Submitted:
04 August 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
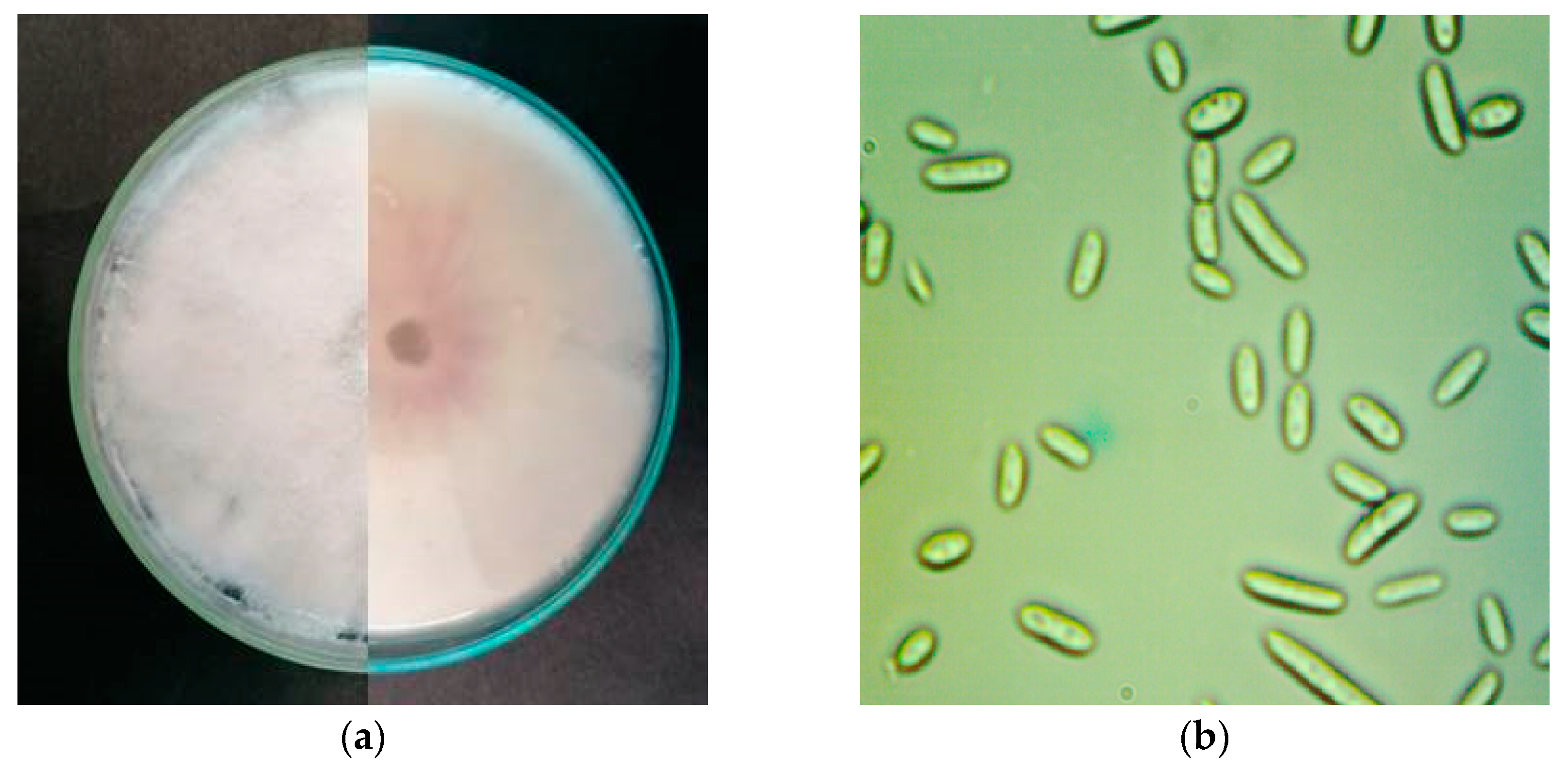
2.1. Fungal Isolate and Identification
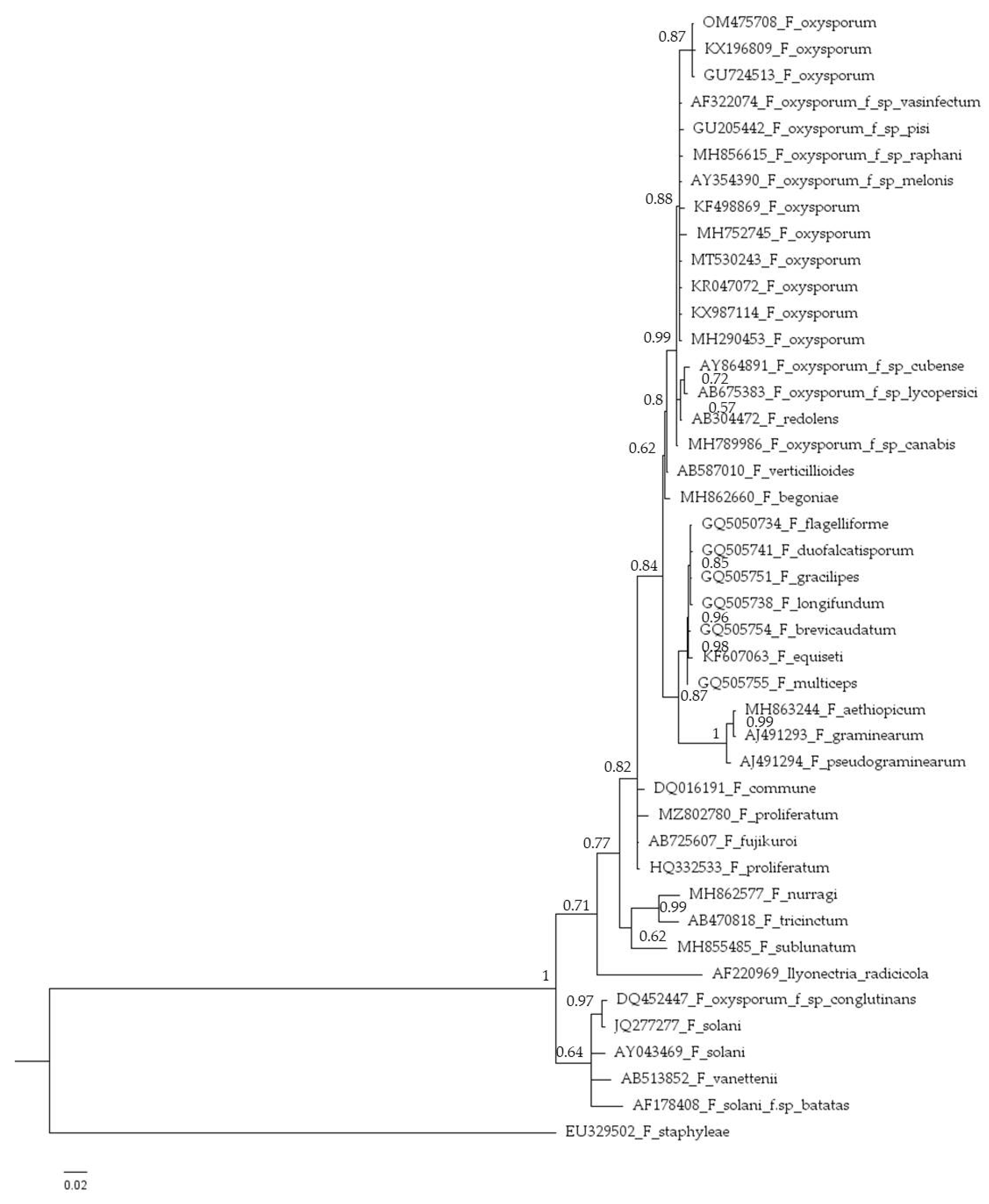
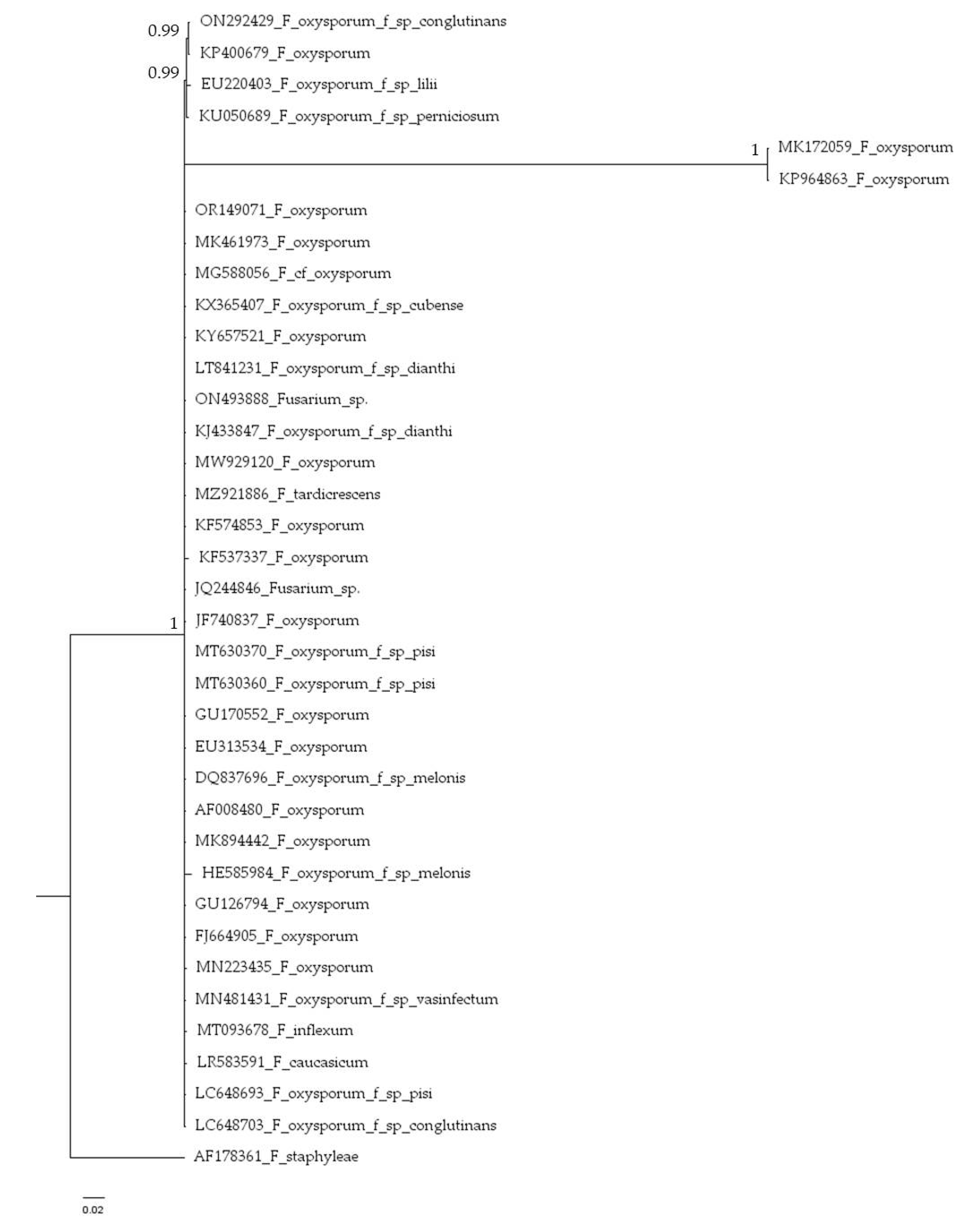
2.2. Molecularal Identification
2.3. Artificial Inoculation
3. Discussion
4. Materials and Methods
4.1. Fungal Strain Collection, Isolation and Growth Conditions
4.2. Fungal Strain Molecular Isolation
4.2.1. Internal Transcribed Spacer Region Analysis
4.2.2. Translation Elongation Factor 1-α Gene Analysis
4.3. Phylogenetic Analysis
4.4. Pathogenicity Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops and Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: an example of taxonomic neglect. Bot. Mus. Leaflets. 1974, 23, 337–367. [Google Scholar] [CrossRef]
- McPartland, J.M.; Guy, G. The evolution of Cannabis and coevolution with the cannabinoid receptor–a hypothesis. In The medicinal use of cannabis; Guy, G., Robson, R., Strong, K., Whittle, B., Eds.; Royal Society of Pharmacists: London, 2004; pp. 71–102. [Google Scholar]
- Thamae, T.; Aghedo, S.; Baillie, C.; Matovic, D. Tensile Properties of Hemp and Agave Americana Fibres. Handb. Tensile Prop. Text. Tech. Fibres. 2009, 73–99. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New Developments in Fiber Hemp (Cannabis Sativa, L.) Breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.P.; Seguin, P.; Mustafa, A.F.; Charron, J.B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Composit. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica. 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L. ) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial Hemp Fibers: An Overview. Fibers. 2019, 7, 106. [Google Scholar] [CrossRef]
- Ranalli, P.; Venturi, G. Hemp as a raw material for industrial applications. Euphytica. 2004, 149, 1–6. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lip. Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Rupasinghe, V.H.P.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.Z. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules. 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K. Epidemiology of Fusarium oxysporum causing root and crown rot of cannabis (Cannabis sativa L., marijuana) plants in commercial greenhouse production. Can. J. Plant Pathol. 2021, 43, 216–235. [Google Scholar] [CrossRef]
- Agrios, G. N. Plant Pathology, 5th ed.; Agrios, G. N., Ed.; Elsevier Academic Press: Burlington, MA, 2005. [Google Scholar]
- Ivić, D.; Domijan, A.M.; Peraica, M.; Miličević, T.; Cvjetković, B. Fusarium spp. on wheat, maize, soybean and pea. Arh Hig Rada Toksikol. 2009, 60, 435–442. [Google Scholar] [CrossRef]
- Duvnjak, T.; Sudaric, A.; Matosa Kocar, M.; Cosic, J.; Vrandecic, K. First report of soybean fusarium wilt caused by Fusarium oxysporum in Croatia. Plan. Dis. 2017, 101, 249. [Google Scholar] [CrossRef]
- Gwinn, K.D.; Hansen, Z.; Kelly, H.; Ownley, B.H. Diseases of Cannabis sativa caused by diverse Fusarium species. Front. Agron. 2022, 3, 796062. [Google Scholar] [CrossRef]
- Patschke, K.; Gottwald, R.; Muller, R. Erste Ergebnisse phytopathologischer Beobachtungen im Hanfanbau im Land Brandenburg. Nach. des Deut. Pflan. 1997, 49, 296–290. [Google Scholar]
- Punja, Z. K.; Collyer; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N. “American weed: a history of Cannabis cultivation in the United States”. EchoGéo. 2019, 48, 17650. [Google Scholar] [CrossRef]
- McCain, A. H.; Noviello, C. “Biological control of Cannabis sativa,” in Proceedings of the VI International Symposium on Biological Control of Weeds (Vancouver, BC). ed E. S. Delfosse. 1985, 635–642. [Google Scholar]
- Noviello, C.; McCain, A. H.; Aloj, B.; Scalcione, M.; Marziano, F. Lotta biologica contro Cannabis sativa medlante l'impiego di Fusarium oxysporum f. sp. cannabis. Annali delta Facolta di Scienze Agrarie della Universita degli Studi di Napoli. Portici. 1990, 24, 33–44. [Google Scholar]
- Rataj, K. Skodivi cinitele pradnych rostlin. Pram. lit. 1957, 2, 1–123. [Google Scholar]
- McPartland, J.M.; Clarke, R.C.; Watson, D.P. Fungal Diseases. In Hemp Diseases and Pests, Management and Biological Control; McPartland, J.M., Clarke, R.C., Watson, D.P., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 93–135. [Google Scholar]
- McPartland, J.M.; Hillig, K.W. Cannabis clinic Fusarium Wilt, J. of Ind. Hemp. 2004, 9, 2–67. [Google Scholar] [CrossRef]
- Noviello, C.; Snyder, W.C. Fusarium wilt of hemp. Phytopathology 1962, 52, 1315–1317. [Google Scholar]
- Summerell, B. A.; Salleh, B. .; Leslie, J. F. A utilitarian approach to Fusarium identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Huber, D. M.; Thompson, I. A. Nitrogen and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L. E., Elmer, W. H., Huber, D. M., Eds.; The American Phytopathological Society: St. Paul, MN, 2009; pp. 31–44. [Google Scholar]
- Lombard, L.; Sandoval-Denis, S. C.; Crous, P. W. Epitypification of Fusarium oxysporum – clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef]
- Bruehl, G. W. Soilborne Plant Pathogens; Macmillan Pub. Co.: New York, NY, 1987. [Google Scholar]
- Tiourebaev, KS.; Pilgeram, A.L.; Anderson, T.W.; Sands, D.C. Soil penetration of a mycoherbicide facilitated by carrier seedlings. Phytopathology. 1997, 87 (6 supplement), S:57. [Google Scholar]
- Agostinelli, A.M.; Clark, A.J.; Brown-Guedira, G.; Van Sanford, D.A. Optimizing phenotypic and genotipis selection for Fusatium head blight resistance in wheat. Euphytica 2012, 186, 115–126. [Google Scholar] [CrossRef]
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.; Riley, R.; Zitomer, N.C.; Colyer, P.; Waalwijk, C.; van der Lee, T.; Moretti, A.; Kang, S.; Kim, H.S.; Geiser, D.M.; Juba, J.H.; Baayen, R.P.; Cromey, M.G.; Sean Bithell, S.; Sutton, D.A.; Skovgaard, K.; Ploetz, R.; Kistler, H.C.; Elliott, M.; Davis, M.; Sarver, B.A.J. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fun. Gen. and Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef]
- Ilić, J.; Ćosić, J.; Jurković, D.; Vrandečić, K. Vegetative compatibility of Fusarium oxysporum isolated from weeds in eastern croatia. Poljoprivreda 2013, 19, 20–24. [Google Scholar]
- European Commission EU Plant Variety Database (v.3.2). Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=240&variety_name=&listed_in=0&show_current=on&show_deleted= (accessed on 15 March 2020).
- Pellegrini, M.; Ercole, C.; Gianchino, C.; Bernardi, M.; Pace, L.; Del Gallo, M. Fusarium oxysporum f. sp. cannabis isolated from Cannabis sativa L.: In vitro and in planta biocontrol by a plant growth promoting-bacteria consortium. Plants. 2021, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Herrer, J. The Emperor Wears No Clothes: The Authoritative Historical Record of Cannabis Conspiracy Against Marijuana; Publishers Group West: New Jersey, 1998; pp. 1–181. [Google Scholar]
- Mihelčić, D. Primjena konoplje u poljoprivredi i tržišne perspektive njezinih prerađevina i proizvoda.Master's thesis / Diplomski rad, 2017; Josip Juraj Strossmayer University of Osijek, Faculty of agriculture / Permanent link https://urn.nsk.hr/urn:nbn:hr:151:098050.
- The Government of the Republic of Croatia https://vlada.gov.hr/UserDocsImages//2016/Sjednice/2019/O%C5%BEujak/148%20sjednica%20VRH//148 %20-%2016.pdf 27 April 2022. 27 April.
- Paying Agency for Agriculture, Fisheries and Rural Development (PAAFRD). Available online: www.apprrr.hr/agronet (accessed on 14 March 2022).
- Leslie, J. L.; Summerell, B. A. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Hoboken, NJ, 2006; p. 212. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, 1990; p. 315. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R. C. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences, 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Edel-Hermann, V.; Gautheron, N.; Durling, M.B.; Kolseth, A.K.; Steinberg, C.; Persson, P.; Friberg, H. Genus-Specific Primers for Study of Fusarium Communities in Field Samples. Appl. Environ. Microbiol. 2015, 82, 491–501. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. and Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Fredrik Ronquist, R.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–10. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v.1.4.4., 2018.
- Matić, M.; Vuković, R.; Vrandečić, K.; Štolfa Čamagajevac, I.; Ćosić, J.; Vuković, A.; Sabljić, K.; Sabo, N.; Dvojković, K.; Novoselović, D. Oxidative status and antioxidative response to Fusarium attack and different nitrogen levels in winter wheat varieties. Plants 2021, 10, 611. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




