1. Introduction
The duck’s vascular endothelium may hold the key to answering an intriguing question for poultry scientists: why are ducks markedly more resilient to highly pathogenic avian influenza virus (
HPAIV) than chickens? HPAIV is frequently fatal to gallinaceous poultry including chickens, but this seems not to be the case for ducks [
5]. Interestingly, according to one report, no wild duck from the genus
Anas experimentally infected with HPAIV showed symptoms or succumbed to the disease [
15]. By contrast, the vascular endothelium has been implicated in fatal HPAIV outcomes in chickens. This lethality has been linked to apoptosis of vascular endothelial cells in the liver, kidney, and brain [
13,
36]. This acute apoptosis is reportedly associated with high levels of endothelial nitric oxide (
NO) release during HPAIV infection, as NO acts with other damaging oxidants to promote excessive inflammation [
2]. From a physiological perspective, HPAIV infection and the associated vascular endothelial apoptosis in chickens may be exacerbated by the high levels of NO released locally. Considering human physiology, NO can promote proinflammatory effects, including increased vascular permeability, cytotoxicity, and inflammatory cell infiltration, when present at high concentrations [
3,
28], and any related loss of endothelial function in vital arteries could quickly prove fatal.
As a potential site of HPAIV-related vascular endothelial injury, the basilar artery (
BA) may provide a fruitful line of research. It is a vital artery that constantly supplies blood to the hindbrain, and a common cerebrovascular feature across vertebrates, contributing to maintenance of cerebral circulatory volume [
26]. Its role in birds appears to be the same as that in mammals, although chickens are the only avian species in which BA reactivity has been studied [
16,
25,
37].
In reports on the vascular response in the BA in chickens, the cerebrovascular endothelium has been identified as a powerful source of spontaneous NO release, based on strong contractions noted after application of
Nω-nitro-L-arginine (
L-NNA), a NO synthase (
NOS) inhibitor [
25]. Furthermore, previous investigations of endothelial cell receptors have indicated that beta-3 adrenergic receptors (
β3-ARs), histamine (
His) H
1 receptors, and muscarinic acetylcholine (
ACh) M
3 receptors are involved in the relaxation of the chicken BA via the NO pathway, suggesting this arterial relaxation is strongly dependent on endothelial release of NO [
16,
25,
37].
Until now, studies on the BA in poultry species have only targeted chickens, but differences in this artery and the local vascular response between chickens and ducks can be hypothesized. Evolutionarily speaking, ducks and chickens are separated by a distance of 90 million years and avian genomes tend to be more strongly conserved than mammalian genomes [
27,
32]. We thus considered that ducks may have a less complex vascular endothelium than their younger avian relatives, chickens. Interestingly, HPAIV reportedly rarely shows endothelial tropism in either wild or domestic ducks [
31,
33]. However, there is a paucity of evidence on the cerebrovascular endothelium in ducks.
Against this background, we aimed to characterize the BA in ducks, by investigating its capacity for endothelial NO release, and its response to a range of vasoactive substances we had previously used in our studies of chicken BA.
2. Materials and Methods
2.1. Tissue preparation
BAs were obtained from freshly slaughtered Aigamo ducks (Anas platyrhynchos / Anas platyrhynchos var. domesticus hybrid; n =71, both sexes, weight: 2.97 ± 0.04 kg). The ducks had been raised for meat and allowed to forage on insects in rice paddies, in farms in Kagoshima prefecture, Japan, and were slaughtered in accordance with the relevant Japanese law on agricultural animals. The sampled arteries were transferred to our laboratory in ice-cold physiological saline (119 mM NaCl, 4.7 mM KCl, 1.6 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 1.2 mM KH2PO4, and 10 mM glucose, pH 7.4) aerated with carbogen [95% (vol/vol) O2, 5% (vol/vol) CO2]. Each artery was immediately dissected free of adherent tissues under a stereomicroscope. All experiments were performed in accordance with the Guidelines for Animal Experiments of Kagoshima University.
2.2. Reagents
The following reagents were used at the described concentrations: His hydrochloride (10-6–10-3 M, Sigma-Aldrich, St. Louis, MO, USA), isoproterenol (10-9–10-5 M, Sigma-Aldrich), ketanserin tartrate (10-6 M, Sigma-Aldrich), methiothepin maleate (10-8–10-6 M, Sigma-Aldrich), diphenhydramine hydrochloride (10-7–10-5 M, Sigma-Aldrich), cimetidine (10-5 M, Sigma-Aldrich), angiotensin (Ang) II acetate salt (10-8–10-4 M, Sigma-Aldrich), butoxamine hydrochloride (10-6 M, Sigma-Aldrich), SR 59230A (10-7–10-6 M, Sigma-Aldrich), L-NNA (10-4 M, Sigma-Aldrich), noradrenaline (NA, 10-9–10-4 M, Tokyo Chemical Industry, Tokyo, Japan), phentolamine mesylate (10-5 M, Tokyo Chemical Industry), 5-hydroxytryptamine (5-HT)-creatinine sulfate (10-8–10-4 M; Merck, Darmstadt, Germany), adrenaline (10-9–10-5 M, Daiichi Sankyo, Tokyo, Japan), ACh chloride (10-9–10-5 M, Daiichi Sankyo), procaterol (10-9 –10-5 M, Fujifilm-Wako, Tokyo, Japan), atenolol (10-6–10-5 M, LKT Laboratories, Tokyo, Japan), hexahydro-sila-difenidol hydrochloride, p-fluoroanalog (pFHHSiD, 10-7, 10-6 M; Research Biochemical, Natick, MA, USA), avian bradykinin ornithokinin (OK, 10-9–10-5 M, BEX Co. Ltd., Tokyo, Japan), and sodium nitroprusside (SNP, 10−4 M; Nacalai Tesque, Kyoto, Japan). All reagents were used in accordance with the relevant manufacturer’s instructions.
2.3. Functional study
Three or four rings, approximately 2 mm wide, were cut from each duck BA. Each ring was mounted horizontally between two L-shaped stainless-steel holders (outer diameter 0.03 mm), with one part fixed to an isometric force transducer. The mounted ring was then immersed in a 4-mL water-jacketed micro-tissue organ bath (UMTB-1, Unique Medical Co. Ltd., Tokyo, Japan), containing oxygenated physiological saline at 41°C (pH 7.4). Each suspended ring was left to equilibrate for at least 30 min under a resting tension of 0.5 mN. This tension was chosen to facilitate induction of maximal contractions in the artery without deflection of the steel. KCl (60 mM) was applied every 30 min until the amplitude of the contraction reached a constant value.
Changes in the KCl concentration of the physiological saline were compensated for by equimolar adjustment of the NaCl concentration. The isometric tension was recorded with an amplifier (AP-621G, Nihon Kohden Kogyo, Tokyo, Japan), digitized with an analog-digital converter (PowerLab/8SP, AD Instruments Co., Castle Hill, NSW, Australia), and stored on the hard disk of a personal computer. In a preliminary experiment, 5-HT induced sufficient contraction and retained the tonic phase, whereas other vasoconstrictors, such as Ang II and NA, failed to induce contractions. Therefore, we used 5-HT to induce the precontracted condition before applying vasodilators. The cumulative concentration-response curve of agonists was obtained by adding a solution of each agonist directly to the fluid in the bath. Antagonists or inhibitors were added to the bathing media 30 min before each agonist. The antagonists had no effect on the resting vascular tone. The log concentration-ratio of EC
50 values (i.e., the concentration producing a half-maximal response) in the absence or presence of an antagonist was calculated and plotted against the logarithm of the antagonist concentration to obtain the pA
2 values [
1].
At the end of the relaxant response, SNP (10−4 M) was applied to produce maximal relaxation, which was taken as 100%.
2.4. Statistical analysis
Results are expressed as the mean ± SEM, and statistical analyses were performed using Student’s t-test or the Bonferroni test after one-way ANOVA (Stat View J-4.5, Abacus Concepts Inc., Berkeley, CA, USA). P-values < 0.05 were considered statistically significant.
4. Discussion
The present study is the first to demonstrate the responsiveness of BAs to vasoactive substances in ducks. We showed that NO is a diminished endothelial mediator in ducks, relative to its reported role in chickens. This information will prove especially useful for comparisons between ducks, which are not prone to disease and death after HPAIV infection, and chickens, which are.
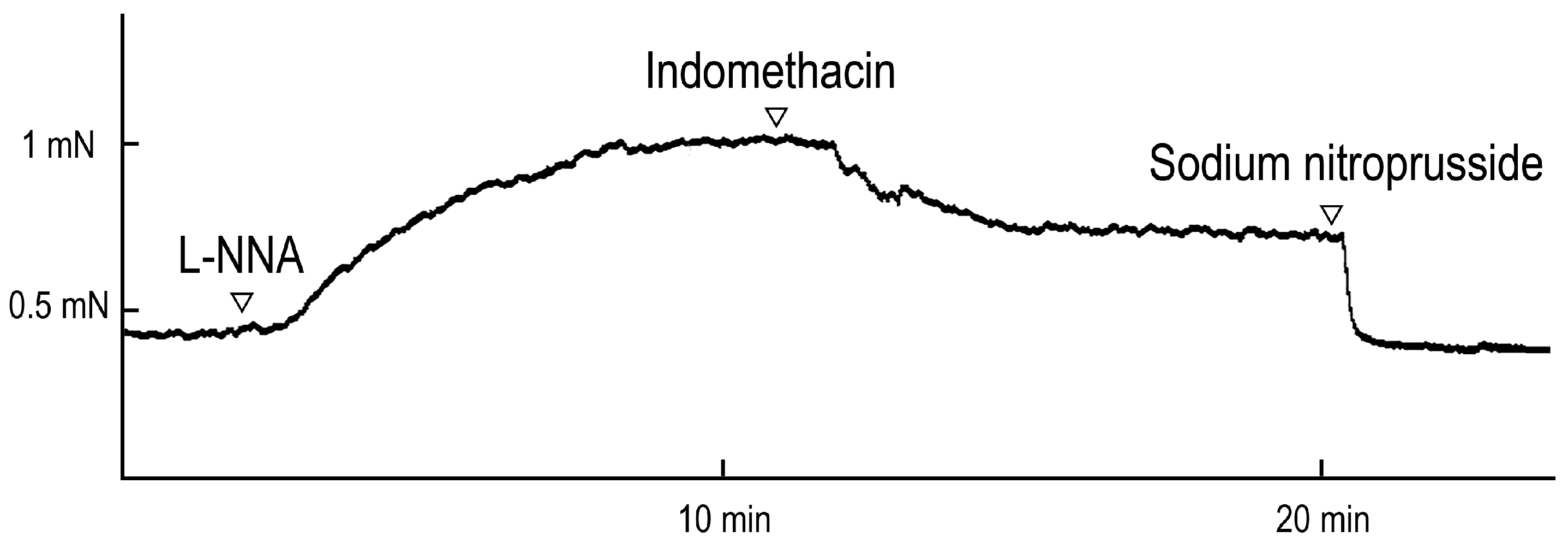
We demonstrated the involvement of spontaneously released endothelial NO, based on the contraction induced by the NOS inhibitor L-NNA, followed by the relaxation induced by indomethacin, which inhibits cyclooxygenase, under resting tension (
Figure 1). Our findings in ducks are similar to results obtained in some mammalian species including dogs [
30], pigs and cattle [
17], horses [
34], and bats [
9]. In porcine BAs, further studies demonstrated that spontaneous NO is released from endothelial cells [
18] and spontaneous thromboxane A
2, which is formed from PGH
2 and inhibited by indomethacin, is released from endothelial and smooth muscle cells [
18,
21]. The mechanism of maintenance of cerebrovascular tone in the duck partially resembles that in the chicken. However, a key difference was that the L-NNA-induced contraction in ducks (30.5%) was markedly weaker than that in chicken (122.1%) [
25], suggesting that duck shows less involvement of spontaneous endothelial NO than chicken.
We also investigated the receptor subtypes involved in contraction and relaxation, and their location (smooth muscle or endothelial cells) in the duck BA.
5-HT receptors appear to play a similar role in ducks and chickens, as the receptor agonist induced a concentration-dependent contraction in isolated duck BAs, in both the avian species we evaluated, and we found that the 5-HT
1 receptor may be the dominant receptor of this subtype (
Figure 3). Based on an evaluation of half-maximal-effect concentrations, we found that the 5-HT receptor antagonist, methiothepin, produced a similar effect in the duck BA to that observed in the chicken BA and the rabbit saphenous vein, where vascular response (vasocontraction) is mediated via activation of 5-HT
1 receptor 16, 35]. Furthermore, the effects of methiothepin and the 5-HT
2 antagonist ketanserin in the duck BA (respective rightward shifts in the concentration-response curve of 35.5-fold and 2.7-fold) were similar to the corresponding shifts (30-fold and 3-fold, respectively) we previously observed in the chicken BA [
16], suggesting ducks and chickens share a similar characterization for this receptor type.
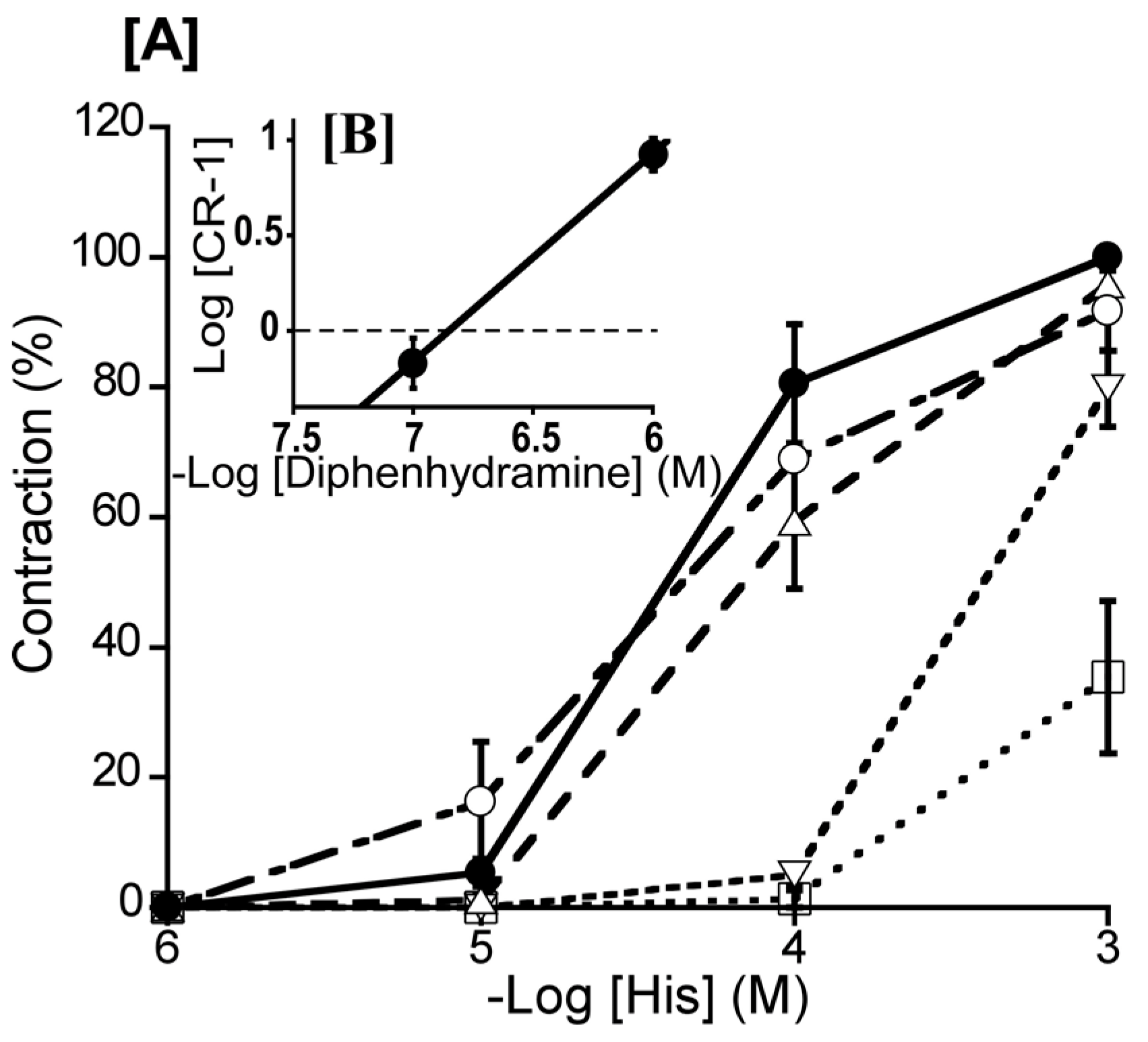
We also profiled the involvement of His receptors. The His-induced concentration-dependent contraction in isolated duck BAs in this study resembled those observed in porcine, bovine, equine, and dolphin BAs, with a similar half-maximal effect to that in the bovine report, but a smaller half-maximal effect than those in the porcine, equine, and cetacean reports [
11,
22,
23]. The H
1-receptor antagonist diphenhydramine (10
-7–10
-5 M) produced an effect of similar extent in duck and dolphin BAs, but its effect in ducks was smaller than in the bovine and porcine reports [
22,
23]. In line with the known vascular response in these mammalian species, we consider that H
1 receptor activation induces the contraction of the duck BA. Our His receptor findings present a contrast to those previously reported in chickens. Okuno et al. (2008) reported that both H
1 and H
2 receptors, which are located on respective endothelial cells and smooth muscle cells, are involved in the relaxation of the chicken BA. However, in ducks, H
1 receptors play a dominant role in His-induced contraction, while H
2 receptor were not involved.
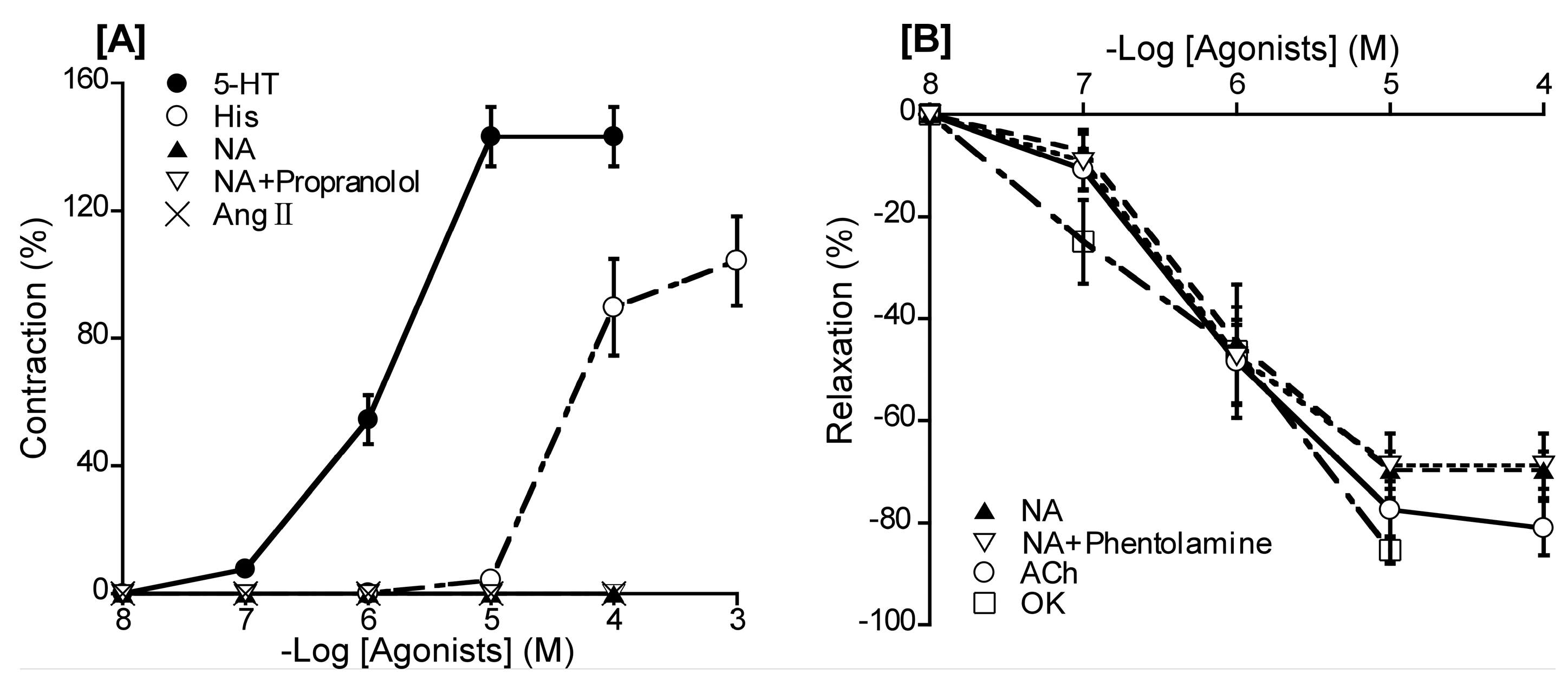
For adrenergic receptors, noradrenaline had no effect on BA at resting tension and phentolamine (a non-selective α-ARs antagonist) had no effect on noradrenaline-induced relaxation under precontraction (
Figure 2), suggesting that α-ARs are not involved in duck BA reactivity. This presents a contrast with the chicken BA, where α-ARs are present and involved in contraction, and phentolamine inhibits this contraction [
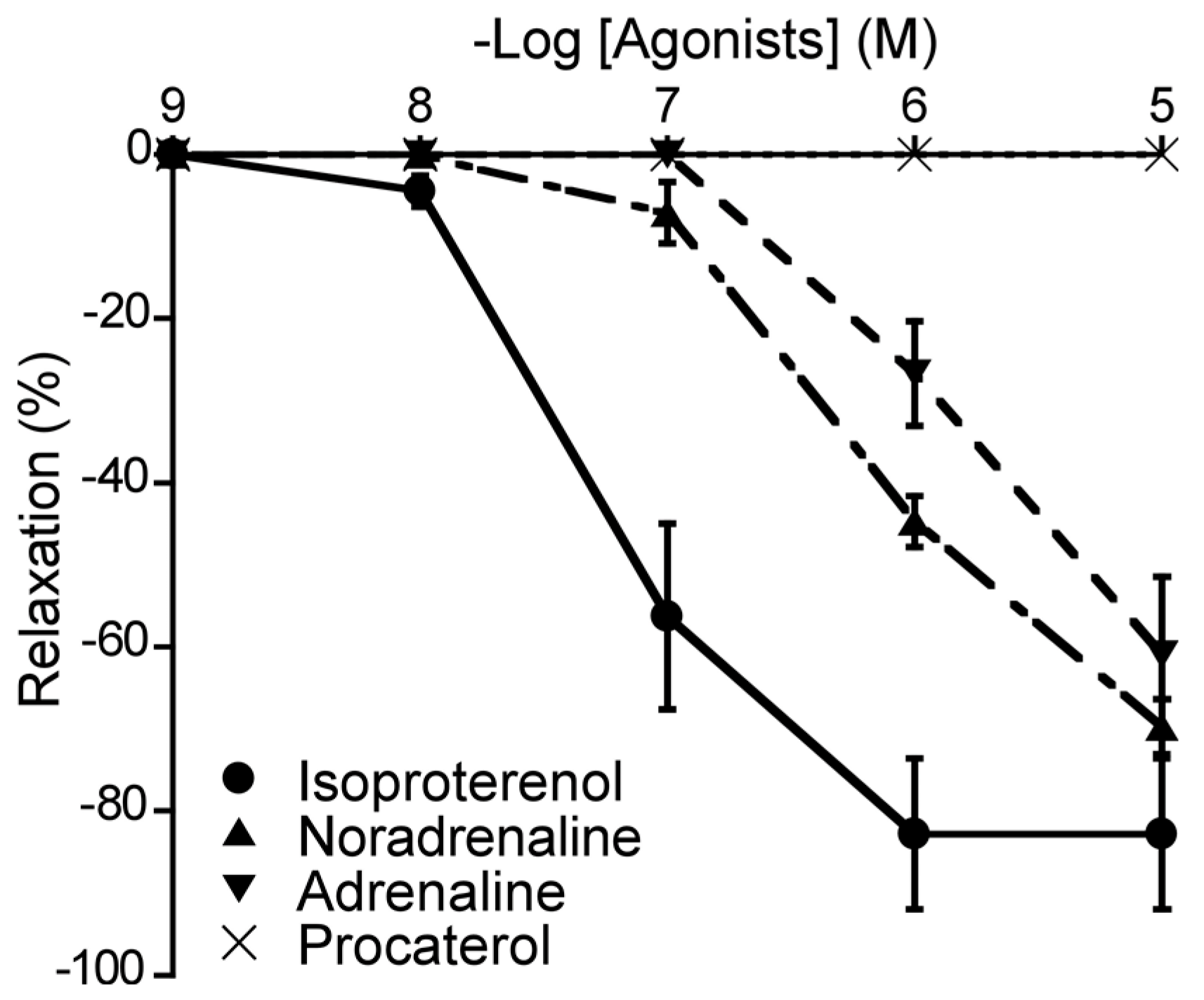
37]. For β-ARs, the duck BA appears to possess β
1-ARs, but with a smaller population for this receptor subtype than in chickens, based on the similar potency ranking but smaller effect size for isoproterenol and NA [
37]. β
2-ARs appear not to play a major role in the duck BA, although they are present as a non-dominant subtype in chickens, based on findings for the effect of procaterol [
37]. Interestingly, the effect of the β-AR agonist isoproterenol-induced relaxation was not inhibited by the NOS inhibitor L-NNA (data not shown); therefore, we suggest that NO plays no role in β-AR mediation in ducks, which represents a physiological difference from chickens.
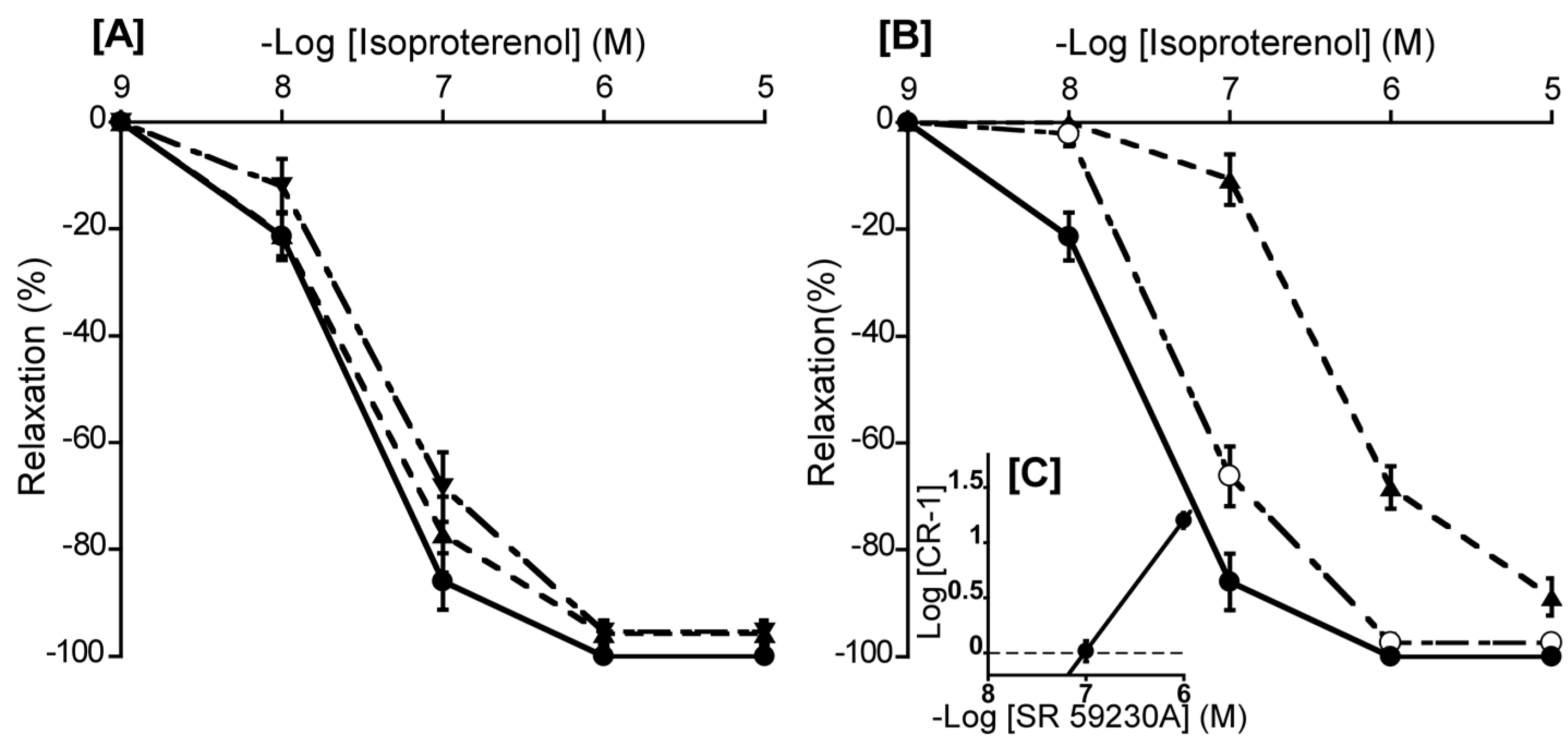
We consider that the β
3-AR is the predominant β-AR subtype in the duck BA, based on results obtained with a range of β-AR antagonists (
Figure 6). Most pertinently, only the β
3-AR antagonist SR 59230A demonstrated an effective antagonistic effect (pA
2 value: 7.03) in duck BAs comparable to that in rat urinary bladder tissue (7.27) and guinea pig gastric fundus (7.35), where β
3-ARs are known to predominate [
8,
24]. Accordingly, we suggest that the β
3-AR is predominantly involved in isoproterenol-induced relaxation in the duck BA, a similar phenomenon to that we reported for chickens. However, this relaxation is endothelium dependent in chickens, but not in ducks.
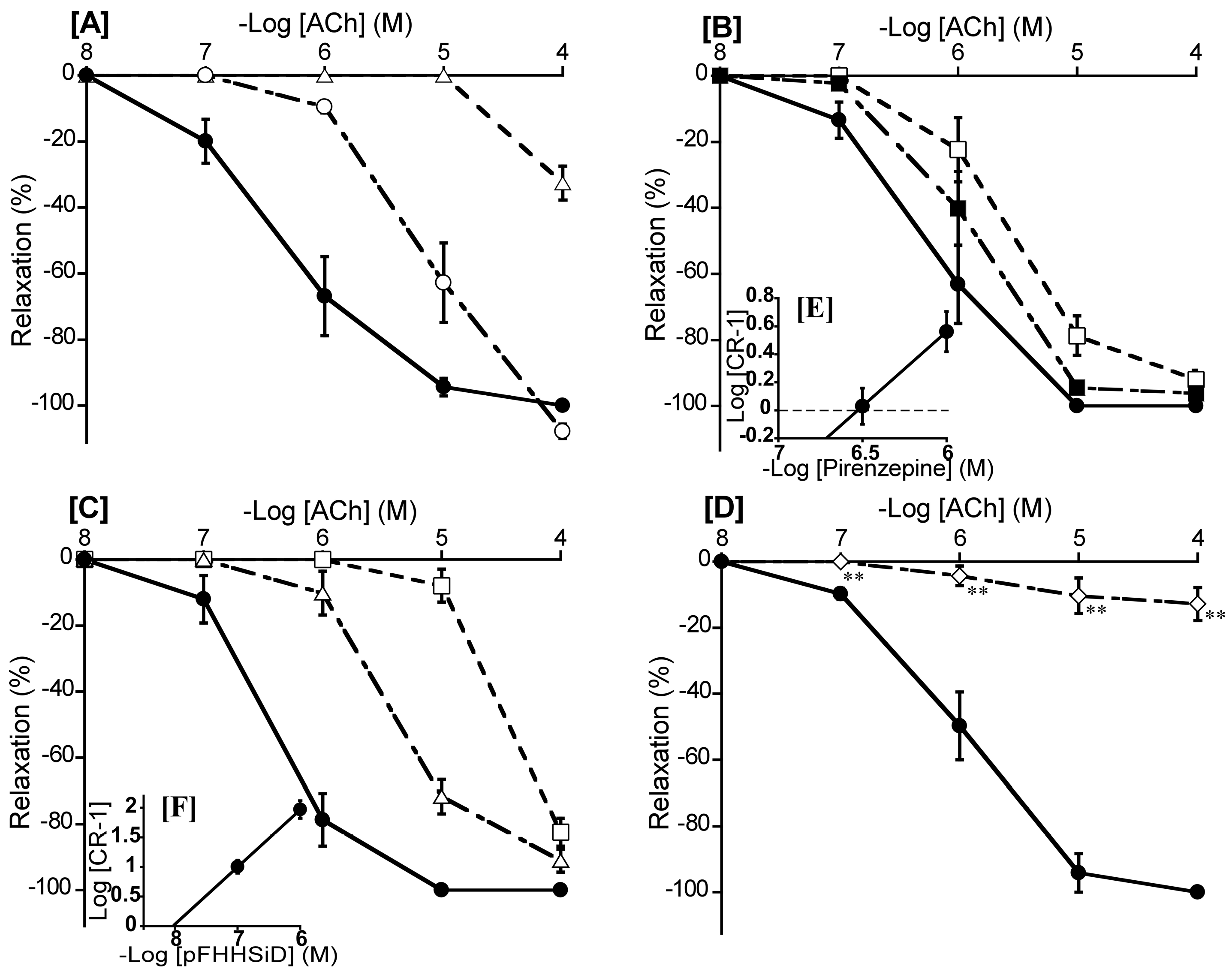
Our findings for muscarinic ACh receptors (
Figure 7) resembled those observed in mouse, chicken, and bat BAs [
9,
12,
16], suggesting their activation is involved in the response to ACh. We further suggest that the M
3 receptor is the predominant subtype involved in ACh-induced relaxation, and M
1 and M
2 receptors may not be involved in duck BAs. We found no effect for the M
2 antagonist methoctramine and only a weak effect for the M
1 antagonist pirenzepine. The latter effect was smaller than that reported in cat cerebral arteries (8.08), where muscarinic M
1 receptor predominate [
4]. However, the M
3 antagonist pFHHSiD yielded a calculated pA
2 value (8.06) similar to those reported in bovine coronary arteries (7.64), human uterine artery (8.17), and chicken BAs (7.55), where the muscarinic M
3 receptor predominate [
6,
14,
16]. In contrast to our findings on β-ARs, we consider that NO is involved in muscarinic M
3 receptor-mediated relaxation in ducks, as it is in other species including mice, bats, and chickens [
9,
12,
37], based on the abolition of the ACh-induced relaxation by the NO inhibitor L-NNA seen in this study (
Figure 7D).
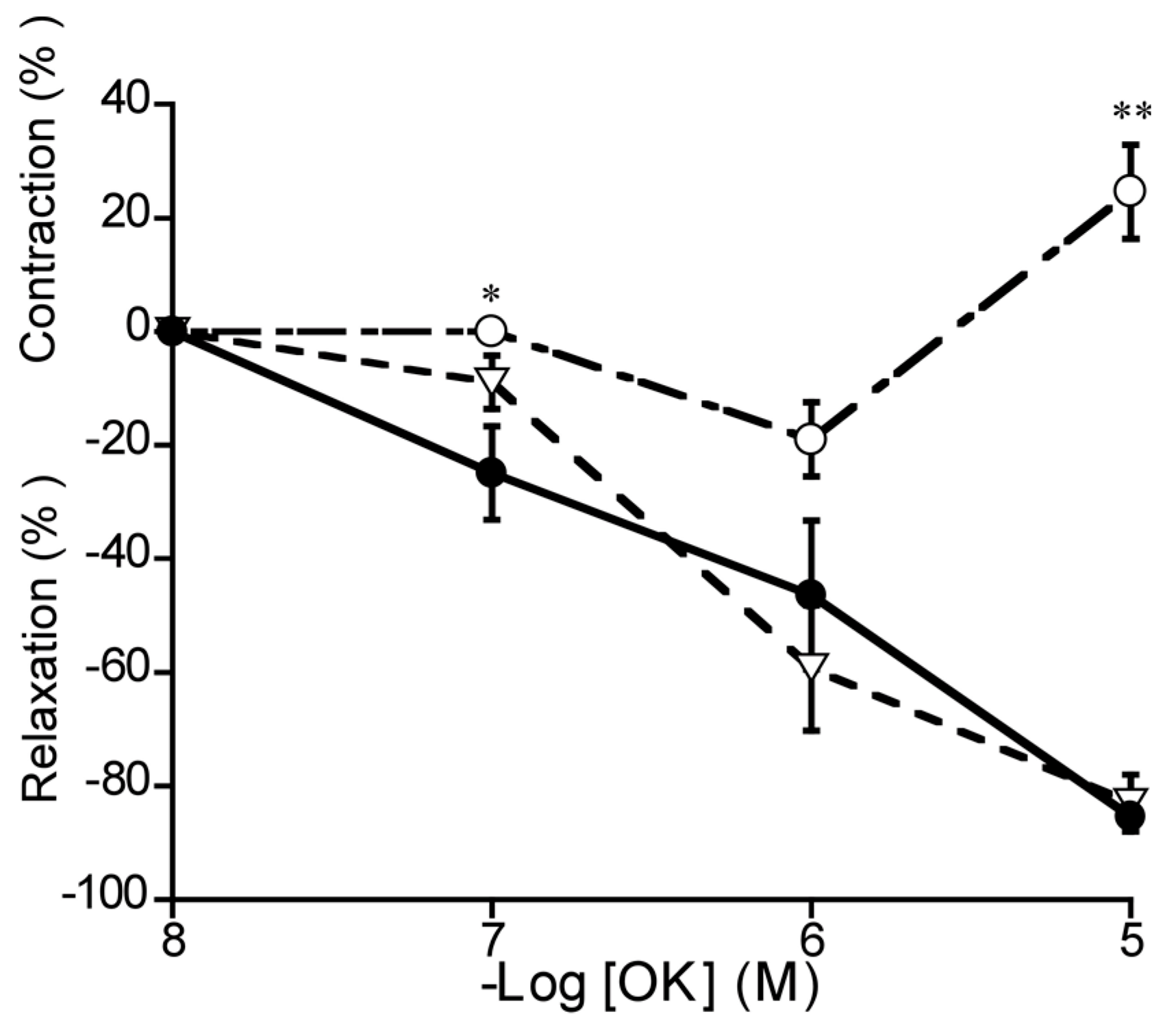
We revealed that avian bradykinin OK induced relaxation and contraction in duck BAs, by regulation of respective NO and cyclooxygenase pathways. In porcine BAs, bradykinin produced NO and prostaglandin F
2α then induced relaxation followed by contraction [
10,
19]. OK receptors are the avian homolog to mammalian bradykinin receptors, and are known to be involved in blood vessel dilatation, smooth muscle contraction, increased vascular permeability, and inflammation [
29]. OK receptors reportedly potentiate pro-inflammatory responses in chicken macrophages [
7]. Pharmacological research on these receptors is hampered by a lack of established antagonists; accordingly, it was not possible to investigate the relevant receptor subtypes in this study. However, we anticipate this line of research will be enabled by advances in cell culture in the future.
BA responsiveness to vasoactive substances is known to differ widely between mammalian species, according to differences in receptor subtypes and their distribution on smooth muscle or endothelial cells. To our knowledge, BA responses have never shown fully identical characteristics in any two mammalian species, and we speculate that similar diversity will be observable across avian species, based on the present study, which is the second characterization of BA response in an avian species. However, one feature common to ducks in this study and to chickens in our previous report was β
3-AR-mediated vasodilation, which has never been reported in mammalian cerebrovascular systems [
20]. We speculate that β
3-ARs may play an important role in the cardiovascular system of birds, and this represents an interesting line of future research.
Figure 1.
Typical contraction induced by Nω-nitro-L-arginine (L-NNA, 10-4 M) and relaxation induced by indomethacin (10-5 M) under precontracted condition induced by L-NNA. Relaxation induced by sodium nitroprusside was taken as 100%, in Figures 2, 5 and 8.
Figure 1.
Typical contraction induced by Nω-nitro-L-arginine (L-NNA, 10-4 M) and relaxation induced by indomethacin (10-5 M) under precontracted condition induced by L-NNA. Relaxation induced by sodium nitroprusside was taken as 100%, in Figures 2, 5 and 8.
Figure 2.
[A] Responsiveness of duck basilar arteries to 5-hydroxytrypatamine (5-HT), histamine (His), noradrenaline (NA), NA in the presence of propranolol, and angiotensin (Ang) II under resting tension. [B]: Responsiveness of isolated duck basilar arteries to NA, NA in the presence of phentolamine (10-5 M) acetylcholine (ACh), and ornithokinin (OK) under precontracted condition induced with 5-HT. The contraction induced by 60 mM KCl [A] or the relaxation induced by 10-4 M sodium nitroprusside [B] were taken as 100% contraction and relaxation, respectively. Each point represents the mean ± SEM of 4–6 ducks.
Figure 2.
[A] Responsiveness of duck basilar arteries to 5-hydroxytrypatamine (5-HT), histamine (His), noradrenaline (NA), NA in the presence of propranolol, and angiotensin (Ang) II under resting tension. [B]: Responsiveness of isolated duck basilar arteries to NA, NA in the presence of phentolamine (10-5 M) acetylcholine (ACh), and ornithokinin (OK) under precontracted condition induced with 5-HT. The contraction induced by 60 mM KCl [A] or the relaxation induced by 10-4 M sodium nitroprusside [B] were taken as 100% contraction and relaxation, respectively. Each point represents the mean ± SEM of 4–6 ducks.
Figure 3.
Effects of methiothepin (Δ: 10-8 M, ▽: 10-7 M, ○: 10-6 M) [A], and ketanserin (○: 10-6 M) [B] on 5-hydroxytrypatamine (5-HT)-induced contraction (●) in duck basilar arteries, with Schild plot for methiothepin [C]. The maximal contraction induced by 5-HT was taken as 100%. Each point represents the mean ± SEM of 6 ducks.
Figure 3.
Effects of methiothepin (Δ: 10-8 M, ▽: 10-7 M, ○: 10-6 M) [A], and ketanserin (○: 10-6 M) [B] on 5-hydroxytrypatamine (5-HT)-induced contraction (●) in duck basilar arteries, with Schild plot for methiothepin [C]. The maximal contraction induced by 5-HT was taken as 100%. Each point represents the mean ± SEM of 6 ducks.
Figure 4.
Effects of diphenhydramine (Δ: 10-7 M, ▽: 10-6 M, ○: 10-5 M) and cimetidine (○: 10-5 M) [A] on histamine (His)-induced contraction (●) in duck basilar arteries, with Schild plot for diphenhydramine [B]. The maximal contraction induced by His was taken as 100%. Each point represents the mean ± SEM of 5 ducks.
Figure 4.
Effects of diphenhydramine (Δ: 10-7 M, ▽: 10-6 M, ○: 10-5 M) and cimetidine (○: 10-5 M) [A] on histamine (His)-induced contraction (●) in duck basilar arteries, with Schild plot for diphenhydramine [B]. The maximal contraction induced by His was taken as 100%. Each point represents the mean ± SEM of 5 ducks.
Figure 5.
Responsiveness of duck basilar arteries to isoproterenol (●), noradrenaline (▲), adrenaline (▼), and procaterol (×) under precontracted condition. The relaxation induced by 10-4 M sodium nitroprusside were taken as 100%. Each point represents the mean ± SEM of 4–6 ducks.
Figure 5.
Responsiveness of duck basilar arteries to isoproterenol (●), noradrenaline (▲), adrenaline (▼), and procaterol (×) under precontracted condition. The relaxation induced by 10-4 M sodium nitroprusside were taken as 100%. Each point represents the mean ± SEM of 4–6 ducks.
Figure 6.
Effects of atenolol (▲: 10-6 M) and butoxamine (▼: 10-6 M) [A], SR 59230A (○: 10-7 M, ▲: 10-6 M) [B] on isoproterenol-induced relaxation (●) in duck basilar arteries, with Schild plot for SR 59230A [C]. The maximal relaxation induced by isoproterenol was taken as 100%. Each point represents the mean ± SEM of 4 or 5 ducks.
Figure 6.
Effects of atenolol (▲: 10-6 M) and butoxamine (▼: 10-6 M) [A], SR 59230A (○: 10-7 M, ▲: 10-6 M) [B] on isoproterenol-induced relaxation (●) in duck basilar arteries, with Schild plot for SR 59230A [C]. The maximal relaxation induced by isoproterenol was taken as 100%. Each point represents the mean ± SEM of 4 or 5 ducks.
Figure 7.
Effects of atropine (○: 10-8 M, Δ: 10-7 M) [A], pirenzepine (■: 10-6.5 M, □: 10-6 M) [B], hexahydro-sila-difenidol hydrochloride, p-fluoroanalog (pFHHSiD; Δ: 10-7 M, □: 10-6 M) [C], and Nω-nitro-L-arginine (◇:10-4 M) [D] on acetylcholine (ACh)-induced relaxation (●) in duck basilar arteries, with Schild plots for pirenzepine [E], and pFHHSiD [F]. The maximal relaxation induced by ACh was taken as 100%. Each point represents the mean ± SEM of 5 ducks. (**p < 0.01 vs. control).
Figure 7.
Effects of atropine (○: 10-8 M, Δ: 10-7 M) [A], pirenzepine (■: 10-6.5 M, □: 10-6 M) [B], hexahydro-sila-difenidol hydrochloride, p-fluoroanalog (pFHHSiD; Δ: 10-7 M, □: 10-6 M) [C], and Nω-nitro-L-arginine (◇:10-4 M) [D] on acetylcholine (ACh)-induced relaxation (●) in duck basilar arteries, with Schild plots for pirenzepine [E], and pFHHSiD [F]. The maximal relaxation induced by ACh was taken as 100%. Each point represents the mean ± SEM of 5 ducks. (**p < 0.01 vs. control).
Figure 8.
Effects of Nω-nitro-L-arginine (○: 10-4 M, L-NNA) and L-NNA plus indomethacin (10-5 M) (▽) on ornithokinin (OK)-induced relaxation (●) in duck basilar arteries. The contraction induced by 60 mM KCl and the relaxation induced by 10-4 M sodium nitroprusside were taken as 100% contraction and relaxation, respectively. Each point represents the mean ± SEM of 4 ducks. (*p < 0.05, **p < 0.01 vs. control).
Figure 8.
Effects of Nω-nitro-L-arginine (○: 10-4 M, L-NNA) and L-NNA plus indomethacin (10-5 M) (▽) on ornithokinin (OK)-induced relaxation (●) in duck basilar arteries. The contraction induced by 60 mM KCl and the relaxation induced by 10-4 M sodium nitroprusside were taken as 100% contraction and relaxation, respectively. Each point represents the mean ± SEM of 4 ducks. (*p < 0.05, **p < 0.01 vs. control).
Table 1.
The pEC50 values and maximal response (Emax) for agonists.
Table 1.
The pEC50 values and maximal response (Emax) for agonists.
| Agonists |
pEC50
|
Emax (%) |
| Resting tension |
|
|
| 5-Hydroxytryptamine |
5.84 ± 0.06 |
143.2 ± 9.3a
|
| Histamine |
4.37 ± 0.09 |
104.3 ± 14.0a
|
| Noradrenaline |
- |
No response |
| Noradrenaline + Propranolol |
- |
No response |
| Angiotesin II |
- |
No response |
| Precontracted condition |
|
|
| Acetylcholine |
6.18 ± 0.17 |
-81.0 ± 5.4b
|
| Noradrenaline |
6.28 ± 0.05 |
-69.7 ± 3.7b
|
| Noradrenaline + Phentolamine |
6.30 ± 0.11 |
-68.8 ± 6.3b
|
| Ornithokinin |
6.20 ± 0.25 |
-85.4 ± 2.7b
|
| Isoproterenol |
7.22 ± 0.11 |
-82.8 ± 9.2b
|
| Adrenaline |
5.89 ± 0.10 |
-60.9 ± 9.5b
|