Submitted:
01 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
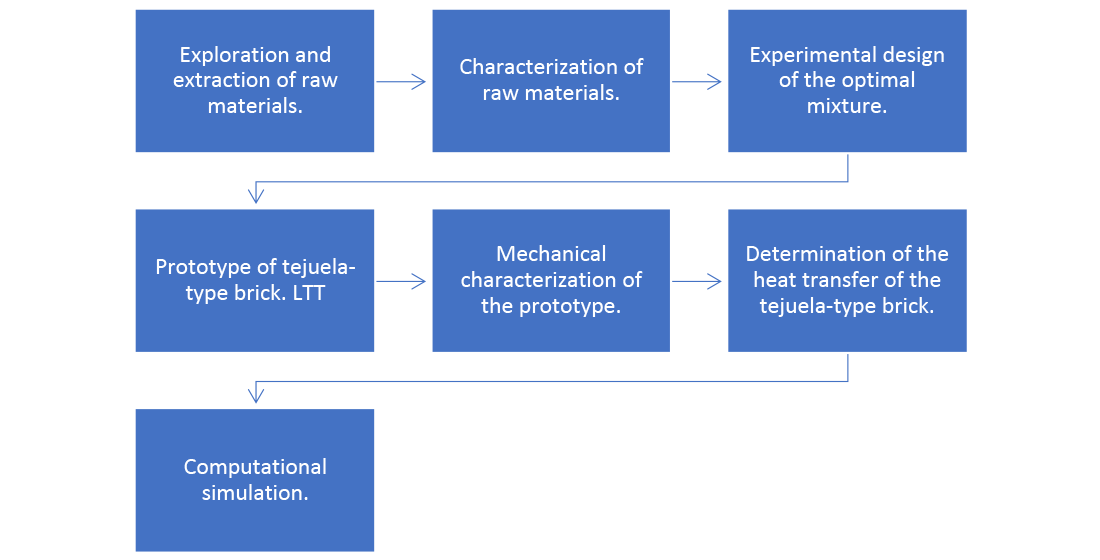
2. Materials y methods
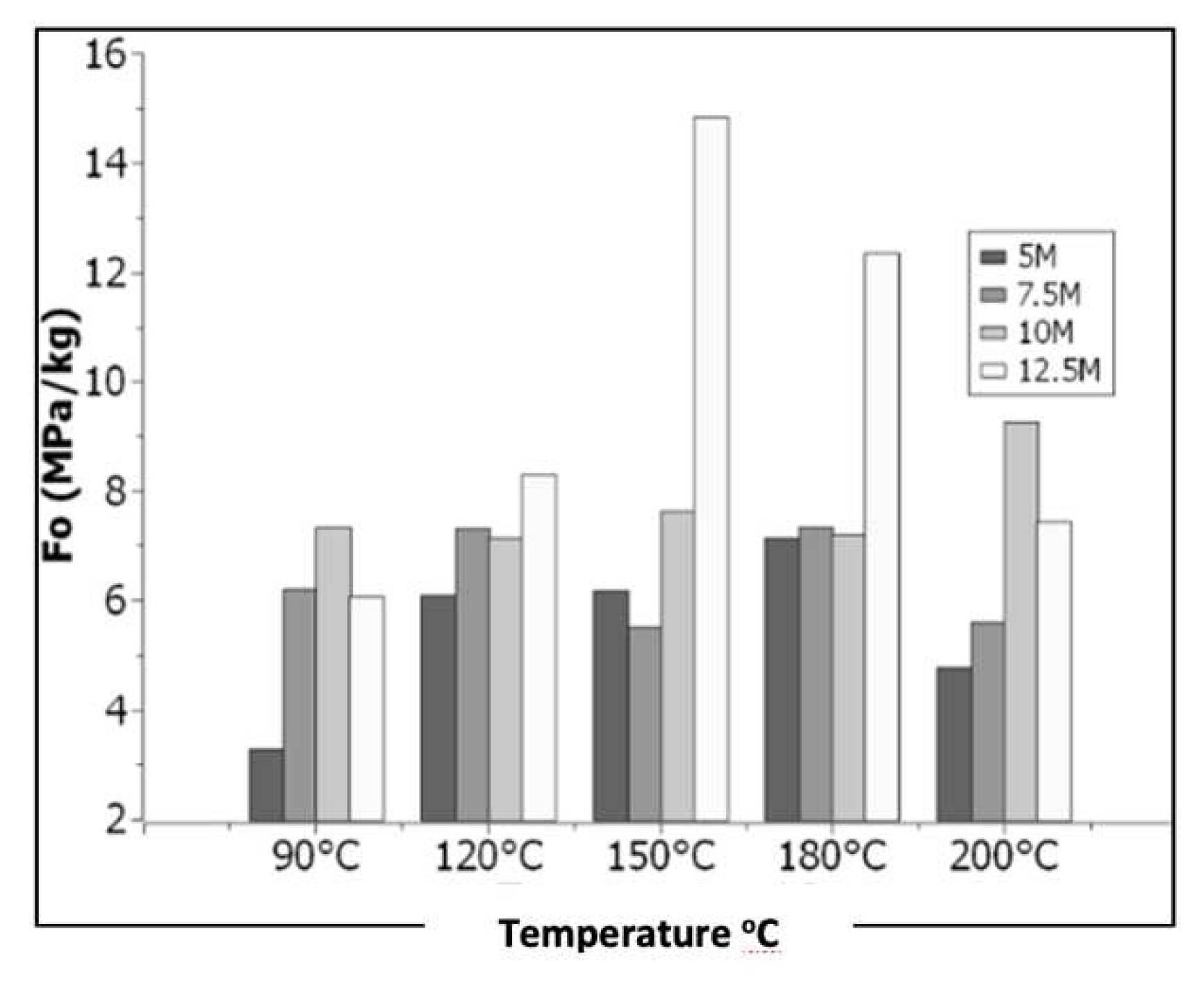
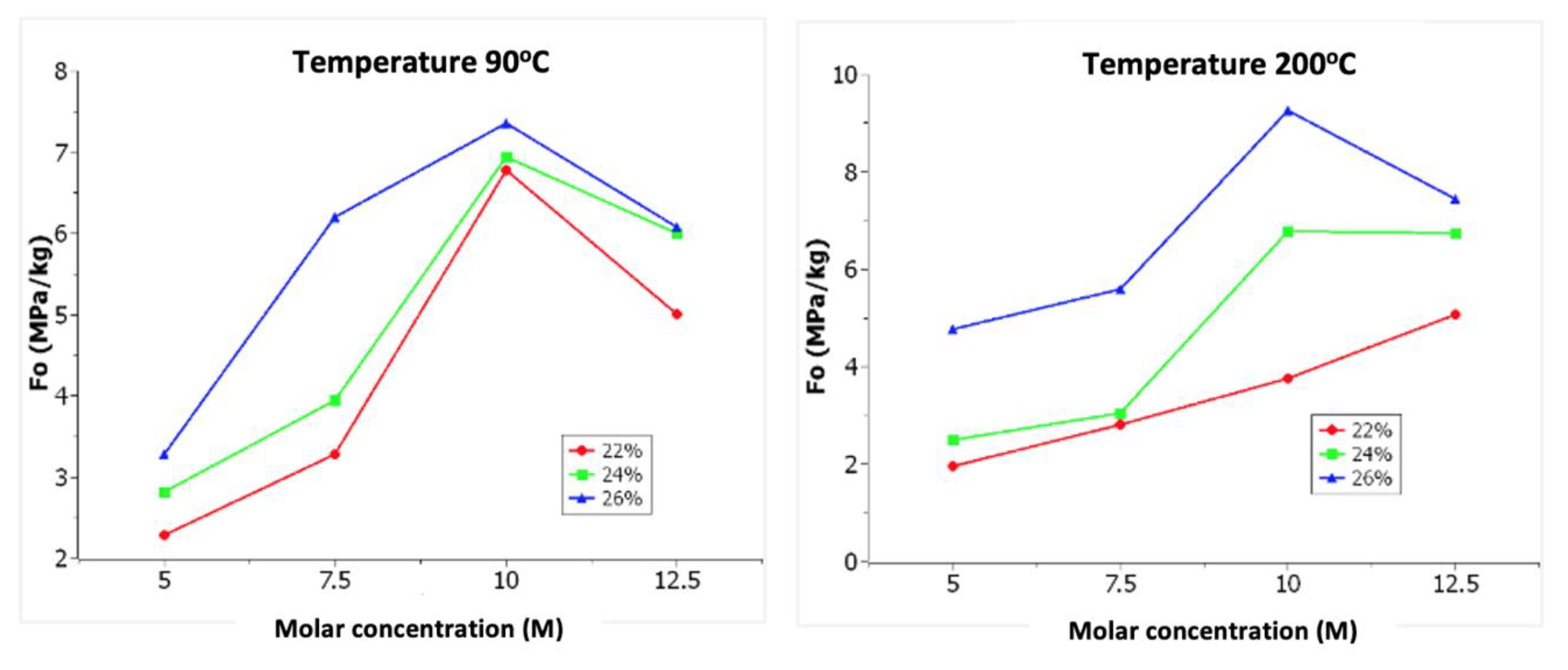
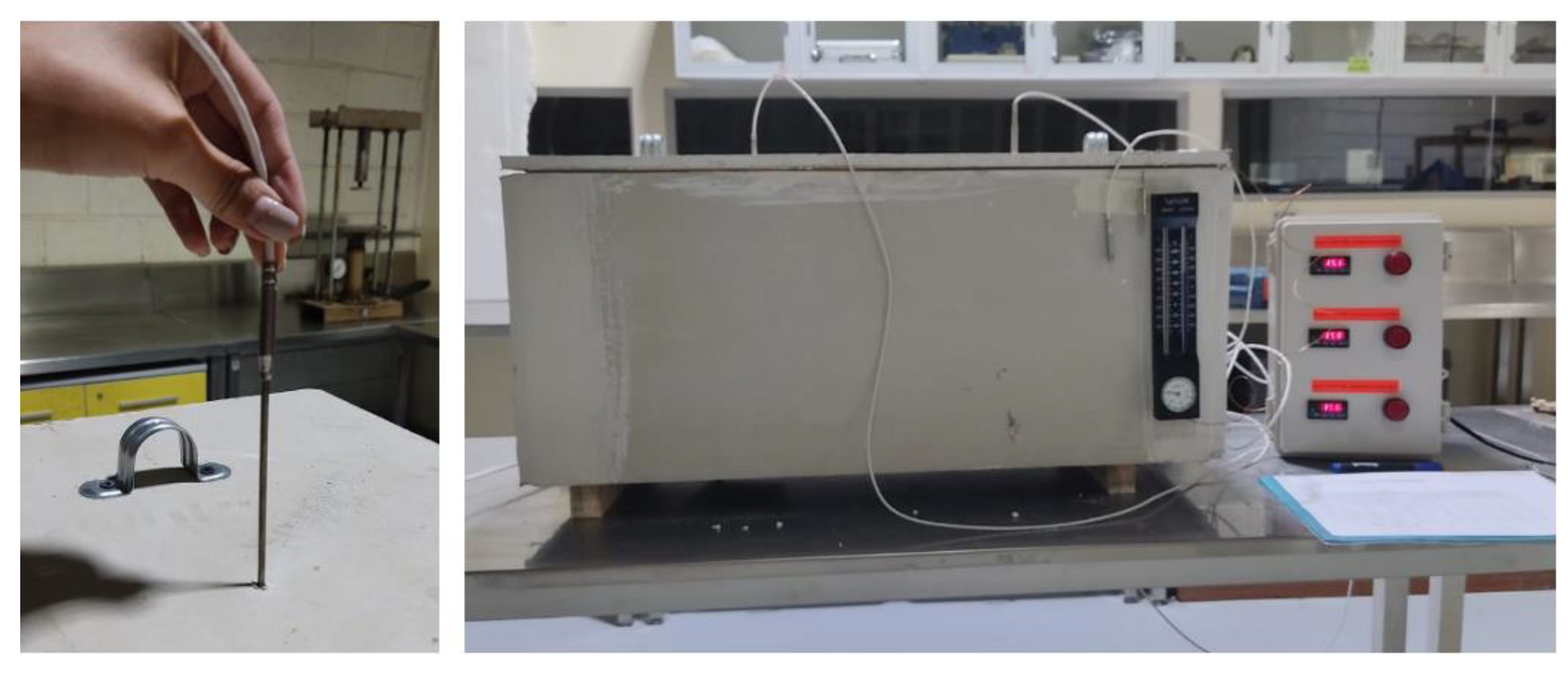
3. Results
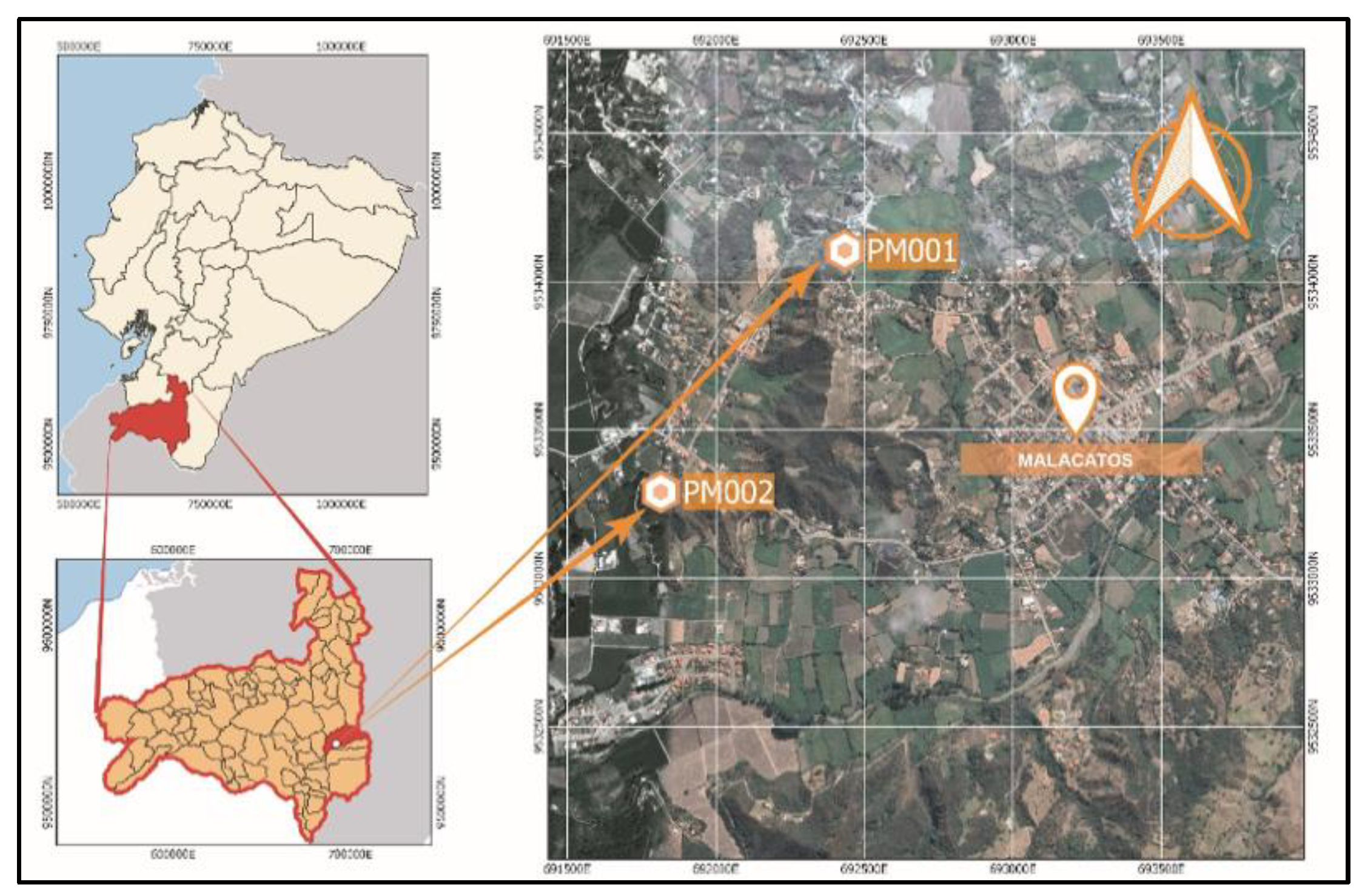
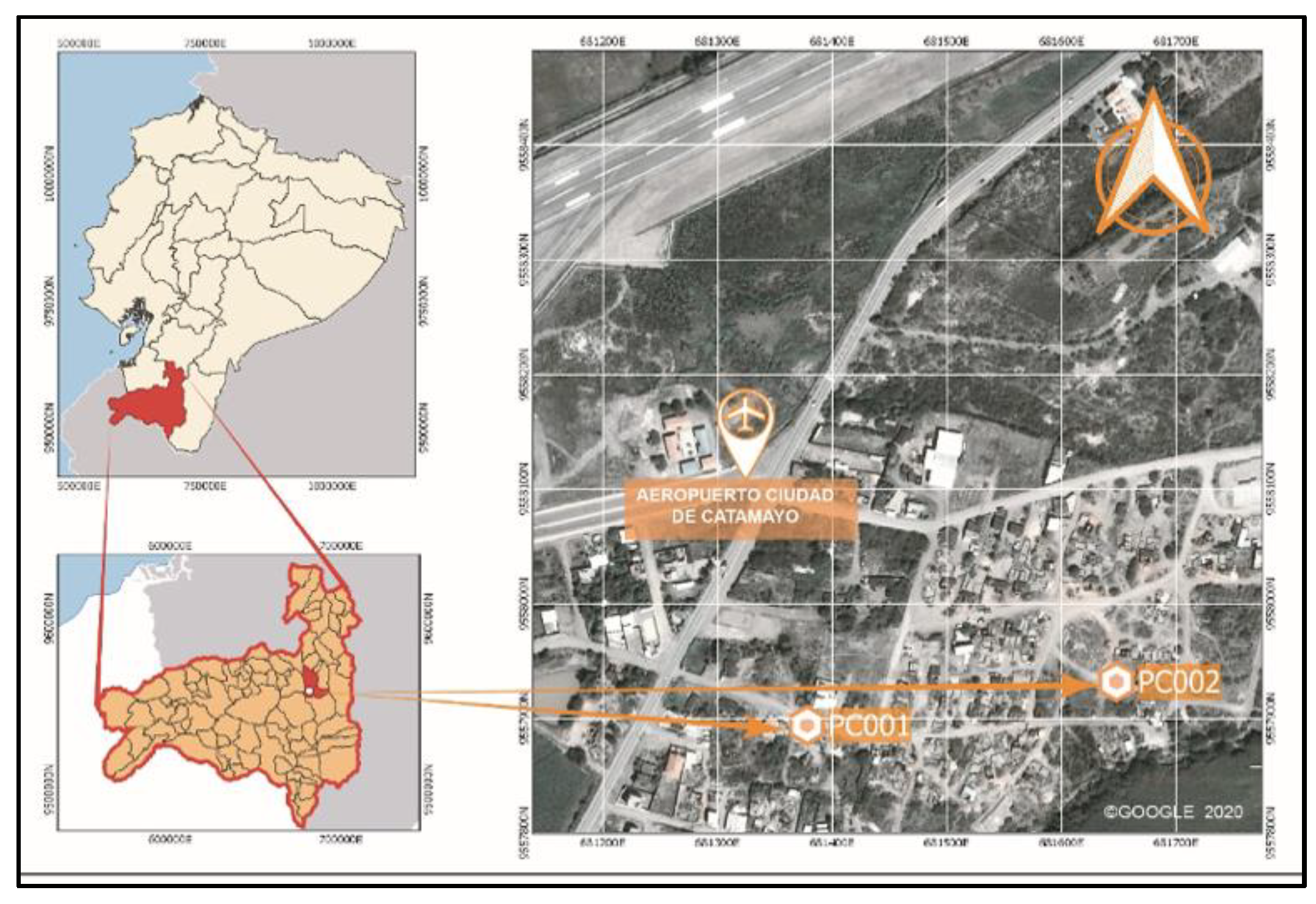
3.1. Experimental Process
3.2. X-ray Fluorescence (XRF)2
3.3. X-ray diffraction

4. Discussion
4.1. Measurement of heat transfer12
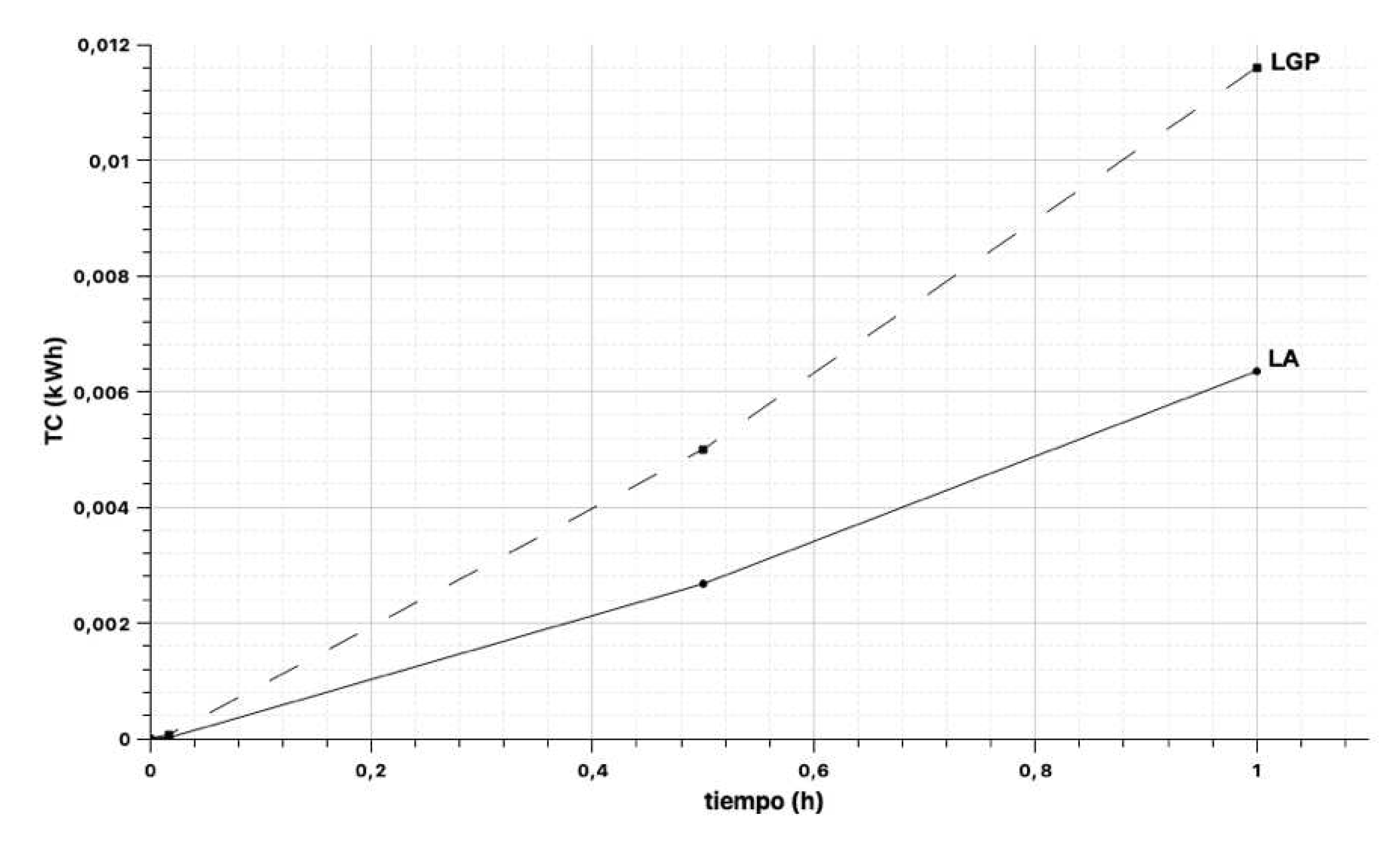
| Sample code | SE-T1 (°C) | SR-T2 (°C) | TE (°C) | time t (h) | Conductivityλ (W/m·K) | Material area A (m2) | Material thickness e (m) | Heat transfer coefficient Q´ (kW) | Heat transfer Q (kWh) |
|---|---|---|---|---|---|---|---|---|---|
| LGP-1 | 16,70 | 15,60 | 16,40 | 0,017 | 0,954 | 0,090 | 0,020 | 0,0047 | 0,000052 |
| LGP-1 | 19,00 | 15,50 | 15,60 | 0,500 | 0,954 | 0,090 | 0,020 | 0,0098 | 0,004883 |
| LGP-1 | 19,80 | 15,60 | 15,50 | 1,000 | 0,954 | 0,090 | 0,020 | 0,0117 | 0,011718 |
| LGP-2 | 17,40 | 15,70 | 15,80 | 0,017 | 0,954 | 0,092 | 0,021 | 0,0046 | 0,000077 |
| LGP-2 | 19,40 | 15,60 | 15,80 | 0,500 | 0,954 | 0,092 | 0,021 | 0,0102 | 0,005088 |
| LGP-2 | 19,90 | 15,60 | 15,70 | 1,000 | 0,954 | 0,092 | 0,021 | 0,0115 | 0,011515 |
| LA-1 | 19,80 | 19,40 | 19,90 | 0,017 | 0,800 | 0,058 | 0,043 | 0,0004 | 0,000007 |
| LA-1 | 25,10 | 19,00 | 19,40 | 0,500 | 0,800 | 0,058 | 0,043 | 0,0066 | 0,003291 |
| LA-1 | 26,10 | 19,00 | 19,00 | 1,000 | 0,800 | 0,058 | 0,043 | 0,0077 | 0,007661 |
| LA-2 | 20,40 | 19,40 | 19,70 | 0,017 | 0,800 | 0,058 | 0,042 | 0,0011 | 0,000019 |
| LA-2 | 23,00 | 19,30 | 19,40 | 0,500 | 0,800 | 0,058 | 0,042 | 0,0041 | 0,002044 |
| LA-2 | 24,00 | 19,50 | 19,30 | 1,000 | 0,800 | 0,058 | 0,042 | 0,0050 | 0,004971 |
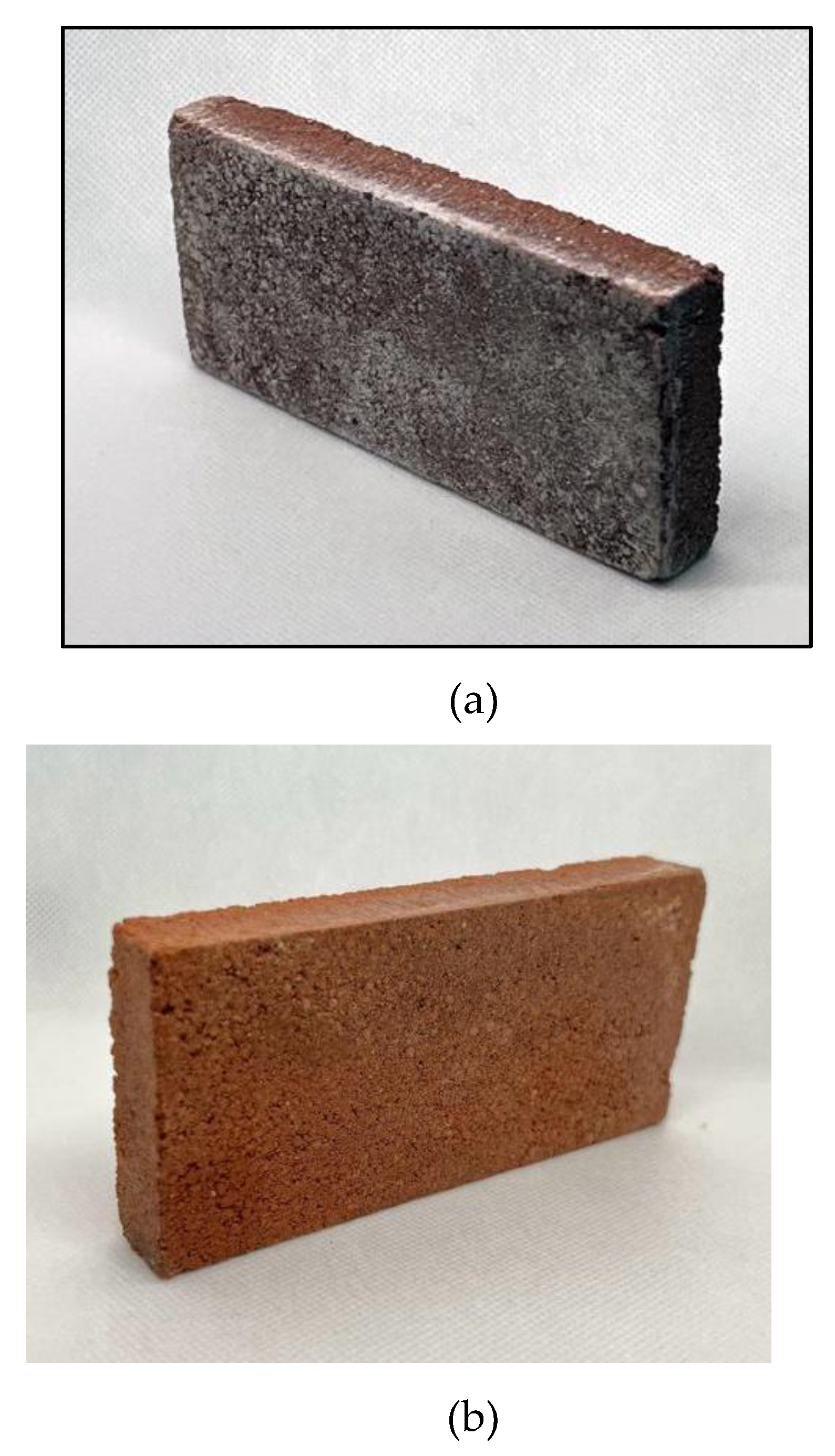
4.2. Analysis of efflorescence in LTT
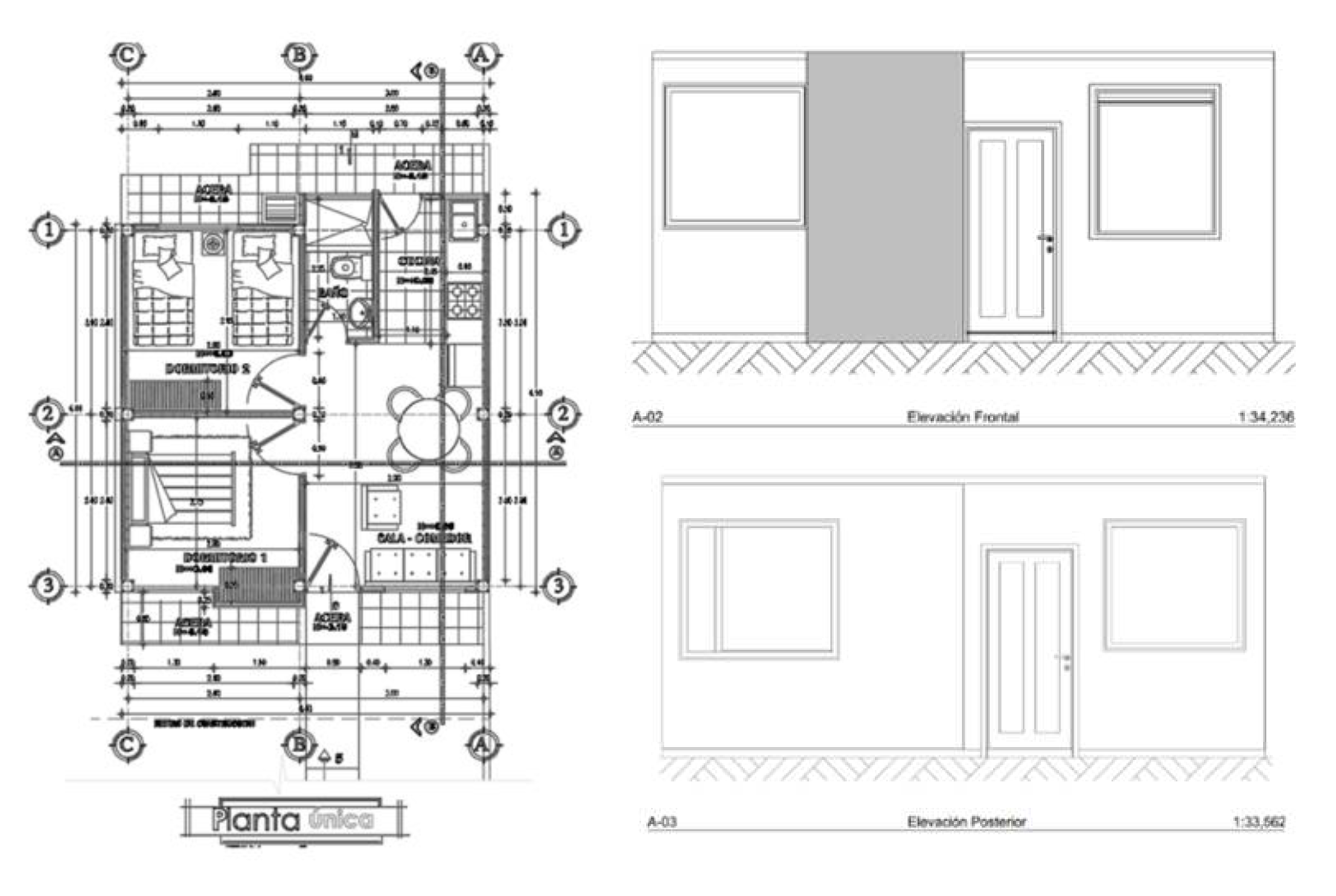
| Typology I | Space distribution | area(m²) |
| Living room–dining room - kitchen | 14.49 | |
| bathroom | 1.87 | |
| bedroom 1 | 9.54 | |
| bedroom 2 | 8.20 |
| Construction package | Components | Thickness(cm) | Conductivity(W/ºK) | Infiltration level(l/sm²) | |
|---|---|---|---|---|---|
| roofing | reinforced concrete slab | reinforced concrete | 7 | 2.3 | 4,40 |
| walls | concrete block | exterior plastering | 1 | 0.5 | 4.40 |
| concrete block | 10 | 0.62 | |||
| interior plastering | 1 | 0.72 | |||
| floor | wood | Hardwood | 0.7 | 0.18 | 1.10 |
| ceramic | Ceramic | 0.5 | 1.75 | 1.10 | |
| doors | metal | Steel | 0.03 | 50 | 4 |
| air (R0.15m²k/w) | 0.1 | ||||
| Steel | 0.03 | ||||
| wood | painted oak | 4.2 | 0.19 | ||
| windows | single-pane glass | clear glass | 0.3 | 0.9 | 4 |
| Construction package | Components | Thickness (cm) | Conductivity (W/m2K) | Current U-value (W/m2K) | Reference U-value (W/m2K) | Compliance with NEC | |
|---|---|---|---|---|---|---|---|
| unconditioned living space ceilings | e=8.2 cm | reinforced concrete | 7.0cm | 2.3 | 4.7 | 2.35 | Does not comply |
| plastering | 1.2cm | 0.81 | |||||
| Walls of habitable spaces- non – conditioned – above ground level | e=12.0cm | plastering | 1.0cm | 0.116 | 2.89 | 2.90 | Complies |
| concrete block | 10cm | 0.62 | |||||
| plastering | 1.0cm | 0.116 | |||||
| Floors of habitable spaces – non - conditioned | e=30cm | ceramic | 0.7cm | 1.75 | 2.82 | 3.2 | Complies |
| subfloor | 8cm | 2.50 |
| elevation | total facade area (m²) | area of glazed surface (m2) | percentage of glazed surface (%) | compliance with NEC–HS-EE |
|---|---|---|---|---|
| Front elevation | 15.12 | 3.29 | 27.1% | Complies |
| Rear elevation | 12.12 | 3.58 | 23.6% | Complies |
 |
| Building envelope - Type I residential unit | |||||
|---|---|---|---|---|---|
| Typology | Component | Current U-value(W/m²K) | Required U-value.(W/m²K) | Compliance | |
| roofing | concrete slab 7cm thick | 4.7 | 2.35 | Does not comply | |
| walls | concrete block | 2.89 | 2.90 | Complies | |
| Intermediate floor | ceramic | 2.82 | 3.20 | Complies | |
4.3. Results of the application

| Building envelope, Typology I Residence | |||||
|---|---|---|---|---|---|
| Typology | Component | Current U value(W/m²k) | Required value.(W/m²k) | Compliance | |
| roofing | concrete slab 7cmthick | 0.643 | U-2.35 | Complies | |
| walls | concrete block | 2.89 | U-2.90 | Complies | |
| floor | ceramic | 2.31 | U-3-20 | Complies | |
5. Conclusions
References
- INEC. Instituo Nacional de Estadisticas y Censos; p. 2013.
- Wong, C.L.; Mo, K.H.; Yap, S.P.; Alengaram, U.J.; Ling, T. C. Potential use of brick waste as alternate concrete-making materials: A review. J Clean Prod 2018, 195, 226–239. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, no. January 2008, 3rd ed.; p. 2011.
- Amin, S.K.; El-Sherbiny, S.A.; El-Magd, A.A.M.A.; Belal, A.; Abadir, M. F. Fabrication of geopolymer bricks using ceramic dust waste. Constr Build Mater 2017, 157, 610–620. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Yap, S.P.; Alengaram, U.J.; Ling, T. C. Potential use of brick waste as alternate concrete-making materials: A review. J Clean Prod 2018, 195, 226–239. [Google Scholar] [CrossRef]
- Sore, S.O.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Synthesis and characterization of geopolymer binders based on local materials from Burkina Faso – Metakaolin and rice husk ash. Constr Build Mater 2016, 124, 301–311. [Google Scholar] [CrossRef]
- Pardo, N.; Penagos, G.; Correa, M.; López, E. Desarrollo de morteros de bajo impacto ambiental a partir de residuos sílico-aluminosos activados alcalinamente del sector minero. Boletín de la Sociedad Española de Cerámica y Vidrio 2021. [Google Scholar] [CrossRef]
- Mustafa al Bakri, A. M.; et al. Geopolymerization method for soil stabilization application. US 2015/0016895 A1, 2015. [Google Scholar]
- García, V.J.; Márquez, C.O.; Zúñiga-Suárez, A.R.; Zuñiga-Torres, B.C.; Villalta-Granda, L. J. Brazilian Test of Concrete Specimens Subjected to Different Loading Geometries: Review and New Insights. Int J Concr Struct Mater 2017, 11. [Google Scholar] [CrossRef]
- Lv, X.; Qin, Y.; Lin, Z.; Tian, Z.; Cui, X. Inhibition of Efflorescence in Na-Based Geopolymer Inorganic Coating. ACS Omega 2020, 5, 14822–14830. [Google Scholar] [CrossRef] [PubMed]
- Faisal, N.H. Diametral compression test method to analyse relative surface stresses in thermally sprayed coated and uncoated circular disc specimens. Surf Coat Technol 2019, 357, 497–514. [Google Scholar] [CrossRef]
| 1 | This test will be based on the technique defined as the "Brazilian disc test," which has been introduced in the literature as a substitute for direct tensile testing in brittle materials [11]. |
| 2 | Reviewed inhttps://hal-univ-tlse2.archives-ouvertes.fr/hal-02017682/document, 16/07/2022. |
| 3 | Rodríguez, Erich, & Bernal, Susan, & Mejía de Gutiérrez, Ruby, & Gordillo, Marisol (2009). Effect of SiO2/Al2O3 and Na2O/SiO2 modules on the properties of geopolymers based on a metakaolin. Revista Facultad de Ingeniería Universidad de Antioquia, (49), 30-41. [Consultation date: July 12, 2022]. ISSN: 0120-6230. Available at: https://www.redalyc.org/articulo.oa?id=43019324003
|
| 4 | Retsch BB100 |
| 5 | Retsch RS 200 |
| 6 | D8-ADVANCE |
| 7 |
To determine the amount of NaOH needed to dissolve for the alkaline solution, we need to calculate it by multiplying 12.5 M by 40 g/mol (molecular weight), considering that it will be dissolved in 1000 ml of distilled water.
|
| 8 | Zúñiga Suárez, Alonso (2018). Science and engineering of new materials in the manufacture of technologically improved bricks. Thesis (Doctoral), E.T.S. Architecture (UPM). https://doi.org/10.20868/UPM.thesis.52643. |
| 9 | To prepare a soil-solution mixture by creating 4 specimens with a diameter of 7 cm and a thickness of 2 cm, which will be subjected to a molding pressure of 39.2266N. The solution content is 22%. It is known that the amount of soil required for the fabrication of the 4 specimens is 411 grams. How to calculate the amount of solution needed? |
| 10 | Reviewed on https://www.normalizacion.gob.ec/buzon/normas/297.pdf, on 16/07/2022. |
| 11 | Reviewed on https://www.normalizacion.gob.ec/buzon/normas/2554.pdf, on 16/07/2022. |
| 12 | Fonseca Diaz, N., & Tibaquirá, J. E. (2008). Method for the experimental estimation of thermal conductivity of some common materials in Colombia for HVAC/R applications. Scientia Et Technica, 2(39). https://doi.org/10.22517/23447214.3185. This article presents the experimental development implemented to estimate the thermal conductivity of some materials used in the field of refrigeration and air conditioning (HVAC/R). |
| 13 | Reviewed on https://www.thermal-engineering.org/es/que-es-la-ley-de-conduccion-termica-de-fourier-definicion/, on 18/10/2022. |
| 14 | To determine the heat transfer experiment, we have based this study on Fourier's law of thermal conduction, which considers a flat wall of thickness e and an average thermal conductivity λ. |
| 15 | Ecuadorian Construction Standard. Habitability and Health. Energy Efficiency. |
| 16 | From the climate file of the city of Loja, obtained from the weather station "Loja La Argelia LJ ECU," it is determined that the Heating Degree Days (HDD) have a value of 588, considering a base temperature for heating of 18°C. On the other hand, the Cooling Degree Days (CDD) have a value of 2339, with a base temperature of 10°C, following the ASHRAE standard as a thermal criterion for climate classification. Analyzing the HDD 18 and CDD 10 data from the weather station and Table 1 of the NEC-HS-EE (page 12), it was determined that the city of Loja belongs to Climate Zone 3, within Zone 3C according to ASHRAE 90.1 standard, referred to as CONTINENTAL LLUVIOSA, meeting the thermal criterion of CDD10°C≤2500 and HDD18°C≤ 2000. |
| 17 | It shows, based on the geographical location, whether the building has heating or cooling requirements, or both. It is also defined as the temperature difference between the average outdoor air temperature over a 24-hour period and a specified base temperature. |
| 18 | |
| 19 | Created by the author using Climate Consultant 6.0 software, based on the ECU_LJ_Loja-La.Argelia.842700_TMYx.2004-2018.epw file. Retrieved from https://climate.onebuilding.org/WMO_Region_3_South_America/ECU_Ecuador/LJ_Loja/ECU_LJ_Loja-La.Argelia.842700_TMYx.2007-2021.zip. |
| 20 | It is considered in spaces with natural ventilation where occupants can open and close windows. Its thermal response depends in part on the external climate and may have a wider comfort range than in buildings with centralized HVAC systems. This model assumes that occupants adapt their clothing to the thermal conditions and are sedentary (1.0 to 1.3 MET). There should not be a mechanical cooling system in place, and this method is not applicable if a mechanical heating system is operating. Additionally, it considers a comfort range of 19.9ºC for lower comfort and 25.5ºC for maximum comfort, according to the climate. |
| 21 | The construction packages used in both housing typologies are summarized in the table, considering the thermal conductivity values taken from the Ecuadorian construction standard NEC (2018) and the infiltration levels from the air tightness manual for buildings in Chile (https://construccionsustentable.uc.cl/images/Documentos/Manual_de_hermeticidad_al_aire_de_edificaciones.pdf). |
| 22 | For the simulation, the Ecodesigner software from Archicad is used. |
| 23 | The thermal conductivity values for each element were obtained from the Ecuadorian Construction Regulations (2018), while the infiltration levels were taken from the air tightness manual for buildings in Chile by Cite & Decon (2011). |
| 24 | According to the NEC_HS_EE (Ecuadorian Construction Standard - Habitability and Health - Energy Efficiency). |
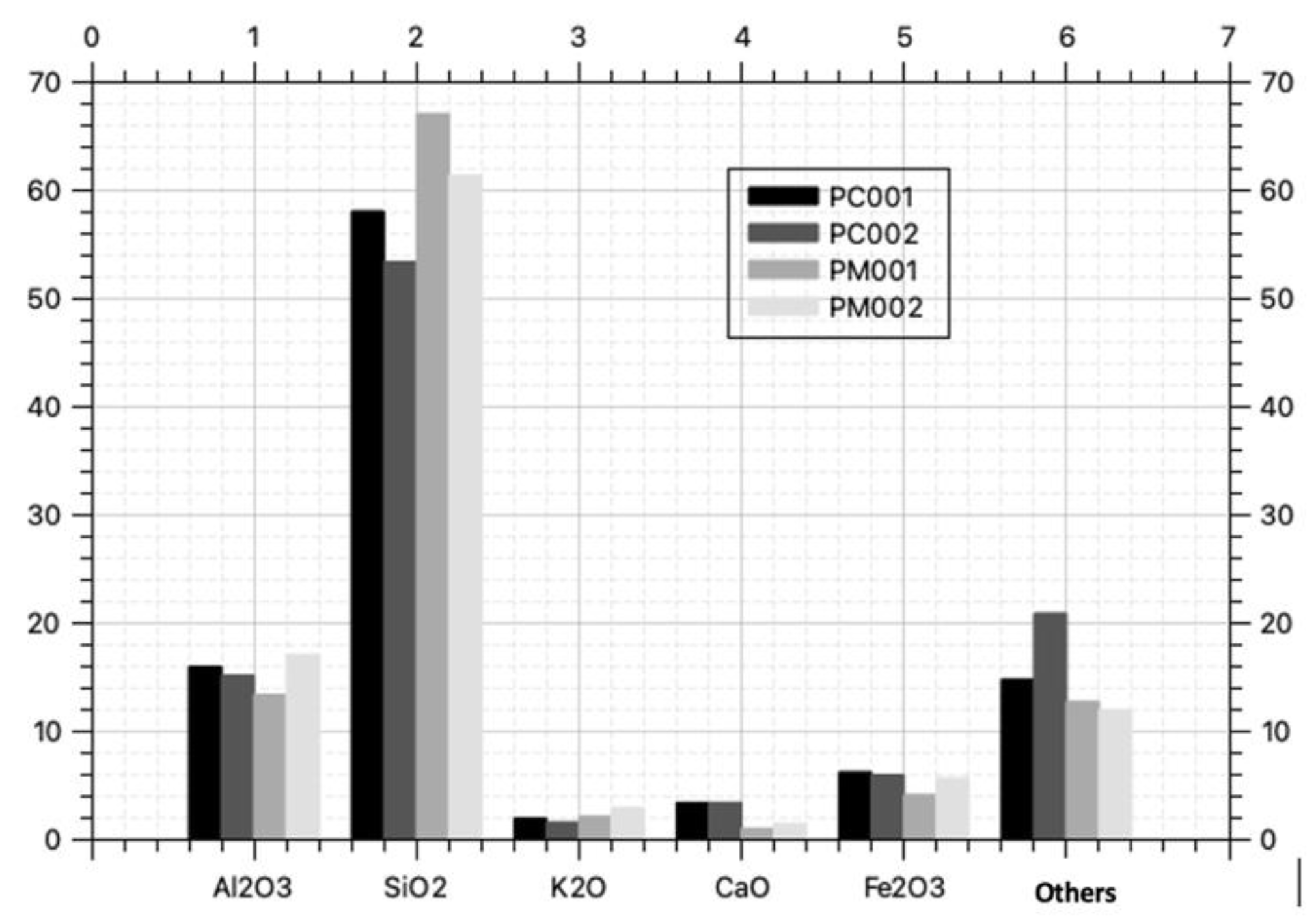





| Sample | AL2O3% | SiO2% | K2O% | CaO% | Fe2O3% | Others% | Total% | ΣOxides >70% |
|---|---|---|---|---|---|---|---|---|
| PC001 | 15.90 | 58.00 | 1.86 | 3.34 | 6.18 | 14.72 | 100.0 | 80.08 |
| PC002 | 15.10 | 53.30 | 1.53 | 3.35 | 5.90 | 20.82 | 100.0 | 74.30 |
| PM001 | 13.30 | 67.00 | 2.04 | 0.95 | 4.04 | 12.67 | 100.0 | 84.34 |
| PM002 | 17.00 | 61.30 | 2.84 | 1.39 | 5.59 | 11.88 | 100.0 | 83.89 |
| Capacity of beaker | Molarity | Amount of NaOH in 1000(ml) | Amount of NaOH in 100(ml) |
|---|---|---|---|
| A(ml) | B(M) | C (g) | D (g) |
| 250 | 12.5 | 500 | 125 |
| 10 | 400 | 100 | |
| 7.5 | 300 | 75 | |
| 5 | 200 | 50 | |
| 100 | 12.5 | 500 | 50 |
| 10 | 400 | 40 | |
| 7.5 | 300 | 30 | |
| 5 | 200 | 20 |
| Solution content | Soil content | Soil-solution content |
|---|---|---|
| (%) | (%) | (%) |
| 22 | 78 | 100 |
| 24 | 76 | 100 |
| 26 | 74 | 100 |
| Mineral compound | Chemical Formula | Semi-quantification (%) |
|---|---|---|
| quartz | SiO2 | 73 |
| hematite | Fe2O3 | 1 |
| albite | NaAlSi3O8 | 1 |
| muscovite | KAl2(Si3Al)O10(OH,F)2 | 20 |
| hydrated aluminum phosphate | Al5[(PO4)2[(P,S)O3(OH,O)]2F2(OH)2(H2O)8•6.48(H2O) | 1 |
| hexahydrated magnesium potassium sulfate | K2Mg (SO4)2•6(H2O) | 4 |
| Ecuador climate zone | JAN | FEB | MAR | APR | MAY | JUN | JUL | AUG | SEP | OCT | NOV | DEC | Unit |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global horizontal radiation* | 372 | 347 | 414 | 407 | 368 | 356 | 302 | 380 | 420 | 404 | 458 | 400 | Wh/m2 |
| Direct normal radiation* | 212 | 154 | 249 | 275 | 270 | 312 | 204 | 309 | 298 | 226 | 319 | 266 | Wh/m2 |
| Diffuse radiation* | 224 | 237 | 240 | 215 | 201 | 160 | 174 | 172 | 224 | 242 | 221 | 215 | Wh/m2 |
| Global horizontal radiation** | 1049 | 1075 | 1077 | 1045 | 988 | 930 | 963 | 995 | 1055 | 1071 | 1091 | 1039 | Wh/m2 |
| Direct normal radiation** | 1001 | 998 | 1011 | 1008 | 984 | 983 | 994 | 988 | 991 | 985 | 1053 | 1019 | Wh/m2 |
| Diffuse radiation** | 538 | 549 | 558 | 532 | 490 | 450 | 458 | 494 | 546 | 567 | 543 | 522 | Wh/m2 |
| Global horizontal radiation*** | 4552 | 4214 | 4981 | 4850 | 4358 | 4197 | 3567 | 4511 | 5031 | 4890 | 5582 | 4898 | Wh/m2 |
| Direct normal radiation*** | 2592 | 1872 | 2995 | 3275 | 3194 | 3683 | 2412 | 3668 | 3577 | 2736 | 3899 | 3262 | Wh/m2 |
| Diffuse radiation*** | 2745 | 2881 | 2895 | 2563 | 2385 | 1885 | 2053 | 2051 | 2691 | 2927 | 2697 | 2638 | Wh/m2 |
| Horizontal global illumination* | 41274 | 38102 | 45280 | 44837 | 41338 | 39798 | 33943 | 41940 | 45970 | 44115 | 50230 | 44414 | lux |
| Direct normal illumination * | 20143 | 14321 | 23495 | 25998 | 26437 | 31269 | 20209 | 30357 | 28057 | 21064 | 29851 | 25404 | lux |
| Dry bulb temperature**** | 14 | 15 | 15 | 14 | 15 | 14 | 14 | 14 | 15 | 15 | 14 | 15 | ºC |
| Dew point temperature**** | 11 | 12 | 12 | 11 | 12 | 11 | 10 | 9 | 12 | 11 | 10 | 12 | ºC |
| Relative humidity**** | 84 | 86 | 83 | 85 | 85 | 81 | 79 | 70 | 80 | 77 | 81 | 82 | % |
| Wind direction***** | 270 | 270 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 270 | 270 | degrees |
| Wind speed**** | 1 | 1 | 2 | 1 | 1 | 2 | 3 | 2 | 2 | 1 | 0 | 1 | m/s |
| Floor temperature**** | 14 | 14 | 14 | 15 | 15 | 15 | 15 | 15 | 15 | 14 | 14 | 14 | ºC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




