1. Introduction
Osteopenia is a condition associated with as many fragility fractures as osteoporosis because of its higher prevalence [
1,
2]. According to World Health Organization criteria, osteopenia is defined as BMD as a T-score -1.0 to -2.5, whereas osteoporosis is defined as a T-score -2.5 or lower [
1,
3]. In the US, 52% of postmenopausal Caucasian woman have osteopenia and 20% have osteoporosis [
4]. In South Korea, 44% of women aged over 66 years had osteopenia based on data of Korea National Screening Program for Transitional Ages 2008-2011 [
5]. Progression of osteopenia and osteoporosis markedly increases the risk of skeletal fractures [
6]. The main goal of screening for and treating osteopenia is to prevent the development of low trauma fractures [
1,
6]. Effective fracture prevention would have a major impact on women’s morbidity and an important impact on mortality [
1,
6].
Osteoporosis may be associated with sarcopenia-a state of low muscle mass and strength, and impaired motor function which increases the risk of falls and fractures. A combination of osteoporosis and sarcopenia is called osteosarcopenia [
7]. Osteoporosis and osteoporotic fractures are prevalent conditions among the elderly, which significantly increase morbidity and mortality, a poor quality of life and a substantial economic burden worldwide [
8,
9,
10,
11]. Direct medical costs of osteoporosis in Americans aged over 50 years was estimated at 13.7–20.3 billion dollars (adjusted to 2005 dollars) [
9]. The prevalence of osteoporosis and osteoporotic fractures is growing more rapidly in South Korea than in other countries [
8]. The elderly population may be 20% of the total population by 2026 in South Korea, leading to a super-aged country in the near future [
12]. In South Korea, a total healthcare expenditure on osteoporosis between 2008 and 2011 increased from
$3,976 million (USD) to
$5,126 million (USD), with an annual increase of 9.2%, which accounted for 16.7% of national healthcare expenditure [
8]. Total healthcare costs for osteoporotic women was 6-fold higher than that for osteoporotic men, while the cost per man was 1.5-fold higher than that for women [
8].
Current therapies including selective estrogen modulators, anti-resorptive pharmacological agents (e.g. bisphosphonates and denosumab), and the bone-forming pharmacological agents (e.g. teriparatide) are expensive, have some side effects and compliance is variable. Lifestyle modifications including diet (calcium, vitamin D, increased protein, reduced salt and increased fruit and vegetables) and physical activity can reduce the risk of osteoporosis and fractures [
13,
14,
15,
16,
17].
In a review [
18,
19] of 5 randomized clinical studies [
20,
21,
22,
23,
24], 100 g/day of whole prune (dried plum) for 1-year improved bone health indices in postmenopausal women [
18,
19]. One study showed 50 g/day of prunes for 6 months also was as effective as 100 g/day of prune in preventing bone loss in older, osteopenic postmenopausal women [
24]. Prunes are a moderate source of vitamin
K (57% of the Daily Value) and prunes contain large amounts of phenolic compounds (184 mg/100 g), mainly as neochlorogenic and chlorogenic acids.. Both of these components may influence bone metabolism. Polyphenols may both inhibit osteoclasts and stimulate osteoblasts[
18,
19]. Currently there are no published clinical data examining the effect of prunes on bone health in Asian women.
Therefore, the aim of this study was to investigate the effects of a 12-month prune consumption on bone mineral density and bone turnover markers in Korean postmenopausal women with osteopenia in a randomized, open-labeled, -controlled design. The primary endpoint was BMD of lumbar spine, forearm, hip and total body using dual-energy X-ray absorptiometry (DXA) at baseline and 12 months. The secondary endpoint was serum vitamin D, serum calcium, serum phosphate, s-CTx and s-P1NP at baseline, 6 and 12 months.
2. Materials and Methods
Subjects
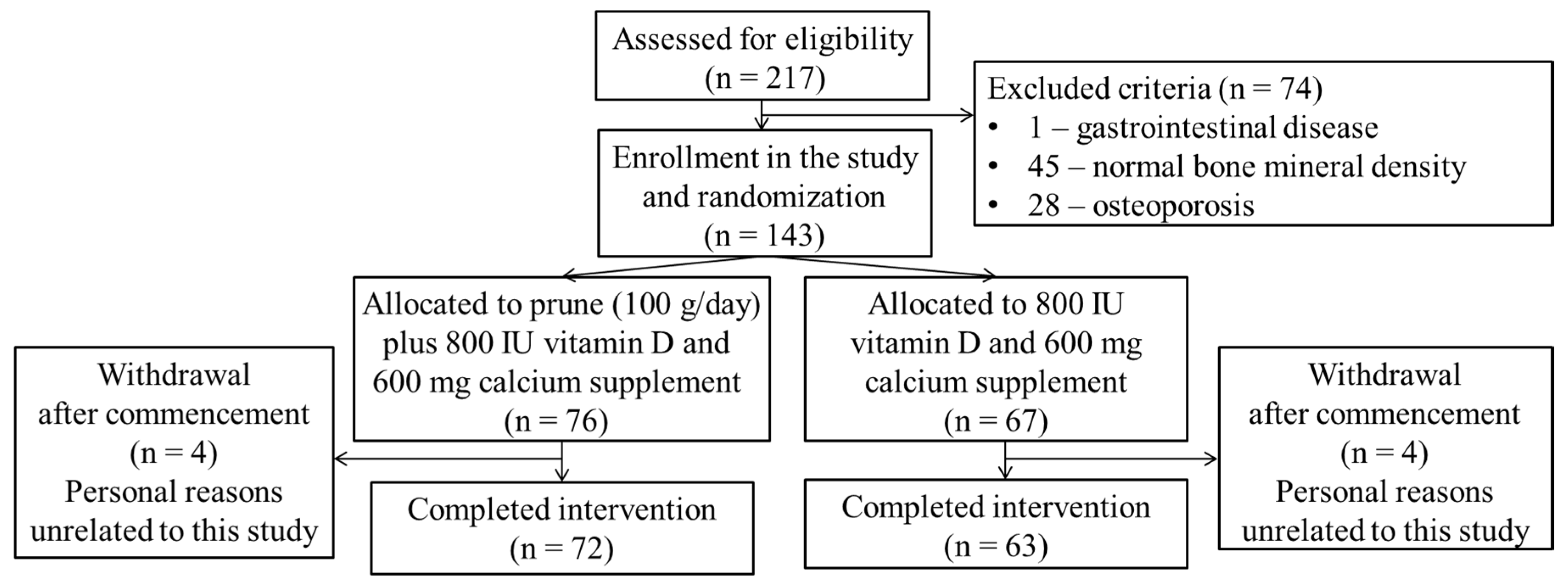
After screening of 217 volunteers, 143 participants with osteopenia were included in this study (
Figure 1). The participants were postmenopausal women with osteopenia, aged 50–75 years, and not on hormone replacement therapy for at least 3 months before initiation of the study. This study was performed in the Gyeongsang national university hospital between June 2020 and December 2022. Recruitment was via flyers at public health centers, and hospitals.
Figure 1.
A flow-diagram of the study participants.
Figure 1.
A flow-diagram of the study participants.
Women with a current or recent use (within the last 3 months) of any medications known to alter bone and calcium metabolism, women with abnormal calcium and phosphate or diseases that alter bone and calcium metabolism and women with low vitamin D with elevated parathyroid hormone (PTH) were excluded. Women who regularly consumed prune or prune juice, women with known intolerances to prunes and women who had particular food allergies, women who did not have the national health insurance and women who smoked were also excluded.
The study was performed according to the guidelines of the Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB) of Gyeongsang National University Hospital (IRB No. GNUH 2020-02-013; web site:
https://www.e-irb.com:3443/index.jsp). This study registered on Clinical Research Information Service (CRIS) (CRIS registration No. KCT0005131; web site:
https://cris.nih.go.kr/cris/search/detailSearch.do?seq=24250&status=5&seq_group=16906&search_page=M). Written informed consent was obtained from all subjects involved in the study. A complete medical history, lifestyle and physical activity surveys were obtained from the participants before commencing the study. The participants were recommended to maintain their usual meal and physical activity pattern.
Study design
Eligible postmenopausal women with osteopenia (n = 143) were randomly allocated into a treatment group or a control group: prunes (100 g/day; n = 76) from the California Prune Board plus 800 IU vitamin D and 600 mg elemental calcium or 800 IU vitamin D and 600 mg elemental calcium (control; n = 67). Randomization was by an online randomization generator. The calcium amount was based on National Osteoporosis Foundation (NOF)’s daily calcium recommendations − women aged over 50 years need a total of 1,200 mg/day assuming 400-600 mg from diet and 600 mg from supplements[
25].
A daily checklist during the intervention was used in order to monitor compliance. Prunes were gradually incorporated into the participant’s diet to ensure tolerance.
Sample size
Determination of the sample size was estimated from the effects on changes in BMD, which was the primary outcome of the trial. Considering several publications [
17,
21,
26], it was expected that a difference between groups of about 1.5% after 12 months with a standard deviation of about 2.5% in total body BMD could be detected between two groups with a power of 80% and two-sided alpha of 0.05 with 45 completers in each group. In terms of absolute changes this represents a difference between groups of 0.01g/cm
2 with a standard deviation of the change of 0.02g/cm
2 Thus we aimed to recruit a total of 120 participants to with an expected rate of dropout of 25% during the trial.
Anthropometric data
A medical history was obtained at the beginning of this study. The international physical activity short survey and anthropometric data were collected at baseline, 6 and 12 months. Body height (cm) was measured to the nearest 0.1 cm. Body weight (kg) was measured to the nearest 0.1 kg with the participant wearing light clothing without shoes. Body height and weight were measured using a stadiometer with scales (DS-103, Dongsahn JENIX, Seoul, Korea). Blood pressure and pulse pressure were measured once on the right upper arm in seated position using an automatic electronic sphygmomanometer (HBP-9020, OMRON Healthcare, Kyoto, Japan).
Measurements of bone mineral density
BMD of lumbar spine (L1-L4), L forearm, R and L hip and total body were assessed at baseline and at the end of the study using dual energy X-ray absorptiometry (DXA; Discovery Wi, Hologic Inc., Bedford, MA, USA). Osteopenia (-2.5 < T-score < -1.0) and osteoporosis (T-score ≤ -2.5) were diagnosed using the World Health Organization (WHO) Asian T-score criteria [
3]. Densitometer accuracy was evaluated by performance of phantom scans on the dates of data acquisition.
Measurements of bone turnover markers
Venous blood samples were collected after an overnight fast at baseline, 6 and 12 months. PTH levels were measured in women with low vitamin D (< 50 nmol/L) at baseline. s-P1NP level was measured using Elecsys total P1NP assay (Roche Diagnostics, Mannheim, Germany) by E170 module immunology analyzer (Roche Diagnostics). s-CTX level was measured using Elecsys β-CrossLaps kit (Roche Diagnostics, Mannheim, Germany) by electrochemiluminescence assay (ECLIA; Roche Diagnostics, Mannheim, Germany). Serum 25(OH)D level was measured using Elecsys vitamin D total electrochemiluminescence binding assay (Roche Diagnostics, Mannheim, Germany) by Cobas 8000 e602 analyzer (Roche Diagnostics). Serum calcium level was measured using colorimetric (NM-BAPTA method) by Roche Cobas 8000 module c701/702 (Roche Diagnostics). Serum phosphorus level was measured using a commercial kit on a Cobas 8000 (Roche Diagnostics, Mannheim, Germany). Serum PTH level was measured using ECLIA (Roche Diagnostics, Mannheim, Germany) on a E170 module immunology analyzer (Roche Diagnostics).
Statistical analysis
The statistical analyses were performed with SPSS 27.0 (IBM, Chicago, IL, USA). BMD (lumbar spine, hip, forearm and total body) (baseline and 12 months), age, height, weight, BMI, blood pressure, pulse rate, physical activity and lab tests (s-CTX, s-P1NP, serum calcium, serum phosphorus and serum vitamin D) (baseline, 6 and 12 months) were tested for normality with the Kolmogorov-Smirnov test, Q-Q plots and histograms. Data was analyzed by repeated measures ANOVA with and without covariates of age, BMI, t score quartile and prune compliance and activity scores. The chi-squared test was used for categorical variables. Change data are presented as means ± standard deviation (SD). Statistical significance level was assumed at P < 0.05.
3. Results
Baseline characteristics of the study participants
As shown in
Figure 1 and
Table 1, of the 217 postmenopausal women who were screened, seventy six participants in test group (dried plum group) and sixty seven participants in the control group were enrolled and four volunteers dropped out of each group over the 12 month period. The mean age of all participants were 60.9 ± 5.8 years. Moderate and high physical activity (5 days per week or more) were non-significantly higher in the prune group while walking 5 days per week or more was similar in both groups. There was no statistically significant difference in demographic characteristics between groups (
Table 1). Compliance to the prune consumption was over 90% in all participants.
Table 1.
Demographic findings of the participants who completed (n = 135).
Table 1.
Demographic findings of the participants who completed (n = 135).
Variables
mean±SD |
Prune group (n = 72)
N (%) |
Control group (n = 63)
N (%) |
P-value |
| Age (year) |
|
60.3 ± 6.1 |
61.5 ± 5.6 |
0.9 |
| BMD L1-L4 g.cm2
|
|
0.885
±0.103 |
0.882
±0.103 |
0.8 |
| BMD Total Hip g.cm2
|
|
0.803
±0.076 |
0.802
±0.066 |
0.9 |
| BMD forearm g.cm2
|
|
0.475
±0.049 |
0.465
±0.052 |
0.3 |
| BMD total body g.cm2
|
|
1.005
±0.056 |
1.003
±0.065 |
0.8 |
| BMI over 25 |
Baseline |
16 (23) |
19 (32) |
0.3 |
| 6 month |
16 (23) |
20 (31) |
0.3 |
| 12 month |
17 (24) |
14 (23) |
0.3 |
| Moderate or high physical activity on 5 or more days per week |
Baseline |
16 (29) |
7 (13) |
0.09 |
| 6 month |
16 (29) |
10 (19) |
0.4 |
| 12 month |
16 (29) |
9 (17) |
0.2 |
| Walking 5 days or more per week |
Baseline |
47 (66) |
45 (73) |
0.4 |
| 6 month |
50 (70) |
42 (68) |
0.4 |
| 12 month |
56 (79) |
43 (70) |
0.2 |
| |
|
|
|
Changes in BMD at 1 year of follow-up for study participants
Table 2 presents the changes of BMD at each site from baseline to 12 months for prune and control group. The change in BMD of total lumbar spine was -0.015 ± 0.024 g/cm² for the prune group and -0.011 ± 0.025 g/cm² for the control group (P = 0.3). The change in BMD of left total hip was -0.007 ± 0.025 g/cm² for the prune group and -0.004 ± 0.033 g/cm² for the control group (P = 0.5). The change in BMD of total forearm was -0.008 ± 0.012 g/cm² for prune group and -0.007 ± 0.015 g/cm² for control group (P = 0.7).
Table 2.
Changes in BMD and T-score at 1 year of follow-.
Table 2.
Changes in BMD and T-score at 1 year of follow-.
| Variables |
Total
(n = 135) |
Prune group
(n = 72) |
Control group
(n = 63) |
P |
| Mean |
SD |
Mean |
SD |
Mean |
SD |
| Lumbar spine BMD |
|
|
|
|
|
|
|
| L1 (g/cm²) |
-0.013 |
0.033 |
-0.013 |
0.036 |
-0.013 |
0.030 |
0.9 |
| L2 (g/cm²) |
-0.012 |
0.039 |
-0.015 |
0.039 |
-0.009 |
0.038 |
0.4 |
| L3 (g/cm²) |
-0.015 |
0.040 |
-0.019 |
0.036 |
-0.010 |
0.043 |
0.2 |
| L4 (g/cm²) |
-0.013 |
0.036 |
-0.014 |
0.031 |
-0.012 |
0.042 |
0.7 |
| Total (g/cm²) |
-0.013 |
0.025 |
-0.015 |
0.024 |
-0.011 |
0.025 |
0.3 |
| Left hip BMD |
|
|
|
|
|
|
|
| Neck (g/cm²) |
-0.004 |
0.024 |
-0.006 |
0.024 |
-0.002 |
0.023 |
0.4 |
| Troch (g/cm²) |
-0.001 |
0.019 |
-0.001 |
0.020 |
-0.001 |
0.018 |
0.9 |
| Inter (g/cm²) |
-0.006 |
0.032 |
-0.007 |
0.031 |
-0.004 |
0.033 |
0.6 |
| Total (g/cm²) |
-0.005 |
0.023 |
-0.007 |
0.025 |
-0.004 |
0.022 |
0.5 |
| Right hip BMD |
|
|
|
|
|
|
|
| Neck (g/cm²) |
-0.004 |
0.028 |
-0.005 |
0.023 |
-0.003 |
0.033 |
0.7 |
| Troch (g/cm²) |
0.000 |
0.018 |
-0.001 |
0.017 |
0.001 |
0.019 |
0.5 |
| Inter (g/cm²) |
0.004 |
0.031 |
0.002 |
0.029 |
0.005 |
0.033 |
0.5 |
| Total (g/cm²) |
0.001 |
0.023 |
0.000 |
0.022 |
0.003 |
0.024 |
0.4 |
| Forearm BMD |
|
|
|
|
|
|
|
| UD (g/cm²) |
-0.005 |
0.016 |
-0.006 |
0.015 |
-0.004 |
0.018 |
0.6 |
| MID (g/cm²) |
-0.008 |
0.014 |
-0.008 |
0.013 |
-0.007 |
0.015 |
0.6 |
| 1/3 (g/cm²) |
-0.011 |
0.017 |
-0.011 |
0.017 |
-0.011 |
0.017 |
0.9 |
| Total (g/cm²) |
-0.008 |
0.013 |
-0.008 |
0.012 |
-0.007 |
0.015 |
0.7 |
|
Total body BMD (g/cm²) |
-0.008 |
0.021 |
-0.008 |
0.020 |
-0.007 |
0.023 |
0.7 |
Laboratory results of the study participants
Table 3 presents the mean and SD of the difference in serum bone biomarkers (CTX, P1NP, vitamin D, calcium and phosphorus) from baseline to 6 months and baseline to 12 months. The change in serum CTX, a marker of bone resorption, was -0.042 ± 0.13 ng/mL for prune group and -0.046 ± 0.16 ng/mL for control group between baseline and 6 months (P = 0.9). Between baseline and 12 months changes in serum CTX were -0.045 ± 0.15 ng/mL for prune group and -0.024 ± 0.17 ng/mL for control group (P = 0.6). Changes in serum P1NP, a marker of bone formation, were -4.03 ± 14.1 ng/mL for prune group and -5.1 ± 14.4 ng/mL for control group between baseline and 6 months (P = 0.7). Changes in serum P1NP were -2.15 ± 14.4 ng/mL for prune group and -2.42 ± 16.0 ng/mL for control group between baseline and 12 months (P = 0.9). Changes in serum vitamin D were 4.7 ± 6.9 ng/mL for prune group and 6.0 ± 6.7 ng/mL for control group between baseline to 6 months (P = 0.4)
Table 3.
Laboratory results of the study participants (n = 135).
Table 3.
Laboratory results of the study participants (n = 135).
| Variables |
Total |
Prune group |
Control group |
P-value |
CTx
(ng/mL) |
6 month - baseline |
-0.044 ± 0.15 |
-0.042 ± 0.13 |
-0.046 ± 0.16 |
0.9 |
| 12 month - baseline |
-0.035 ± 0.16 |
-0.045 ± 0.15 |
-0.024 ± 0.17 |
0. 6 |
P1NP
(ng/mL) |
6 month - baseline |
-4.60 ± 14.2 |
-4.03 ± 14.1 |
-5.14 ± 14.4 |
0.7 |
| 12 month - baseline |
-2.3 ± 15.0 |
-2.15 ± 14.4 |
-2.4 ± 16.0 |
0.9 |
Vit D
(ng/mL) |
6 month - baseline |
5.3 ± 6.7 |
4.7 ± 6.8 |
6.0 ± 6.7 |
0.4 |
| 12 month - baseline |
5.5 ± 7.3 |
4.7 ± 8.4 |
6.3 ± 5.9 |
0.3 |
Ca
(mg/dL) |
6 month - baseline |
0.06 ± 0.4 |
-0.01 ± 0.3 |
0.1 ± 0.4 |
0.08 |
| 12 month - baseline |
-0.1 ± 0.4 |
-0.2 ± 0.4 |
-0.05 ± 0.42 |
0.2 |
P
(mg/dL) |
6 month - baseline |
0.03 ± 0.6 |
0.06 ± 0.6 |
-0.01 ± 0. |
0.6 |
| 12 month - baseline |
-0.06 ± 0.5 |
-0.08 ± 0.5 |
-0.04 ± 0.5 |
0.7 |
4. Discussion
This study examined the effect of 100 g/day of prunes for 12 months on BMD and bone turnover markers of Korean postmenopausal women with osteopenia in a randomized, open-labeled, controlled design. No effects were seen with prunes in either serum markers or BMD at any site.
In the first human studies using prunes Arjmandi et al. [
20] randomly assigned fifty-eight postmenopausal women not on hormone replacement therapy (HRT) to consume either 100 g dried plums or 75 g dried apples daily for 3 months. In comparison with corresponding baseline values, only dried plums significantly increased serum levels of insulin-like growth factor-I (IGF-I) and bone-specific alkaline phosphatase (BSAP) activity. The two groups however were not significantly different from each other. Hooshmand et al. [
21] investigated the effect of prune intake (100 g/day) on BMD in postmenopausal women with osteopenia compared to dried apple intake for 12 months in 162 women. Both groups received 500 mg calcium plus 400 IU vitamin D daily. A total of 100 participants completed the study, with 45 in the prune group and 55 in the dried apple group. In this study [
21], bone biomarkers [bone-specific alkaline phosphatase (BALP), osteocalcin (OC), tartrate-resistant acid phosphatase-5b (TRAB-5b) and C-reactive protein (CRP) at baseline, 3, 6 and 12 months] and BMD (whole body, lumbar spine, hip and femoral neck at baseline and at the end of the study) were measured. This study showed that BMD in the prune group significantly increased at the ulna and spine BMD compared to the dried apple group. No differences were seen between groups in serum markers [
21]. In a second paper RANKL and sclerostin were measured but there was no difference between groups [
22]. In a small study prunes had no additional effect to resistance training in bone turnover markers over 6 months in breast cancer survivors [
23].
De Souza et al. study [
27,
28] evaluated the effects of prune intakes (50 g/day and 100 g/day) on BMD and bone turnover in postmenopausal women for 12 months. A total of 183 participants completed, 46 subjects in the 100 g/day prune group, 67 subjects in the 50 g/day prune group and 70 subjects in the control group (0 g/day prune) were completed. All study participants received 1200 mg calcium plus 800 IU vitamin D3 daily. This study measured BMD (lumbar spine, hip and total body) at screening, 6 and 12 months and also assessed bone biomarkers (P1NP, CTx, IGF-1 and vitamin D) at baseline, 6 and 12 months [
27]. Using 50 g of prunes per day a diet by time effect was seen in total hip BMD (P = 0.017) but no other bone area with an intention to treat analysis. Using a completors analysis at 12 months the effect on % change in BMD was stronger with a 1.1% fall in the control group and a 0.27% fall in the prune group (P = 0.011). With 100 g/day power was reduced by dropouts and a significant variation in the response so that the diet by time interaction was P = 0.287 despite the mean difference between groups being similar (0.005) in the 50 and 100 g groups. In the participants who completed the full 12-mo intervention, there was no difference in percent change in total hip BMD for the control compared with the 100-g prune groups (– 1.1 ± 0.2% compared with –0.23 ± 0.4%, P = 0.131).
With both prune groups combined a group × time interaction was borderline significant for total hip BMD (P = 0.051). In the participants who completed the full 12-mo intervention, the control group lost 1.1 ± 0.2% BMD at the total hip compared with the pooled prune group, who lost 0.25 ± 0.2% (P = 0.007). No significant effects were seen with prune supplementation in blood biomarkers.
Given the negative results from this study in Korean women and the negative results with the 100 g supplementation in the De Souza study we have a quandary. Previous studies using 100 g of prunes have shown no ill effects of this amount American women while in the De Souza study it appears some volunteers have no response to it while others had a large response given the mean change and the SD in this group. Does this variation in response to the higher dose reflect pre-existing intake of polyphenols with no response (or a negative response) in those individuals with a high intake of fruits and vegetables and/or tea and coffee? Does this response also explain the negative effect in Korean women who would be expected to have a much higher intake of polyphenols than American women. We assume that Korean women have quite different microbiota to Caucasian women in the USA given the geographical microbiome data [
29] and the microbiome in Japanese [
30] and this may influence the response to prunes. Increased plant intake and polyphenol-rich green tea intake in addition to a Mediterranean diet has been shown to modulate the microbiome [
31]. The microbiome has been related to bone density and susceptibility to osteoporosis in women [
32]. Prunes appear to modulate inflammation in preclinical studies [
33] as well as change the gut microbiota [
34]. Polyphenol intake is related to bone density while coffee intake is related to bone density in Korean [
35] and Chinese women [
36] with polyphenol metabolites also related to bone density in the latter study. One particular polyphenol, resveratrol, increases bone density in Australian women but this may be partly due to its estrogenic action [
37]. One area of inconsistency in the high plant, high polyphenol hypothesis is the finding that vegetarian and especially vegan women in the Oxford EPIC study have a higher fracture rate than omnivores even after adjusting for BMI, protein and calcium intake [
38].
It is difficult to understand the varying effects of prunes on different regions in different studies with no consistency apparent. Certainly in the De Souza study where only hip BMD was different there is no suggestion of an effect on total body or lumbar spine BMD nor is any effect on hip BMD seen in the other studies.
Conclusions
In conclusion, in Asian women 100 g/day of prunes is not beneficial. This is the first study in non Caucasian women. Given the results for the 50 g/day group in American women it is possible this dose of prunes may be beneficial in Korean women and we recommend further research be conducted in Asian women with a lower dose of prunes.
Author Contributions
Formal analysis, Peter Clifton; Funding acquisition, Yoona Kim; Investigation, Minkyung Je Je; Project administration, Yoona Kim; Resources, Jun-il Yoo and Minkyung Je Je; Supervision, Yoona Kim; Writing – original draft, Jun-il Yoo; Writing – review & editing, Yoona Kim and Peter Clifton., KY. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by CALIFORNIA PRUNE BOARD, grant number-not applicable Prunes were aupplied by the California Prune Board. The funders had no role in study design, analysis or publication.
Institutional Review Board Statement
The study was performed according to the guidelines of the Declaration of Helsinki. This study was approved by the Institutional Review Board (IRB) of Gyeongsang National University Hospital (IRB No. GNUH 2020-02-013). The trial was registered with the Korean clinical trial registry number KCT0005131.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We thank all participants for their time and efforts to contribute to this project. We thank Hyelim Chin, Somin Lee, Subin Kim, Kyeonghoon Kang, Yeonjeong Choi and Seoyoung Oh for assistance with the study.
Conflicts of Interest
There was no financial relationship between the authors and the funder. The California Prune Board provided the funding only. It had no role in study design, study analysis or publication.
References
- Karaguzel, G.; Holick, M.F. Diagnosis and treatment of osteopenia. Rev Endocr Metab Disord 2010, 11, 237–251. [Google Scholar] [CrossRef]
- Cranney, A.; Jamal, S.A.; Tsang, J.F.; Josse, R.G.; Leslie, W.D. Low bone mineral density and fracture burden in postmenopausal women. Cmaj 2007, 177, 575–580. [Google Scholar] [CrossRef] [PubMed]
- WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organization: Geneva, 1994.
- Neda Sarafrazi, P.D., Edwina A. Wambogo, Ph.D., M.S., M.P.H., R.D., and John A. Shepherd, . Osteoporosis or low bone mass in older adults: United states, 2017–2018. NCHS Data Brief No. 405 March 2021 2021.
- Baek, Y.H.; Cho, S.W.; Jeong, H.E.; Kim, J.H.; Hwang, Y.; Lange, J.L.; Shin, J.Y. 10-year fracture risk in postmenopausal women with osteopenia and osteoporosis in south korea.
- Khosla, S.; Melton, L.J., 3rd. Clinical practice. Osteopenia. N Engl J Med 2007, 356, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Gregson, C.L.; Bruyère, O.; Cooper, C.; Dennison, E.M. Osteosarcopenia: Where osteoporosis and sarcopenia collide.
- Ha, Y.C.; Kim, H.Y.; Jang, S.; Lee, Y.K.; Kim, T.Y. Economic burden of osteoporosis in south korea: Claim data of the national health insurance service from 2008 to 2011. Calcif Tissue Int 2017, 101, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the united states, 2005-2025. J Bone Miner Res 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Tatangelo, G.; Watts, J.; Lim, K.; Connaughton, C.; Abimanyi-Ochom, J.; Borgström, F.; Nicholson, G.C.; Shore-Lorenti, C.; Stuart, A.L.; Iuliano-Burns, S. , et al. The cost of osteoporosis, osteopenia, and associated fractures in australia in 2017. J Bone Miner Res 2019, 34, 616–625. [Google Scholar] [CrossRef]
- Zanker, J.; Duque, G. Osteoporosis in older persons: Old and new players. J Am Geriatr Soc 2019, 67, 831–840. [Google Scholar] [CrossRef]
- Ha, Y.C.; Park, Y.G.; Nam, K.W.; Kim, S.R. Trend in hip fracture incidence and mortality in korea: A prospective cohort study from 2002 to 2011. J Korean Med Sci 2015, 30, 483–488. [Google Scholar] [CrossRef]
- Liu, Z.M.; Leung, J.; Wong, S.Y.; Wong, C.K.; Chan, R.; Woo, J. Greater fruit intake was associated with better bone mineral status among chinese elderly men and women: Results of hong kong mr. Os and ms. Os studies. J Am Med Dir Assoc 2015, 16, 309–315. [Google Scholar] [CrossRef]
- Hannan, M.T.; Tucker, K.L.; Dawson-Hughes, B.; Cupples, L.A.; Felson, D.T.; Kiel, D.P. Effect of dietary protein on bone loss in elderly men and women: The framingham osteoporosis study. J Bone Miner Res 2000, 15, 2504–2512. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C. , et al. Dietary protein and bone health: A systematic review and meta-analysis from the national osteoporosis foundation. Am J Clin Nutr 2017, 105, 1528–1543. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.L.; Devine, A.; Dhaliwal, S.S.; Dick, I.M. Effects of calcium supplementation on clinical fracture and bone structure: Results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med 2006, 166, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Dallal, G.E.; Krall, E.A.; Sadowski, L.; Sahyoun, N.; Tannenbaum, S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990, 323, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Johnson, S.A.; Pourafshar, S.; Navaei, N.; George, K.S.; Hooshmand, S.; Chai, S.C.; Akhavan, N.S. Bone-protective effects of dried plum in postmenopausal women: Efficacy and possible mechanisms. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Wallace, T.C. Dried plums, prunes and bone health: A comprehensive review. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H.; Khalil, D.A.; Lucas, E.A.; Georgis, A.; Stoecker, B.J.; Hardin, C.; Payton, M.E.; Wild, R.A. Dried plums improve indices of bone formation in postmenopausal women. J Womens Health Gend Based Med 2002, 11, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br J Nutr 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Hooshmand, S.; Brisco, J.R.; Arjmandi, B.H. The effect of dried plum on serum levels of receptor activator of nf-κb ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: A randomised controlled trial. Br J Nutr 2014, 112, 55–60. [Google Scholar] [CrossRef]
- Simonavice, E.; Liu, P.Y.; Ilich, J.Z.; Kim, J.S.; Arjmandi, B.; Panton, L.B. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl Physiol Nutr Metab 2014, 39, 730–739. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos Int 2016, 27, 2271–2279. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O'Karma, M.; Wallace, T.C.; Zemel, B.S. The national osteoporosis foundation's position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations.
- Harris, S.; Dallal, G.E.; Dawson-Hughes, B. Influence of body weight on rates of change in bone density of the spine, hip, and radius in postmenopausal women. Calcif Tissue Int 1992, 50, 19–23. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Strock, N.C.; Rogers, C.J.; Williams, N.I.; Ferruzzi, M.G.; Nakatsu, C.H.; Simpson, A.M.; Weaver, C. Rationale and study design of randomized controlled trial of dietary supplementation with prune (dried plums) on bone density, geometry, and estimated bone strength in postmenopausal women: The prune study. Contemporary Clinical Trials Communications 2022, 28, 100941. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Strock, N.A.-O.X.; Williams, N.I.; Lee, H.; Koltun, K.A.-O.; Rogers, C.; Ferruzzi, M.G.; Nakatsu, C.H.; Weaver, C. Prunes preserve hip bone mineral density in a 12-month randomized controlled trial in postmenopausal women: The prune study.
- Yatsunenko, T.; Rey Fe Fau - Manary, M.J.; Manary Mj Fau - Trehan, I.; Trehan I Fau - Dominguez-Bello, M.G.; Dominguez-Bello Mg Fau - Contreras, M.; Contreras M Fau - Magris, M.; Magris M Fau - Hidalgo, G.; Hidalgo G Fau - Baldassano, R.N.; Baldassano Rn Fau - Anokhin, A.P.; Anokhin Ap Fau - Heath, A.C., et al. Human gut microbiome viewed across age and geography.
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy japanese and its microbial and functional uniqueness.
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J., et al. The effects of the green-mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial.
- Cronin, O.; Lanham-New, S.A.; Corfe, B.M.; Gregson, C.L.; Darling, A.L.; Ahmadi, K.R.; Gibson, P.S.; Tobias, J.H.; Ward, K.A.; Traka, M.H. , et al. Role of the microbiome in regulating bone metabolism and susceptibility to osteoporosis.
- Damani, J.J.; De Souza, M.J.; VanEvery, H.L.; Strock, N.C.A.; Rogers, C.A.-O. The role of prunes in modulating inflammatory pathways to improve bone health in postmenopausal women.
- Simpson, A.A.-O.; De Souza, M.J.; Damani, J.A.-O.; Rogers, C.; Williams, N.I.; Weaver, C.; Ferruzzi, M.A.-O.; Chadwick-Corbin, S.; Nakatsu, C.A.-O.X. Prune supplementation for 12 months alters the gut microbiome in postmenopausal women.
- Choi, E.; Choi, K.H.; Park, S.M.; Shin, D.; Joh, H.K.; Cho, E. The benefit of bone health by drinking coffee among korean postmenopausal women: A cross-sectional analysis of the fourth & fifth korea national health and nutrition examination surveys.
- Chau, Y.P.; Au, P.C.M.; Li, G.H.Y.; Sing, C.W.; Cheng, V.K.F.; Tan, K.C.B.; Kung, A.W.C.; Cheung, C.L. Serum metabolome of coffee consumption and its association with bone mineral density: The hong kong osteoporosis study. Lid - dgz210 [pii] lid - 10.1210/clinem/dgz210 [doi].
- Wong, R.A.-O.; Thaung Zaw, J.J.; Xian, C.A.-O.; Howe, P.A.-O. Regular supplementation with resveratrol improves bone mineral density in postmenopausal women: A randomized, placebo-controlled trial.
- Tong, T.A.-O.; Appleby, P.N.; Armstrong, M.E.G.; Fensom, G.K.; Knuppel, A.; Papier, K.; Perez-Cornago, A.; Travis, R.C.; Key, T.J. Vegetarian and vegan diets and risks of total and site-specific fractures: Results from the prospective epic-oxford study.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).