Submitted:
03 August 2023
Posted:
03 August 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethanolic Extracts Propolis (EEP)
2.2. Gas Chromatographic-Mass Spectrometry (GC-MS)
2.3. Evaluation of antimycotic activity:
2.3.1. Inoculum preparation
2.3.2. Determination of Minimum Inhibitory Concentration and minimum fungicidal concentration
2.4. Structural damage
3. Results
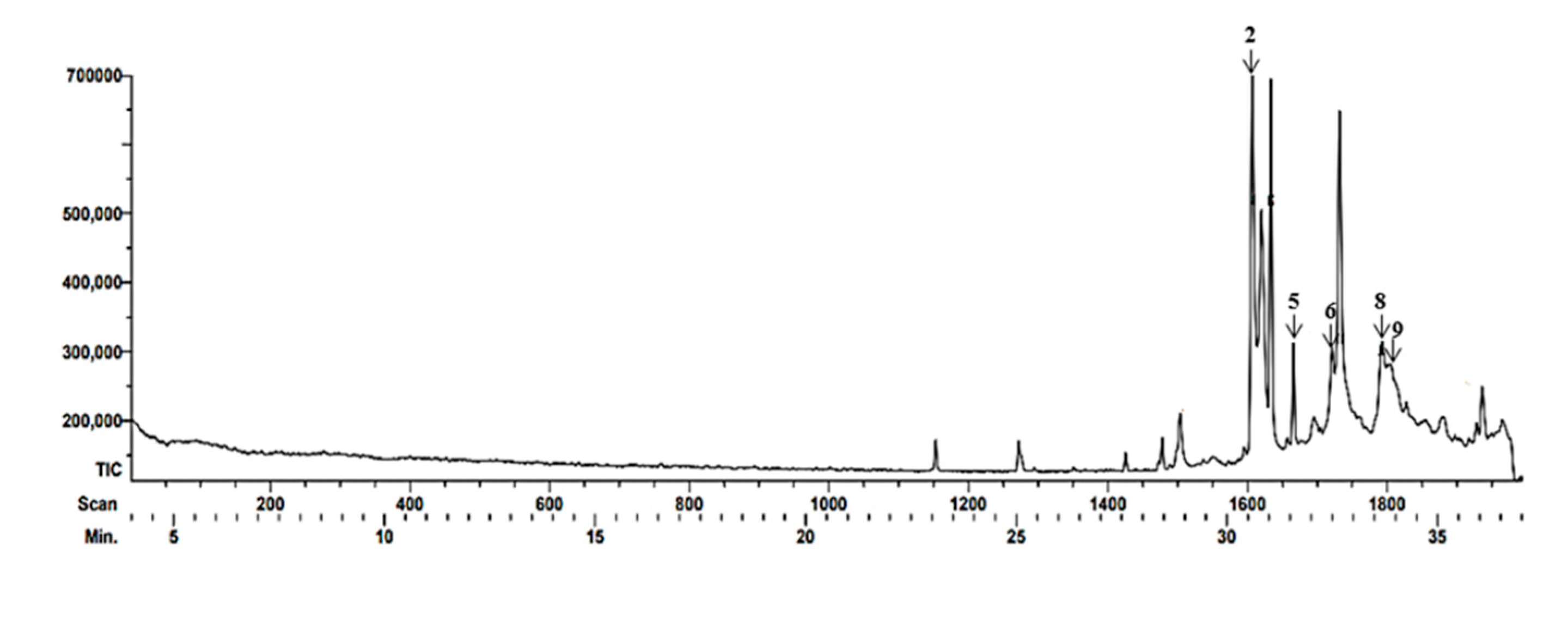
3.1. Gas Chromatographic-Mass Spectrometry (GC-MS)
| Bak | Time Retention (TR) |
Composite proposed by the database |
Chemical classification |
Biological activity | Reference |
|---|---|---|---|---|---|
| 2 | 30.6 | 1,4-Methanecycloocta[d]piri-dazine, 1,4,4a,5,6,9,10,10a-octahydro-11,11-dimethyl-, (1.alpha.,4.alpha.,4a.alfa. ,10a.alfa.) - | Pyridazine (heterocyclic compound) | Antioxidant | [27] |
| 5 | 31.58 | Fanersol Isomer a | Sesquiterpene | Antimicrobian | [28] |
| 6 | 32.48 | Ethanone, 1-(1,3a,4,5,6,7-hexahydro-4-hydroxy-3,8-dimethyl-5-azulenyl)- | Ketone Sesquiterpene |
Antimicrobian | [29] |
| 8 | 33.68 | 2H-1-Benzoxacyclohexadecin-16(18aH)-one,3,4,5,6,7,8,9,10,11,12,13,14-dodecahydro-18,18a-dihdroxy-2-methyl | Macrocycle | Activity Not reported | [30,31] |
| 9 | 33.7 | Furan-2,5-dicarbaldhyde 2,5-Furandicarboxaldehydo | Heterocyclic compound with aldehyde groups | Antioxidant Antimicrobian |
[32,33] |
3.2. Evaluation of the antimycotic activity
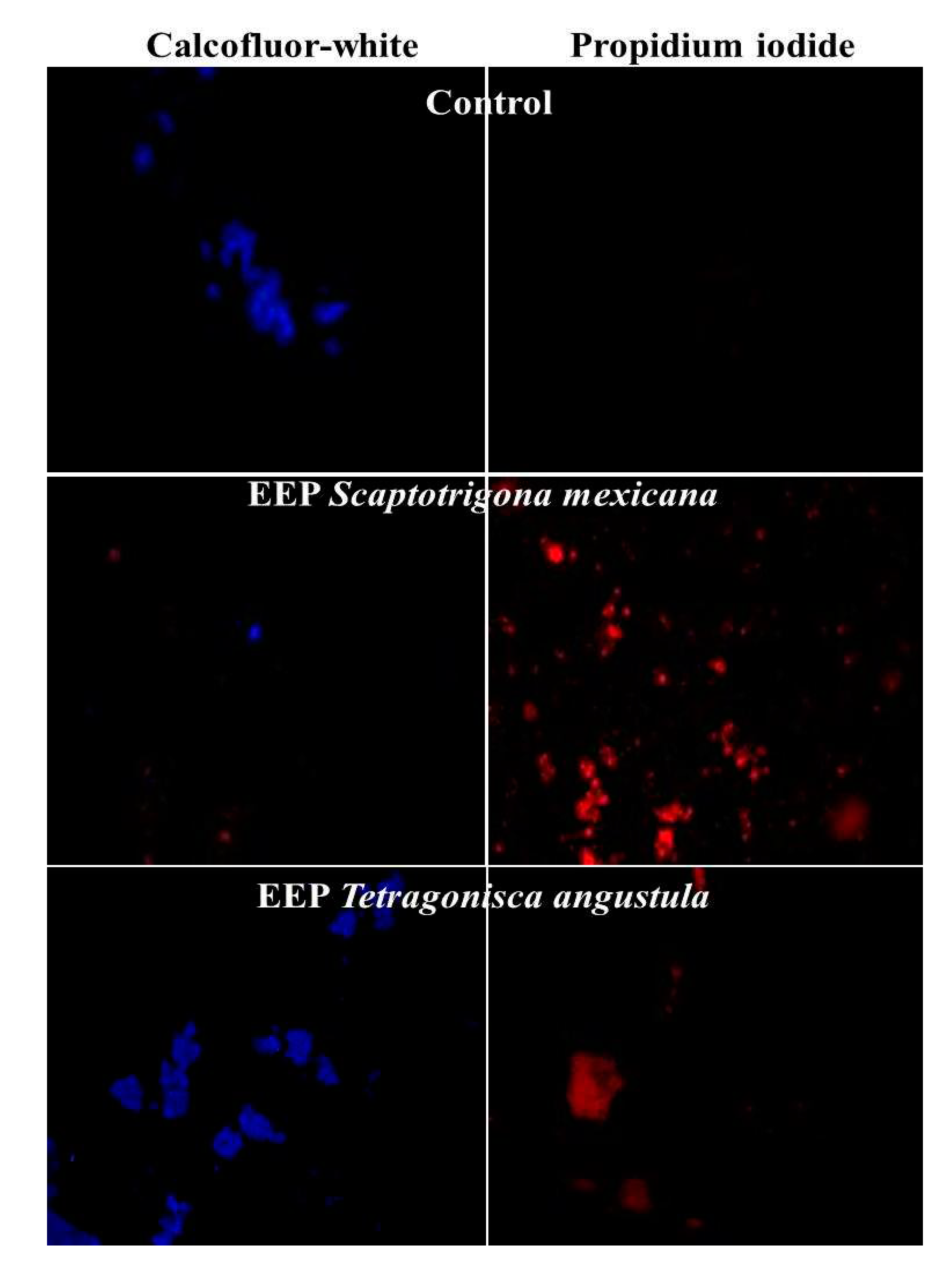
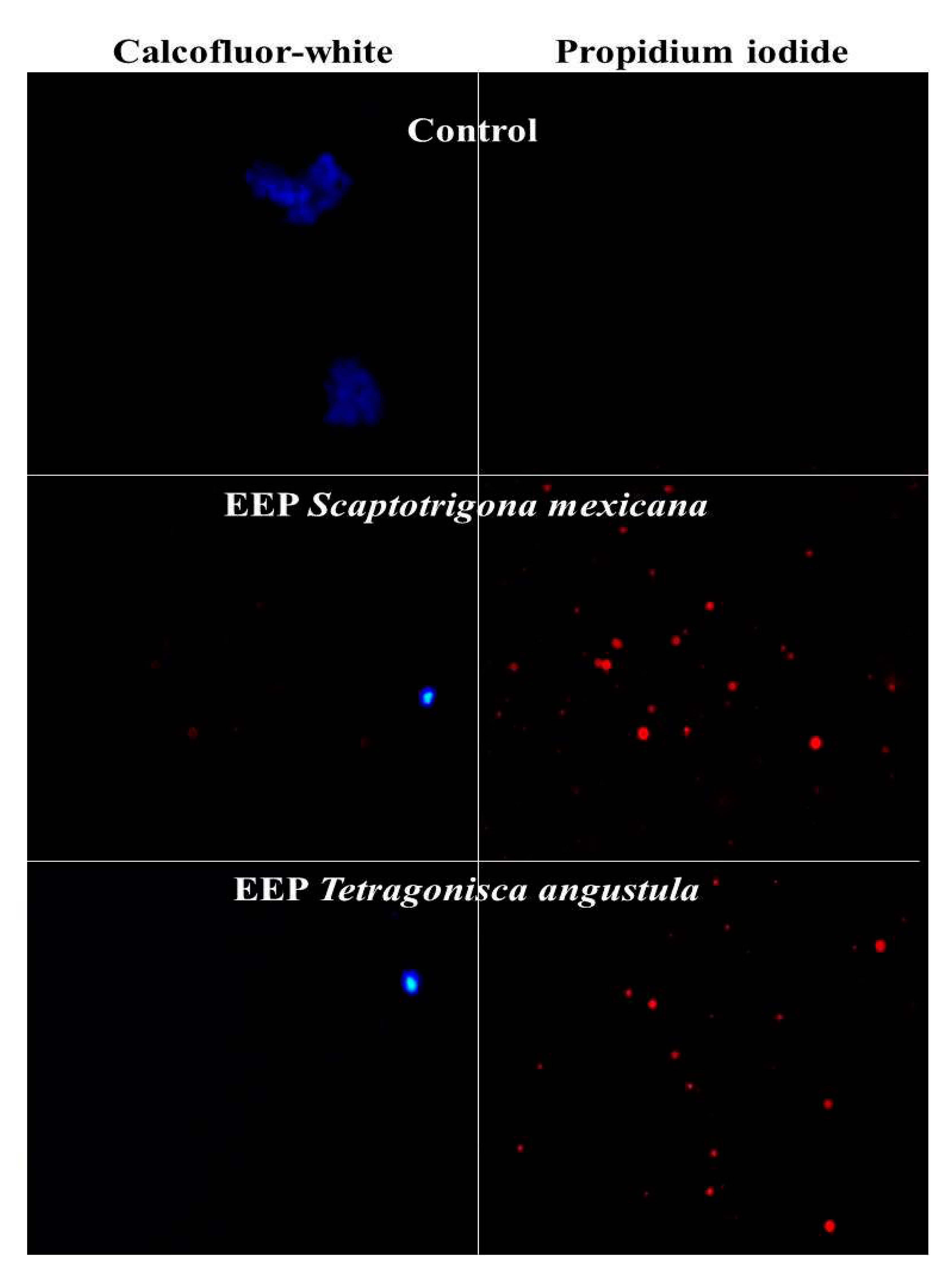
3.3. Structural damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakchiche, B.; Temizer, I.K.; Aytaç,G. A.; Çelemli, G.; Yegin, C.B.; Ghareeb, M.A.; Chemical Composition and Biological Activities of Honeybee Products From Algeria. J. Appl. Biotechnol. Rep. 2020, 7, 93–103. [Google Scholar]
- Bankova, V.; Popova, M. Propolis of Stingless Bees: A Promising Source of Biologically Active Compounds. Pharmacogn Rev. 2007, 1, 88–92. [Google Scholar]
- Ramon, S.J.; Peraz, L.E.; Rodríguez, B.R.; Yam, P.A.; Madera, S.T.; Ortiz, VE. Partial characterization of ethanolic extract of Melipona beecheii propolis and in vitro evaluation of its antifungal activity. Rev. Bras. Farmacogn. 2019, 29, 319–324. [Google Scholar] [CrossRef]
- Arnold N, Zepeda R, Vázquez M, Aldasoro, M. Las abejas sin aguijón y su cultivo en Oaxaca, México: Con catálogo de especies. 1a ed; ECOSUR-CONABIO, San Cristóbal de Las Casas. México, 2018, 10-58.
- Ayala, R. Revisión de las abejas sin aguijón de México (Hymenoptera: Apidae: Meliponini). Folia Entomol. Mex. Mex. 1999, 106–123. [Google Scholar]
- Pardini, R.; da Rocha, P.L.B.; El-Hani, C.; Pardini, F. Desafíos y Oportunidades para Cerrar la Brecha entre Investigación e Implementación en Ciencias y Gestión Ecológicas en Brasil. Biología de la conservación: Voces de los trópicos. PH Raven, NS Sodhi & L. Gibson : John Wiley & Sons, Ltd., Reino Unido, 2013, 75-85.
- Hurtado, M.M.; Jara, L.; May, I.J.; Quezada, E.G.; Ruiz, C.P.; De la Rúa, P. A geometric morphometric and microsatellite analyses of Scaptotrigona mexicana and S. pectoralis (Apidae: Meliponini) sheds light on the biodiversity of Mesoamerican stingless bees. J. Insect Conserv. 2016, 20, 753–763. [Google Scholar]
- Mateo, A.J.; Rodríguez, P.B.; González, G.S.; Cruz, T.A. Structural damage in Cryptococcus neoformans caused by a Mexican propolis. Nova Scientia. 2020, 12, 1–17. [Google Scholar]
- Quintero, M.M.L.; Londoño, O.A.; Manzano, G.P.; López, M.R.; Soto, Z.C.I.; Carrillo, M.L.; Penieres, C.J.G.; García, T.C. G,.;Cruz-Sánchez, T.A. Effect of Mexican propolis extracts from Apis mellifera on Candida albicans in vitro growth. Rev Iberoam Micol. 2008, 25, 22–26. [Google Scholar] [CrossRef]
- Rodríguez, P.B.; Canales, M.M.; Penieres, C.J.; Cruz, S.T. Chemical composition, antioxidant properties and antimicrobial activity of Mexican propolis. Acta Universitaria. 2019, 30, 1–29. [Google Scholar] [CrossRef]
- Flores, I.S.; Moreno, M.; Londoño, A. ; Cruz. TA. Use of mexican propolis for the tropical treatment of dermatomycosis in horses. Open J. Vet. Med. 2016, 6, 1–8. [Google Scholar]
- Tovar, N.; García,L. ; Cruz TA. Propolis in dogs: Clinical experiences and perspectives (a brief review). Open J. Vet. Med. 2016, 5, 11–17. [Google Scholar]
- Girãoa, M.R.N.; Brilhanteab, RA.; Cordeirob, A.J.; Monteiroc, J.J.C.; Sidrimb,M. F.G.; Rochaab, P.RS. Malassezia pachydermatis isolated from normal and diseased external ear canals in dogs: A comparative analysis. Vet. J. 2006, 172, 544–548. [Google Scholar]
- Lozina, L.; Boehringer, S.; D’Aquino, M.; Acosta, O. Eficacia del propóleos Sobre Malassezia pachydermatis. Correlación de Diferentes Técnicas in vitro. Acta Farm. Bonaerense. 2006, 25, 560–563. [Google Scholar]
- Cabañes, F. J. Diagnosis of Malassezia pachidermatitis and otitis in dogs and cats, is it just a matter of counting? Rev Iberoam Micol. 2021, 38, 3–4. [Google Scholar] [CrossRef]
- Deegan, K.R.; Fonseca, M.S.; Oliveira, D.C.P.; Santos,L. M.; Fernández, C.C.; Hanna,S.A.; Machado, B.A.S.; Umsa-Guez, M.A.;Meyer,; Portela,R.W. Susceptibility of Malassezia pachydermatis clinical isolates to allopatic antifungals and Brazilian red, green, brown propolis extracts. Front. Vet. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Lozina, L.A.; Peichoto, M.E.; Boehringer, S.I.; Koscinczuk, P.; Granero, G.E.; Acosta, O.C. Efficacy of Argentine propolis formulation for tropical treatment of canine otitis externa. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1359–1366. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación. Norma Oficial Mexicana NOM-003-AG/GAN-2017: Propóleos, producción y especificaciones para su Procesamiento. 2017. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5500103&fecha=06/10/2017#gsc.tab=0.
- Dos Santos, L.; Hochheim, S.; Boeder, A.M.; Kroger, A.; Tomazzoli, M.M.; Dal Pai,R. ; Maraschin, M.; Guedes, A.; de Cordova, C.M.M.; Chemical characterization, antioxidant, cytotoxic and antibacterial activity of propolis extracts and isolated compounds from the Brazilian stingless bees Melipona quadrifasciata and Tetragonisca angustula. J. Apic. Res. 2017, 56, 543–558. [Google Scholar] [CrossRef]
- Rivera; Y. C.R., Terrazas, L.I.; Jiménez, E.; Campos, J.E.; Flores, O.C.M.; Hernández, L.B.; Cruz, S.T.; Garrido, F.G.I.; Rodríguez-Monroy, M.A.;Canales M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, α-Pinene and γ-Terpinene-An in vitro study. Molecules. 2017, 22, 1–13. [Google Scholar]
- Kaneko, T.; Makimura, K.; Abe, M.; Shiota, R.; Nakamura, Y.; Kano, R.; Hasegawa, A.; Sugita, T.; Shibuya, S.; Watanabe, S.; Yamaguchi, H.; Abe, S.; Okamura, N. Revised Culture-Based System for Identification of Malassezia Species. J. Clin. Microbiol. 2007, 45, 3737–3742. [Google Scholar] [CrossRef]
- Aspiroz, C.; Gilaberte, Y.; Rezusta, A.; Boekhout, T. ; Rubio. M.C. Gentamycin inhibits the growth of Malassezia pachydermatis in culture. Rev. Iberoam. Micol. 2010, 27, 20–21. [Google Scholar]
- Kaneko, T.; Makimura, K.; Abe, M.; Shiota, R.; Nakamura, Y.; Kano, R.; Hasegawa, A.; Sugita, T.; Shibuya, S.; Watanabe, S.; Yamaguchi, H.; Abe, S.; Okamura, N. Revised Culture-Based System for Identification of Malassezia Species. J. Clin. Microbiol. 2007, 45, 3737–3742. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- K. Sánchez, A.K.;Fernández M.R.F.; Moreno M. M. ;Villegas A.L.; Meneses G.F.; Arenas G.R. Sensitivity and specificity of mycological direct examination with calcofluor hite for the diagnosis of onychomycosis. Med. Cutan. Iber. Lat. Am. 2013, 41, 261–266. [Google Scholar]
- Phillips, A.J. ; Ian Sudbery,I. ;Ramsdale,M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. PNAS. 2003, 100, 14327–14332. [Google Scholar] [PubMed]
- Urbizu, G.A.L.; Castillo, R.O.; Martínez, A.G.C.; Torres,C. J.A. Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac. J. Trop. Biomed. 2017, 10, 121–125. [Google Scholar]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.A.; Silva, J.R.; da Silva, D.F.; a Rocha, J. .LC.; Neves,B.H.; Borges, M.M.;Umzza, G.M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE. 2018, 3, 1–20. [Google Scholar]
- Fatnassi, S.; Zarrouk, H.; Chatti, S. Chemical composition and antimicrobial activity of volatile fraction of the peel of Maclura pomifera fruit growing in Tunisia. J. Soc. Chim. Tunis. 2011, 13, 1–6. [Google Scholar]
- Delmondes, G.A.; Santiago, L.I.C.; Días, D.Q.; Cunha, G.L.D.; Araújo, I.M.; Barbosa, R.; Coutinho, H.D.M.; Felipe, CF.B.; Barbosa, F.J.M.; Lima, N.T.R.; De Menezes, I.R.A.; Kerntopf, M.R. Pharmacological Applications of Farnesol (C15H26O): A patent review. Expert Opin Ther Pat. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Chen, C.; Change, H.; Kirk, K. Betulachrysoquinone hemiketal: A p-benzoquinone hemiketal macrocyclic compound produced by Phanerochaete chrysospor. Phytochemistry. 1977, 16, 1983–1985. [Google Scholar] [CrossRef]
- Muriira, K.G. : Nyagah, M.N.E.; Machocho, A.K.; Nyawira, W.L., Mungiria, J.N.M. Chemical Composition and in vitro Antioxidant Activities of Ocimum americanum. Adv. Anal. Chem. 2015, 5, 42–49. [Google Scholar]
- Otieno, A.J. Antimicrobial activity and phytochemical profiles of Warburgia ugandensis sprague (canellaceae) extracts from different populations across the Kenyan Rift Valley a Thesis Submitted in Partial Fulfillment of the Requirements for the Award of the Degree of Master of Science (Biotechnology) in the School of Pure and Applied Sciences of Kenyatta University. Kenia 2016.
- Peng, D.; Yu, Z.X.; Wang, C.H.; Gong,B. ; Liu,Y.Y.; Wei, J.H. Chemical Constituents and Anti-Inflammatory Effect of Incense Smoke from Agarwood Determined by GC-MS. Int. J. Anal. Chem. 2020, 4575030, 1–19. [Google Scholar]
- Satyala, P.; Maharjanb, S.; Setzera,W. N. Volatile Constituents from the Leaves, Fruits (Berries), Stems and Roots of Solanum xanthocarpum from Nepal. Nat. Prod. Commun. 2015, 10, 361–364. [Google Scholar] [CrossRef]
- Takahashi, S.; Yeo, Y.; Zhao, Y.; O’Maille, P.E.; Greenhagen, B.T.; Noel, J.P.; Coates, M.R.; Chappell, J. Functional Characterization of Premnaspirodiene Oxygenase, a Cytochrome P450 Catalyzing Regio- and Stereo-specific Hydroxylations of Diverse Sesquiterpene Substrates. Int. J. Biol. Chem. 2007, 282, 31744–31754. [Google Scholar] [CrossRef]
- Bruzua, V.H.; Henríquez, G.W.; Crescente, O; Lanza, J. G. Aceite esencial de Wedelia calycina (Asteraceae): Composición química, actividad antibacteriana y antifúngica. Saber. 2015, 27, 87–93. [Google Scholar]
- Mohd, B.A.A.; Zin, M.; Annisava, A.R.N.; Nafi, N.N.E.; Mohd, K.S. Phytochemical screening and antioxidant properties of stingless bee Geniotrigona thoracica propolis. Mal. J. Fund. Appl. Sci. 2019, 15, 330–335. [Google Scholar] [CrossRef]
- Surek, M.; Fachi. , M.M.;De Fátima, C.A.; De Oliveira, F.F.; Pontarolo, R.; Crisma,A.R.; De Souza, M.W.; Felipe, K.B. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 2021, 269, 1–14. [Google Scholar] [CrossRef]
- Torres, A.R.; Sandjo, L.P.; Friedemann, M.T.; Tomazzoli, M.M.; Maraschin, M.; Mello, C.F.; Santos, A.R.S. Chemical characterization, antioxidant and antimicrobial activity of propolis obtained from Melipona quadrifasciata quadrifasciata and Tetragonisca angustula stingless bees. Braz. J. Med. Biol. Res. 2018, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.A.; Santana, H.A.A.; Yah, P.N.; Ramón, S.J.M.; Cáceres, M.R.; Borges, A.R.L.; Ortiz, VE. Pentacyclic triterpenes and other constituents in propolis extract from Melipona beecheii collected in Yucatan, Mexico. Rev. Bras. Farmacogn. 2019, 29, 358–363. [Google Scholar]
- Grajales, C.J.; Elías, C.J.; Lozano, G.E.; Albores, F.V.; López, G.A. Actividad antimicrobiana de propóleos de abejas sin aguijón en combinación con ajo, Allium sativum (Amaryllidaceae). Rev. Biol. Trop. 2021, 69, 23–35. [Google Scholar]
- Bakchiche B, Temizer IK, Aytaç GA, Çelemli G, Yegin CB, Ghareeb MA. Chemical Composition and Biological Activities of Honeybee Products from Algeria. J. Appl. Biotechnol. Rep. 2020, 7, 93–103. [Google Scholar]
- Ramon, S.J.; Peraz, L.E.; Rodríguez, B.R.; Yam, P.A.; Madera, S.T.; Ortiz, VE. Partial characterization of ethanolic extract of Melipona beecheii propolis and in vitro evaluation of its antifungal activity. Rev. Bras. Farmacogn. 2019, 29, 319–324. [Google Scholar] [CrossRef]
- Peng, D.; Yu,Z. X.;, Wang, C.H.; Gong,B.;Liu,Y.Y.; Wei, J.H. Chemical Constituents and Anti-Inflammatory Effect of Incense Smoke from Agarwood Determined by GC-MS. Int. J. Anal. Chem. 2020, 4575030, 1–19. [Google Scholar]
- Sarvesan, R.; Eganathan, P.; Saranya, J.; Sujanapa, P. Chemical composition and antibacterial activity of leaf essential oil of Eugenia cotinifolia ssp. Codyensis (Munro ex wight) Ashton. Int. J. Pharm. Sci. 2015, 9, 3981–3985. [Google Scholar]
- Xie, C.; Wang, S. , Cao, M.Y.; Xiong, W.;Wu, L. (E)-9-Octadecenoic Acid Ethyl Ester Derived from Lotus Seedpod Ameliorates Inflammatory Responses by Regulating MAPKs and NF-κB Signalling Pathways in LPS-Induced RAW264.7 Macrophages. Evid. Based Complementary Altern. Med. 2022, 2022, 6731360. [Google Scholar] [CrossRef]
- Lotti, C.; Castro, G.M.M.; Sá, LFR. Inhibition of Saccharomyces cerevisiae Pdr5p by a natural compound extracted from Brazilian Red Propolis. Braz. J. Pharmacogn. 2011, 21, 21,901–907. [Google Scholar] [CrossRef]
- Hee, Y.C. In vitro evaluation of the antifungal activity of propolis extract on Cryptococcus neoformans and Candida albicans. Mycobiology. 2002, 30, 93–95. [Google Scholar]
- Campos, J.F.; Dos Santos, U.P.; Da Rocha, P.; Dos S Damião, M.J.; Balestieri, J.B.; Cardoso, C.A.; Paredes-Gamero, E.J.; Estevinho, L.M.; De Picoli,Souza K. ; Dos Santos E.L. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca fiebrigi (Jataí). Evid. Based Complementary Altern. Med. 2015, 2015, 296186. [Google Scholar]
- Shehu, A.; Ismail, S.; Rohin, M.A.K.; Harun, A.; Aziz, A.; Haque, M. Antifungal Properties of Malaysian Tualang Honey and Stingless Bee Propolis against Candida albicans and Cryptococcus neoformans. Appl. Pharm. Sci. 2016, 6, 44–50. [Google Scholar] [CrossRef]
- Farida. S.; Sahlan, M.; Rohmatin, E.; Adawiyah R. The beneficial effect of Indonesian propolis wax from Tetragonula sp. as a therapy in limited vaginal candidiasis patients. Saudi J. Biol. Sci. 2020, 27, 142–146. [Google Scholar]
- Deegan KR, Fonseca MS, Oliveira DCP, Santos LM, Fernández CC, Hanna SA, Machado, BAS, Umsa-Guez MA, Meyer R, Portela RW. Susceptibility of Malassezia pachydermatis clinical isolates to allopatic antifungals and Brazilian red, green, brown propolis extracts. Front. Vet. Sci. 2019, 6, 1–12. [Google Scholar]
- Lozina LA, Peichoto ME, Boehringer SI, Koscinczuk P, Granero GE, Acosta OC. Efficacy of Argentine propolis formulation for tropical treatment of canine otitis externa. Arq. Bras. Med.Vet. Zootec. 2010, 62, 1359–1366. [Google Scholar] [CrossRef]
- Cardoso, R.L.; Maboni, F; Machado, G; Alves S. H.; de Vargas A.C. Antimicrobial activity of propolis extract against Staphylococcus coagulase positive and Malassezia pachydermatis of canine otitis. Vet. Microbiol. 2010, 142, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Hageage, G.J.; Harrington, B.J. Use of calcofluor white in clinical mycology. Lab. Med. 1984, 15, 109–112. [Google Scholar] [CrossRef]
- Ram, A.F.J.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Mihai, A.L.; Popa, M.E. In vitro activity of natural antimicrobial compounds against Aspergillus strains. Agric. Agric. Sci. Proc. 2015, 6, 585–592. [Google Scholar] [CrossRef]
- Pei, S.; Liu, R.; Gao, H.; Chen, H.; Wu, W.; Fang, X.; Han, Y. Inhibitory effect and possible mechanism of carvacrol against Colletotrichum fructicola. Postharvest Biol. Technol. 2020, 163, 111126. [Google Scholar] [CrossRef]
- Medina-Romero, Y.M.; Hernandez-Hernandez, A.B.; Rodriguez-Monroy, M.A.; Canales-Martínez, M.M. Essential oils of Bursera morelensis and Lippia graveolens for the development of a new biopesticides in postharvest control. Sci. Rep. 2021, 11, 20135. [Google Scholar] [CrossRef]
- de Castro, P.A.; Bom, V.L.; Brown, N.A.; de Almeida, R.S.; Ramalho, L.N.; Savoldi, M.; Goldman, M.H.; Berretta, A.A.; Goldman, G.H. ; Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genet. Biol. 2023, 60, 74–86. [Google Scholar] [CrossRef]
- Adomavičiūtė,E. ; Pupkevičiūtė,S.; Juškaitė, V.; Žilius,M.; Stanys,S.; Pavilonis, A.; Briedis, Vitalis. Formation and Investigation of Electrospun PLA Materials with Propolis Extracts and Silver Nanoparticles for Biomedical Applications. J. Nanomaterials. 2017, 8612819. [Google Scholar]
- Alfarrayeh, I.; Pollák, E.; Czéh, Á.; Vida, A.; Das, S.; Papp, G. Antifungal and Anti-Biofilm Effects of Caffeic Acid Phenethyl Ester on Different Candida Species. Antibiotics. 2021, 10, 1359. [Google Scholar] [CrossRef]
- Grecka, K.; Xiong, Z.R.; Chen, H.; Pełka, K.; Worobo, R.W.; Szweda, P. Effect of Ethanol Extracts of Propolis (EEPs) against Staphylococcal Biofilm—Microscopic Studies. Pathogens. 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
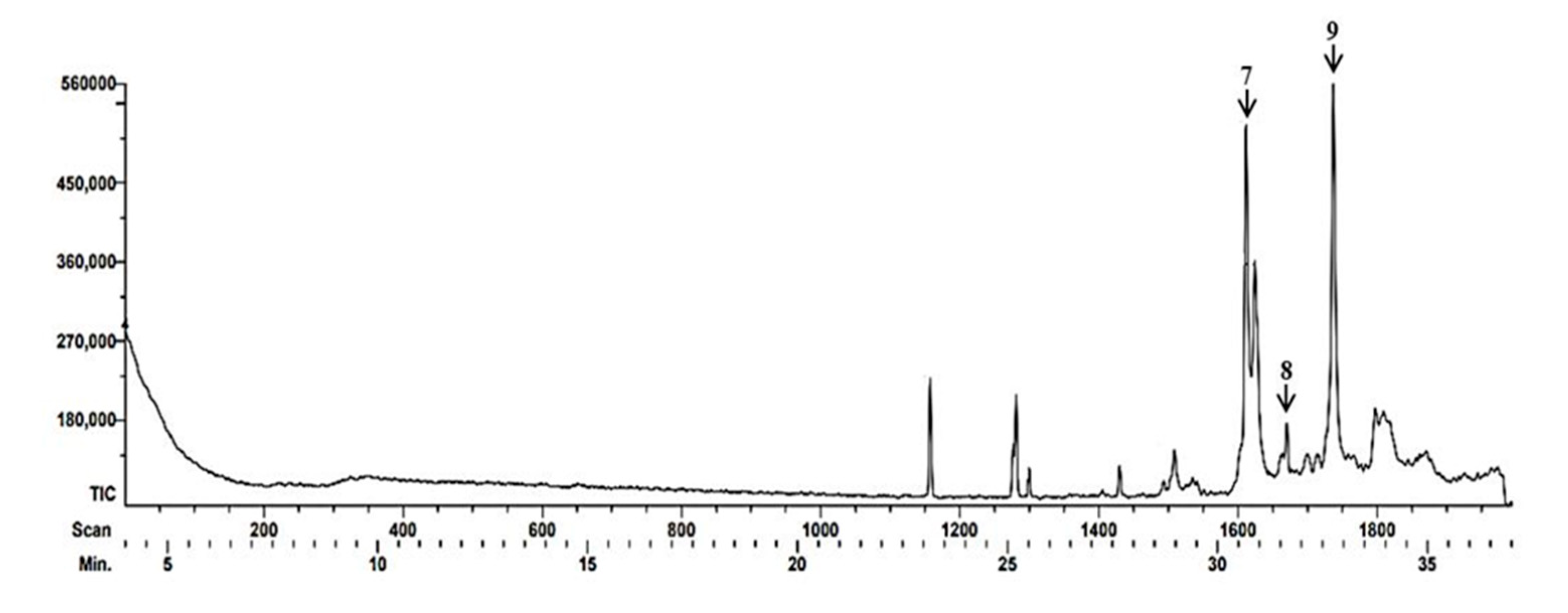
| Beak | Time Retention (TR) |
Composite proposed by the database |
Chemical classification |
Biological activity |
Reference |
|---|---|---|---|---|---|
| 7 | 30.68 |
Solavetivona | Sesquiterpenoid and a cyclic ketone | Antifungal anti-inflammatory |
[34,35,36] |
| 8 | 31.65 | 2-Methyl-3-(3-methyl-but-2-enyl)-2-(4-methyl-pent-3-enyl)-oxethane |
Heterocycle compound | Not found Information | |
| 9 | 32.75 | (1S,6R,9S)-5,5,9,10-Tetramethyltricycle [7.3.0.0(1,6)]dodec-10(11)-ene | Sesquiterpene | Antibacterian antifúngal |
[37] |
| Origen | MIC (mg/ml) M. pachydermatis ATCC 14522 | MFC(mg/ml) M. pachydermatis ATCC 14522 |
Media CMI (mg/ml ) in isolation Clinical (n = 3) |
Media CMF (mg/ml) in isolation Clinical (n = 3) |
Number of Isolates Clinical Inhibited (n = 3) |
|
|---|---|---|---|---|---|---|
| Scaptotrigona mexicana | Municipio de Yecuatla Veracruz |
32 | 64 | 32 | 64 | 3 |
| Tetragonisca angustula | Municipio de Chalchihuitan Chiapas |
32 | 64 | 32 | 64 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




