Submitted:
02 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Definition of defective CNs
- (a)
-
zero-dimensional (0D) point defects (e.g., doping, vacancy, reconstruction), which can be further divided to:
- (b)
- one-dimensional (1D) line defects (e.g., dislocation);
- (c)
- two-dimensional (2D) planar defects (e.g., grain boundary)
- (d)
- three-dimensional (3D) volume defects (e.g., spatial lattice disorder).
3. Physical and chemical properties of defective CNs with relevance in catalysis, electrocatalysis and electrochemistry
- (i).
- (ii).
- (i).
- micro-pores allow more active sites into the electrolyte,
- (ii).
- meso-pores can facilitate the mass transport in the catalyst layer,
- (iii).
- macro-pores ensure the long-term stability of the catalyst [53].
4. MW-assisted synthesis for the preparation and modification of materials containing defective CNs
4.1. Definition of MW-assisted synthesis
- ✓ contactless heating;
- ✓ a direct transfer of energy to the reactants;
- ✓ independence from heat convection;
- ✓ rapid heating rates;
- ✓ easy control of irradiation parameters;
- ✓ selectivity of heating, the possibility of conducting the reaction locally and volumetrically.
- ✓ top-down; methods, which include the transformation of solid materials into carbon nanomaterials;
- ✓ bottom-up; methods, which include the preparation of carbon nanomaterials from liquid or gaseous carbonaceous precursors.
4.2. MW-assisted synthesis for the preparation and modification of defective CNs: implications on properties and applications
4.3. MW-assisted synthesis for the preparation of hybrid materials containing defective CNs: implications on properties and applications
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yu, S.-S.; Zheng, W.-T. Effect of N/B Doping on the Electronic and Field Emission Properties for Carbon Nanotubes, Carbon Nanocones, and Graphene Nanoribbons. Nanoscale 2010, 2, 1069. [Google Scholar] [CrossRef] [PubMed]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Robertson, J. Comparison of Diamond-like Carbon to Diamond for Applications. Phys. Status Solidi A 2008, 205, 2233–2244. [Google Scholar] [CrossRef]
- Robertson, J. Electronic Structure of Diamond-like Carbon. Diam. Relat. Mater. 1997, 6, 212–218. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon Nano-Onions: Unique Carbon Nanostructures with Fascinating Properties and Their Potential Applications. Inorganica Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of Graphene and Its Disorders: A Review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, Properties, and Applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Iqbal, M.; Shi, Z.; Zhang, H.; Guo, Z. Novel Emerging Graphdiyne Based Two Dimensional Materials: Synthesis, Properties and Renewable Energy Applications. Nano Today 2021, 39, 101207. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, N.; Yu, R.; Zhang, Y.; Zhang, J.; Li, Y.; Wang, D. Unique Structural Advances of Graphdiyne for Energy Applications. EnergyChem 2020, 2, 100041. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yan, M.; Yu, J. Carbon Nanostructures in Biology and Medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Hu, Y.; Shenderova, O.A.; Hu, Z.; Padgett, C.W.; Brenner, D.W. Carbon Nanostructures for Advanced Composites. Rep. Prog. Phys. 2006, 69, 1847–1895. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of Nanostructured Carbon Materials for Electrochemical Capacitor Applications: Advantages and Limitations of Activated Carbon, Carbide-Derived Carbon, Zeolite-Templated Carbon, Carbon Aerogels, Carbon Nanotubes, Onion-like Carbon, and Graphene: Nanostructured Carbon Materials for Electrochemical Capacitor Applications. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Bobrowska, D.M.; Olejnika, P.; Echegoyen, L.; Brzezinska, M. Onion-like Carbon Nanostructures: An Overview of Bio-Applications. Curr. Med. Chem. 2018, 25. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon Nanotubes: A Novel Material for Multifaceted Applications in Human Healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef] [PubMed]
- Sek, S.; Breczko, J.; Plonska-Brzezinska, M.E.; Wilczewska, A.Z.; Echegoyen, L. STM-Based Molecular Junction of Carbon Nano-Onion. ChemPhysChem 2013, 14, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-S.; Golberg, D.; Bando, Y. Carbon “Onions” as Point Electron Sources. ACS Nano 2010, 4, 4396–4402. [Google Scholar] [CrossRef]
- Keller, N.; Maksimova, N.I.; Roddatis, V.V.; Schur, M.; Mestl, G.; Butenko, Y.V.; Kuznetsov, V.L.; Schlögl, R. The Catalytic Use of Onion-like Carbon Materials for Styrene Synthesis by Oxidative Dehydrogenation of Ethylbenzene. Angew. Chem. Int. Ed Engl. 2002, 41, 1885–1888. [Google Scholar] [CrossRef]
- Su, D.; Maksimova, N.I.; Mestl, G.; Kuznetsov, V.L.; Keller, V.; Schlögl, R.; Keller, N. Oxidative Dehydrogenation of Ethylbenzene to Styrene over Ultra-Dispersed Diamond and Onion-like Carbon. Carbon 2007, 45, 2145–2151. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E.; Echegoyen, L. Carbon Nano-Onions for Supercapacitor Electrodes: Recent Developments and Applications. J. Mater. Chem. A 2013, 1, 13703. [Google Scholar] [CrossRef]
- Luszczyn, J.; Plonska-Brzezinska, M.E.; Palkar, A.; Dubis, A.T.; Simionescu, A.; Simionescu, D.T.; Kalska-Szostko, B.; Winkler, K.; Echegoyen, L. Small Noncytotoxic Carbon Nano-Onions: First Covalent Functionalization with Biomolecules. Chem. - Eur. J. 2010, 16, 4870–4880. [Google Scholar] [CrossRef]
- Breczko, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Electrochemical Oxidation and Determination of Dopamine in the Presence of Uric and Ascorbic Acids Using a Carbon Nano-Onion and Poly(Diallyldimethylammonium Chloride) Composite. Electrochimica Acta 2012, 72, 61–67. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. The Application of Graphene Based Materials for Actuators. J. Mater. Chem. 2012, 22, 3671. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and Its Derivatives as Biomedical Materials: Future Prospects and Challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Giordani, S. Carbon Nano-Onions (Multi-Layer Fullerenes): Chemistry and Applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Sonkar, S.K.; Saxena, M.; Sarkar, S. Carbon Nano-Onions for Imaging the Life Cycle of Drosophila Melanogaster. Small 2011, 7, 3170–3177. [Google Scholar] [CrossRef]
- Giordani, S.; Bartelmess, J.; Frasconi, M.; Biondi, I.; Cheung, S.; Grossi, M.; Wu, D.; Echegoyen, L.; O’Shea, D.F. NIR Fluorescence Labelled Carbon Nano-Onions: Synthesis, Analysis and Cellular Imaging. J Mater Chem B 2014, 2, 7459–7463. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Brzezinski, K.; Sulikowski, B.; Olejniczak, Z.; Gras, M.; Lota, G.; Molina-Ontoria, A.; Jakubczyk, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Boron-Doped Polygonal Carbon Nano-Onions: Synthesis and Applications in Electrochemical Energy Storage. Chem. - Eur. J. 2017, 23, 7132–7141. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for Electrochemical Capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Li, D.; Jia, Y.; Chang, G.; Chen, J.; Liu, H.; Wang, J.; Hu, Y.; Xia, Y.; Yang, D.; Yao, X. A Defect-Driven Metal-Free Electrocatalyst for Oxygen Reduction in Acidic Electrolyte. Chem 2018, 4, 2345–2356. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant Contribution of Intrinsic Carbon Defects to Oxygen Reduction Activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.-F.; Chen, X.; Li, B.-Q.; Hou, T.-Z.; Zhang, B.; Zhang, Q.; Titirici, M.-M.; Wei, F. Topological Defects in Metal-Free Nanocarbon for Oxygen Electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Banham, D.; Ye, S.; Pei, K.; Ozaki, J.; Kishimoto, T.; Imashiro, Y. A Review of the Stability and Durability of Non-Precious Metal Catalysts for the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2015, 285, 334–348. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Cai, Q.; Vasileff, A.; Li, L.H.; Han, Y.; Chen, Y.; Qiao, S.-Z. Molecule-Level g-C 3 N 4 Coordinated Transition Metals as a New Class of Electrocatalysts for Oxygen Electrode Reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jiang, K.; Wang, H.; Yao, X. The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Yao, X. Defects on Carbons for Electrocatalytic Oxygen Reduction. Chem. Soc. Rev. 2018, 47, 7628–7658. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct Atomic-Level Insight into the Active Sites of a High-Performance PGM-Free ORR Catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Pan, X.; Wang, S.; Chen, X.; Hu, P.; Sun, L.; Bao, X. Size Effect of Graphene on Electrocatalytic Activation of Oxygen. Chem. Commun. 2011, 47, 10016. [Google Scholar] [CrossRef]
- Liu, D.; Ni, K.; Ye, J.; Xie, J.; Zhu, Y.; Song, L. Tailoring the Structure of Carbon Nanomaterials toward High-End Energy Applications. Adv. Mater. 2018, 30, 1802104. [Google Scholar] [CrossRef]
- Kotakoski, J.; Krasheninnikov, A.V.; Kaiser, U.; Meyer, J.C. From Point Defects in Graphene to Two-Dimensional Amorphous Carbon. Phys. Rev. Lett. 2011, 106. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of Catalytic Sites for Oxygen Reduction and Oxygen Evolution in N-Doped Graphene Materials: Development of Highly Efficient Metal-Free Bifunctional Electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, J.; Yao, X. Defect Electrocatalytic Mechanism: Concept, Topological Structure and Perspective. Mater. Chem. Front. 2018, 2, 1250–1268. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Yao, X. Defects on Carbons for Electrocatalytic Oxygen Reduction. Chem. Soc. Rev. 2018, 47, 7628–7658. [Google Scholar] [CrossRef] [PubMed]
- Frauenheim, Th.; Blaudeck, P.; Stephan, U.; Jungnickel, G. Atomic Structure and Physical Properties of Amorphous Carbon and Its Hydrogenated Analogs. Phys. Rev. B 1993, 48, 4823–4834. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.-H.; Krishnan, K.M.; Veirs, D.K.; Rubin, M.D.; Hopper, C.B.; Bhushan, B.; Bogy, D.B. Chemical Structure and Physical Properties of Diamond-like Amorphous Carbon Films Prepared by Magnetron Sputtering. J. Mater. Res. 1990, 5, 2543–2554. [Google Scholar] [CrossRef]
- Mounier, E.; Bertin, F.; Adamik, M.; Pauleau, Y.; Barna, P.B. Effect of the Substrate Temperature on the Physical Characteristics of Amorphous Carbon Films Deposited by d.c. Magnetron Sputtering. Diam. Relat. Mater. 1996, 5, 1509–1515. [Google Scholar] [CrossRef]
- Zhou, H.; Sheng, X.; Xiao, J.; Ding, Z.; Wang, D.; Zhang, X.; Liu, J.; Wu, R.; Feng, X.; Jiang, L. Increasing the Efficiency of Photocatalytic Reactions via Surface Microenvironment Engineering. J. Am. Chem. Soc. 2020, 142, 2738–2743. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, W.; Li, Z.; Xiong, Y. Surface and Interface Engineering in Photocatalysis. ChemNanoMat 2015, 1, 223–239. [Google Scholar] [CrossRef]
- Li, J.; Abbas, S.U.; Wang, H.; Zhang, Z.; Hu, W. Recent Advances in Interface Engineering for Electrocatalytic CO2 Reduction Reaction. Nano-Micro Lett. 2021, 13, 216. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Xiong, J.; Mi, L.; Song, X.-Z.; Li, Y. Defect and Interface Engineering in Metal Sulfide Catalysts for the Electrocatalytic Nitrogen Reduction Reaction: A Review. J. Mater. Chem. A 2022, 10, 6927–6949. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. The Chalkboard: Fundamentals of Electrochemical Capacitor Design and Operation. Electrochem. Soc. Interface 2008, 17, 31–32. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ni, K.; Ye, J.; Xie, J.; Zhu, Y.; Song, L. Carbon Nanomaterials: Tailoring the Structure of Carbon Nanomaterials toward High-End Energy Applications (Adv. Mater. 48/2018). Adv. Mater. 2018, 30, 1870371. [Google Scholar] [CrossRef]
- Deng, J.-H.; Cheng, L.; Wang, F.-J.; Yu, B.; Li, G.-Z.; Li, D.-J.; Cheng, G.-A. Ultralow Field Emission from Thinned, Open-Ended, and Defected Carbon Nanotubes by Using Microwave Hydrogen Plasma Processing. Appl. Surf. Sci. 2015, 324, 293–299. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Electron Emission in Intense Electric Fields. Proc. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character 1928, 119, 173–181. [CrossRef]
- An, Y.; Li, C.; Sun, X.; Wang, K.; Su, F.; Liu, F.; Zhang, X.; Ma, Y. Deoxygenated Porous Carbon with Highly Stable Electrochemical Reaction Interface for Practical High-Performance Lithium-Ion Capacitors. J. Phys. Appl. Phys. 2022, 55, 045501. [Google Scholar] [CrossRef]
- Wang, C.; Chi, M.; Wang, G.; van der Vliet, D.; Li, D.; More, K.; Wang, H.-H.; Schlueter, J.A.; Markovic, N.M.; Stamenkovic, V.R. Correlation Between Surface Chemistry and Electrocatalytic Properties of Monodisperse PtxNi1-x Nanoparticles. Adv. Funct. Mater. 2011, 21, 147–152. [Google Scholar] [CrossRef]
- Trasatti, S. Oxide/Aqueous Solution Interfaces, Interplay of Surface Chemistry and Electrocatalysis. Mater. Chem. Phys. 1987, 16, 157–174. [Google Scholar] [CrossRef]
- Chen, D.; Zou, Y.; Wang, S. Surface Chemical-Functionalization of Ultrathin Two-Dimensional Nanomaterials for Electrocatalysis. Mater. Today Energy 2019, 12, 250–268. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom Doped Graphene Engineering for Energy Storage and Conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Basivi, P.K.; Pasupuleti, K.S.; Gelija, D.; Kim, M.-D.; Pasupuleti, V.R.; Kim, C.W. UV-Light-Enhanced Room Temperature NO 2 Gas-Sensing Performances Based on Sulfur-Doped Graphitic Carbon Nitride Nanoflakes. New J. Chem. 2022, 46, 19254–19262. [Google Scholar] [CrossRef]
- Fortunato, G.V.; Kronka, M.S.; Cardoso, E.S.F.; dos Santos, A.J.; Roveda, A.C.; Lima, F.H.B.; Ledendecker, M.; Maia, G.; Lanza, M.R.V. A Comprehensive Comparison of Oxygen and Nitrogen Functionalities in Carbon and Their Implications for the Oxygen Reduction Reaction. J. Catal. 2022, 413, 1034–1047. [Google Scholar] [CrossRef]
- Szymański, G.S.; Wiśniewski, M.; Olejnik, P.; Koter, S.; Castro, E.; Echegoyen, L.; Terzyk, A.P.; Plonska-Brzezinska, M.E. Correlation between the Catalytic and Electrocatalytic Properties of Nitrogen-Doped Carbon Nanoonions and the Polarity of the Carbon Surface: Experimental and Theoretical Investigations. Carbon 2019, 151, 120–129. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward Design of Synergistically Active Carbon-Based Catalysts for Electrocatalytic Hydrogen Evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active Sites of Nitrogen-Doped Carbon Materials for Oxygen Reduction Reaction Clarified Using Model Catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design Principles for Heteroatom-Doped Carbon Nanomaterials as Highly Efficient Catalysts for Fuel Cells and Metal-Air Batteries. Adv. Mater. 2015, 27, 6834–6840. [Google Scholar] [CrossRef]
- Pinto, H.; Markevich, A. Electronic and Electrochemical Doping of Graphene by Surface Adsorbates. Beilstein J. Nanotechnol. 2014, 5, 1842–1848. [Google Scholar] [CrossRef]
- Kepaptsoglou, D.; Hardcastle, T.P.; Seabourne, C.R.; Bangert, U.; Zan, R.; Amani, J.A.; Hofsäss, H.; Nicholls, R.J.; Brydson, R.M.D.; Scott, A.J.; et al. Electronic Structure Modification of Ion Implanted Graphene: The Spectroscopic Signatures of p- and n-Type Doping. ACS Nano 2015, 9, 11398–11407. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Srivastava, P.; Shrivastava, A.K. Electronic and Transport Properties of Boron and Nitrogen Doped Graphene Nanoribbons: An Ab Initio Approach. Appl. Nanosci. 2014, 4, 461–467. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ashokkumar, M.; Gullapalli, H.; Gong, Y.; Vajtai, R.; Thanikaivelan, P.; Ajayan, P.M. Nitrogen-Rich Carbon Nano-Onions for Oxygen Reduction Reaction. Carbon 2018, 130, 645–651. [Google Scholar] [CrossRef]
- Sa, Y.J.; Park, C.; Jeong, H.Y.; Park, S.-H.; Lee, Z.; Kim, K.T.; Park, G.-G.; Joo, S.H. Carbon Nanotubes/Heteroatom-Doped Carbon Core-Sheath Nanostructures as Highly Active, Metal-Free Oxygen Reduction Electrocatalysts for Alkaline Fuel Cells. Angew. Chem. Int. Ed. 2014, 53, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, Y.; Kong, X.; Deng, X.; Park, H.J.; Guo, Y.; Jin, S.; Qi, Z.; Lee, Z.; Qiao, Z.; et al. The Origin of Improved Electrical Double-Layer Capacitance by Inclusion of Topological Defects and Dopants in Graphene for Supercapacitors. Angew. Chem. Int. Ed. 2016, 55, 13822–13827. [Google Scholar] [CrossRef] [PubMed]
- Sikeyi, L.L.; Ntuli, T.D.; Mongwe, T.H.; Maxakato, N.W.; Carleschi, E.; Doyle, B.P.; Coville, N.J.; Maubane-Nkadimeng, M.S. Microwave Assisted Synthesis of Nitrogen Doped and Oxygen Functionalized Carbon Nano Onions Supported Palladium Nanoparticles as Hybrid Anodic Electrocatalysts for Direct Alkaline Ethanol Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 10862–10875. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Fedoseeva, Yu.V.; Romanenko, A.I.; Gusel’nikov, A.V.; Vilkov, O.Yu.; Maksimovskiy, E.A.; Bychanok, D.S.; Kuzhir, P.P.; Bulusheva, L.G.; Okotrub, A.V. Effect of Boron and Nitrogen Additives on Structure and Transport Properties of Arc-Produced Carbon. Carbon 2019, 143, 660–668. [Google Scholar] [CrossRef]
- Umrao, S.; Gupta, T.K.; Kumar, S.; Singh, V.K.; Sultania, M.K.; Jung, J.H.; Oh, I.-K.; Srivastava, A. Microwave-Assisted Synthesis of Boron and Nitrogen Co-Doped Reduced Graphene Oxide for the Protection of Electromagnetic Radiation in Ku-Band. ACS Appl. Mater. Interfaces 2015, 7, 19831–19842. [Google Scholar] [CrossRef]
- Shaikh, A.; Singh, B.K.; Mohapatra, D.; Parida, S. Nitrogen-Doped Carbon Nano-Onions as a Metal-Free Electrocatalyst. Electrocatalysis 2019, 10, 222–231. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Xiao, Z.; Mou, X.; Meng, X.; Yang, Q.; Ma, Y.; Zhao, N.; Huang, X.; Shaislamov, U.; Kong, D.; Zhi, L. Electrifying Schiff-Based Networks as Model Catalysts towards Deeply Understanding the Crucial Role of Sp2-Carbon in Nitrogen-Doped Carbocatalyst for Oxygen Reduction Reaction. Appl. Surf. Sci. 2022, 599, 153961. [Google Scholar] [CrossRef]
- Masemola, C.M.; Moloto, N.; Tetana, Z.N.; Gqoba, S.S.; Mubiayi, P.K.; Linganiso, E.C. N-Doped Graphene Quantum Dot-Modified Polyaniline for Room-Temperature Sensing of Alcohol Vapors. Mater. Chem. Phys. 2022, 287, 126229. [Google Scholar] [CrossRef]
- Savilov, S.V.; Arkhipova, E.A.; Ivanov, A.S.; Maslakov, K.I.; Shen, Z.; Aldoshin, S.M.; Lunin, V.V. Pyrolytic Synthesis and Characterization of N-Doped Carbon Nanoflakes for Electrochemical Applications. Mater. Res. Bull. 2015, 69, 7–12. [Google Scholar] [CrossRef]
- Kakaei, K.; Balavandi, A. Synthesis of Halogen-Doped Reduced Graphene Oxide Nanosheets as Highly Efficient Metal-Free Electrocatalyst for Oxygen Reduction Reaction. J. Colloid Interface Sci. 2016, 463, 46–54. [Google Scholar] [CrossRef]
- Pham, C.V.; Britton, B.; Böhm, T.; Holdcroft, S.; Thiele, S. Doped, Defect-Enriched Carbon Nanotubes as an Efficient Oxygen Reduction Catalyst for Anion Exchange Membrane Fuel Cells. Adv. Mater. Interfaces 2018, 5, 1800184. [Google Scholar] [CrossRef]
- Lai, Q.; Wei, K.; Tang, Z.; Liu, X.; Zheng, J.; Liang, Y. Edge Reconfiguration of N, P-Codoped Carbon Boosts Oxygen Reduction Electrocatalysis. J. Mater. Sci. 2021, 56, 19577–19588. [Google Scholar] [CrossRef]
- Xia, C.; Hai, X.; Chen, X.-W.; Wang, J.-H. Simultaneously Fabrication of Free and Solidified N, S-Doped Graphene Quantum Dots via a Facile Solvent-Free Synthesis Route for Fluorescent Detection. Talanta 2017, 168, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Camisasca, A.; Sacco, A.; Brescia, R.; Giordani, S. Boron/Nitrogen-Codoped Carbon Nano-Onion Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2018, 1, 5763–5773. [Google Scholar] [CrossRef]
- Domga; Karnan, M. ; Oladoyinbo, F.; Noumi, G.B.; Tchatchueng, J.B.; Sieliechi, M.J.; Sathish, M.; Pattanayak, D.K. A Simple, Economical One-Pot Microwave Assisted Synthesis of Nitrogen and Sulfur Co-Doped Graphene for High Energy Supercapacitors. Electrochimica Acta 2020, 341, 135999. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Chen, S.; Wang, X.; Ma, Y.; Wu, Q.; Jiang, Y.; Qian, W.; Hu, Z. Can Boron and Nitrogen Co-Doping Improve Oxygen Reduction Reaction Activity of Carbon Nanotubes? J. Am. Chem. Soc. 2013, 135, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S.Z. Two-Step Boron and Nitrogen Doping in Graphene for Enhanced Synergistic Catalysis. Angew. Chem. Int. Ed. 2013, 52, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. Building a Project Work Breakdown Structure: Visualizing Objectives, Deliverables, Activities, and Schedules; ESI International Project Management Series; Auerbach Publications, 2008; Vol. 20085940; ISBN 978-1-4200-6969-3.
- Portet, C.; Yushin, G.; Gogotsi, Y. Electrochemical Performance of Carbon Onions, Nanodiamonds, Carbon Black and Multiwalled Nanotubes in Electrical Double Layer Capacitors. Carbon 2007, 45, 2511–2518. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-Power Micrometre-Sized Supercapacitors Based on Onion-like Carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.; Jäckel, N.; Aslan, M.; Weingarth, D.; Presser, V. Understanding Structure and Porosity of Nanodiamond-Derived Carbon Onions. Carbon 2015, 84, 584–598. [Google Scholar] [CrossRef]
- Bandaru, P.R.; Yamada, H.; Narayanan, R.; Hoefer, M. Charge Transfer and Storage in Nanostructures. Mater. Sci. Eng. R Rep. 2015, 96, 1–69. [Google Scholar] [CrossRef]
- Bagge-Hansen, M.; Bastea, S.; Hammons, J.A.; Nielsen, M.H.; Lauderbach, L.M.; Hodgin, R.L.; Pagoria, P.; May, C.; Aloni, S.; Jones, A.; et al. Detonation Synthesis of Carbon Nano-Onions via Liquid Carbon Condensation. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Moussa, G.; Matei Ghimbeu, C.; Taberna, P.-L.; Simon, P.; Vix-Guterl, C. Relationship between the Carbon Nano-Onions (CNOs) Surface Chemistry/Defects and Their Capacitance in Aqueous and Organic Electrolytes. Carbon 2016, 105, 628–637. [Google Scholar] [CrossRef]
- Borgohain, R.; Li, J.; Selegue, J.P.; Cheng, Y.-T. Electrochemical Study of Functionalized Carbon Nano-Onions for High-Performance Supercapacitor Electrodes. J. Phys. Chem. C 2012, 116, 15068–15075. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Kar, K.K. Microwave as a Tool for Synthesis of Carbon-Based Electrodes for Energy Storage. ACS Appl. Mater. Interfaces 2022, 14, 20306–20325. [Google Scholar] [CrossRef]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and Modification of Carbon Nanomaterials Utilizing Microwave Heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, K.-H. Microwave Heating of Carbon-Based Solid Materials. Carbon Lett. 2014, 15, 15–24. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave Heating Processes Involving Carbon Materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Poyraz, S.; Smith, J.; Kushvaha, V.; Tippur, H.; Zhang, X. An Ultrafast Microwave Approach towards Multi-Component and Multi-Dimensional Nanomaterials. RSC Adv. 2014, 4, 9308. [Google Scholar] [CrossRef]
- Slocombe, D.; Porch, A.; Bustarret, E.; Williams, O.A. Microwave Properties of Nanodiamond Particles. Appl. Phys. Lett. 2013, 102, 244102. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Zhou, Z.; Jiang, M.; Lu, J.; Wang, Y.; Wang, L. Effective Improvement in Microwave Absorption by Uniform Dispersion of Nanodiamond in Polyaniline through in-Situ Polymerization. Appl. Phys. Lett. 2015, 106, 233103. [Google Scholar] [CrossRef]
- Yoon, B.-J.; Hong, E.H.; Jee, S.E.; Yoon, D.-M.; Shim, D.-S.; Son, G.-Y.; Lee, Y.J.; Lee, K.-H.; Kim, H.S.; Park, C.G. Fabrication of Flexible Carbon Nanotube Field Emitter Arrays by Direct Microwave Irradiation on Organic Polymer Substrate. J. Am. Chem. Soc. 2005, 127, 8234–8235. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of Inorganic Materials Using Ionic Liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, C.; Jin, Z.; Wang, D.-W.; Yan, X.; Chen, Z.; Zhu, G.; Yao, X. Carbon for the Oxygen Reduction Reaction: A Defect Mechanism. J. Mater. Chem. A 2015, 3, 11736–11739. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Gordeev, E.G.; Ananikov, V.P. Noninnocent Nature of Carbon Support in Metal/Carbon Catalysts: Etching/Pitting vs Nanotube Growth under Microwave Irradiation. ACS Catal. 2014, 4, 3806–3814. [Google Scholar] [CrossRef]
- Bajpai, R.; Wagner, H.D. Fast Growth of Carbon Nanotubes Using a Microwave Oven. Carbon 2015, 82, 327–336. [Google Scholar] [CrossRef]
- Hsin, Y.-L.; Lin, C.-F.; Liang, Y.-C.; Hwang, K.C.; Horng, J.-C.; Ho, J.A.; Lin, C.-C.; Hwu, J.R. Microwave Arcing Induced Formation and Growth Mechanisms of Core/Shell Metal/Carbon Nanoparticles in Organic Solutions: Formation and Growth Mechanisms of Core/Shell Nanoparticles. Adv. Funct. Mater. 2008, 18, 2048–2056. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Kushvaha, V.; Poyraz, S.; Tippur, H.; Park, S.; Kim, M.; Liu, Y.; Bar, J.; Chen, H.; et al. Poptube Approach for Ultrafast Carbon Nanotube Growth. Chem. Commun. 2011, 47, 9912. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Tauchi, L.; Nguyen-Tran, H.-D.; Ohta, T.; Shimizu, M.; Ohta, K. Development of a Novel Method to Synthesize Carbon Nanotubes from Granulated Polystyrene and Nickel Nanoparticles by Microwave Heating. J. Mater. Chem. 2011, 21, 14569. [Google Scholar] [CrossRef]
- Li, S.; Guo, C. ; Qiao, yuqing Microwave-Assisted Synthesis of Functionalized Graphene Hydrogels for High Performance Supercapacitors. Mater. Sci. Eng. B 2021, 273, 115407. [Google Scholar] [CrossRef]
- Rosli, N.H.A.; Lau, K.S.; Winie, T.; Chin, S.X.; Chia, C.H. Synergistic Effect of Sulfur-Doped Reduced Graphene Oxide Created via Microwave-Assisted Synthesis for Supercapacitor Applications. Diam. Relat. Mater. 2021, 120, 108696. [Google Scholar] [CrossRef]
- Ma, J.; Yamamoto, Y.; Su, C.; Badhulika, S.; Fukuhara, C.; Kong, C.Y. One-Pot Microwave-Assisted Synthesis of Porous Reduced Graphene Oxide as an Electrode Material for High Capacitance Supercapacitor. Electrochimica Acta 2021, 386, 138439. [Google Scholar] [CrossRef]
- Kim, T.; Chang Kang, H.; Thanh Tung, T.; Don Lee, J.; Kim, H.; Seok Yang, W.; Gyu Yoon, H.; Suh, K.S. Ionic Liquid-Assisted Microwave Reduction of Graphite Oxide for Supercapacitors. RSC Adv. 2012, 2, 8808. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Shah, V.; Nizamuddin, S.; Mubarak, N.M.; Karri, R.R.; Dehghani, M.H.; Ramesh, S.; Khalid, M.; Rahman, M.E. Microwave-Assisted Synthesis of Carbon Nanotubes for the Removal of Toxic Cationic Dyes from Textile Wastewater. J. Mol. Liq. 2022, 356, 119045. [Google Scholar] [CrossRef]
- Ali, M.; Riaz, R.; Anjum, A.S.; Sun, K.C.; Li, H.; Ahn, S.; Jeong, S.H.; Ko, M.J. Microwave-Assisted Ultrafast in-Situ Growth of N-Doped Carbon Quantum Dots on Multiwalled Carbon Nanotubes as an Efficient Electrocatalyst for Photovoltaics. J. Colloid Interface Sci. 2021, 586, 349–361. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Jin, R.; Meng, Q.; Chen, Y.; Long, X.; Wang, L.; Guo, H.; Zhang, S. Ultrasonic-Microwave Assisted Synthesis of GO/g-C3N4 Composites for Efficient Photocatalytic H2 Evolution. Solid State Sci. 2019, 97, 105990. [Google Scholar] [CrossRef]
- Wang, W.; Jin, J.; Wu, Y.; Zhang, W.; Jiang, H.; Li, X.; Wang, G. Unique Holey Graphene/Carbon Dots Frameworks by Microwave-Initiated Chain Reduction for High-Performance Compressible Supercapacitors and Reusable Oil/Water Separation. J. Mater. Chem. A 2019, 7, 22054–22062. [Google Scholar] [CrossRef]
- Jessl, S.; Copic, D.; Engelke, S.; Ahmad, S.; De Volder, M. Hydrothermal Coating of Patterned Carbon Nanotube Forest for Structured Lithium-Ion Battery Electrodes. Small 2019, 15, 1901201. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Tiwari, V.S.; Yadav, A.; Savu, R.; Vaz, A.R.; Moshkalev, S.A. Enhanced Magnetic Performance of Iron Oxide Nanoparticles Anchored Pristine/ N-Doped Multi-Walled Carbon Nanotubes by Microwave-Assisted Approach. J. Alloys Compd. 2017, 695, 1793–1801. [Google Scholar] [CrossRef]
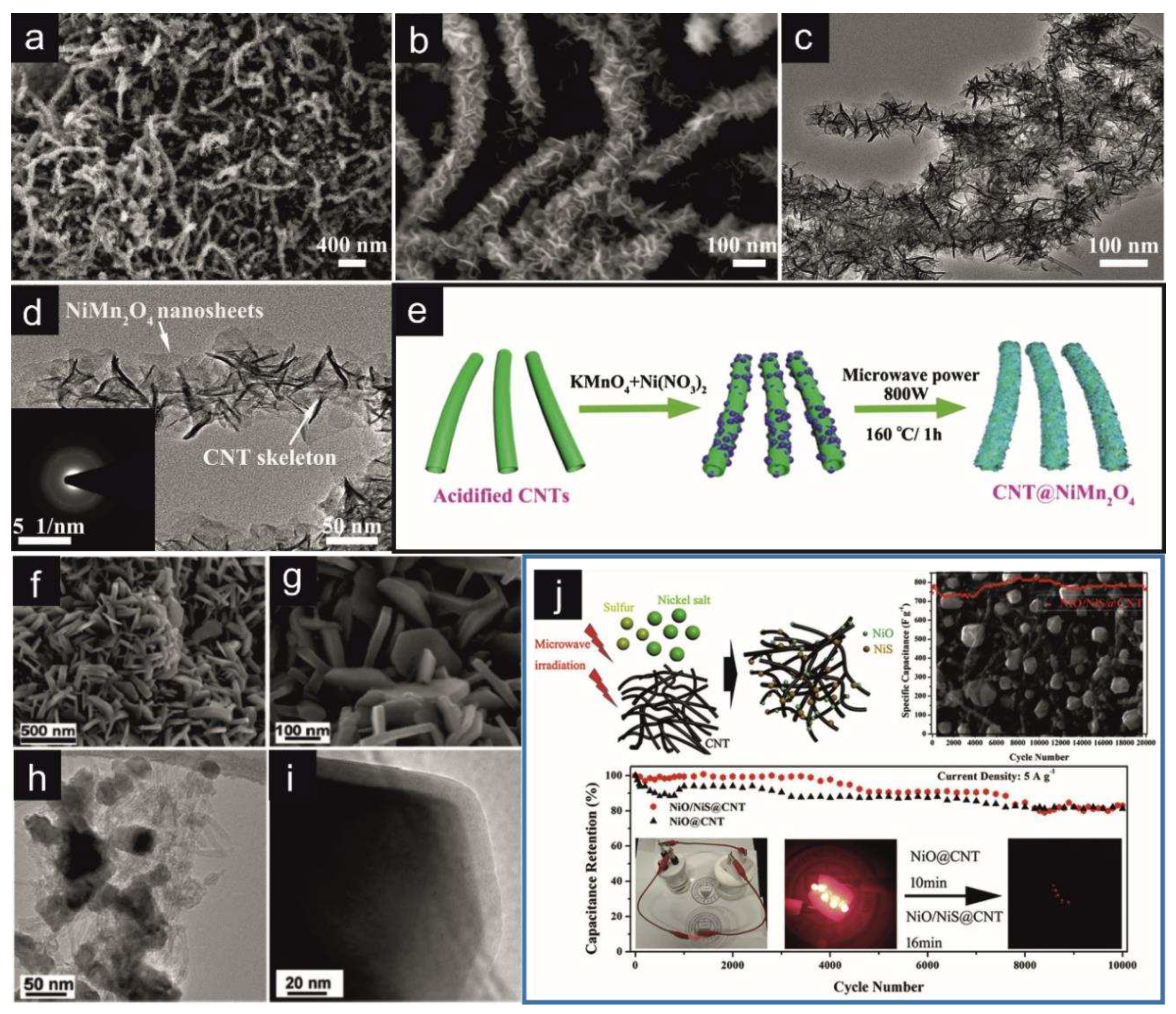
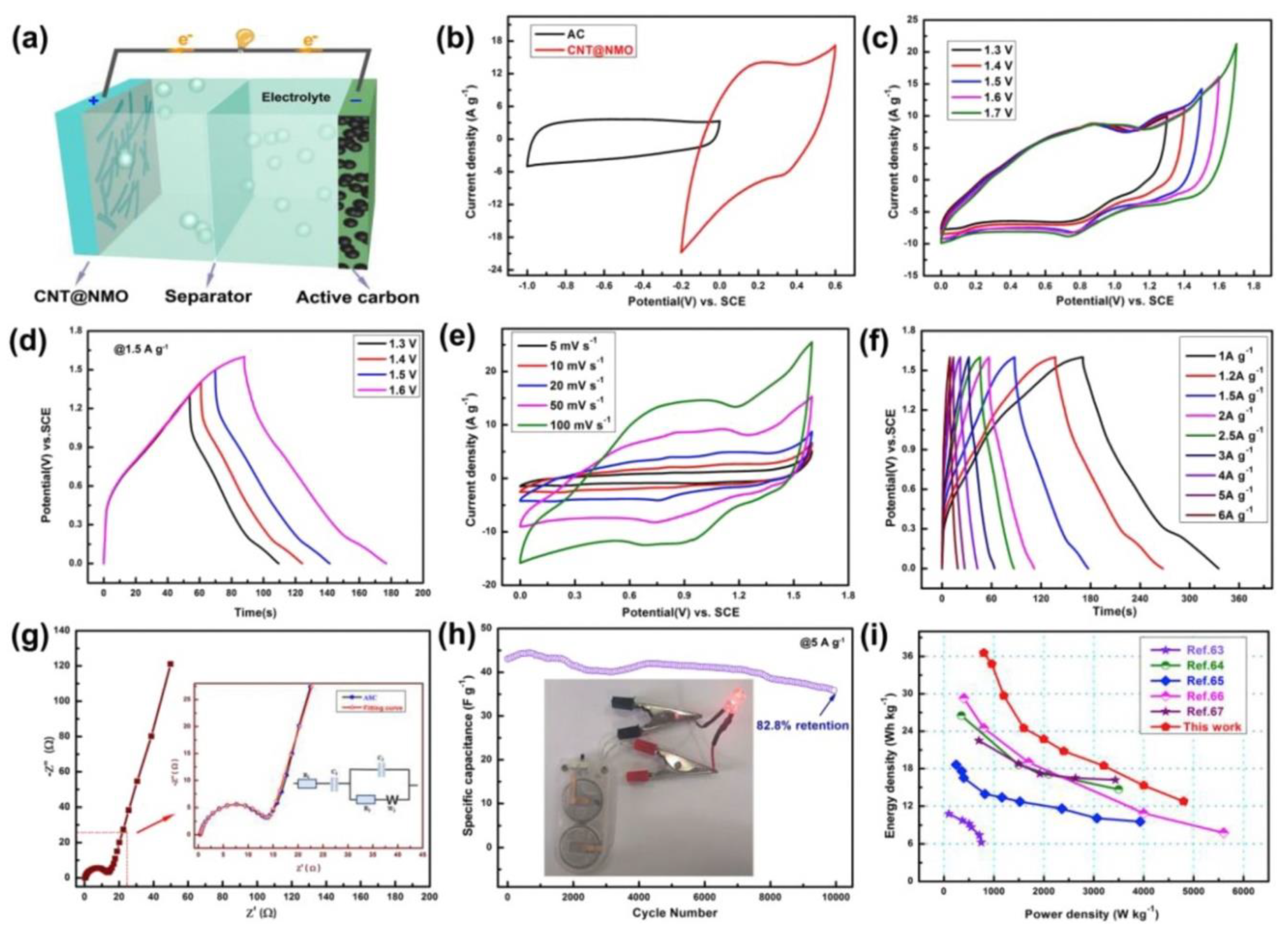
- Sun, Y.; Du, X.; Zhang, J.; Huang, N.; Yang, L.; Sun, X. Microwave-Assisted Preparation and Improvement Mechanism of Carbon Nanotube@NiMn2O4 Core-Shell Nanocomposite for High Performance Asymmetric Supercapacitors. J. Power Sources 2020, 473, 228609. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Tan, X.; Holt, C.M.B.; Zhang, L.; Amirkhiz, B.S.; Mitlin, D. Supercapacitive Carbon Nanotube-Cobalt Molybdate Nanocomposites Prepared via Solvent-Free Microwave Synthesis. RSC Adv. 2012, 2, 2753. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, Y.; Liu, S.; Tan, X.; Wang, S.; Guo, Q.; Luo, J.; Li, Z. One-Step Microwave Synthesis of NiO/NiS@CNT Nanocomposites for High-Cycling-Stability Supercapacitors. J. Alloys Compd. 2019, 806, 170–179. [Google Scholar] [CrossRef]
- Chakraborty, S.; Simon, R.; Vadakkekara, A.; N. L., M. Microwave Assisted Synthesis of Poly(Ortho-Phenylenediamine-Co-Aniline) and Functionalised Carbon Nanotube Nanocomposites for Fabric-Based Supercapacitors. Electrochimica Acta 2022, 403, 139678. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Guo, X.; Liu, Y.; Zheng, Y.; Zhang, M.; Li, R.; Peng, Z.; Zhang, Y.; Zhang, T. One-Step Microwave-Hydrothermal Preparation of NiS/RGO Hybrid for High-Performance Symmetric Solid-State Supercapacitor. Appl. Surf. Sci. 2020, 514, 146080. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Alaferdov, A.V.; Moshkalev, S.A. Rapid and Controllable Synthesis of Fe3O4 Octahedral Nanocrystals Embedded-Reduced Graphene Oxide Using Microwave Irradiation for High Performance Lithium-Ion Batteries. Electrochimica Acta 2018, 281, 78–87. [Google Scholar] [CrossRef]
- Bae, S.-H.; Karthikeyan, K.; Lee, Y.-S.; Oh, I.-K. Microwave Self-Assembly of 3D Graphene-Carbon Nanotube-Nickel Nanostructure for High Capacity Anode Material in Lithium Ion Battery. Carbon 2013, 64, 527–536. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Savu, R.; Dubey, P.K.; Kumar, P.; Moshkalev, S.A. Microwave-Assisted Synthesis of Void-Induced Graphene-Wrapped Nickel Oxide Hybrids for Supercapacitor Applications. RSC Adv. 2016, 6, 26612–26620. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Soe, H.M.; Abdel-Galeil, M.M.; Kawamura, G.; Matsuda, A. Honeycomb-like Open-Edged Reduced-Graphene-Oxide-Enclosed Transition Metal Oxides (NiO/Co3O4) as Improved Electrode Materials for High-Performance Supercapacitor. J. Energy Storage 2020, 30, 101539. [Google Scholar] [CrossRef]
- Fang, J.; Li, M.; Li, Q.; Zhang, W.; Shou, Q.; Liu, F.; Zhang, X.; Cheng, J. Microwave-Assisted Synthesis of CoAl-Layered Double Hydroxide/Graphene Oxide Composite and Its Application in Supercapacitors. Electrochimica Acta 2012, 85, 248–255. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.P.; Fang, J.H.; Yang, Y.; Liu, F.; Zhang, X.B. NiAl-Layered Double Hydroxide/Reduced Graphene Oxide Composite: Microwave-Assisted Synthesis and Supercapacitive Properties. Electrochimica Acta 2014, 134, 309–318. [Google Scholar] [CrossRef]
- Liu, T.; Chai, H.; Jia, D.; Su, Y.; Wang, T.; Zhou, W. Rapid Microwave-Assisted Synthesis of Mesoporous NiMoO4 Nanorod/Reduced Graphene Oxide Composites for High-Performance Supercapacitors. Electrochimica Acta 2015, 180, 998–1006. [Google Scholar] [CrossRef]
- Reddy, B.J.; Vickraman, P.; Justin, A.S. Asymmetric Supercapacitor Device Performance Based on Microwave Synthesis of N-Doped Graphene/Nickel Sulfide Nanocomposite. J. Mater. Sci. 2019, 54, 6361–6373. [Google Scholar] [CrossRef]
- Kumar, R.; Abdel-Galeil, M.M.; Ya, K.Z.; Fujita, K.; Tan, W.K.; Matsuda, A. Facile and Fast Microwave-Assisted Formation of Reduced Graphene Oxide-Wrapped Manganese Cobaltite Ternary Hybrids as Improved Supercapacitor Electrode Material. Appl. Surf. Sci. 2019, 481, 296–306. [Google Scholar] [CrossRef]
- Nagaraju, P.; Arivanandhan, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Enhanced Electrochemical Performance of α-MoO 3 /Graphene Nanocomposites Prepared by an in Situ Microwave Irradiation Technique for Energy Storage Applications. RSC Adv. 2020, 10, 22836–22847. [Google Scholar] [CrossRef]
- Miao, C.; Yin, X.; Xia, G.; Zhu, K.; Ye, K.; Wang, Q.; Yan, J.; Cao, D.; Wang, G. Facile Microwave-Assisted Synthesis of Cobalt Diselenide/Reduced Graphene Oxide Composite for High-Performance Supercapacitors. Appl. Surf. Sci. 2021, 543, 148811. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Madito, M.J.; Bello, A.; Lekitima, J.; Manyala, N. Microwave-Assisted Synthesis of Cobalt Sulphide Nanoparticle Clusters on Activated Graphene Foam for Electrochemical Supercapacitors. RSC Adv. 2017, 7, 20231–20240. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Manganese Nitride Stabilized on Reduced Graphene Oxide Substrate for High Performance Sodium Ion Batteries, Super-Capacitors and EMI Shielding. J. Alloys Compd. 2019, 808, 151748. [Google Scholar] [CrossRef]
- Vimuna, V.M.; Athira, A.R.; Dinesh Babu, K.V.; Xavier, T.S. Simultaneous Stirring and Microwave Assisted Synthesis of Nanoflakes MnO2/RGO Composite Electrode Material for Symmetric Supercapacitor with Enhanced Electrochemical Performance. Diam. Relat. Mater. 2020, 110, 108129. [Google Scholar] [CrossRef]
- Kumar, R.; da Silva, E.T.S.G.; Singh, R.K.; Savu, R.; Alaferdov, A.V.; Fonseca, L.C.; Carossi, L.C.; Singh, A.; Khandka, S.; Kar, K.K.; et al. Microwave-Assisted Synthesis of Palladium Nanoparticles Intercalated Nitrogen Doped Reduced Graphene Oxide and Their Electrocatalytic Activity for Direct-Ethanol Fuel Cells. J. Colloid Interface Sci. 2018, 515, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Savu, R.; Singh, R.K.; Joanni, E.; Singh, D.P.; Tiwari, V.S.; Vaz, A.R.; da Silva, E.T.S.G.; Maluta, J.R.; Kubota, L.T.; et al. Controlled Density of Defects Assisted Perforated Structure in Reduced Graphene Oxide Nanosheets-Palladium Hybrids for Enhanced Ethanol Electro-Oxidation. Carbon 2017, 117, 137–146. [Google Scholar] [CrossRef]
- Ma, T.; Liu, H.; Wang, Y.; Zhang, M. Rapid Construction of Three-Dimensional Sulfur Doped Graphene Supported by NiFeS2 Interconnected Networks as Convenient Electron/Ion Transport Channels for Flexible Supercapacitors. Electrochimica Acta 2019, 309, 1–10. [Google Scholar] [CrossRef]
- Kumar, R.; Oh, J.-H.; Kim, H.-J.; Jung, J.-H.; Jung, C.-H.; Hong, W.G.; Kim, H.-J.; Park, J.-Y.; Oh, I.-K. Nanohole-Structured and Palladium-Embedded 3D Porous Graphene for Ultrahigh Hydrogen Storage and CO Oxidation Multifunctionalities. ACS Nano 2015, 9, 7343–7351. [Google Scholar] [CrossRef]
- Khamlich, S.; Mokrani, T.; Dhlamini, M.S.; Mothudi, B.M.; Maaza, M. Microwave-Assisted Synthesis of Simonkolleite Nanoplatelets on Nickel Foam–Graphene with Enhanced Surface Area for High-Performance Supercapacitors. J. Colloid Interface Sci. 2016, 461, 154–161. [Google Scholar] [CrossRef]
- Zhao, F.; Xie, D.; Huang, W.; Song, X.; Aurang Zeb Gul Sial, M.; Wu, H.; Deng, F.; Zhang, Q.; Zou, J.; Zeng, X. Defect-Rich Honeycomb-like Nickel Cobalt Sulfides on Graphene through Rapid Microwave-Induced Synthesis for Ultrahigh Rate Supercapacitors. J. Colloid Interface Sci. 2020, 580, 160–170. [Google Scholar] [CrossRef]
- Sridhar, V.; Kim, H.-J.; Jung, J.-H.; Lee, C.; Park, S.; Oh, I.-K. Defect-Engineered Three-Dimensional Graphene–Nanotube–Palladium Nanostructures with Ultrahigh Capacitance. ACS Nano 2012, 6, 10562–10570. [Google Scholar] [CrossRef]
- Yan, K.; Sun, X.; Ying, S.; Cheng, W.; Deng, Y.; Ma, Z.; Zhao, Y.; Wang, X.; Pan, L.; Shi, Y. Ultrafast Microwave Synthesis of Rambutan-like CMK-3/Carbon Nanotubes Nanocomposites for High-Performance Supercapacitor Electrode Materials. Sci. Rep. 2020, 10, 6227. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Meléndrez, M.; Fernández, K. Development of Gelatin Aerogels Reinforced with Graphene Oxide by Microwave-Assisted Synthesis: Influence of the Synthesis Conditions on Their Physicochemical Properties. Polymer 2020, 208, 122951. [Google Scholar] [CrossRef]
- Cuenca, J.A.; Thomas, E.; Mandal, S.; Williams, O.; Porch, A. Microwave Determination of Sp2 Carbon Fraction in Nanodiamond Powders. Carbon 2015, 81, 174–178. [Google Scholar] [CrossRef]
- Adam, M.; Hart, A.; Stevens, L.A.; Wood, J.; Robinson, J.P.; Rigby, S.P. Microwave Synthesis of Carbon Onions in Fractal Aggregates Using Heavy Oil as a Precursor. Carbon 2018, 138, 427–435. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; Laíño, R.B.; Calzón, J.A.G.; García, M.E.D. Facile Synthesis of Water-Soluble Carbon Nano-Onions under Alkaline Conditions. Beilstein J. Nanotechnol. 2016, 7, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Vadahanambi, S.; Jung, J.-H.; Kumar, R.; Kim, H.-J.; Oh, I.-K. An Ionic Liquid-Assisted Method for Splitting Carbon Nanotubes to Produce Graphene Nano-Ribbons by Microwave Radiation. Carbon 2013, 53, 391–398. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Joanni, E.; Sahoo, S.; Kawamura, G.; Matsuda, A. Microwave-Assisted Synthesis of Iron Oxide Homogeneously Dispersed on Reduced Graphene Oxide for High-Performance Supercapacitor Electrodes. J. Energy Storage 2022, 56, 105896. [Google Scholar] [CrossRef]
- Yang, S.; Li, S.; Song, L.; Lv, Y.; Duan, Z.; Li, C.; Praeg, R.F.; Gao, D.; Chen, G. Defect-Density Control of Platinum-Based Nanoframes with High-Index Facets for Enhanced Electrochemical Properties. Nano Res. 2019, 12, 2881–2888. [Google Scholar] [CrossRef]
- Lu, J.; Luo, L.; Yin, S.; Hasan, S.W.; Tsiakaras, P. Oxygen Reduction Reaction over PtFeM (M = Mo, V, W) Alloy Electrocatalysts: Role of the Compressive Strain Effect on Pt. ACS Sustain. Chem. Eng. 2019, 7, 16209–16214. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.; et al. PdMo Bimetallene for Oxygen Reduction Catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Back, S.; Kim, J.-H.; Kim, Y.-T.; Jung, Y. Bifunctional Interface of Au and Cu for Improved CO 2 Electroreduction. ACS Appl. Mater. Interfaces 2016, 8, 23022–23027. [Google Scholar] [CrossRef]
- Rondinini, S.; Aricci, G.; Krpetić, Ž.; Locatelli, C.; Minguzzi, A.; Porta, F.; Vertova, A. Electroreductions on Silver-Based Electrocatalysts: The Use of Ag Nanoparticles for CHCl 3 to CH 4 Conversion. Fuel Cells 2009, 9, 253–263. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yin, K.; Zhang, J.; Gao, H.; Liu, N.; Peng, Z.; Zhang, Z. Nanoporous Iridium-Based Alloy Nanowires as Highly Efficient Electrocatalysts Toward Acidic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 39728–39736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Z.; Xie, M.; Lyu, Z.; Chi, M.; Mavrikakis, M.; Jin, W.; Xia, Y. Iridium-Based Cubic Nanocages with 1.1-Nm-Thick Walls: A Highly Efficient and Durable Electrocatalyst for Water Oxidation in an Acidic Medium. Angew. Chem. Int. Ed. 2019, 58, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, L. Carbon-Based Metal-Free Catalysts. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]

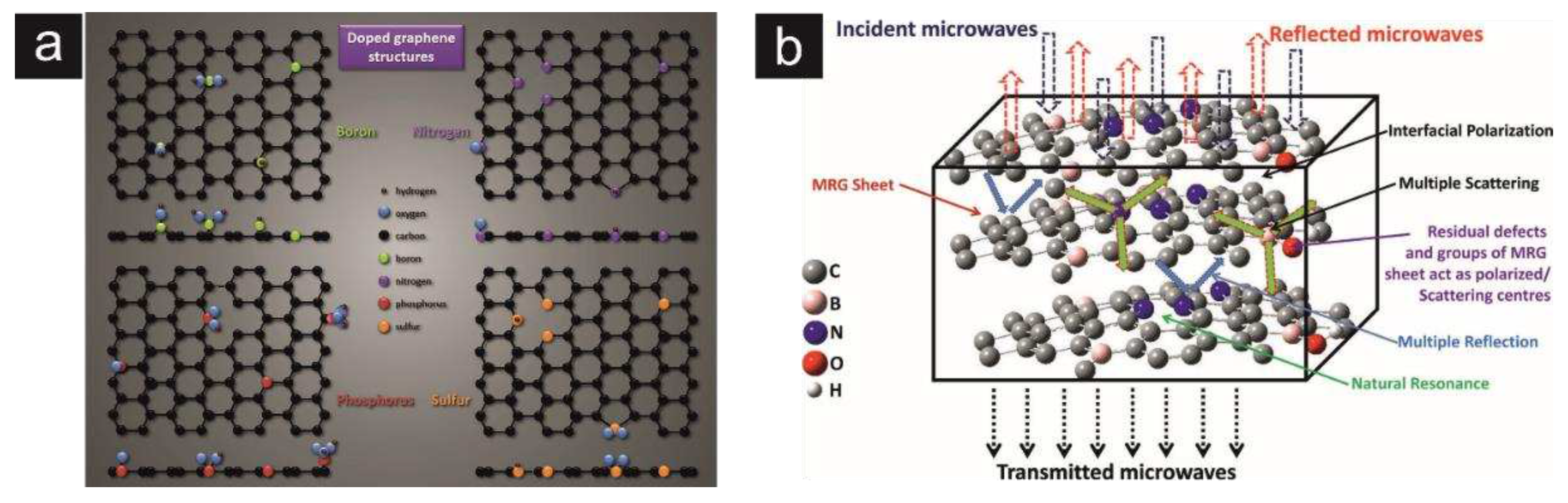
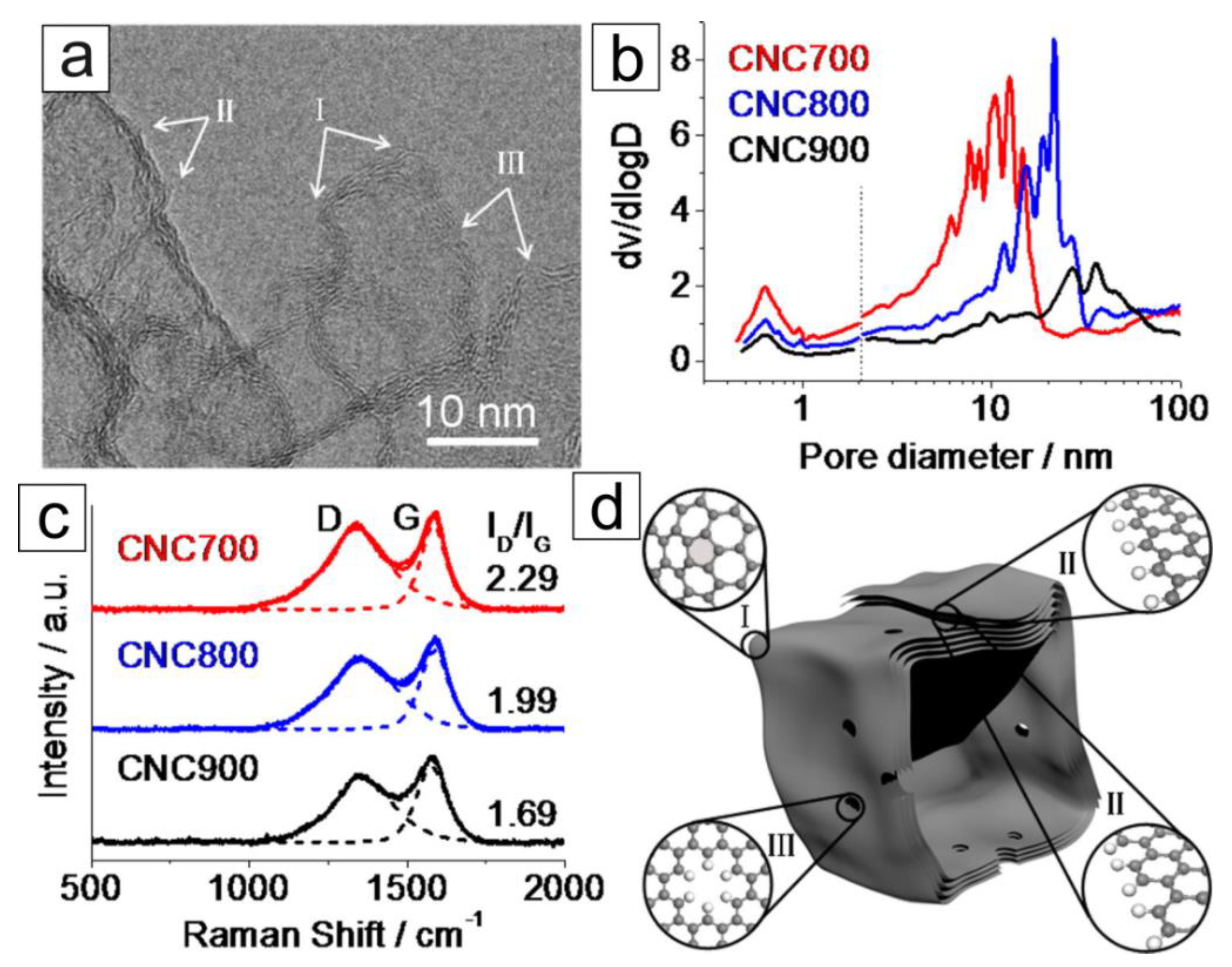
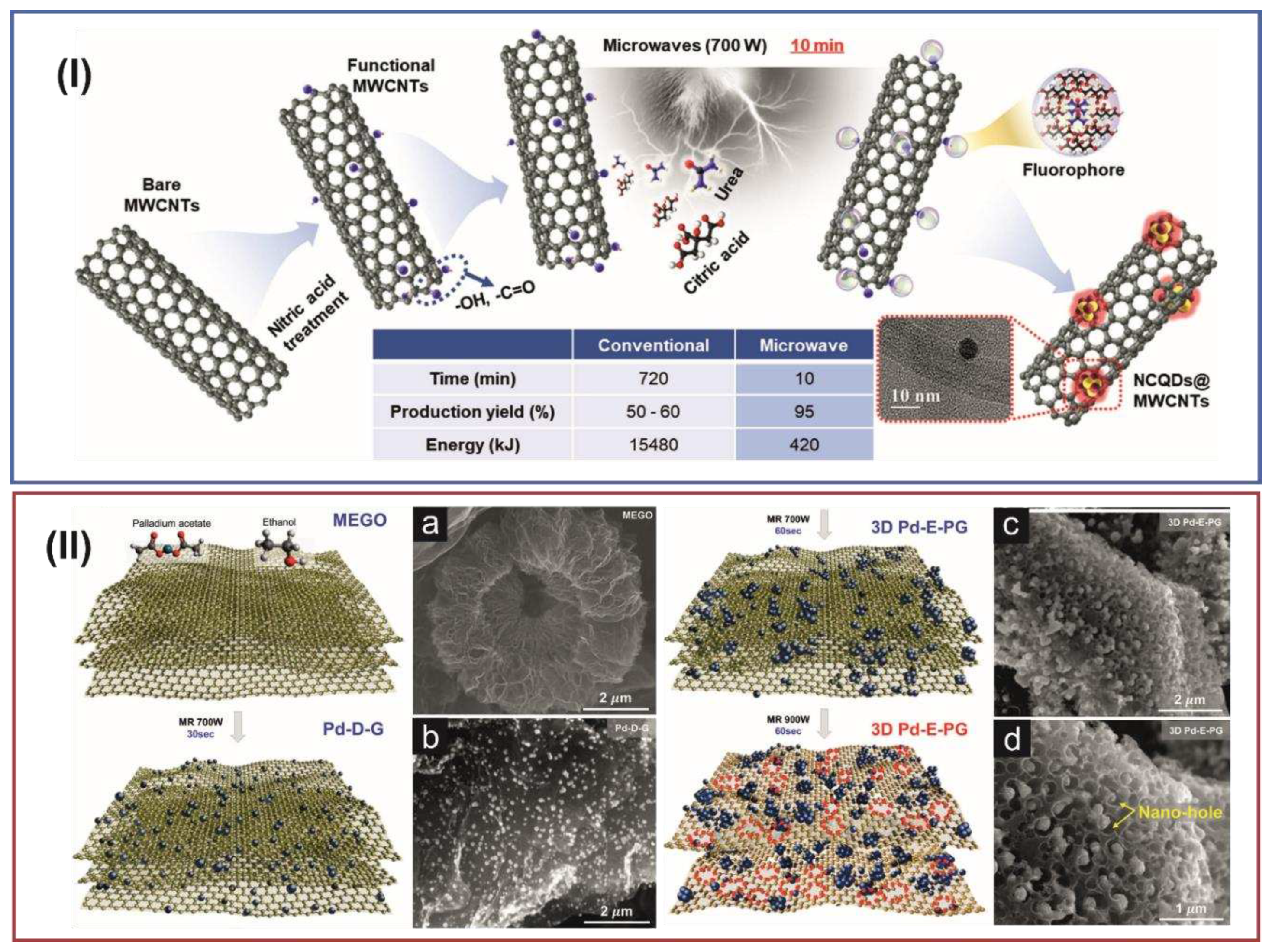
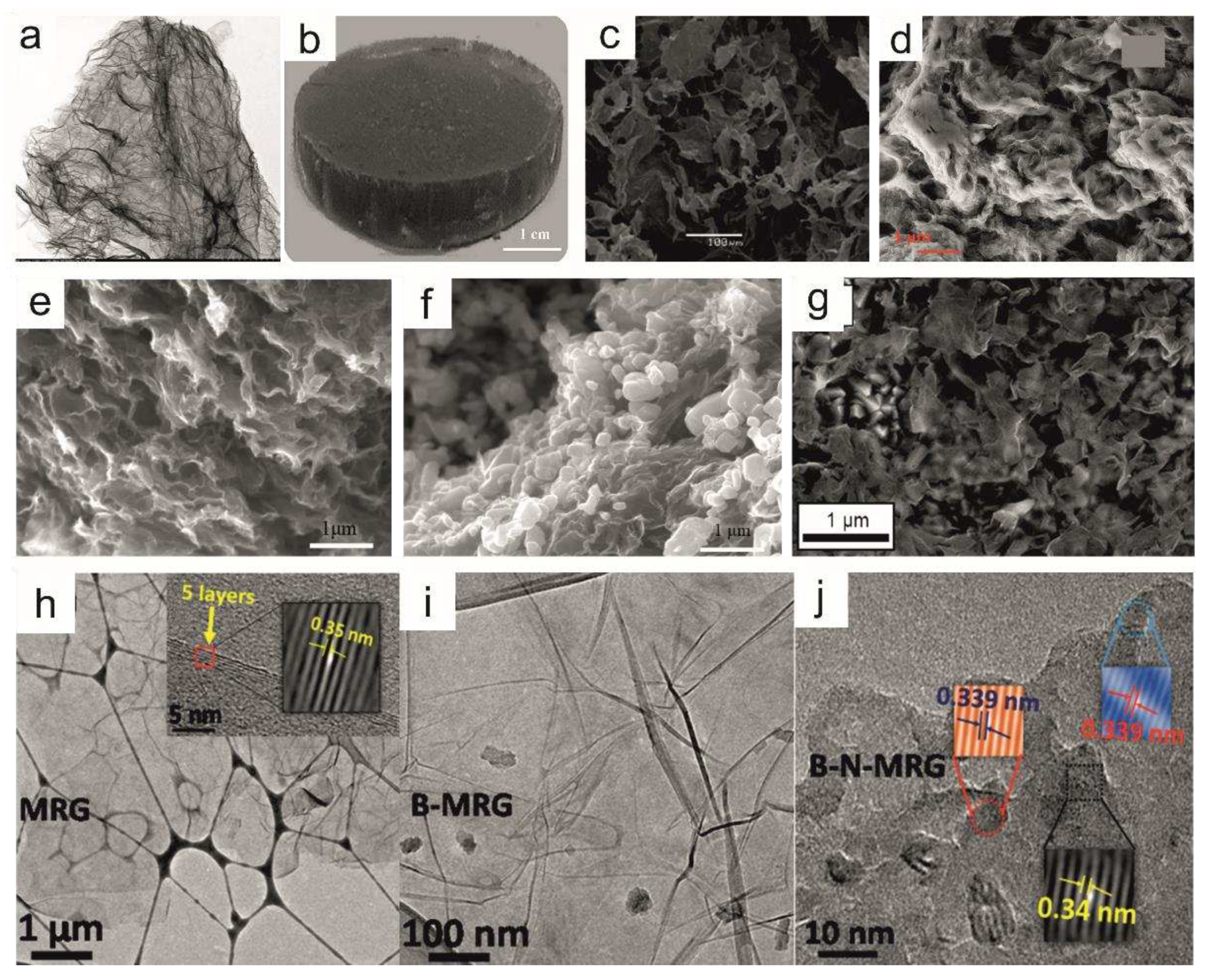
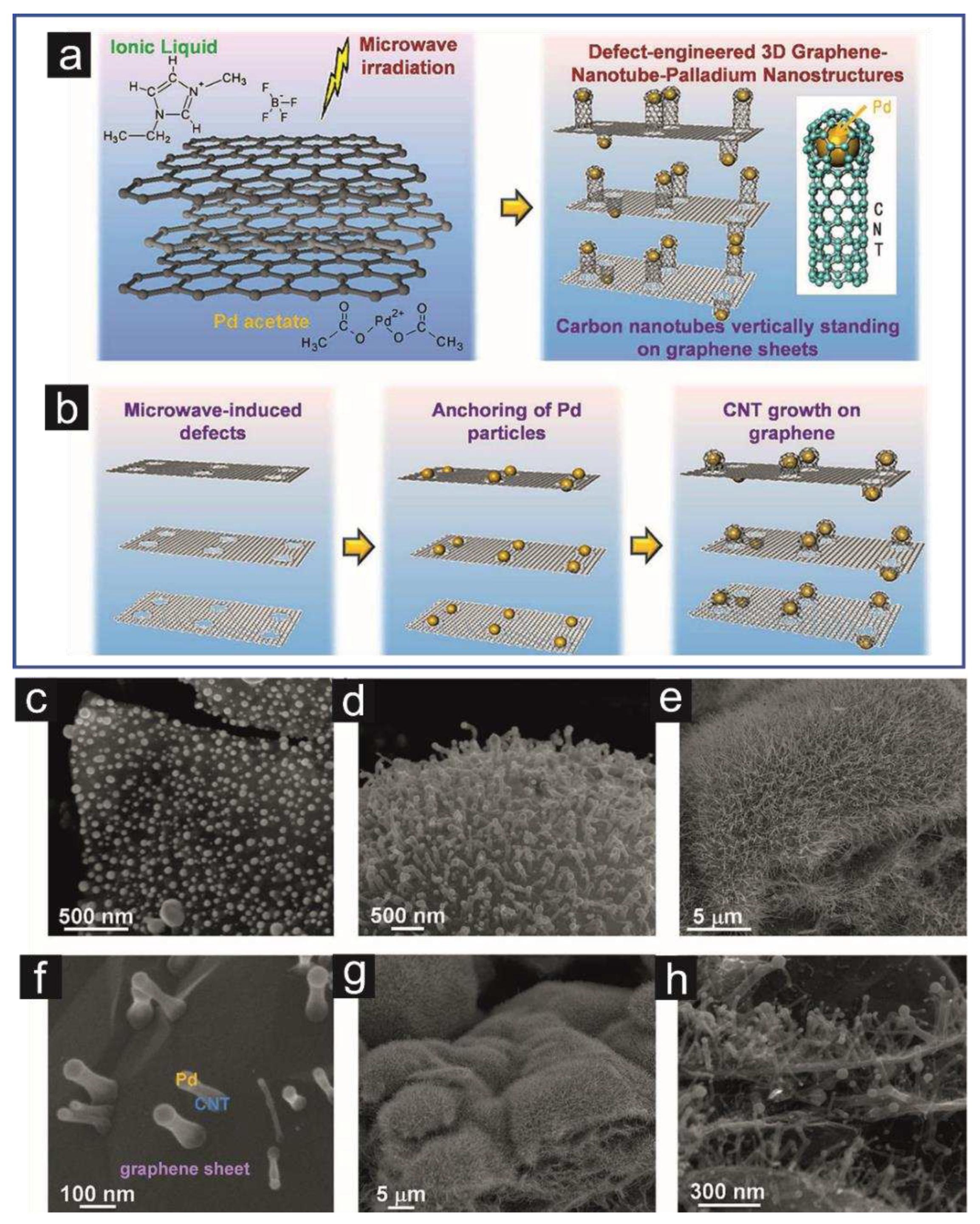
| Heteroatom doping |
Substrates | Carbon nanostructures |
Methods | Applications | Ref. |
|---|---|---|---|---|---|
| Nitrogen | Collagen | OLC | Thermal annealing | ORR catalysis | [72] |
| Acetonitrile | CNO | Pyrolysis | ORR catalysis Nitride sensor |
[78] | |
| Graphene Acetonitrile |
Graphene | CVD | Supercapacitor | [74] | |
| Graphene Melamine |
Graphene | Thermal annealing | ORR, OER, HER catalysis | [79] | |
| Melamine L-cysteine |
Graphene | Polymerization Pyrolysis |
ORR and OER catalysis Zn-air batteries |
[41] | |
| CNT Ionic liquid |
CNT/porous carbon (Core-sheath) |
Carbonization Post-modification |
ORR catalysis | [73] | |
| CNT Melamine |
CNT/porous carbon | Pyrolysis Polymerization Post-modification |
ORR catalysis Zn-air batteries |
[80] | |
| Citric acid Urea Aniline |
GQD | MW-assisted hydrothermal process Polymerization |
Gas sensing | [81] | |
| Acetonitrile, pyridine, amine N2 gas atmosphere |
Carbon nanoflakes |
Hard-templating Pyrolysis |
Electrochemistry | [82] | |
|
Halogen (F, Cl, Br, I) |
Graphite Halogen-containing acids |
rGO | Electrochemical exfoliation/post-modification GO | ORR catalysis | [83] |
| Sulfur | Melamine Thiourea |
Graphitic carbon nitride (g-C3N4) nanoflakes | Pyrolysis | NO2 gas sensors | [63] |
| Multi-heteroatoms | Compounds containing S, O, N, C | CNT (S, O, N) |
Post-modification | ORR catalysis | [84] |
| Compounds containing O, N, C | CNT (N, O) |
Post-modification | ORR catalysis | [64] | |
| Solid graphite rod Nitrogen atmosphere Amorphous boron |
CNT (N, B) |
Arc-discharge evaporation | Conductors Magnetoconductors |
[76] | |
| Melamine Phosphoric acid |
Graphitic carbon nitride (P) | Thermal annealing | ORR catalysis | [85] | |
| Citric acid L-cysteine |
GQD (N, S) |
Hard-templating Carbonization |
Fluorescence detection of Fe3+ | [86] | |
| ND Boric acid |
CNO (N, B) |
Thermal annealing | ORR catalysis | [87] | |
| Olive oil Nitric acid |
CNO (N, O) |
Pyrolysis CVD |
Fuel cells | [75] | |
| Benzene | Carbon nanocages (O, N) | Hard-templating Thermal annealing |
ORR catalysis | [31] |
| Defective CNs | Materials containing d-CNs | Methods Parameters |
Applications | Refs. |
|---|---|---|---|---|
| d-G (hydrogel) | MW-assisted synthesis (800 W; 5 min.) Hydrothermal process |
Supercapacitors (340 F/g at 0.5 A/g) |
[115] | |
| Pristine d-CNs | N,S-GO | MW-assisted synthesis (800 W; 5 min.) |
Supercapacitors | [88] |
| S-rGO | MW-assisted synthesis (140°C; 30 min.) |
Supercapacitors (238 F/g) |
[116] | |
| N,B-rGO | MW-assisted synthesis |
EMI shielding devices | [77] | |
| rGO (porous) | MW-assisted synthesis (700 W; 180°C; 6 min.) |
Supercapacitors (568 F/g at 1 A/g) |
[117] | |
| rGO | IL-assisted MW synthesis (700 W; 15 s.) |
Supercapacitors (135 F/g; 58 Wh/kg; 246 kW/kg) |
[118] | |
| d-CNT | MW hydrogen plasma processing | Vacuum electron sources | [54] | |
| d-CNT | MW-assisted synthesis (C2H2/H2, 0.6 ratio; 900 W; 35 min.) |
Sorbents (removing organic pollutants from wastewater) | [119] | |
| N-CQD/ox-MWCNT | MW-assisted synthesis (700 W; 10 min.) |
Electrocatalysis DSSC |
[120] | |
| GO/g-C3N4 | Ultrasonic-MW-assisted synthesis (700 W; 5 min.) |
Photocatalytic H2 evolution |
[121] | |
| N-PGF | MW-assisted synthesis (800 W; 4 s.) |
Supercapacitors (12.3 mW h/cm; 0.42 W/cm) Sorbents |
[122] | |
|
Hybrid materials containing d-CNs |
CNT/Fe2O3 | CVD growth of CNTs Hard templating Hydrothermal method MW-assisted synthesis |
Lithium-Ion Battery Electrodes | [123] |
| N-MWCNT/Fe3O4 | CVD growth of MWCNTs MW-assisted solvothermal synthesis (800 W; 1.5 min.) |
Superparamagnetic materials | [124] | |
| CNT/NiMn2O4 | MW-assisted hydrothermal synthesis (800 W; 160°C; 1 h) |
Supercapacitors (916 F/g at 1 A/g; 36.5 Wh/kg; 800 W/kg) |
[125] | |
| MWCNT/CoMoO4 | MW-assisted solid-state synthesis (480 W; 8 min.; 720 W; 7 min.) |
Supercapacitors (170 F/g at 0.1 A/g) |
[126] | |
| NiS@CNT/NiO | MW-assisted solid-state synthesis (1000 W; 60 s.) |
Supercapacitors (810 F/g at 1 A/g) |
[127] | |
| ox-CNT/o-PDA-co-ANI | MW-assisted synthesis | Supercapacitors (147 F/g at 0.5 A/g) |
[128] | |
| rGO/NiS | MW-assisted hydrothermal synthesis (700 W; 4 min.) |
Supercapacitors (1746 F/g at 1 A/g) Solid-state Supercapacitors (14.20 F/g; 7.1 Wh/kg; 1836 W/kg) |
[129] | |
| rGO/Fe3O4 | Chemical exfoliation MW-assisted synthesis (700 W; 1.25-1.75 min.) |
Lithium-Ion Battery Electrodes | [130] | |
| rGO/CNT/NiNP | Thermal exfoliation MW-assisted synthesis (700 W; 5 min.) |
Lithium-Ion Battery Electrodes | [131] | |
| GO/NiO | Exfoliation MW-assisted synthesis |
Energy storage devices (549 F/g at 10 mV/s) |
[132] | |
| rGO/NiO/Co3O4 | MW-assisted synthesis (700 W; 45 sec.) |
Supercapacitors | [133] | |
| rGO/CoAl-LDH | MW-assisted reflux synthesis (1000 W; 100°C; 2 h) |
Supercapacitors | [134] | |
| rGO/NiAl-LDH | MW-assisted reflux synthesis (1000 W; 100°C; 2 h) |
Supercapacitors | [135] | |
| rGO/NiMoO4 | MW-solvothermal synthesis (200 W; 115°C; 25 min.) Thermal annealing |
Supercapacitors | [136] | |
| N-G/NiS | MW-assisted synthesis |
Supercapacitors (1468 F/g at 1 A/g; 66.6 Wh/kg; 405.8 W/kg) |
[137] | |
| rGO/MnCo2O4 | Exfoliation Reduction MW-assisted synthesis (900 W; 45, 55 and 70 s) |
Supercapacitors (562 F/g at 20 mV/s) |
[138] | |
| G/α-MoO3 | MW-assisted synthesis (700 W; 7 min.) |
Supercapacitors (483 F/g at 1 A/g) |
[139] | |
| rGO/CoSe2 | MW-assisted synthesis (700 W; 7 min.) Thermal annealing |
Supercapacitors (761 F/g at 1 A/g; 43.1 Wh/kg) LED |
[140] | |
| G/Co9S8 | MW-assisted hydrothermal synthesis (700 W; 160°C; 30 min.; 8·106 Pa) |
Supercapacitors (1150 F/g at 5 mV/s) |
[141] | |
| rGO/MnN | MW-assisted synthesis (900 W; 1 min.) |
Sodium ion batteries Supercapacitors EMI shielding |
[142] | |
| rGO/MnO2 | Conventional synthesis MW-assisted synthesis (700 W; 2 min.; 21 cycles) |
Supercapacitors (140 F/g at 1 A/g) |
[143] | |
| N-rGO/Pd | MW-assisted synthesis (900 W; 1 min.) |
Direct-Ethanol Fuel Cells | [144] | |
| rGO/Pd | MW-assisted synthesis | Electrocatalysis (Ethanol Oxidation) |
[145] | |
| S-rGO/NiFeS2 | MW-assisted synthesis | Supercapacitors (1073 F/g at 1 A/g; 45.7 Wh/kg; 222 W/kg) |
[146] | |
| 3D Pd-E-PG | MW-assisted synthesis (700÷900 W; 30÷60 sec.) |
H2 storage CO oxidation |
[147] | |
| NiF-G/SimonK | MW-hydrothermal synthesis | Supercapacitors (836 F/g at 1 A/g) |
[148] | |
| G/NiCoS | MW-assisted synthesis (600 W; 20 min.) |
Supercapacitors (1186 F/g at 1 A/g; 46.4 Wh/kg) |
[149] | |
| G/CNT/Pd | IL-assisted MW synthesis |
Energy storage systems (1615 F/g at 10 mV/s) |
[150] | |
| CMK-3/CNT | Hard-templating method MW-assisted synthesis (700 W; 30 s.) |
Supercapacitors (315 F/g at 1 A/g) |
[151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





