1. Introduction
Two-dimensional (2D) transition metal dichalcogenides (TMDCs) materials are considered to have great potential for application in fields such as integrated circuits, field-effect transistors (FET), and optoelectronic devices[1-3]. In order to expand its application circumstance, doping can be used to regulate the performance of TMDC materials. For instance, doping in TMDCs has the potential to modulate their charge carrier concentration, phase, electronic and optical band structures, and magnetic properties and may give TMDCs new properties and functionalities for various applications[4-8].
However, the current doping method of monolayer MoS
2 will cause lattice distortion and uneven doping
[9-11]. For example, Physical vapor deposition (PVD) and chemical vapor deposition (CVD) have poor crystallization quality and uniformity. On the other hand, note that previous achievements in metal substitutional doping of TMDCs are primarily limited in the metal substitutional doping of TMDCs is mainly limited to part of the transition metal elements (e.g., Mo, W, Re, Nb, which can be referred to like-elements) that form corresponding species in the TMDC family, such as MoS
2, WSe
2, ReS
2, and NbS
2[12-13]. For example, Suh et al[
14]. found that the substitutional doping of MoS
2 with Nb leads to its structural transformation from the 2H to the 3R phase.
Nevertheless, the doping of unlike elements still poses significant challenges. On the contrary, recent theoretical research has found that elements such as Cr, Mn, Fe, etc., if doped into TMDC materials, will greatly alter the properties of TMDC and endow the materials with unique characteristics, such as dilute magnetism and self-spintronic applications
[15-17]. But successful examples are rarely reported. For example, it was reported that Er-doped MoS
2 thin films were obtained by depositing Er onto Mo-thin films and then vulcanizing them, and their optical properties were improved[
18]. However, this method is not generalizable and requires strict experimental conditions. The domain size of Er-doped MoS
2 thin films grown through sulfurization is usually relatively small. This requires a doping method that can have universality and ensure the production of high-quality TMDC materials, opening up ideas for this field.
Here, we use MoS
2 as the representative material of TMDCs and synthesize high-quality monolayer MoS
2 using chemical vapor transport (CVT) in a high vacuum-sealed environment. Based on further improving the process, a one-step CVT method was implemented to achieve the doping of IVB and VB transition metal elements, introducing foreign defects to enrich the electronic energy level structure of MoS
2 itself and improving the luminescence performance of monolayer MoS
2 crystal. On the other hand, the doping of lanthanide elements was also achieved using this method, and fluorescence-enhanced MoS
2 crystals doped with lanthanide elements were obtained. The band structure of MoS
2 was changed by using the rich electronic energy level of rare earth elements. It finds that the fluorescence of MoS
2 markedly enhances after doping
[19-21]. Moreover, the carrier lifetime was substantially prolonged by the kinetic test. These works are of great significance for further research on 2D MoS
2 optoelectronic devices and FET transistors[
22].
2. Materials and Methods
Sample preparation: The Zr, Hf, Gd, and Dy-doped MoS2 monolayers were synthesized by the CVT growth method. First, MoO3 (Alfa Aesar, 99.7%, ≈ 2.3 mg), ZrO2 (Alfa Aesar, 99.99%, ≈ 0.60 mg), HfO2 (Alfa Aesar, 99.99%, ≈ 1.01 mg), GdCl3 (Alfa Aesar, 99.99%, ≈ 1.22 mg), DyCl3 (Alfa Aesar, 99.99%, ≈ 1.25 mg), S (Alfa Aesar, 99.7%, ≈ 0.40 mg), and transport agent of iodine (Alfa Aesar, 99.7%, ≈ 3.00 mg) powers were mixed and loaded into a quartz ampule at one end. The mica substrate and raw materials are placed at both ends of the quartz tube and separated by the necking of the quartz tube. Afterwards, vacuum the quartz tube to 3-5×10-4 Pa and sealed. The experiment used a single temperature zone tube furnace, with a high temperature zone in the middle and low temperature zones at both ends. The raw material area in the sealed quartz tube is placed in the high-temperature zone, and the substrate area (growth zone) is placed in the low-temperature zone. Set the procedure to heat up to 850 ℃ at 40 ℃/min, hold for 0.5-2 h, and then air cooled to room temperature.
Transfer Method: Use a homogenizer to adsorb the doped monolayer MoS2 film and rotate it at 3000 rpm for 20 seconds while dripping PMMA solution onto the surface of the film. Then remove the sample and place it on a heating plate to heat at 80 ℃ for 8-10 minutes to cure. After cooling the sample to room temperature, the PMMA-deposited MoS2 film and substrate were separated by soaking in deionized water. Finally, the thin film is transferred to the desired substrate and annealed at 70 ℃ for 2 h to firmly adhere to the substrate. The PMMA film is then washed or soaked with acetone to dissolve and release the MoS2 film onto the target substrate.
Optical Spectroscopy Measurement: Commercial Confocal microscopy (WITec Alfa-300) is used to test PL and Raman data. The testing conditions are room temperature, laser wavelength of 532 nm, and spatial resolution of 2 μm. This microscope worked in reflection mode, and the signal was detected using an electrically cooled charge-coupled device camera.
The TR spectrum was obtained through testing using a self-built ultra-fast pump-probe spectroscopy system in the laboratory. A homemade microscope was connected to the system to realize micro-resolution. A Yb: KGW laser (Pharos, Light Conversion Ltd., a repetition rate of 100 kHz; pulse width of 120 fs) with a working wavelength of 1030 nm was used as the light source. The laser output beam is divided into pump light and probe light during the testing process. Pump light is used to generate pump pulses (315-2600 nm) to pump the sample. The probe light passes through the time delay line and focuses on the YAG crystal to produce a continuum pulse to probe the dynamics after pumping. After filtering the pump pulse using a long-wave filter, the reflection spectrum is collected with a complementary metal-oxide-semiconductor detector. The TR signal is calculated as ΔR/R = (Rpump-on-Rpump-off)/Rpump-off, where Rpump-on and Rpump-off are the reflection detection signals with and without pump excitation.
Characterization: The optical micrograph of Hf, Zr, Dy, and Gd-doped MoS2 was taken and observed through Olympus BX 51M. XPS data were collected using Thermo Scientific ESCALAB250Xi, and all peaks would be calibrated with C1s peak binding energy at 284.8 eV for adventitious carbon. STEM images of doped MoS2 were obtained using Titan 60-300 at 200 kV.
3. Results and Discussion
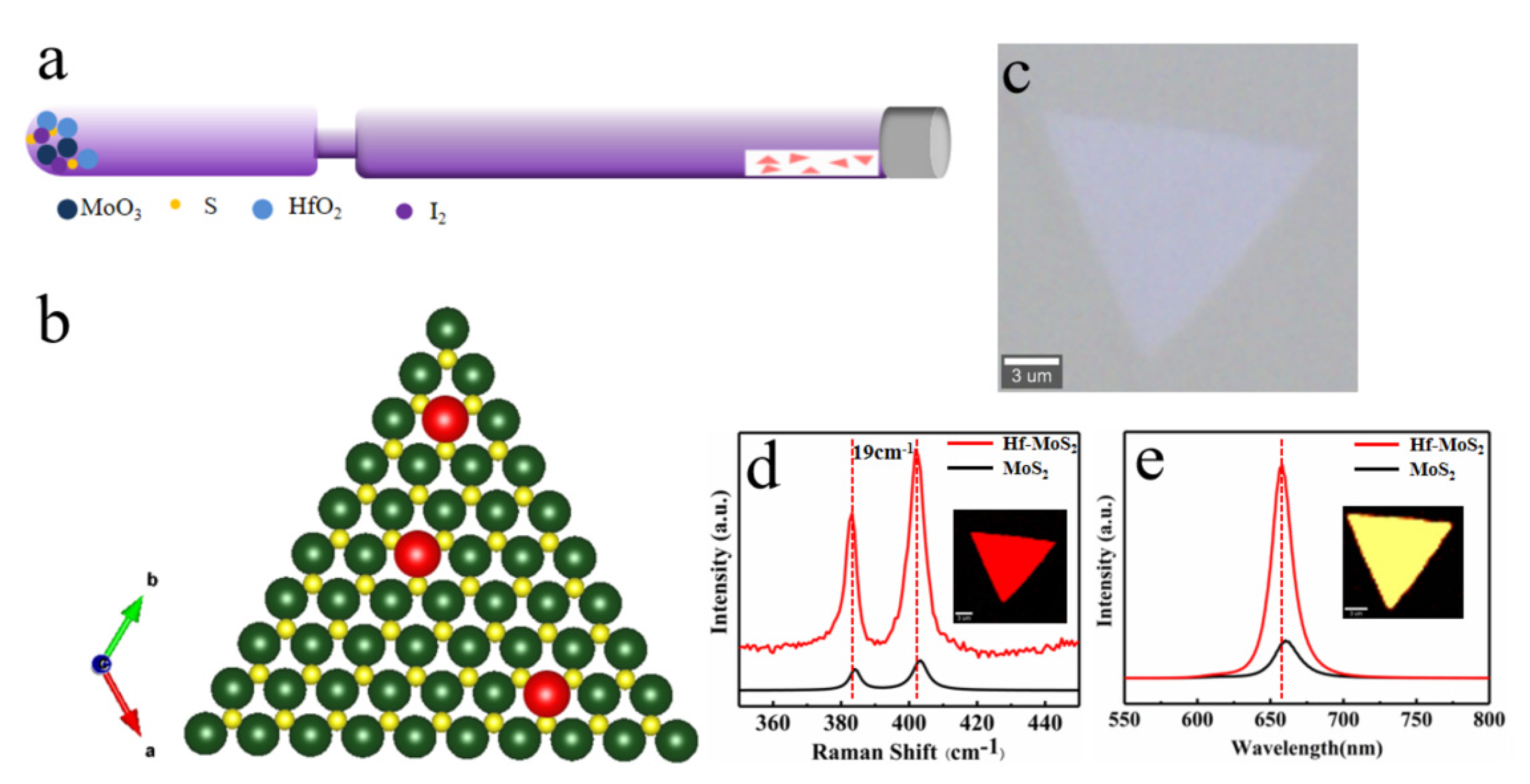
We chose the Hf element as the representative element for monolayer MoS
2 crystals doped with transition metal materials, using the CVT method of a sealed quartz tube. The mixture of MoO
3/HfO
2/S/I
2 powers and growth mica substrate were placed on both ends of the quartz tube, as shown in
Figure 1a. When using CVT method to obtain ultra-thin MoS
2, it is essential to use a way to reduce the transport efficiency of the transport agent. The specific implementation method is to reduce the size of the transport channel between the raw material and substrate (as shown in
Figure 1a). This design can separate the substrate and raw materials and slow down the transport rate, thereby controlling the film size and thickness[
23]. The final obtained model structure of Hf-doped MoS
2 is a 2H-MoS
2 structure where Hf replaces Mo atoms.
Figure 1c,d, and e show optical micrographs, Raman, and PL spectra of Hf-doped monolayer MoS
2 crystal, respectively. From the optical photo, it can be seen morphology of MoS
2 after doping remains almost unchanged, maintaining the growth morphology of pure MoS
2. In addition,
Figure 1d shows the two characteristic Raman vibration modes of E
12g and A
1g mode related to the out-of-plane vibration. The similar Raman spectra also confirm that the lattice has no significant changes after doping, except for the collective red-shift of Raman peak positions caused by lattice distortion after atomic substitution. The Raman spectrum characterization measured a 19 cm
-1 difference in the A
1g and E
12g vibration peak position of doped MoS
2[24-25], consistent with the Raman peak position difference between monolayer MoS
2. The Raman Mapping diagram in the illustration also indicates the uniformity of crystal quality after doping. The steady-state PL spectra were collected on the pristine and Hf-doped MoS
2 monolayers under the same conditions at room temperature, as listed in
Figure 1e. The test shows that the PL intensity of Hf-doped monolayer MoS
2 is stronger than that of pure MoS
2, which is about five times stronger. Additionally, the PL peak position is slightly blue-shifted due to the lattice distortion and bandgap changes caused by doping. Simultaneously, the luminescence peak of PL also has a narrow half-peak width, indicating the uniformity of luminescence quality
[26-27]. There, we believe that doping the Hf element introduces the electronic energy level of the Hf element, which enriches the electronic structure of MoS
2.
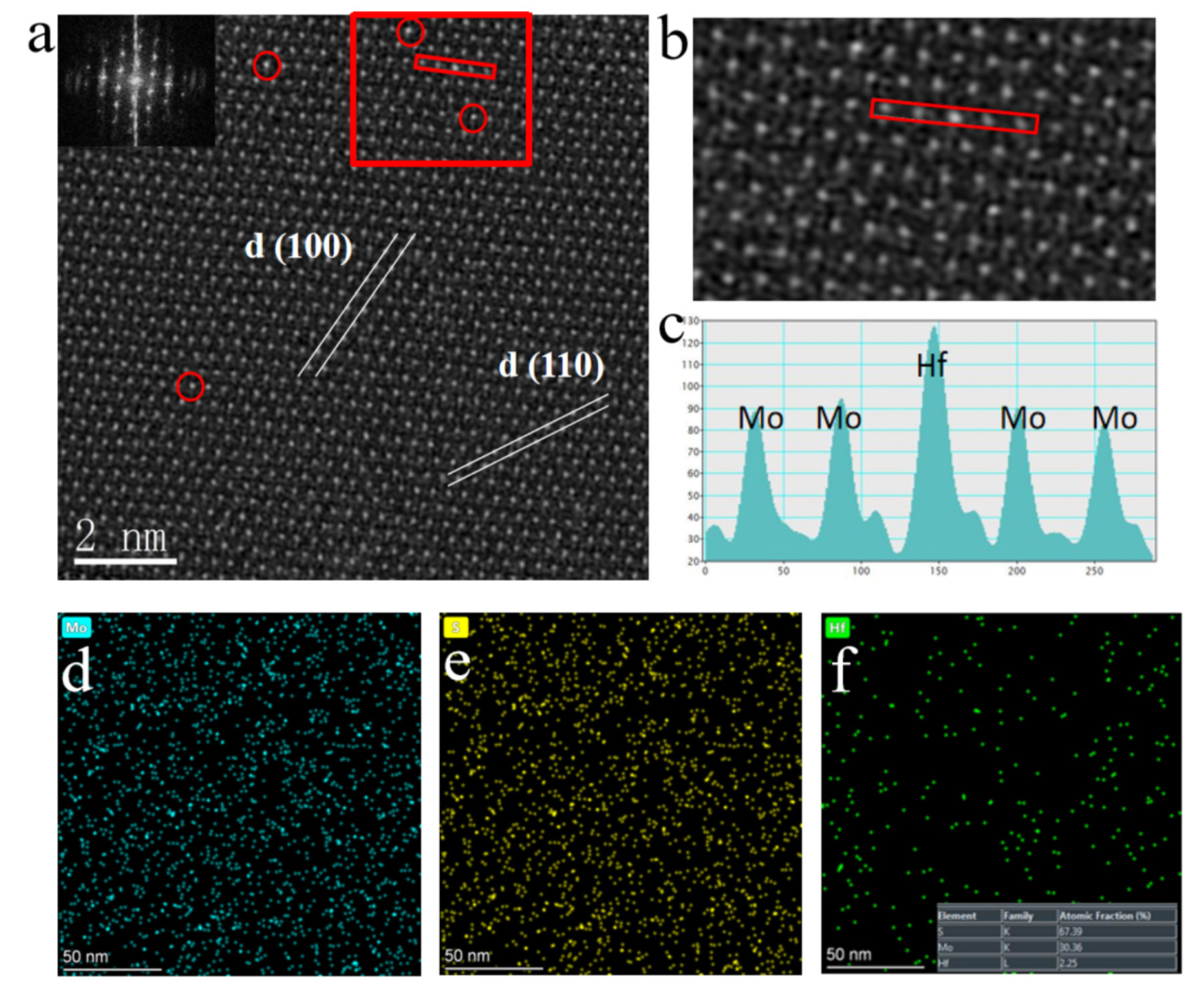
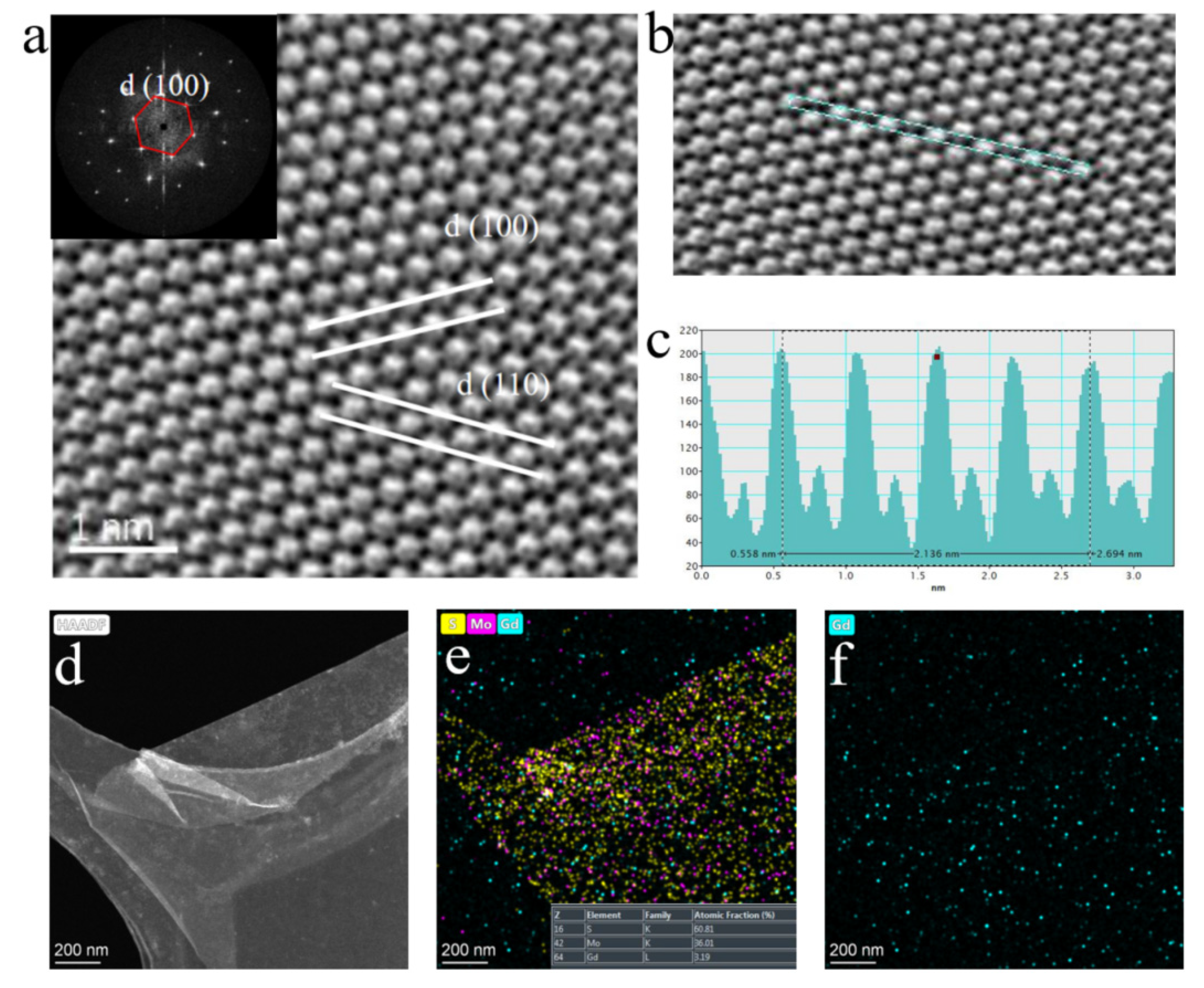
HAAD-STEM can distinguish different atoms according to different brightness, which is caused by different atomic numbers (S (Z=16), Mo (Z=42), and Hf (Z=72)). Based on this, we marked the position occupied by Hf doping in the MoS
2 monolayer with red circles in
Figure 2a. Simultaneously, the plane spacing between two adjacent (100) faces is 0.28 nm, which is consistent with the data of pure monolayer MoS
2. But, measurement found that the distance between two adjacent Mo atoms was 0.32 nm, and compared with pure phase MoS
2, the distance between faces and atoms increased. This is because the Hf atom is much larger than the Mo atom, leading to slight lattice distortion in MoS
2.
On the other hand, it proves that the Hf atom has successfully embedded in the lattice of MoS
2, replacing the position of Mo atoms, which is consistent with HAADF-STEM testing. Furthermore, in
Figure 2b, the highlighted area in
Figure 1a was enlarged, and a clear distinction between light and dark can be seen. In addition, the illustration in
Figure 2a also shows the uniformity and high quality of the thin film material[
28].
The profile of the intensity line in
Figure 2c corresponds to the selected area in
Figure 2b, where Hf and Mo atoms are marked. All the dots at Mo atom sites show similar contrast except for the bright sites for Hf atoms, suggesting that almost no Mo atom vacancies are found on the measured surface[
7,
29]. Another noteworthy phenomenon is that the strength near the Hf atom substituting the Mo atom is different from that near the Mo atom in other regions (
Figure 2c). We supposed that this is due to the generation of new bonding oxygen atoms near the doped Hf atom and the formation of a new O-Hf-S structure by replacing the S atom. Furthermore, EDS spectrum mapping shows that Hf is evenly distributed among atoms in the sample, which a doping amount of approximately 1:15 compared to the Mo element (
Figure 2f, illustration). Moreover, the sum of the ratio of the Mo element to the S element is about 1:2, proving that the Hf element replaces the Mo element. Moreover, the absence of noticeable lattice distortion and the presence of Mo vacancies are necessary proof for proving the feasibility of this doping process
[30-31].
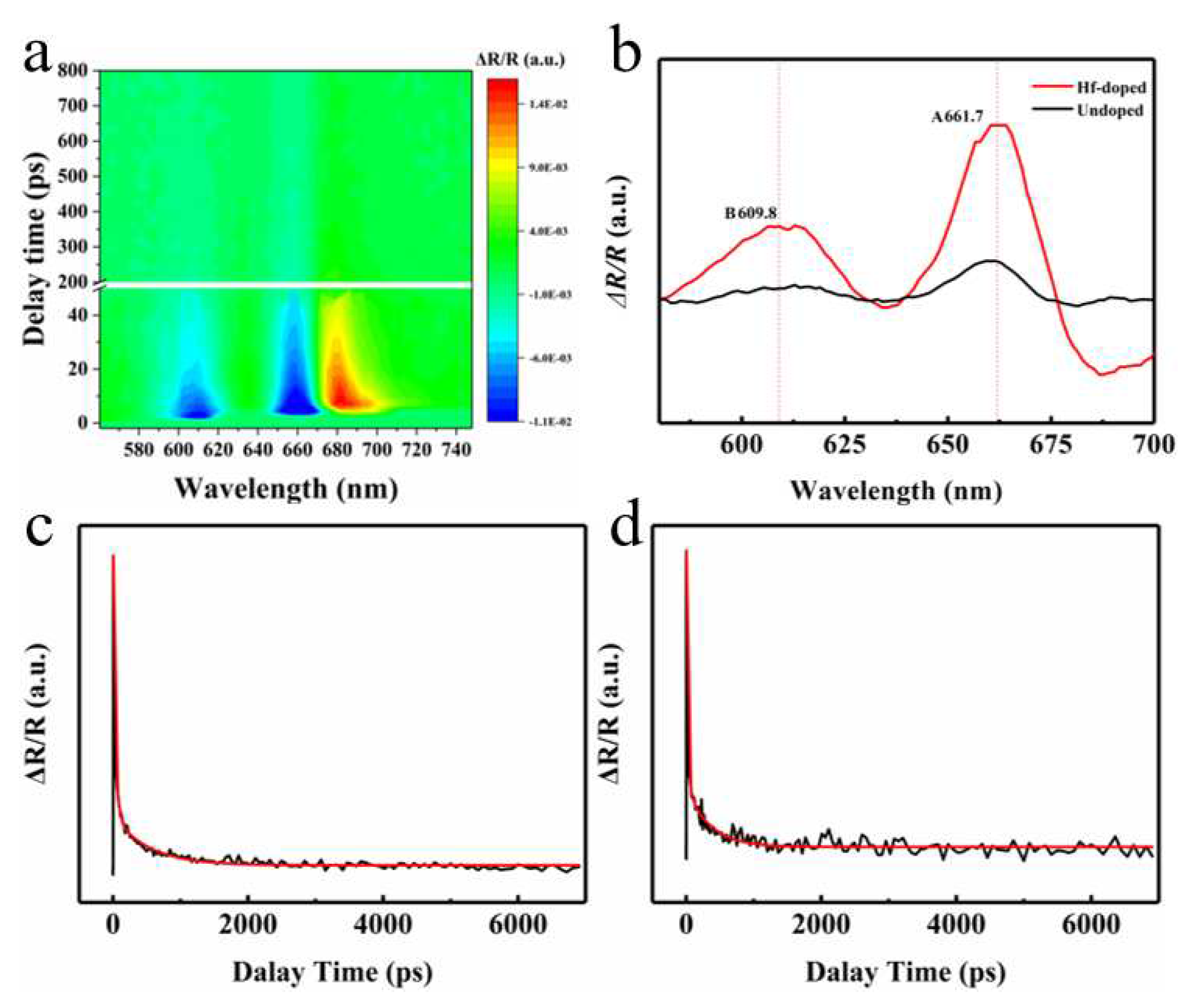
The femtosecond pump-probe is an essential tool for studying the carrier dynamics of Hf-doped MoS
2 monolayers (please refer to the Materials and Methods section for detailed testing information). Firstly, we tested the 2D profile of transient reflection (TR) spectra of the Hf-doped MoS
2 monolayer at room temperature with the excitation power of 2 μw under a 530 nm laser.
Figure 3b shows the TR spectrum of A exciton resonance with a 4.2 ps time delay extracted from
Figure 3a. Two peaks in each TR spectrum correspond to the A and B excitons of the Hf-doped MoS
2 monolayer, which are much stronger than those of pure MoS
2, corresponding to the phenomenon of luminescence enhancement in the PL spectrum. Therefore, we collected the TR kinetic decay curves of A-exciton resonance in Hf-doped MoS
2, as shown in
Figure 3c. Analyze the kinetic decay curves through tri-exponentially fitted (the results are listed in
Table 1). The fitting results indicate that the relaxation lifetime τ
1 has increased from 0.91 ps to 2.14 ps after doping, which is believed to be due to the additional defect scattering of hot carriers caused by Hf doping, thereby prolonging the relaxation time. More importantly, the lifetime τ
2 representing the radiative recombination process has also significantly increased. According to the previous report
[32-34], the photoluminescence quantum yield (PLQY) enhancement of two different radiation processes can be roughly estimated by the following equation:
Here,
and
correspond to the average exciton lifetime of doped and undoped monolayer MoS
2, respectively. Based on the previous work of our research group[
7], substituting τ
2 to calculate PLQY enhancement instead of average lifetime. The calculated value of η is approximately 1.67, confirming the previously observed phenomenon of PL enhancement after Hf doping. It is worth noting that the calculated value of η based on this formula is an estimated value, far less than the five times luminescence enhancement obtained through PL testing, which is related to the testing mechanism. TR spectrum is an absorption spectrum collected through a pump probe, while PL spectrum is based on the spectrum obtained by a streak camera, which provides more accurate data compared to the former. On the other hand, the TR detection system is a platform built by our laboratory, and its collection efficiency is weaker than commercial confocal microscopy detection platforms. Thus, the PLQY enhancement, η is underestimated.
Furthermore, the radiation efficiency is positively correlated to the densities of electrons in the conduction band and holes in the valance band. During the EEA process (τ
3), the exciton-scattering makes one non-radiatively recombine, passing the energy to the other exciton. The additional energy promotes the residual exciton to become a free carrier. Therefore, the increase in carrier density caused by doping is another key factor that enhances PL in addition to PLQY. From
Table 1, we can intuitively see that the lifetime τ
3 of the doped EEA process is much enhanced compared to the original monolayer MoS
2, and a longer lifetime promotes radiation recombination and improves PL intensity
[35-37]. Based on the above discussion, we believe that the enhancement of PL by decay lifetime is crucial.
From the perspective of material structure, combining STEM images and XPS test results (
Figure S1, supporting literature), we assume that the doping process introduces doping elements and some oxygen atoms, which can repair vacancies in monolayer MoS
2 while suppressing nonradiative recombination, improving carrier lifetime and enhancing PL. The absence of Mo and S vacancies in the STEM test image in
Figure 2a–c is also substantial proof. Moreover, the O-Hf-S unit and Hf atoms mentioned earlier can also improve the interaction between monolayer MoS
2 and mica substrate, stabilizing excitons and leading to longer exciton lifetimes, ultimately achieving enhanced PL luminescence[
38].
Unlike the IVB group elements, the sizeable atomic radius of rare earth elements will cause more significant lattice distortion of monolayer MoS
2 crystals after doping. On this basis, high-quality lanthanide (Gd, Dy) doped monolayer MoS
2 crystals were obtained through a one-step CVT process
[39-41]. Among them, the Atomic radius of rare earth elements is large and difficult to be doped. The content of rare earth elements in the system is increased by using chlorides with low melting points to improve the success rate of doping. And when the amount of chloride increases, it will cause the doped MoS
2 film to grow thinner and smaller in size. (As shown in
Figure S3). Here, we take Gd-doped monolayer MoS
2 as an example.
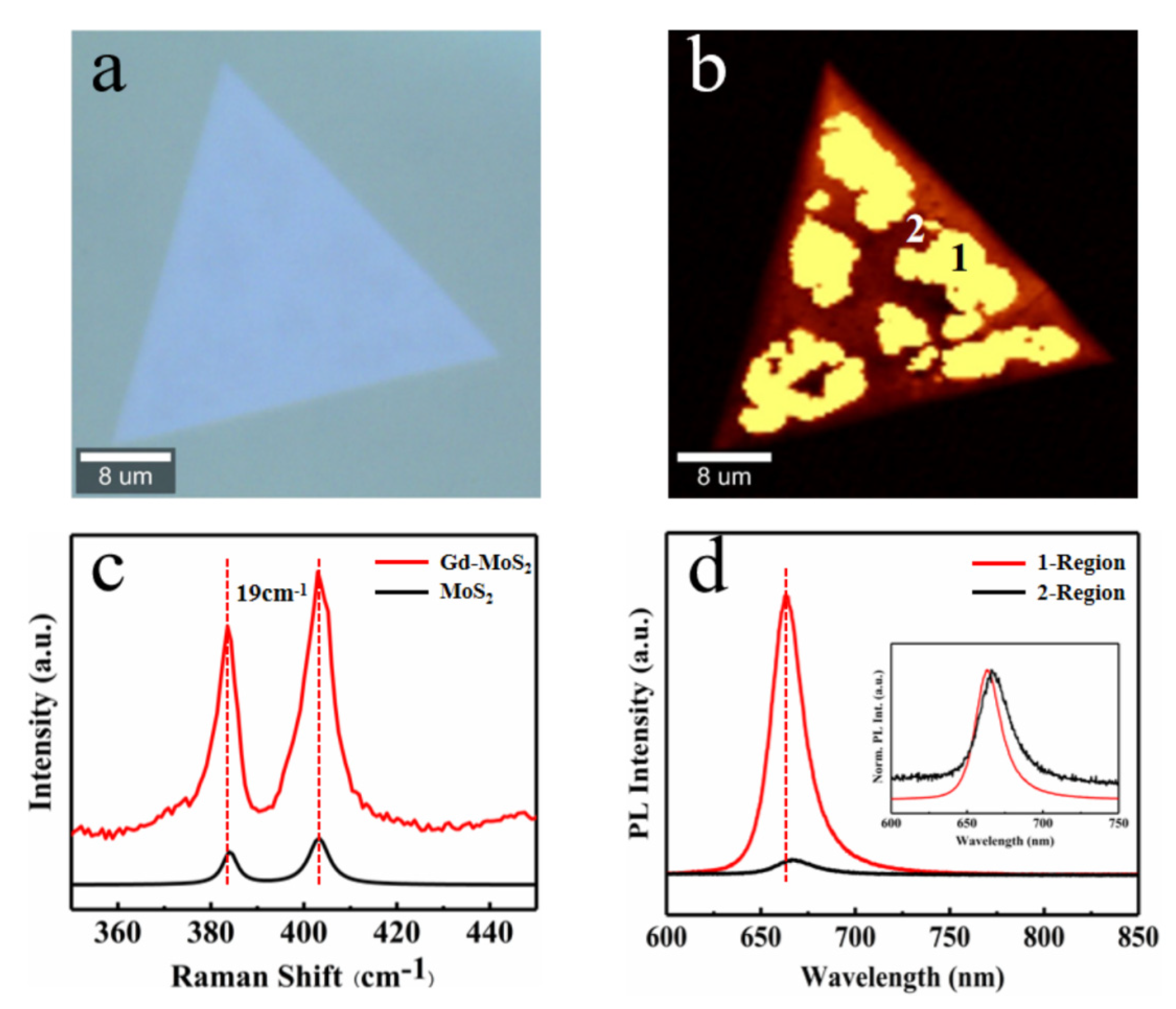
Figure 4a presents the optical microscope image of a typical Gd-MoS
2 monolayer. It exhibits a well-defined triangular shape without grain boundaries, indicating the high quality of the as-grown Gd-MoS
2 monolayer. (The microscope image of Dy-MoS
2 monolayer in
Figure S4 of the Supporting Information). Two characteristic Raman vibration modes of E
12g and A
1g of monolayer Gd-MoS
2 were collected by Raman spectroscopy using a 532 nm laser shown in
Figure 4c. The E
12g mode represents the in-plane vibration of Mo-S atoms, while the A
1g mode is related to the out-plane vibration. The vibration peak position is almost consistent with that of undoped MoS
2, indicating that the doped ions have little effect on the lattice of the material, and the distance between the two vibration peaks is 19 cm
-1, which also indicates that a monolayer Gd doped MoS
2 has been prepared. Simultaneously, the Raman mapping in the illustration also shows the high-quality crystallinity of the material.
Analyze and characterize the PL performance of monolayer MoS
2 before and after Gd doping using a Raman spectrometer. It was found that the fluorescence luminescence of Gd-doped MoS
2 is not uniform (
Figure 4d). In the PL mapping of monolayer MoS
2, there is a difference of more than ten times in the luminescence intensity of the two PL regions, bright region 1 and dark region 2. The luminescence intensity of the second region is consistent with the luminescence peak position and intensity of the undoped MoS
2. Due to the difference in the size of doped ions, it can affect the coordination environment in the matrix, leading to the formation of asymmetric fields
[41-43]. The lower the symmetry of the substrate, the more uneven the crystal field environment around the doped ions, leading to an increase in electron coupling between the 4f energy level and higher electron configurations, resulting in an increase in the probability of f-f transition, specifically manifested in the significant luminescence intensity within the same triangular crystal.
However, due to the resolution of HR-TEM, the difference between Gd atoms and Mo cannot be diffraction in STEM images (
Figure 5a). On the other hand, diffraction patterns indicate that the doped MoS
2 is a single crystal with high crystallinity, and the (100) plane spacing is about 0.271 nm, with a distance between two Mo atoms of about 0.316 nm. Compared to the plane spacing and atomic spacing of pure MoS
2, this confirms the lattice distortion phenomenon caused by doping large Gd atoms into the MoS
2 lattice.
Figure 5d,e, and f show the elemental analysis results of the EDS spectrum, where Gd is uniformly distributed in the 2D MoS
2 crystal. The quantitative test results indicate that the doping ratio of Gd is about 3.19%.
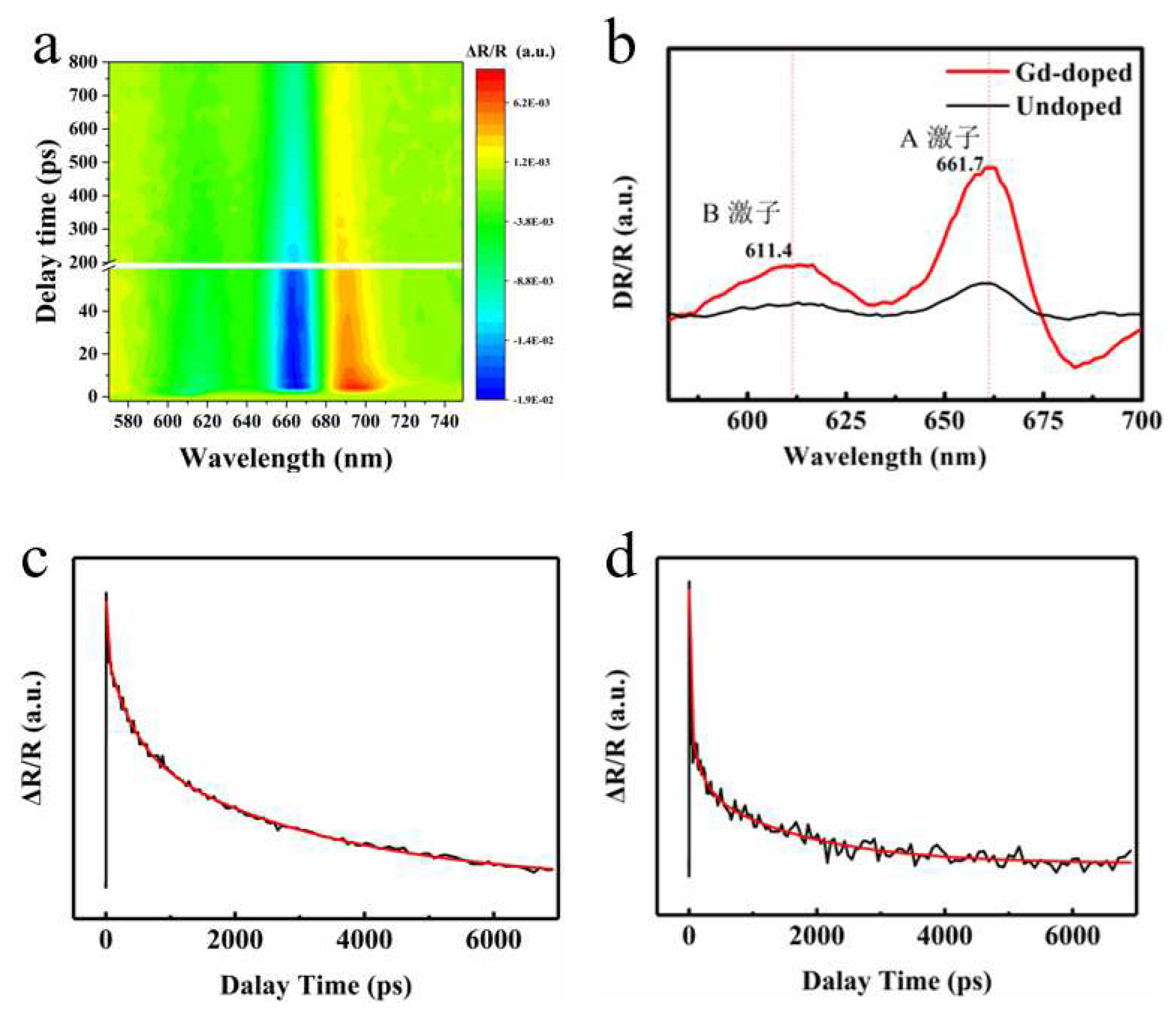
Furthermore, in order to investigate the reasons for the enhancement of fluorescence intensity after Gd doping and the changing behavior of exciton transitions, transient absorption spectra and exciton lifetime tests were conducted on the sample using a pump detection system to analyze the luminescence kinetics of the sample. As shown in
Figure 6b, we found that the absorption peaks of A and B excitons in MoS
2 doped with Gd shifted red by about 2 nm, and the intensity was significantly enhanced, consistent with the luminescence intensity and peak position changes of the PL spectrum.
Figure 6c, d shows that the lifetimes of excitons A and B are much longer than those of pure MoS
2, corresponding to the significant enhancement of PL strength after Gd doping. The defect capture process in various stages of exciton lifetime τ
1 has also significantly increased in length due to introducing more defects after doping and more electrons in rare earth elements. Exciton recombination process τ
2 and exciton annihilation process τ
3. Compared to before doping, the intensity of PL increased by many times, corresponding to a significant increase in PL intensity. However, due to the large size of rare earth elements, more defects are introduced after doping, resulting in poor crystal quality and lower PL intensity to Ti doping.
In addition, we also selected Ti (Our research group has previously reported the relevant work), Zr as the IVB group element (
Figure S2, Supporting Information), and Dy as the rare earth element for monolayer MoS
2 doping experiments. It was found that the doping of the Zr element resulted in a two-fold decrease in PL performance. Tests showed that doping with electrons of different orbital energy levels affected their internal electronic transition behavior and enhanced their luminescence performance. Zr atoms had the same electronic energy levels as Mo atoms, with no transition behavior energy levels. After doping, only the effect of crystal quality deterioration resulted in a decrease in their luminescence performance
[44-46]. As for the other rare earth element Dy, the doping effect is similar to that of Gd, but the luminescence effect of Dy-doped monolayer MoS
2 is only three times enhanced, which also indicates that the luminescence effect of Gd element doped with F orbitals in a semi-full state is evident. The data can be found in supporting literature.