Submitted:
03 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Phytoestrogens: Chemical Classification and General Aspects
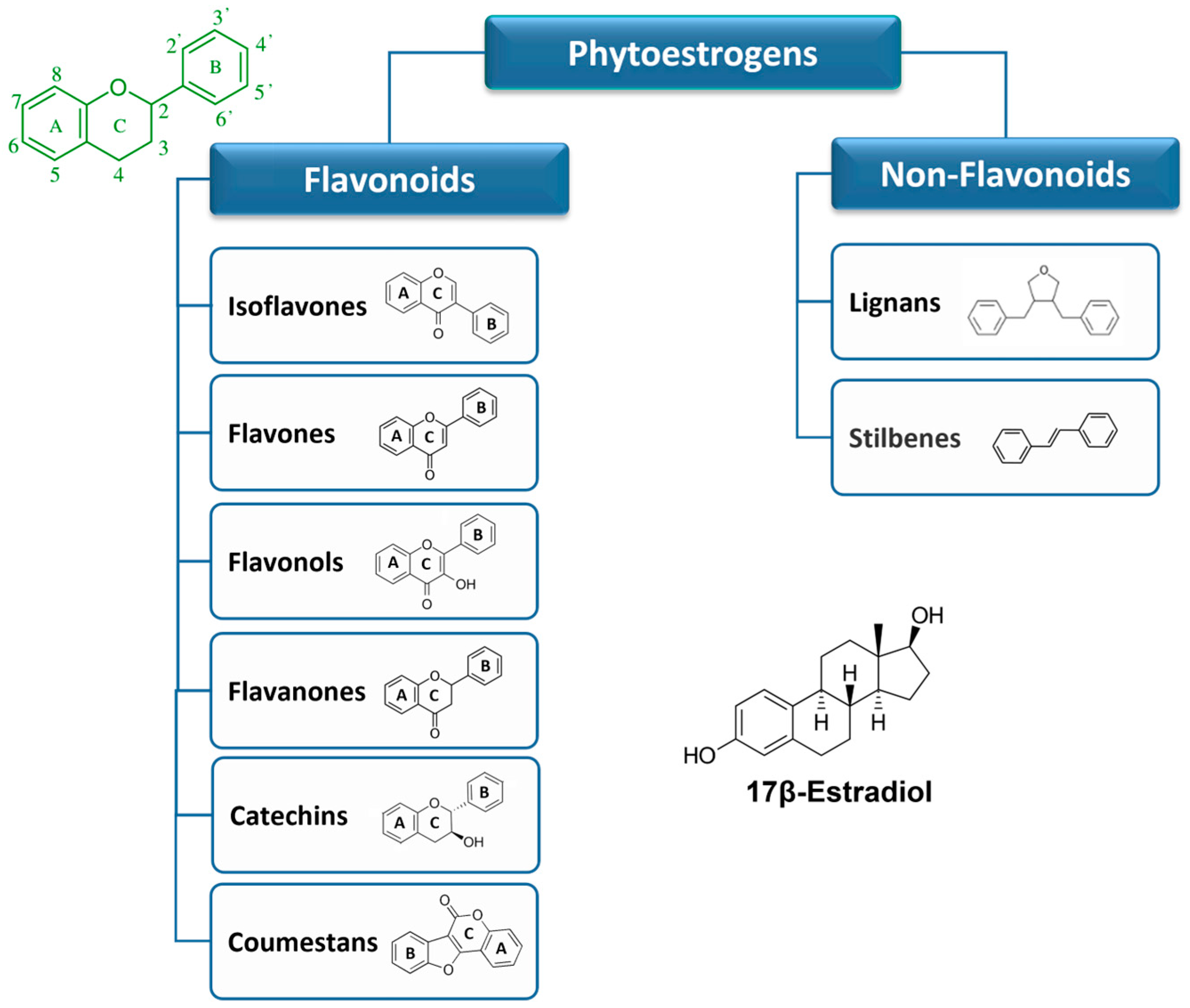
2.1. Flavonoids
2.2. Non-flavonoids
2.3. Metabolism of Dietary Phytoestrogens
3. Phytoestrogen Mechanisms of Action - Anticancer Related Effects
| Phytoestrogen | Relative binding affinity | References | |
|---|---|---|---|
| ERα | ERβ | ||
|
Isoflavones: Genistein Daidzein |
|||
| 4 | 87 | ||
| 0.1 | 0.5 | [19] | |
| Biochanin A | < 0.01 | < 0.01 | |
| Formononetin | < 0.01 | < 0.01 | |
| Flavonols: | |||
| Quercetin | 0.01 | 0.04 | [19] |
| Galangin | ND | ND | |
| Flavones: | |||
| Apigenin | 0.3 | 0.6 | [19] |
| Flavanones: | |||
| Naringenin | 0.01 | 0.11 | [19] |
| Stilbenes: | |||
| Resveratrol | 6.11–11.2a | 4.7–15.66a | [53] |
| Polydatin | ND | ND | |
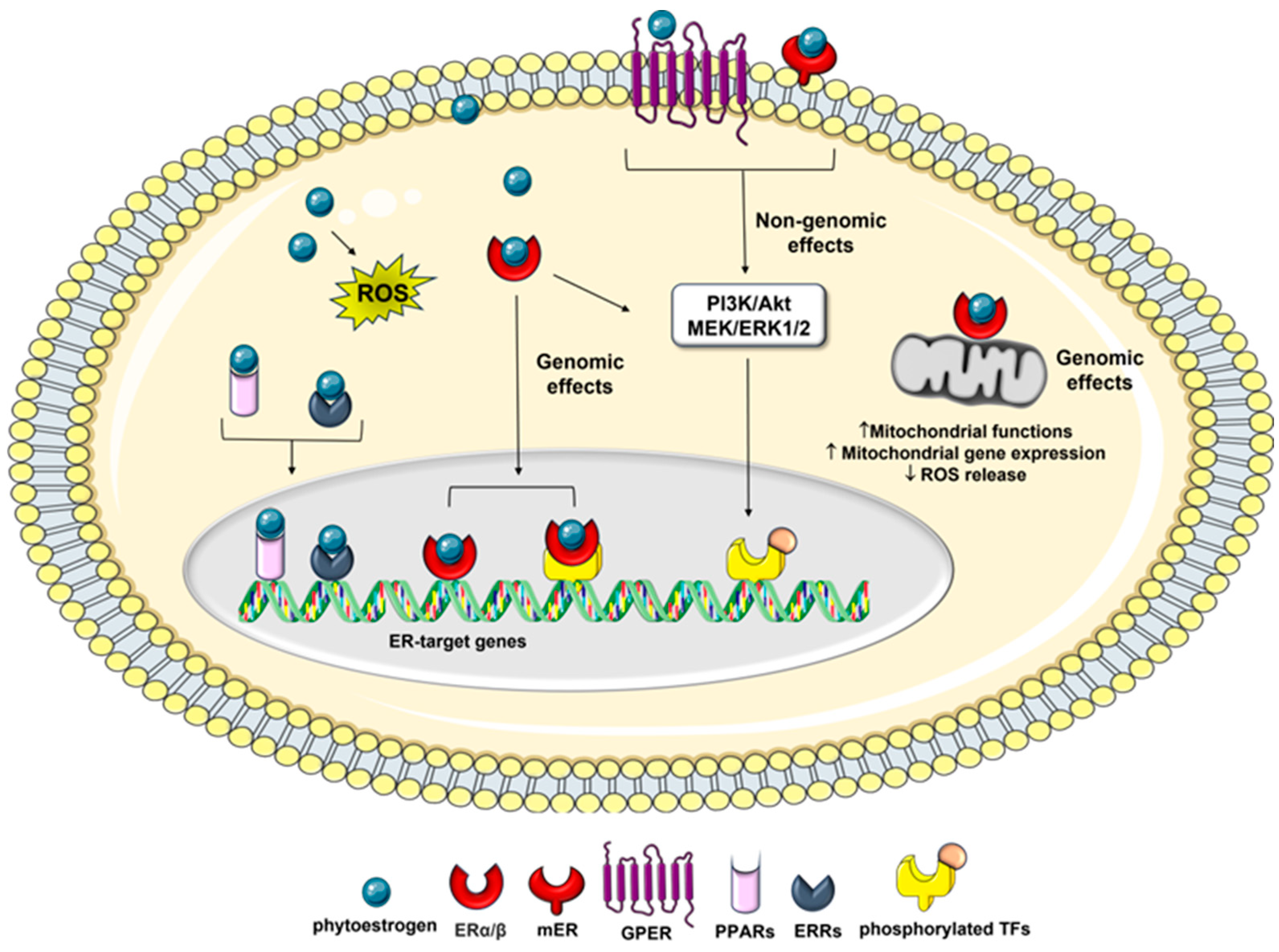
4. Molecular Basis of Osteosarcoma Pathogeneses
5. Estrogen Receptors as a Potential Target for the Treatment of Osteosarcoma
6. Anti-Osteosarcoma Effects of Flavonoids
6.1. Genistein and Related Isoflavones
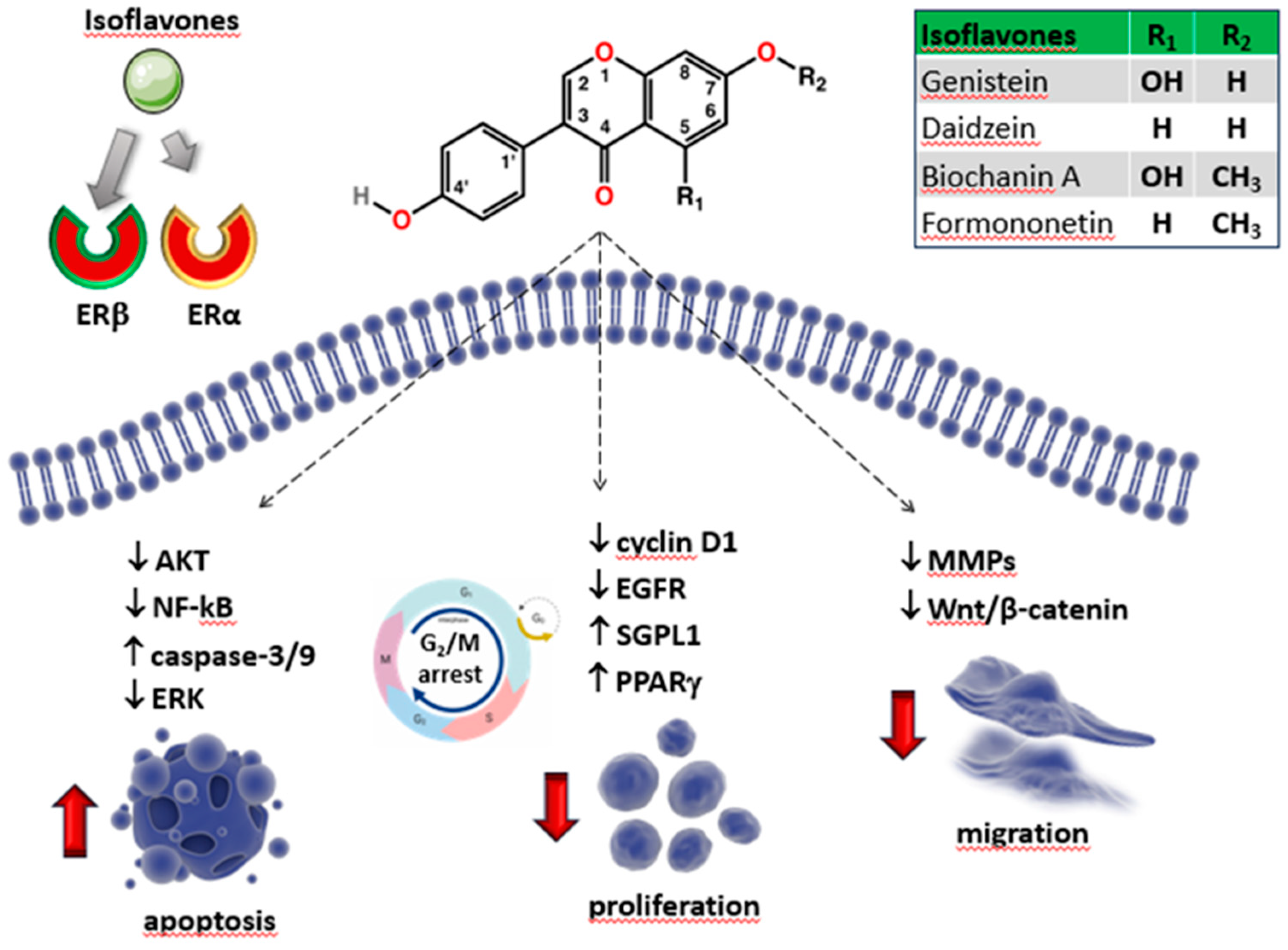
6.1.1. Daidzein
6.1.2. Biochanin A
6.1.3. Formononetin
6.2. Flavonols
6.2.1. Quercetin
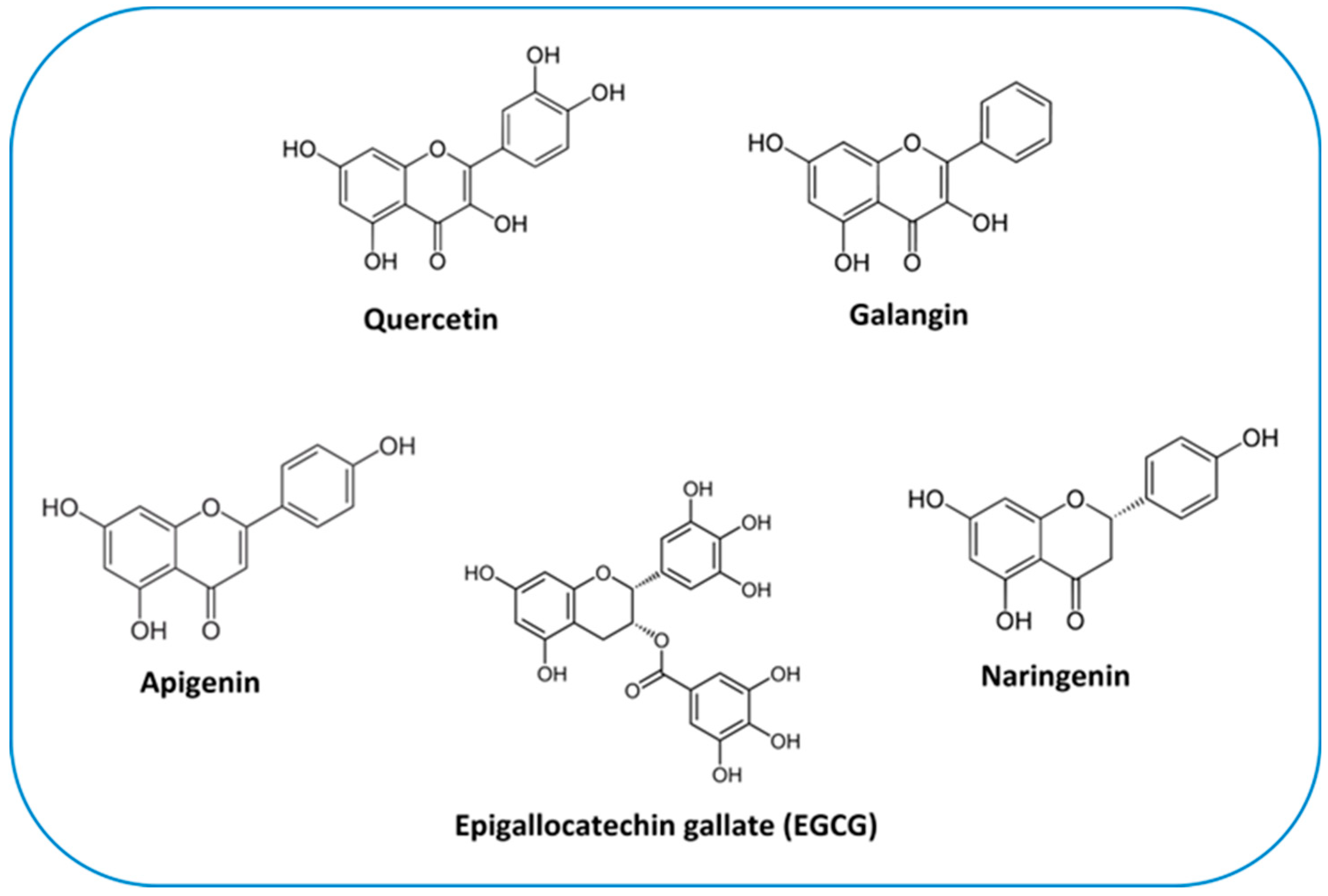
6.2.2. Galangin
6.3. Apigenin
6.4. Naringenin
6.5. Catechins
| Phytoestrogen | Cell Line/ in vivo model |
Concentrations | Combined treatment |
Molecular Mechanism | Observed Effects | References |
|---|---|---|---|---|---|---|
| Genistein | U2OS | 1µM | ER-mediated down-regulation of EGFR |
↑ differentiation ↑ apoptosis ↑ cell cycle arrest |
[156] | |
| MG-63 | 2.5 – 30 μmol/L | ↑ matrix vesicles, ↑ ALP activity |
↑differentiation ↑mineralized bone noduli ↓ proliferation |
[160] | ||
| MG-63, SaOS-2 | 10 – 30 µM | ↓ PTK, ↑ ER | ↓ synthesis and secretion of GAGs/PGs | [164] | ||
| MG-63 | 40 – 80 µM | ↑ PPARγ pathway | ↑cell cycle arrest ↓ proliferation |
[166] | ||
| SaOS-2, MG-63 | 1 – 100 µM | calcitriol 10 nM for 48 h |
↑ SGPL1, VDR, ERβ | ↑ calcitriol sensitivity ↓ proliferation ↓ extracellular acidification ↓ mitochondrial respiration |
[167] | |
| MG-63, U2OS | 10 - 100 μmol/L | gemcitabine 0.5 µmol/l for 72 h |
↓ Akt/NF-κB pathway | ↑ gemcitabine sensitivity ↑ apoptosis |
[270] | |
| Daidzein | U2OS | 1 µM | ER-mediated down-regulation of EGFR |
↑ differentiation ↑ apoptosis ↑cell cycle arrest |
[156] | |
| 143B, U2OS xenograft mouse model |
10 - 500 μmol/L 20 mg/kg every 2 days |
↓ Src-ERK pathway | ↑ apoptosis ↑ cell cycle arrest ↓ migration ↓ tumour weights |
[179] | ||
| Biochanin A | MG-63, U2OS | 5 - 30 μg/mL | DOX 1μg/mL for 24 h |
↑ caspase-3/9, ↑ Bax: Bcl-2/Bcl-XL ratio |
↑ DOX sensitivity ↓ proliferation ↑ apoptosis |
[185] |
| MG-63, U2OS | 5 - 80 μM | ↓ PCNA, cyclin D1, Bcl-2, ↑ Bax, caspase-3; ↓ MMP-9, N-cadherin, ↑ E-cadherin |
↑ cell cycle arrest ↑ apoptosis ↓ migration, invation |
[186] | ||
| Formononetin | U2OS, tumor-bearing nude mice |
20 - 80 μM, 80 mg/kg/d |
↓ Bcl-2, miR-375, ↑ Bax | ↑ apoptosis ↓ tumour weights |
[193] | |
| U2OS | 5 - 100 μM |
↑ caspase-3 and Bax, ↓ Bcl-2 ↓ ERK and PI3K/AKT pathway |
↑ apoptosis |
[192] | ||
| MG-63 | 15 - 45 μM |
↑ miR-214-3p/PTEN pathway | ↓ proliferation ↑ apoptosis |
[194] | ||
| tumor-bearing nude mice |
25-100mg/kg/d | ↓ ESR1, p53, ERBB2 | ↓ tumour weights | [196] | ||
|
Quercetin |
MG-63 | 20 - 320 μM |
↑ cytochrome C, caspase-3/9, Bax, ↓ Bcl-2 |
↑ apoptosis |
[209] | |
| HOS, ATCC 1543 |
10 - 1000 µM | ↓ cyclin D1, ↑ caspase-3, cleaved PARP |
↑ cell cycle arrest ↓ proliferation ↑ apoptosis |
[212] | ||
| U2OS/MTX300 | 10 - 50 µM | ↑ cytochrome C, caspase-3, Bax, cleaved PARP; ↓Bcl-2, Akt | ↑ apoptosis ↓ proliferation |
[214] | ||
| 143B | 10 - 100 µM | ↑ caspase-3, cleaved PARP | ↑ cell cycle arrest ↑ apoptosis ↓ adhesion ↓ migration |
[207] | ||
| MG-63 | 50 - 200 µM | ↑ LC3B-II/LC3B-I ratio, ↓ ROS - NUPR1 pathway |
↑ autophagy | [218] | ||
| HOS, MG-63, tumor-bearing nude mice |
20 - 100 µM 25-100 mg/kg/d |
↓ HIF-1α, VEGF, MMP-2/9 | ↓ migration, invasion ↓ tumor growth |
[210] | ||
| U2OS, SaOS-2 | ↓PTHR1 | ↓ proliferation ↓ adhesion ↓ migration, invasion |
[211] | |||
| 143B | 5 μM | CDDP 5 μM for 24 h |
↑ miR-217- KRAS axis | ↑ CDDP sensitivity ↓ proliferation ↓ migration, invasion |
[208] | |
| SaOS-2 | 10 - 200 μM |
MTX 10-200 μM for 48 h |
↑ p53, CBX7, CYLD, ↓ Bcl-2, miR-223 |
↑ MTX sensitivity ↓ proliferation ↑ apoptosis |
[229] | |
| Galangin | MG-63, U2OS | 5 - 300 µM | ↓ PI3K/Akt pathway ↓ cyclin D1, MMP-2/9, ↑ p27Kip1, caspase-3/8 |
↓ proliferation ↑ apoptosis ↓ invasion |
[236] | |
| MG-63, U2OS tumor xenograft mouse |
25 - 100 μM 50, 100 mg/kg/d |
↑ TGF-β1/Smad2/3 pathway ↑Col I, ALP, OPN, OCN |
↑ differentiation ↓ tumor growth |
[237] | ||
| Apigenin | U2OS tumor xenograft mouse |
50 - 200 μM 2 mg/kg every 3 days |
↑ caspase-3/8/9, BAX, AIF |
↑ apoptosis ↓ tumor growth |
[243] | |
| U2OS, MG-63 |
20 - 100 μg/ml |
↓ Wnt/β-catenin pathway | ↓ proliferation ↓ invasion |
[244] | ||
| Naringenin | HOS, U2OS | 100 - 500 μM | ↑ ROS-Mediated ER Stress | ↑ autophagy ↑ apoptosis |
[253] | |
|
EGCG |
MG-63, 143B, SaOS-2 tumor-bearing nude mice |
10 - 50 μM 10 - 40 mg/kg every 2 days |
↓ Wnt/β-catenin pathway | ↓ proliferation ↓ migration, invasion ↓ tumor growth |
[268] | |
| MG-63, U2OS tumor-bearing nude mice |
0.0125 - 0.1 g/L 30 mg/kg/d |
↑ miR-1 | ↓ proliferation ↓ tumor growth |
[267] | ||
| U2OS | 5 - 50 μM | IL-1Ra1 ng/ml | ↓ MMP-2, VEGF ↓ IL-6/8 |
↓ proliferation ↓ invasion |
[266] | |
| U2OS, SaOS-2 | 20 μg/ml | DOX 1 - 2.5 μM | ↓ SOX2OT | ↑ autophagy | [269], p. 7 |
7. Anti-Osteosarcoma Effects of Non-Flavonoids
7.1. Stilbenes
7.1.1. Resveratrol
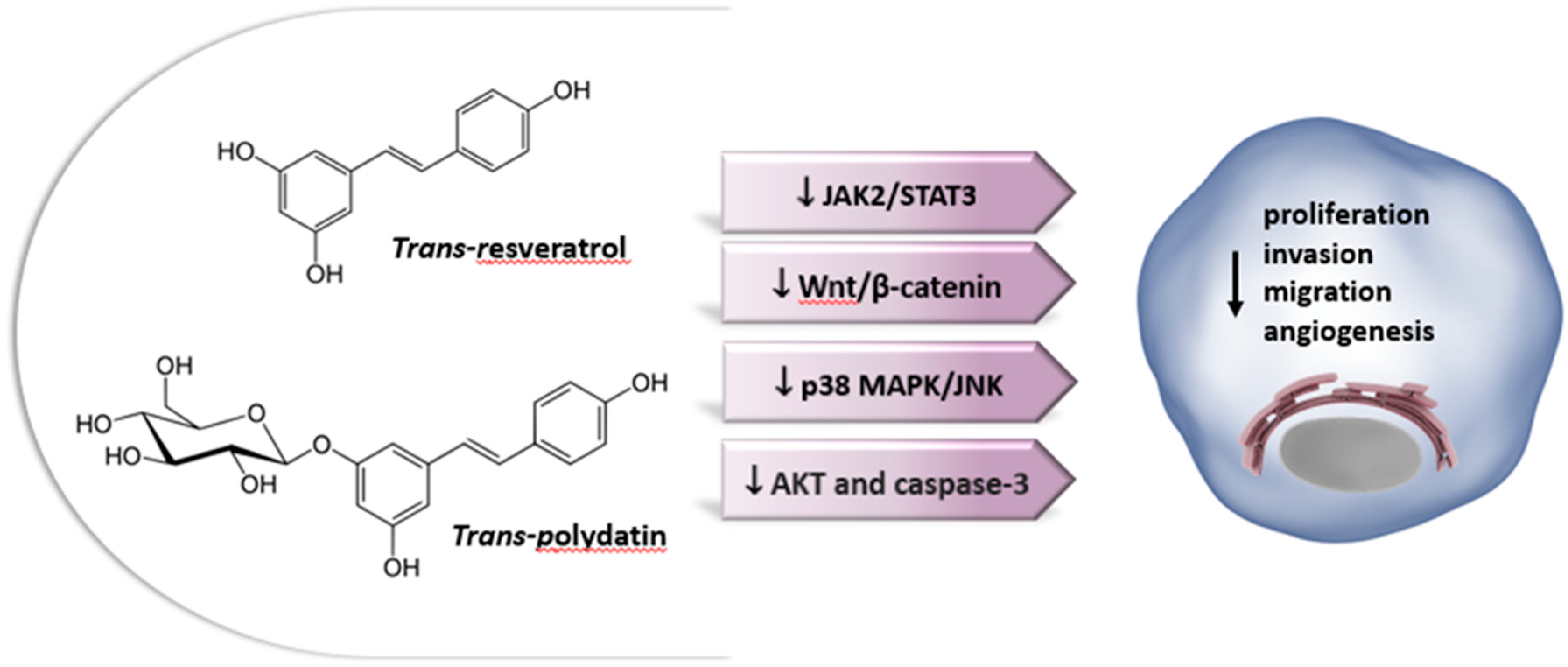
7.1.2. Polydatin
7.2. Lignans
| Phytoestrogen | Cell Line/ in vivo model |
Concentrations | Combined treatment | Molecular Mechanism | Observed Effects | References |
|---|---|---|---|---|---|---|
| Resveratrol | MNNG/HOS, MG-63 tumor xenograft mouse |
10 - 40 μM 100 mg/kg/d |
↑caspase-3, Bax, cleaved PARP, ↓ Bcl-2, Bcl-xL; ↓ cytokines ↓ JAK2/STAT3 pathway |
↑ apoptosis ↓ CSCs survival ↓ tumor growth |
[289] | |
| U2OS | 6 - 24 μg/ml | ↓ Wnt/β-catenin pathway ↓ β-catenin, c-myc, cyclin D1, ↓MMP-2/9 ↑ Cx43, E-cadherin |
↓ proliferation ↑ apoptosis ↓ migration, invasion |
[295] | ||
| MG-63 | 10 - 40 µg/ml | ↓ β-catenin signaling | ↓ proliferation | [288] | ||
| HOS, MG-63, U2OS, SaOS-2, 143B HOS orthotopic graft model |
25 - 200 μM 40, 100-mg/kg/d |
↓ p38 MAPK/JNK pathways ↓ CREB, ↓ MMP-2, ↑ miR-328 |
↓ migration, invasion, adhesion ↓ tumor growth, metastasis |
[301] | ||
| U2OS | 10 - 40 μM | ↓ VEGF | [304] | |||
| MG-63, SaOS-2, KHOS, U2OS | 50 -100 µM | DOX 0.1-10 µM or CDDP 0.2-2 µg/mL for 24 h |
↓ pAKT, ↑ caspase-3 ↓ IL-6/8 ↑ Osx, OPN, ALP, Col I, OCN |
↓ proliferation ↑ apoptosis ↑ differentiation ↑ DOX/CDDP sensitivity |
[291] | |
| Polydatin | 143B, MG-63 | 1 - 100 µM | ↓ β-catenin signaling ↑ Bax/Bcl-2, caspase-3 |
↓ proliferation ↑ apoptosis |
[326] | |
| MG-63 | 10 -160 µM | ↓ STAT3 signaling | ↑ apoptosis, ↑ autophagy | [327,328] | ||
| Polydatin | SaOS-2/ DOX, MG-63/DO xMG-63/ DOX xenograft model |
50 - 250 μM 150 mg/kg/d |
↓ TUG1/Akt signaling | ↓ proliferation ↑ apoptosis ↓ tumor growth |
[329] | |
| U2OS, MG-63 | paclitaxel | ↓ proliferation ↓ migration ↑cell-cycle arrest |
[328] | |||
| SaOS-2 | 1 - 150 μM |
ionizing radiation | ↓ Wnt/β-catenin pathway ↑ lipid metabolite secretion |
↑ differentiation ↑cell-cycle arrest ↑radiation sensitivity |
[330] | |
| Enterodiol, Enterolactone | MG-63 | 0.1–10 mg/ml | ↑ALP activity ↑ ON, Col I |
↓ proliferation ↑ differentiation |
[342] |
8. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- G. Ottaviani e N. Jaffe, «The Epidemiology of Osteosarcoma», in Pediatric and Adolescent Osteosarcoma, N. Jaffe, O. S. Bruland, e S. Bielack, A c. di, in Cancer Treatment and Research, vol. 152. Boston, MA: Springer US, 2009, pp. 3–13. doi: 10.1007/978-1-4419-0284-9_1. [CrossRef]
- S. K. Denduluri et al., «Molecular pathogenesis and therapeutic strategies of human osteosarcoma», J Biomed Res, vol. 30, ago. 2015, doi: 10.7555/JBR.29.20150075. [CrossRef]
- B. Zhang, Y. Zhang, R. Li, J. Li, X. Lu, e Y. Zhang, «The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: a network meta-analysis», J Orthop Surg Res, vol. 15, fasc. 1, p. 51, dic. 2020, doi: 10.1186/s13018-020-1576-0. [CrossRef]
- L. Marchandet, M. Lallier, C. Charrier, M. Baud’huin, B. Ory, e F. Lamoureux, «Mechanisms of Resistance to Conventional Therapies for Osteosarcoma», Cancers, vol. 13, fasc. 4, p. 683, feb. 2021, doi: 10.3390/cancers13040683. [CrossRef]
- Y. Arima, H. Nobusue, e H. Saya, «Targeting of cancer stem cells by differentiation therapy», Cancer Sci, vol. 111, fasc. 8, pp. 2689–2695, ago. 2020, doi: 10.1111/cas.14504. [CrossRef]
- S. Shukla, S. Ohnuma, e S. V. Ambudkar, «Improving Cancer Chemotherapy with Modulators of ABC Drug Transporters», CDT, vol. 12, fasc. 5, pp. 621–630, mag. 2011, doi: 10.2174/138945011795378540. [CrossRef]
- J.-J. Monsuez, J.-C. Charniot, N. Vignat, e J.-Y. Artigou, «Cardiac side-effects of cancer chemotherapy», International Journal of Cardiology, vol. 144, fasc. 1, pp. 3–15, set. 2010, doi: 10.1016/j.ijcard.2010.03.003. [CrossRef]
- M. Torrens-Mas e P. Roca, «Phytoestrogens for Cancer Prevention and Treatment», Biology (Basel), vol. 9, fasc. 12, p. 427, nov. 2020, doi: 10.3390/biology9120427. [CrossRef]
- X. J. Hu, W. R. Song, L. Y. Gao, S. P. Nie, G. Eisenbrand, e M. Y. Xie, «Assessment of dietary phytoestrogen intake via plant-derived foods in China», Food Addit Contam Part A Chem Anal Control Expo Risk Assess, vol. 31, fasc. 8, pp. 1325–1335, 2014, doi: 10.1080/19440049.2014.930562. [CrossRef]
- M. Cipolletti, V. Solar Fernandez, E. Montalesi, M. Marino, e M. Fiocchetti, «Beyond the Antioxidant Activity of Dietary Polyphenols in Cancer: the Modulation of Estrogen Receptors (ERs) Signaling», Int J Mol Sci, vol. 19, fasc. 9, p. 2624, set. 2018, doi: 10.3390/ijms19092624. [CrossRef]
- C. R. Sirtori, A. Arnoldi, e S. K. Johnson, «Phytoestrogens: end of a tale?», Ann Med, vol. 37, fasc. 6, pp. 423–438, 2005, doi: 10.1080/07853890510044586. [CrossRef]
- V. S. Ionescu, A. Popa, A. Alexandru, E. Manole, M. Neagu, e S. Pop, «Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health», Antioxidants (Basel), vol. 10, fasc. 12, p. 1893, nov. 2021, doi: 10.3390/antiox10121893. [CrossRef]
- Z. Yang, M. Yang, W. Yu, e H. Tao, «Molecular mechanisms of estrogen receptor β -induced apoptosis and autophagy in tumors: implication for treating osteosarcoma», J Int Med Res, vol. 47, fasc. 10, pp. 4644–4655, ott. 2019, doi: 10.1177/0300060519871373. [CrossRef]
- S. C. Manolagas, C. A. O’Brien, e M. Almeida, «The role of estrogen and androgen receptors in bone health and disease», Nat Rev Endocrinol, vol. 9, fasc. 12, pp. 699–712, dic. 2013, doi: 10.1038/nrendo.2013.179. [CrossRef]
- M. Tobeiha et al., «Potential of natural products in osteosarcoma treatment: Focus on molecular mechanisms», Biomedicine & Pharmacotherapy, vol. 144, p. 112257, dic. 2021, doi: 10.1016/j.biopha.2021.112257. [CrossRef]
- T. P. Kondratyuk e J. M. Pezzuto, «Natural Product Polyphenols of Relevance to Human Health», Pharmaceutical Biology, vol. 42, fasc. sup1, pp. 46–63, gen. 2004, doi: 10.3109/13880200490893519. [CrossRef]
- P. Vuorela et al., «Natural Products in the Process of Finding New Drug Candidates», CMC, vol. 11, fasc. 11, pp. 1375–1389, giu. 2004, doi: 10.2174/0929867043365116. [CrossRef]
- R. J. Miksicek, «Estrogenic Flavonoids: Structural Requirements for Biological Activity», Experimental Biology and Medicine, vol. 208, fasc. 1, pp. 44–50, gen. 1995, doi: 10.3181/00379727-208-43830. [CrossRef]
- G. G. Kuiper et al., «Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta», Endocrinology, vol. 139, fasc. 10, pp. 4252–4263, ott. 1998, doi: 10.1210/endo.139.10.6216. [CrossRef]
- J. C. Le Bail, Y. Champavier, A. J. Chulia, e G. Habrioux, «Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells», Life Sci, vol. 66, fasc. 14, pp. 1281–1291, feb. 2000, doi: 10.1016/s0024-3205(00)00435-5. [CrossRef]
- I. M. C. M. Rietjens, A. M. Sotoca, J. Vervoort, e J. Louisse, «Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks», Mol. Nutr. Food Res., vol. 57, fasc. 1, pp. 100–113, gen. 2013, doi: 10.1002/mnfr.201200439. [CrossRef]
- P. Roca, J. Sastre-Serra, M. Nadal-Serrano, D. G. Pons, M. del M. Blanquer-Rosselló, e J. Oliver, «Phytoestrogens and mitochondrial biogenesis in breast cancer. Influence of estrogen receptors ratio», Curr Pharm Des, vol. 20, fasc. 35, pp. 5594–5618, 2014, doi: 10.2174/1381612820666140306100709. [CrossRef]
- I. Nikolić, I. Savić-Gajić, A. Tačić, e I. Savić, «Classification and biological activity of phytoestrogens: A review», Adv Techol, vol. 6, fasc. 2, pp. 96–106, 2017, doi: 10.5937/savteh1702096N. [CrossRef]
- R. E. Mutha, A. U. Tatiya, e S. J. Surana, «Flavonoids as natural phenolic compounds and their role in therapeutics: an overview», Futur J Pharm Sci, vol. 7, fasc. 1, p. 25, 2021, doi: 10.1186/s43094-020-00161-8. [CrossRef]
- T.-Y. Wang, Q. Li, e K.-S. Bi, «Bioactive flavonoids in medicinal plants: Structure, activity and biological fate», Asian J Pharm Sci, vol. 13, fasc. 1, pp. 12–23, gen. 2018, doi: 10.1016/j.ajps.2017.08.004. [CrossRef]
- B. A. Graf, P. E. Milbury, e J. B. Blumberg, «Flavonols, flavones, flavanones, and human health: epidemiological evidence», J Med Food, vol. 8, fasc. 3, pp. 281–290, 2005, doi: 10.1089/jmf.2005.8.281. [CrossRef]
- A. N. Panche, A. D. Diwan, e S. R. Chandra, «Flavonoids: an overview», J Nutr Sci, vol. 5, p. e47, 2016, doi: 10.1017/jns.2016.41. [CrossRef]
- M.-J. Xu et al., «Simultaneous characterization of prenylated flavonoids and isoflavonoids in Psoralea corylifolia L. by liquid chromatography with diode-array detection and quadrupole time-of-flight mass spectrometry», Rapid Commun Mass Spectrom, vol. 26, fasc. 19, pp. 2343–2358, ott. 2012, doi: 10.1002/rcm.6361. [CrossRef]
- C. Manach, A. Scalbert, C. Morand, C. Rémésy, e L. Jiménez, «Polyphenols: food sources and bioavailability», The American Journal of Clinical Nutrition, vol. 79, fasc. 5, pp. 727–747, mag. 2004, doi: 10.1093/ajcn/79.5.727. [CrossRef]
- A. Crozier, I. B. Jaganath, e M. N. Clifford, «Dietary phenolics: chemistry, bioavailability and effects on health», Nat Prod Rep, vol. 26, fasc. 8, pp. 1001–1043, ago. 2009, doi: 10.1039/b802662a. [CrossRef]
- A. Durazzo et al., «Dietary Lignans: Definition, Description and Research Trends in Databases Development», Molecules, vol. 23, fasc. 12, p. 3251, dic. 2018, doi: 10.3390/molecules23123251. [CrossRef]
- C. Rodríguez-García, C. Sánchez-Quesada, E. Toledo, M. Delgado-Rodríguez, e J. J. Gaforio, «Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion?», Molecules, vol. 24, fasc. 5, p. 917, mar. 2019, doi: 10.3390/molecules24050917. [CrossRef]
- C. Rivière, A. D. Pawlus, e J.-M. Mérillon, «Natural stilbenoids: distribution in the plant kingdom and chemotaxonomic interest in Vitaceae», Nat Prod Rep, vol. 29, fasc. 11, pp. 1317–1333, nov. 2012, doi: 10.1039/c2np20049j. [CrossRef]
- J. A. Sirerol, M. L. Rodríguez, S. Mena, M. A. Asensi, J. M. Estrela, e A. L. Ortega, «Role of Natural Stilbenes in the Prevention of Cancer», Oxid Med Cell Longev, vol. 2016, p. 3128951, 2016, doi: 10.1155/2016/3128951. [CrossRef]
- J. Burns, T. Yokota, H. Ashihara, M. E. J. Lean, e A. Crozier, «Plant foods and herbal sources of resveratrol», J Agric Food Chem, vol. 50, fasc. 11, pp. 3337–3340, mag. 2002, doi: 10.1021/jf0112973. [CrossRef]
- T. Clavel e J. O. Mapesa, «Phenolics in Human Nutrition: Importance of the Intestinal Microbiome for Isoflavone and Lignan Bioavailability», in Natural Products, K. G. Ramawat e J.-M. Mérillon, A c. di, Berlin, Heidelberg: Springer Berlin Heidelberg, 2013, pp. 2433–2463. doi: 10.1007/978-3-642-22144-6_94. [CrossRef]
- M. T. Viggiani, L. Polimeno, A. Di Leo, e M. Barone, «Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions», Nutrients, vol. 11, fasc. 8, p. 1709, lug. 2019, doi: 10.3390/nu11081709. [CrossRef]
- S. V. Luca et al., «Bioactivity of dietary polyphenols: The role of metabolites», Critical Reviews in Food Science and Nutrition, vol. 60, fasc. 4, pp. 626–659, feb. 2020, doi: 10.1080/10408398.2018.1546669. [CrossRef]
- A. Sarfraz et al., «Biochanin A: A novel bioactive multifunctional compound from nature», Sci Total Environ, vol. 722, p. 137907, giu. 2020, doi: 10.1016/j.scitotenv.2020.137907. [CrossRef]
- E. Bowey, H. Adlercreutz, e I. Rowland, «Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats», Food Chem Toxicol, vol. 41, fasc. 5, pp. 631–636, mag. 2003, doi: 10.1016/s0278-6915(02)00324-1. [CrossRef]
- A. V. Sirotkin e A. H. Harrath, «Phytoestrogens and their effects», Eur J Pharmacol, vol. 741, pp. 230–236, ott. 2014, doi: 10.1016/j.ejphar.2014.07.057. [CrossRef]
- I. Paterni, C. Granchi, J. A. Katzenellenbogen, e F. Minutolo, «Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential», Steroids, vol. 90, pp. 13–29, nov. 2014, doi: 10.1016/j.steroids.2014.06.012. [CrossRef]
- I. M. C. M. Rietjens, J. Louisse, e K. Beekmann, «The potential health effects of dietary phytoestrogens», Br J Pharmacol, vol. 174, fasc. 11, pp. 1263–1280, giu. 2017, doi: 10.1111/bph.13622. [CrossRef]
- V. C. Jordan, «SERMs: meeting the promise of multifunctional medicines», J Natl Cancer Inst, vol. 99, fasc. 5, pp. 350–356, mar. 2007, doi: 10.1093/jnci/djk062. [CrossRef]
- S. Bedell, M. Nachtigall, e F. Naftolin, «The pros and cons of plant estrogens for menopause», J Steroid Biochem Mol Biol, vol. 139, pp. 225–236, gen. 2014, doi: 10.1016/j.jsbmb.2012.12.004. [CrossRef]
- M. G. M. van de Schans, J.-P. Vincken, P. de Waard, A. R. M. Hamers, T. F. H. Bovee, e H. Gruppen, «Glyceollins and dehydroglyceollins isolated from soybean act as SERMs and ER subtype-selective phytoestrogens», J Steroid Biochem Mol Biol, vol. 156, pp. 53–63, feb. 2016, doi: 10.1016/j.jsbmb.2015.11.020. [CrossRef]
- G.-A. Lee, K.-A. Hwang, e K.-C. Choi, «Roles of Dietary Phytoestrogens on the Regulation of Epithelial-Mesenchymal Transition in Diverse Cancer Metastasis», Toxins (Basel), vol. 8, fasc. 6, p. 162, mag. 2016, doi: 10.3390/toxins8060162. [CrossRef]
- M. F. McCarty, «Isoflavones made simple - genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits», Med Hypotheses, vol. 66, fasc. 6, pp. 1093–1114, 2006, doi: 10.1016/j.mehy.2004.11.046. [CrossRef]
- C. Thomas e J.-Å. Gustafsson, «The different roles of ER subtypes in cancer biology and therapy», Nat Rev Cancer, vol. 11, fasc. 8, pp. 597–608, ago. 2011, doi: 10.1038/nrc3093. [CrossRef]
- P. Jonsson, A. Katchy, e C. Williams, «Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells», Endocrine-Related Cancer, vol. 21, fasc. 2, pp. 143–160, apr. 2014, doi: 10.1530/ERC-13-0444. [CrossRef]
- B. Huang, M. Warner, e J.-Å. Gustafsson, «Estrogen receptors in breast carcinogenesis and endocrine therapy», Molecular and Cellular Endocrinology, vol. 418, pp. 240–244, dic. 2015, doi: 10.1016/j.mce.2014.11.015. [CrossRef]
- H. van der Woude, M. G. R. Ter Veld, N. Jacobs, P. T. van der Saag, A. J. Murk, e I. M. C. M. Rietjens, «The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor», Mol Nutr Food Res, vol. 49, fasc. 8, pp. 763–771, ago. 2005, doi: 10.1002/mnfr.200500036. [CrossRef]
- J. L. Bowers, V. V. Tyulmenkov, S. C. Jernigan, e C. M. Klinge, «Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta», Endocrinology, vol. 141, fasc. 10, pp. 3657–3667, ott. 2000, doi: 10.1210/endo.141.10.7721. [CrossRef]
- S. O. Mueller, S. Simon, K. Chae, M. Metzler, e K. S. Korach, «Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells», Toxicol Sci, vol. 80, fasc. 1, pp. 14–25, lug. 2004, doi: 10.1093/toxsci/kfh147. [CrossRef]
- J. An, C. Tzagarakis-Foster, T. C. Scharschmidt, N. Lomri, e D. C. Leitman, «Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens», J Biol Chem, vol. 276, fasc. 21, pp. 17808–17814, mag. 2001, doi: 10.1074/jbc.M100953200. [CrossRef]
- D. P. McDonnell, «The molecular determinants of estrogen receptor pharmacology», Maturitas, vol. 48 Suppl 1, pp. S7-12, ago. 2004, doi: 10.1016/j.maturitas.2004.03.006. [CrossRef]
- G. G. Kuiper et al., «Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta», Endocrinology, vol. 138, fasc. 3, pp. 863–870, mar. 1997, doi: 10.1210/endo.138.3.4979. [CrossRef]
- E. L. Robb e J. A. Stuart, «Resveratrol interacts with estrogen receptor-β to inhibit cell replicative growth and enhance stress resistance by upregulating mitochondrial superoxide dismutase», Free Radic Biol Med, vol. 50, fasc. 7, pp. 821–831, apr. 2011, doi: 10.1016/j.freeradbiomed.2010.12.038. [CrossRef]
- D. Surico et al., «Modulation of Oxidative Stress by 17 β-Estradiol and Genistein in Human Hepatic Cell Lines In Vitro», Cell Physiol Biochem, vol. 42, fasc. 3, pp. 1051–1062, 2017, doi: 10.1159/000478752. [CrossRef]
- A. K. Tanwar, N. Dhiman, A. Kumar, e V. Jaitak, «Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach», Eur J Med Chem, vol. 213, p. 113037, mar. 2021, doi: 10.1016/j.ejmech.2020.113037. [CrossRef]
- Y. Yamaguchi, «Microenvironmental regulation of estrogen signals in breast cancer», Breast Cancer, vol. 14, fasc. 2, pp. 175–181, 2007, doi: 10.2325/jbcs.975. [CrossRef]
- E. R. Prossnitz e M. Barton, «The G-protein-coupled estrogen receptor GPER in health and disease», Nat Rev Endocrinol, vol. 7, fasc. 12, pp. 715–726, ago. 2011, doi: 10.1038/nrendo.2011.122. [CrossRef]
- R. Viñas, Y.-J. Jeng, e C. S. Watson, «Non-Genomic Effects of Xenoestrogen Mixtures», IJERPH, vol. 9, fasc. 8, pp. 2694–2714, lug. 2012, doi: 10.3390/ijerph9082694. [CrossRef]
- L. Molina, F. A. Bustamante, K. D. Bhoola, C. D. Figueroa, e P. Ehrenfeld, «Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling», Clin Sci (Lond), vol. 132, fasc. 24, pp. 2583–2598, dic. 2018, doi: 10.1042/CS20180885. [CrossRef]
- M. Razandi, A. Pedram, I. Merchenthaler, G. L. Greene, e E. R. Levin, «Plasma membrane estrogen receptors exist and functions as dimers», Mol Endocrinol, vol. 18, fasc. 12, pp. 2854–2865, dic. 2004, doi: 10.1210/me.2004-0115. [CrossRef]
- D. C. Márquez, J. Lee, T. Lin, e R. J. Pietras, «Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor», Endocrine, vol. 16, fasc. 2, pp. 73–81, nov. 2001, doi: 10.1385/ENDO:16:2:073. [CrossRef]
- P. Gong, Z. Madak-Erdogan, J. A. Flaws, D. J. Shapiro, J. A. Katzenellenbogen, e B. S. Katzenellenbogen, «Estrogen receptor-α and aryl hydrocarbon receptor involvement in the actions of botanical estrogens in target cells», Mol Cell Endocrinol, vol. 437, pp. 190–200, dic. 2016, doi: 10.1016/j.mce.2016.08.025. [CrossRef]
- Q. Huang e Q. Chen, «Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids», PPAR Res, vol. 2017, p. 3203161, 2017, doi: 10.1155/2017/3203161. [CrossRef]
- K. Kumari, A. K. Adhya, A. K. Rath, P. B. Reddy, e S. K. Mishra, «Estrogen-related receptors alpha, beta and gamma expression and function is associated with transcriptional repressor EZH2 in breast carcinoma», BMC Cancer, vol. 18, fasc. 1, p. 690, dic. 2018, doi: 10.1186/s12885-018-4586-0. [CrossRef]
- S. Barnes, «The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products», Lymphat Res Biol, vol. 8, fasc. 1, pp. 89–98, mar. 2010, doi: 10.1089/lrb.2009.0030. [CrossRef]
- S. Lecomte, F. Demay, F. Ferrière, e F. Pakdel, «Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects?», Int J Mol Sci, vol. 18, fasc. 7, p. 1381, giu. 2017, doi: 10.3390/ijms18071381. [CrossRef]
- K. Pandima Devi, T. Rajavel, M. Daglia, S. F. Nabavi, A. Bishayee, e S. M. Nabavi, «Targeting miRNAs by polyphenols: Novel therapeutic strategy for cancer», Semin Cancer Biol, vol. 46, pp. 146–157, ott. 2017, doi: 10.1016/j.semcancer.2017.02.001. [CrossRef]
- C.-J. Hsieh, Y.-L. Hsu, Y.-F. Huang, e E.-M. Tsai, «Molecular Mechanisms of Anticancer Effects of Phytoestrogens in Breast Cancer», Curr Protein Pept Sci, vol. 19, fasc. 3, pp. 323–332, 2018, doi: 10.2174/1389203718666170111121255. [CrossRef]
- G. L. Russo, I. Tedesco, C. Spagnuolo, e M. Russo, «Antioxidant polyphenols in cancer treatment: Friend, foe or foil?», Seminars in Cancer Biology, vol. 46, pp. 1–13, ott. 2017, doi: 10.1016/j.semcancer.2017.05.005. [CrossRef]
- C. Park et al., «Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway», Antioxidants (Basel), vol. 8, fasc. 9, p. 327, ago. 2019, doi: 10.3390/antiox8090327. [CrossRef]
- Y.-C. Hsiao et al., «Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo», Environmental Toxicology, vol. 34, fasc. 4, pp. 443–456, apr. 2019, doi: 10.1002/tox.22698. [CrossRef]
- S. Rodríguez-Enríquez et al., «Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress», Toxicol Appl Pharmacol, vol. 370, pp. 65–77, mag. 2019, doi: 10.1016/j.taap.2019.03.008. [CrossRef]
- A. Scalbert, C. Manach, C. Morand, C. Rémésy, e L. Jiménez, «Dietary Polyphenols and the Prevention of Diseases», Critical Reviews in Food Science and Nutrition, vol. 45, fasc. 4, pp. 287–306, giu. 2005, doi: 10.1080/1040869059096. [CrossRef]
- F. Virgili e M. Marino, «Regulation of cellular signals from nutritional molecules: a specific role for phytochemicals, beyond antioxidant activity», Free Radical Biology and Medicine, vol. 45, fasc. 9, pp. 1205–1216, nov. 2008, doi: 10.1016/j.freeradbiomed.2008.08.001. [CrossRef]
- M. Asensi, A. Ortega, S. Mena, F. Feddi, e J. M. Estrela, «Natural polyphenols in cancer therapy», Critical Reviews in Clinical Laboratory Sciences, vol. 48, fasc. 5–6, pp. 197–216, dic. 2011, doi: 10.3109/10408363.2011.631268. [CrossRef]
- J. W. Martin, J. A. Squire, e M. Zielenska, «The genetics of osteosarcoma», Sarcoma, vol. 2012, p. 627254, 2012, doi: 10.1155/2012/627254. [CrossRef]
- J. W. V. de Azevedo et al., «Biology and pathogenesis of human osteosarcoma», Oncol Lett, vol. 19, fasc. 2, pp. 1099–1116, feb. 2020, doi: 10.3892/ol.2019.11229. [CrossRef]
- A. M. Czarnecka et al., «Molecular Biology of Osteosarcoma», Cancers (Basel), vol. 12, fasc. 8, p. 2130, lug. 2020, doi: 10.3390/cancers12082130. [CrossRef]
- M. Hameed e D. Mandelker, «Tumor Syndromes Predisposing to Osteosarcoma», Adv Anat Pathol, vol. 25, fasc. 4, pp. 217–222, lug. 2018, doi: 10.1097/PAP.0000000000000190. [CrossRef]
- X. Chen et al., «Recurrent Somatic Structural Variations Contribute to Tumorigenesis in Pediatric Osteosarcoma», Cell Reports, vol. 7, fasc. 1, pp. 104–112, apr. 2014, doi: 10.1016/j.celrep.2014.03.003. [CrossRef]
- L. L. Wang et al., «Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund-Thomson syndrome», J Natl Cancer Inst, vol. 95, fasc. 9, pp. 669–674, mag. 2003, doi: 10.1093/jnci/95.9.669. [CrossRef]
- R. A. Kleinerman, S. J. Schonfeld, e M. A. Tucker, «Sarcomas in hereditary retinoblastoma», Clin Sarcoma Res, vol. 2, fasc. 1, p. 15, dic. 2012, doi: 10.1186/2045-3329-2-15. [CrossRef]
- K. Rickel, F. Fang, e J. Tao, «Molecular genetics of osteosarcoma», Bone, vol. 102, pp. 69–79, set. 2017, doi: 10.1016/j.bone.2016.10.017. [CrossRef]
- M. Fiedorowicz, E. Bartnik, P. Sobczuk, P. Teterycz, e A. M. Czarnecka, «Molecular biology of sarcoma», Oncol Clin Pract, vol. 14, fasc. 6, pp. 307–330, mar. 2019, doi: 10.5603/OCP.2018.0045. [CrossRef]
- D. Chen et al., «Super enhancer inhibitors suppress MYC driven transcriptional amplification and tumor progression in osteosarcoma», Bone Res, vol. 6, fasc. 1, p. 11, apr. 2018, doi: 10.1038/s41413-018-0009-8. [CrossRef]
- L. C. Kim, L. Song, e E. B. Haura, «Src kinases as therapeutic targets for cancer», Nat Rev Clin Oncol, vol. 6, fasc. 10, pp. 587–595, ott. 2009, doi: 10.1038/nrclinonc.2009.129. [CrossRef]
- P. Hingorani, W. Zhang, R. Gorlick, e E. A. Kolb, «Inhibition of Src Phosphorylation Alters Metastatic Potential of Osteosarcoma In vitro but not In vivo», Clinical Cancer Research, vol. 15, fasc. 10, pp. 3416–3422, mag. 2009, doi: 10.1158/1078-0432.CCR-08-1657. [CrossRef]
- M. L. Broadhead, J. C. M. Clark, D. E. Myers, C. R. Dass, e P. F. M. Choong, «The molecular pathogenesis of osteosarcoma: a review», Sarcoma, vol. 2011, p. 959248, 2011, doi: 10.1155/2011/959248. [CrossRef]
- S. Gagiannis et al., «Parathyroid hormone-related protein confers chemoresistance by blocking apoptosis signaling via death receptors and mitochondria», Int. J. Cancer, vol. 125, fasc. 7, pp. 1551–1557, ott. 2009, doi: 10.1002/ijc.24471. [CrossRef]
- S. Sharma, T. K. Kelly, e P. A. Jones, «Epigenetics in cancer», Carcinogenesis, vol. 31, fasc. 1, pp. 27–36, gen. 2010, doi: 10.1093/carcin/bgp220. [CrossRef]
- K. B. Jones et al., «miRNA Signatures Associate with Pathogenesis and Progression of Osteosarcoma», Cancer Research, vol. 72, fasc. 7, pp. 1865–1877, apr. 2012, doi: 10.1158/0008-5472.CAN-11-2663. [CrossRef]
- G. B. Andersen, A. Knudsen, H. Hager, L. L. Hansen, e J. Tost, «miRNA profiling identifies deregulated miRNAs associated with osteosarcoma development and time to metastasis in two large cohorts», Mol Oncol, vol. 12, fasc. 1, pp. 114–131, gen. 2018, doi: 10.1002/1878-0261.12154. [CrossRef]
- S. Zhou et al., «miR-421 is a diagnostic and prognostic marker in patients with osteosarcoma», Tumour Biol, vol. 37, fasc. 7, pp. 9001–9007, lug. 2016, doi: 10.1007/s13277-015-4578-5. [CrossRef]
- Z. Ren et al., «MiR-421 promotes the development of osteosarcoma by regulating MCPIP1 expression», Cancer Biol Ther, vol. 21, fasc. 3, pp. 231–240, 2020, doi: 10.1080/15384047.2019.1683331. [CrossRef]
- J. PosthumaDeBoer, M. A. Witlox, G. J. L. Kaspers, e B. J. van Royen, «Molecular alterations as target for therapy in metastatic osteosarcoma: a review of literature», Clin Exp Metastasis, vol. 28, fasc. 5, pp. 493–503, giu. 2011, doi: 10.1007/s10585-011-9384-x. [CrossRef]
- M. Felx et al., «Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma», Clin Sci (Lond), vol. 110, fasc. 6, pp. 645–654, giu. 2006, doi: 10.1042/CS20050286. [CrossRef]
- J. Cui, D. Dean, F. J. Hornicek, Z. Chen, e Z. Duan, «The role of extracelluar matrix in osteosarcoma progression and metastasis», J Exp Clin Cancer Res, vol. 39, fasc. 1, p. 178, dic. 2020, doi: 10.1186/s13046-020-01685-w. [CrossRef]
- C. Chen, M. Zhao, A. Tian, X. Zhang, Z. Yao, e X. Ma, «Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells», Oncotarget, vol. 6, fasc. 19, pp. 17570–17583, lug. 2015, doi: 10.18632/oncotarget.4100. [CrossRef]
- F. Fang et al., «Targeting the Wnt/β-catenin pathway in human osteosarcoma cells», Oncotarget, vol. 9, fasc. 95, pp. 36780–36792, dic. 2018, doi: 10.18632/oncotarget.26377. [CrossRef]
- L. L. Worth, E. A. Lafleur, S.-F. Jia, e E. S. Kleinerman, «Fas expression inversely correlates with metastatic potential in osteosarcoma cells», Oncol Rep, vol. 9, fasc. 4, pp. 823–827, 2002. [CrossRef]
- E. A. Lafleur et al., «Increased Fas expression reduces the metastatic potential of human osteosarcoma cells», Clin Cancer Res, vol. 10, fasc. 23, pp. 8114–8119, dic. 2004, doi: 10.1158/1078-0432.CCR-04-0353. [CrossRef]
- G. Alloisio et al., «Effects of Extracellular Osteoanabolic Agents on the Endogenous Response of Osteoblastic Cells», Cells, vol. 10, fasc. 9, p. 2383, set. 2021, doi: 10.3390/cells10092383. [CrossRef]
- B. Navet et al., «The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases», Cancers (Basel), vol. 10, fasc. 11, p. 398, ott. 2018, doi: 10.3390/cancers10110398. [CrossRef]
- K. S. Nørregaard, H. J. Jürgensen, H. Gårdsvoll, L. H. Engelholm, N. Behrendt, e K. Søe, «Osteosarcoma and Metastasis Associated Bone Degradation—A Tale of Osteoclast and Malignant Cell Cooperativity», IJMS, vol. 22, fasc. 13, p. 6865, giu. 2021, doi: 10.3390/ijms22136865. [CrossRef]
- E. Grimaud et al., «Receptor Activator of Nuclear Factor κB Ligand (RANKL)/Osteoprotegerin (OPG) Ratio Is Increased in Severe Osteolysis», The American Journal of Pathology, vol. 163, fasc. 5, pp. 2021–2031, nov. 2003, doi: 10.1016/S0002-9440(10)63560-2. [CrossRef]
- Y. Chen et al., «RANKL blockade prevents and treats aggressive osteosarcomas», Sci Transl Med, vol. 7, fasc. 317, p. 317ra197, dic. 2015, doi: 10.1126/scitranslmed.aad0295. [CrossRef]
- E. R. Wagner et al., «Defective osteogenic differentiation in the development of osteosarcoma», Sarcoma, vol. 2011, p. 325238, 2011, doi: 10.1155/2011/325238. [CrossRef]
- E. R. Wagner et al., «Therapeutic Implications of PPARgamma in Human Osteosarcoma», PPAR Res, vol. 2010, p. 956427, 2010, doi: 10.1155/2010/956427. [CrossRef]
- L. Carpio, J. Gladu, D. Goltzman, e S. A. Rabbani, «Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways», Am J Physiol Endocrinol Metab, vol. 281, fasc. 3, pp. E489-499, set. 2001, doi: 10.1152/ajpendo.2001.281.3.E489. [CrossRef]
- A. Kallio et al., «Estrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cells», Mol Cell Endocrinol, vol. 289, fasc. 1–2, pp. 38–48, lug. 2008, doi: 10.1016/j.mce.2008.03.005. [CrossRef]
- S. J. Cotterill, C. M. Wright, M. S. Pearce, A. W. Craft, e UKCCSG/MRC Bone Tumour Working Group, «Stature of young people with malignant bone tumors», Pediatr Blood Cancer, vol. 42, fasc. 1, pp. 59–63, gen. 2004, doi: 10.1002/pbc.10437. [CrossRef]
- S.-M. Wu, L.-H. Shih, J.-Y. Lee, Y.-J. Shen, e H.-H. Lee, «Estrogen enhances activity of Wnt signaling during osteogenesis by inducing Fhl1 expression», J Cell Biochem, vol. 116, fasc. 7, pp. 1419–1430, lug. 2015, doi: 10.1002/jcb.25102. [CrossRef]
- A. B. Khalid e S. A. Krum, «Estrogen receptors alpha and beta in bone», Bone, vol. 87, pp. 130–135, giu. 2016, doi: 10.1016/j.bone.2016.03.016. [CrossRef]
- P. V. Bodine et al., «Estrogen receptor-alpha is developmentally regulated during osteoblast differentiation and contributes to selective responsiveness of gene expression», Endocrinology, vol. 139, fasc. 4, pp. 2048–2057, apr. 1998, doi: 10.1210/endo.139.4.5897. [CrossRef]
- T. C. Spelsberg, M. Subramaniam, B. L. Riggs, e S. Khosla, «The Actions and Interactions of Sex Steroids and Growth Factors/Cytokines on the Skeleton», Molecular Endocrinology, vol. 13, fasc. 6, pp. 819–828, giu. 1999, doi: 10.1210/mend.13.6.0299. [CrossRef]
- D. G. Monroe, B. J. Getz, S. A. Johnsen, B. L. Riggs, S. Khosla, e T. C. Spelsberg, «Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERalpha or ERbeta», J Cell Biochem, vol. 90, fasc. 2, pp. 315–326, ott. 2003, doi: 10.1002/jcb.10633. [CrossRef]
- M. K. Tee et al., «Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta», Mol Biol Cell, vol. 15, fasc. 3, pp. 1262–1272, mar. 2004, doi: 10.1091/mbc.e03-06-0360. [CrossRef]
- R. D. Roberts, «Is Estrogen the Answer for Osteosarcoma?», Cancer Research, vol. 79, fasc. 6, pp. 1034–1035, mar. 2019, doi: 10.1158/0008-5472.CAN-19-0209. [CrossRef]
- M. A. Lillo Osuna et al., «Activation of Estrogen Receptor Alpha by Decitabine Inhibits Osteosarcoma Growth and Metastasis», Cancer Res, vol. 79, fasc. 6, pp. 1054–1068, mar. 2019, doi: 10.1158/0008-5472.CAN-18-1255. [CrossRef]
- F. Stossi, D. H. Barnett, J. Frasor, B. Komm, C. R. Lyttle, e B. S. Katzenellenbogen, «Transcriptional Profiling of Estrogen-Regulated Gene Expression via Estrogen Receptor (ER) α or ERβ in Human Osteosarcoma Cells: Distinct and Common Target Genes for These Receptors», Endocrinology, vol. 145, fasc. 7, pp. 3473–3486, lug. 2004, doi: 10.1210/en.2003-1682. [CrossRef]
- O. Dohi et al., «Sex steroid receptors expression and hormone-induced cell proliferation in human osteosarcoma», Cancer Sci, vol. 99, fasc. 3, pp. 518–523, mar. 2008, doi: 10.1111/j.1349-7006.2007.00673.x. [CrossRef]
- B. J. Deroo e K. S. Korach, «Estrogen receptors and human disease», J Clin Invest, vol. 116, fasc. 3, pp. 561–570, mar. 2006, doi: 10.1172/JCI27987. [CrossRef]
- K. L. Auld et al., «Estrogen-related receptor α regulates osteoblast differentiation via Wnt/β-catenin signaling», J Mol Endocrinol, vol. 48, fasc. 2, pp. 177–191, apr. 2012, doi: 10.1530/JME-11-0140. [CrossRef]
- P.-I. Lin, Y.-T. Tai, W. P. Chan, Y.-L. Lin, M.-H. Liao, e R.-M. Chen, «Estrogen/ERα signaling axis participates in osteoblast maturation via upregulating chromosomal and mitochondrial complex gene expressions», Oncotarget, vol. 9, fasc. 1, pp. 1169–1186, gen. 2018, doi: 10.18632/oncotarget.23453. [CrossRef]
- J.-Y. Wang, C.-M. Chen, C.-F. Chen, P.-K. Wu, e W.-M. Chen, «Suppression of Estrogen Receptor Alpha Inhibits Cell Proliferation, Differentiation and Enhances the Chemosensitivity of P53-Positive U2OS Osteosarcoma Cell», IJMS, vol. 22, fasc. 20, p. 11238, ott. 2021, doi: 10.3390/ijms222011238. [CrossRef]
- Z.-X. Ouyang e X.-A. Li, «Inhibitory effects of tamoxifen and doxorubicin, alone and in combination, on the proliferation of the MG63 human osteosarcoma cell line», Oncol Lett, vol. 6, fasc. 4, pp. 970–976, ott. 2013, doi: 10.3892/ol.2013.1487. [CrossRef]
- T. Quist, H. Jin, J.-F. Zhu, K. Smith-Fry, M. R. Capecchi, e K. B. Jones, «The impact of osteoblastic differentiation on osteosarcomagenesis in the mouse», Oncogene, vol. 34, fasc. 32, pp. 4278–4284, ago. 2015, doi: 10.1038/onc.2014.354. [CrossRef]
- G. Lazennec, «Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis», Cancer Lett, vol. 231, fasc. 2, pp. 151–157, gen. 2006, doi: 10.1016/j.canlet.2005.01.021. [CrossRef]
- M. Gorska, R. M. Wyszkowska, A. Kuban-Jankowska, e M. Wozniak, «Impact of Apparent Antagonism of Estrogen Receptor β by Fulvestrant on Anticancer Activity of 2-Methoxyestradiol», Anticancer Res, vol. 36, fasc. 5, pp. 2217–2226, mag. 2016.
- M. Yang, B. Liu, L. Jin, H. Tao, e Z. Yang, «Estrogen receptor β exhibited anti-tumor effects on osteosarcoma cells by regulating integrin, IAP, NF-kB/BCL-2 and PI3K/Akt signal pathway», J Bone Oncol, vol. 9, pp. 15–20, nov. 2017, doi: 10.1016/j.jbo.2017.09.005. [CrossRef]
- Y. Zhang, C. Yin, X. Zhou, Y. Wu, e L. Wang, «Silencing of estrogen receptor β promotes the invasion and migration of osteosarcoma cells through activating Wnt signaling pathway», OTT, vol. Volume 12, pp. 6779–6788, ago. 2019, doi: 10.2147/OTT.S219222. [CrossRef]
- Z. Yang, W. Yu, B. Liu, M. Yang, e H. Tao, «Estrogen receptor β induces autophagy of osteosarcoma through the mTOR signaling pathway», J Orthop Surg Res, vol. 15, fasc. 1, p. 50, feb. 2020, doi: 10.1186/s13018-020-1575-1. [CrossRef]
- K. Polkowski e A. P. Mazurek, «Biological properties of genistein. A review of in vitro and in vivo data», Acta Pol Pharm, vol. 57, fasc. 2, pp. 135–155, 2000.
- H. S. Tuli et al., «Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances», Front. Pharmacol., vol. 10, p. 1336, dic. 2019, doi: 10.3389/fphar.2019.01336. [CrossRef]
- K. L. Chan, M. K. Y. Siu, Y.-X. Jiang, J.-J. Wang, T. H. Y. Leung, e H. Y. S. Ngan, «Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer», Cancer Cell Int, vol. 18, p. 65, 2018, doi: 10.1186/s12935-018-0559-2. [CrossRef]
- Cassidy Aedin, «Bioavailability of isoflavones in humans. In Flavonoids and Related Compounds: Bioavail-ability and Function», CRC Press: Boca Raton, 2012.
- K. Polkowski et al., «Cytostatic and cytotoxic activity of synthetic genistein glycosides against human cancer cell lines», Cancer Lett, vol. 203, fasc. 1, pp. 59–69, gen. 2004, doi: 10.1016/j.canlet.2003.08.023. [CrossRef]
- C. Spagnuolo et al., «Genistein and cancer: current status, challenges, and future directions», Adv Nutr, vol. 6, fasc. 4, pp. 408–419, lug. 2015, doi: 10.3945/an.114.008052. [CrossRef]
- L. Křížová, K. Dadáková, J. Kašparovská, e T. Kašparovský, «Isoflavones», Molecules, vol. 24, fasc. 6, p. 1076, mar. 2019, doi: 10.3390/molecules24061076. [CrossRef]
- T. Hertrampf, M. J. Gruca, J. Seibel, U. Laudenbach, K. H. Fritzemeier, e P. Diel, «The bone-protective effect of the phytoestrogen genistein is mediated via ER alpha-dependent mechanisms and strongly enhanced by physical activity», Bone, vol. 40, fasc. 6, pp. 1529–1535, giu. 2007, doi: 10.1016/j.bone.2007.02.006. [CrossRef]
- M. S. Kurzer, «Hormonal effects of soy in premenopausal women and men», J Nutr, vol. 132, fasc. 3, pp. 570S-573S, mar. 2002, doi: 10.1093/jn/132.3.570S. [CrossRef]
- F. H. Sarkar e Y. Li, «Mechanisms of cancer chemoprevention by soy isoflavone genistein», Cancer Metastasis Rev, vol. 21, fasc. 3–4, pp. 265–280, 2002, doi: 10.1023/a:1021210910821. [CrossRef]
- H. Jiang, J. Fan, L. Cheng, P. Hu, e R. Liu, «The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells», OTT, vol. Volume 11, pp. 8153–8163, nov. 2018, doi: 10.2147/OTT.S182239. [CrossRef]
- A. Ranjan et al., «Role of Phytochemicals in Cancer Prevention», IJMS, vol. 20, fasc. 20, p. 4981, ott. 2019, doi: 10.3390/ijms20204981. [CrossRef]
- D. J. Rickard, D. G. Monroe, T. J. Ruesink, S. Khosla, B. L. Riggs, e T. C. Spelsberg, «Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors alpha and beta», J Cell Biochem, vol. 89, fasc. 3, pp. 633–646, giu. 2003, doi: 10.1002/jcb.10539. [CrossRef]
- G. Lambrinidis, M. Halabalaki, E. S. Katsanou, A.-L. Skaltsounis, M. N. Alexis, e E. Mikros, «The estrogen receptor and polyphenols: molecular simulation studies of their interactions, a review», Environ Chem Lett, vol. 4, fasc. 3, pp. 159–174, ago. 2006, doi: 10.1007/s10311-006-0065-y. [CrossRef]
- S. Banerjee, Y. Li, Z. Wang, e F. H. Sarkar, «Multi-targeted therapy of cancer by genistein», Cancer Lett, vol. 269, fasc. 2, pp. 226–242, ott. 2008, doi: 10.1016/j.canlet.2008.03.052. [CrossRef]
- S. Nakashima, T. Koike, e Y. Nozawa, «Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses», Mol Pharmacol, vol. 39, fasc. 4, pp. 475–480, apr. 1991.
- Z. M. Shao, M. L. Alpaugh, J. A. Fontana, e S. H. Barsky, «Genistein inhibits proliferation similarly in estrogen receptor-positive and negative human breast carcinoma cell lines characterized by P21WAF1/CIP1 induction, G2/M arrest, and apoptosis», J Cell Biochem, vol. 69, fasc. 1, pp. 44–54, apr. 1998.
- S. Djiogue et al., «Estrogenic properties of naturally occurring prenylated isoflavones in U2OS human osteosarcoma cells: Structure-activity relationships», J Steroid Biochem Mol Biol, vol. 120, fasc. 4–5, pp. 184–191, giu. 2010, doi: 10.1016/j.jsbmb.2010.04.014. [CrossRef]
- L. Salvatori et al., «Down-regulation of epidermal growth factor receptor induced by estrogens and phytoestrogens promotes the differentiation of U2OS human osteosarcoma cells», J. Cell. Physiol., vol. 220, fasc. 1, pp. 35–44, lug. 2009, doi: 10.1002/jcp.21724. [CrossRef]
- X. W. Chen, S. C. Garner, e J. J. B. Anderson, «Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway», Biochem Biophys Res Commun, vol. 295, fasc. 2, pp. 417–422, lug. 2002, doi: 10.1016/s0006-291x(02)00667-8. [CrossRef]
- A. De Wilde, M. Lieberherr, C. Colin, e A. Pointillart, «A low dose of daidzein acts as an ERbeta-selective agonist in trabecular osteoblasts of young female piglets», J Cell Physiol, vol. 200, fasc. 2, pp. 253–262, ago. 2004, doi: 10.1002/jcp.20008. [CrossRef]
- T.-L. Jia, H.-Z. Wang, L.-P. Xie, X.-Y. Wang, e R.-Q. Zhang, «Daidzein enhances osteoblast growth that may be mediated by increased bone morphogenetic protein (BMP) production», Biochem Pharmacol, vol. 65, fasc. 5, pp. 709–715, mar. 2003, doi: 10.1016/s0006-2952(02)01585-x. [CrossRef]
- C. Morris, J. Thorpe, L. Ambrosio, e M. Santin, «The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts», J Nutr, vol. 136, fasc. 5, pp. 1166–1170, mag. 2006, doi: 10.1093/jn/136.5.1166. [CrossRef]
- A. Nakamura et al., «Genistein inhibits cell invasion and motility by inducing cell differentiation in murine osteosarcoma cell line LM8», BMC Cell Biol, vol. 13, fasc. 1, p. 24, dic. 2012, doi: 10.1186/1471-2121-13-24. [CrossRef]
- J. Wei, M. Hu, K. Huang, S. Lin, e H. Du, «Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression», Int J Mol Sci, vol. 21, fasc. 17, p. 5983, ago. 2020, doi: 10.3390/ijms21175983. [CrossRef]
- M. A. Birch e T. M. Skerry, «Differential regulation of syndecan expression by osteosarcoma cell lines in response to cytokines but not osteotropic hormones», Bone, vol. 24, fasc. 6, pp. 571–578, giu. 1999, doi: 10.1016/s8756-3282(99)00088-5. [CrossRef]
- D. Nikitovic, A. M. Tsatsakis, N. K. Karamanos, e G. N. Tzanakakis, «The effects of genistein on the synthesis and distribution of glycosaminoglycans/proteoglycans by two osteosarcoma cell lines depends on tyrosine kinase and the estrogen receptor density», Anticancer Res, vol. 23, fasc. 1A, pp. 459–464, 2003.
- Z.-C. Dang, V. Audinot, S. E. Papapoulos, J. A. Boutin, e C. W. G. M. Löwik, «Peroxisome Proliferator-activated Receptor γ (PPARγ) as a Molecular Target for the Soy Phytoestrogen Genistein», Journal of Biological Chemistry, vol. 278, fasc. 2, pp. 962–967, gen. 2003, doi: 10.1074/jbc.M209483200. [CrossRef]
- M. Song et al., «Genistein exerts growth inhibition on human osteosarcoma MG-63 cells via PPARγ pathway», Int J Oncol, vol. 46, fasc. 3, pp. 1131–1140, mar. 2015, doi: 10.3892/ijo.2015.2829. [CrossRef]
- N. Engel et al., «Synergistic Action of Genistein and Calcitriol in Immature Osteosarcoma MG-63 Cells by SGPL1 Up-Regulation», PLoS One, vol. 12, fasc. 1, p. e0169742, 2017, doi: 10.1371/journal.pone.0169742. [CrossRef]
- W. B. Grant e C. F. Garland, «Vitamin D has a greater impact on cancer mortality rates than on cancer incidence rates», BMJ, vol. 348, p. g2862, apr. 2014, doi: 10.1136/bmj.g2862. [CrossRef]
- J. Lappe et al., «Effect of a combination of genistein, polyunsaturated fatty acids and vitamins D3 and K1 on bone mineral density in postmenopausal women: a randomized, placebo-controlled, double-blind pilot study», Eur J Nutr, vol. 52, fasc. 1, pp. 203–215, feb. 2013, doi: 10.1007/s00394-012-0304-x. [CrossRef]
- E. Degagné et al., «Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs», J Clin Invest, vol. 124, fasc. 12, pp. 5368–5384, dic. 2014, doi: 10.1172/JCI74188. [CrossRef]
- T. Ando, J. Ichikawa, A. Okamoto, K. Tasaka, A. Nakao, e Y. Hamada, «Gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines», J Orthop Res, vol. 23, fasc. 4, pp. 964–969, lug. 2005, doi: 10.1016/j.orthres.2005.01.010. [CrossRef]
- B. Zhang, Z.-L. Shi, B. Liu, X.-B. Yan, J. Feng, e H.-M. Tao, «Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: the role of Akt and nuclear factor-kappaB», Anticancer Drugs, vol. 21, fasc. 3, pp. 288–296, mar. 2010, doi: 10.1097/CAD.0b013e328334da17. [CrossRef]
- C. Nakanishi e M. Toi, «Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs», Nat Rev Cancer, vol. 5, fasc. 4, pp. 297–309, apr. 2005, doi: 10.1038/nrc1588. [CrossRef]
- C. Liang et al., «Genistein potentiates the anti-cancer effects of gemcitabine in human osteosarcoma via the downregulation of Akt and nuclear factor-κB pathway», Anticancer Agents Med Chem, vol. 12, fasc. 5, pp. 554–563, giu. 2012, doi: 10.2174/187152012800617867. [CrossRef]
- M. M. Alshehri et al., «Therapeutic Potential of Isoflavones with an Emphasis on Daidzein», Oxid Med Cell Longev, vol. 2021, p. 6331630, 2021, doi: 10.1155/2021/6331630. [CrossRef]
- P. J. Magee, P. Allsopp, A. Samaletdin, e I. R. Rowland, «Daidzein, R-(+)equol and S-(-)equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2», Eur J Nutr, vol. 53, fasc. 1, pp. 345–350, feb. 2014, doi: 10.1007/s00394-013-0520-z. [CrossRef]
- V. Singh-Gupta et al., «Daidzein Effect on Hormone Refractory Prostate Cancer In Vitro and In Vivo Compared to Genistein and Soy Extract: Potentiation of Radiotherapy», Pharm Res, vol. 27, fasc. 6, pp. 1115–1127, giu. 2010, doi: 10.1007/s11095-010-0107-9. [CrossRef]
- Y.-S. Liang, W.-T. Qi, W. Guo, C.-L. Wang, Z.-B. Hu, e A.-K. Li, «Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets», Food & Nutrition Research, vol. 62, fasc. 0, mag. 2018, doi: 10.29219/fnr.v62.1384. [CrossRef]
- Y. Zhu et al., «Investigation of inhibition effect of daidzein on osteosarcoma cells based on experimental validation and systematic pharmacology analysis», PeerJ, vol. 9, p. e12072, 2021, doi: 10.7717/peerj.12072. [CrossRef]
- G. Renda et al., «Comparative assessment of dermal wound healing potentials of various Trifolium L. extracts and determination of their isoflavone contents as potential active ingredients», J Ethnopharmacol, vol. 148, fasc. 2, pp. 423–432, lug. 2013, doi: 10.1016/j.jep.2013.04.031. [CrossRef]
- C. Yu, P. Zhang, L. Lou, e Y. Wang, «Perspectives Regarding the Role of Biochanin A in Humans», Front. Pharmacol., vol. 10, p. 793, lug. 2019, doi: 10.3389/fphar.2019.00793. [CrossRef]
- Z.-J. Feng e W.-F. Lai, «Chemical and Biological Properties of Biochanin A and Its Pharmaceutical Applications», Pharmaceutics, vol. 15, fasc. 4, p. 1105, mar. 2023, doi: 10.3390/pharmaceutics15041105. [CrossRef]
- W.-Y. Wu et al., «Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells», Int J Mol Med, vol. 35, fasc. 2, pp. 391–398, feb. 2015, doi: 10.3892/ijmm.2014.2020. [CrossRef]
- A. M. Jalaludeen et al., «Biochanin A Ameliorates Arsenic-Induced Hepato- and Hematotoxicity in Rats», Molecules, vol. 21, fasc. 1, p. 69, gen. 2016, doi: 10.3390/molecules21010069. [CrossRef]
- Y.-N. Hsu et al., «Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis», Food Chem Toxicol, vol. 112, pp. 194–204, feb. 2018, doi: 10.1016/j.fct.2017.12.062. [CrossRef]
- Y. Zhao, L. Wang, X. Zhai, T. Cui, G. Wang, e Q. Pang, «The effect of biochanin A on cell growth, apoptosis, and migration in osteosarcoma cells», Pharmazie, vol. 73, fasc. 6, pp. 335–341, giu. 2018, doi: 10.1691/ph.2018.7351. [CrossRef]
- A. Caley e R. Jones, «The principles of cancer treatment by chemotherapy», Surgery (Oxford), vol. 30, fasc. 4, pp. 186–190, apr. 2012, doi: 10.1016/j.mpsur.2012.01.004. [CrossRef]
- M.-H. Lu, M.-F. Fan, e X.-D. Yu, «NSD2 promotes osteosarcoma cell proliferation and metastasis by inhibiting E-cadherin expression», Eur Rev Med Pharmacol Sci, vol. 21, fasc. 5, pp. 928–936, mar. 2017.
- A. Labernadie et al., «A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion», Nat Cell Biol, vol. 19, fasc. 3, pp. 224–237, mar. 2017, doi: 10.1038/ncb3478. [CrossRef]
- S. Ong et al., «Focus on Formononetin: Anticancer Potential and Molecular Targets», Cancers, vol. 11, fasc. 5, p. 611, mag. 2019, doi: 10.3390/cancers11050611. [CrossRef]
- J. Machado Dutra, P. J. P. Espitia, e R. Andrade Batista, «Formononetin: Biological effects and uses - A review», Food Chem, vol. 359, p. 129975, ott. 2021, doi: 10.1016/j.foodchem.2021.129975. [CrossRef]
- Y. Liu et al., «The Proapoptotic Effect of Formononetin in Human Osteosarcoma Cells: Involvement of Inactivation of ERK and Akt Pathways», Cell Physiol Biochem, vol. 34, fasc. 3, pp. 637–645, 2014, doi: 10.1159/000363029. [CrossRef]
- W. Hu e Z. Xiao, «Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo», Cell Physiol Biochem, vol. 37, fasc. 3, pp. 933–939, 2015, doi: 10.1159/000430220. [CrossRef]
- K. Li, H. Shen, M. Lu, J. Chen, Q. Yin, e P. Li, «Formononetin inhibits osteosarcoma cell proliferation and promotes apoptosis by regulating the miR-214-3p/phosphatase and tensin homolog pathway», Transl Cancer Res, vol. 9, fasc. 8, pp. 4914–4921, ago. 2020, doi: 10.21037/tcr-20-2296. [CrossRef]
- X. Zhao et al., «RNA Sequencing of Osteosarcoma Gene Expression Profile Revealed that miR-214-3p Facilitates Osteosarcoma Cell Proliferation via Targeting Ubiquinol-Cytochrome c Reductase Core Protein 1 (UQCRC1)», Med Sci Monit, vol. 25, pp. 4982–4991, lug. 2019, doi: 10.12659/MSM.917375. [CrossRef]
- W. Hu et al., «Anti-cancer targets of formononetin and molecular mechanisms in osteosarcoma: Findings of bioinformatic and experimental assays», J Cell Mol Med, vol. 23, fasc. 5, pp. 3505–3511, mag. 2019, doi: 10.1111/jcmm.14248. [CrossRef]
- I. Domínguez-López, M. Yago-Aragón, A. Salas-Huetos, A. Tresserra-Rimbau, e S. Hurtado-Barroso, «Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review», Nutrients, vol. 12, fasc. 8, p. 2456, ago. 2020, doi: 10.3390/nu12082456. [CrossRef]
- M. G. Hertog, P. C. Hollman, M. B. Katan, e D. Kromhout, «Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands», Nutr Cancer, vol. 20, fasc. 1, pp. 21–29, 1993, doi: 10.1080/01635589309514267. [CrossRef]
- G. D’Andrea, «Quercetin: A flavonol with multifaceted therapeutic applications?», Fitoterapia, vol. 106, pp. 256–271, ott. 2015, doi: 10.1016/j.fitote.2015.09.018. [CrossRef]
- M. Harwood, B. Danielewska-Nikiel, J. F. Borzelleca, G. W. Flamm, G. M. Williams, e T. C. Lines, «A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties», Food Chem Toxicol, vol. 45, fasc. 11, pp. 2179–2205, nov. 2007, doi: 10.1016/j.fct.2007.05.015. [CrossRef]
- X. Wang, D. Ha, R. Yoshitake, Y. S. Chan, D. Sadava, e S. Chen, «Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts», Int J Mol Sci, vol. 22, fasc. 16, p. 8798, ago. 2021, doi: 10.3390/ijms22168798. [CrossRef]
- D. Yang, T. Wang, M. Long, e P. Li, «Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine», Oxid Med Cell Longev, vol. 2020, p. 8825387, 2020, doi: 10.1155/2020/8825387. [CrossRef]
- F. Virgili, F. Acconcia, R. Ambra, A. Rinna, P. Totta, e M. Marino, «Nutritional flavonoids modulate estrogen receptor alpha signaling», IUBMB Life, vol. 56, fasc. 3, pp. 145–151, mar. 2004, doi: 10.1080/15216540410001685083. [CrossRef]
- M. Marino, M. Pellegrini, P. La Rosa, e F. Acconcia, «Susceptibility of estrogen receptor rapid responses to xenoestrogens: Physiological outcomes», Steroids, vol. 77, fasc. 10, pp. 910–917, ago. 2012, doi: 10.1016/j.steroids.2012.02.019. [CrossRef]
- P. Galluzzo et al., «Quercetin-induced apoptotic cascade in cancer cells: antioxidant versus estrogen receptor alpha-dependent mechanisms», Mol Nutr Food Res, vol. 53, fasc. 6, pp. 699–708, giu. 2009, doi: 10.1002/mnfr.200800239. [CrossRef]
- P. Bulzomi e M. Marino, «Environmental endocrine disruptors: does a sex-related susceptibility exist?», Front Biosci (Landmark Ed), vol. 16, fasc. 7, pp. 2478–2498, giu. 2011, doi: 10.2741/3867. [CrossRef]
- K. Berndt, C. Campanile, R. Muff, E. Strehler, W. Born, e B. Fuchs, «Evaluation of quercetin as a potential drug in osteosarcoma treatment», Anticancer Res, vol. 33, fasc. 4, pp. 1297–1306, apr. 2013.
- X. Zhang, Q. Guo, J. Chen, e Z. Chen, «Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating microRNA-217-KRAS Axis», Mol Cells, vol. 38, fasc. 7, pp. 638–642, lug. 2015, doi: 10.14348/molcells.2015.0037. [CrossRef]
- W. Liang et al., «Quercetin-mediated apoptosis via activation of the mitochondrial-dependent pathway in MG-63 osteosarcoma cells», Mol Med Rep, vol. 4, fasc. 5, pp. 1017–1023, 2011, doi: 10.3892/mmr.2011.533. [CrossRef]
- H. Lan, W. Hong, P. Fan, D. Qian, J. Zhu, e B. Bai, «Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells», Cell Physiol Biochem, vol. 43, fasc. 2, pp. 553–567, 2017, doi: 10.1159/000480528. [CrossRef]
- S. Li, Y. Pei, W. Wang, F. Liu, K. Zheng, e X. Zhang, «Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1», Biomed Pharmacother, vol. 114, p. 108839, giu. 2019, doi: 10.1016/j.biopha.2019.108839. [CrossRef]
- D. K. Suh, E. J. Lee, H. C. Kim, e J. H. Kim, «Induction of G(1)/S phase arrest and apoptosis by quercetin in human osteosarcoma cells», Arch Pharm Res, vol. 33, fasc. 5, pp. 781–785, mag. 2010, doi: 10.1007/s12272-010-0519-4. [CrossRef]
- N. Delepine, G. Delepine, G. Bacci, G. Rosen, e J. C. Desbois, «Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. Analysis of the literature», Cancer, vol. 78, fasc. 10, pp. 2127–2135, nov. 1996.
- X. Xie et al., «Quercetin induces apoptosis in the methotrexate-resistant osteosarcoma cell line U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of Akt», Oncol Rep, vol. 26, fasc. 3, pp. 687–693, set. 2011, doi: 10.3892/or.2011.1328. [CrossRef]
- J. Yin et al., «[Effect and mechanism of quercetin on proliferation and apoptosis of human osteosarcoma cell U-2OS/MTX300]», Zhongguo Zhong Yao Za Zhi, vol. 37, fasc. 5, pp. 611–614, mar. 2012.
- J. Yuan et al., «Osteoblastic and osteolytic human osteosarcomas can be studied with a new xenograft mouse model producing spontaneous metastases», Cancer Invest, vol. 27, fasc. 4, pp. 435–442, mag. 2009, doi: 10.1080/07357900802491477. [CrossRef]
- K. Husmann et al., «Matrix Metalloproteinase 1 promotes tumor formation and lung metastasis in an intratibial injection osteosarcoma mouse model», Biochim Biophys Acta, vol. 1832, fasc. 2, pp. 347–354, feb. 2013, doi: 10.1016/j.bbadis.2012.11.006. [CrossRef]
- B. Wu et al., «Quercetin induced NUPR1-dependent autophagic cell death by disturbing reactive oxygen species homeostasis in osteosarcoma cells», J. Clin. Biochem. Nutr., vol. 67, fasc. 2, pp. 137–145, 2020, doi: 10.3164/jcbn.19-121. [CrossRef]
- C. W. Yun e S. H. Lee, «The Roles of Autophagy in Cancer», Int J Mol Sci, vol. 19, fasc. 11, p. 3466, nov. 2018, doi: 10.3390/ijms19113466. [CrossRef]
- P. Bhat, J. Kriel, B. Shubha Priya, null Basappa, N. S. Shivananju, e B. Loos, «Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization», Biochem Pharmacol, vol. 147, pp. 170–182, gen. 2018, doi: 10.1016/j.bcp.2017.11.021. [CrossRef]
- T. Martin et al., «NUPR1 and its potential role in cancer and pathological conditions (Review)», Int J Oncol, vol. 58, fasc. 5, p. 21, mar. 2021, doi: 10.3892/ijo.2021.5201. [CrossRef]
- D. Hanahan e R. A. Weinberg, «Hallmarks of cancer: the next generation», Cell, vol. 144, fasc. 5, pp. 646–674, mar. 2011, doi: 10.1016/j.cell.2011.02.013. [CrossRef]
- H.-Y. Ren et al., «Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: a meta-analysis», OTT, p. 1477, mar. 2016, doi: 10.2147/OTT.S95490. [CrossRef]
- D. Nikitovic et al., «Parathyroid hormone/parathyroid hormone-related peptide regulate osteosarcoma cell functions: Focus on the extracellular matrix (Review)», Oncology Reports, vol. 36, fasc. 4, pp. 1787–1792, ott. 2016, doi: 10.3892/or.2016.4986. [CrossRef]
- N. Oršolić e N. Car, «Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo», Tumour Biol, vol. 35, fasc. 7, pp. 6445–6454, lug. 2014, doi: 10.1007/s13277-014-1843-y. [CrossRef]
- L. Yi, Y. Zongyuan, G. Cheng, Z. Lingyun, Y. GuiLian, e G. Wei, «Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway», Cancer Sci, vol. 105, fasc. 5, pp. 520–527, mag. 2014, doi: 10.1111/cas.12395. [CrossRef]
- G. Scambia et al., «Synergistic antiproliferative activity of quercetin and cisplatin on ovarian cancer cell growth», Anticancer Drugs, vol. 1, fasc. 1, pp. 45–48, ott. 1990, doi: 10.1097/00001813-199010000-00008. [CrossRef]
- J. Guo, Z. Feng, Z. Huang, H. Wang, e W. Lu, «MicroRNA-217 functions as a tumour suppressor gene and correlates with cell resistance to cisplatin in lung cancer», Mol Cells, vol. 37, fasc. 9, pp. 664–671, set. 2014, doi: 10.14348/molcells.2014.0121. [CrossRef]
- E. Mohammadi, F. Alemi, M. Maleki, F. Malakoti, N. Farsad-Akhtar, e B. Yousefi, «Quercetin and Methotrexate in Combination have Anticancer Activity in Osteosarcoma Cells and Repress Oncogenic MicroRNA-223», Drug Res (Stuttg), vol. 72, fasc. 4, pp. 226–233, apr. 2022, doi: 10.1055/a-1709-0658. [CrossRef]
- Y. C. Jung et al., «Anti-inflammatory effects of galangin on lipopolysaccharide-activated macrophages via ERK and NF-κB pathway regulation», Immunopharmacol Immunotoxicol, vol. 36, fasc. 6, pp. 426–432, dic. 2014, doi: 10.3109/08923973.2014.968257. [CrossRef]
- T. P. T. Cushnie e A. J. Lamb, «Assessment of the antibacterial activity of galangin against 4-quinolone resistant strains of Staphylococcus aureus», Phytomedicine, vol. 13, fasc. 3, pp. 187–191, feb. 2006, doi: 10.1016/j.phymed.2004.07.003. [CrossRef]
- J. J. Meyer, A. J. Afolayan, M. B. Taylor, e D. Erasmus, «Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens», J Ethnopharmacol, vol. 56, fasc. 2, pp. 165–169, apr. 1997, doi: 10.1016/s0378-8741(97)01514-6. [CrossRef]
- L. Zhu, Q. Luo, J. Bi, J. Ding, S. Ge, e F. Chen, «Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo», Chem Biol Interact, vol. 224, pp. 149–156, dic. 2014, doi: 10.1016/j.cbi.2014.10.027. [CrossRef]
- J. Cao et al., «Galangin inhibits cell invasion by suppressing the epithelial-mesenchymal transition and inducing apoptosis in renal cell carcinoma», Mol Med Rep, vol. 13, fasc. 5, pp. 4238–4244, mag. 2016, doi: 10.3892/mmr.2016.5042. [CrossRef]
- W.-W. Zou e S.-P. Xu, «Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma», Biomedicine & Pharmacotherapy, vol. 97, pp. 851–863, gen. 2018, doi: 10.1016/j.biopha.2017.09.144. [CrossRef]
- Z. Yang et al., «Galangin suppresses human osteosarcoma cells: An exploration of its underlying mechanism», Oncology Reports, vol. 37, fasc. 1, pp. 435–441, gen. 2017, doi: 10.3892/or.2016.5224. [CrossRef]
- C. Liu, M. Ma, J. Zhang, S. Gui, X. Zhang, e S. Xue, «Galangin inhibits human osteosarcoma cells growth by inducing transforming growth factor-β1-dependent osteogenic differentiation», Biomed Pharmacother, vol. 89, pp. 1415–1421, mag. 2017, doi: 10.1016/j.biopha.2017.03.030. [CrossRef]
- K. Janssens, P. ten Dijke, S. Janssens, e W. Van Hul, «Transforming growth factor-beta1 to the bone», Endocr Rev, vol. 26, fasc. 6, pp. 743–774, ott. 2005, doi: 10.1210/er.2004-0001. [CrossRef]
- D. Patel, S. Shukla, e S. Gupta, «Apigenin and cancer chemoprevention: progress, potential and promise (review)», Int J Oncol, vol. 30, fasc. 1, pp. 233–245, gen. 2007. [CrossRef]
- Cos et al., «Structure−Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers», J. Nat. Prod., vol. 61, fasc. 1, pp. 71–76, gen. 1998, doi: 10.1021/np970237h. [CrossRef]
- T.-D. Way, M.-C. Kao, e J.-K. Lin, «Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway», J Biol Chem, vol. 279, fasc. 6, pp. 4479–4489, feb. 2004, doi: 10.1074/jbc.M305529200. [CrossRef]
- M. E. Gonzalez-Mejia, O. H. Voss, E. J. Murnan, e A. I. Doseff, «Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein–27», Cell Death Dis, vol. 1, fasc. 8, pp. e64–e64, ago. 2010, doi: 10.1038/cddis.2010.41. [CrossRef]
- C.-C. Lin et al., «Apigenin induces apoptosis through mitochondrial dysfunction in U-2 OS human osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth in vivo», J Agric Food Chem, vol. 60, fasc. 45, pp. 11395–11402, nov. 2012, doi: 10.1021/jf303446x. [CrossRef]
- X. Liu, L. Li, L. Lv, D. Chen, L. Shen, e Z. Xie, «Apigenin inhibits the proliferation and invasion of osteosarcoma cells by suppressing the Wnt/β-catenin signaling pathway», Oncol Rep, vol. 34, fasc. 2, pp. 1035–1041, ago. 2015, doi: 10.3892/or.2015.4022. [CrossRef]
- X. Du, J. Yang, D. Yang, W. Tian, e Z. Zhu, «The genetic basis for inactivation of Wnt pathway in human osteosarcoma», BMC Cancer, vol. 14, fasc. 1, p. 450, dic. 2014, doi: 10.1186/1471-2407-14-450. [CrossRef]
- F. Zhang, A. Chen, J. Chen, T. Yu, e F. Guo, «SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells», Asian Pac J Cancer Prev, vol. 12, fasc. 1, pp. 239–245, 2011.
- B. Salehi et al., «The Therapeutic Potential of Naringenin: A Review of Clinical Trials», Pharmaceuticals, vol. 12, fasc. 1, p. 11, gen. 2019, doi: 10.3390/ph12010011. [CrossRef]
- A. Arafah et al., «Multi-Therapeutic Potential of Naringenin (4’,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms», Plants (Basel), vol. 9, fasc. 12, p. 1784, dic. 2020, doi: 10.3390/plants9121784. [CrossRef]
- G.-Y. Liou e P. Storz, «Reactive oxygen species in cancer», Free Radic Res, vol. 44, fasc. 5, pp. 479–496, mag. 2010, doi: 10.3109/10715761003667554. [CrossRef]
- L. Zhang et al., «Citrus aurantium Naringenin Prevents Osteosarcoma Progression and Recurrence in the Patients Who Underwent Osteosarcoma Surgery by Improving Antioxidant Capability», Oxidative Medicine and Cellular Longevity, vol. 2018, pp. 1–16, 2018, doi: 10.1155/2018/8713263. [CrossRef]
- S.-I. Kanno et al., «Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice», Biol Pharm Bull, vol. 28, fasc. 3, pp. 527–530, mar. 2005, doi: 10.1248/bpb.28.527. [CrossRef]
- P. Totta, F. Acconcia, S. Leone, I. Cardillo, e M. Marino, «Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor alpha and beta signalling», IUBMB Life, vol. 56, fasc. 8, pp. 491–499, ago. 2004, doi: 10.1080/15216540400010792. [CrossRef]
- C.-W. Lee et al., «Naringenin Induces ROS-Mediated ER Stress, Autophagy, and Apoptosis in Human Osteosarcoma Cell Lines», Molecules, vol. 27, fasc. 2, p. 373, gen. 2022, doi: 10.3390/molecules27020373. [CrossRef]
- L.-P. Xiang et al., «Suppressive Effects of Tea Catechins on Breast Cancer», Nutrients, vol. 8, fasc. 8, p. 458, lug. 2016, doi: 10.3390/nu8080458. [CrossRef]
- X. Chen et al., «Effects of Tea-Polysaccharide Conjugates and Metal Ions on Precipitate Formation by Epigallocatechin Gallate and Caffeine, the Key Components of Green Tea Infusion», J Agric Food Chem, vol. 67, fasc. 13, pp. 3744–3751, apr. 2019, doi: 10.1021/acs.jafc.8b06681. [CrossRef]
- Y. Shirakami e M. Shimizu, «Possible Mechanisms of Green Tea and Its Constituents against Cancer», Molecules, vol. 23, fasc. 9, p. 2284, set. 2018, doi: 10.3390/molecules23092284. [CrossRef]
- C.-Y. Tsai et al., «Epigallocatechin-3-Gallate Suppresses Human Herpesvirus 8 Replication and Induces ROS Leading to Apoptosis and Autophagy in Primary Effusion Lymphoma Cells», IJMS, vol. 19, fasc. 1, p. 16, dic. 2017, doi: 10.3390/ijms19010016. [CrossRef]
- S. Stadlbauer et al., «Impact of Green Tea Catechin ECG and Its Synthesized Fluorinated Analogue on Prostate Cancer Cells and Stimulated Immunocompetent Cells», Planta Med, vol. 84, fasc. 11, pp. 813–819, lug. 2018, doi: 10.1055/s-0044-102099. [CrossRef]
- J. Wang, G. C. W. Man, T. H. Chan, J. Kwong, e C. C. Wang, «A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer», Cancer Lett, vol. 412, pp. 10–20, gen. 2018, doi: 10.1016/j.canlet.2017.09.054. [CrossRef]
- J.-W. Oh, M. Muthu, S. S. C. Pushparaj, e J. Gopal, «Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components», Molecules, vol. 28, fasc. 5, p. 2151, feb. 2023, doi: 10.3390/molecules28052151. [CrossRef]
- A. Kale et al., «Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract: QUERCETIN/RED ONIONS INCREASE BIOAVAILABILITY OF EGCG», Phytother. Res., vol. 24, fasc. S1, pp. S48–S55, gen. 2010, doi: 10.1002/ptr.2899. [CrossRef]
- Y. Wang et al., «In Vitro and in Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles», ACS Nano, vol. 10, fasc. 11, pp. 9927–9937, nov. 2016, doi: 10.1021/acsnano.6b03835. [CrossRef]
- W. Aljohani, M. W. Ullah, X. Zhang, e G. Yang, «Bioprinting and its applications in tissue engineering and regenerative medicine», International Journal of Biological Macromolecules, vol. 107, pp. 261–275, feb. 2018, doi: 10.1016/j.ijbiomac.2017.08.171. [CrossRef]
- L. P. Gabriel et al., «Bio-based polyurethane for tissue engineering applications: How hydroxyapatite nanoparticles influence the structure, thermal and biological behavior of polyurethane composites», Nanomedicine: Nanotechnology, Biology and Medicine, vol. 13, fasc. 1, pp. 201–208, gen. 2017, doi: 10.1016/j.nano.2016.09.008. [CrossRef]
- S. Khan et al., «Catechins-Modified Selenium-Doped Hydroxyapatite Nanomaterials for Improved Osteosarcoma Therapy Through Generation of Reactive Oxygen Species», Front Oncol, vol. 9, p. 499, 2019, doi: 10.3389/fonc.2019.00499. [CrossRef]
- A.-S. Hönicke, S. A. Ender, e J. Radons, «Combined administration of EGCG and IL-1 receptor antagonist efficiently downregulates IL-1-induced tumorigenic factors in U-2 OS human osteosarcoma cells», Int J Oncol, vol. 41, fasc. 2, pp. 753–758, ago. 2012, doi: 10.3892/ijo.2012.1498. [CrossRef]
- K. Zhu e W. Wang, «Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1», Tumour Biol, vol. 37, fasc. 4, pp. 4373–4382, apr. 2016, doi: 10.1007/s13277-015-4187-3. [CrossRef]
- C. Dong, Z. Wang, P. Shen, Y. Chen, J. Wang, e H. Wang, «Epigallocatechin-3-gallate suppresses the growth of human osteosarcoma by inhibiting the Wnt/β-catenin signalling pathway», Bioengineered, vol. 13, fasc. 4, pp. 8490–8502, apr. 2022, doi: 10.1080/21655979.2022.2051805. [CrossRef]
- W. Wang, D. Chen, e K. Zhu, «SOX2OT variant 7 contributes to the synergistic interaction between EGCG and Doxorubicin to kill osteosarcoma via autophagy and stemness inhibition», J Exp Clin Cancer Res, vol. 37, fasc. 1, p. 37, feb. 2018, doi: 10.1186/s13046-018-0689-3. [CrossRef]
- B. Zhang, Z.-L. Shi, B. Liu, X.-B. Yan, J. Feng, e H.-M. Tao, «Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: the role of Akt and nuclear factor-kappaB», Anticancer Drugs, vol. 21, fasc. 3, pp. 288–296, mar. 2010, doi: 10.1097/CAD.0b013e328334da17. [CrossRef]
- A. Y. Berman, R. A. Motechin, M. Y. Wiesenfeld, e M. K. Holz, «The therapeutic potential of resveratrol: a review of clinical trials», NPJ Precis Oncol, vol. 1, p. 35, 2017, doi: 10.1038/s41698-017-0038-6. [CrossRef]
- P. Jeandet, A.-C. Douillet-Breuil, R. Bessis, S. Debord, M. Sbaghi, e M. Adrian, «Phytoalexins from the Vitaceae: Biosynthesis, Phytoalexin Gene Expression in Transgenic Plants, Antifungal Activity, and Metabolism», J. Agric. Food Chem., vol. 50, fasc. 10, pp. 2731–2741, mag. 2002, doi: 10.1021/jf011429s. [CrossRef]
- X. Chen et al., «Stereospecific determination ofcis- andtrans-resveratrol in rat plasma by HPLC: application to pharmacokinetic studies», Biomed. Chromatogr., vol. 21, fasc. 3, pp. 257–265, mar. 2007, doi: 10.1002/bmc.747. [CrossRef]
- B. B. Aggarwal, Y. Takada, e O. V. Oommen, «From chemoprevention to chemotherapy: common targets and common goals», Expert Opinion on Investigational Drugs, vol. 13, fasc. 10, pp. 1327–1338, ott. 2004, doi: 10.1517/13543784.13.10.1327. [CrossRef]
- C.-H. Cottart, V. Nivet-Antoine, e J.-L. Beaudeux, «Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans», Mol Nutr Food Res, vol. 58, fasc. 1, pp. 7–21, gen. 2014, doi: 10.1002/mnfr.201200589. [CrossRef]
- F. Hajizadeh-Sharafabad, A. Sahebkar, F. Zabetian-Targhi, e V. Maleki, «The impact of resveratrol on toxicity and related complications of advanced glycation end products: A systematic review», BioFactors, vol. 45, fasc. 5, pp. 651–665, set. 2019, doi: 10.1002/biof.1531. [CrossRef]
- Q. Xiao et al., «A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy», Front Pharmacol, vol. 9, p. 1534, 2018, doi: 10.3389/fphar.2018.01534. [CrossRef]
- C. Cal, H. Garban, A. Jazirehi, C. Yeh, Y. Mizutani, e B. Bonavida, «Resveratrol and cancer: chemoprevention, apoptosis, and chemo-immunosensitizing activities», Curr Med Chem Anticancer Agents, vol. 3, fasc. 2, pp. 77–93, mar. 2003, doi: 10.2174/1568011033353443. [CrossRef]
- J.-H. Ko et al., «The Role of Resveratrol in Cancer Therapy», IJMS, vol. 18, fasc. 12, p. 2589, dic. 2017, doi: 10.3390/ijms18122589. [CrossRef]
- K. T. Noh, S. H. Chae, S. H. Chun, I. D. Jung, H. K. Kang, e Y.-M. Park, «Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase», Biochem Biophys Res Commun, vol. 431, fasc. 2, pp. 348–353, feb. 2013, doi: 10.1016/j.bbrc.2012.12.093. [CrossRef]
- Z.-H. Xin et al., «Finding an efficient tetramethylated hydroxydiethylene of resveratrol analogue for potential anticancer agent», BMC Chem, vol. 14, fasc. 1, p. 13, dic. 2020, doi: 10.1186/s13065-020-00667-5. [CrossRef]
- T. Sakamoto, H. Horiguchi, E. Oguma, e F. Kayama, «Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells», J Nutr Biochem, vol. 21, fasc. 9, pp. 856–864, set. 2010, doi: 10.1016/j.jnutbio.2009.06.010. [CrossRef]
- E. Pozo-Guisado, A. Alvarez-Barrientos, S. Mulero-Navarro, B. Santiago-Josefat, e P. M. Fernandez-Salguero, «The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle», Biochem Pharmacol, vol. 64, fasc. 9, pp. 1375–1386, nov. 2002, doi: 10.1016/s0006-2952(02)01296-0. [CrossRef]
- J. C. Tou, «Resveratrol supplementation affects bone acquisition and osteoporosis: Pre-clinical evidence toward translational diet therapy», Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, vol. 1852, fasc. 6, pp. 1186–1194, giu. 2015, doi: 10.1016/j.bbadis.2014.10.003. [CrossRef]
- D. Bellavia et al., «Non-flavonoid polyphenols in osteoporosis: preclinical evidence», Trends Endocrinol Metab, vol. 32, fasc. 7, pp. 515–529, lug. 2021, doi: 10.1016/j.tem.2021.03.008. [CrossRef]
- Y. Li, C.-M. Bäckesjö, L.-A. Haldosén, e U. Lindgren, «Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells», Eur J Pharmacol, vol. 609, fasc. 1–3, pp. 13–18, mag. 2009, doi: 10.1016/j.ejphar.2009.03.004. [CrossRef]
- Y. Liu et al., «Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway», Toxicology, vol. 304, pp. 120–131, feb. 2013, doi: 10.1016/j.tox.2012.12.018. [CrossRef]
- Y. Zou, J. Yang, e D. Jiang, «Resveratrol inhibits canonical Wnt signaling in human MG-63 osteosarcoma cells», Molecular Medicine Reports, vol. 12, fasc. 5, pp. 7221–7226, nov. 2015, doi: 10.3892/mmr.2015.4338. [CrossRef]
- L. Peng e D. Jiang, «Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition», PLoS ONE, vol. 13, fasc. 10, p. e0205918, ott. 2018, doi: 10.1371/journal.pone.0205918. [CrossRef]
- N. Xu, L. Wang, S. Fu, e B. Jiang, «Resveratrol is cytotoxic and acts synergistically with NF-κB inhibition in osteosarcoma MG-63 cells», Arch Med Sci, vol. 17, fasc. 1, pp. 166–176, gen. 2021, doi: 10.5114/aoms.2020.100777. [CrossRef]
- A. De Luca et al., «Multiple Effects of Resveratrol on Osteosarcoma Cell Lines», Pharmaceuticals (Basel), vol. 15, fasc. 3, p. 342, mar. 2022, doi: 10.3390/ph15030342. [CrossRef]
- T. Mengie Ayele, Z. Tilahun Muche, A. Behaile Teklemariam, A. Bogale Kassie, e E. Chekol Abebe, «Role of JAK2/STAT3 Signaling Pathway in the Tumorigenesis, Chemotherapy Resistance, and Treatment of Solid Tumors: A Systemic Review», J Inflamm Res, vol. 15, pp. 1349–1364, 2022, doi: 10.2147/JIR.S353489. [CrossRef]
- C.-F. Chiu et al., «NF-κB-driven suppression of FOXO3a contributes to EGFR mutation-independent gefitinib resistance», Proc Natl Acad Sci U S A, vol. 113, fasc. 18, pp. E2526-2535, mag. 2016, doi: 10.1073/pnas.1522612113. [CrossRef]
- A. Mitra, L. Mishra, e S. Li, «EMT, CTCs and CSCs in tumor relapse and drug-resistance», Oncotarget, vol. 6, fasc. 13, pp. 10697–10711, mag. 2015, doi: 10.18632/oncotarget.4037. [CrossRef]
- D. Xie et al., «Antitumor activity of resveratrol against human osteosarcoma cells: a key role of Cx43 and Wnt/β-catenin signaling pathway», Oncotarget, vol. 8, fasc. 67, pp. 111419–111432, dic. 2017, doi: 10.18632/oncotarget.22810. [CrossRef]
- Y. Shen, P. R. Khusial, X. Li, H. Ichikawa, A. P. Moreno, e G. S. Goldberg, «Src Utilizes Cas to Block Gap Junctional Communication Mediated by Connexin43», Journal of Biological Chemistry, vol. 282, fasc. 26, pp. 18914–18921, giu. 2007, doi: 10.1074/jbc.M608980200. [CrossRef]
- H. Clevers, «Wnt/beta-catenin signaling in development and disease», Cell, vol. 127, fasc. 3, pp. 469–480, nov. 2006, doi: 10.1016/j.cell.2006.10.018. [CrossRef]
- P. J. Morin et al., «Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC», Science, vol. 275, fasc. 5307, pp. 1787–1790, mar. 1997, doi: 10.1126/science.275.5307.1787. [CrossRef]
- V. Korinek et al., «Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma», Science, vol. 275, fasc. 5307, pp. 1784–1787, mar. 1997, doi: 10.1126/science.275.5307.1784. [CrossRef]
- S.-O. Yoon, S.-J. Park, C.-H. Yun, e A.-S. Chung, «Roles of matrix metalloproteinases in tumor metastasis and angiogenesis», J Biochem Mol Biol, vol. 36, fasc. 1, pp. 128–137, gen. 2003, doi: 10.5483/bmbrep.2003.36.1.128. [CrossRef]
- S.-F. Yang et al., «Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas», Oncotarget, vol. 6, fasc. 5, pp. 2736–2753, feb. 2015, doi: 10.18632/oncotarget.3088. [CrossRef]
- G. Ranieri, R. Patruno, E. Ruggieri, S. Montemurro, P. Valerio, e D. Ribatti, «Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic», Curr Med Chem, vol. 13, fasc. 16, pp. 1845–1857, 2006, doi: 10.2174/092986706777585059. [CrossRef]
- L. C. J. Baker et al., «The HIF-pathway inhibitor NSC-134754 induces metabolic changes and anti-tumour activity while maintaining vascular function», Br J Cancer, vol. 106, fasc. 10, pp. 1638–1647, mag. 2012, doi: 10.1038/bjc.2012.131. [CrossRef]
- Z. Liu, Y. Li, e R. Yang, «Effects of resveratrol on vascular endothelial growth factor expression in osteosarcoma cells and cell proliferation», Oncol Lett, vol. 4, fasc. 4, pp. 837–839, ott. 2012, doi: 10.3892/ol.2012.824. [CrossRef]
- Q. Liu, M. Li, S. Wang, Z. Xiao, Y. Xiong, e G. Wang, «Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation», Front. Cell Dev. Biol., vol. 8, p. 601224, dic. 2020, doi: 10.3389/fcell.2020.601224. [CrossRef]
- V. T. Baron, R. Pio, Z. Jia, e D. Mercola, «Early Growth Response 3 regulates genes of inflammation and directly activates IL6 and IL8 expression in prostate cancer», Br J Cancer, vol. 112, fasc. 4, pp. 755–764, feb. 2015, doi: 10.1038/bjc.2014.622. [CrossRef]
- H. Jayatilaka et al., «Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration», Nat Commun, vol. 8, fasc. 1, p. 15584, mag. 2017, doi: 10.1038/ncomms15584. [CrossRef]
- T. H. Kim et al., «Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin», Biochimica et Biophysica Acta (BBA) - General Subjects, vol. 1840, fasc. 1, pp. 615–625, gen. 2014, doi: 10.1016/j.bbagen.2013.10.023. [CrossRef]
- A. S. Barros, E. C. Costa, A. S. Nunes, D. De Melo-Diogo, e I. J. Correia, «Comparative study of the therapeutic effect of Doxorubicin and Resveratrol combination on 2D and 3D (spheroids) cell culture models», International Journal of Pharmaceutics, vol. 551, fasc. 1–2, pp. 76–83, nov. 2018, doi: 10.1016/j.ijpharm.2018.09.016. [CrossRef]
- M. Ren et al., «Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest», Oncol Rep, lug. 2020, doi: 10.3892/or.2020.7708. [CrossRef]
- D. Şöhretoğlu, M. Y. Baran, R. Arroo, e A. Kuruüzüm-Uz, «Recent advances in chemistry, therapeutic properties and sources of polydatin», Phytochem Rev, vol. 17, fasc. 5, pp. 973–1005, ott. 2018, doi: 10.1007/s11101-018-9574-0. [CrossRef]
- P. Jeandet et al., «Phytostilbenes as agrochemicals: biosynthesis, bioactivity, metabolic engineering and biotechnology», Nat Prod Rep, vol. 38, fasc. 7, pp. 1282–1329, lug. 2021, doi: 10.1039/d0np00030b. [CrossRef]
- S. Chen et al., «Polydatin down-regulates the phosphorylation level of Creb and induces apoptosis in human breast cancer cell», PLoS ONE, vol. 12, fasc. 5, p. e0176501, mag. 2017, doi: 10.1371/journal.pone.0176501. [CrossRef]
- J. Ye et al., «Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways», Sci Rep, vol. 7, fasc. 1, p. 11895, set. 2017, doi: 10.1038/s41598-017-12252-3. [CrossRef]
- Y.-S. Shen et al., «Polydatin improves osteogenic differentiation of human bone mesenchymal stem cells by stimulating TAZ expression via BMP2-Wnt/β-catenin signaling pathway», Stem Cell Res Ther, vol. 11, fasc. 1, p. 204, mag. 2020, doi: 10.1186/s13287-020-01705-8. [CrossRef]
- A. Karami, S. Fakhri, L. Kooshki, e H. Khan, «Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits», Molecules, vol. 27, fasc. 19, p. 6474, ott. 2022, doi: 10.3390/molecules27196474. [CrossRef]
- Y. Chen et al., «Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease», Mol Neurodegeneration, vol. 10, fasc. 1, p. 4, dic. 2015, doi: 10.1186/1750-1326-10-4. [CrossRef]
- M. Chen, «Enzymatic transformation of polydatin to resveratrol by piceid-β-d-glucosidase from Aspergillus oryzae».
- W.-J. Cao, K. Wu, C. Wang, e D.-M. Wan, «Polydatin-induced cell apoptosis and cell cycle arrest are potentiated by Janus kinase 2 inhibition in leukemia cells», Mol Med Rep, vol. 13, fasc. 4, pp. 3297–3302, apr. 2016, doi: 10.3892/mmr.2016.4909. [CrossRef]
- H.-L. Wang et al., «Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo», Phytomedicine, vol. 22, fasc. 5, pp. 553–559, mag. 2015, doi: 10.1016/j.phymed.2015.03.014. [CrossRef]
- H. Zhang et al., «Anticancer effects and potential mechanisms of ginsenoside Rh2 in various cancer types (Review)», Oncol Rep, vol. 45, fasc. 4, p. 33, feb. 2021, doi: 10.3892/or.2021.7984. [CrossRef]
- M. A. Shah et al., «Uncovering the Anticancer Potential of Polydatin: A Mechanistic Insight», Molecules, vol. 27, fasc. 21, p. 7175, ott. 2022, doi: 10.3390/molecules27217175. [CrossRef]
- B. Wang, Y. Zhang, Z. Mao, D. Yu, e C. Gao, «Toxicity of ZnO nanoparticles to macrophages due to cell uptake and intracellular release of zinc ions», J Nanosci Nanotechnol, vol. 14, fasc. 8, pp. 5688–5696, ago. 2014, doi: 10.1166/jnn.2014.8876. [CrossRef]
- C. Hong et al., «Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer», Theranostics, vol. 9, fasc. 15, pp. 4437–4449, 2019, doi: 10.7150/thno.34953. [CrossRef]
- L. Mele et al., «A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo», Cell Death Dis, vol. 9, fasc. 5, p. 572, mag. 2018, doi: 10.1038/s41419-018-0635-5. [CrossRef]
- G. Xu, G. Kuang, W. Jiang, R. Jiang, e D. Jiang, «Polydatin promotes apoptosis through upregulation the ratio of Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin signaling in human osteosarcoma cells», Am J Transl Res, vol. 8, fasc. 2, pp. 922–931, 2016.
- C.-Q. Jiang, L.-L. Ma, Z.-D. Lv, F. Feng, Z. Chen, e Z.-D. Liu, «Polydatin induces apoptosis and autophagy via STAT3 signaling in human osteosarcoma MG-63 cells», J Nat Med, vol. 74, fasc. 3, pp. 533–544, giu. 2020, doi: 10.1007/s11418-020-01399-5. [CrossRef]
- W. Zhao, Z. Chen, e M. Guan, «Polydatin enhances the chemosensitivity of osteosarcoma cells to paclitaxel», J Cell Biochem, vol. 120, fasc. 10, pp. 17481–17490, ott. 2019, doi: 10.1002/jcb.29012. [CrossRef]
- T. Hu, Z. Fei, H. Su, R. Xie, e L. Chen, «Polydatin inhibits proliferation and promotes apoptosis of doxorubicin-resistant osteosarcoma through LncRNA TUG1 mediated suppression of Akt signaling», Toxicol Appl Pharmacol, vol. 371, pp. 55–62, mag. 2019, doi: 10.1016/j.taap.2019.04.005. [CrossRef]
- A. Luce et al., «Polydatin Induces Differentiation and Radiation Sensitivity in Human Osteosarcoma Cells and Parallel Secretion through Lipid Metabolite Secretion», Oxidative Medicine and Cellular Longevity, vol. 2021, pp. 1–11, lug. 2021, doi: 10.1155/2021/3337013. [CrossRef]
- V. Kota e H. Hama, «2′-Hydroxy ceramide in membrane homeostasis and cell signaling», Advances in Biological Regulation, vol. 54, pp. 223–230, gen. 2014, doi: 10.1016/j.jbior.2013.09.012. [CrossRef]
- E. Matsuzaki et al., «Sphingosine-1-phosphate promotes the nuclear translocation of β-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines», Bone, vol. 55, fasc. 2, pp. 315–324, ago. 2013, doi: 10.1016/j.bone.2013.04.008. [CrossRef]
- F. Mostafa, A. Abdel-Moneim, M. Abdul-Hamid, S. R. Galaly, e H. M. Mohamed, «Polydatin and polydatin-loaded chitosan nanoparticles attenuate diabetic cardiomyopathy in rats», J Mol Histol, vol. 52, fasc. 2, pp. 135–152, apr. 2021, doi: 10.1007/s10735-020-09930-4. [CrossRef]
- Q. Guan, W. Chen, X. Hu, X. Wang, e L. Li, «Novel nanoliposomal delivery system for polydatin: preparation, characterization, and in vivo evaluation», DDDT, p. 1805, mar. 2015, doi: 10.2147/DDDT.S77615. [CrossRef]
- L. Lin et al., «Nanodrug with ROS and pH Dual-Sensitivity Ameliorates Liver Fibrosis via Multicellular Regulation», Adv Sci (Weinh), vol. 7, fasc. 7, p. 1903138, apr. 2020, doi: 10.1002/advs.201903138. [CrossRef]
- J. Xiao, F. Wang, J. Liu, L. Wang, G. Kai, e X. Yu, «Effect of ZnO#ZnS QDs heterojunctures on the stilbenes–plasma proteins interactions», Mol. BioSyst., vol. 7, fasc. 8, p. 2452, 2011, doi: 10.1039/c1mb05087g. [CrossRef]
- A. Basta-Kaim et al., «Protective effects of polydatin in free and nanocapsulated form on changes caused by lipopolysaccharide in hippocampal organotypic cultures», Pharmacol Rep, vol. 71, fasc. 4, pp. 603–613, ago. 2019, doi: 10.1016/j.pharep.2019.02.017. [CrossRef]
- A. M. Abd El-Hameed, A. I. Yousef, S. M. Abd El-Twab, A. A. G. El-Shahawy, e A. Abdel-Moneim, «Hepatoprotective Effects of Polydatin-Loaded Chitosan Nanoparticles in Diabetic Rats: Modulation of Glucose Metabolism, Oxidative Stress, and Inflammation Biomarkers», Biochemistry (Mosc), vol. 86, fasc. 2, pp. 179–189, feb. 2021, doi: 10.1134/S0006297921020061. [CrossRef]
- E. D. Moyers-Montoya, R. G. Escobedo-González, C. L. Vargas-Requena, P. E. Garcia-Casillas, e C. A. Martínez-Pérez, «Epithelial Growth Factor-Anchored on Polycaprolactone/6-deoxy-6-amino-β-cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation», Polymers, vol. 13, fasc. 8, p. 1303, apr. 2021, doi: 10.3390/polym13081303. [CrossRef]
- S. Lama et al., «Polydatin Incorporated in Polycaprolactone Nanofibers Improves Osteogenic Differentiation», Pharmaceuticals (Basel), vol. 15, fasc. 6, p. 727, giu. 2022, doi: 10.3390/ph15060727. [CrossRef]
- Á. Peirotén, D. Bravo, e J. M. Landete, «Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health», Crit Rev Food Sci Nutr, vol. 60, fasc. 11, pp. 1922–1937, 2020, doi: 10.1080/10408398.2019.1622505. [CrossRef]
- J. Feng, Z. Shi, e Z. Ye, «Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells», Biol Pharm Bull, vol. 31, fasc. 6, pp. 1067–1070, giu. 2008, doi: 10.1248/bpb.31.1067. [CrossRef]
- S. C. Thomasset, D. P. Berry, G. Garcea, T. Marczylo, W. P. Steward, e A. J. Gescher, «Dietary polyphenolic phytochemicals—promising cancer chemopreventive agents in humans? A review of their clinical properties», Int. J. Cancer, vol. 120, fasc. 3, pp. 451–458, feb. 2007, doi: 10.1002/ijc.22419. [CrossRef]
- R. H. Dashwood, «Frontiers in Polyphenols and Cancer Prevention3», The Journal of Nutrition, vol. 137, fasc. 1, pp. 267S-269S, gen. 2007, doi: 10.1093/jn/137.1.267S. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





