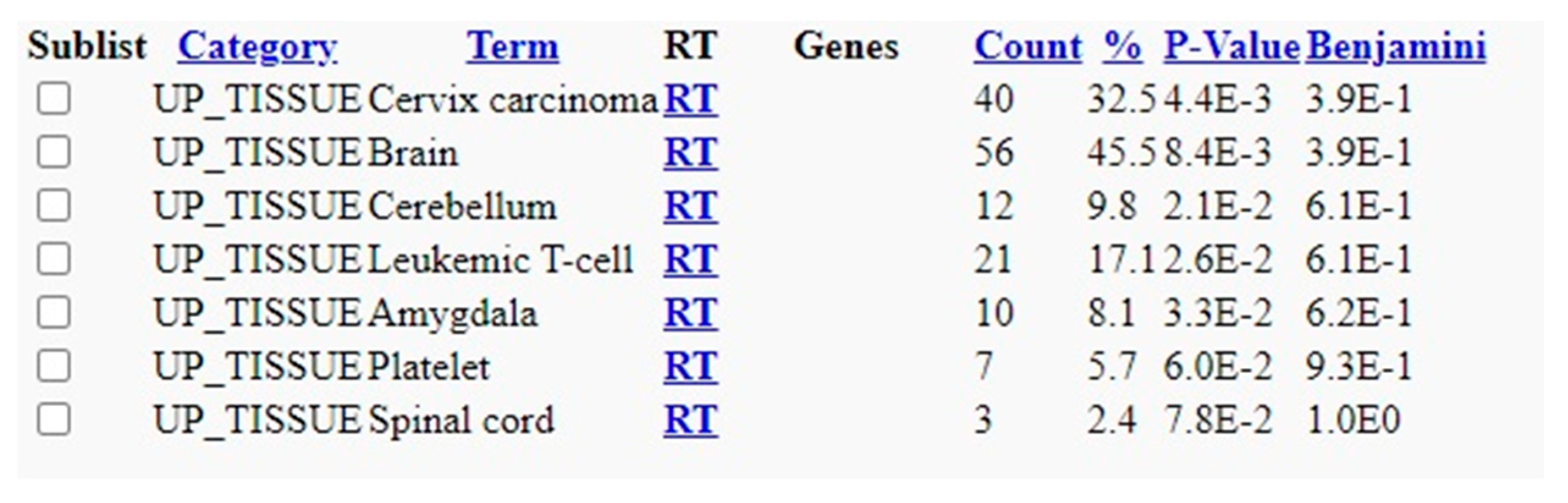
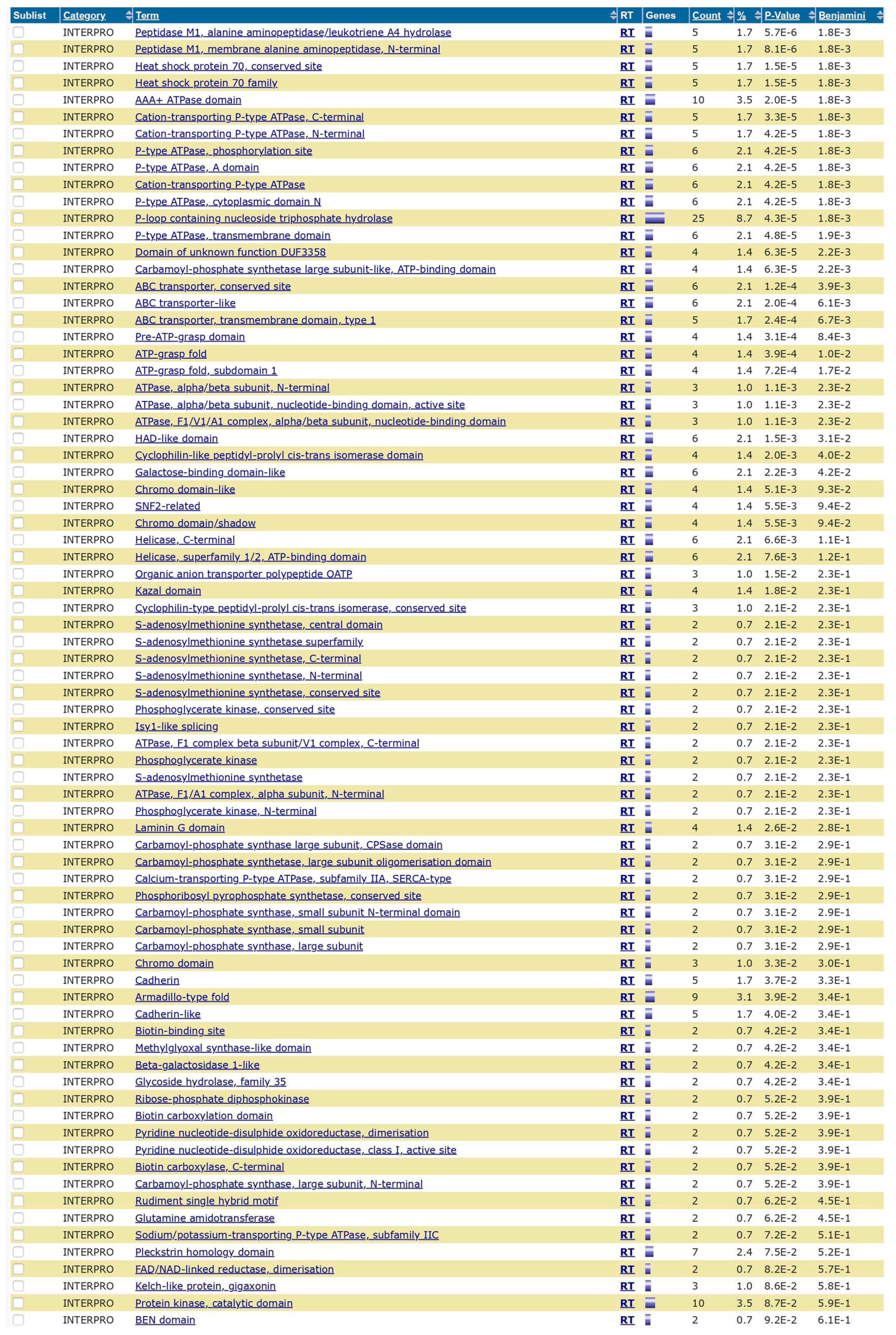
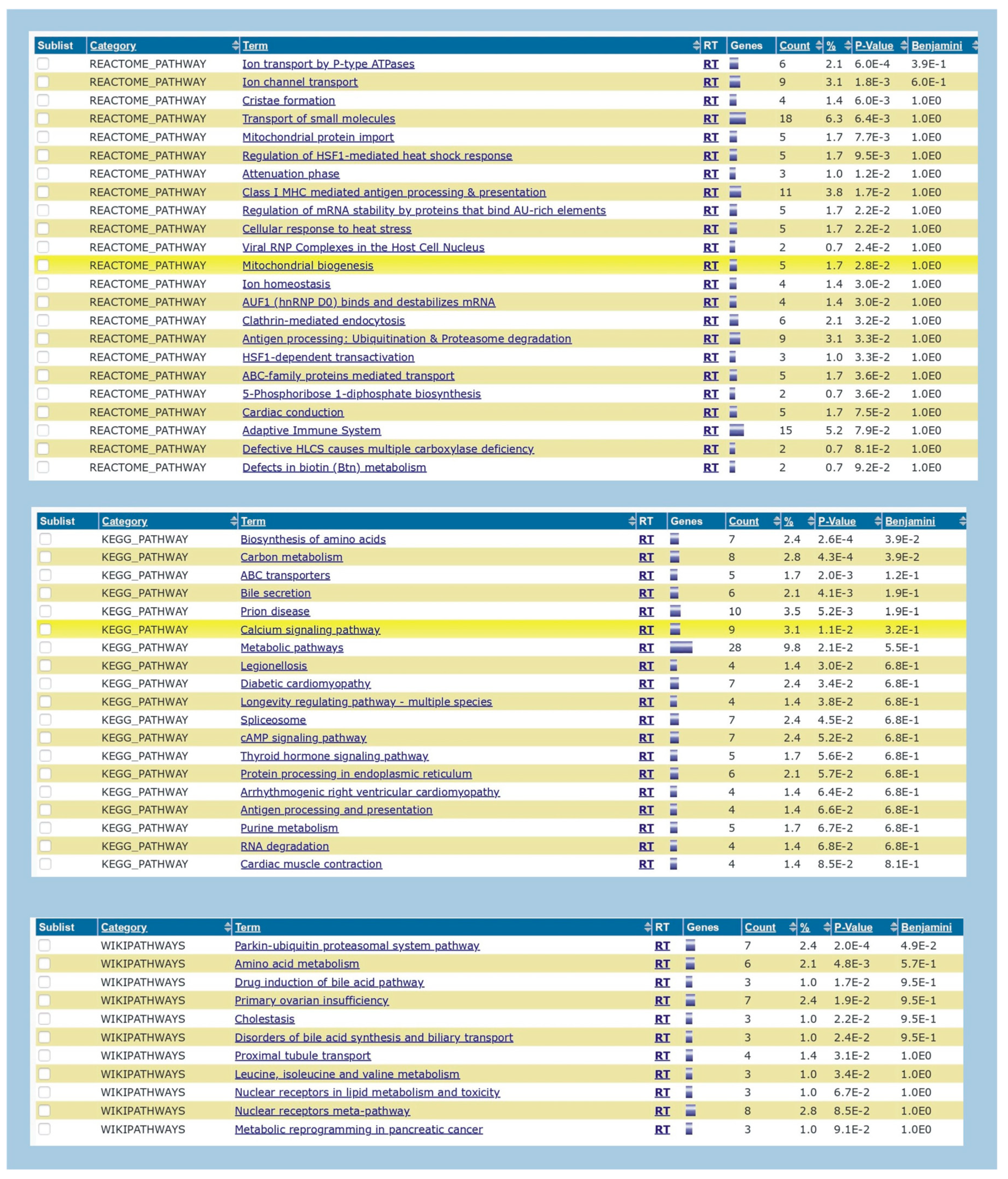
Based on the Blastn analysis, 124 hits or overlapping sequences were observed in the analysis of H1N1 vs. Homosapiens. 287 hits were observed between the overlapping segments of Streptococcus pneumoniae and Homo sapiens. The results are published in the data repository (supplement data 1 and 2). DAVID analysis showed various gene enrichment related to functions with statistical significance. The results are published in the supplement data repository (supplement data 3). The enrichment values and the level of significance of the genes are also shown in the results. A wide range of enrichment and functional annotations are observed, which give insights into the background processes after the vaccinations or infections with these organisms. The functional annotation chart with biogrid interactions (
Figure 1), genes and pathway map (
Figure 2), protein domain (
Figure 3) and tissue characterisation (
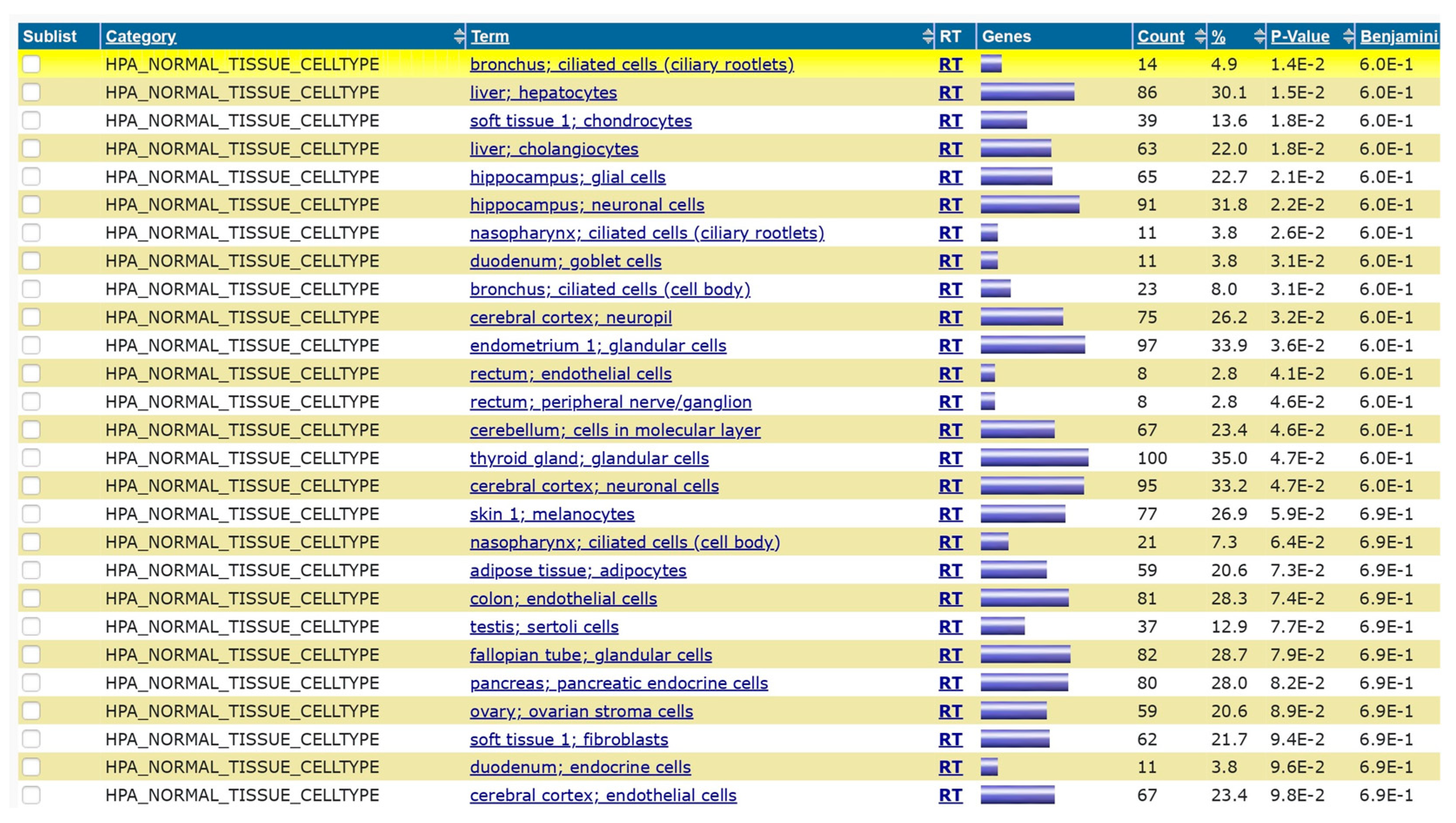
Figure 4) derived from DAVID analysis between influenza H1N1 and Homo sapiens indicate the various functional associations with associated genes. Similarly the results of DAVID analysis with Streptococcus pneumoniae and Homo sapiens show the associated protein domain (
Figure 5), pathways (
Figure 6), genes associated with tissue expression (
Figure 7) and biogrid interactions (Supplement
Figure S1).
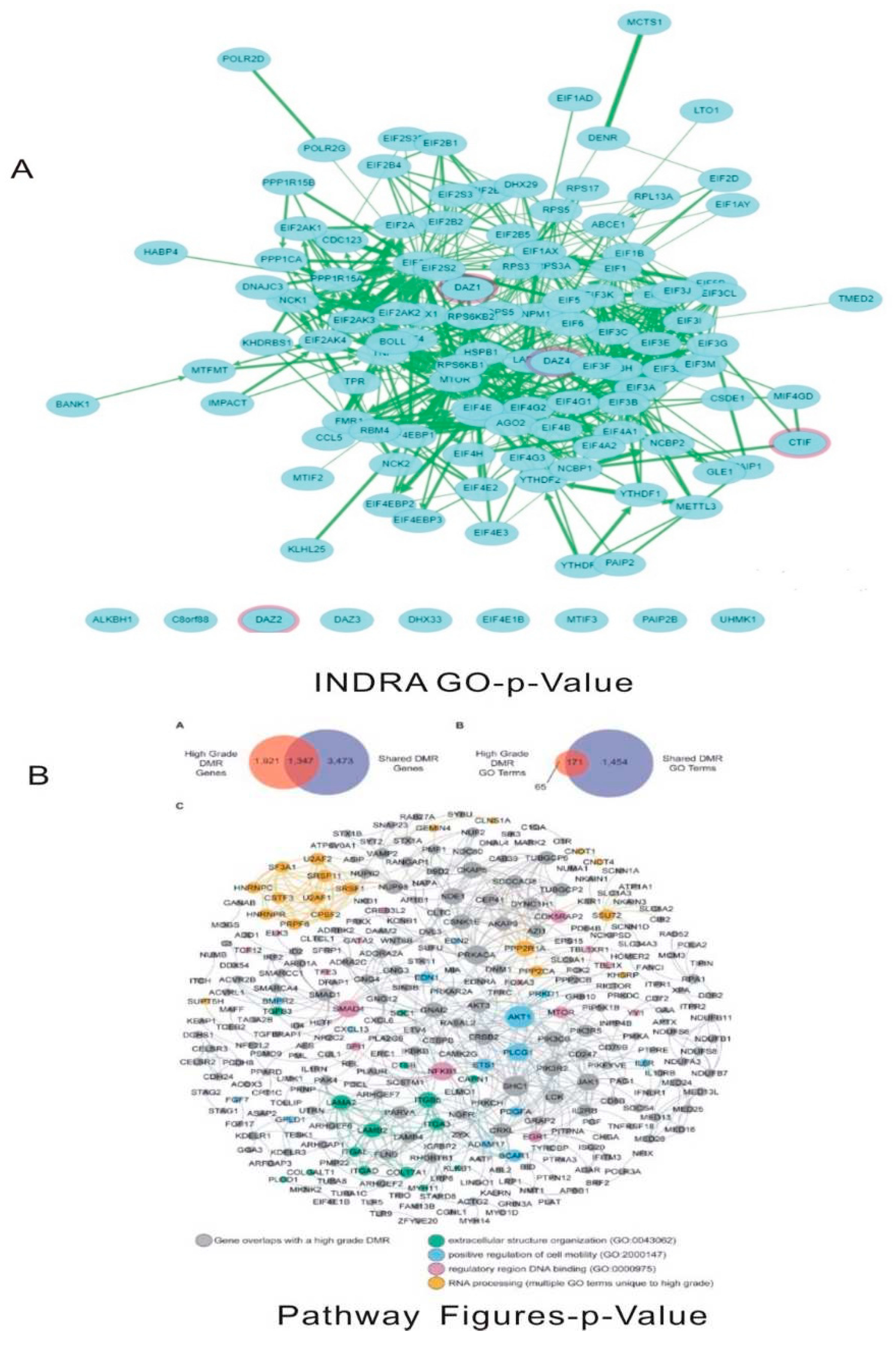
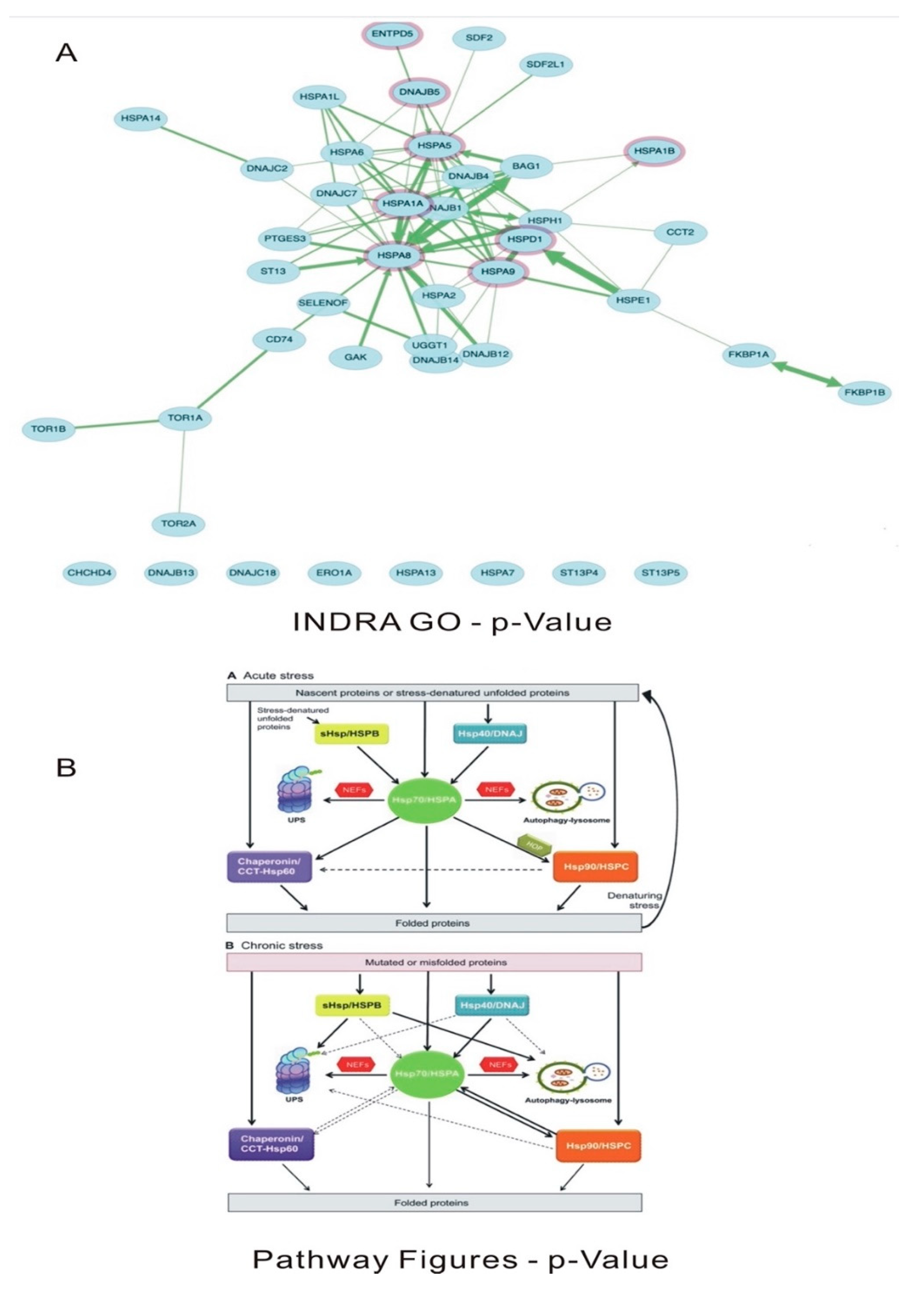
Numerically streptococcus pneumoniae has more functional interactions than influenza H1N1 virus though the extent of biological outcomes is difficult to quantify. NDEx analysis of the overlapping genes showed the pathways associated with the genes and gene ontology analysis (
Figure 8 and
Figure 9) showed the relevant associated genes (Supplement
Figures S2 and S3). Heat shock related pathways and signalling was predominantly involved with S. pneumoniae-homo sapiens overlapping genes (
Figure 9). Genes related to histone modifications, myc pathways, DNA damage check point signalling, FGF/IGF, notch signalling were associated with influenza – Homo sapiens overlap related genes (Supplement
Figure S2). PRKN, SNCA, STUB1, GPR37, SEPT, TUBA, TUBB genes related pathways concerning proteasomal degradation were associated with S. pneumonia-Homo sapiens overlapping genes (Supplement
Figure S3). Some of the other seen by pathway figures similarity were KRAS, EGFR and by INDRO GO similarity ELAVL1 and MAPKAPK2 were some of the pathways identified (Supplement
Figure S4) when analysed between influenza H1N1 Vs Homosapiens. Genes like HSF1, GCase and HSPA9 were some of the pathways identified when analysed between Streptococcus pneumoniae Vs Homosapiens (Supplement
Figure S5).
Discussion
In the comparison of the nucleotide sequences, there were many overlapping significant alignments between the influenza (H1N1) and Streptococcus pneumonia and the human genome. This could be an evolutionary or a survival process where the organisms function and induce immunological processes by molecular mimicry. This can lead to the induction of cellular processes – cytoplasmic, nuclear, and various levels as seen through functional analysis with DAVID. These vaccinations are associated with various benefits, as reported by previous studies. These vaccinations are protective against diseases like Covid-19 through changes in adaptive immunity and are also reported to have cardioprotective effects though the exact mechanisms are not understood [
9]. The countries which had high influenza or lower respiratory tract infections burden illnesses and countries which had higher influenza vaccinations, like South Korea, had significantly lower Covid 19 mortality during the pandemic [
10]. Various pathways and genes are associated with the overlapping gene. Predominantly these are inflammation, death signalling and pathways related to epigenetic modifications. Heat shock proteins are involved in normal and other cellular activities during stress, inflammation, etc. They are targets for cancer therapy, inflammation, myocardial ischemia, transplantation, and neurodegenerative diseases’ activity modulation [
11].
The PRKN gene is actively involved in the Parkin protein, which actively breaks down unnecessary proteins by tagging the damaged and excessive proteins with ubiquitin. Mutations in PRKN genes are well-established causes of early-onset parkinsonism [
12]. TUBB, TUBA are microtubule related genes, SEPT genes are related to specific polymerisation during mitosis. The CHIP protein coded by the STUB1 gene binds and inhibits ATPase activity of the chaperone proteins HSC70 and HSP70 and prevent their forward reactions [
13]. Alpha-synuclein is a protein encoded by SNCA and defects in this are associated with parkinsonism disease.GPR37 encodes [
14] G-protein coupled receptor protein and it has been shown to interact with HSPA1A and Parkin(ligase). GPR37 are receptors for glial and neuroprotective factors [
15].
As seen in
Figure 8, extracellular structure organization ITGBF, LAMA2, LAMB2, ITG A3 and ITG B5; positive regulation of cell motility through Akt1, PLCG1 pathways; regulatory region DNA binding through NFKB1, mTOR, SMAD4, EGR1 pathways; and PRPF, SRSF 1/11, CSTF pathways associated with RNA processing are some of the actively involved in cellular pathways related genes associated with influenza-homo sapiens overlap associated genes. The AKT pathway has a significant role in interacting with oncogenes and also metabolic pathways [
16]. Higher PLCG1 is associated with tumour growth and poor survival [
17].
MAPKAPK2 is involved in various cellular pathways involved in stress and inflammation, nuclear export, and gene expression regulation. It plays an important role in tumour regulation [
18,
19]. ELAVL1 primarily couples mRNA stability with the 3’ UTSs of interferon-stimulated genes [
20]. The EGFR signalling pathway is one of the most important pathways in mammalian cells, which regulates a series of important events, including proliferation, migration, differentiation, and apoptosis, as well as those that regulate intercellular communication during development. EGFR is a major gene in the pathogenesis of lung cancer [
21].
KRAS-related genes are involved in cellular growth, division, survival, and death. KRAS is also a target of active research for its regulatory molecular identification [
22]. HSF1 is a major transcription factor for heat shock proteins. HSPA9 belongs to HSP 70 gene family. This plays a role in cellular proliferation, stress response, and maintenance of mitochondria. HSPA9 is active in regulating apoptosis also. HSP 27 was shown to be a substrate of this kinase in vivo [
23]. GCase catalyzes the cleavage of major glycolipid glucosylceramide (GL-1) into glucose and ceramide and the minor lipid glucosphingosine into sphingosine and water [
24].
Further studies involving the protein encoded by these overlapping nucleotide sequences would provide more information about the changes or induction of the cellular functions. Similarly, other closely associated microorganisms like commensals can also be studied to better understand the cause-effect/associations of various disease pathology in humans, such as autoimmune disorders. Incidence of non-communicable diseases like coronary artery disease, diabetes, etc., is also interesting to study if there is any influence by the microbiological changes.
Conclusion
The study shows overlapping nucleotide sequences between the human genome and Streptococcus pneumoniae and Influenza (H1N1) virus genome sequences. These overlapping sequences are also associated with various functional annotations. Further evaluations can help to understand the modifications of the encoded proteins and metabolism during these infections.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
MCA conceived the idea, performed the analysis in DAVID and NDEx, and wrote the paper. JW, JL, RH performed the Blastn analysis and derived the results in Blastn.
Conflicts of Interest
None.
References
- Arokiaraj, MC. Correlation of influenza vaccination and influenza incidence on COVID-19 severity and other perspectives. Available at SSRN 3572814. 2020 Apr 10.
- Behrouzi B, Bhatt DL, Cannon CP, et al. Association of Influenza Vaccination With Cardiovascular Risk: A Meta-analysis. JAMA Netw Open. 2022;5(4):e228873. [CrossRef]
- Jaiswal V, Ang SP, Lnu K, Ishak A, Pokhrel NB, Chia JE, Hajra A, Biswas M, Matetic A, Dhatt R, Mamas MA. Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J Clin Med. 2022 Jun 30;11(13):3799. [CrossRef]
- Hannah Chung and others, Influenza Vaccine Effectiveness Against All-Cause Mortality Following Laboratory-Confirmed Influenza in Older Adults, 2010–2011 to 2015–2016 Seasons in Ontario, Canada, Clinical Infectious Diseases, Volume 73, Issue 5, 1 September 2021, Pages e1191–e1199. [CrossRef]
- Debisarun PA, Gössling KL, Bulut O, Kilic G, Zoodsma M, Liu Z, Oldenburg M, Rüchel N, Zhang B, Xu CJ, Struycken P. Induction of trained immunity by influenza vaccination-impact on COVID-19. PLoS pathogens. 2021 Oct 25;17(10):e1009928.
- https://guides.lib.berkeley.edu/ncbi/blast.
- Lobo, I. (2008) Basic Local Alignment Search Tool (BLAST). Nature Education 1(1):21.
- DAVID Brad T Sherman and others, DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update), Nucleic Acids Research, Volume 50, Issue W1, 5 July 2022, Pages W216–W221. 5 July. [CrossRef]
- Wilcox CR, Islam N, Dambha-Miller H. Association between influenza vaccination and hospitalisation or all-cause mortality in people with covid-19: A retrospective cohort study. BMJ Open Respiratory Research. 2021;8(1). [CrossRef]
- Arokiaraj, MC. Considering Interim Interventions to Control COVID-19 Associated Morbidity and Mortality-Perspectives. Front Public Health. 2020 Sep 22;8:444. [CrossRef]
- Dubey A, Prajapati KS, Swamy M, Pachauri V. Heat shock proteins: a therapeutic target worth to consider. Vet World. 2015 Jan;8(1):46-51. [CrossRef]
- Castelo Rueda MP, Raftopoulou A, Gögele M, Borsche M, Emmert D, Fuchsberger C, Hantikainen EM, Vukovic V, Klein C, Pramstaller PP, Pichler I and Hicks AA (2021). Frequency of Heterozygous Parkin (PRKN) Variants and Penetrance of Parkinson's Disease Risk Markers in the Population-Based CHRIS Cohort. Front. Neurol. 12:706145. [CrossRef]
- Zhang, S. , Hu, Zw., Mao, Cy. et al. CHIP as a therapeutic target for neurological diseases. Cell Death Dis 11, 727 (2020). [CrossRef]
- Guo Y, Sun Y, Song Z, Zheng W, Xiong W, Yang Y, Yuan L, Deng H. Genetic Analysis and Literature Review of SNCA Variants in Parkinson's Disease. Front Aging Neurosci. 2021 Aug 12;13:648151. [CrossRef]
- Meyer RC, Giddens MM, Schaefer SA, Hall RA. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proceedings of the National Academy of Sciences. 2013;110(23):9529–34. [CrossRef]
- Nitulescu G, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, et al. The AKT pathway in oncology therapy and beyond (review). International Journal of Oncology. 2018; [CrossRef]
- Li, T. , Yang, Z., Li, H. et al. Phospholipase Cγ1 (PLCG1) overexpression is associated with tumor growth and poor survival in IDH wild-type lower-grade gliomas in adult patients. Lab Invest 102, 143–153 (2022). [CrossRef]
- Yang L, Liu B, Qiu F, Huang B, Li Y, Huang D, et al. The effect of functionalmapkapk2copy number variation CNV-30450 on elevating nasopharyngeal carcinoma risk is modulated by EBV infection. Carcinogenesis. 2013;35(1):46–52. [CrossRef]
- Soni, S. , Anand, P. & Padwad, Y.S. MAPKAPK2: the master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J Exp Clin Cancer Res 38, 121 (2019). [CrossRef]
- Rothamel K, Arcos S, Kim B, Reasoner C, Lisy S, Mukherjee N, et al. ELAVL1 primarily couples mRNA stability with the 3′ utrs of interferon-stimulated genes. Cell Reports. 2021;35(8):109178. [CrossRef]
- Carcereny E, Morán T, Capdevila L, Cros S, Vilà L, de Los Llanos Gil M, Remón J, Rosell R. The epidermal growth factor receptor (EGRF) in lung cancer. Transl Respir Med. 2015 Feb 24;3:1. [CrossRef]
- Huang, L. , Guo, Z., Wang, F. et al. KRAS mutation: from undruggable to druggable in cancer. Sig Transduct Target Ther 6, 386 (2021). [CrossRef]
- Liu T, Krysiak K, Shirai CL, Kim S, Shao J, Ndonwi M, et al. (2017) Knockdown of HSPA9 induces TP53-dependent apoptosis in human hematopoietic progenitor cells. PLoS ONE 12(2): e0170470. [CrossRef]
- HSC 70 Wang Z, Li Y, Yang X, Zhao J, Cheng Y and Wang J (2020) Mechanism and Complex Roles of HSC70 in Viral Infections. Front. Microbiol.11:1577. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).