Submitted:
02 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
Chapter I
INTRODUCTION
Objectives of the Study
Significance of the Study
Scope and Limitations
Chapter II
REVIEW OF RELATED LITERATURE AND STUDIES
2.1. Kaingen Riverine Ecosystem



2.2. Mangrove Ecosystem
2.3. Carbon Sequestration
2.4. Water Quality
2.4.1. Temperature
2.4.2. Turbidity
2.4.3. Total Dissolved Solids
2.4.4. Salinity
2.4.5. Conductivity
2.4.6. pH
2.4.7. Dissolved Oxygen
2.4.8. Phosphates and Nitrates
2.5. Soil Quality
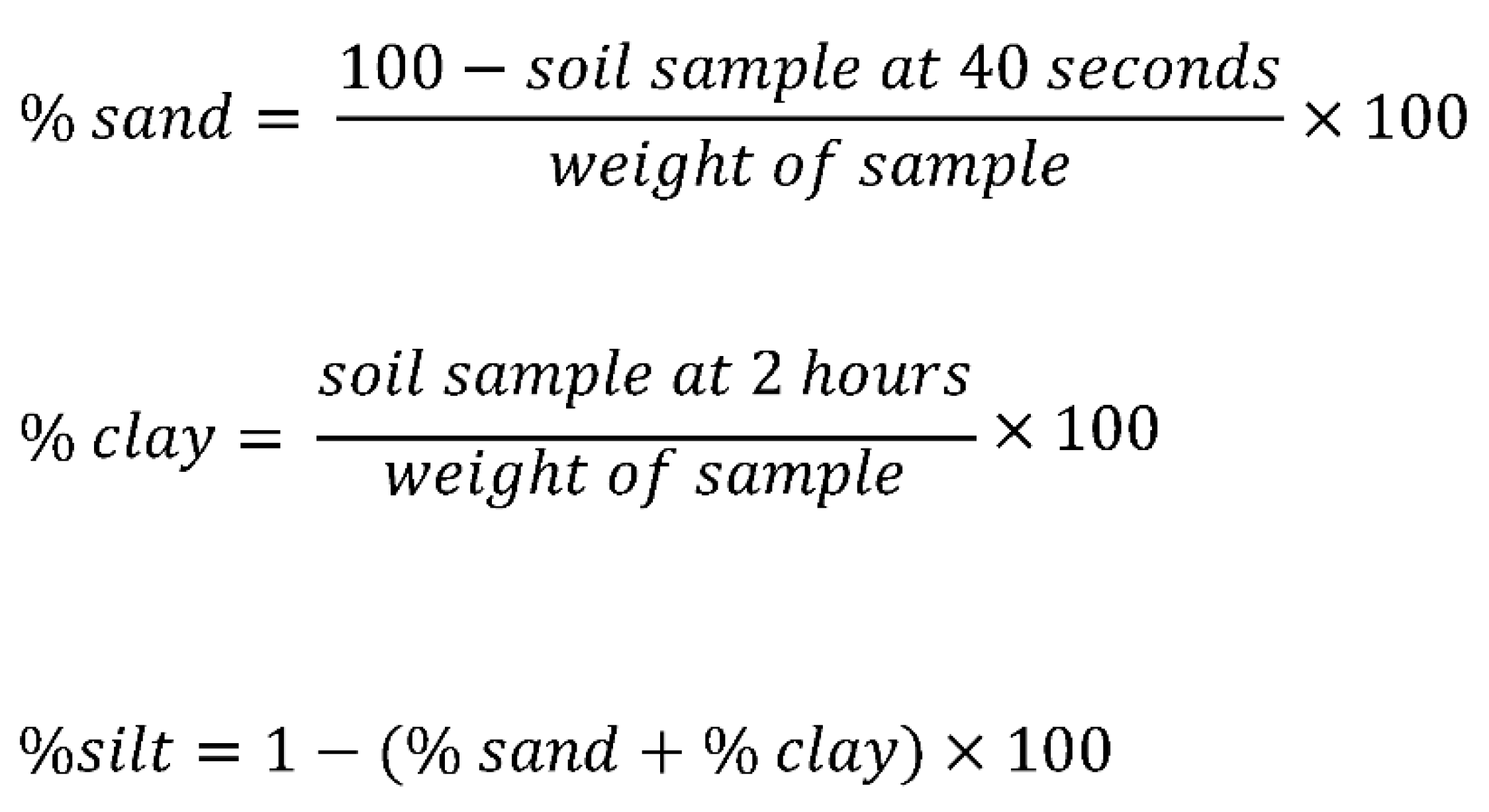
2.5.1. Soil texture
2.5.2. Water Holding Capacity
2.5.3. Soil Temperature
2.5.4. Soil pH
2.5.5. Organic matter
2.5.6. Organic Carbon
2.5.7. Nitrogen, Phosphorus, and Potassium (NPK)
2.6. Related Studies
2.6.1. Local Studies
2.6.2. Foreign Studies
2.6.3. Synthesis
Chapter III
METHODOLOGY
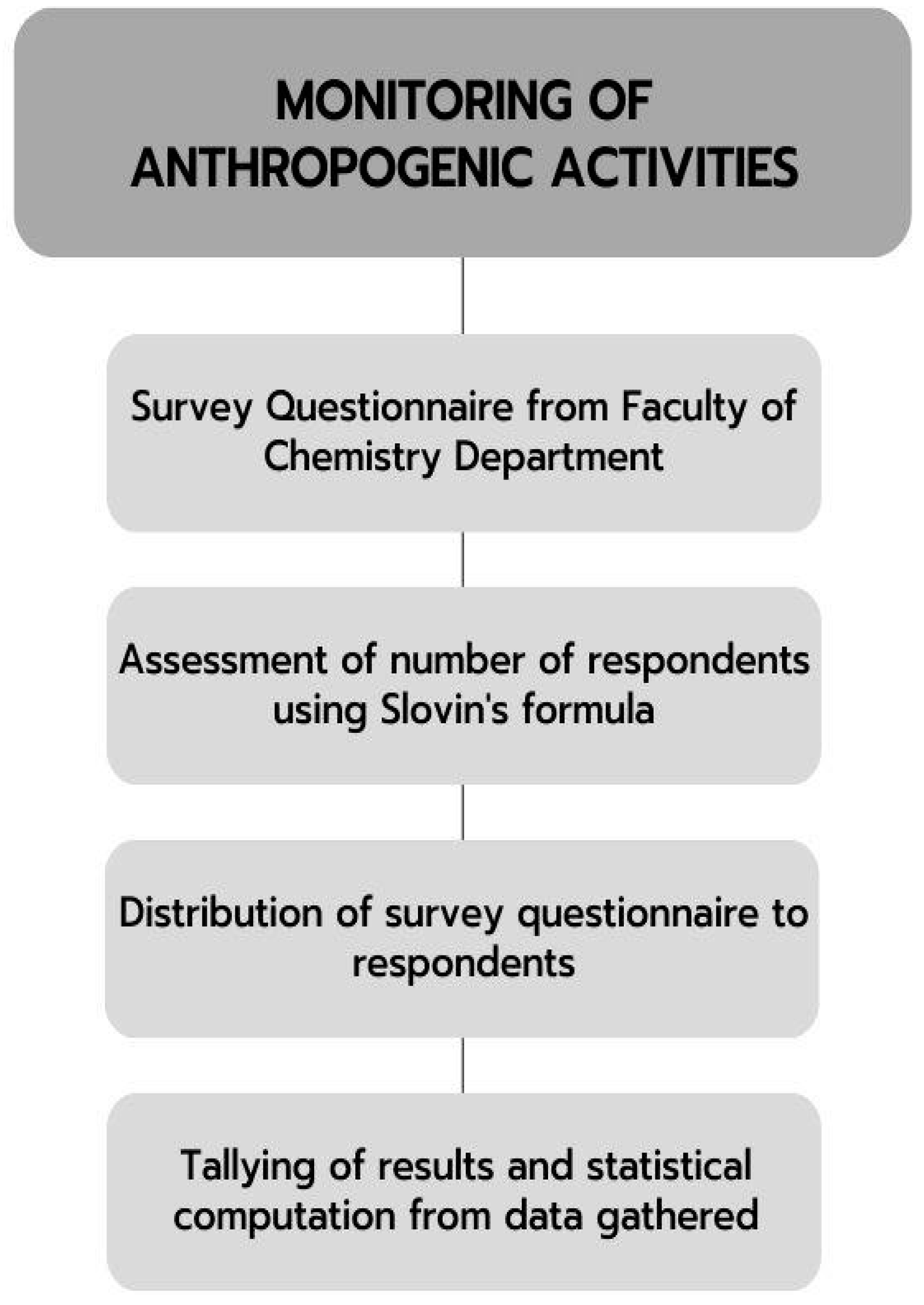
3.1. Administration of Anthropogenic Activities Questionnaire
- N = the size of the population
- i>e = the desired margin of error
3.2. Water Quality Analysis
3.2.1. Temperature
3.2.2. Turbidity
3.2.3. Total Dissolved Solids (TDS)
3.2.4. Salinity
3.2.5. Conductivity
3.2.6. pH
3.2.7. Dissolved Oxygen (DO)
3.2.8. Phosphates and Nitrates
3.3. Determining the Water Quality Index
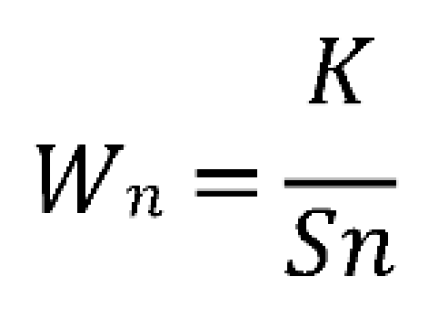
- K = Proportionality constant; is obtained using the following formula:
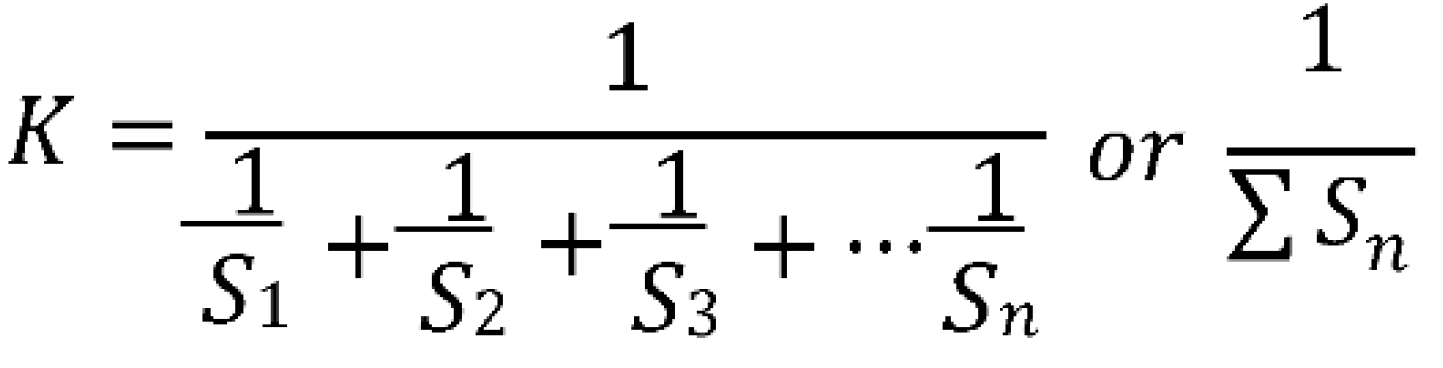
- Sn = Standard desirable value of the nth parameters
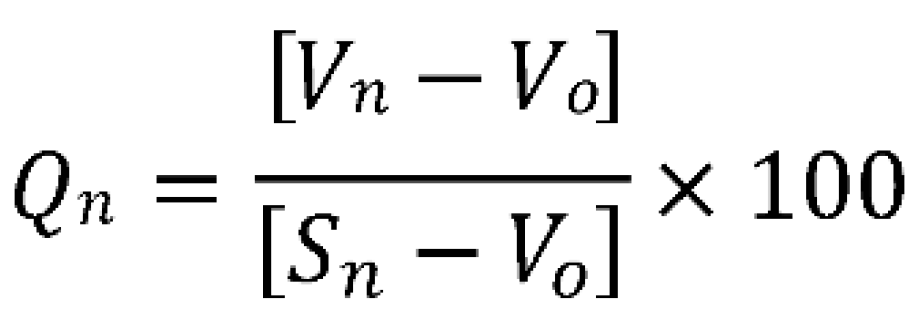
- Vn = monthly data of each site per nth parameter
- Sn = Standard acceptable value of the nth parameter
- VO = The parameters’ ideal value in pure water
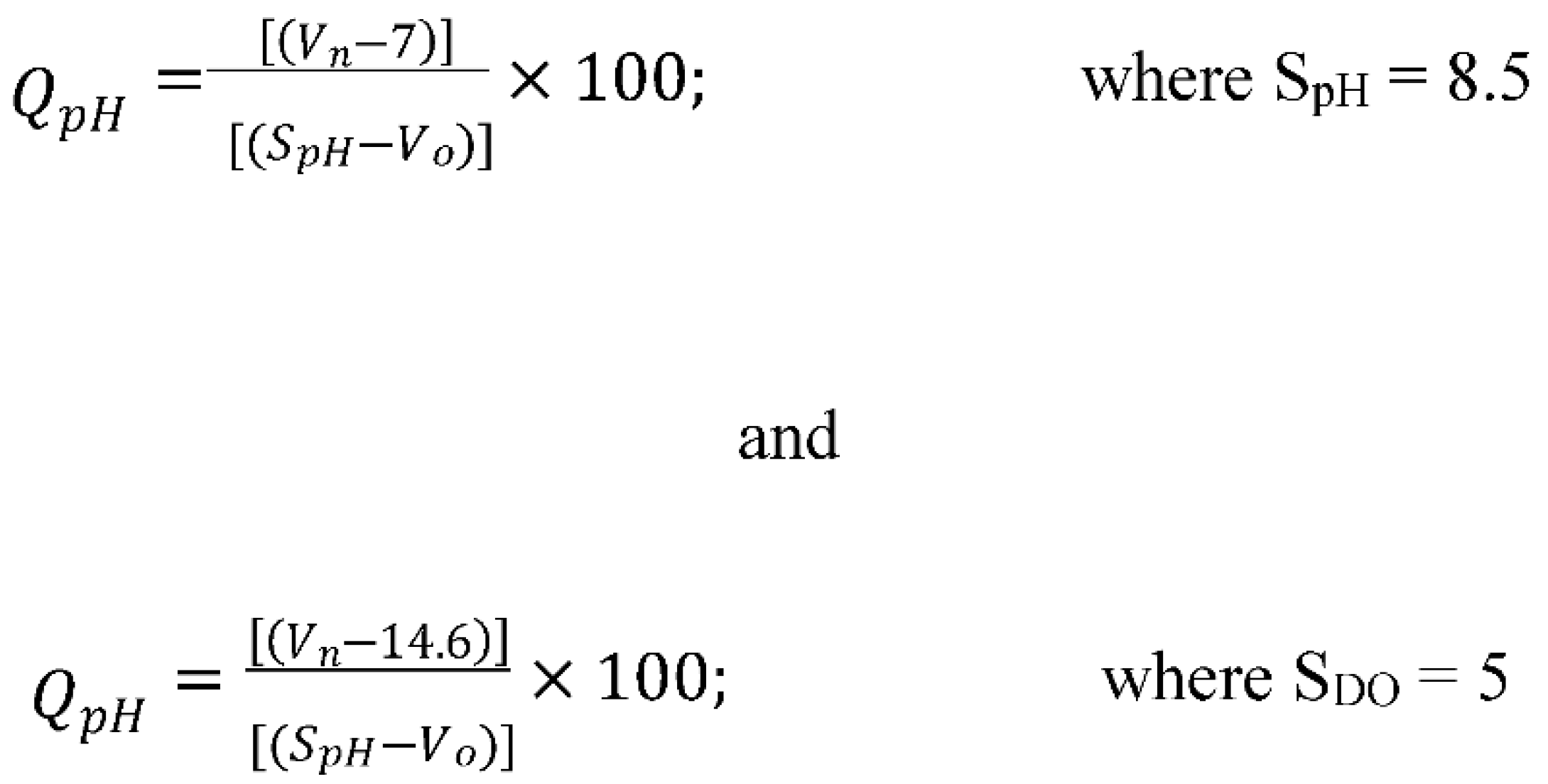
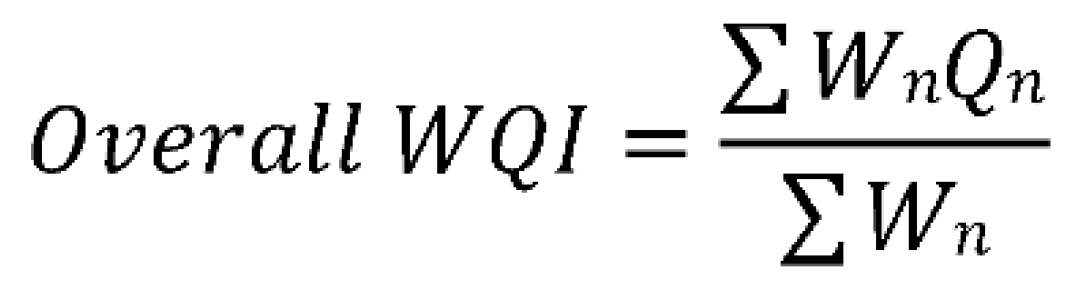
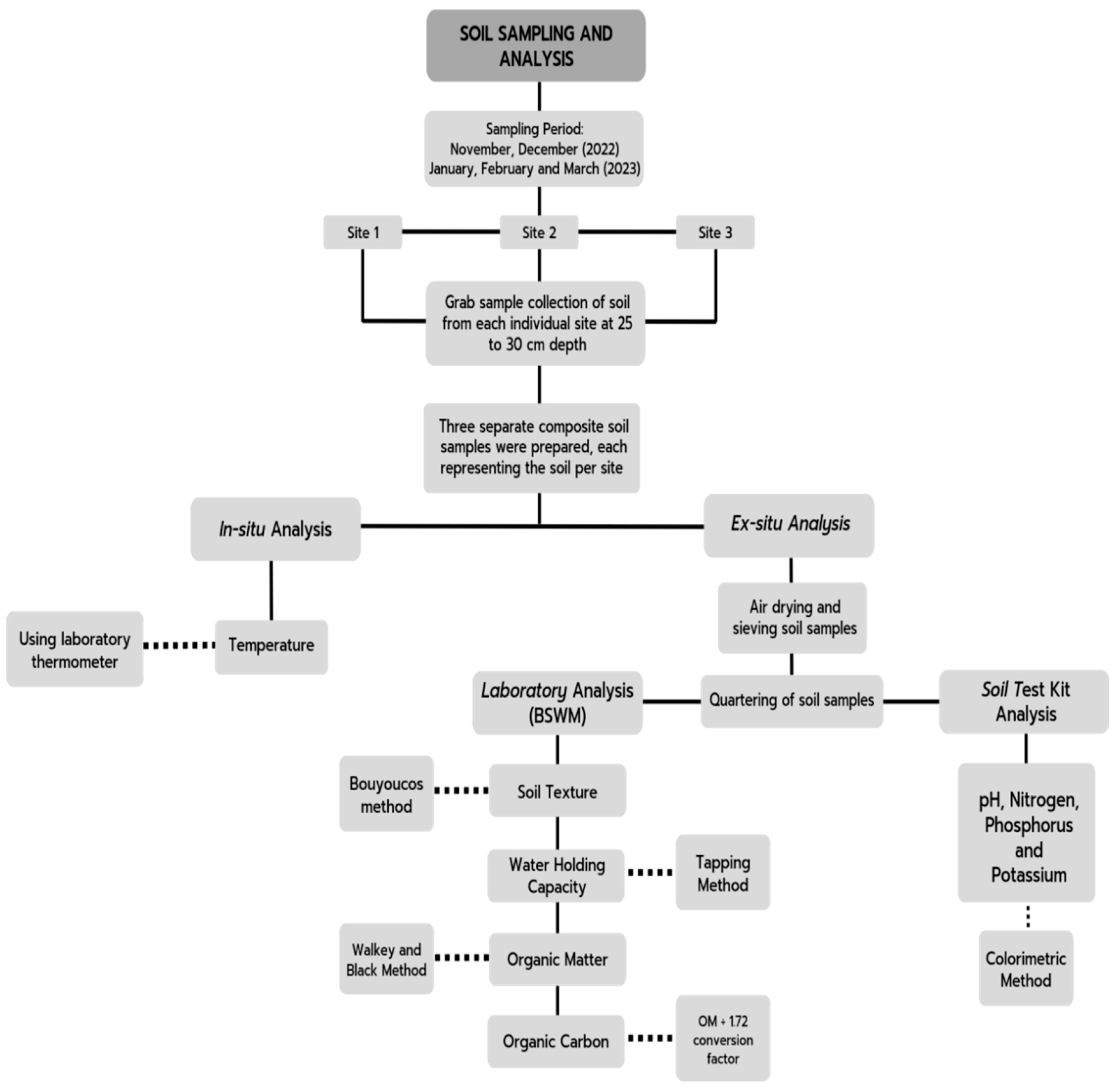
3.4. Soil Quality Analysis
3.4.1. Test for Soil Texture
3.4.2. Test for Water Holding Capacity
3.4.3. Test for Soil Temperature
3.4.4. Test for Soil pH
3.4.5. Test for Soil Organic Matter
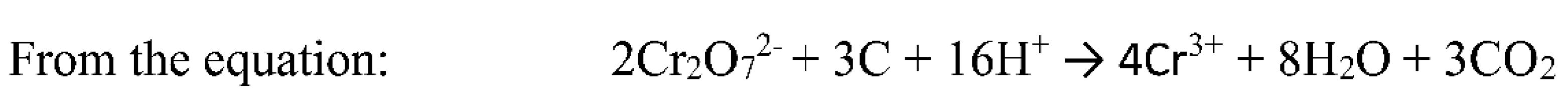
- N=Normality of K2Cr2O7 solution
- T=Volume of FeSO4 used in the sample titration (mL)
- S=Volume of FeSO4 used in the blank titration (mL)
- OSDW=Oven-dry sample weight (g)
3.4.6. Test for Soil Organic Carbon
3.4.7. Test for Nitrogen (N)
3.4.8. Test for Phosphorus (P)
3.4.9. Test for Potassium (K)
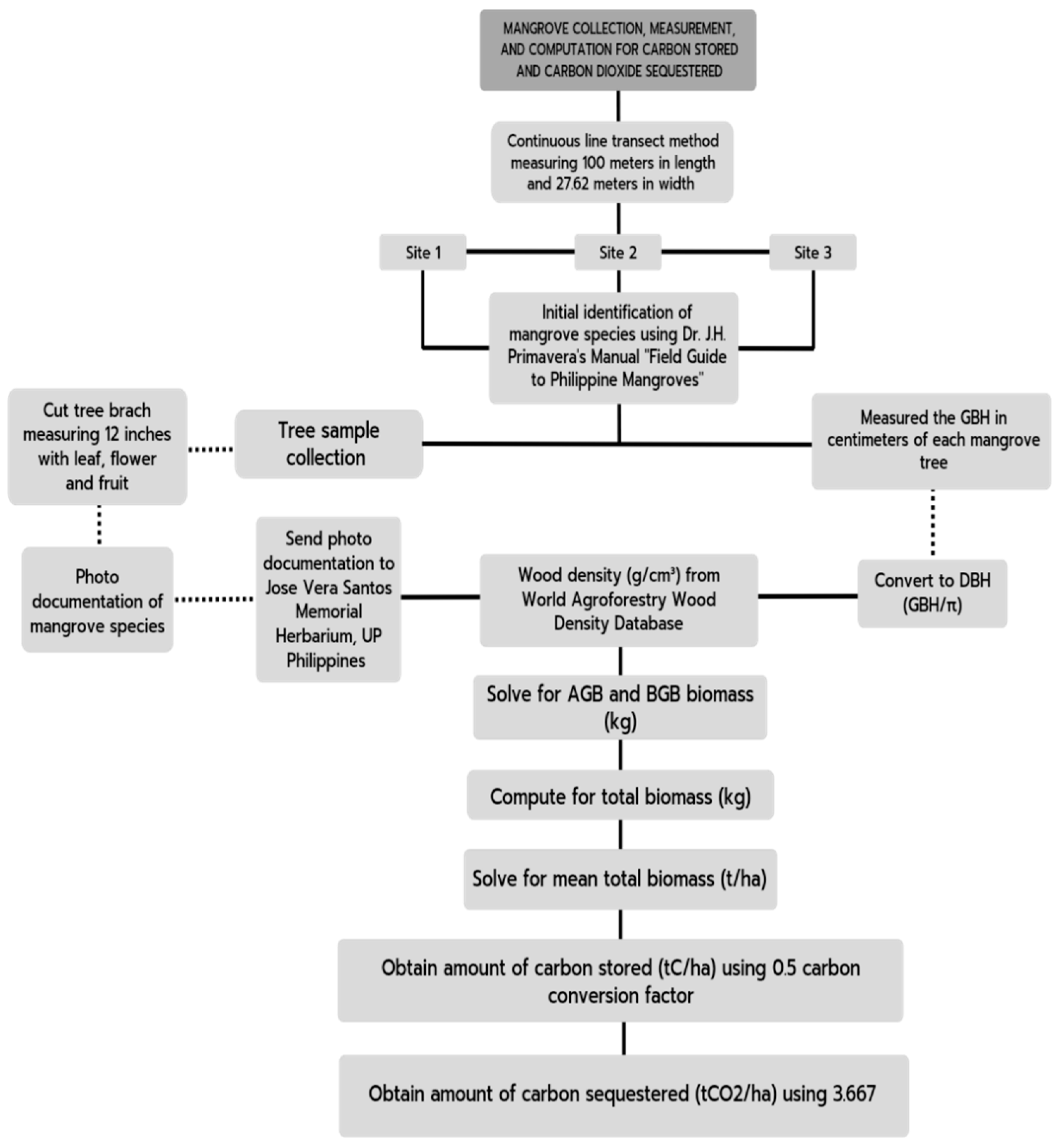
3.5. Aboveground Biomass, Belowground Biomass, and Total Carbon Biomass of Mangrove Trees
- ρ = wood density in g/cm3 of each species of mangroves based on the database of World of Agroforestry for Wood Density (2017)
- D = diameter of each tree in cm
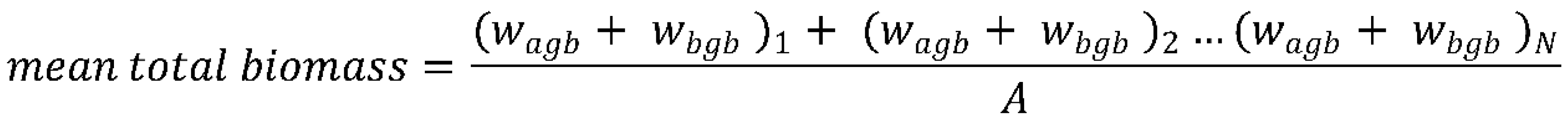
- N = number of trees per site
- A = Area per site = 2,762 m2
3.6. Carbon Stored and Carbon Sequestered by Mangrove Trees
| Amount of Carbon Stored (tC/ha) | Amount of Carbon dioxide Sequestered (tCO2/ha) |
| (Mean total carbon biomass) (0.50) | (tC/ha) (3.667) |
3.7. Mangrove Species Inventory
3.8. Species Diversity Indices
3.8.1. Shannon-Wiener Index
- H = depicts the symbol for the Shannon Index of Diversity,
- 𝑝i= pertains to the individual’s proportion based on the ith species; and
- ln = refers to the natural logarithm
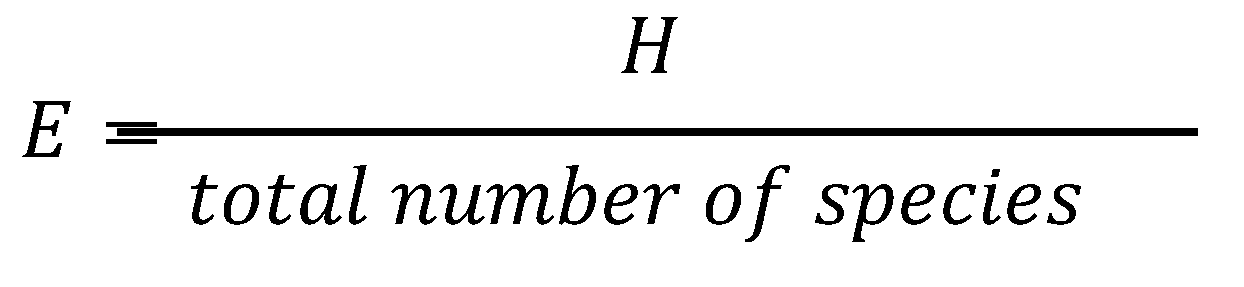
- E = depicts the symbol for species evenness; and
- H = pertains to the calculated species richness
3.8.2. Simpson’s Diversity Index
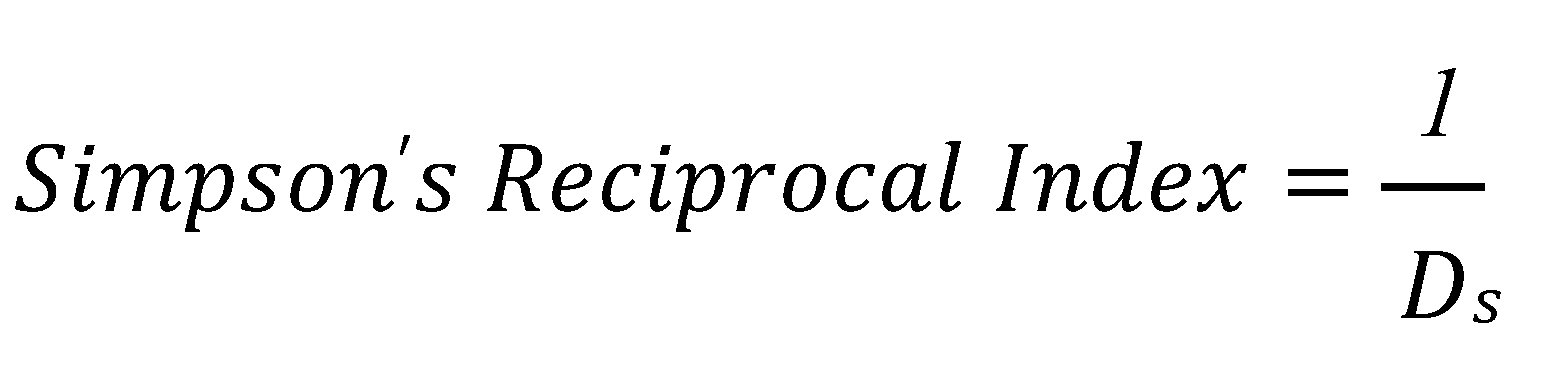
3.8.3. Sorensen Index of Similarity
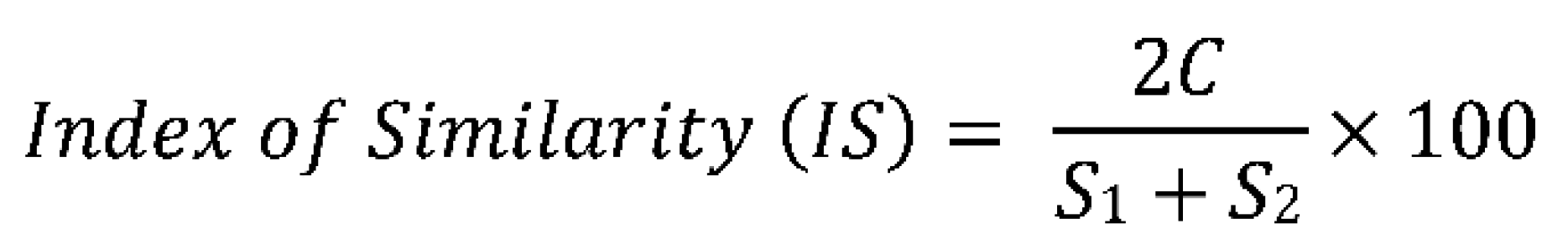
- C = species present in both communities
- S1 = number of species found in the community I
- S2 = number of species found in the community II
3.9. Species Importance Value
3.9.1. Relative Frequency
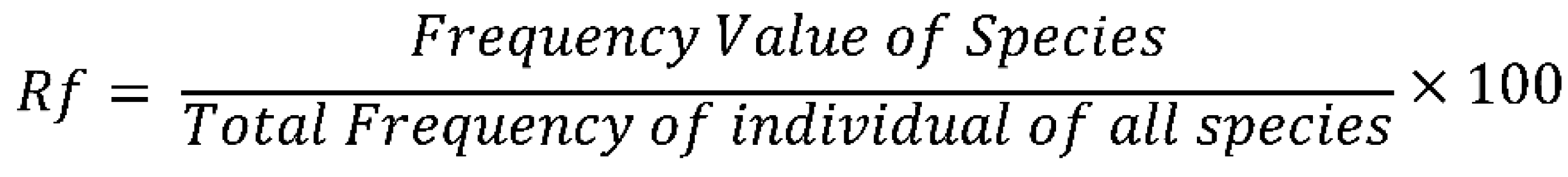
3.9.2. Relative Abundance
3.9.3. Relative Dominance
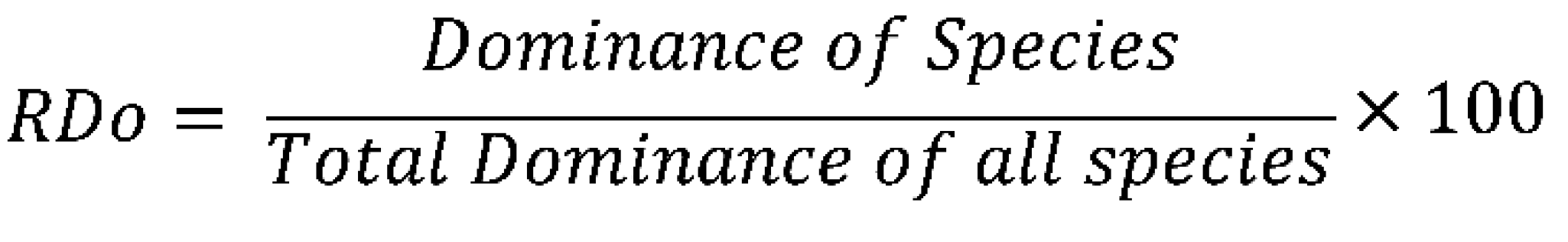
3.10. Statistical Treatment
Kruskal-Wallis
Friedman Test
Pearson's r Correlation
Chapter IV
RESULTS AND DISCUSSION
4.1. Anthropogenic Activities in Kaingen River
4.2. Protection and Conservation Measures of Kaingen River
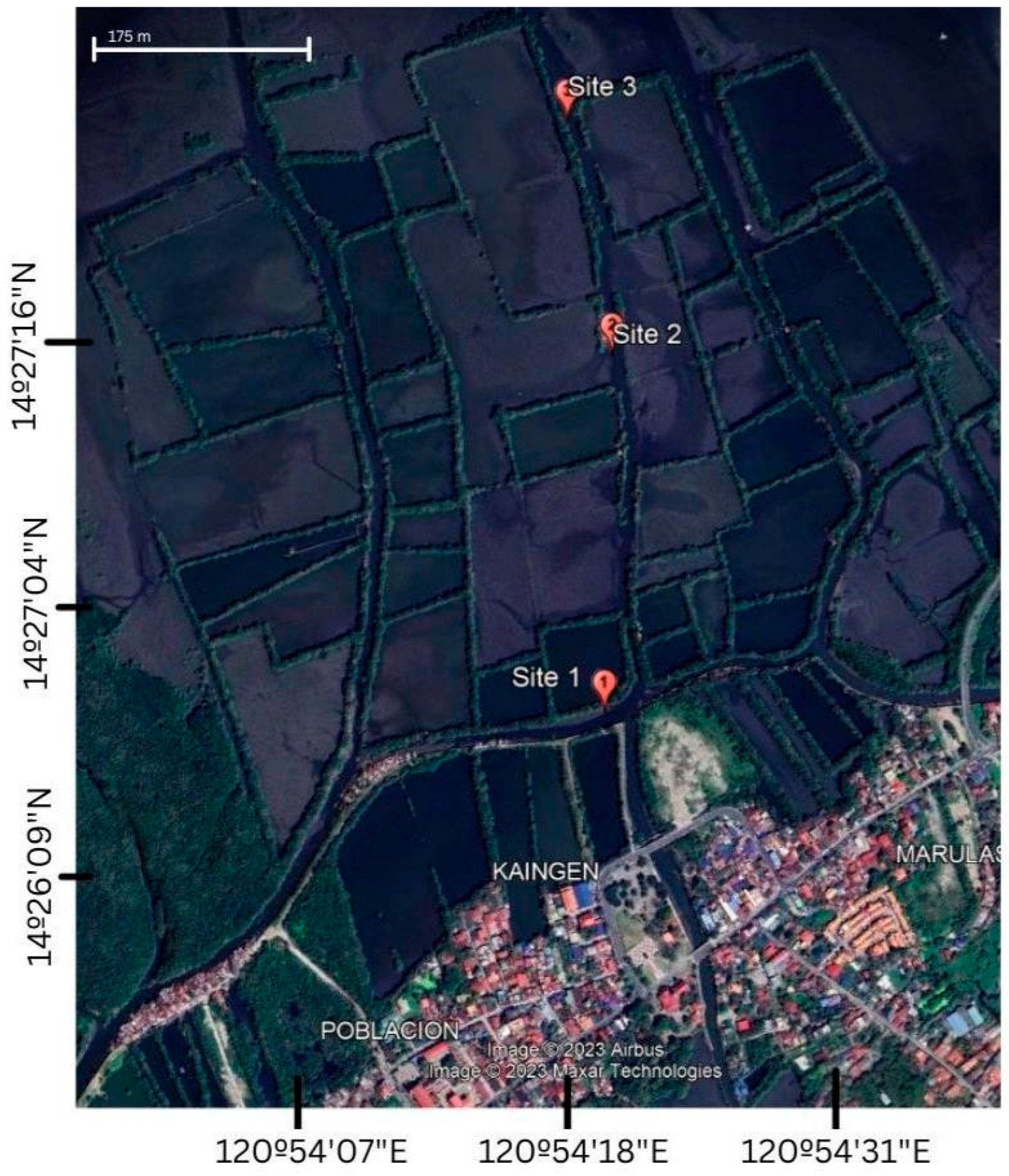
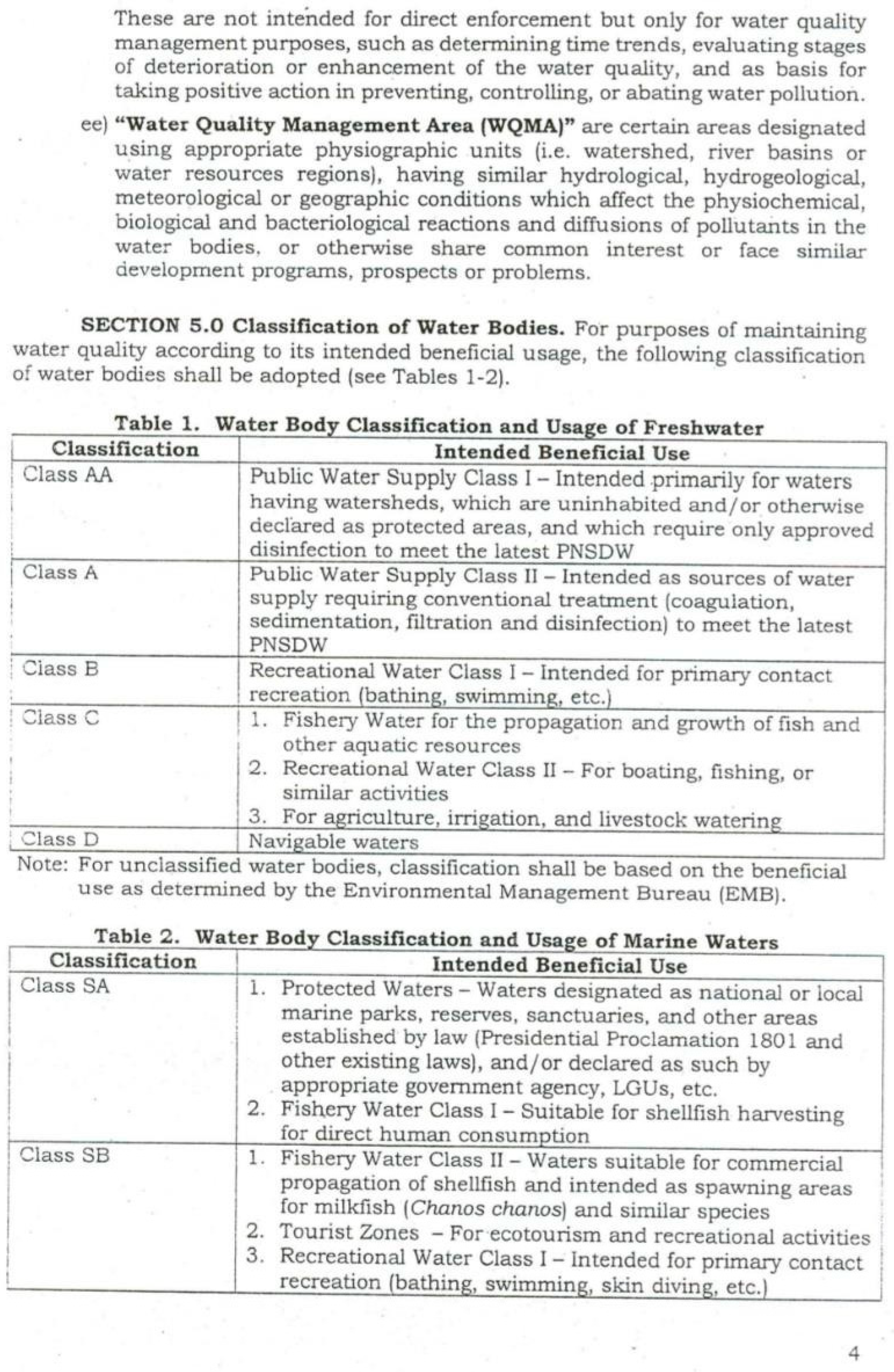
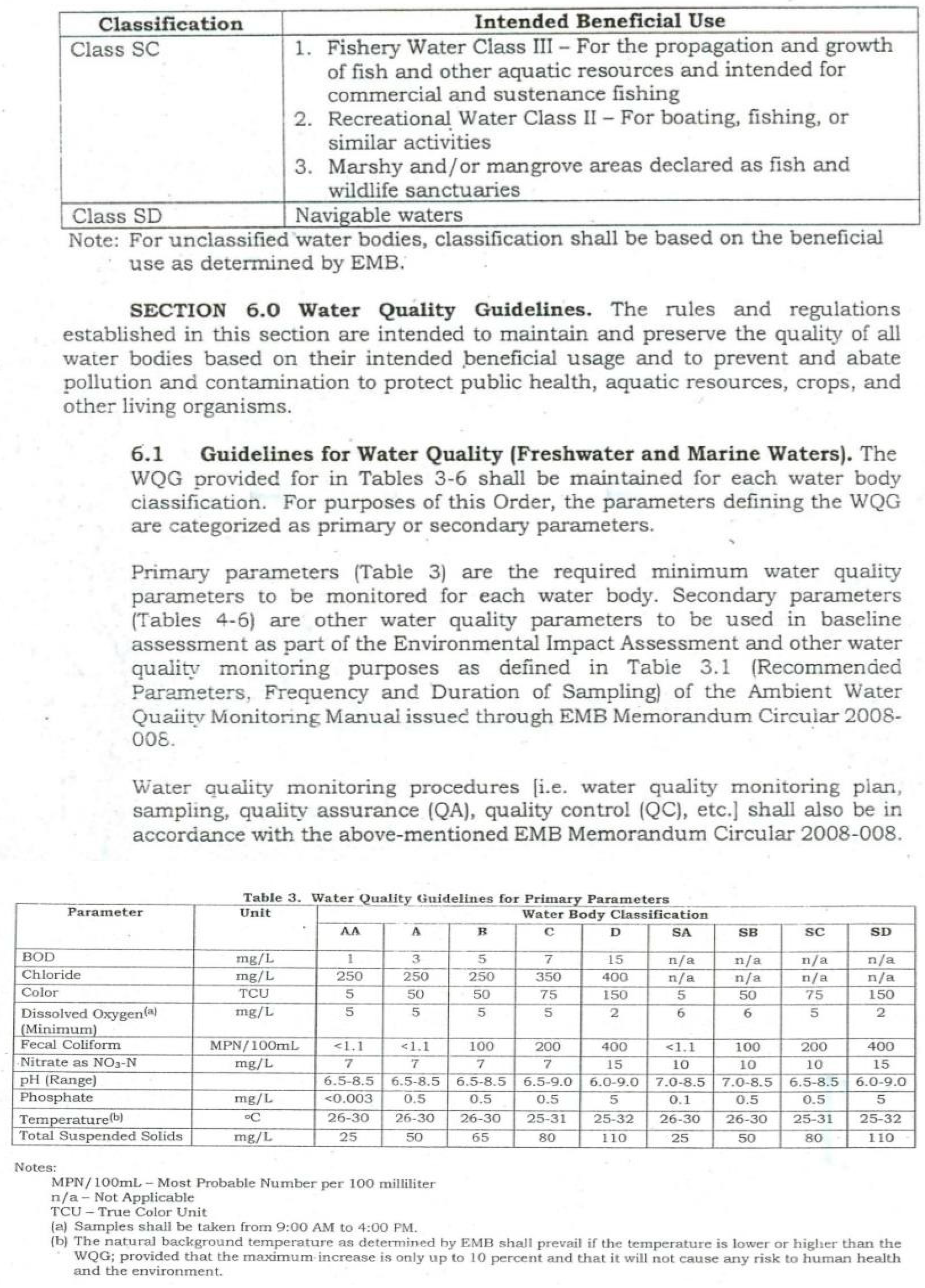
4.3. Water Quality Parameters of the Three Sampling Sites in Kaingen River, Kawit, Cavite during the Sampling Period
4.3.1. Water Temperature
4.3.2. Turbidity
4.3.3. Total Dissolved Solids (TDS)
4.3.4. Salinity
4.3.5.Conductivity
4.3.6. Water pH
4.3.7. Dissolved Oxygen
4.3.8. Phosphates and Nitrates
4.4. Water Quality Index
4.5. Soil Texture and Water Holding Capacity of the Three Sampling Sites in Kaingen River, Kawit, Cavite during the Sampling Period.
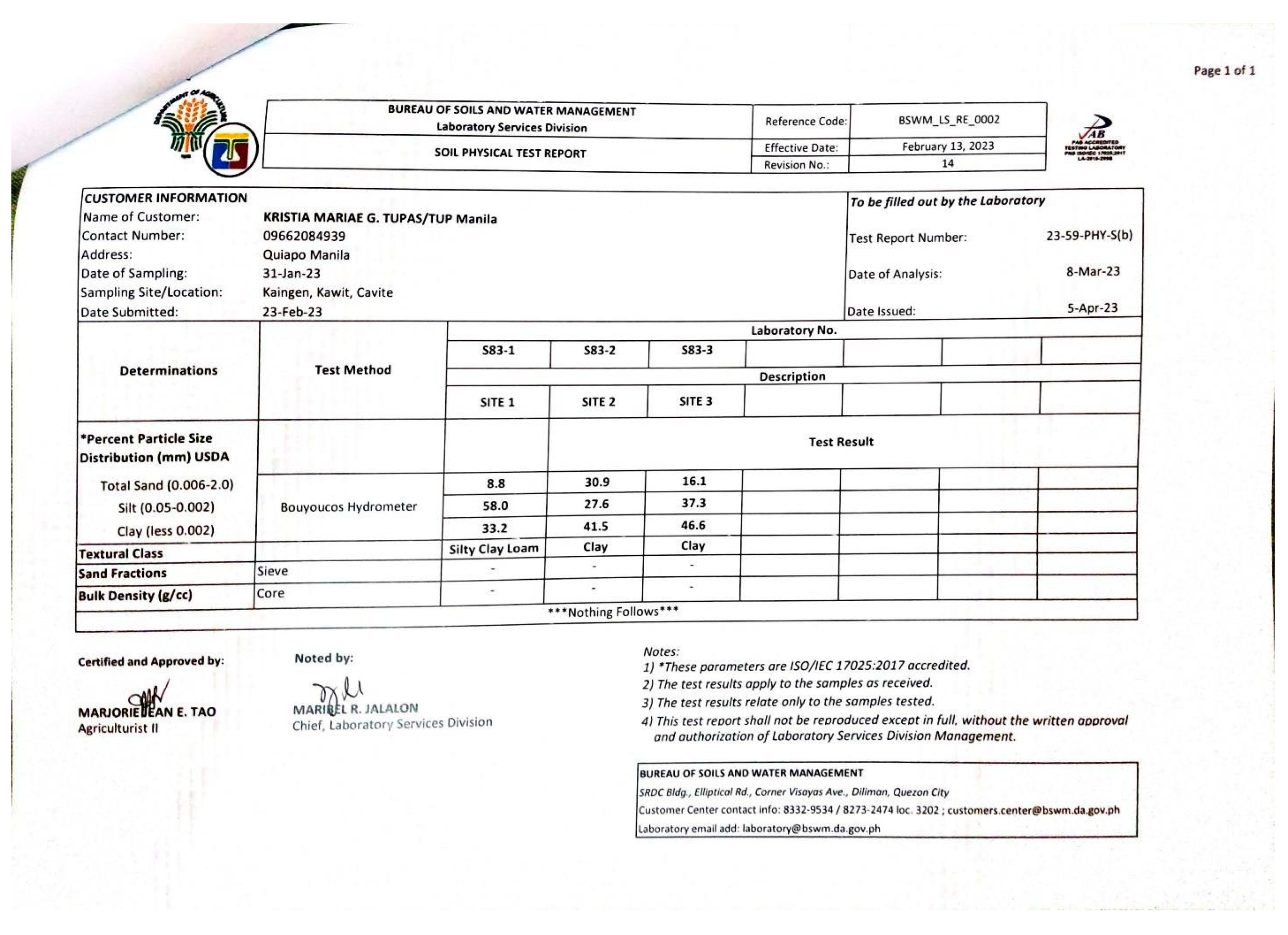
4.5.1. Soil Texture
4.5.2. Water Holding Capacity
4.6. Soil Physicochemical Characteristics of the Three Sampling Sites in Kaingen River, Kawit, Cavite.
4.6.1. Soil Temperature
4.6.2. Soil pH
4.6.3. Organic Matter
4.6.4. Organic Carbon
| Parameters | Sampling Period | Sampling Sites | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
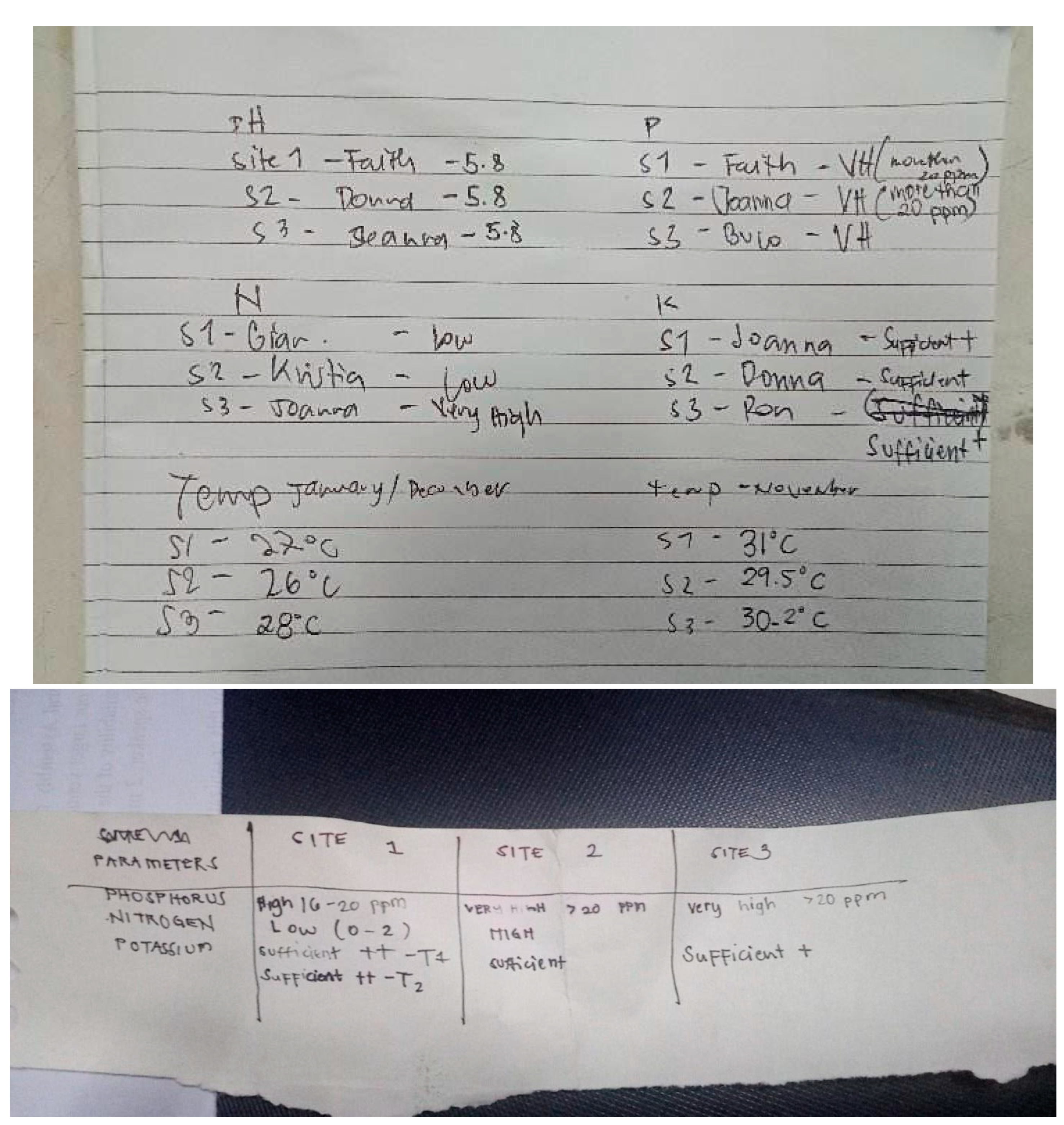
| November | Low | High | Very High | |
| December | Low | Low | Very High | |
| Nitrogen (N) | January | Very High | Low | High |
| February | Very High | Very High | Very High | |
| March | Very High | Low | Very High | |
| November | High | Very High | Very High | |
| December | Very High | Very High | Very High | |
| Phosphorus (P) | January | Very High | Very High | Very High |
| February | Very High | Very High | Very High | |
| March | Very High | Very High | Very High | |
| November | Sufficient ++ | Sufficient | Sufficient + | |
| December | Sufficient + | Sufficient | Sufficient + | |
| Potassium (K) | January | Sufficient ++ | Low | Sufficient |
| February | Low | Sufficient | Sufficient | |
| March | Sufficient | Sufficient | Low | |
4.6.5. Nitrogen (N)
4.6.6. Phosphorus (P)
4.6.7. Potassium (K)
4.7. Relationship of Water Quality to the Abundance of Mangrove Trees Found in Kaingen River, Kawit, Cavite
4.8. Relationship of Soil Quality to the Abundance of Mangrove Trees Found in Kaingen River, Kawit, Cavite
4.9. Mangrove Trees Inventory in Kaingen River, Kawit, Cavite, Philippines
4.10. Diversity Indices of Mangrove Trees Found in Kaingen River
4.11. Amount of Carbon Stored and Sequestered Per Mangrove Species
4.12. Calculated Carbon Stored and Sequestered of Mangrove Trees found in Kaingen River
Chapter V
SUMMARY, CONCLUSION, AND RECOMMENDATIONS
5.1. Summary
5.2. Conclusion
- Different anthropogenic activities in the area may affect the Kaingen Riverine system. Some of these activities include but are not limited to bathing, washing clothes, disposal of household and chemical waste, excretion, and bathing of animals.
- There are varying trends in the result of water quality in terms of temperature, turbidity, pH, DO, phosphate, and nitrates based on the DENR Standard for Class C Waters and TDS, salinity, and conductivity.
- There are varying trends in the result of soil quality in terms of soil texture, water holding capacity, soil temperature, soil pH, organic matter, organic carbon, and NPK.
- Three species of mangroves were identified in the Kaingen River: Avicennia alba, Rhizophora mucronata, and Xylocarpus granatum.
- A total of 71.89 tC/ha stored, and 263.62 tCO2/ha sequestered by the mangrove trees in Kaingen River were calculated using allometric equations.
- There is no significant relationship between water and soil quality collected in three (3) sampling sites per sample month and five (5) sampling periods per site.
- The results show no correlation between water quality and the abundance of mangroves, but there was a correlation between soil quality and the abundance in distribution of mangrove trees species in the Kaingen River.
5.3. Recommendations
- Use of mangrove plant parts such as leaves and seedlings to determine concentrations of pollutants.
- Include a survey on threats to mangroves based on the activities in the Kaingen River.
- Include carbon footprint and/or carbon emission in terms of carbon generated by vehicles, electricity consumption, and use of fuel of the entire barangay Kaingen, Kawit, Cavite, Philippines.
- Conduct air quality monitoring in the Kaingen River.
- Conduct a survey of Avifauna species in Kaingen River.
- Analyze heavy metals of mangrove roots and water in Kaingen River.
- Test for organophosphates and their relationship to mangroves.
- Conduct economic valuation of the mangrove ecosystem in Kaingen River.
Appendix A. Sampling Site/Station




Appendix B. Schematic Diagram

Appendix C. Survey Questionnaire


Appendix D. Survey Results
| Total No. of Respondents | % | ||
|---|---|---|---|
| Gender | Male | 164 | 48.81 |
| Female | 172 | 51.19 | |
| Age | 18 – 30 | 172 | 51.19 |
| 31 – 40 | 51 | 15.18 | |
| 41 – 50 | 39 | 11.61 | |
| 51 – above | 74 | 22.02 | |
| Civil Status | Single | 189 | 56.25 |
| Married | 111 | 33.04 | |
| Widowed | 25 | 7.44 | |
| Separated | 11 | 3.27 | |
| Educational Attainment | Elementary | 35 | 10.42 |
| High School | 160 | 47.62 | |
| College | 133 | 39.58 | |
| Vocational | 8 | 2.38 | |
| Members of the Family | 0 – 1 | 1 | 0.30 |
| 2 – 4 | 135 | 40.18 | |
| 5 – 7 | 167 | 49.70 | |
| 8 – 10 | 24 | 7.14 | |
| 11 – more | 9 | 2.68 | |
| Proper Sewage System | Yes | 221 | 65.77 |
| No | 115 | 34.23 |
| 1 | 2 | 3 | 4 | Respondents | X | REMARKS | |
|---|---|---|---|---|---|---|---|
| Fishing | 194 | 44 | 46 | 52 | 336 | 1.87 | Seldom |
| Bathing | 202 | 45 | 38 | 51 | 336 | 1.82 | Seldom |
| Washing of clothes | 258 | 29 | 21 | 27 | 335 | 1.45 | Seldom |
| Throwing garbage | 220 | 46 | 27 | 42 | 335 | 1.67 | Seldom |
| Disposing of chemical waste | 286 | 22 | 12 | 16 | 336 | 1.28 | Seldom |
| Excreting | 263 | 29 | 23 | 21 | 336 | 1.41 | Seldom |
| Bathing of Animals | 276 | 30 | 16 | 14 | 336 | 1.31 | Seldom |
| 1 | 2 | 3 | 4 | Respondents | X | REMARKS | |
|---|---|---|---|---|---|---|---|
| Preventing people | 17 | 10 | 71 | 238 | 336 | 1.87 | Strongly Agree |
| Environmental groups | 16 | 14 | 91 | 215 | 336 | 1.82 | Strongly Agree |
| Creating groups | 16 | 15 | 106 | 198 | 335 | 1.45 | Strongly Agree |
| River policies | 17 | 8 | 88 | 223 | 336 | 1.67 | Strongly Agree |
| Sustainable methods | 17 | 14 | 81 | 224 | 336 | 1.28 | Strongly Agree |
| River management | 21 | 25 | 81 | 209 | 336 | 1.41 | Strongly Agree |
| Deposits | 9 | 8 | 52 | 266 | 335 | 1.31 | Strongly Agree |
| 1 | 2 | 3 | 4 | 5 | Respondents | X | REMARKS | |
|---|---|---|---|---|---|---|---|---|
| Precipitation Runoff | 53 | 42 | 68 | 71 | 102 | 336 | 3.38 | Often |
| Fishing | 73 | 36 | 59 | 60 | 108 | 336 | 3.28 | Often |
| Tides | 44 | 57 | 82 | 74 | 79 | 336 | 3.26 | Often |
| Weathering and erosion | 169 | 71 | 37 | 35 | 24 | 336 | 2.03 | Seldom |
| Debris and leaf decomposition | 144 | 61 | 56 | 36 | 39 | 336 | 2.30 | Seldom |
| Precipitation Runoff | 53 | 42 | 68 | 71 | 102 | 336 | 3.38 | Often |
| Fishing | 73 | 36 | 59 | 60 | 108 | 336 | 3.28 | Often |
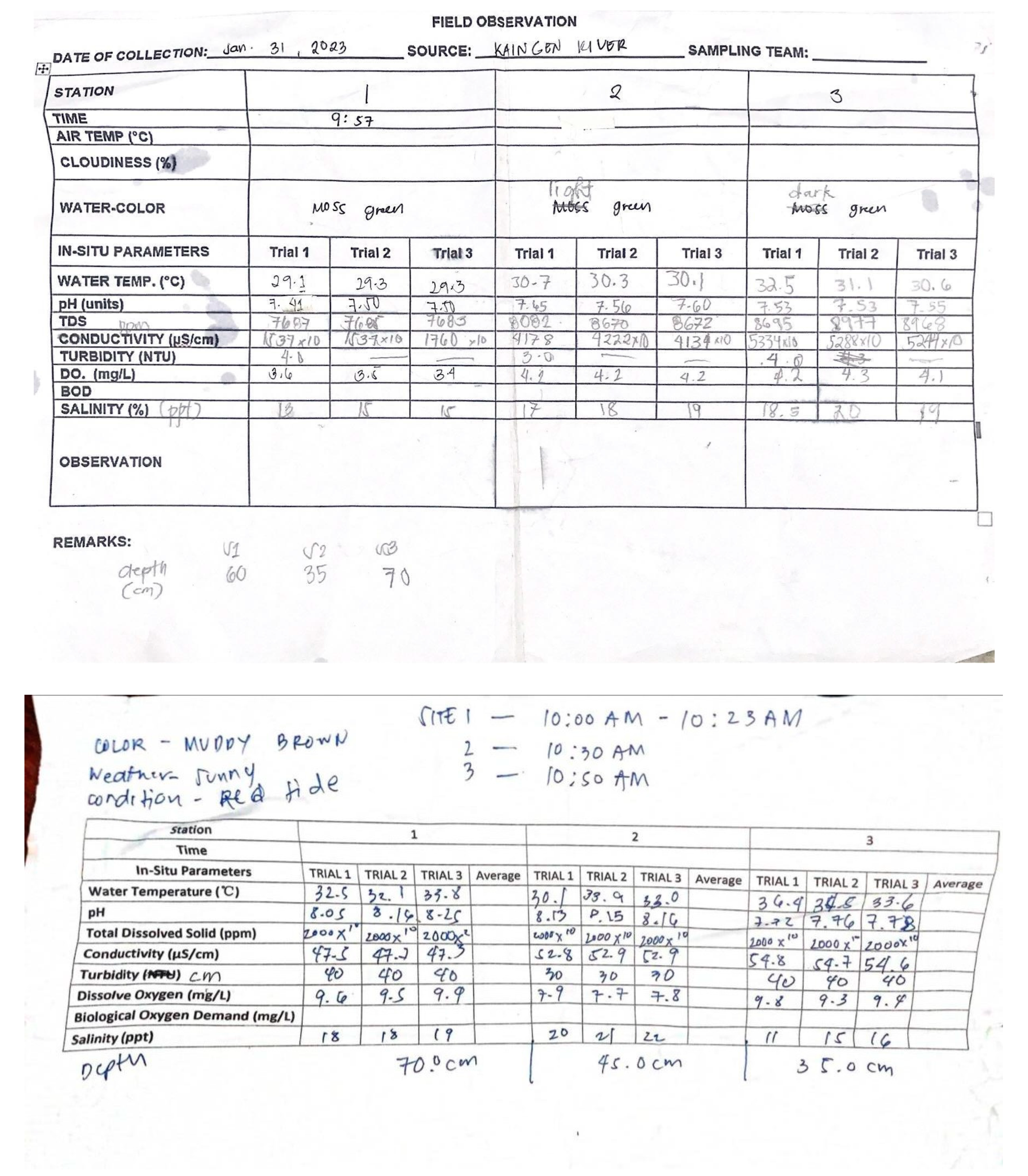
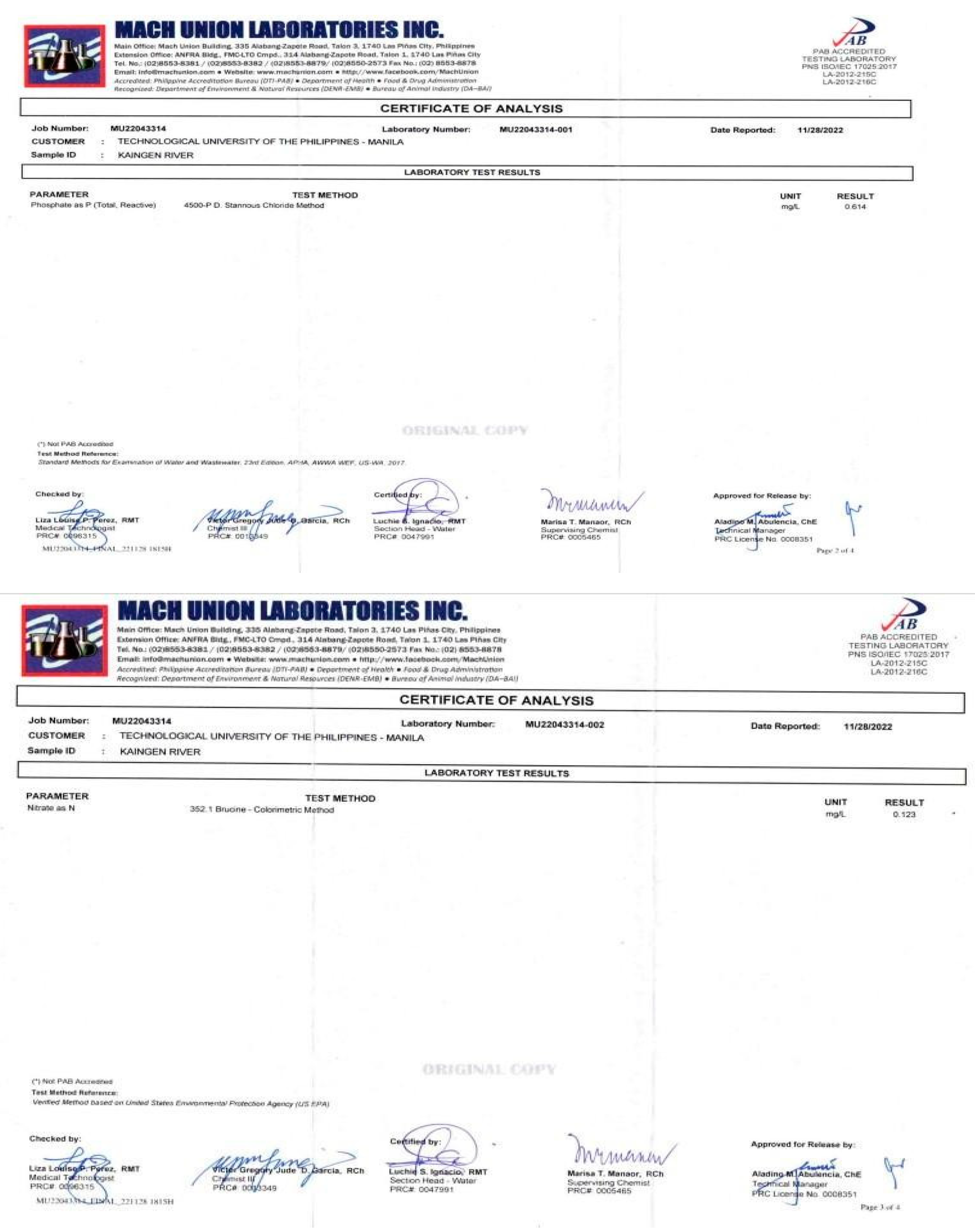
Appendix E. Physicochemical and Biological Properties of Water and Soil



 
|
| Parameter | Sites | Sampling Period | ||||
|---|---|---|---|---|---|---|
| November | December | January | February | March | ||
| 1 | 16.5203 | 12.0147 | 22.5276 | 12.0147 | 22.5276 | |
| Turbidity | 2 | 12.0147 | 22.5276 | 36.0442 | 36.0442 | 22.5276 |
| 3 | 22.5276 | 22.5276 | 22.5276 | 16.5203 | 36.0442 | |
| 1 | 0.0234 | 0.0640 | 0.0269 | 0.0668 | 0.0793 | |
| Conductivity | 2 | 0.0230 | 0.1414 | 0.0697 | 0.0692 | 0.0882 |
| 3 | 0.0234 | 0.1548 | 0.0883 | 0.0684 | 0.0913 | |
| Total Dissolved Solids | 1 | 0.0360 | 0.0984 | 0.0414 | 0.1028 | 0.1219 |
| 2 | 0.0354 | 0.2175 | 0.1073 | 0.1065 | 0.1357 | |
| 3 | 0.0360 | 0.2382 | 0.1358 | 0.1053 | 0.1404 | |
| 1 | 0.0263 | 0.0720 | 0.0303 | 0.0752 | 0.0892 | |
| Salinity | 2 | 0.0259 | 0.1591 | 0.0784 | 0.0778 | 0.0992 |
| 3 | 0.0263 | 0.1741 | 0.0993 | 0.0770 | 0.1027 | |
| 1 | 3.9183 | 3.9287 | 3.8819 | 4.0690 | 4.2353 | |
| pH | 2 | 4.1002 | 4.0066 | 3.9495 | 4.0222 | 4.2353 |
| 3 | 4.0534 | 3.9910 | 3.9183 | 3.9443 | 4.0274 | |
| Dissolved Oxygen | 1 | 4.5055 | 8.1099 | 5.2564 | 6.9085 | 14.2675 |
| 2 | 7.6594 | 9.7620 | 6.3077 | 6.0074 | 11.7144 | |
| 3 | 6.3077 | 8.4103 | 6.3077 | 5.8572 | 14.2675 | |
| 1 | 0.0942 | 0.2222 | 0.1303 | 0.0766 | 0.1073 | |
| Nitrates | 2 | 0.0942 | 0.2222 | 0.1303 | 0.0766 | 0.1073 |
| 3 | 0.0942 | 0.2222 | 0.1303 | 0.0766 | 0.1073 | |
| 1 | 92.2131 | 122.40 | 109.184 | 107.832 | 69.5353 | |
| Phosphates | 2 | 92.2131 | 122.40 | 109.184 | 107.832 | 69.5353 |
| 3 | 92.2131 | 122.40 | 109.184 | 107.832 | 69.5353 | |
| Total Coliform | 1 | 0.3604 | 0.0020 | 0.0360 | 0.0036 | 0.0050 |
| 2 | 0.3604 | 0.0020 | 0.0360 | 0.0036 | 0.0050 | |
| 3 | 0.3604 | 0.0020 | 0.0360 | 0.0036 | 0.0050 | |
| Ave. WQI in Five Months | 1 | 129.57 (UNSUITABLE) | ||||
| 2 | 138.91 (UNSUITABLE) | |||||
| 3 | 137.00 (UNSUITABLE) | |||||
| Ave. WQI per Month | 119.96 (US) | 154.82 (US) | 146.48 (US) | 139.96 (US) | 114.58 (US) | |

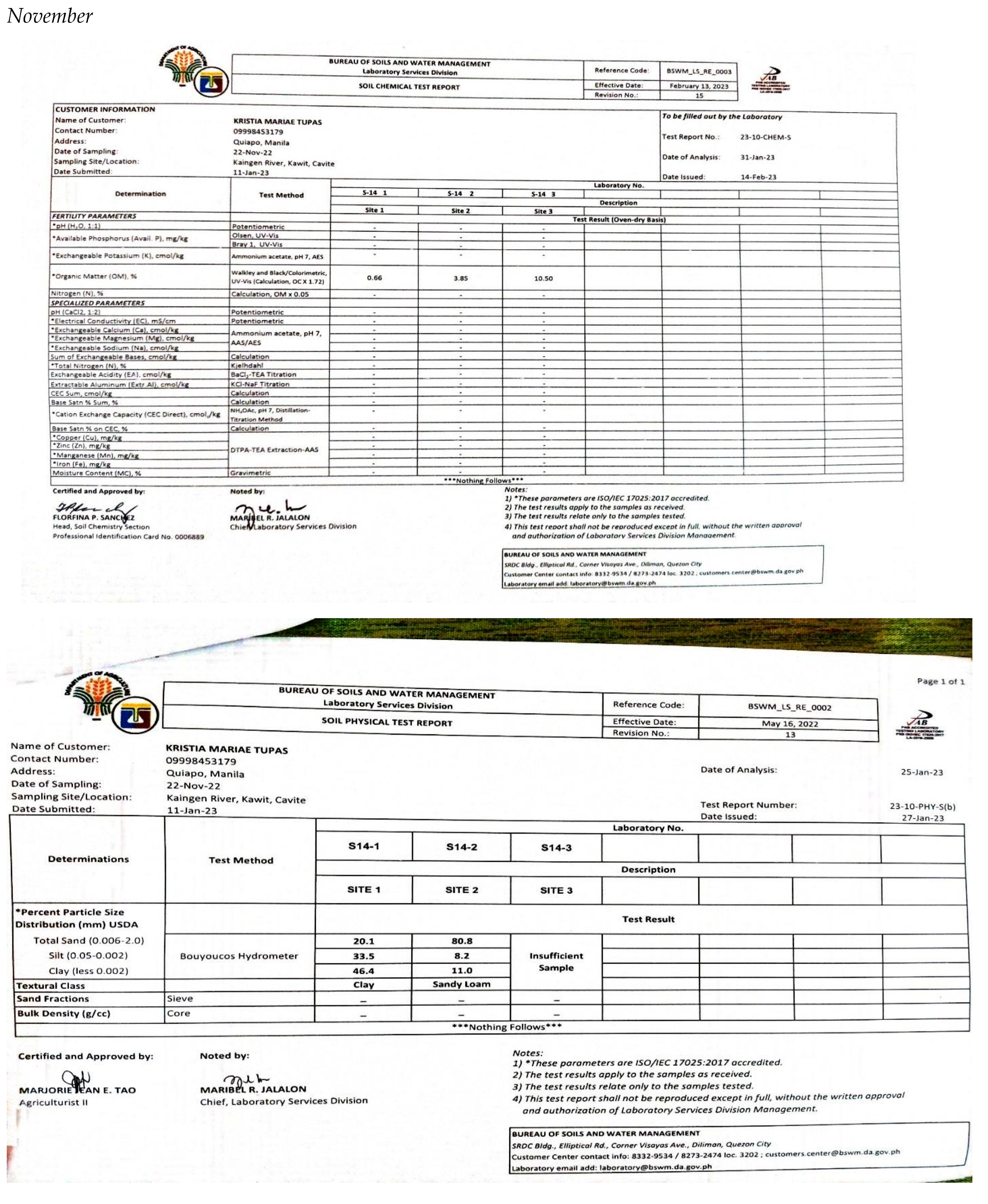
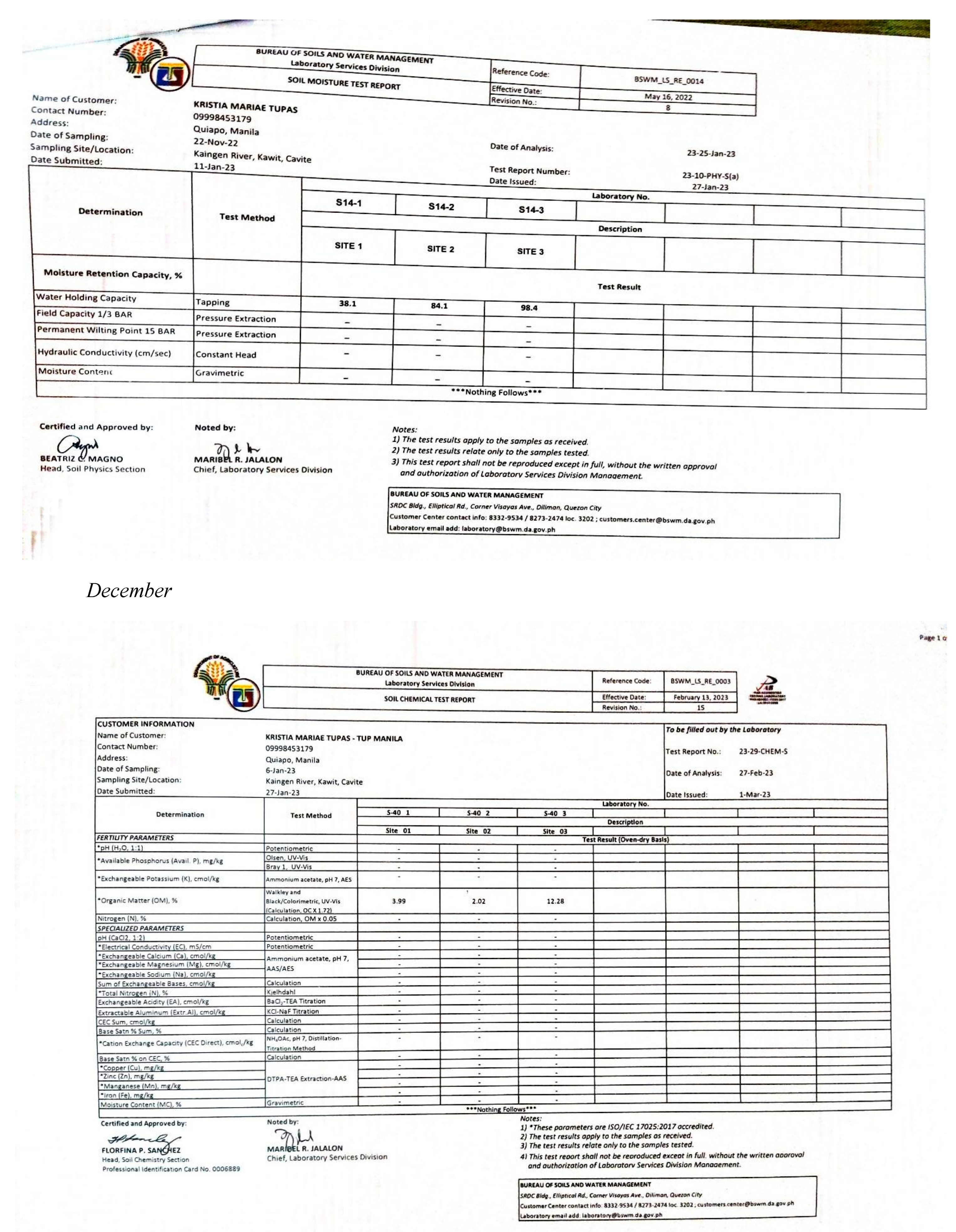
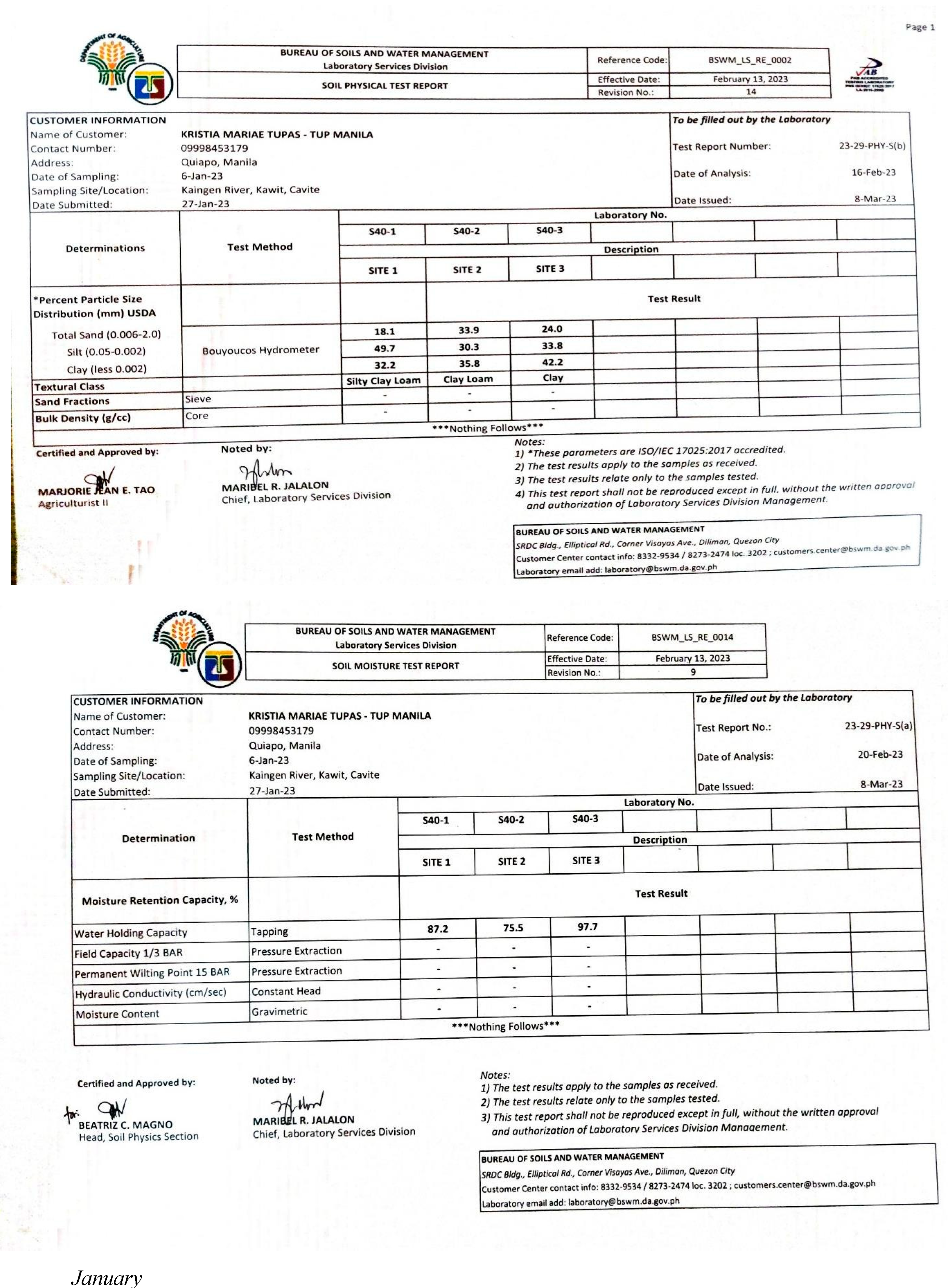
Appendix F. Abundance of Species
| Tree No. | Scientific Name | Local name | GBH (cm) |
DBH (cm) | Wood Density (g/cm3) | AGB (kg) | BGB (kg) | Total Biomass (kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | Avicennia alba | Bungalon | 91.9 | 29.25261013 | 0.6987 | 709.1358437 | 259.2721792 | 968.4080229 |
| 2 | Avicennia alba | Bungalon | 162.6 | 51.75706646 | 0.6987 | 2886.222521 | 920.2018123 | 3806.424333 |
| 3 | Avicennia alba | Bungalon | 35.6 | 11.33180545 | 0.6987 | 68.79237045 | 31.58017106 | 100.3725415 |
| 4 | Avicennia alba | Bungalon | 61 | 19.41685765 | 0.6987 | 258.752571 | 104.3825053 | 363.1350764 |
| 5 | Avicennia alba | Bungalon | 80.8 | 25.71937866 | 0.6987 | 516.660461 | 194.8268028 | 711.4872638 |
| 6 | Avicennia alba | Bungalon | 172.7 | 54.9719888 | 0.6987 | 3347.437138 | 1051.924262 | 4399.3614 |
| 7 | Avicennia alba | Bungalon | 71.1 | 22.63177998 | 0.6987 | 377.2007064 | 146.6715887 | 523.8722952 |
| 8 | Avicennia alba | Bungalon | 128.3 | 40.8390629 | 0.6987 | 1611.42781 | 543.824363 | 2155.252173 |
| 9 | Avicennia alba | Bungalon | 62.7 | 19.95798319 | 0.6987 | 276.8544 | 110.9505408 | 387.8049408 |
| 10 | Avicennia alba | Bungalon | 56.4 | 17.9526356 | 0.6987 | 213.3633765 | 87.70716907 | 301.0705455 |
| 11 | Avicennia alba | Bungalon | 94.7 | 30.14387573 | 0.6987 | 763.4739581 | 277.1356756 | 1040.609634 |
| 12 | Avicennia alba | Bungalon | 68.6 | 21.83600713 | 0.6987 | 345.4065781 | 135.4674699 | 480.874048 |
| 13 | Avicennia alba | Bungalon | 38.1 | 12.1275783 | 0.6987 | 81.29217058 | 36.71545619 | 118.0076268 |
| 14 | Avicennia alba | Bungalon | 30.5 | 9.708428826 | 0.6987 | 47.02739088 | 22.40480281 | 69.4321937 |
| 15 | Avicennia alba | Bungalon | 26.7 | 8.498854087 | 0.6987 | 33.89931968 | 16.67441027 | 50.57372995 |
| 16 | Rhizophora mucronata | Bakhaw babae | 45.7 | 14.54672778 | 0.8483 | 154.3923777 | 65.45729277 | 219.8496705 |
| 17 | Rhizophora mucronata | Bakhaw babae | 48.3 | 15.37433155 | 0.8483 | 176.9057483 | 74.01277804 | 250.9185264 |
| 18 | Rhizophora mucronata | Bakhaw babae | 26.4 | 8.403361345 | 0.8483 | 40.02926939 | 19.35997504 | 59.38924442 |
| 19 | Rhizophora mucronata | Bakhaw babae | 33.02 | 10.51056786 | 0.8483 | 69.41024749 | 31.81479787 | 101.2250454 |
| 20 | Rhizophora mucronata | Bakhaw babae | 38.6 | 12.28673287 | 0.8483 | 101.9146865 | 44.99537666 | 146.9100632 |
| 21 | Rhizophora mucronata | Bakhaw babae | 32.3 | 10.28138528 | 0.8483 | 65.74613214 | 30.2951896 | 96.04132175 |
| Total Biomass of Site 1 (kg) | Total Biomass (t/ha) | C. Stored (tC/ha) | CO2 Sequestered (tCO2/ha) | |||||
| 5.919992648 | 59.19992648 | 29.59996324 | 108.5430652 |
| Tree No. | Scientific Name | Local name | GBH (cm) |
DBH (cm) | Wood Density (g/cm3) | AGB (kg) | BGB (kg) | Total Biomass (kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | Avicennia alba | Bungalon | 48.0 | 15.27883881 | 0.6987 | 143.4916811 | 61.31278305 | 204.8044641 |
| 2 | Avicennia alba | Bungalon | 27.0 | 8.59434683 | 0.6987 | 34.84401078 | 17.09318681 | 51.93719759 |
| 3 | Avicennia alba | Bungalon | 24.0 | 7.639419404 | 0.6987 | 26.07912009 | 13.16025908 | 39.23937918 |
| 4 | Avicennia alba | Bungalon | 27.0 | 8.59434683 | 0.6987 | 34.84401078 | 17.09318681 | 51.93719759 |
| 5 | Avicennia alba | Bungalon | 33.5 | 10.66335625 | 0.6987 | 59.23569727 | 27.59274798 | 86.82844524 |
| 6 | Avicennia alba | Bungalon | 33.0 | 10.50420168 | 0.6987 | 57.08441723 | 26.68679559 | 83.77121282 |
| 7 | Avicennia alba | Bungalon | 40.0 | 12.73236567 | 0.6987 | 91.63066919 | 40.9042716 | 132.5349408 |
| 8 | Avicennia alba | Bungalon | 32.0 | 10.18589254 | 0.6987 | 52.92272845 | 24.92461361 | 77.84734206 |
| 9 | Xylocarpus granatum | Tabigi | 25.0 | 7.957728546 | 0.6721 | 27.73632089 | 13.91451251 | 41.6508334 |
| 10 | Xylocarpus granatum | Tabigi | 8.5 | 2.705627706 | 0.6721 | 1.952032252 | 1.268677365 | 3.220709617 |
| 11 | Xylocarpus granatum | Tabigi | 9.0 | 2.864782277 | 0.6721 | 2.246740971 | 1.440321844 | 3.687062815 |
| 12 | Rhizophora mucronata | Bakhaw babae | 16.0 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 |
| 13 | Rhizophora mucronata | Bakhaw babae | 39.0 | 12.41405653 | 0.8483 | 104.5324036 | 46.03705171 | 150.5694553 |
| 14 | Rhizophora mucronata | Bakhaw babae | 40.0 | 12.73236567 | 0.8483 | 111.2498879 | 48.69868745 | 159.9485753 |
| 15 | Rhizophora mucronata | Bakhaw babae | 37.8 | 12.03208556 | 0.8483 | 96.79698516 | 42.95125627 | 139.7482414 |
| 16 | Rhizophora mucronata | Bakhaw babae | 11.0 | 3.50140056 | 0.8483 | 4.645773248 | 2.772271986 | 7.418045234 |
| 17 | Rhizophora mucronata | Bakhaw babae | 6.5 | 2.069009422 | 0.8483 | 1.27349974 | 0.862207363 | 2.135707103 |
| 18 | Rhizophora mucronata | Bakhaw babae | 12.0 | 3.819709702 | 0.8483 | 5.754636138 | 3.362996703 | 9.11763284 |
| 19 | Rhizophora mucronata | Bakhaw babae | 9.0 | 2.864782277 | 0.8483 | 2.835754152 | 1.775670772 | 4.611424924 |
| 20 | Rhizophora mucronata | Bakhaw babae | 5.0 | 1.591545709 | 0.8483 | 0.667879655 | 0.48156785 | 1.149447504 |
| 21 | Rhizophora mucronata | Bakhaw babae | 7.0 | 2.228163993 | 0.8483 | 1.528175321 | 1.016393138 | 2.544568458 |
| 22 | Rhizophora mucronata | Bakhaw babae | 7.0 | 2.228163993 | 0.8483 | 1.528175321 | 1.016393138 | 2.544568458 |
| 23 | Rhizophora mucronata | Bakhaw babae | 10.0 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 |
| 24 | Rhizophora mucronata | Bakhaw babae | 9.0 | 2.864782277 | 0.8483 | 2.835754152 | 1.775670772 | 4.611424924 |
| 25 | Rhizophora mucronata | Bakhaw babae | 9.0 | 2.864782277 | 0.8483 | 2.835754152 | 1.775670772 | 4.611424924 |
| 26 | Rhizophora mucronata | Bakhaw babae | 10.0 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 |
| 27 | Rhizophora mucronata | Bakhaw babae | 5.5 | 1.75070028 | 0.8483 | 0.844353328 | 0.595044227 | 1.439397555 |
| 28 | Rhizophora mucronata | Bakhaw babae | 10.0 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 |
| 29 | Rhizophora mucronata | Bakhaw babae | 5.6 | 1.782531194 | 0.8483 | 0.882621596 | 0.619329113 | 1.501950709 |
| 30 | Rhizophora mucronata | Bakhaw babae | 8.0 | 2.546473135 | 0.8483 | 2.122429979 | 1.367111216 | 3.489541195 |
| 31 | Rhizophora mucronata | Bakhaw babae | 55.0 | 17.5070028 | 0.8483 | 243.5141598 | 98.75276082 | 342.2669206 |
| 32 | Rhizophora mucronata | Bakhaw babae | 7.0 | 2.228163993 | 0.8483 | 1.528175321 | 1.016393138 | 2.544568458 |
| 33 | Rhizophora mucronata | Bakhaw babae | 7.0 | 2.228163993 | 0.8483 | 1.528175321 | 1.016393138 | 2.544568458 |
| 34 | Rhizophora mucronata | Bakhaw babae | 21.0 | 6.684491979 | 0.8483 | 22.79772278 | 11.64852429 | 34.44624707 |
| 35 | Rhizophora mucronata | Bakhaw babae | 20.5 | 6.525337408 | 0.8483 | 21.48555129 | 11.04174331 | 32.5272946 |
| 36 | Rhizophora mucronata | Bakhaw babae | 10.5 | 3.342245989 | 0.8483 | 4.143407798 | 2.500255085 | 6.643662883 |
| 37 | Rhizophora mucronata | Bakhaw babae | 5.5 | 1.75070028 | 0.8483 | 0.844353328 | 0.595044227 | 1.439397555 |
| 38 | Rhizophora mucronata | Bakhaw babae | 21.5 | 6.84364655 | 0.8483 | 24.15631114 | 12.27318997 | 36.4295011 |
| 39 | Rhizophora mucronata | Bakhaw babae | 14.0 | 4.456327986 | 0.8483 | 8.408276237 | 4.735308897 | 13.14358513 |
| 40 | Rhizophora mucronata | Bakhaw babae | 15.5 | 4.933791698 | 0.8483 | 10.80060245 | 5.935816207 | 16.73641866 |
| 41 | Rhizophora mucronata | Bakhaw babae | 5.5 | 1.75070028 | 0.8483 | 0.844353328 | 0.595044227 | 1.439397555 |
| 42 | Rhizophora mucronata | Bakhaw babae | 6.0 | 1.909854851 | 0.8483 | 1.045885349 | 0.721838183 | 1.767723532 |
| 43 | Rhizophora mucronata | Bakhaw babae | 8.5 | 2.705627706 | 0.8483 | 2.463783603 | 1.564062453 | 4.027846056 |
| 44 | Rhizophora mucronata | Bakhaw babae | 10.0 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 |
| 45 | Rhizophora mucronata | Bakhaw babae | 15.0 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 |
| 46 | Rhizophora mucronata | Bakhaw babae | 16.9 | 5.379424497 | 0.8483 | 13.36082565 | 7.192047707 | 20.55287336 |
| 47 | Rhizophora mucronata | Bakhaw babae | 12.2 | 3.88337153 | 0.8483 | 5.993454143 | 3.488694169 | 9.482148312 |
| 48 | Rhizophora mucronata | Bakhaw babae | 14.0 | 4.456327986 | 0.8483 | 8.408276237 | 4.735308897 | 13.14358513 |
| 49 | Rhizophora mucronata | Bakhaw babae | 6.0 | 1.909854851 | 0.8483 | 1.045885349 | 0.721838183 | 1.767723532 |
| 50 | Rhizophora mucronata | Bakhaw babae | 8.0 | 2.546473135 | 0.8483 | 2.122429979 | 1.367111216 | 3.489541195 |
| 51 | Rhizophora mucronata | Bakhaw babae | 10.0 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 |
| 52 | Rhizophora mucronata | Bakhaw babae | 9.0 | 2.864782277 | 0.8483 | 2.835754152 | 1.775670772 | 4.611424924 |
| 53 | Rhizophora mucronata | Bakhaw babae | 6.7 | 2.13267125 | 0.8483 | 1.372069208 | 0.922210659 | 2.294279867 |
| 54 | Rhizophora mucronata | Bakhaw babae | 10.2 | 3.246753247 | 0.8483 | 3.858232774 | 2.344425609 | 6.202658383 |
| 55 | Rhizophora mucronata | Bakhaw babae | 8.0 | 2.546473135 | 0.8483 | 2.122429979 | 1.367111216 | 3.489541195 |
| 56 | Rhizophora mucronata | Bakhaw babae | 16.6 | 5.283931755 | 0.8483 | 12.78491662 | 6.911686493 | 19.69660311 |
| 57 | Rhizophora mucronata | Bakhaw babae | 15.8 | 5.029284441 | 0.8483 | 11.32213782 | 6.193880303 | 17.51601813 |
| 58 | Rhizophora mucronata | Bakhaw babae | 15.5 | 4.933791698 | 0.8483 | 10.80060245 | 5.935816207 | 16.73641866 |
| 59 | Rhizophora mucronata | Bakhaw babae | 16.0 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 |
| 60 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 |
| 61 | Rhizophora mucronata | Bakhaw babae | 18.0 | 5.729564553 | 0.8483 | 15.60279369 | 8.272733549 | 23.87552723 |
| 62 | Rhizophora mucronata | Bakhaw babae | 19.7 | 6.270690094 | 0.8483 | 19.4813442 | 10.10785719 | 29.5892014 |
| 63 | Rhizophora mucronata | Bakhaw babae | 19.1 | 6.079704609 | 0.8483 | 18.05402697 | 9.437091167 | 27.49111813 |
| 64 | Rhizophora mucronata | Bakhaw babae | 16.2 | 5.156608098 | 0.8483 | 12.04034522 | 6.547377891 | 18.58772311 |
| 65 | Rhizophora mucronata | Bakhaw babae | 20.3 | 6.461675579 | 0.8483 | 20.97356501 | 10.80401751 | 31.77758253 |
| 66 | Rhizophora mucronata | Bakhaw babae | 14.9 | 4.742806213 | 0.8483 | 9.800996264 | 5.437729392 | 15.23872566 |
| 67 | Rhizophora mucronata | Bakhaw babae | 15.0 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 |
| 68 | Rhizophora mucronata | Bakhaw babae | 16.9 | 5.379424497 | 0.8483 | 13.36082565 | 7.192047707 | 20.55287336 |
| 69 | Rhizophora mucronata | Bakhaw babae | 19.4 | 6.175197352 | 0.8483 | 18.75962959 | 9.76931056 | 28.52894015 |
| 70 | Rhizophora mucronata | Bakhaw babae | 17.0 | 5.411255411 | 0.8483 | 13.55614951 | 7.28686428 | 20.84301379 |
| 71 | Rhizophora mucronata | Bakhaw babae | 17.8 | 5.665902725 | 0.8483 | 15.17977062 | 8.070054737 | 23.24982536 |
| 72 | Rhizophora mucronata | Bakhaw babae | 15.0 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 |
| 73 | Rhizophora mucronata | Bakhaw babae | 18.3 | 5.825057296 | 0.8483 | 16.25031127 | 8.581940408 | 24.83225167 |
| 74 | Rhizophora mucronata | Bakhaw babae | 19.0 | 6.047873695 | 0.8483 | 17.82238667 | 9.327753688 | 27.15014035 |
| 75 | Rhizophora mucronata | Bakhaw babae | 20.6 | 6.557168322 | 0.8483 | 21.74429671 | 11.16167325 | 32.90596995 |
| 76 | Rhizophora mucronata | Bakhaw babae | 3.2 | 1.018589254 | 0.8483 | 0.222792697 | 0.178803916 | 0.401596613 |
| 77 | Rhizophora mucronata | Bakhaw babae | 5.3 | 1.687038452 | 0.8483 | 0.770815861 | 0.5480706 | 1.318886461 |
| 78 | Rhizophora mucronata | Bakhaw babae | 2.0 | 0.636618284 | 0.8483 | 0.070107712 | 0.062984062 | 0.133091774 |
| 79 | Rhizophora mucronata | Bakhaw babae | 5.0 | 1.591545709 | 0.8483 | 0.667879655 | 0.48156785 | 1.149447504 |
| 80 | Rhizophora mucronata | Bakhaw babae | 4.9 | 1.559714795 | 0.8483 | 0.635498252 | 0.460446709 | 1.095944961 |
| 81 | Rhizophora mucronata | Bakhaw babae | 3.7 | 1.177743825 | 0.8483 | 0.318425797 | 0.246803834 | 0.565229631 |
| 82 | Rhizophora mucronata | Bakhaw babae | 3.4 | 1.082251082 | 0.8483 | 0.258624783 | 0.204563088 | 0.463187871 |
| 83 | Rhizophora mucronata | Bakhaw babae | 4.5 | 1.432391138 | 0.8483 | 0.515388575 | 0.381132388 | 0.896520963 |
| 84 | Rhizophora mucronata | Bakhaw babae | 17.8 | 5.665902725 | 0.8483 | 15.17977062 | 8.070054737 | 23.24982536 |
| 85 | Rhizophora mucronata | Bakhaw babae | 15.0 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 |
| 86 | Rhizophora mucronata | Bakhaw babae | 17.2 | 5.47491724 | 0.8483 | 13.95185592 | 7.478547001 | 21.43040292 |
| 87 | Rhizophora mucronata | Bakhaw babae | 16.0 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 |
| 88 | Rhizophora mucronata | Bakhaw babae | 19.2 | 6.111535523 | 0.8483 | 18.2874447 | 9.54712927 | 27.83457397 |
| 89 | Rhizophora mucronata | Bakhaw babae | 20.3 | 6.461675579 | 0.8483 | 20.97356501 | 10.80401751 | 31.77758253 |
| 90 | Rhizophora mucronata | Bakhaw babae | 16.7 | 5.315762669 | 0.8483 | 12.97521379 | 7.004459705 | 19.9796735 |
| 91 | Rhizophora mucronata | Bakhaw babae | 15.4 | 4.901960784 | 0.8483 | 10.62999295 | 5.851134422 | 16.48112737 |
| 92 | Rhizophora mucronata | Bakhaw babae | 19.0 | 6.047873695 | 0.8483 | 17.82238667 | 9.327753688 | 27.15014035 |
| 93 | Rhizophora mucronata | Bakhaw babae | 15.3 | 4.87012987 | 0.8483 | 10.46099328 | 5.767120844 | 16.22811413 |
| 94 | Rhizophora mucronata | Bakhaw babae | 16.8 | 5.347593583 | 0.8483 | 13.16718194 | 7.097913145 | 20.26509508 |
| 95 | Rhizophora mucronata | Bakhaw babae | 16.4 | 5.220269926 | 0.8483 | 12.40931673 | 6.728177154 | 19.13749389 |
| 96 | Rhizophora mucronata | Bakhaw babae | 15.5 | 4.933791698 | 0.8483 | 10.80060245 | 5.935816207 | 16.73641866 |
| 97 | Rhizophora mucronata | Bakhaw babae | 18.9 | 6.016042781 | 0.8483 | 17.59251952 | 9.219116022 | 26.81163554 |
| 98 | Rhizophora mucronata | Bakhaw babae | 20.1 | 6.398013751 | 0.8483 | 20.46889059 | 10.56913202 | 31.03802261 |
| 99 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 |
| 100 | Rhizophora mucronata | Bakhaw babae | 15.9 | 5.061115355 | 0.8483 | 11.49923447 | 6.281244391 | 17.78047886 |
| 101 | Rhizophora mucronata | Bakhaw babae | 16.7 | 5.315762669 | 0.8483 | 12.97521379 | 7.004459705 | 19.9796735 |
| 102 | Rhizophora mucronata | Bakhaw babae | 20.0 | 6.366182837 | 0.8483 | 20.21928494 | 10.45275246 | 30.67203741 |
| 103 | Rhizophora mucronata | Bakhaw babae | 17.0 | 5.411255411 | 0.8483 | 13.55614951 | 7.28686428 | 20.84301379 |
| 104 | Rhizophora mucronata | Bakhaw babae | 14.9 | 4.742806213 | 0.8483 | 9.800996264 | 5.437729392 | 15.23872566 |
| 105 | Rhizophora mucronata | Bakhaw babae | 19.6 | 6.23885918 | 0.8483 | 19.23897538 | 9.994303962 | 29.23327934 |
| 106 | Rhizophora mucronata | Bakhaw babae | 21.8 | 6.939139292 | 0.8483 | 24.99395466 | 12.65661271 | 37.65056737 |
| 107 | Rhizophora mucronata | Bakhaw babae | 20.7 | 6.588999236 | 0.8483 | 22.00488246 | 11.28231555 | 33.28719802 |
| 108 | Rhizophora mucronata | Bakhaw babae | 24.2 | 7.703081232 | 0.8483 | 32.3160152 | 15.95931283 | 48.27532803 |
| 109 | Rhizophora mucronata | Bakhaw babae | 23.0 | 7.321110262 | 0.8483 | 28.51559776 | 14.25541521 | 42.77101297 |
| 110 | Rhizophora mucronata | Bakhaw babae | 25.1 | 7.98955946 | 0.8483 | 35.35325113 | 17.30691804 | 52.66016917 |
| 111 | Rhizophora mucronata | Bakhaw babae | 24.8 | 7.894066718 | 0.8483 | 34.32283387 | 16.85104377 | 51.17387764 |
| 112 | Rhizophora mucronata | Bakhaw babae | 22.7 | 7.22561752 | 0.8483 | 27.60931375 | 13.84590917 | 41.45522292 |
| 113 | Rhizophora mucronata | Bakhaw babae | 22.2 | 7.066462949 | 0.8483 | 26.1372758 | 13.17794463 | 39.31522043 |
| 114 | Rhizophora mucronata | Bakhaw babae | 21.7 | 6.907308378 | 0.8483 | 24.71285657 | 12.5280848 | 37.24094137 |
| 115 | Rhizophora mucronata | Bakhaw babae | 20.8 | 6.62083015 | 0.8483 | 22.26731267 | 11.40367099 | 33.67098366 |
| 116 | Rhizophora mucronata | Bakhaw babae | 25.0 | 7.957728546 | 0.8483 | 35.0077682 | 17.1542168 | 52.161985 |
| 117 | Rhizophora mucronata | Bakhaw babae | 24.3 | 7.734912147 | 0.8483 | 32.6455084 | 16.10608559 | 48.75159399 |
| 118 | Rhizophora mucronata | Bakhaw babae | 24.9 | 7.925897632 | 0.8483 | 34.66429701 | 17.00225893 | 51.66655595 |
| 119 | Rhizophora mucronata | Bakhaw babae | 20.9 | 6.652661064 | 0.8483 | 22.53159141 | 11.52574032 | 34.05733173 |
| 120 | Rhizophora mucronata | Bakhaw babae | 16.8 | 5.347593583 | 0.8483 | 13.16718194 | 7.097913145 | 20.26509508 |
| 121 | Rhizophora mucronata | Bakhaw babae | 16.4 | 5.220269926 | 0.8483 | 12.40931673 | 6.728177154 | 19.13749389 |
| 122 | Rhizophora mucronata | Bakhaw babae | 19.8 | 6.302521008 | 0.8483 | 19.72551594 | 10.22211583 | 29.94763177 |
| 123 | Rhizophora mucronata | Bakhaw babae | 20.0 | 6.366182837 | 0.8483 | 20.21928494 | 10.45275246 | 30.67203741 |
| 124 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 |
| 125 | Rhizophora mucronata | Bakhaw babae | 15.2 | 4.838298956 | 0.8483 | 10.29359862 | 5.683774517 | 15.97737314 |
| 126 | Rhizophora mucronata | Bakhaw babae | 18.3 | 5.825057296 | 0.8483 | 16.25031127 | 8.581940408 | 24.83225167 |
| 127 | Rhizophora mucronata | Bakhaw babae | 19.7 | 6.270690094 | 0.8483 | 19.4813442 | 10.10785719 | 29.5892014 |
| 128 | Rhizophora mucronata | Bakhaw babae | 16.5 | 5.25210084 | 0.8483 | 12.59628581 | 6.81959261 | 19.41587842 |
| 129 | Rhizophora mucronata | Bakhaw babae | 19.1 | 6.079704609 | 0.8483 | 18.05402697 | 9.437091167 | 27.49111813 |
| 130 | Rhizophora mucronata | Bakhaw babae | 18.7 | 5.952380952 | 0.8483 | 17.13808748 | 9.00393688 | 26.14202436 |
| 131 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 |
| 132 | Rhizophora mucronata | Bakhaw babae | 15.6 | 4.965622613 | 0.8483 | 10.97282658 | 6.021167151 | 16.99399373 |
| 133 | Rhizophora mucronata | Bakhaw babae | 17.7 | 5.634071811 | 0.8483 | 14.9708424 | 7.969750518 | 22.94059292 |
| 134 | Rhizophora mucronata | Bakhaw babae | 19.9 | 6.334351923 | 0.8483 | 19.97149479 | 10.33708066 | 30.30857544 |
| 135 | Rhizophora mucronata | Bakhaw babae | 23.6 | 7.512095747 | 0.8483 | 30.38054292 | 15.0941521 | 45.47469501 |
| 136 | Rhizophora mucronata | Bakhaw babae | 22.0 | 7.00280112 | 0.8483 | 25.56182152 | 12.91583317 | 38.47765469 |
| 137 | Rhizophora mucronata | Bakhaw babae | 24.8 | 7.894066718 | 0.8483 | 34.32283387 | 16.85104377 | 51.17387764 |
| 138 | Rhizophora mucronata | Bakhaw babae | 18.6 | 5.920550038 | 0.8483 | 16.91351396 | 8.89739377 | 25.81090773 |
| 139 | Rhizophora mucronata | Bakhaw babae | 22.6 | 7.193786606 | 0.8483 | 27.31107304 | 13.7108636 | 41.02193663 |
| 140 | Rhizophora mucronata | Bakhaw babae | 15.3 | 4.87012987 | 0.8483 | 10.46099328 | 5.767120844 | 16.22811413 |
| 141 | Rhizophora mucronata | Bakhaw babae | 16.5 | 5.25210084 | 0.8483 | 12.59628581 | 6.81959261 | 19.41587842 |
| 142 | Rhizophora mucronata | Bakhaw babae | 23.7 | 7.543926662 | 0.8483 | 30.69820165 | 15.23650658 | 45.93470823 |
| 143 | Rhizophora mucronata | Bakhaw babae | 25.2 | 8.021390374 | 0.8483 | 35.70074949 | 17.4603633 | 53.16111279 |
| 144 | Rhizophora mucronata | Bakhaw babae | 18.0 | 5.729564553 | 0.8483 | 15.60279369 | 8.272733549 | 23.87552723 |
| 145 | Rhizophora mucronata | Bakhaw babae | 23.0 | 7.321110262 | 0.8483 | 28.51559776 | 14.25541521 | 42.77101297 |
| 146 | Rhizophora mucronata | Bakhaw babae | 24.7 | 7.862235803 | 0.8483 | 33.98337504 | 16.70057067 | 50.68394571 |
| 147 | Rhizophora mucronata | Bakhaw babae | 20.1 | 6.398013751 | 0.8483 | 20.46889059 | 10.56913202 | 31.03802261 |
| 148 | Rhizophora mucronata | Bakhaw babae | 23.0 | 7.321110262 | 0.8483 | 28.51559776 | 14.25541521 | 42.77101297 |
| 149 | Rhizophora mucronata | Bakhaw babae | 19.4 | 6.175197352 | 0.8483 | 18.75962959 | 9.76931056 | 28.52894015 |
| 150 | Rhizophora mucronata | Bakhaw babae | 18.3 | 5.825057296 | 0.8483 | 16.25031127 | 8.581940408 | 24.83225167 |
| 151 | Rhizophora mucronata | Bakhaw babae | 22.7 | 7.22561752 | 0.8483 | 27.60931375 | 13.84590917 | 41.45522292 |
| 152 | Rhizophora mucronata | Bakhaw babae | 24.6 | 7.830404889 | 0.8483 | 33.64591681 | 16.55083896 | 50.19675578 |
| 153 | Rhizophora mucronata | Bakhaw babae | 20.1 | 6.398013751 | 0.8483 | 20.46889059 | 10.56913202 | 31.03802261 |
| 154 | Rhizophora mucronata | Bakhaw babae | 16.8 | 5.347593583 | 0.8483 | 13.16718194 | 7.097913145 | 20.26509508 |
| 155 | Rhizophora mucronata | Bakhaw babae | 19.7 | 6.270690094 | 0.8483 | 19.4813442 | 10.10785719 | 29.5892014 |
| 156 | Rhizophora mucronata | Bakhaw babae | 24.9 | 7.925897632 | 0.8483 | 34.66429701 | 17.00225893 | 51.66655595 |
| 157 | Rhizophora mucronata | Bakhaw babae | 25.0 | 7.957728546 | 0.8483 | 35.0077682 | 17.1542168 | 52.161985 |
| 158 | Rhizophora mucronata | Bakhaw babae | 15.2 | 4.838298956 | 0.8483 | 10.29359862 | 5.683774517 | 15.97737314 |
| 159 | Rhizophora mucronata | Bakhaw babae | 17.8 | 5.665902725 | 0.8483 | 15.17977062 | 8.070054737 | 23.24982536 |
| 160 | Rhizophora mucronata | Bakhaw babae | 21.6 | 6.875477464 | 0.8483 | 24.4336434 | 12.40027746 | 36.83392086 |
| 161 | Rhizophora mucronata | Bakhaw babae | 24.6 | 7.830404889 | 0.8483 | 33.64591681 | 16.55083896 | 50.19675578 |
| 162 | Rhizophora mucronata | Bakhaw babae | 23.8 | 7.575757576 | 0.8483 | 31.01782331 | 15.37959574 | 46.39741905 |
| 163 | Rhizophora mucronata | Bakhaw babae | 19.8 | 6.302521008 | 0.8483 | 19.72551594 | 10.22211583 | 29.94763177 |
| 164 | Rhizophora mucronata | Bakhaw babae | 22.3 | 7.098293863 | 0.8483 | 26.42785811 | 13.3100863 | 39.73794441 |
| 165 | Rhizophora mucronata | Bakhaw babae | 16.9 | 5.379424497 | 0.8483 | 13.36082565 | 7.192047707 | 20.55287336 |
| 166 | Rhizophora mucronata | Bakhaw babae | 26 | 8.276037688 | 0.8483 | 38.55373335 | 18.71478783 | 57.26852118 |
| 167 | Rhizophora mucronata | Bakhaw babae | 22.4 | 7.130124777 | 0.8483 | 26.72034914 | 13.44295287 | 40.16330201 |
| 168 | Rhizophora mucronata | Bakhaw babae | 24.8 | 7.894066718 | 0.8483 | 34.32283387 | 16.85104377 | 51.17387764 |
| 169 | Rhizophora mucronata | Bakhaw babae | 17.0 | 5.411255411 | 0.8483 | 13.55614951 | 7.28686428 | 20.84301379 |
| 170 | Rhizophora mucronata | Bakhaw babae | 15.2 | 4.838298956 | 0.8483 | 10.29359862 | 5.683774517 | 15.97737314 |
| 171 | Rhizophora mucronata | Bakhaw babae | 23.7 | 7.543926662 | 0.8483 | 30.69820165 | 15.23650658 | 45.93470823 |
| 172 | Rhizophora mucronata | Bakhaw babae | 26.7 | 8.498854087 | 0.8483 | 41.15756818 | 19.85176272 | 61.0093309 |
| 173 | Rhizophora mucronata | Bakhaw babae | 18.8 | 5.984211867 | 0.8483 | 17.36442123 | 9.111177358 | 26.47559859 |
| 174 | Rhizophora mucronata | Bakhaw babae | 15.9 | 5.061115355 | 0.8483 | 11.49923447 | 6.281244391 | 17.78047886 |
| 175 | Rhizophora mucronata | Bakhaw babae | 23.3 | 7.416603005 | 0.8483 | 29.43930609 | 14.67148993 | 44.11079602 |
| 176 | Rhizophora mucronata | Bakhaw babae | 18.1 | 5.761395467 | 0.8483 | 15.8168974 | 8.375109839 | 24.19200724 |
| 177 | Rhizophora mucronata | Bakhaw babae | 16 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 |
| 178 | Rhizophora mucronata | Bakhaw babae | 18 | 5.729564553 | 0.8483 | 15.60279369 | 8.272733549 | 23.87552723 |
| 179 | Rhizophora mucronata | Bakhaw babae | 19.8 | 6.302521008 | 0.8483 | 19.72551594 | 10.22211583 | 29.94763177 |
| 180 | Rhizophora mucronata | Bakhaw babae | 20.5 | 6.525337408 | 0.8483 | 21.48555129 | 11.04174331 | 32.5272946 |
| 181 | Rhizophora mucronata | Bakhaw babae | 23.6 | 7.512095747 | 0.8483 | 30.38054292 | 15.0941521 | 45.47469501 |
| 182 | Xylocarpus granatum | Tabigi | 25.0 | 7.957728546 | 0.6721 | 27.73632089 | 13.91451251 | 41.6508334 |
| 183 | Xylocarpus granatum | Tabigi | 15.5 | 4.933791698 | 0.6721 | 8.55721432 | 4.814792179 | 13.3720065 |
| 184 | Xylocarpus granatum | Tabigi | 47.0 | 14.96052967 | 0.6721 | 131.062112 | 56.50650169 | 187.5686137 |
| 185 | Xylocarpus granatum | Tabigi | 13.4 | 4.265342501 | 0.6721 | 5.981281409 | 3.485088643 | 9.466370052 |
| 186 | Xylocarpus granatum | Tabigi | 21.0 | 6.684491979 | 0.6721 | 18.06241835 | 9.448611899 | 27.51103024 |
| 187 | Xylocarpus granatum | Tabigi | 6.0 | 1.909854851 | 0.6721 | 0.828644988 | 0.585513553 | 1.414158541 |
| 188 | Xylocarpus granatum | Tabigi | 39.5 | 12.5732111 | 0.6721 | 85.45653647 | 38.41375204 | 123.8702885 |
| 189 | Xylocarpus granatum | Tabigi | 25.0 | 7.957728546 | 0.6721 | 27.73632089 | 13.91451251 | 41.6508334 |
| 190 | Xylocarpus granatum | Tabigi | 40.0 | 12.73236567 | 0.6721 | 88.14222522 | 39.50157001 | 127.6437952 |
| 191 | Xylocarpus granatum | Tabigi | 55.0 | 17.5070028 | 0.6721 | 192.9339465 | 80.10255099 | 273.0364975 |
| 192 | Xylocarpus granatum | Tabigi | 25.2 | 8.021390374 | 0.6721 | 28.28536336 | 14.16284092 | 42.44820427 |
| Total Biomass of Site 2 (kg) | Total Biomass (t/ha) | C. Stored (tC/ha) | CO2 Sequestered (tCO2/ha) | |||||
| 2.305169919 | 23.05169919 | 11.52584959 | 42.26529046 | |||||
| Tree No. | Scientific Name | Local Name | GBH (cm) | DBH (cm) | Wood Density (g/cm3) | AGB (kg) | BGB (kg) | Total Biomass (kg) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Avicennia alba | Tabigi | 149 | 47.42806213 | 0.6987 | 2328.152905 | 757.9996385 | 3086.152544 | ||
| 2 | Rhizophora mucronata | Bakhaw babae | 35 | 11.14081996 | 0.8483 | 80.10126827 | 36.20554871 | 116.306817 | ||
| 3 | Rhizophora mucronata | Bakhaw babae | 37.6 | 11.96842373 | 0.8483 | 95.54195021 | 42.44837712 | 137.9903273 | ||
| 4 | Rhizophora mucronata | Bakhaw babae | 11 | 3.50140056 | 0.8483 | 4.645773248 | 2.772271986 | 7.418045234 | ||
| 5 | Rhizophora mucronata | Bakhaw babae | 73 | 23.23656735 | 0.8483 | 488.6593796 | 185.1487672 | 673.8081468 | ||
| 6 | Rhizophora mucronata | Bakhaw babae | 74 | 23.5548765 | 0.8483 | 505.291548 | 190.8264157 | 696.1179638 | ||
| 7 | Rhizophora mucronata | Bakhaw babae | 32.5 | 10.34504711 | 0.8483 | 66.75212126 | 30.71320487 | 97.46532613 | ||
| 8 | Rhizophora mucronata | Bakhaw babae | 62 | 19.73516679 | 0.8483 | 326.9757612 | 128.8408633 | 455.8166245 | ||
| 9 | Rhizophora mucronata | Bakhaw babae | 55 | 17.5070028 | 0.8483 | 243.5141598 | 98.75276082 | 342.2669206 | ||
| 10 | Rhizophora mucronata | Bakhaw babae | 62.5 | 19.89432136 | 0.8483 | 333.5007739 | 131.1588843 | 464.6596582 | ||
| 11 | Rhizophora mucronata | Bakhaw babae | 37.2 | 11.84110008 | 0.8483 | 93.06098296 | 41.45237342 | 134.5133564 | ||
| 12 | Rhizophora mucronata | Bakhaw babae | 10 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||
| 13 | Rhizophora mucronata | Bakhaw babae | 57 | 18.14362108 | 0.8483 | 265.8790683 | 106.9021045 | 372.7811728 | ||
| 14 | Rhizophora mucronata | Bakhaw babae | 46.6 + 49.2 | 15.24700789 | 0.8483 | 173.3234665 | 72.65891613 | 245.9823826 | ||
| 15 | Rhizophora mucronata | Bakhaw babae | 25 | 7.957728546 | 0.8483 | 35.0077682 | 17.1542168 | 52.161985 | ||
| 16 | Rhizophora mucronata | Bakhaw babae | 59.4 | 18.90756303 | 0.8483 | 294.2704531 | 117.1520744 | 411.4225276 | ||
| 17 | Rhizophora mucronata | Bakhaw babae | 11 | 3.50140056 | 0.8483 | 4.645773248 | 2.772271986 | 7.418045234 | ||
| 18 | Rhizophora mucronata | Bakhaw babae | 53.5 | 17.02953909 | 0.8483 | 227.5004747 | 92.87299935 | 320.373474 | ||
| 19 | Rhizophora mucronata | Bakhaw babae | 70.7 | 22.50445633 | 0.8483 | 451.6518165 | 172.4468135 | 624.0986301 | ||
| 20 | Rhizophora mucronata | Bakhaw babae | 40.4 | 12.85968933 | 0.8483 | 114.006644 | 49.78639791 | 163.7930419 | ||
| 21 | Rhizophora mucronata | Bakhaw babae | 42 | 13.36898396 | 0.8483 | 125.4368842 | 54.26970989 | 179.7065941 | ||
| 22 | Rhizophora mucronata | Bakhaw babae | 51.2 | 16.29742806 | 0.8483 | 204.1907702 | 84.24097577 | 288.431746 | ||
| 23 | Rhizophora mucronata | Bakhaw babae | 22.4 | 7.130124777 | 0.8483 | 26.72034914 | 13.44295287 | 40.16330201 | ||
| 24 | Rhizophora mucronata | Bakhaw babae | 33.7 | 10.72701808 | 0.8483 | 72.97961358 | 33.28759779 | 106.2672114 | ||
| 25 | Rhizophora mucronata | Bakhaw babae | 47 | 14.96052967 | 0.8483 | 165.4217968 | 69.66286313 | 235.08466 | ||
| 26 | Rhizophora mucronata | Bakhaw babae | 55.9 | 17.79348103 | 0.8483 | 253.4341007 | 102.3760311 | 355.8101318 | ||
| 27 | Rhizophora mucronata | Bakhaw babae | 42 | 13.36898396 | 0.8483 | 125.4368842 | 54.26970989 | 179.7065941 | ||
| 28 | Rhizophora mucronata | Bakhaw babae | 41.4 | 13.17799847 | 0.8483 | 121.0745441 | 52.56356743 | 173.6381116 | ||
| 29 | Rhizophora mucronata | Bakhaw babae | 60 | 19.09854851 | 0.8483 | 301.6366295 | 119.7953198 | 421.4319493 | ||
| 30 | Rhizophora mucronata | Bakhaw babae | 57.1 | 18.175452 | 0.8483 | 267.0280164 | 107.3189057 | 374.3469221 | ||
| 31 | Rhizophora mucronata | Bakhaw babae | 58 | 18.46193023 | 0.8483 | 277.5012013 | 111.1102746 | 388.6114759 | ||
| 32 | Rhizophora mucronata | Bakhaw babae | 64.7 | 20.59460148 | 0.8483 | 363.1253211 | 141.628801 | 504.7541221 | ||
| 33 | Rhizophora mucronata | Bakhaw babae | 49.2 | 15.66080978 | 0.8483 | 185.1254623 | 77.1092721 | 262.2347344 | ||
| 34 | Rhizophora mucronata | Bakhaw babae | 75.6 | 24.06417112 | 0.8483 | 532.5932038 | 200.107083 | 732.7002868 | ||
| 35 | Rhizophora mucronata | Bakhaw babae | 58 | 18.46193023 | 0.8483 | 277.5012013 | 111.1102746 | 388.6114759 | ||
| 36 | Rhizophora mucronata | Bakhaw babae | 65.2 + 70.7 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||
| 37 | Rhizophora mucronata | Bakhaw babae | 98.5 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||
| 38 | Rhizophora mucronata | Bakhaw babae | 61 | 19.41685765 | 0.8483 | 314.1545814 | 124.2728645 | 438.4274459 | ||
| 39 | Rhizophora mucronata | Bakhaw babae | 28 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||
| 40 | Rhizophora mucronata | Bakhaw babae | 68 | 21.64502165 | 0.8483 | 410.3967649 | 158.1662659 | 568.5630307 | ||
| 41 | Rhizophora mucronata | Bakhaw babae | 19 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||
| 42 | Rhizophora mucronata | Bakhaw babae | 33 | 10.50420168 | 0.8483 | 69.30687152 | 31.77203423 | 101.0789058 | ||
| 43 | Rhizophora mucronata | Bakhaw babae | 40 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||
| Total Biomass of Site 3(kg) | Total Biomass (t/ha) | C. Stored (tC/ha) | CO2 Sequestered (tCO2/ha) | |||||||
| 6.152733237 | 61.52733237 | 30.76366618 | 112.8103639 | |||||||
| Tree No. | Scientific Name | Local name | GBH (cm) |
DBH (cm) | Wood Density (g/cm3) | AGB (kg) | BGB (kg) | Total Biomass (kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | Avicennia alba | Bungalon | 91.9 | 29.25261013 | 0.6987 | 709.1358437 | 259.2721792 | 968.4080229 |
| 2 | Avicennia alba | Bungalon | 162.6 | 51.75706646 | 0.6987 | 2886.222521 | 920.2018123 | 3806.424333 |
| 3 | Avicennia alba | Bungalon | 35.6 | 11.33180545 | 0.6987 | 68.79237045 | 31.58017106 | 100.3725415 |
| 4 | Avicennia alba | Bungalon | 61 | 19.41685765 | 0.6987 | 258.752571 | 104.3825053 | 363.1350764 |
| 5 | Avicennia alba | Bungalon | 80.8 | 25.71937866 | 0.6987 | 516.660461 | 194.8268028 | 711.4872638 |
| 6 | Avicennia alba | Bungalon | 172.7 | 54.9719888 | 0.6987 | 3347.437138 | 1051.924262 | 4399.3614 |
| 7 | Avicennia alba | Bungalon | 71.1 | 22.63177998 | 0.6987 | 377.2007064 | 146.6715887 | 523.8722952 |
| 8 | Avicennia alba | Bungalon | 128.3 | 40.8390629 | 0.6987 | 1611.42781 | 543.824363 | 2155.252173 |
| 9 | Avicennia alba | Bungalon | 62.7 | 19.95798319 | 0.6987 | 276.8544 | 110.9505408 | 387.8049408 |
| 10 | Avicennia alba | Bungalon | 56.4 | 17.9526356 | 0.6987 | 213.3633765 | 87.70716907 | 301.0705455 |
| 11 | Avicennia alba | Bungalon | 94.7 | 30.14387573 | 0.6987 | 763.4739581 | 277.1356756 | 1040.609634 |
| 12 | Avicennia alba | Bungalon | 68.6 | 21.83600713 | 0.6987 | 345.4065781 | 135.4674699 | 480.874048 |
| 13 | Avicennia alba | Bungalon | 38.1 | 12.1275783 | 0.6987 | 81.29217058 | 36.71545619 | 118.0076268 |
| 14 | Avicennia alba | Bungalon | 30.5 | 9.708428826 | 0.6987 | 47.02739088 | 22.40480281 | 69.4321937 |
| 15 | Avicennia alba | Bungalon | 26.7 | 8.498854087 | 0.6987 | 33.89931968 | 16.67441027 | 50.57372995 |
| 16 | Avicennia alba | Bungalon | 48.0 | 15.27883881 | 0.6987 | 143.4916811 | 61.31278305 | 204.8044641 |
| 17 | Avicennia alba | Bungalon | 27.0 | 8.59434683 | 0.6987 | 34.84401078 | 17.09318681 | 51.93719759 |
| 18 | Avicennia alba | Bungalon | 24.0 | 7.639419404 | 0.6987 | 26.07912009 | 13.16025908 | 39.23937918 |
| 19 | Avicennia alba | Bungalon | 27.0 | 8.59434683 | 0.6987 | 34.84401078 | 17.09318681 | 51.93719759 |
| 20 | Avicennia alba | Bungalon | 33.5 | 10.66335625 | 0.6987 | 59.23569727 | 27.59274798 | 86.82844524 |
| 21 | Avicennia alba | Bungalon | 33.0 | 10.50420168 | 0.6987 | 57.08441723 | 26.68679559 | 83.77121282 |
| 22 | Avicennia alba | Bungalon | 40.0 | 12.73236567 | 0.6987 | 91.63066919 | 40.9042716 | 132.5349408 |
| 23 | Avicennia alba | Bungalon | 32.0 | 10.18589254 | 0.6987 | 52.92272845 | 24.92461361 | 77.84734206 |
| 24 | Avicennia alba | Bungalon | 149 | 47.42806213 | 0.6987 | 2328.152905 | 757.9996385 | 3086.152544 |
| 25 | Avicennia alba | Bungalon | 48.0 | 15.27883881 | 0.6987 | 143.4916811 | 61.31278305 | 0.074150784 |
| 26 | Avicennia alba | Bungalon | 27.0 | 8.59434683 | 0.6987 | 34.84401078 | 17.09318681 | 0.018804199 |
| 27 | Avicennia alba | Bungalon | 24.0 | 7.639419404 | 0.6987 | 26.07912009 | 13.16025908 | 0.014206872 |
| 28 | Avicennia alba | Bungalon | 27.0 | 8.59434683 | 0.6987 | 34.84401078 | 17.09318681 | 0.018804199 |
| 29 | Avicennia alba | Bungalon | 33.5 | 10.66335625 | 0.6987 | 59.23569727 | 27.59274798 | 0.031436801 |
| 30 | Avicennia alba | Bungalon | 33.0 | 10.50420168 | 0.6987 | 57.08441723 | 26.68679559 | 0.030329911 |
| 31 | Avicennia alba | Bungalon | 40.0 | 12.73236567 | 0.6987 | 91.63066919 | 40.9042716 | 0.047985134 |
| 32 | Avicennia alba | Bungalon | 32.0 | 10.18589254 | 0.6987 | 52.92272845 | 24.92461361 | 0.028185135 |
| Total Biomass of Avicennia alba (kg) | Total Biomass (t/ha) | C. Stored (tC/ha) | CO2 Sequestered (tCO2/ha) | |||||
| 6.984794515 | 69.84794515 | 34.92397257 | 128.0662074 | |||||
| Tree No. | Scientific Name | Local Name | GBH (cm) |
DBH (cm) | Wood Density (g/cm3) | AGB (kg) | BGB (kg) | Total Biomass (kg) |
|---|---|---|---|---|---|---|---|---|
| 1 | Xylocarpus granatum | Tabigi | 25.0 | 7.957728546 | 0.6721 | 27.73632089 | 13.91451251 | 41.6508334 |
| 2 | Xylocarpus granatum | Tabigi | 15.5 | 4.933791698 | 0.6721 | 8.55721432 | 4.814792179 | 13.3720065 |
| 3 | Xylocarpus granatum | Tabigi | 47.0 | 14.96052967 | 0.6721 | 131.062112 | 56.50650169 | 187.5686137 |
| 4 | Xylocarpus granatum | Tabigi | 13.4 | 4.265342501 | 0.6721 | 5.981281409 | 3.485088643 | 9.466370052 |
| 5 | Xylocarpus granatum | Tabigi | 21.0 | 6.684491979 | 0.6721 | 18.06241835 | 9.448611899 | 27.51103024 |
| 6 | Xylocarpus granatum | Tabigi | 39.5 | 12.5732111 | 0.6721 | 85.45653647 | 38.41375204 | 123.8702885 |
| 7 | Xylocarpus granatum | Tabigi | 25.0 | 7.957728546 | 0.6721 | 27.73632089 | 13.91451251 | 41.6508334 |
| 8 | Xylocarpus granatum | Tabigi | 40.0 | 12.73236567 | 0.6721 | 88.14222522 | 39.50157001 | 127.6437952 |
| 9 | Xylocarpus granatum | Tabigi | 55.0 | 17.5070028 | 0.6721 | 192.9339465 | 80.10255099 | 273.0364975 |
| 10 | Xylocarpus granatum | Tabigi | 25.2 | 8.021390374 | 0.6721 | 28.28536336 | 14.16284092 | 42.44820427 |
| 11 | Xylocarpus granatum | Tabigi | 25.0 | 7.957728546 | 0.6721 | 27.73632089 | 13.91451251 | 41.6508334 |
| Total Biomass of Xylocarpus granatum (kg) | Total Biomass (t/ha) | C. Stored (tC/ha) | CO2 Sequestered (tCO2/ha) | |||||
| 0.336665209 | 3.366652086 | 1.683326043 | 6.1727566 | |||||
| Tree No. | Scientific Name | Local name | GBH (cm) | DBH (cm) | Wood Density (g/cm3) | AGB (kg) | BGB (kg) | Total Biomass (kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rhizophora mucronata | Bakhaw babae | 45.7 | 14.54672778 | 0.8483 | 154.3923777 | 65.45729277 | 219.8496705 | ||||||
| 2 | Rhizophora mucronata | Bakhaw babae | 48.3 | 15.37433155 | 0.8483 | 176.9057483 | 74.01277804 | 250.9185264 | ||||||
| 3 | Rhizophora mucronata | Bakhaw babae | 26.4 | 8.403361345 | 0.8483 | 40.02926939 | 19.35997504 | 59.38924442 | ||||||
| 4 | Rhizophora mucronata | Bakhaw babae | 33.02 | 10.51056786 | 0.8483 | 69.41024749 | 31.81479787 | 101.2250454 | ||||||
| 5 | Rhizophora mucronata | Bakhaw babae | 38.6 | 12.28673287 | 0.8483 | 101.9146865 | 44.99537666 | 146.9100632 | ||||||
| 6 | Rhizophora mucronata | Bakhaw babae | 32.3 | 10.28138528 | 0.8483 | 65.74613214 | 30.2951896 | 96.04132175 | ||||||
| 7 | Rhizophora mucronata | Bakhaw babae | 16 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 | ||||||
| 8 | Rhizophora mucronata | Bakhaw babae | 39 | 12.41405653 | 0.8483 | 104.5324036 | 46.03705171 | 150.5694553 | ||||||
| 9 | Rhizophora mucronata | Bakhaw babae | 40 | 12.73236567 | 0.8483 | 111.2498879 | 48.69868745 | 159.9485753 | ||||||
| 10 | Rhizophora mucronata | Bakhaw babae | 37.8 | 12.03208556 | 0.8483 | 96.79698516 | 42.95125627 | 139.7482414 | ||||||
| 11 | Rhizophora mucronata | Bakhaw babae | 11 | 3.50140056 | 0.8483 | 4.645773248 | 2.772271986 | 7.418045234 | ||||||
| 12 | Rhizophora mucronata | Bakhaw babae | 6.5 | 2.069009422 | 0.8483 | 1.27349974 | 0.862207363 | 2.135707103 | ||||||
| 13 | Rhizophora mucronata | Bakhaw babae | 12 | 3.819709702 | 0.8483 | 5.754636138 | 3.362996703 | 9.11763284 | ||||||
| 14 | Rhizophora mucronata | Bakhaw babae | 10 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||||||
| 15 | Rhizophora mucronata | Bakhaw babae | 10 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||||||
| 16 | Rhizophora mucronata | Bakhaw babae | 10 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||||||
| 17 | Rhizophora mucronata | Bakhaw babae | 55 | 17.5070028 | 0.8483 | 243.5141598 | 98.75276082 | 342.2669206 | ||||||
| 18 | Rhizophora mucronata | Bakhaw babae | 21 | 6.684491979 | 0.8483 | 22.79772278 | 11.64852429 | 34.44624707 | ||||||
| 19 | Rhizophora mucronata | Bakhaw babae | 20.5 | 6.525337408 | 0.8483 | 21.48555129 | 11.04174331 | 32.5272946 | ||||||
| 20 | Rhizophora mucronata | Bakhaw babae | 10.5 | 3.342245989 | 0.8483 | 4.143407798 | 2.500255085 | 6.643662883 | ||||||
| 21 | Rhizophora mucronata | Bakhaw babae | 21.5 | 6.84364655 | 0.8483 | 24.15631114 | 12.27318997 | 36.4295011 | ||||||
| 22 | Rhizophora mucronata | Bakhaw babae | 14 | 4.456327986 | 0.8483 | 8.408276237 | 4.735308897 | 13.14358513 | ||||||
| 23 | Rhizophora mucronata | Bakhaw babae | 15.5 | 4.933791698 | 0.8483 | 10.80060245 | 5.935816207 | 16.73641866 | ||||||
| 24 | Rhizophora mucronata | Bakhaw babae | 10 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||||||
| 25 | Rhizophora mucronata | Bakhaw babae | 15 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 | ||||||
| 26 | Rhizophora mucronata | Bakhaw babae | 16.9 | 5.379424497 | 0.8483 | 13.36082565 | 7.192047707 | 20.55287336 | ||||||
| 27 | Rhizophora mucronata | Bakhaw babae | 12.2 | 3.88337153 | 0.8483 | 5.993454143 | 3.488694169 | 9.482148312 | ||||||
| 28 | Rhizophora mucronata | Bakhaw babae | 14 | 4.456327986 | 0.8483 | 8.408276237 | 4.735308897 | 13.14358513 | ||||||
| 29 | Rhizophora mucronata | Bakhaw babae | 10 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||||||
| 30 | Rhizophora mucronata | Bakhaw babae | 10.2 | 3.246753247 | 0.8483 | 3.858232774 | 2.344425609 | 6.202658383 | ||||||
| 31 | Rhizophora mucronata | Bakhaw babae | 16.6 | 5.283931755 | 0.8483 | 12.78491662 | 6.911686493 | 19.69660311 | ||||||
| 32 | Rhizophora mucronata | Bakhaw babae | 15.8 | 5.029284441 | 0.8483 | 11.32213782 | 6.193880303 | 17.51601813 | ||||||
| 33 | Rhizophora mucronata | Bakhaw babae | 15.5 | 4.933791698 | 0.8483 | 10.80060245 | 5.935816207 | 16.73641866 | ||||||
| 34 | Rhizophora mucronata | Bakhaw babae | 16 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 | ||||||
| 35 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 | ||||||
| 36 | Rhizophora mucronata | Bakhaw babae | 18 | 5.729564553 | 0.8483 | 15.60279369 | 8.272733549 | 23.87552723 | ||||||
| 37 | Rhizophora mucronata | Bakhaw babae | 19.7 | 6.270690094 | 0.8483 | 19.4813442 | 10.10785719 | 29.5892014 | ||||||
| 38 | Rhizophora mucronata | Bakhaw babae | 19.1 | 6.079704609 | 0.8483 | 18.05402697 | 9.437091167 | 27.49111813 | ||||||
| 39 | Rhizophora mucronata | Bakhaw babae | 16.2 | 5.156608098 | 0.8483 | 12.04034522 | 6.547377891 | 18.58772311 | ||||||
| 40 | Rhizophora mucronata | Bakhaw babae | 20.3 | 6.461675579 | 0.8483 | 20.97356501 | 10.80401751 | 31.77758253 | ||||||
| 41 | Rhizophora mucronata | Bakhaw babae | 14.9 | 4.742806213 | 0.8483 | 9.800996264 | 5.437729392 | 15.23872566 | ||||||
| 42 | Rhizophora mucronata | Bakhaw babae | 15 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 | ||||||
| 43 | Rhizophora mucronata | Bakhaw babae | 16.9 | 5.379424497 | 0.8483 | 13.36082565 | 7.192047707 | 20.55287336 | ||||||
| 44 | Rhizophora mucronata | Bakhaw babae | 19.4 | 6.175197352 | 0.8483 | 18.75962959 | 9.76931056 | 28.52894015 | ||||||
| 45 | Rhizophora mucronata | Bakhaw babae | 17 | 5.411255411 | 0.8483 | 13.55614951 | 7.28686428 | 20.84301379 | ||||||
| 46 | Rhizophora mucronata | Bakhaw babae | 17.8 | 5.665902725 | 0.8483 | 15.17977062 | 8.070054737 | 23.24982536 | ||||||
| 47 | Rhizophora mucronata | Bakhaw babae | 15 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 | ||||||
| 48 | Rhizophora mucronata | Bakhaw babae | 18.3 | 5.825057296 | 0.8483 | 16.25031127 | 8.581940408 | 24.83225167 | ||||||
| 49 | Rhizophora mucronata | Bakhaw babae | 19 | 6.047873695 | 0.8483 | 17.82238667 | 9.327753688 | 27.15014035 | ||||||
| 50 | Rhizophora mucronata | Bakhaw babae | 20.6 | 6.557168322 | 0.8483 | 21.74429671 | 11.16167325 | 32.90596995 | ||||||
| 51 | Rhizophora mucronata | Bakhaw babae | 17.8 | 5.665902725 | 0.8483 | 15.17977062 | 8.070054737 | 23.24982536 | ||||||
| 52 | Rhizophora mucronata | Bakhaw babae | 15 | 4.774637128 | 0.8483 | 9.963604971 | 5.51907976 | 15.48268473 | ||||||
| 53 | Rhizophora mucronata | Bakhaw babae | 17.2 | 5.47491724 | 0.8483 | 13.95185592 | 7.478547001 | 21.43040292 | ||||||
| 54 | Rhizophora mucronata | Bakhaw babae | 16 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 | ||||||
| 55 | Rhizophora mucronata | Bakhaw babae | 19.2 | 6.111535523 | 0.8483 | 18.2874447 | 9.54712927 | 27.83457397 | ||||||
| 56 | Rhizophora mucronata | Bakhaw babae | 20.3 | 6.461675579 | 0.8483 | 20.97356501 | 10.80401751 | 31.77758253 | ||||||
| 57 | Rhizophora mucronata | Bakhaw babae | 16.7 | 5.315762669 | 0.8483 | 12.97521379 | 7.004459705 | 19.9796735 | ||||||
| 58 | Rhizophora mucronata | Bakhaw babae | 15.4 | 4.901960784 | 0.8483 | 10.62999295 | 5.851134422 | 16.48112737 | ||||||
| 59 | Rhizophora mucronata | Bakhaw babae | 19 | 6.047873695 | 0.8483 | 17.82238667 | 9.327753688 | 27.15014035 | ||||||
| 60 | Rhizophora mucronata | Bakhaw babae | 15.3 | 4.87012987 | 0.8483 | 10.46099328 | 5.767120844 | 16.22811413 | ||||||
| 61 | Rhizophora mucronata | Bakhaw babae | 16.8 | 5.347593583 | 0.8483 | 13.16718194 | 7.097913145 | 20.26509508 | ||||||
| 62 | Rhizophora mucronata | Bakhaw babae | 16.4 | 5.220269926 | 0.8483 | 12.40931673 | 6.728177154 | 19.13749389 | ||||||
| 63 | Rhizophora mucronata | Bakhaw babae | 15.5 | 4.933791698 | 0.8483 | 10.80060245 | 5.935816207 | 16.73641866 | ||||||
| 64 | Rhizophora mucronata | Bakhaw babae | 18.9 | 6.016042781 | 0.8483 | 17.59251952 | 9.219116022 | 26.81163554 | ||||||
| 65 | Rhizophora mucronata | Bakhaw babae | 20.1 | 6.398013751 | 0.8483 | 20.46889059 | 10.56913202 | 31.03802261 | ||||||
| 66 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 | ||||||
| 67 | Rhizophora mucronata | Bakhaw babae | 15.9 | 5.061115355 | 0.8483 | 11.49923447 | 6.281244391 | 17.78047886 | ||||||
| 68 | Rhizophora mucronata | Bakhaw babae | 16.7 | 5.315762669 | 0.8483 | 12.97521379 | 7.004459705 | 19.9796735 | ||||||
| 69 | Rhizophora mucronata | Bakhaw babae | 20 | 6.366182837 | 0.8483 | 20.21928494 | 10.45275246 | 30.67203741 | ||||||
| 70 | Rhizophora mucronata | Bakhaw babae | 17 | 5.411255411 | 0.8483 | 13.55614951 | 7.28686428 | 20.84301379 | ||||||
| 71 | Rhizophora mucronata | Bakhaw babae | 14.9 | 4.742806213 | 0.8483 | 9.800996264 | 5.437729392 | 15.23872566 | ||||||
| 72 | Rhizophora mucronata | Bakhaw babae | 19.6 | 6.23885918 | 0.8483 | 19.23897538 | 9.994303962 | 29.23327934 | ||||||
| 73 | Rhizophora mucronata | Bakhaw babae | 21.8 | 6.939139292 | 0.8483 | 24.99395466 | 12.65661271 | 37.65056737 | ||||||
| 74 | Rhizophora mucronata | Bakhaw babae | 20.7 | 6.588999236 | 0.8483 | 22.00488246 | 11.28231555 | 33.28719802 | ||||||
| 75 | Rhizophora mucronata | Bakhaw babae | 24.2 | 7.703081232 | 0.8483 | 32.3160152 | 15.95931283 | 48.27532803 | ||||||
| 76 | Rhizophora mucronata | Bakhaw babae | 23 | 7.321110262 | 0.8483 | 28.51559776 | 14.25541521 | 42.77101297 | ||||||
| 77 | Rhizophora mucronata | Bakhaw babae | 25.1 | 7.98955946 | 0.8483 | 35.35325113 | 17.30691804 | 52.66016917 | ||||||
| 78 | Rhizophora mucronata | Bakhaw babae | 24.8 | 7.894066718 | 0.8483 | 34.32283387 | 16.85104377 | 51.17387764 | ||||||
| 79 | Rhizophora mucronata | Bakhaw babae | 22.7 | 7.22561752 | 0.8483 | 27.60931375 | 13.84590917 | 41.45522292 | ||||||
| 80 | Rhizophora mucronata | Bakhaw babae | 22.2 | 7.066462949 | 0.8483 | 26.1372758 | 13.17794463 | 39.31522043 | ||||||
| 81 | Rhizophora mucronata | Bakhaw babae | 21.7 | 6.907308378 | 0.8483 | 24.71285657 | 12.5280848 | 37.24094137 | ||||||
| 82 | Rhizophora mucronata | Bakhaw babae | 20.8 | 6.62083015 | 0.8483 | 22.26731267 | 11.40367099 | 33.67098366 | ||||||
| 83 | Rhizophora mucronata | Bakhaw babae | 25 | 7.957728546 | 0.8483 | 35.0077682 | 17.1542168 | 52.161985 | ||||||
| 84 | Rhizophora mucronata | Bakhaw babae | 24.3 | 7.734912147 | 0.8483 | 32.6455084 | 16.10608559 | 48.75159399 | ||||||
| 85 | Rhizophora mucronata | Bakhaw babae | 24.9 | 7.925897632 | 0.8483 | 34.66429701 | 17.00225893 | 51.66655595 | ||||||
| 86 | Rhizophora mucronata | Bakhaw babae | 20.9 | 6.652661064 | 0.8483 | 22.53159141 | 11.52574032 | 34.05733173 | ||||||
| 87 | Rhizophora mucronata | Bakhaw babae | 16.8 | 5.347593583 | 0.8483 | 13.16718194 | 7.097913145 | 20.26509508 | ||||||
| 88 | Rhizophora mucronata | Bakhaw babae | 16.4 | 5.220269926 | 0.8483 | 12.40931673 | 6.728177154 | 19.13749389 | ||||||
| 89 | Rhizophora mucronata | Bakhaw babae | 19.8 | 6.302521008 | 0.8483 | 19.72551594 | 10.22211583 | 29.94763177 | ||||||
| 90 | Rhizophora mucronata | Bakhaw babae | 20 | 6.366182837 | 0.8483 | 20.21928494 | 10.45275246 | 30.67203741 | ||||||
| 91 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 | ||||||
| 92 | Rhizophora mucronata | Bakhaw babae | 15.2 | 4.838298956 | 0.8483 | 10.29359862 | 5.683774517 | 15.97737314 | ||||||
| 93 | Rhizophora mucronata | Bakhaw babae | 18.3 | 5.825057296 | 0.8483 | 16.25031127 | 8.581940408 | 24.83225167 | ||||||
| 94 | Rhizophora mucronata | Bakhaw babae | 19.7 | 6.270690094 | 0.8483 | 19.4813442 | 10.10785719 | 29.5892014 | ||||||
| 95 | Rhizophora mucronata | Bakhaw babae | 16.5 | 5.25210084 | 0.8483 | 12.59628581 | 6.81959261 | 19.41587842 | ||||||
| 96 | Rhizophora mucronata | Bakhaw babae | 19.1 | 6.079704609 | 0.8483 | 18.05402697 | 9.437091167 | 27.49111813 | ||||||
| 97 | Rhizophora mucronata | Bakhaw babae | 18.7 | 5.952380952 | 0.8483 | 17.13808748 | 9.00393688 | 26.14202436 | ||||||
| 98 | Rhizophora mucronata | Bakhaw babae | 17.4 | 5.538579068 | 0.8483 | 14.35433753 | 7.672968345 | 22.02730588 | ||||||
| 99 | Rhizophora mucronata | Bakhaw babae | 15.6 | 4.965622613 | 0.8483 | 10.97282658 | 6.021167151 | 16.99399373 | ||||||
| 100 | Rhizophora mucronata | Bakhaw babae | 17.7 | 5.634071811 | 0.8483 | 14.9708424 | 7.969750518 | 22.94059292 | ||||||
| 101 | Rhizophora mucronata | Bakhaw babae | 19.9 | 6.334351923 | 0.8483 | 19.97149479 | 10.33708066 | 30.30857544 | ||||||
| 102 | Rhizophora mucronata | Bakhaw babae | 23.6 | 7.512095747 | 0.8483 | 30.38054292 | 15.0941521 | 45.47469501 | ||||||
| 103 | Rhizophora mucronata | Bakhaw babae | 22 | 7.00280112 | 0.8483 | 25.56182152 | 12.91583317 | 38.47765469 | ||||||
| 104 | Rhizophora mucronata | Bakhaw babae | 24.8 | 7.894066718 | 0.8483 | 34.32283387 | 16.85104377 | 51.17387764 | ||||||
| 105 | Rhizophora mucronata | Bakhaw babae | 18.6 | 5.920550038 | 0.8483 | 16.91351396 | 8.89739377 | 25.81090773 | ||||||
| 106 | Rhizophora mucronata | Bakhaw babae | 22.6 | 7.193786606 | 0.8483 | 27.31107304 | 13.7108636 | 41.02193663 | ||||||
| 107 | Rhizophora mucronata | Bakhaw babae | 15.3 | 4.87012987 | 0.8483 | 10.46099328 | 5.767120844 | 16.22811413 | ||||||
| 108 | Rhizophora mucronata | Bakhaw babae | 16.5 | 5.25210084 | 0.8483 | 12.59628581 | 6.81959261 | 19.41587842 | ||||||
| 109 | Rhizophora mucronata | Bakhaw babae | 23.7 | 7.543926662 | 0.8483 | 30.69820165 | 15.23650658 | 45.93470823 | ||||||
| 110 | Rhizophora mucronata | Bakhaw babae | 25.2 | 8.021390374 | 0.8483 | 35.70074949 | 17.4603633 | 53.16111279 | ||||||
| 111 | Rhizophora mucronata | Bakhaw babae | 18 | 5.729564553 | 0.8483 | 15.60279369 | 8.272733549 | 23.87552723 | ||||||
| 112 | Rhizophora mucronata | Bakhaw babae | 23 | 7.321110262 | 0.8483 | 28.51559776 | 14.25541521 | 42.77101297 | ||||||
| 113 | Rhizophora mucronata | Bakhaw babae | 24.7 | 7.862235803 | 0.8483 | 33.98337504 | 16.70057067 | 50.68394571 | ||||||
| 114 | Rhizophora mucronata | Bakhaw babae | 20.1 | 6.398013751 | 0.8483 | 20.46889059 | 10.56913202 | 31.03802261 | ||||||
| 115 | Rhizophora mucronata | Bakhaw babae | 23 | 7.321110262 | 0.8483 | 28.51559776 | 14.25541521 | 42.77101297 | ||||||
| 116 | Rhizophora mucronata | Bakhaw babae | 19.4 | 6.175197352 | 0.8483 | 18.75962959 | 9.76931056 | 28.52894015 | ||||||
| 117 | Rhizophora mucronata | Bakhaw babae | 18.3 | 5.825057296 | 0.8483 | 16.25031127 | 8.581940408 | 24.83225167 | ||||||
| 118 | Rhizophora mucronata | Bakhaw babae | 22.7 | 7.22561752 | 0.8483 | 27.60931375 | 13.84590917 | 41.45522292 | ||||||
| 119 | Rhizophora mucronata | Bakhaw babae | 24.6 | 7.830404889 | 0.8483 | 33.64591681 | 16.55083896 | 50.19675578 | ||||||
| 120 | Rhizophora mucronata | Bakhaw babae | 20.1 | 6.398013751 | 0.8483 | 20.46889059 | 10.56913202 | 31.03802261 | ||||||
| 121 | Rhizophora mucronata | Bakhaw babae | 16.8 | 5.347593583 | 0.8483 | 13.16718194 | 7.097913145 | 20.26509508 | ||||||
| 122 | Rhizophora mucronata | Bakhaw babae | 19.7 | 6.270690094 | 0.8483 | 19.4813442 | 10.10785719 | 29.5892014 | ||||||
| 123 | Rhizophora mucronata | Bakhaw babae | 24.9 | 7.925897632 | 0.8483 | 34.66429701 | 17.00225893 | 51.66655595 | ||||||
| 124 | Rhizophora mucronata | Bakhaw babae | 25 | 7.957728546 | 0.8483 | 35.0077682 | 17.1542168 | 52.161985 | ||||||
| 125 | Rhizophora mucronata | Bakhaw babae | 15.2 | 4.838298956 | 0.8483 | 10.29359862 | 5.683774517 | 15.97737314 | ||||||
| 126 | Rhizophora mucronata | Bakhaw babae | 17.8 | 5.665902725 | 0.8483 | 15.17977062 | 8.070054737 | 23.24982536 | ||||||
| 127 | Rhizophora mucronata | Bakhaw babae | 21.6 | 6.875477464 | 0.8483 | 24.4336434 | 12.40027746 | 36.83392086 | ||||||
| 128 | Rhizophora mucronata | Bakhaw babae | 24.6 | 7.830404889 | 0.8483 | 33.64591681 | 16.55083896 | 50.19675578 | ||||||
| 129 | Rhizophora mucronata | Bakhaw babae | 23.8 | 7.575757576 | 0.8483 | 31.01782331 | 15.37959574 | 46.39741905 | ||||||
| 130 | Rhizophora mucronata | Bakhaw babae | 19.8 | 6.302521008 | 0.8483 | 19.72551594 | 10.22211583 | 29.94763177 | ||||||
| 131 | Rhizophora mucronata | Bakhaw babae | 22.3 | 7.098293863 | 0.8483 | 26.42785811 | 13.3100863 | 39.73794441 | ||||||
| 132 | Rhizophora mucronata | Bakhaw babae | 16.9 | 5.379424497 | 0.8483 | 13.36082565 | 7.192047707 | 20.55287336 | ||||||
| 133 | Rhizophora mucronata | Bakhaw babae | 26 | 8.276037688 | 0.8483 | 38.55373335 | 18.71478783 | 57.26852118 | ||||||
| 134 | Rhizophora mucronata | Bakhaw babae | 22.4 | 7.130124777 | 0.8483 | 26.72034914 | 13.44295287 | 40.16330201 | ||||||
| 135 | Rhizophora mucronata | Bakhaw babae | 24.8 | 7.894066718 | 0.8483 | 34.32283387 | 16.85104377 | 51.17387764 | ||||||
| 136 | Rhizophora mucronata | Bakhaw babae | 17 | 5.411255411 | 0.8483 | 13.55614951 | 7.28686428 | 20.84301379 | ||||||
| 137 | Rhizophora mucronata | Bakhaw babae | 15.2 | 4.838298956 | 0.8483 | 10.29359862 | 5.683774517 | 15.97737314 | ||||||
| 138 | Rhizophora mucronata | Bakhaw babae | 23.7 | 7.543926662 | 0.8483 | 30.69820165 | 15.23650658 | 45.93470823 | ||||||
| 139 | Rhizophora mucronata | Bakhaw babae | 26.7 | 8.498854087 | 0.8483 | 41.15756818 | 19.85176272 | 61.0093309 | ||||||
| 140 | Rhizophora mucronata | Bakhaw babae | 18.8 | 5.984211867 | 0.8483 | 17.36442123 | 9.111177358 | 26.47559859 | ||||||
| 141 | Rhizophora mucronata | Bakhaw babae | 15.9 | 5.061115355 | 0.8483 | 11.49923447 | 6.281244391 | 17.78047886 | ||||||
| 142 | Rhizophora mucronata | Bakhaw babae | 23.3 | 7.416603005 | 0.8483 | 29.43930609 | 14.67148993 | 44.11079602 | ||||||
| 143 | Rhizophora mucronata | Bakhaw babae | 18.1 | 5.761395467 | 0.8483 | 15.8168974 | 8.375109839 | 24.19200724 | ||||||
| 144 | Rhizophora mucronata | Bakhaw babae | 16 | 5.092946269 | 0.8483 | 11.67796477 | 6.3692814 | 18.04724617 | ||||||
| 145 | Rhizophora mucronata | Bakhaw babae | 18 | 5.729564553 | 0.8483 | 15.60279369 | 8.272733549 | 23.87552723 | ||||||
| 146 | Rhizophora mucronata | Bakhaw babae | 19.8 | 6.302521008 | 0.8483 | 19.72551594 | 10.22211583 | 29.94763177 | ||||||
| 147 | Rhizophora mucronata | Bakhaw babae | 20.5 | 6.525337408 | 0.8483 | 21.48555129 | 11.04174331 | 32.5272946 | ||||||
| 148 | Rhizophora mucronata | Bakhaw babae | 23.6 | 7.512095747 | 0.8483 | 30.38054292 | 15.0941521 | 45.47469501 | ||||||
| 149 | Rhizophora mucronata | Bakhaw babae | 35 | 11.14081996 | 0.8483 | 80.10126827 | 36.20554871 | 116.306817 | ||||||
| 150 | Rhizophora mucronata | Bakhaw babae | 37.6 | 11.96842373 | 0.8483 | 95.54195021 | 42.44837712 | 137.9903273 | ||||||
| 151 | Rhizophora mucronata | Bakhaw babae | 11 | 3.50140056 | 0.8483 | 4.645773248 | 2.772271986 | 7.418045234 | ||||||
| 152 | Rhizophora mucronata | Bakhaw babae | 73 | 23.23656735 | 0.8483 | 488.6593796 | 185.1487672 | 673.8081468 | ||||||
| 153 | Rhizophora mucronata | Bakhaw babae | 74 | 23.5548765 | 0.8483 | 505.291548 | 190.8264157 | 696.1179638 | ||||||
| 154 | Rhizophora mucronata | Bakhaw babae | 32.5 | 10.34504711 | 0.8483 | 66.75212126 | 30.71320487 | 97.46532613 | ||||||
| 155 | Rhizophora mucronata | Bakhaw babae | 62 | 19.73516679 | 0.8483 | 326.9757612 | 128.8408633 | 455.8166245 | ||||||
| 156 | Rhizophora mucronata | Bakhaw babae | 55.0 | 17.5070028 | 0.8483 | 243.5141598 | 98.75276082 | 342.2669206 | ||||||
| 157 | Rhizophora mucronata | Bakhaw babae | 62.5 | 19.89432136 | 0.8483 | 333.5007739 | 131.1588843 | 464.6596582 | ||||||
| 158 | Rhizophora mucronata | Bakhaw babae | 37.2 | 11.84110008 | 0.8483 | 93.06098296 | 41.45237342 | 134.5133564 | ||||||
| 159 | Rhizophora mucronata | Bakhaw babae | 10.0 | 3.183091418 | 0.8483 | 3.674785579 | 2.243592995 | 5.918378573 | ||||||
| 160 | Rhizophora mucronata | Bakhaw babae | 57.0 | 18.14362108 | 0.8483 | 265.8790683 | 106.9021045 | 372.7811728 | ||||||
| 161 | Rhizophora mucronata | Bakhaw babae | 46.6 + 49.2 | 15.24700789 | 0.8483 | 173.3234665 | 72.65891613 | 245.9823826 | ||||||
| 162 | Rhizophora mucronata | Bakhaw babae | 25.0 | 7.957728546 | 0.8483 | 35.0077682 | 17.1542168 | 52.161985 | ||||||
| 163 | Rhizophora mucronata | Bakhaw babae | 59.4 | 18.90756303 | 0.8483 | 294.2704531 | 117.1520744 | 411.4225276 | ||||||
| 164 | Rhizophora mucronata | Bakhaw babae | 11.0 | 3.50140056 | 0.8483 | 4.645773248 | 2.772271986 | 7.418045234 | ||||||
| 165 | Rhizophora mucronata | Bakhaw babae | 53.5 | 17.02953909 | 0.8483 | 227.5004747 | 92.87299935 | 320.373474 | ||||||
| 166 | Rhizophora mucronata | Bakhaw babae | 70.7 | 22.50445633 | 0.8483 | 451.6518165 | 172.4468135 | 624.0986301 | ||||||
| 167 | Rhizophora mucronata | Bakhaw babae | 40.4 | 12.85968933 | 0.8483 | 114.006644 | 49.78639791 | 163.7930419 | ||||||
| 168 | Rhizophora mucronata | Bakhaw babae | 42.0 | 13.36898396 | 0.8483 | 125.4368842 | 54.26970989 | 179.7065941 | ||||||
| 169 | Rhizophora mucronata | Bakhaw babae | 51.2 | 16.29742806 | 0.8483 | 204.1907702 | 84.24097577 | 288.431746 | ||||||
| 170 | Rhizophora mucronata | Bakhaw babae | 22.4 | 7.130124777 | 0.8483 | 26.72034914 | 13.44295287 | 40.16330201 | ||||||
| 171 | Rhizophora mucronata | Bakhaw babae | 33.7 | 10.72701808 | 0.8483 | 72.97961358 | 33.28759779 | 106.2672114 | ||||||
| 172 | Rhizophora mucronata | Bakhaw babae | 47.0 | 14.96052967 | 0.8483 | 165.4217968 | 69.66286313 | 235.08466 | ||||||
| 173 | Rhizophora mucronata | Bakhaw babae | 55.9 | 17.79348103 | 0.8483 | 253.4341007 | 102.3760311 | 355.8101318 | ||||||
| 174 | Rhizophora mucronata | Bakhaw babae | 42.0 | 13.36898396 | 0.8483 | 125.4368842 | 54.26970989 | 179.7065941 | ||||||
| 175 | Rhizophora mucronata | Bakhaw babae | 41.4 | 13.17799847 | 0.8483 | 121.0745441 | 52.56356743 | 173.6381116 | ||||||
| 176 | Rhizophora mucronata | Bakhaw babae | 60.0 | 19.09854851 | 0.8483 | 301.6366295 | 119.7953198 | 421.4319493 | ||||||
| 177 | Rhizophora mucronata | Bakhaw babae | 57.1 | 18.175452 | 0.8483 | 267.0280164 | 107.3189057 | 374.3469221 | ||||||
| 178 | Rhizophora mucronata | Bakhaw babae | 58.0 | 18.46193023 | 0.8483 | 277.5012013 | 111.1102746 | 388.6114759 | ||||||
| 179 | Rhizophora mucronata | Bakhaw babae | 64.7 | 20.59460148 | 0.8483 | 363.1253211 | 141.628801 | 504.7541221 | ||||||
| 180 | Rhizophora mucronata | Bakhaw babae | 49.2 | 15.66080978 | 0.8483 | 185.1254623 | 77.1092721 | 262.2347344 | ||||||
| 181 | Rhizophora mucronata | Bakhaw babae | 75.6 | 24.06417112 | 0.8483 | 532.5932038 | 200.107083 | 732.7002868 | ||||||
| 182 | Rhizophora mucronata | Bakhaw babae | 58.0 | 18.46193023 | 0.8483 | 277.5012013 | 111.1102746 | 388.6114759 | ||||||
| 183 | Rhizophora mucronata | Bakhaw babae | 65.2 + 70.7 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||||||
| 184 | Rhizophora mucronata | Bakhaw babae | 98.5 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||||||
| 185 | Rhizophora mucronata | Bakhaw babae | 61.0 | 19.41685765 | 0.8483 | 314.1545814 | 124.2728645 | 438.4274459 | ||||||
| 186 | Rhizophora mucronata | Bakhaw babae | 28.0 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||||||
| 187 | Rhizophora mucronata | Bakhaw babae | 68.0 | 21.64502165 | 0.8483 | 410.3967649 | 158.1662659 | 568.5630307 | ||||||
| 188 | Rhizophora mucronata | Bakhaw babae | 19.0 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||||||
| 189 | Rhizophora mucronata | Bakhaw babae | 33.0 | 10.50420168 | 0.8483 | 69.30687152 | 31.77203423 | 101.0789058 | ||||||
| 190 | Rhizophora mucronata | Bakhaw babae | 40.0 | 21.62910619 | 0.8483 | 409.654828 | 157.9081985 | 567.5630265 | ||||||
| Total Biomass of Rhizophora mucronata (kg) | Total Biomass (t/ha) | C. Stored (tC/ha) | CO2 Sequestered (tCO2/ha) | |||||||||||
| 7.031091513 | 70.31091513 | 35.15545757 | 128.9150629 | |||||||||||
| Mangrove Species | Site 1 | Site 2 | Site 3 | Total No. of Species | Total Occurrences (%) |
|---|---|---|---|---|---|
| Acanthaceae | |||||
| Avicennia alba | 15 | 8 | 1 | 24 | 9.38 |
| Meliaceae | |||||
| Xylocarpus granatum | - | 14 | - | 14 | 5.47 |
| Rhizophoraceae | |||||
| Rhizophora mucronata | 6 | 170 | 42 | 218 | 85.16 |
Appendix G. Statistical Treatment
| Parameters | November | December | January | |||
| p-value | Remarks | p-value | Remarks | p-value | Remarks | |
| Temperature | 0.023 | S | 0.066 | NS | 0.038 | S |
| Turbidity | 0.018 | S | 0.018 | S | 0.018 | S |
| TDS | 0.414 | NS | 0.027 | S | 0.027 | S |
| Salinity | 0.414 | NS | 0.027 | S | 0.027 | S |
| Conductivity | 0.414 | NS | 0.027 | S | 0.027 | S |
| pH | 0.026 | S | 0.106 | NS | 0.026 | S |
| DO | 0.026 | S | 0.063 | NS | 0.053 | NS |
| Parameters | February | March | ||||
| p-value | Remarks | p-value | Remarks | |||
| Temperature | 0.054 | NS | 0.193 | NS | ||
| Turbidity | 0.018 | S | 0.018 | S | ||
| TDS | 0.05 | S | 0.027 | S | ||
| Salinity | 0.05 | S | 0.027 | S | ||
| Conductivity | 0.05 | S | 0.027 | S | ||
| pH | 0.027 | S | 0.063 | NS | ||
| DO | 0.048 | S | 0.063 | NS | ||
| Parameters | SITE 1 | SITE 2 | SITE 3 | ||||
|---|---|---|---|---|---|---|---|
| p - value | Remarks | p - value | Remarks | p - value | Remarks | ||
| Temperature | 0.031 | S | 0.189 | NS | 0.017 | S | |
| Turbidity | 0.017 | S | 0.017 | S | 0.017 | S | |
| TDS | 0.017 | S | 0.022 | S | 0.017 | S | |
| Salinity | 0.017 | S | 0.022 | S | 0.017 | S | |
| Conductivity | 0.017 | S | 0.022 | S | 0.017 | S | |
| pH | 0.022 | S | 0.017 | S | 0.017 | S | |
| DO | 0.018 | S | 0.017 | S | 0.022 | S | |
| Nitrate | 0.406 | NS | 0.406 | NS | 0.406 | NS | |
| Phosphate | 0.406 | NS | 0.406 | NS | 0.406 | NS | |
| Parameters | p - value | Remarks |
|---|---|---|
| Soil Temperature | 0.398 | Not Significant |
| Soil pH | 1.000 | Not Significant |
| Organic Matter | 0.008 | Significant |
| Organic Carbon | 0.008 | Significant |
| Water Holding Capacity | 0.027 | Significant |
| Nitrogen | 0.135 | Not Significant |
| Phosphorus | 0.368 | Not Significant |
| Potassium | 0.323 | Not Significant |
| Parameters | p - value | Remarks |
|---|---|---|
| Soil Temperature | 0.137 | Not Significant |
| Soil pH | 1.000 | Not Significant |
| Organic Matter | 0.308 | Not Significant |
| Organic Carbon | 0.308 | Not Significant |
| Water Holding Capacity | 0.339 | Not Significant |
| Nitrogen | 0.406 | Not Significant |
| Phosphorus | 0.406 | Not Significant |
| Potassium | 0.322 | Not Significant |
Appendix H. Species Diversity Computation
| Species | n | pi | In(pi) | PiInPi | |
|---|---|---|---|---|---|
| Site 1 | |||||
| Avicennia alba | 15 | 0.71429 | -0.33647 | -0.24034 | |
| Xylocarpus granatum | 0 | 0 | 0 | 0 | |
| Rhizophora mucronata | 6 | 0.28571 | -1.25276 | -0.35793 | |
| Total | 21 | ||||
| Shannon-Weiner (H) | 0.5983 | ||||
| Shannon-Weiner Evenness (E) | 0.1994 | ||||
| Species Richness(S) | 2 | ||||
| Site 2 | |||||
| Avicennia alba | 8 | 0.04167 | -3.17805 | -0.13242 | |
| Xylocarpus granatum | 14 | 0.07292 | -2.61844 | -0.19093 | |
| Rhizophora mucronata | 170 | 0.88542 | -0.12170 | -0.10775 | |
| Total | 192 | ||||
| Shannon-Weiner (H) | 0.4311 | ||||
| Shannon-Weiner Evenness (E) | 0.1437 | ||||
| Species Richness (S) | 3 | ||||
| Site 3 | |||||
| Avicennia alba | 1 | 0.02326 | -3.76120 | -0.08747 | |
| Xylocarpus granatum | 0 | 0 | 0 | 0 | |
| Rhizophora mucronata | 42 | 0.97674 | -0.02353 | -0.02298 | |
| Total | 43 | ||||
| Shannon-Weiner (H) | 0.1105 | ||||
| Shannon-Weiner Evenness (E) | 0.0368 | ||||
| Species Richness (S) | 2 | ||||
| Site 1 Species | n | n-1 | n(n-1) | Dominance (C) |
Diversity (1-D) |
Reciprocal (1/D) |
| Avicennia alba | 15 | 14 | 210 | |||
| Xylocarpus granatum | 0 | 5 | 30 | 0.5714 | 0.4286 | 1.750 |
| Rhizophora mucronata | 6 | 0 | 40 | |||
| Site 2 Species | n | n-1 | n(n-1) | Dominance (C) |
Diversity (1-D) |
Reciprocal (1/D) |
| Avicennia alba | 8 | 7 | 56 | |||
| Xylocarpus granatum | 14 | 169 | 28730 | 0.7899 | 0.2101 | 1.266 |
| Rhizophora mucronata | 170 | 13 | 182 | |||
| Site 3 Species | n | n-1 | n(n-1) | Dominance (C) | Diversity (1-D) |
Reciprocal (1/D) |
| Avicennia alba | 1 | 0 | 0 | |||
| Xylocarpus granatum | 0 | 41 | 1722 | 0.9318 | 0.06818 | 1.073 |
| Rhizophora mucronata | 42 | 0 | 1722 |
Appendix I. Photo-documentations
| Local Name | Family Name | Scientific Name | No. of Trees per Species |
|---|---|---|---|
| Bakhaw Babae | Rhizophoreceae | Rhizophora mucronata Poir | 24 |
| Bungalon | Acanthaceae | Avicennia alba Blume | 14 |
| Tabigi | Meliaceae | Xylocarpus granatum J.Koenig | 218 |
| Banago | Malvaceae | Thespesia populneoides (Roxb.) Kostel. | 1 |












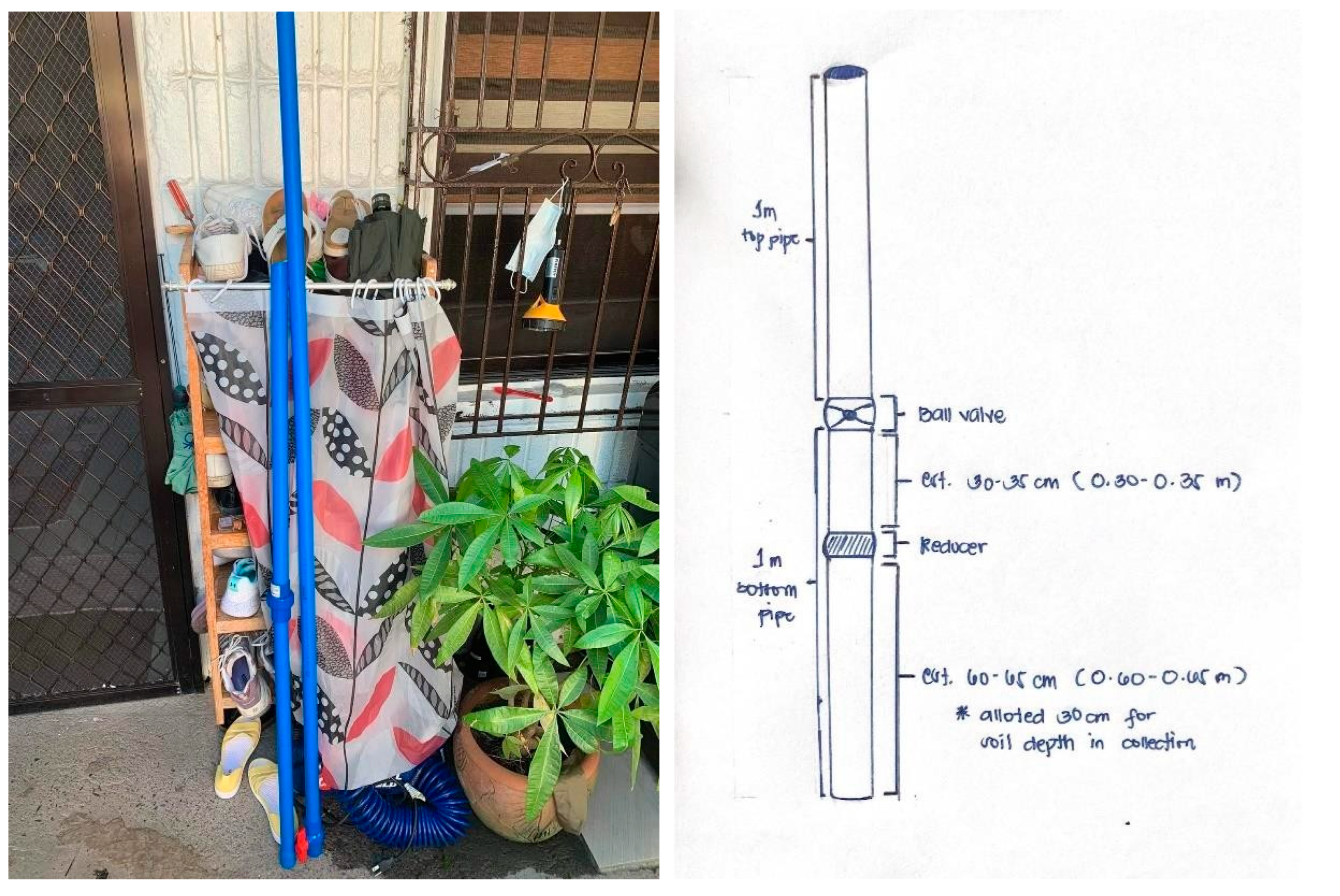
Appendix J. Letters and Certifications



















Appendix K. Line of Budget
| Unit (PHP) | Quantity | Total (PHP) | ||
| I. | Material/s | |||
| Life Vest | 200 | 5 pcs | 1000 | |
| Nylon Rope | 200 | 2 pcs | 400 | |
| Sola Bottle | 21 | 2 pcs | 42 | |
| Electrical Tape | 110 | 1 pc | 110 | |
| Black Cartolina | 25 | 2 pcs | 50 | |
| Waterproof Phone Case | 150 | 5 pcs | 750 | |
| Field Tape Measure | 250 | 2 pcs | 500 | |
| Meter Stick | 42 | 1 pc | 42 | |
| Zip Lock Bags | 88 | 1 pack | 88 | |
| Masking Tape | 30 | 3 pcs | 90 | |
| Clear Packaging Tape | 100 | 1 pc | 100 | |
| Raincoat | 100 | 5 pcs | 500 | |
| Pail | 26 | 1 pc | 26 | |
| Clearbook | 53 | 2 pcs | 106 | |
| Metal Soil Probe | 877 | 1 pc | 877 | |
| DIY Soil Probe (PVC) | 676 | 1 pc | 676 | |
| Field Guide Book | 700 | 5 pcs | 3500 | |
| Binder Clip | 228/box | 2 boxes | 456 | |
| Folder | 7 | 7 pcs | 49 | |
| Puncher | 190 | 1 pc | 190 | |
| Expanding Folder | 252 | 1 set | 252 | |
| Fastener | 59 | 7 pcs | 49 | |
| SUBTOTAL | 9853 | |||
| II. | Acquired In-Situ Device and Other Equipment | |||
| DO Analyzer | 3365 | 1 | 3365 | |
| Salinity Refractor | 537 | 1 | 537 | |
| Portable pH Meter with TDS | 774 | 1 | 774 | |
| 5-in-1 pH Meter Water Quality Tester | 1560 | 1 | 1560 | |
| BSWM Soil Test Kit | 1500 | 1 | 1500 | |
| SUBTOTAL | 7736 | |||
| III. | Laboratory Expenses | |||
| Mangrove Species ID | 200 | 7 | 1400 | |
| 1st Soil Parameter Testing BSWM | 2250 | 1 | 2250 | |
| 2nd Soil Parameter Testing BSWM | 2250 | 1 | 2250 | |
| 3rd Soil Parameter Testing BSWM | 2250 | 1 | 2250 | |
| 4th Soil Parameter Testing BSWM | 2250 | 1 | 2250 | |
| 5th Soil Parameter Testing BSWM | 2250 | 1 | 2250 | |
| Laboratory Expenses for Nitrates (Mach Union Laboratories Inc.) | 2500 | 1 | 2500 | |
| Laboratory Expenses for Phosphates (Mach Union Laboratories Inc.) | 2500 | 1 | 2500 | |
| SUBTOTAL | 17650 | |||
| IV. | Transportation | |||
| 1st Ocular Inspection | 350 | 2 | 700 | |
| 2nd Ocular Inspection | 300 | 3 | 900 | |
| 3rd Ocular Inspection | 300 | 5 | 1500 | |
| November Sampling | 300 | 5 | 1500 | |
| December Sampling | 300 | 5 | 1500 | |
| January Sampling | 300 | 5 | 1500 | |
| February Sampling | 300 | 5 | 1500 | |
| March Sampling | 300 | 5 | 1500 | |
| Total Travel Expense for Gratuitous Permit (4 days) | 300/day | 3 | 3600 | |
| January Thesis Survey | 300 | 5 | 1500 | |
| February Thesis Survey | 300 | 5 | 1500 | |
| Boat Rent (November Sampling) | 2000 | 1 | 2000 | |
| Boat Rent (December Sampling) | 2000 | 1 | 2000 | |
| Boat Rent (January Sampling) | 2000 | 1 | 2000 | |
| Boat Rent (February Sampling) | 2000 | 1 | 2000 | |
| Boat Rent (March Sampling) | 2000 | 1 | 2000 | |
| Travel expense to BSWM Laboratory (November) | 250 | 1 | 250 | |
| Travel expense to BSWM Laboratory (December) | 250 | 1 | 250 | |
| Travel expense to BSWM Laboratory (January) | 250 | 1 | 250 | |
| Travel expense to BSWM Laboratory (February) | 250 | 2 | 500 | |
| Travel expense to BSWM Laboratory (March) | 250 | 1 | 250 | |
| Travel expense to BSWM Laboratory (April) | 250 | 1 | 250 | |
| Travel expense to BSWM Laboratory (May) | 250 | 2 | 500 | |
| June Travel Expenses | 4800 | 4 | 19200 | |
| July Travel Expenses | 4800 | 4 | 19200 | |
| SUBTOTAL | 67850 | |||
| V. | Other Expenses | Unit (PHP) | Quantity | Total (PHP) |
| TURNITIN Account | 300 | 1 | 300 | |
| Gratuitous Permit | 200 | 1 | 200 | |
| November Printing Expenses | 470 | 1 | 470 | |
| December Printing Expenses | 585 | 1 | 585 | |
| January Printing Expenses | 380 | 1 | 380 | |
| February Printing Expenses | 440 | 1 | 440 | |
| March Printing Expenses | 270 | 1 | 270 | |
| April Printing Expenses | 300 | 1 | 300 | |
| May Printing Expenses | 2574 | 1 | 2574 | |
| Food for Panel | 143 | 5 | 715 | |
| June Printing Expense | 2500 | 1 | 2500 | |
| July Printing Expense (For book bind) | 800 | 10 | 8000 | |
| Grammarian | 1500 | 1 | 1500 | |
| Notary | 50 | 4 | 200 | |
| Book bind | 250 | 10 | 2500 | |
| Tarpaulin Printing | 300 | 1 | 300 | |
| CD Burn | 80 | 5 | 400 | |
| SUBTOTAL | 21634 | |||
| TOTAL | PHP 124,723.00 |
References
- Abino, A. C., Castillo, J. A. A., & Lee, Y. J. (2013). Assessment of species diversity, biomass and carbon sequestration potential of a natural mangrove stand in Samar, the Philippines. Forest Science and Technology, 10(1), 2–8. [CrossRef]
- Ahmed, S., Sarker, S. K., Friess, D. A., Kamruzzaman, N. M., Jacobs, M. A., Islam, A., Alam, A., Suvo, M. S. H., Sani, N. H., Dey, T. K., Naabeh, C. S. S., & Pretzsch, H. (2022). Salinity reduces site quality and mangrove forest functions. From monitoring to understanding. Science of the Total Environment, 853, 158662. [CrossRef]
- Alhassan, A. B., & Aljahdali, M. O. (2021). Nutrient and physicochemical properties as potential causes of stress in mangroves of the central Red Sea. PLOS ONE, 16(12), e0261620. [CrossRef]
- Alongi, D. M. (2014). Carbon Cycling and Storage in Mangrove Forests. Annual Review of Marine Science, 6(1), 195–219. [CrossRef]
- Alsumaiti, T. S., & Shahid, S. A. (2018). Comprehensive analysis of mangrove soil in Eastern Lagoon National Park of Abu Dhabi Emirate. International Journal of Business and Applied Social Science, 4(5), 39-56. https://nbnresolving.org/urn:nbn:de:0168-ssoar-57449-3.
- Arnaud, M., Baird, A. J., Morris, P. J., Dang, T. H., & Nguyen, T. T. (2020). Sensitivity of mangrove soil organic matter decay to warming and sea level change. Global Change Biology, 26(3), 1899–1907. [CrossRef]
- Baird, R. B., Eaton, A. D., & Rice, E. W. (Eds.). (2017, June 15). Standard methods for the examination of water and wastewater. Standard Methods for the Examination of Water and Wastewater. https://www.standardmethods.org/doi/abs/10.2105/SMWW.2882.
- Basyuni, M., Putri, L. a. P., Nainggolan, B., & Sihaloho, P. K. (2014). Growth and biomass in response to salinity and subsequent fresh water in mangrove seedlings Avicennia marina and Rhizophora stylosa. Jurnal Manajemen Hutan Tropika, 20(1), 17–25. [CrossRef]
- Basyuni, M., Ramayani, Hayullah, A., Prayunita, Hamka, M., Putri, L. A. P., & Baba, S. (2019). Growth of salt-secretor and non-salt secretor mangrove seedlings with varying salinity and their relations to habitat zonation. IOP Conference Series. [CrossRef]
- Bayabil, H. K., Li, Y., Tong, Z., & Gao, B. (2021). Potential management practices of saltwater intrusion impacts on soil health and water quality: a review. Journal of Water and Climate Change, 12(5), 1327–1343. [CrossRef]
- Bonner, M. T., Herbohn, J., Gregorio, N., Pasa, A., Avela, M. S., Solano, C., Moreno, M.O. M., Almendras-Ferraren, A., Wills, J., Shoo, L. P., & Schmidt, S. (2019). Soil organic carbon recovery in tropical tree plantations may depend on restoration of soil microbial composition and function. Geoderma, 353, 70– 80. [CrossRef]
- Cañizares, L.P. & Seronay, R.A. (2016). Diversity and species composition of mangroves in Barangay Imelda, Dinagat Island, Philippines. Volume 9, Issue 3. AACL Bioflux. 9(3):518-526. Retrieved on June 1, 2022, from http://www.bioflux.com.ro/docs/2016.518-527.pdf.
- Carnell, P. E., Palacios, M. M., Waryszak, P., Trevathan-Tackett, S. M., Masqué, P., & Macreadie, P. I. (2022). Blue carbon drawdown by restored mangrove forests improves with age. Research Online. https://ro.ecu.edu.au/ecuworks2022-2026/136/.
- Chen, G., & Fang, X. (2015). Accuracy of Hourly Water Temperatures in Rivers Calculated from Air Temperatures. Water, 7(12), 1068–1087. [CrossRef]
- Chen, S., Xie, J., & Wen, Z. (2021). Microalgae-based wastewater treatment and utilization of microalgae biomass. Elsevier EBooks, 165–198. [CrossRef]
- Chen, H., Hsu, L., Huang, S., & Liao, X. (2020). Assessment of the Components and Sources of Acid Deposition in Northeast Asia: A Case Study of the Coastal and Metropolitan Cities in Northern Taiwan. Atmosphere, 11(9), 983. [CrossRef]
- Chen, Z., Lum, S., & Srikanth, S. (2015). Mangrove root: Adaptations and ecological importance. Trees, 30(2), 451–465. [CrossRef]
- Chorchuhirun, B. (2020). Comparative Anatomy of Two Mangrove Species, Xylocarpus granatum and Xylocarpus moluccensis (Meliaceae). Li01.tci-thaijo.org. [CrossRef]
- Chunkao, K., Jitthaisong, O., Dhanmanonda, P., & Teejuntuk, S. (2012). Water quality from mangrove forest: The King’s Royally Initiated Laem Phak Bia Environmental Research and Development Project, Phetchaburi Province, Thailand. Modern Applied Science, 6(8). [CrossRef]
- Christina Curell, Michigan State University Extension. (2022, January 21). Why is soil water holding capacity important? MSU Extension. Retrieved October 10, 2022, from https://www.canr.msu.edu/news/why_is_soil_water_holding_capacity_impo rtant.
- Collins, M., Knutti, R., Arblaster, J., Dufresne, J.-L., Fichefet, T., Friedlingstein, P., et al. (2013). Long-term climate change, projections, commitments and irreversibility in Climate Change 2013, The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley. New York, NY: Cambridge University Press, 1029–1136.
- Dissolved Oxygen. (2023, May 18). US EPA. https://www.epa.gov/caddis-vol2/dissolved- oxygen#checklist.
- Dolorosa, R. G., Dangan-Galon, F., Mendoza, N. I., & Sespeñe, J. S. (2016). Diversity and structural complexity of mangrove forest along Puerto Princesa Bay, Palawan Island, Philippines. Journal of Marine and Island Cultures, 5(2), 118–125. [CrossRef]
- Dror, I., Yaron, B., & Berkowitz, B. (2021). The Human Impact on All Soil-Forming Factors during the Anthropocene. ACS Environmental Au. [CrossRef]
- Easton, Z., & Bock, E. (2016). Soil and soil water relationships. VCE publication. https://ext.vt.edu/content/dam/ext_vt_edu/topics/agriculture/water/document s/Soil-and-Soil-Water-Relationships.pdf.
- Feller, C. (2018). Mangroves. Smithsonian Ocean. https://ocean.si.edu/ocean-life/plants- algae/mangroves.
- Fukumasu, J., Jarvis, N., Koestel, J., Kätterer, T., & Larsbo, M. (2022). Relations between soil organic carbon content and the pore size distribution for an arable topsoil with large variations in soil properties. European Journal of Soil Science, 73(1). [CrossRef]
- Gangopadhyay, S., Bhattacharyya, T., Mishra, T. K., & Banerjee, S. (2021). Organic carbon stock in the forest soils of Himalayas and other areas in India. Forest Resources Resilience and Conflicts, 93–116. [CrossRef]
- Garcia, K., Malabrigo, P. L., & Gevaña, D. T. (2014). Philippines’ Mangrove Ecosystem: Status, Threats and Conservation. In Springer eBooks (pp. 81–94). [CrossRef]
- Geng, Y., Baumann, F., Song, C., Zhang, M., Shi, Y., Kuhn, P., Scholten, T., & He, J. (2017). Increasing temperature reduces the coupling between available nitrogen and phosphorus in soils of Chinese grasslands. Scientific Reports, 7(1). [CrossRef]
- Ghosh, A., Mukherjee, S., & Sarkar, N. S. (2018). Influence of soil texture on nature of mangrove vegetation in Sundarbans Tiger Reserve forest of India. International Journal of Environment, Agriculture and Biotechnology, 3(2), 656–626. [CrossRef]
- Greenhouse Gases Equivalencies Calculator - Calculations and References. (2022, June 23). US EPA. Retrieved October 9, 2022, from https://www.epa.gov/energy/greenhouse-gases-equivalencies-calculator- calculations-and-references.
- Halder, B. (2023). Mapping and monitoring land dynamics using geospatial techniques on Pathar Pratima Block, South 24 Parganas, India. Elsevier EBooks, 299–324. [CrossRef]
- Hancock, G., Turner, L., & Webb, A. (2022). Organic carbon export in steep forested catchments – An assessment of scale and disturbance. Journal of Hydrology, 612, 128011. [CrossRef]
- Harishma, K. M., Sandeep, S., & Sreekumar, V. B. (2020). Biomass and carbon stocks in mangrove ecosystems of Kerala, southwest coast of India. Ecological Processes, 9(31). [CrossRef]
- Irvine, K. (2017). Teacher Handbook of Water Quality for the Singapore Geography Curriculum. National Institute of Education. 10.13140/RG.2.2.25193.80486.
- Jimenez, L. C. Z., Queiroz, H. M., Cherubin, M. R., & Ferreira, T. O. (2022, March 7). Applying the Soil Management Assessment Framework (SMAF) to assess mangrove soil quality. Sustainability, 14(5), 3085. [CrossRef]
- Jimenez, L. C. Z., Queiroz, H. M., Otero, X. L., Nóbrega, G. N., & Ferreira, T. O. (2021, August 26). Soil organic matter responses to mangrove restoration: A replanting experience in Northeast Brazil. International Journal of Environmental Research and Public Health, 18(17), 8981. [CrossRef]
- Jiao, S., Liu, S., Li, Y., Xu, Z., Kong, B., Li, Y., & Shen, Y. (2020). Variation of soil organic carbon and physical properties in relation to land uses in the Yellow River Delta, China. Scientific Reports, 10(1). [CrossRef]
- Joshi, P., & Beck, K. A. (2015). Biological oxygen demand and economic growth: An empirical investigation. Water Economics and Policy, 01(02), 1550001. [CrossRef]
- Kadarsah, A., Salim, D., & Husain, D. S. (2020). Study of Water and Sediment Quality and Heavy Metal Pollution (Pb) at South Kalimantan Mangrove Ecosystem. IOP Conference Series. [CrossRef]
- Kumar, M., Krishi, J., Jabalpur, J., & Pradesh, M. (2021). Pharmacy journal Pharmaceutical journal. The Pharma Innovation Journal. https://www.thepharmajournal.com/archives/2022/vol11issue2S/PartD/S-11-1-275-433.pdf.
- Kiernan, D. (2021, May 1). 10.1: Introduction, Simpson’s index and Shannon-Weiner index. Statistics LibreTexts. Retrieved June 21, 2022, from https://stats.libretexts.org/Bookshelves/Applied_Statistics/Book%3A_Natur al_Resources_Biometrics_(Kiernan)/10%3A_Quantitative_Measures_of_Di versity_Site_Similarity_and_Habitat_Suitability/10.01%3A_IntroductionSimpsons_Index_and_Shannon-Weiner_Index.
- Kiselev, M. V., Voropay, N. N., & Cherkashina, A. A. (2019). Influence of anthropogenic activities on the temperature regime of soils of the South-Western Baikal region. IOP Conference Series, 381(1), 012043. [CrossRef]
- Lal, G., & Kumar, M. (2022). Effect of soil temperature gradient on potassium movement in controlled Paddy Field. The Pharma Innovation Journal. https://www.thepharmajournal.com/special- issue?year=2022&vol=11&issue=2S&ArticleId=10542.
- Liu, F., Zhang, H., Ming, J., Zheng, J., Tian, D., & Chen, D. (2020b). Importance of precipitation on the upper ocean salinity response to Typhoon Kalmaegi (2014). Water, 12(2), 614. [CrossRef]
- Liu, S., Wang, D., Miao, W., Wang, Z., Zhang, P., & Li, D. (2023). Characteristics of runoff and Sediment Load during Flood Events in the Upper Yangtze River, China. Journal of Hydrology, 129433. [CrossRef]
- McFadden, T. N., Kauffman, J. B., & Bhomia, R. K. (2016). Effects of nesting waterbirds on nutrient levels in mangroves, Gulf of Fonseca, Honduras. Wetlands Ecology and Management, 24(2), 217–229. [CrossRef]
- Muliawan, R., Prartono, T., & Bengen, D. G. (2020). Productivity and decomposition rate of Rhizophora mucronata and Avicennia alba litter based on environment characteristics in Muara Gembong. IOP Conference Series. [CrossRef]
- Nesperos, V. C., Villanueva, C. M., Garcia, J. E., & Gevaña, D. T. (2021). Assessment of blue carbon stock of mangrove vegetation in Infanta, Quezon, Philippines. Ecosystems and Development Journal, 11(1 & 2), 48–60. https://ovcre.uplb.edu.ph/journalsuplb/index.php/EDJ/article/download/618/ 595/.
- Noor, Tahira & Batool, Nazima & Mazhar, Roomina & Ilyas, Noshin. (2015). Effects of Siltation, Temperature and Salinity on Mangrove Plants.
- Onwuka, B. M., & Mang, B. (2018). Effects of Soil Temperature on Some Soil Properties and Plant Growth. Advances in Plants and Agriculture Research, 8(1). [CrossRef]
- Pawar, P. R. (2013). Monitoring the impact of anthropogenic inputs on water quality of the mangrove ecosystem of Uran, Navi Mumbai, west coast of India. Marine Pollution Bulletin, 75(1–2), 291–300. [CrossRef]
- Pototan, B., Capin, N., Delima, A. G., & Novero, A. (2020). Assessment of mangrove species diversity in Banaybanay, Davao Oriental, Philippines, 22(1). [CrossRef]
- Pradipta, N., Alamsjah, M. A., & Masithah, E. D. (2021, February 1). Study of nitrogen (N) and phosphorus (P) in the land of mangrove sediments in ecotourism area Wonorejo Surabaya and coastal area of Jenu Tuban. IOP Conference Series: Earth and Environmental Science, 679(1), 012048. [CrossRef]
- Primavera, J. H., & Dianala, R. D. B. (2009). Field guide to Philippine mangroves. Philippine Tropical Forest Conservation Inc.
- Primavera, J., Friess, D., Lavieren, H.V., Lee, S. et al. (2018). The mangrove ecosystem. 10.1016/B978-0-12-805052-1.00001-2.
- Rastegar, S., Gozari, M. (2017). Effect of mangrove plant extract on growth of four fungal pathogens. J. Paramed. Sci. 8 (1), 1-6.
- Reef, R., & Lovelock, C. E. (2015). Regulation of water balance in mangroves. Annals of Botany, 115(3), 385–395. https://www.jstor.org/stable/26525623.
- Rugebregt, M. J., & Nurhati, I. S. (2020). Preliminary Study of Ocean Acidification: Relationship of pH, Temperature, and Salinity in Ohoililir, Southeast Maluku. IOP Conference Series, 618(1), 012004. [CrossRef]
- Rusydi, A. F. (2018). Correlation between conductivity and total dissolved solid in various types of water: A review. IOP Conference Series, 118, 012019. [CrossRef]
- Sahu, S. K., & Kathiresan, K. (2019). The age and species composition of mangrove forest directly influence the net primary productivity and carbon sequestration potential. Biocatalysis and Agricultural Biotechnology, 20, 101235. [CrossRef]
- Salcedo, A. J. M., Estévez, E., Salvadó, H., Barquín, J., & Cañedo-Argüelles, M. (2022). Human activities disrupt the temporal dynamics of salinity in Spanish rivers. Hydrobiologia. [CrossRef]
- Santos-Andrade, M., Hatje, V., Arias-Ortiz, A., Patire, V. F., & Da Silva, L. A. (2021). Human disturbance drives loss of soil organic matter and changes its stability and sources in mangroves. Environmental Research, 202, 111663. [CrossRef]
- Sari, K., & Soeprobowati, T. R. (2021). Impact of Water Quality Detorioration in Mangrove Forest in Semarang Coastal Area. Indonesian Journal of Limnology, 2(2), 37–48. [CrossRef]
- Shi, M., Ma, J., & Zhang, K. (2022). The Impact of Water Temperature on In-Line Turbidity Detection. Water, 14(22), 3720. [CrossRef]
- Siddique, M. R. H., Saha, S., Salekin, S., & Mahmood, H. (2017). Salinity strongly drives the survival, growth, leaf demography, and nutrient partitioning in seedlings of Xylocarpus granatum J. König. iForest Research Article Biogeosciences and Forestry. https://iforest.sisef.org/pdf/?id=ifor2382-010.
- Sofawi, A. B., Nazri, M. N., & Rozainah, M.Z. (2017). Nutrient variability in mangrove soil: anthropogenic, seasonal and depth variation factors. Applied Ecology and Environmental Research, 15(4), 1983–1998. [CrossRef]
- Supriyantini, E., Santoso, A., & Soenardjo, N. (2018). Nitrate and Phosphate Contents on Sediments Related to The Density Levels of MangroveRhizophoraSp. in Mangrove Park Waters of Pekalongan, Central Java. IOP Conference Series, 116, 012013. [CrossRef]
- Tomotsune, M., Yoshitake, S., Iimura, Y., Kida, M., Fujitake, N., Koizumi, H., & Ohtsuka, T. (2018, July). Effects of soil temperature and tidal condition on variation in carbon dioxide flux from soil sediment in a subtropical mangrove forest. Journal of Tropical Ecology, 34(4), 268–275. [CrossRef]
- Wang, Q., Wen, Y., Zhao, B., Hong, H., Liao, R., Li, J., Liu, J., Lu, H., & Yan, C. (2021). Coastal soil texture controls soil organic carbon distribution and storage of mangroves in China. CATENA, 207, 105709. [CrossRef]
- Ward, R. D., Friess, D. A., Day, R. H., & Mackenzie, R. A. (2016, April). Impacts of climate change on mangrove ecosystems: a region-by-region overview. Ecosystem Health and Sustainability, 2(4), e01211. [CrossRef]
- Wdowczyk, A., & Szymańska-Pulikowska, A. (2021). Analysis of the possibility of conducting a comprehensive assessment of landfill leachate contamination using physicochemical indicators and toxicity test. Ecotoxicology and Environmental Safety, 221, 112434. [CrossRef]
- Water quality guidelines and general effluent standards of 2016. (2018, May 4). Department of Environment and Natural Resources. https://emb.gov.ph/wp- content/uploads/2019/04/DAO-2016-08_WATER-QUALITY- GUIDELINES-AND-GENERAL-EFFLUENT-STANDARDS.pdf.
- Water Science School. (2019, October 22). Turbidity and water U.S. Geological Survey. U.S. Geological Survey. https://www.usgs.gov/special-topics/water- science-school/science/turbidity-and-water.
- Wei, H., Guenet, B., Vicca, S., Nunan, N., Asard, H., AbdElgawad, H., Shen, W., & Janssens, I. A. (2014). High clay content accelerates the decomposition of fresh organic matter in artificial soils. Soil Biology & Biochemistry, 77, 100– 108. [CrossRef]
- Wiarta, R. (2019). Carbon sequestration by young Rhizophora apiculata plants in Kubu Raya District, West Kalimantan, Indonesia. https://www.semanticscholar.org/paper/Carbon-sequestration-by-young- Rhizophora-apiculata-Wiarta- Indrayani/fbba8e640f6042fd1537c35b0b348715e64a57e5.
- Wibowo, H., & Kasno, A. (2021). Soil organic carbon and total nitrogen dynamics in paddy soils on the Java Island, Indonesia. IOP Conference Series, 648(1), 012192. [CrossRef]
- Wood Density Database. (2017). World of Agroforestry. Retrieved October 1, 2022, from http://db.worldagroforestry.org//wd.
- Yang, P., Hu, Z., & Shu, Q. (2021). Factors Affecting Soil Organic Carbon Content between Natural and Reclaimed Sites in Rudong Coast, Jiangsu Province, China. Journal of Marine Science and Engineering, 9(12), 1453. [CrossRef]
- Zhang, X., Li, Q., Gao, J., Hu, Y., Song, M., & Yue, Y. (2020). Effects of rainfall amount and frequency on soil nitrogen mineralization in Zoigê alpine wetland. European Journal of Soil Biology, 97, 103170. [CrossRef]
- Zhang, Y., Wu, W., & Liu, H. (2019). Factors affecting variations of soil pH in different horizons in hilly regions. PLOS ONE, 14(6), e0218563. [CrossRef]
- Zhang, Y., Wang, K., Wang, J., Liu, C., & Shangguan, Z. (2021). Changes in soil water holding capacity and water availability following vegetation restoration on the Chinese Loess Plateau. Scientific Reports, 11(1). [CrossRef]
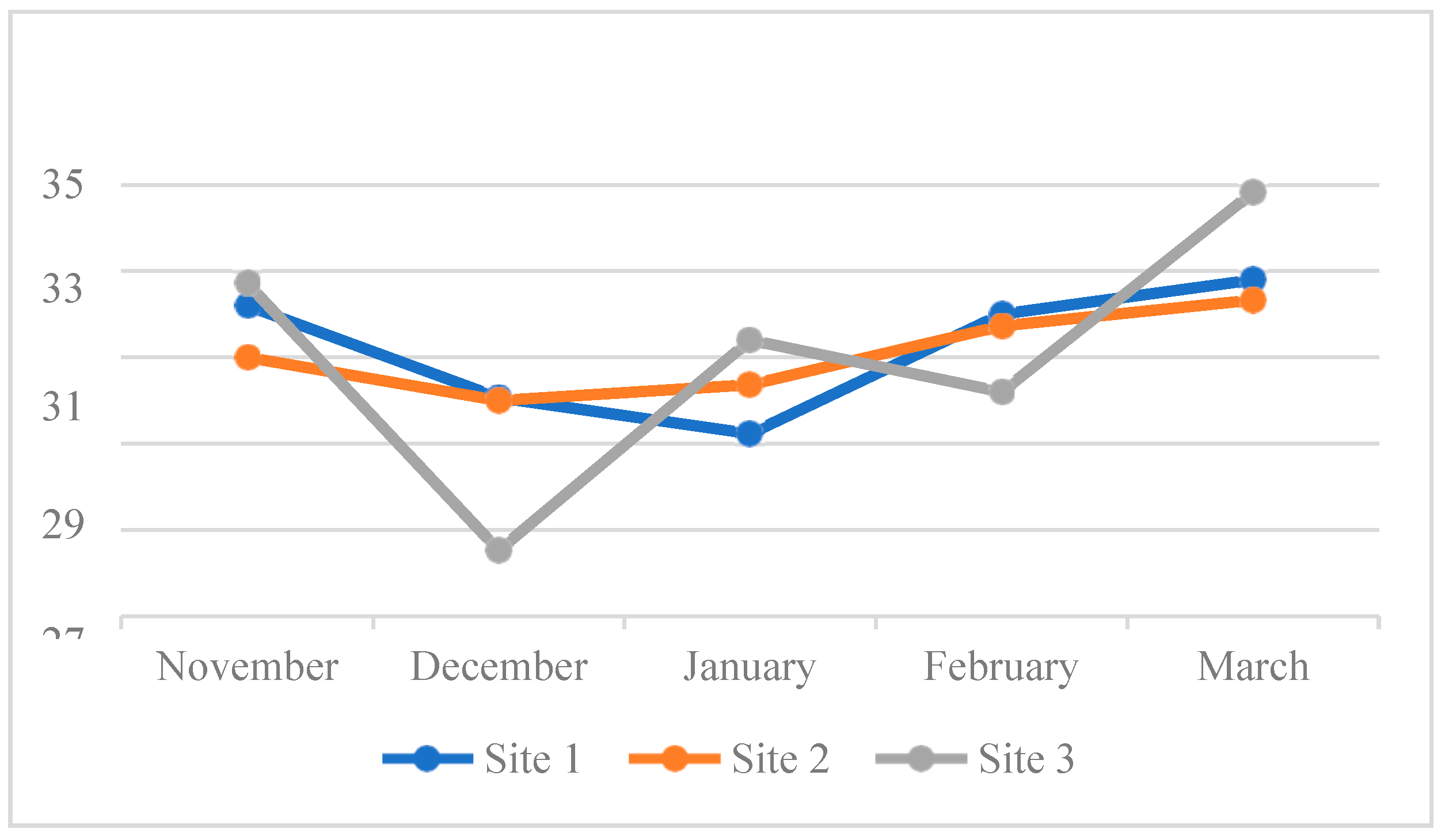
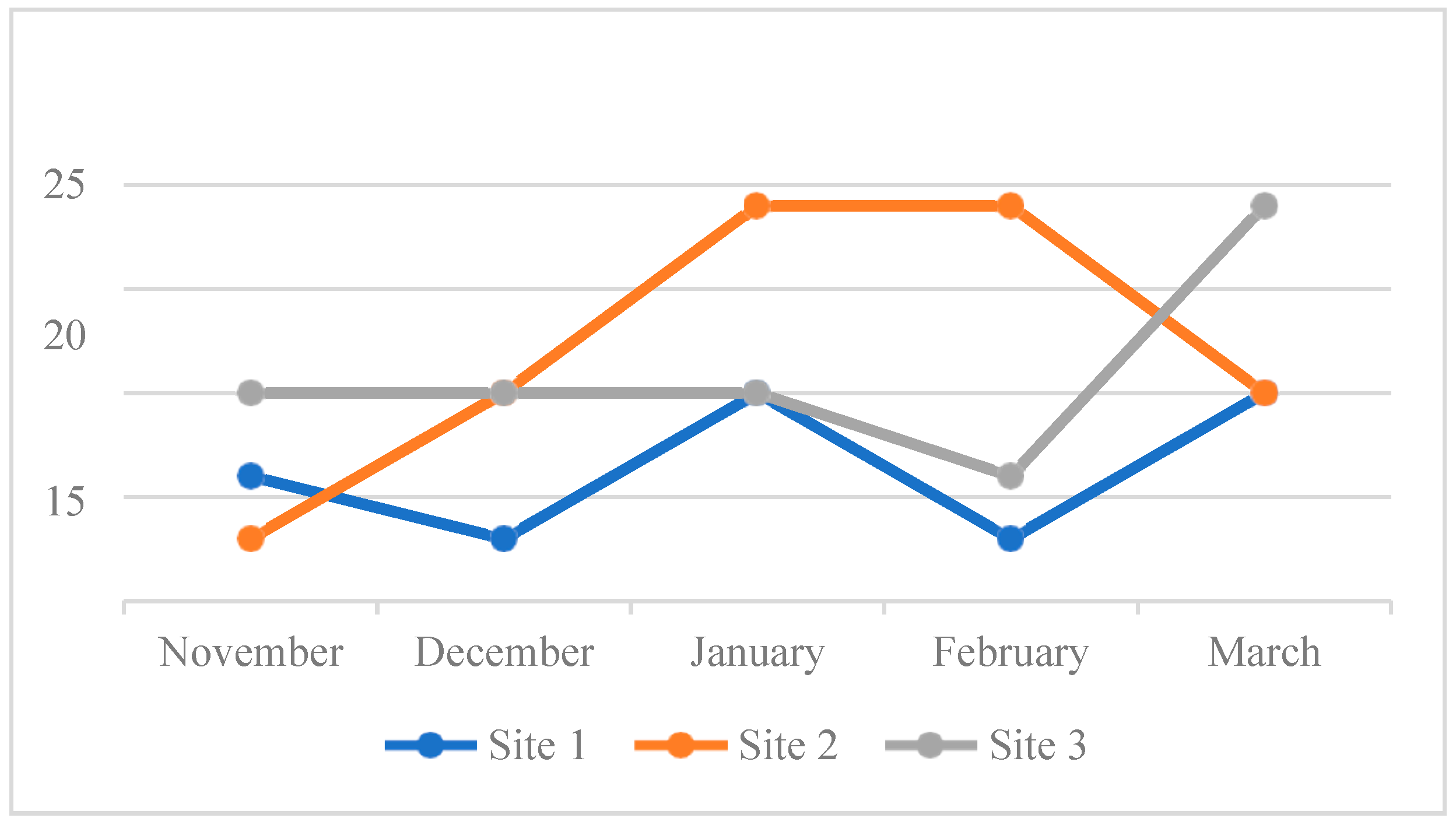
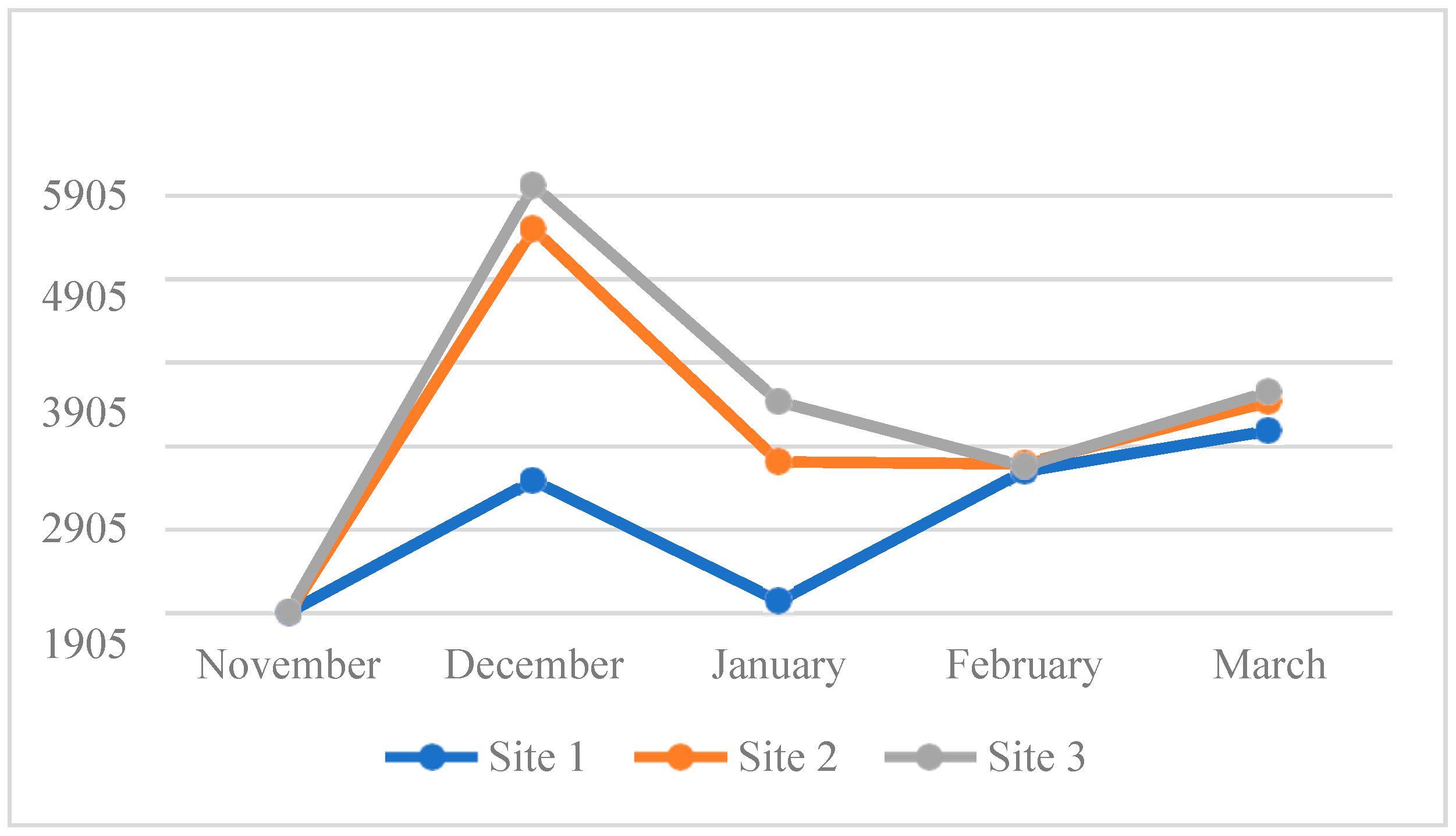
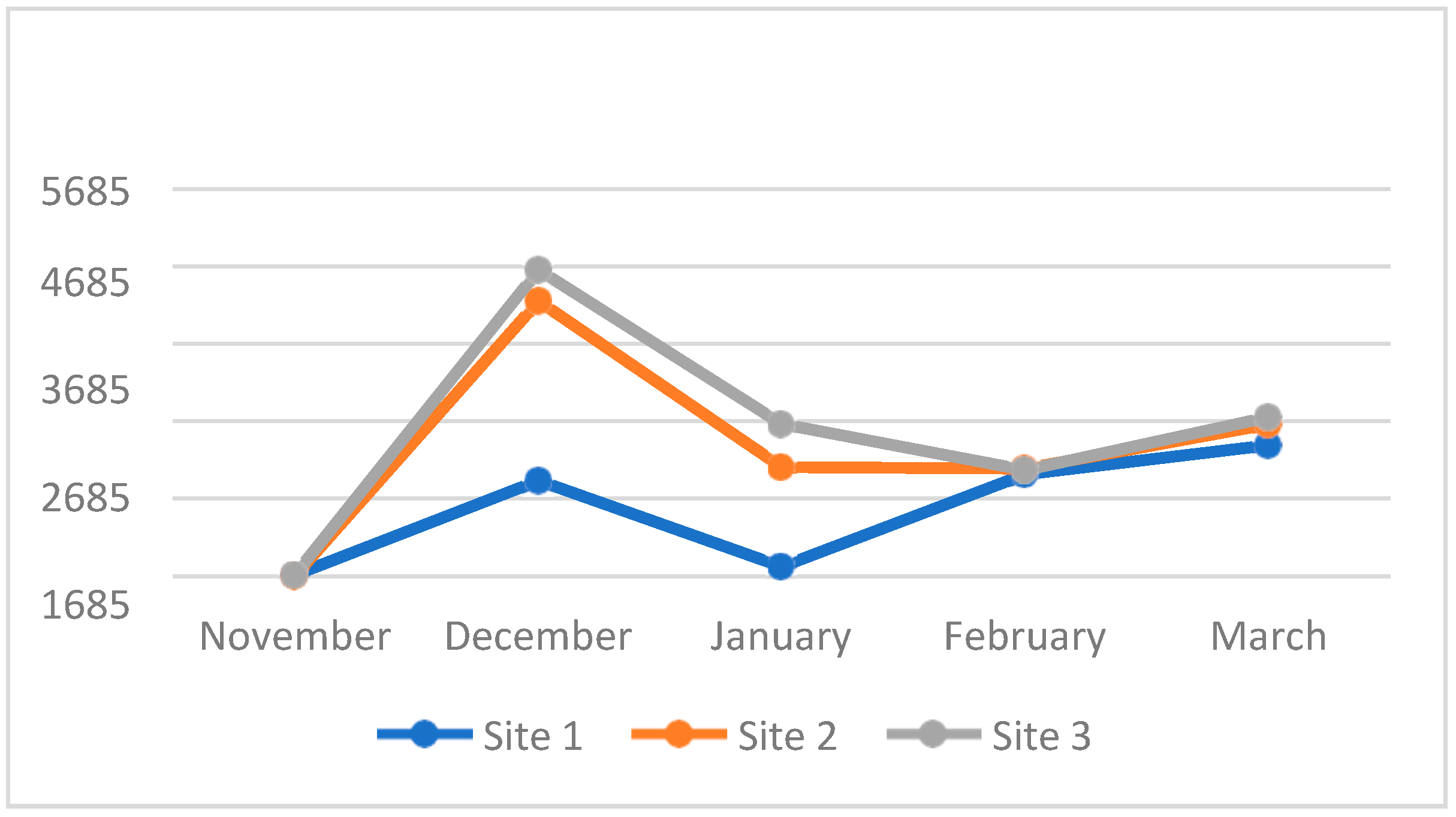
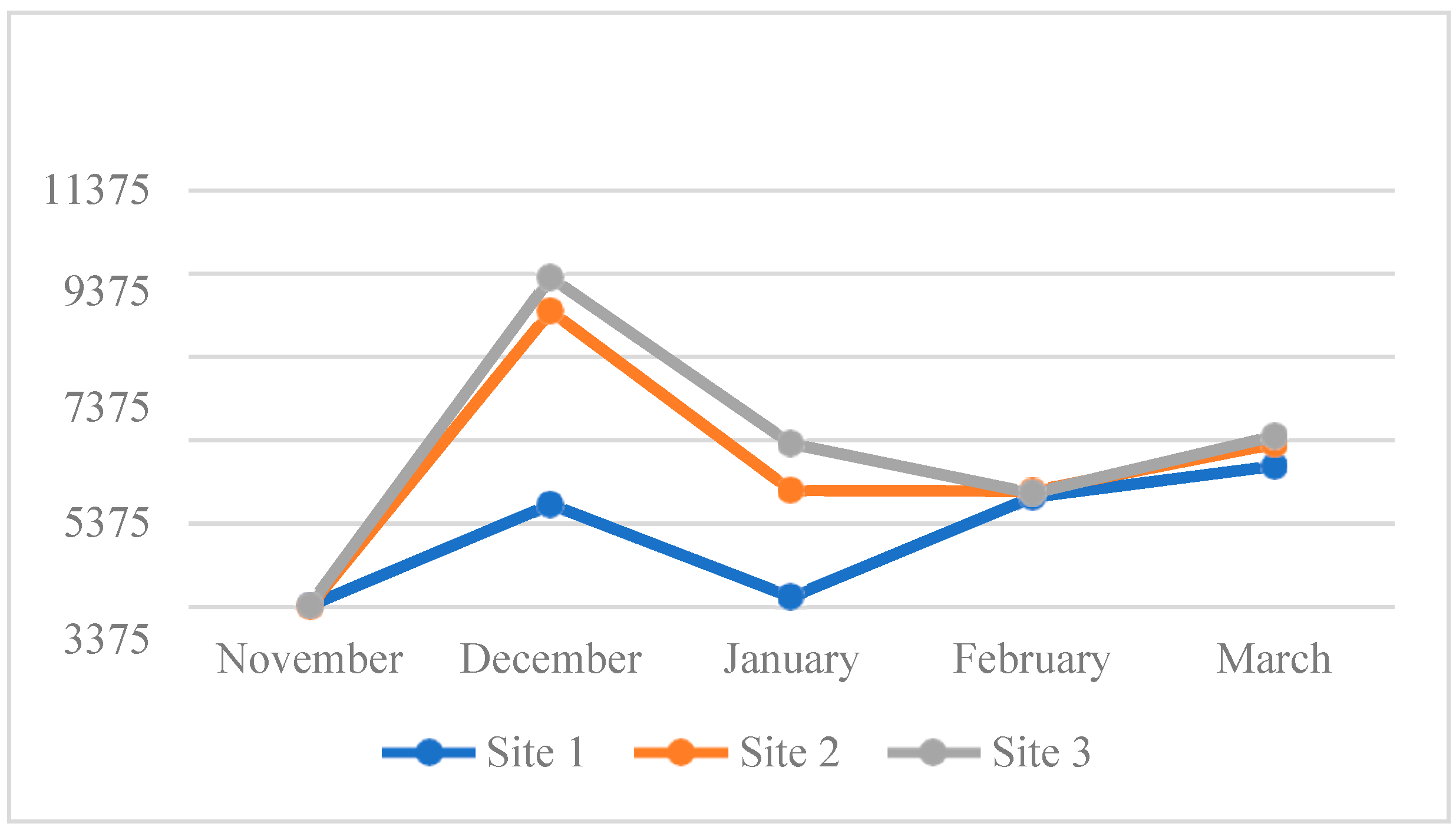
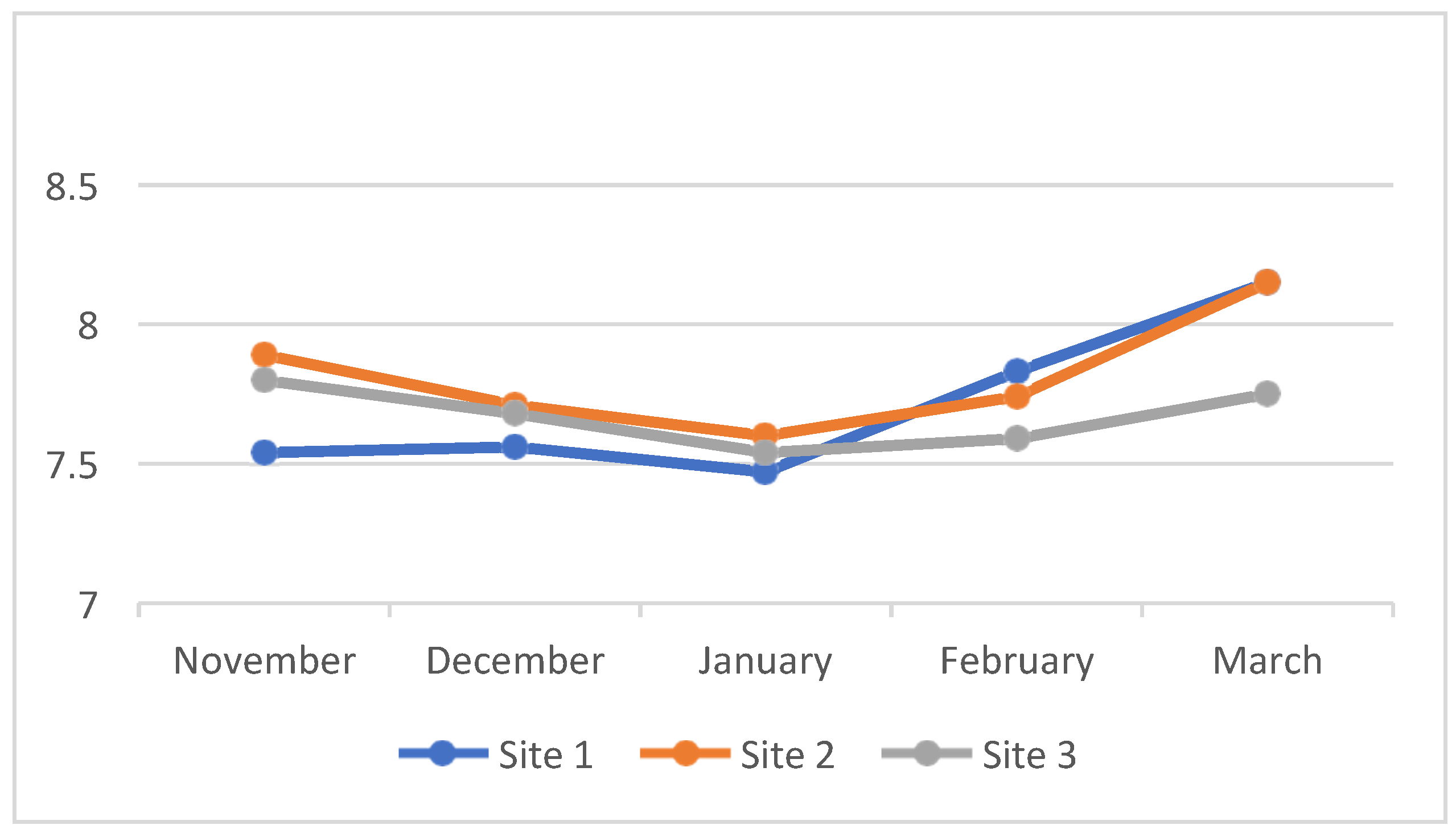

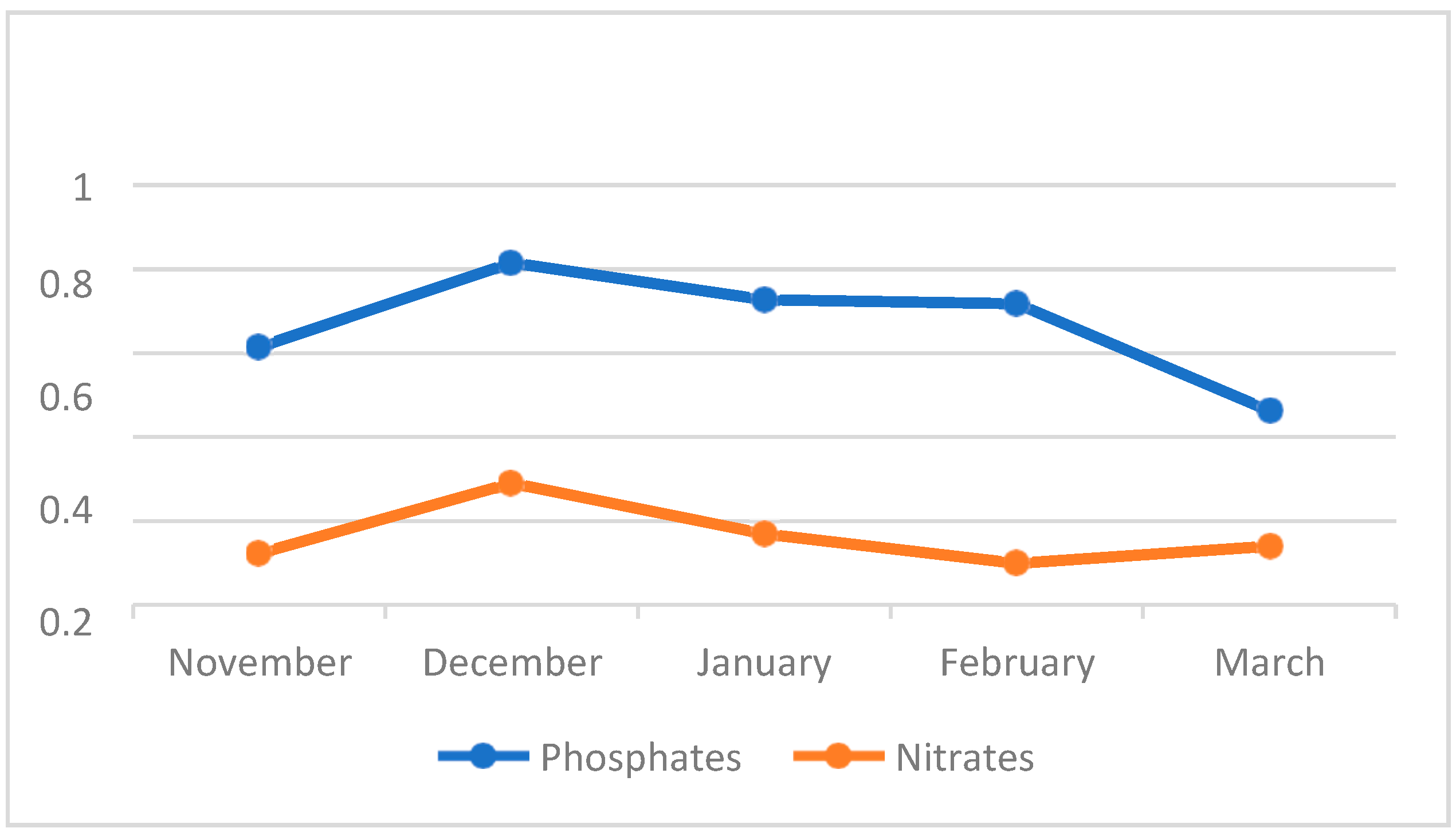
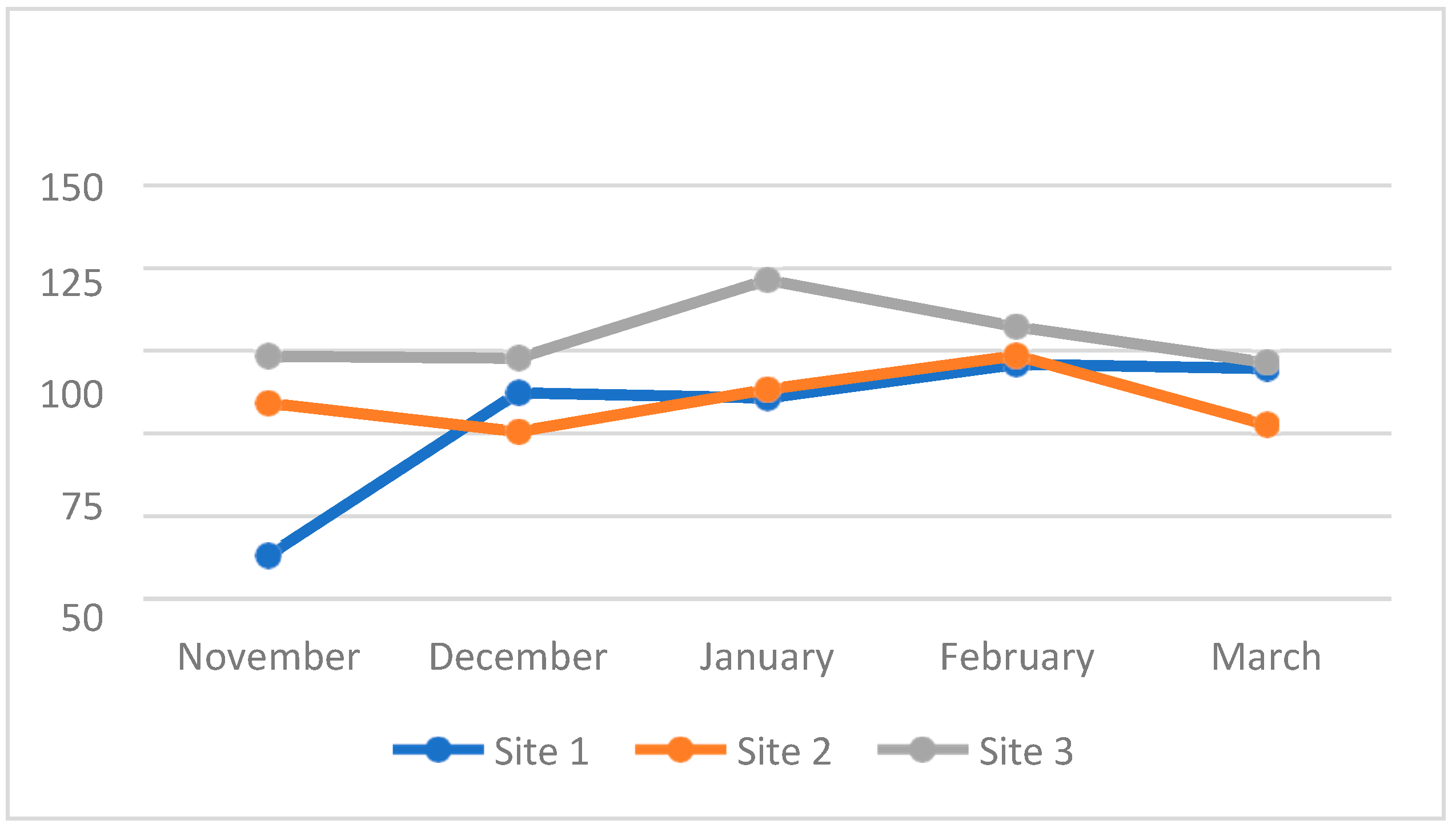
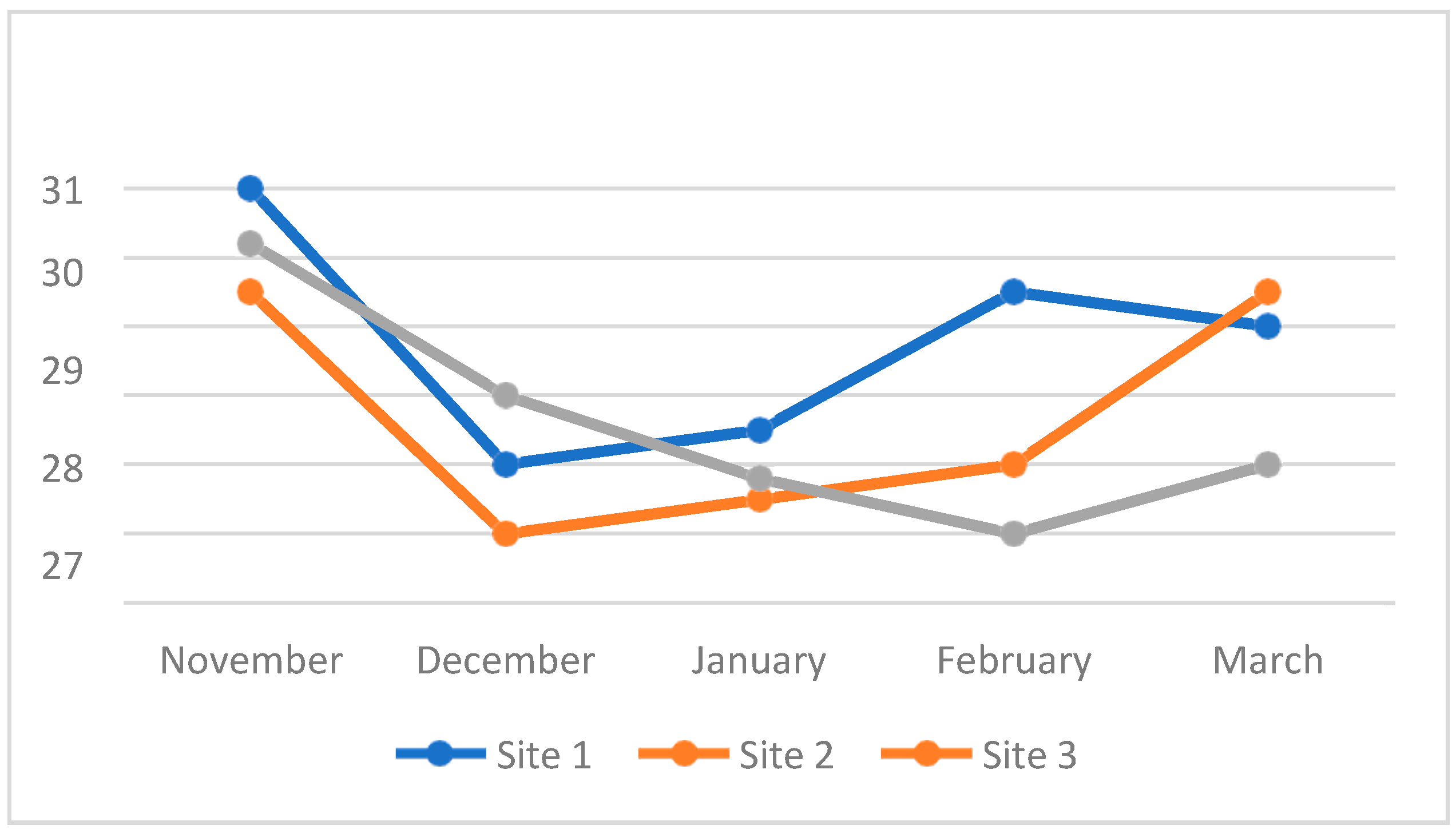
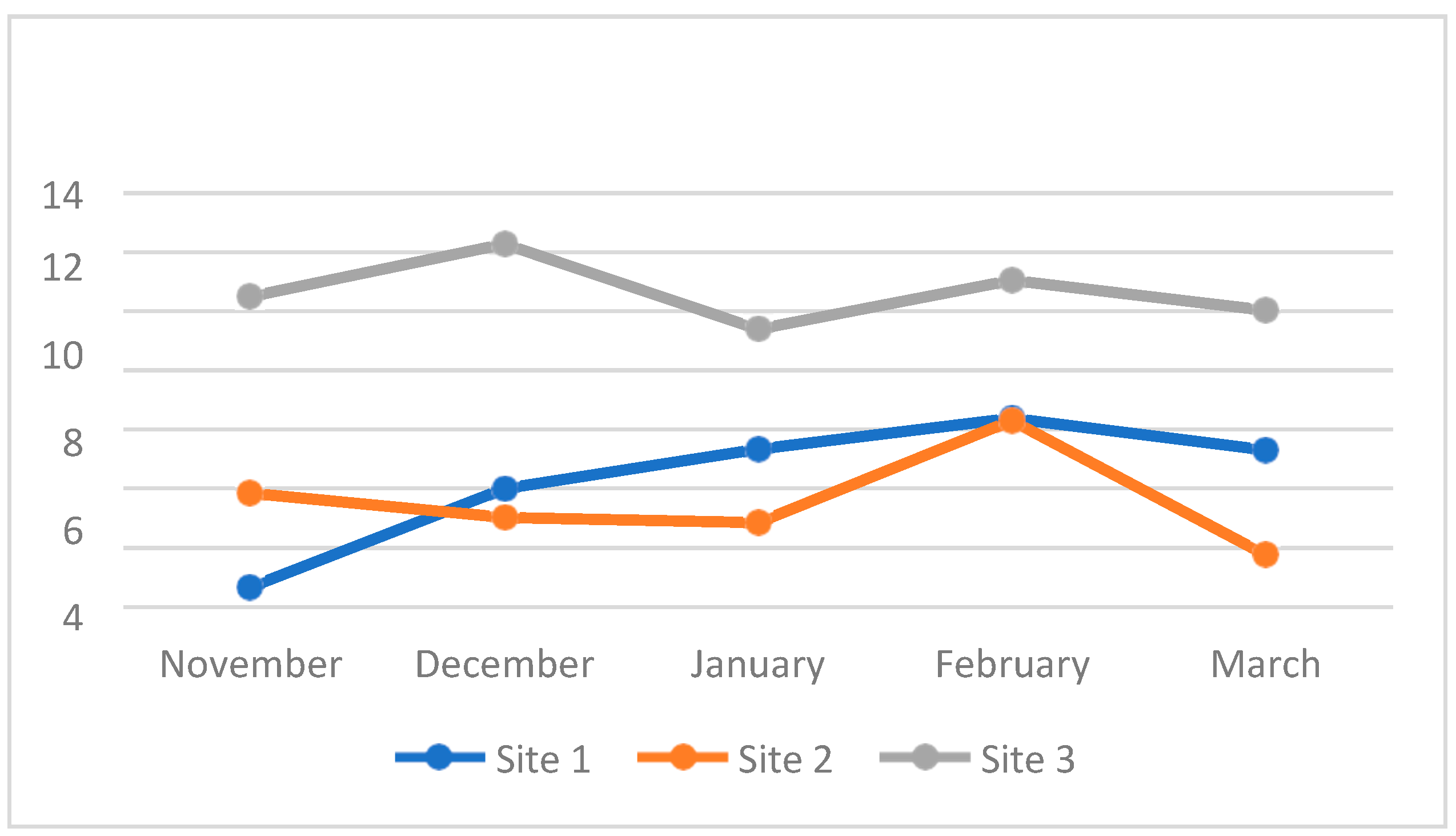
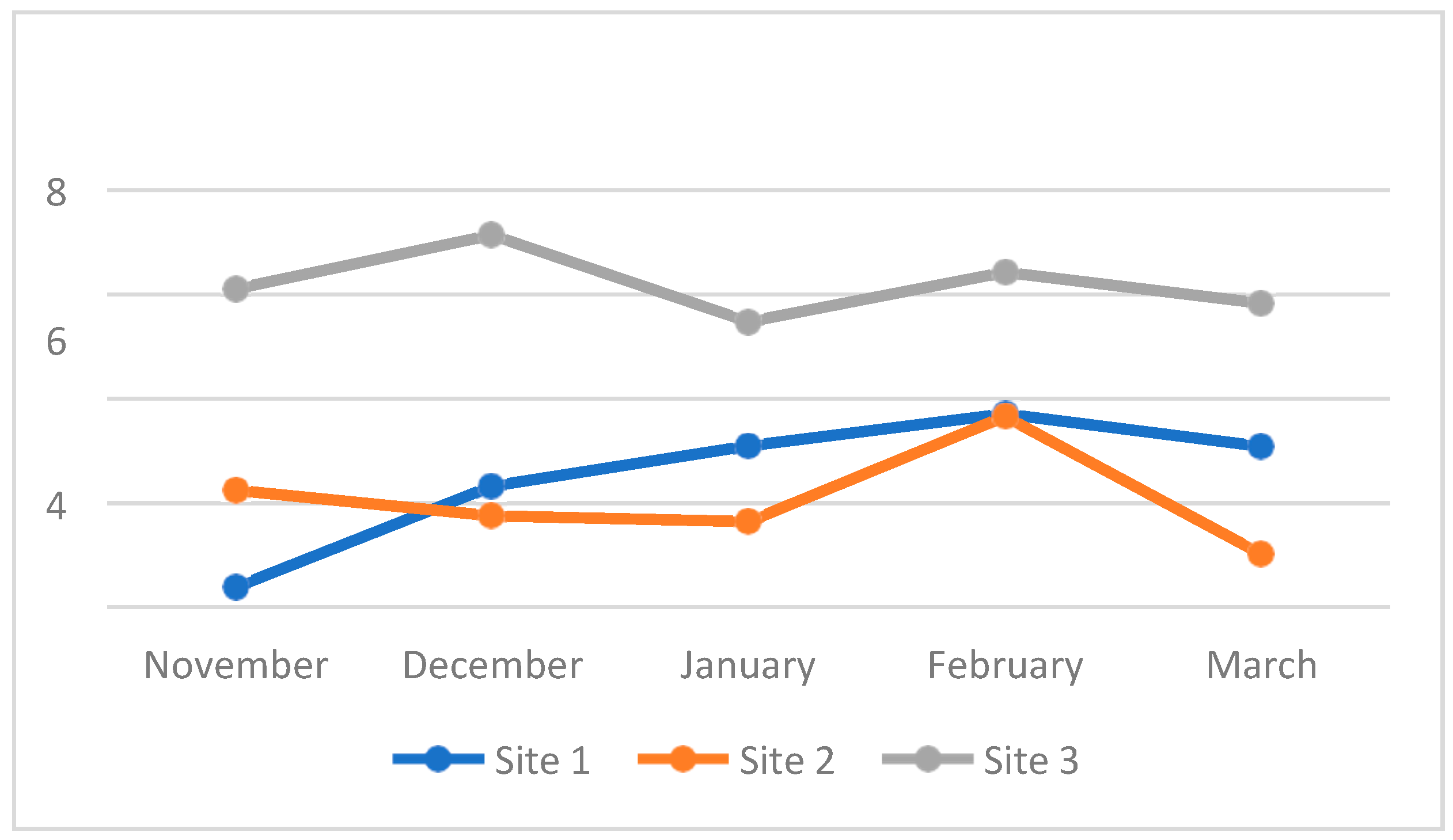
| Anthropogenic Activities | Mean | Remarks |
|---|---|---|
| Fishing | 1.87 | Seldom |
| Bathing | 1.82 | Seldom |
| Washing of clothes | 1.45 | Never |
| Throwing garbage | 1.67 | Never |
| Disposing of chemical waste | 1.28 | Never |
| Excreting | 1.41 | Never |
| Bathing of Animals | 1.31 | Never |
| Protection and Conservation Measures | Mean | Remarks |
|---|---|---|
| Preventing people from committing unlawful behavior in the river | 3.58 | Strongly Agree |
| Taking part in environmental groups | 3.50 | Strongly Agree |
| Creating groups and organizations | 3.45 | Strongly Agree |
| River conservation guidelines | 3.54 | Strongly Agree |
| Promoting sustainable methods | 3.52 | Strongly Agree |
| River management | 3.42 | Strongly Agree |
| Preventing depositing wastes | 3.72 | Strongly Agree |
| Supporting environmental programs | 3.67 | Strongly Agree |
| Parameters | Site | November | December | January | February | March | DENR Standard for Class C Water |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 1 2 3 |
32.20 31.00 32.73 |
30.07 30.00 26.53 |
29.23 30.37 31.40 |
32.00 31.73 30.2 |
32.80 32.33 34.83 |
25-31 |
| Turbidity (NTU) | 1 2 3 |
11.00 8.000 15.00 |
8.000 15.00 15.00 |
15.00 24.00 15.00 |
8.000 24.00 11.00 |
15.00 15.00 24.00 |
- |
| Total Dissolved Solid (mg/L) | 1 2 3 |
910.5 896.5 911.8 |
2491.6 5507.6 6029.9 |
1047.3 2715.7 3437.7 |
2602.1 2695.4 2665.0 |
3087.5 3436.4 3555.5 |
- |
| Salinity (mg/L) | 1 2 3 |
700.3 689.7 701.4 |
1916.7 4236.7 4638.4 |
805.7 2089.0 2644.4 |
2001.7 2073.4 2050.0 |
2375.0 2643.4 2735.0 |
- |
| Conductivity (µS/cm) | 1 2 3 |
1400.7 1379.3 1402.7 |
3833.3 8473.3 9276.7 |
1611.3 4178.0 5288.7 |
4003.3 4146.7 4100.0 |
4750.0 5286.7 5470.0 |
- |
| pH | 1 2 3 |
7.540 7.890 7.800 |
7.560 7.710 7.680 |
7.470 7.600 7.540 |
7.830 7.740 7.590 |
8.150 8.150 7.750 |
6.5-9.0 |
| Dissolved Oxygen (mg/L) | 1 2 3 |
2.970 5.130 4.170 |
5.400 6.530 5.570 |
3.500 4.200 4.200 |
4.600 4.000 3.900 |
9.500 7.800 9.500 |
≥ 5 |
| Phosphates (mg/L) | 0.6140 | 0.8150 | 0.7270 | 0.7180 | 0.4630 | ≤ 0.025 | |
| Nitrates (mg/L) | 0.1230 | 0.2900 | 0.1700 | 0.1000 | 0.1400 | ≤ 7 |
| Sampling Sites | |||||
|---|---|---|---|---|---|
| Sampling Periods | 1 | 2 | 3 | Average WQI | Remarks |
| WQI | WQI | WQI | |||
| November | 117.7 | 116.53 | 125.64 | 119.96 | Unsuitable |
| December | 146.91 | 159.44 | 158.12 | 154.82 | Unsuitable |
| January | 141.11 | 155.91 | 142.43 | 146.48 | Unsuitable |
| February | 131.15 | 154.24 | 134.48 | 139.96 | Unsuitable |
| March | 110.97 | 108.45 | 124.32 | 114.58 | Unsuitable |
| Average WQI | 129.57 | 138.91 | 137.00 | - | Unsuitable |
| Soil Parameters | Site | Sampling Period | ||||
|---|---|---|---|---|---|---|
| November | December | January | February | March | ||
| 1 | 20.10 | 18.10 | 8.800 | 17.70 | 12.80 | |
| Total Sand | 2 | 80.80 | 33.90 | 30.90 | 29.00 | 31.50 |
| 3 | - | 24.00 | 16.10 | 16.10 | 11.70 | |
| 1 | 33.50 | 49.70 | 58.00 | 51.10 | 53.00 | |
| Total Silt | 2 | 8.200 | 30.30 | 27.60 | 25.50 | 25.70 |
| 3 | - | 24.00 | 37.30 | 41.90 | 44.10 | |
| 1 | 46.40 | 33.80 | 33.30 | 31.20 | 34.20 | |
| Total Clay | 2 | 11.00 | 35.80 | 41.50 | 45.50 | 42.80 |
| 3 | - | 42.20 | 46.60 | 42.00 | 44.20 | |
| 1 | Clay | Silty Clay Loam | Silty Clay Loam | Silty Clay Loam | Silty Clay Loam | |
| Texture Class | 2 | Sandy Loam |
Clay Loam | Clay | Loam | Clay |
| 3 | - | Clay | Clay | Silty Clay | Silty Clay | |
| Water Holding Capacity (WHC) |
1 | 38.10 | 87.20 | 85.70 | 95.90 | 94.60 |
| 2 | 84.10 | 75.50 | 88.30 | 98.50 | 77.60 | |
| 3 | 98.40 | 97.70 | 121.4 | 107.2 | 96.50 | |
| Parameters | Sampling Period | Sampling Sites | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| November | 31.00 | 29.50 | 30.20 | |
| December | 27.00 | 26.00 | 28.00 | |
| Temperature (°C) | January | 27.50 | 26.50 | 26.80 |
| February | 29.50 | 27.00 | 26.00 | |
| March | 29.00 | 29.50 | 27.00 | |
| November | 5.800 | 5.800 | 5.800 | |
| December | 5.800 | 5.800 | 5.800 | |
| pH | January February |
5.800 5.800 |
5.800 5.800 |
5.800 5.800 |
| March | 5.800 | 5.800 | 5.800 | |
| November | 0.6600 | 3.850 | 10.50 | |
| December | 3.990 | 2.020 | 12.28 | |
| Organic Matter (%) | January February |
5.320 6.380 |
2.840 6.300 |
9.400 11.05 |
| March | 5.300 | 1.760 | 10.03 | |
| November | 0.3800 | 2.240 | 6.100 | |
| December | 2.320 | 1.170 | 7.140 | |
| Organic Carbon (%) | January | 3.090 | 1.650 | 5.470 |
| February | 3.710 | 3.660 | 6.420 | |
| March | 3.080 | 1.020 | 5.830 |
| Parameters | r-value | p-value | Remarks |
|---|---|---|---|
| Temperature | -0.032 | 0.911 | Negligible Correlation/ Not Significant |
| Turbidity | -0.298 | 0.28 | Negligible Correlation/Not Significant |
| Total Dissolved Solids | 0.16 | 0.568 | Negligible Correlation/ Not Significant |
| Salinity | 0.16 | 0.568 | Negligible Correlation/ Not Significant |
| Conductivity | 0.16 | 0.568 | Negligible Correlation/ Not Significant |
| pH | 0.054 | 0.849 | Negligible Correlation/Not Significant |
| Dissolved Oxygen | 0.021 | 0.987 | Negligible Correlation/Not Significant |
| Parameters | r-value | p-value | Remarks |
|---|---|---|---|
| Water Holding Capacity | -0.132 | 0.639 | Negligible correlation/Not significant |
| Soil Temperature | -0.187 | 0.504 | Negligible correlation/Not significant |
| pH | a | a | Cannot be computed (variable is constant) |
| Organic Matter | -0.452 | 0.090 | Low negative correlation/Not Significant |
| Organic Carbon | -0.452 | 0.090 | Low negative correlation/Not Significant |
| N | -0.414 | 0.127 | Low negative correlation/Not Significant |
| P | 0.226 | 0.417 | Negligible correlation/ Not Significant |
| K | -0.384 | 0.158 | Low negative correlation/Not Significant |
| Mangrove Species | Site 1 | Site 2 | Site 3 | Total No. of Species | % Occurrences |
|---|---|---|---|---|---|
| Acanthaceae | |||||
| Avicennia alba | 15 | 8 | 1 | 24 | 9.38 |
| Meliaceae | |||||
| Xylocarpus granatum | - | 14 | - | 14 | 5.47 |
| Rhizophoraceae | |||||
| Rhizophora mucronata | 6 | 170 | 42 | 218 | 85.16 |
| Family Name | Scientific Name | Common Name | Biological Status | IUCN Status |
|---|---|---|---|---|
| Acanthaceae | Avicennia alba | Bungalon | Introduced | Least Concern |
| Meliaceae | Xylocarpus granatum | Tabigi | Native | Least Concern |
| Rhizophoraceae | Rhizophora mucronata | Bakhaw babae | Native | Least Concern |
| Malvaceae | Thespesia populneoides | Banago | Introduced | Least Concern |
| Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | 1 | 2 | 3 | f | Rf | RA | RD | IV | Rank |
| Rhizophora mucronata | 6 | 170 | 42 | 218 | 85.16 | 1.172 | 1.172 | 87.50 | 1 |
| Avicennia alba | 15 | 8 | 1 | 24 | 9.375 | 1.172 | 1.172 | 11.72 | 2 |
| Xylocarpus granatum | 0 | 14 | 0 | 14 | 5.469 | 1.172 | 1.172 | 7.813 | 3 |
| Diversity Indices | Site 1 | Site 2 | Site 3 |
|---|---|---|---|
| Shannon-Weiner Index | |||
| Diversity (H) | 0.5983 | 0.4311 | 0.1105 |
| Evenness (E) | 0.1994 | 0.1437 | 0.0368 |
| Simpson’s Index | |||
| Diversity (Ds) | 0.5714 | 0.7899 | 0.9318 |
| Dominance (1-Ds) | 0.4286 | 0.2101 | 0.0682 |
| Reciprocal (1/Ds) | 1.750 | 1.266 | 1.073 |
| Sites | Index of Similarity (%) |
|---|---|
| Sites 1 and Site 2 | 80.00 |
| Site 2 and Site 3 | 80.00 |
| Site 1 and Site 3 | 100.0 |
| Mangrove Species | Abundanc e per Species | GBH Range (cm) | DBH Range (cm) |
Mean Total Biomass (t/ha) | Carbon Stored (tC/ha) | CO2 Sequestered (tCO2/ha) |
|---|---|---|---|---|---|---|
| Avicennia alba | 32 | 24.00 – 172.7 | 7.640-54.97 | 69.85 | 34.92 | 128.06 |
| Xylocarpus granatum | 11 | 10.00 – 47.00 | 4.270-14.96 | 3.367 | 1.683 | 6.173 |
| Rhizophora mucronata | 190 | 10.00 – 67.95 | 3.180-24.06 | 70.31 | 35.16 | 128.92 |
| Site | Aboveground Biomass (kg) | Belowground Biomass (kg) | Mean Total Biomass (tC/ha) | Carbon Stored (tC/ha) | CO2 Sequestered (tCO2/ha) |
|---|---|---|---|---|---|
| 1 | 12145.35 | 4205.67 | 59.20 | 29.60 | 108.54 |
| 2 | 4276.35 | 2090.53 | 23.05 | 11.53 | 42.27 |
| 3 | 12277.50 | 4716.35 | 61.53 | 30.76 | 112.81 |
| TOTAL | 71.89 | 263.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





