1. Introduction
The brewing of vinegar mainly includes two major fermentation processes: alcohol fermentation and acetic acid fermentation. The alcohol fermentation stage mainly completes the saccharification and alcoholic fermentation of raw materials through saccharifying enzymes and other fungal strains produced by yeast and molds. The acetic acid fermentation stage mainly metabolizes alcohol into acetic acid through bacterial strains such as acetic acid bacteria and lactic acid bacteria[
1]. The complicated composition of Chinese vinegars reflects the raw materials and production processes used in their manufacture. Vinegar takes a long time to produce; the most timeconsuming stage is the maturation process. The traditional production process involves a maturation period during which many substances that impart Chinese vinegar has its own distinct sensory characteristics[
2]. The quality of Cuqu and vinegar mash plays a decisive role in the final quality of aged vinegar products. Accurately grasping and understanding the changes in physicochemical characteristics during the fermentation process of aged vinegar is conducive to reasonable control of the production process of aged vinegar, optimization and improvement of the production process, and also provides certain theoretical support for the transformation of the aged vinegar industry to mechanized production. Vinegar brewing is an open and multi microbial fermentation process, in which a large number of microorganisms participate and continuously succession, playing an important role in the quality of aged vinegar[
1]. Some reports have pointed out that analyzing the structure and diversity of microbial communities is an important means to reveal the metabolic mechanism of vinegar brewing process[
3]. Therefore, studying the dynamic changes of microorganisms during vinegar brewing is of great significance for improving the quality of vinegar.
This experiment tracked and sampled the vinegar mash during the brewing process of aged vinegar, and studied the dynamic changes in physicochemical characteristics such as moisture content, pH value, and total acid during the fermentation process, in order to provide systematic experimental research data for production control and process transformation. Through high-throughput sequencing, the microbial diversity and change rule in the fermentation process of Shanxi aged vinegar were studied, so as to obtain the microbial succession rule in the fermentation process of Shanxi aged vinegar and the dominant bacteria in different fermentation stages, and the correlation analysis of microorganisms and physicochemical characteristics was carried out, not only to provide data support for identifying the different microorganisms in the alcohol fermentation stage and acetic acid fermentation stage of Shanxi aged vinegar, It also provides a theoretical basis for establishing corresponding rapid detection methods and microbial standard systems in the next step to test whether there are any abnormalities in the fermentation of aged vinegar.
2. Material and method
2.1. Reagents and instruments
2.1.1. Reagents and Materials
Sodium hydroxide, formaldehyde, DNS reagent, acetic acid, and sodium acetate were purchased from Solebao (Beijing, China); Glucose and casein were purchased from Tianjin Guangfu Technology Development Co., Ltd; NaH2PO4 and Na2HPO4 were purchased from Tianjin Bodi Chemical Co., Ltd; The GeneJET gel recycling kit was purchased from Thermo Scientific; CTAB Genomic DNA Extraction Kit DPPH purchased from Beijing Baiao Leibo Technology Co., Ltd.
2.1.2. Instruments and equipment
PH meter (Shanghai Yuejin Medical Instrument Co., Ltd.), UV spectrophotometer (Shanghai Spectral Instrument Co., Ltd.), moisture content analyzer (Shenzhen Guanya Moisture Instrument Technology Co., Ltd.), H2100R desktop high-speed frozen centrifuge (Hunan Xiangyi Laboratory Instrument Development Co., Ltd.), MX-S vortex mixer (Beijing Jiahang Bochuang Technology Co., Ltd.), NanoBio 200 ultra micro spectrophotometer (Aopu Tiancheng Technology Co., Ltd.), gel imaging system (Beijing Boao Jingdian Biotechnology Co., Ltd.), T100 PCR amplification instrument (Bio rad Company of the United States).
2.2. Experimental methods
2.2.1. Sample
From April to May 2021, samples of Cuqu and vinegar mash used in the same production batch were collected from a vinegar factory in Yangquan City, Shanxi Province.
Cuqu sample (C0): After crushing the koji blocks used in production, the 5-point sampling method is used. After thoroughly mixing, 500g is taken and placed in a sterile sealed bag. Store in a refrigerator at -80 ℃ for future use.
Vinegar mash samples: Take samples from the 1st day (C1), 3rd day (C2), 5th day (C3), 7th day (C4), 10th day (C5), 13th day (C6), 16th day (C7), 20th day (C8), and 25th day (C9) of fermentation, respectively. The sampling point is 35 cm below the surface of the vinegar mash. Take 500 g of samples and place them in a sterile sealed bag. Store them in a refrigerator at -80 ℃ for future use.
2.2.2. Method for determining physicochemical characteristics
2.2.2.1. Sample pretreatment
In addition to measuring the moisture content, when measuring its remaining physicochemical characteristics, weigh 10 g of the sample and add 30 mL of distilled water to soak for 1 hour. Filter with filter paper, and take the filtrate for subsequent measurement.
2.2.2.2. Determination method
Determination of moisture content: Weigh 2g of Cuqu and vinegar mash samples and use a moisture content analyzer for measurement; Determination of pH, total acid, reducing sugar, saccharification power, and amino acid nitrogen: refer to reference for the specific operation process[
2]; Determination of soluble salt free solids: Refer to the method in GB/T 18186-2000 “Brewing Soy Sauce”. Each sample is measured three times in parallel.
2.2.3. Research methods for microbial community dynamics
2.2.3.1. Sample pretreatment
The sample pre-treatment method is based on reference[
4], with 3 parallel treatments set for each sample.
2.2.3.2. Total DNA extraction of samples
CTAB method is used to extract the total DNA of the sample, and the total DNA obtained is detected by electrophoresis on 1.0% agarose gel. The agarose gel electrophoresis detection parameters are as follows: Marker sample loading 2 μ L. Sample loading volume 3 μ L. Electrophoresis time 40 minutes, agarose concentration 1.0%, voltage 100 V.
2.2.3.3. PCR amplification and high-throughput sequencing
After testing the purity and concentration of DNA, using genomic DNA as a template, PCR amplification was performed using primers 341F (5 ‘- GCTACGGGNGGWGCAG-3’)/805R (5 ‘- GATACHVGGGTATCTAATCC-3’) and 1737F (5 ‘- GAAGTAAAAGTCGTAACAG-3’)/2043R (5 ‘- GCTGTGTTCATCGATGC-3’) to amplify the gene sequences of bacterial 16S rDNA V3-V4 region and fungal ITS1 region, respectively. PCR amplification system 30 μ L:Phusion Master Mix(2 ×) 15 µL,Primer(2 µmol/L)3µL,gDNA(1 ng/µL)10µL,ddH2O 2µL. Reaction procedure: pre denaturation at 98 ℃ for 1 minute; 98 ℃ for 10 seconds, 50 ℃ for 30 seconds, 72 ℃ for 30 seconds, 30 cycles; 72 ℃,5 min。 After amplification, 2% agarose gel electrophoresis was used for detection, and the product was recovered with GeneJET gel recovery kit. The qualified amplification products were high-throughput sequencing through the Illumina NovaSeq 6000 platform of Beijing Nuohe Zhiyuan Technology Co., Ltd.
2.3. Data analysis
First, remove the barcode and primer sequence from the offline data, and use FLASH (V1.2.11) software for double-ended sequencing reads splicing. Fastp (V0.23.0) software for quality control; Usearch (10.0.259) software removes chimeras; Using QIIME2 (version 2021.8) software for OTU (Operational Taxonomic Unit) partitioning and species annotation and alpha diversity analysis; T-test is implemented through R software (3.6.3); SPSS (IBM23) software was used to calculate Spearman correlation coefficients and perform significance analysis of differences. Origin8.5 software was used to analyze the data and plot it.
3. Result and Analysis
3.1. Analysis of physicochemical characteristics
3.1.1. Analysis of the physicochemical characteristics of Cuqu
From
Table 1, it can be seen that the moisture content of Cuqu used for the fermentation of Shanxi aged vinegar is 13.71 ± 0.36%. Excessive moisture content of koji can easily lead to secondary mold formation and affect the quality of koji[
5]. Acidity is also one of the key indicators for evaluating the quality of Daqu, the acidity of Cuqu used in this experiment is 1.49 ± 0.11g/100g, Organic acids in vinegar, such as Tartaric acid, contribute greatly to the overall sensory flavor[
6]. The content of amino acid nitrogen will affect the aroma of Cuqu, and the higher the content, the stronger the flavor[
7]. The experimental determination showed that the amino acid nitrogen content of Shanxi aged vinegar was 0.42 ± 0.05g/100g. Rong et al found that the amino acid nitrogen content of Daqu used in Shanxi aged vinegar was 0.16 g/100g[
8]. Amino acids not only endow brewing wine with various taste characteristics such as freshness, sweetness, bitterness, and astringency, but also provide nitrogen sources for the growth and metabolism of microorganisms such as yeast during the fermentation process, promoting fermentation effect[9, 10]. The saccharification power of Cuqu measured in this experiment is 1811.30 ± 2.31U/g. It is speculated that the number of mold microorganisms in Cuqu in this experiment is more than that of “traditional Chinese medicine Cuqu” in Baoning vinegar.
3.1.2. Analysis of changes in physicochemical characteristics during the fermentation process of aged vinegar
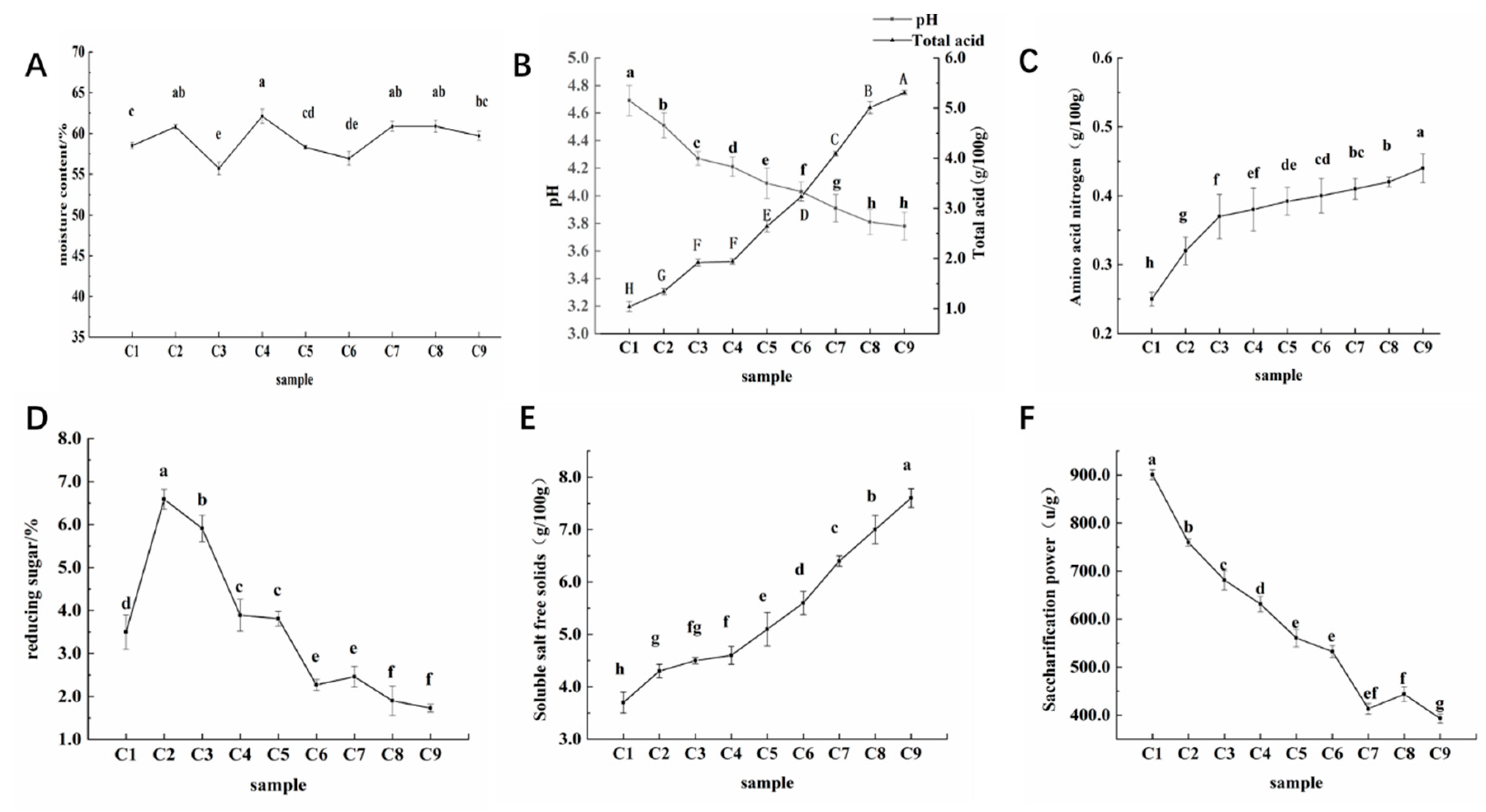
The changes in moisture content during the fermentation process of aged vinegar are shown in
Figure 1, and the moisture content remains between 55.72 ± 2.17% and 62.11 ± 1.98% throughout the entire fermentation stage. PH and total acid exhibit distinct changes during the fermentation process, with pH consistently decreasing to a minimum of 3.78 ± 0.08, while total acid continues to rise from the initial 1.04 ± 0.12g/100g to 5.31 ± 0.20g/100g. During the fermentation process of aged vinegar, the amino acid nitrogen content increased from the initial 0.25 ± 0.05g/100g to the final 0.44 ± 0.08g/100g. Reducing sugar shows a trend of first increasing and then decreasing during the fermentation process of aged vinegar. The soluble salt free solids showed a continuous upward trend throughout the fermentation process of aged vinegar, with the final content of 7.6 ± 0.62g/100g, which is more than twice that of 3.7 ± 0.77g/100g in the C1 period. Except during the fermentation period of C6~C8, the saccharification power fluctuated slightly, showing a state of first decreasing and then increasing. The remaining fermentation periods showed a downward trend, from 900.61 ± 2.41U/g at the beginning to 393.18 ± 1.96U/g at the end of fermentation.
During the reproduction and metabolism of microorganisms, a portion of water is generated, and at the same time, the daily turning of fermented grains during the acetic acid fermentation stage causes a portion of water to be lost, resulting in fluctuating changes in water content. In the early stage of fermentation, with sufficient raw materials, a large number of microorganisms begin to accumulate and metabolize, including acid-producing bacteria such as acetic acid bacteria, which can oxidize ethanol, propanol, and butanol to acetic acid, pyruvate, and butyric acid[
11], respectively. Therefore, pH changes are significant. C4~C8 is the acetification stage. Acetic acid bacteria metabolize a large amount of ethanol accumulated in the Ethanol fermentation stage into acetic acid, resulting in an increase in the total acid content. In addition to acetic acid production, there are also organic acids such as succinic acid, oxalic acid, lactic acid, etc. These acids have the ability to buffer H+in acetic acid[12, 13], so the total acid increases sharply, but the pH change is relatively gentle. In the early stage of fermentation, the content of amino acid nitrogen significantly increases, possibly due to the protein in the raw material being decomposed by microorganisms into amino acids and peptides[
14], and the yeast will self dissolve and release peptide substances[
15] in the later stage of alcohol fermentation. During the C1~C2 period, reducing sugars significantly increase, and mold decomposes a large amount of starch in the raw material into reducing sugars[
16]. Afterwards, the consumption rate of reducing sugars is greater than the generation rate, mainly reflected in the utilization of reducing sugars by yeast to produce alcohol[
17]. In the early stage of fermentation, the starch in the bran is decomposed by Cuqu and the amylase contained in the bran itself. The results of this study are similar to the trend of changes in saccharification capacity during the fermentation process of Sichuan bran vinegar. It is speculated that the reason may be the accumulation of alcohol and acid substances during the fermentation process, which inhibits microbial metabolism and leads to a decrease in saccharification capacity[
18].
3.2. Analysis of microbial community changes during the fermentation process of aged vinegar
3.2.1. Dilution curve analysis
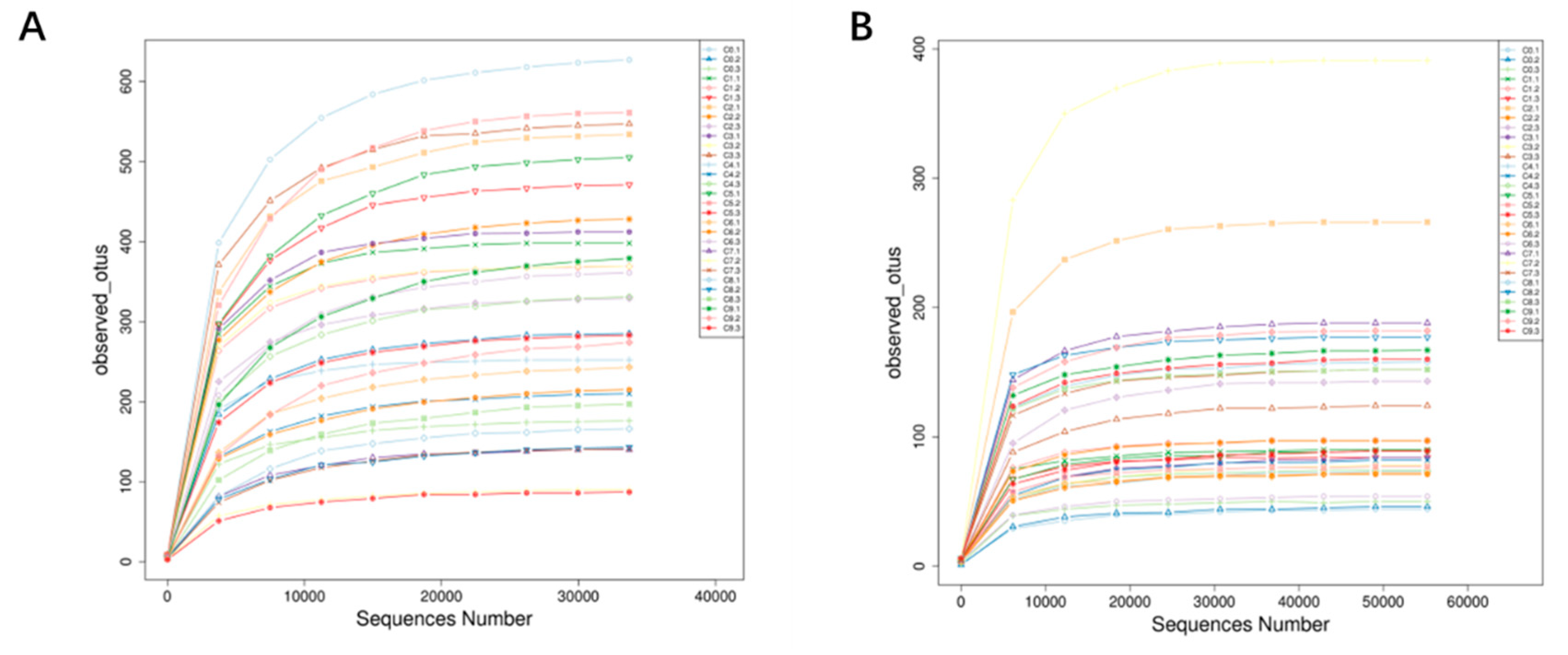
From
Figure 2, it can be seen that as the sequencing depth continues to increase, the dilution curves of bacteria and fungi eventually tend to flatten, indicating a high coverage of sequencing quantity, which can reflect the microbial information of most bacteria and fungi in Cuqu and vinegar mash samples, and the sequencing results are reliable.
3.2.2. Alpha Diversity Analysis
The Chao1 index and Simpson index were used to analyze the microbial diversity during the fermentation process of Shanxi aged vinegar. As shown in
Table 2, analyzing and comparing the Simpson index, it can be seen that the Simpson index of bacteria fluctuates between 0.536 ± 0.151 and 0.960 ± 0.054, while the Simpson index of fungi fluctuates between 0.539 ± 0.072 and 0.868 ± 0.011, indicating that the diversity of fungi and bacterial communities is constantly changing during the fermentation process. From these two indices, it can be seen that there are differences in the diversity and richness of microbial communities in Cuqu and vinegar mash at different stages used in Shanxi’s aged vinegar fermentation.
3.2.3. Venn plot analysis of different fermentation stages
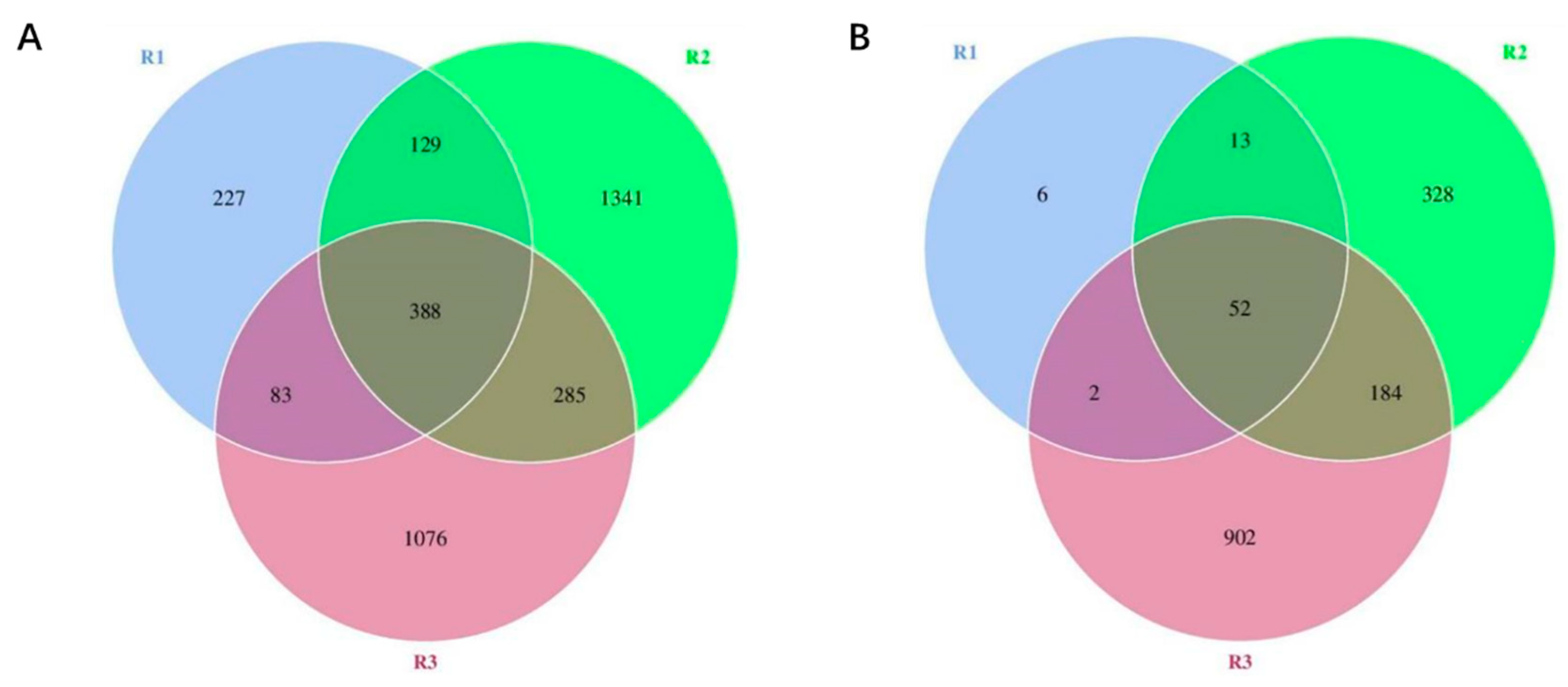
Set Cuqu (C0) as group R1, alcohol fermentation stage (C1~C4) as group R2, and acetic acid fermentation stage (C5~C9) as group R3. From
Figure 3, it can be seen that a total of 3529 bacterial OTUs were detected in the Cuqu and vinegar mash samples. The OTU numbers of bacteria in different stages were R1 (827)<R3 (1832)<R2 (2143), and a total of 388 bacterial OTUs were detected in the three stages. Among them, the OTU number of R2 bacteria reached more than twice that of R1, which may be due to the addition of other raw materials during alcohol fermentation, thereby introducing new strains [
27]. In addition, the number of OTUs of bacteria in R2 group is the largest, which indicates that the types of bacteria involved in the alcohol fermentation stage are the most abundant. It may be that as the fermentation proceeds, some microorganisms with a low number gradually adapt to the fermentation environment and continue to enrich, reaching the threshold of high-throughput sequencing detection. During the entire fermentation process, a total of 1487 fungal OTUs were detected, with R1 (73)<R2 (577)<R3 (1140) at different stages. The total number of fungal OTUs in the three stages was 52. And the number of fungal OTUs in R3 reaches its peak at most.
3.2.4. Analysis of the Succession of Bacterial Community Structure
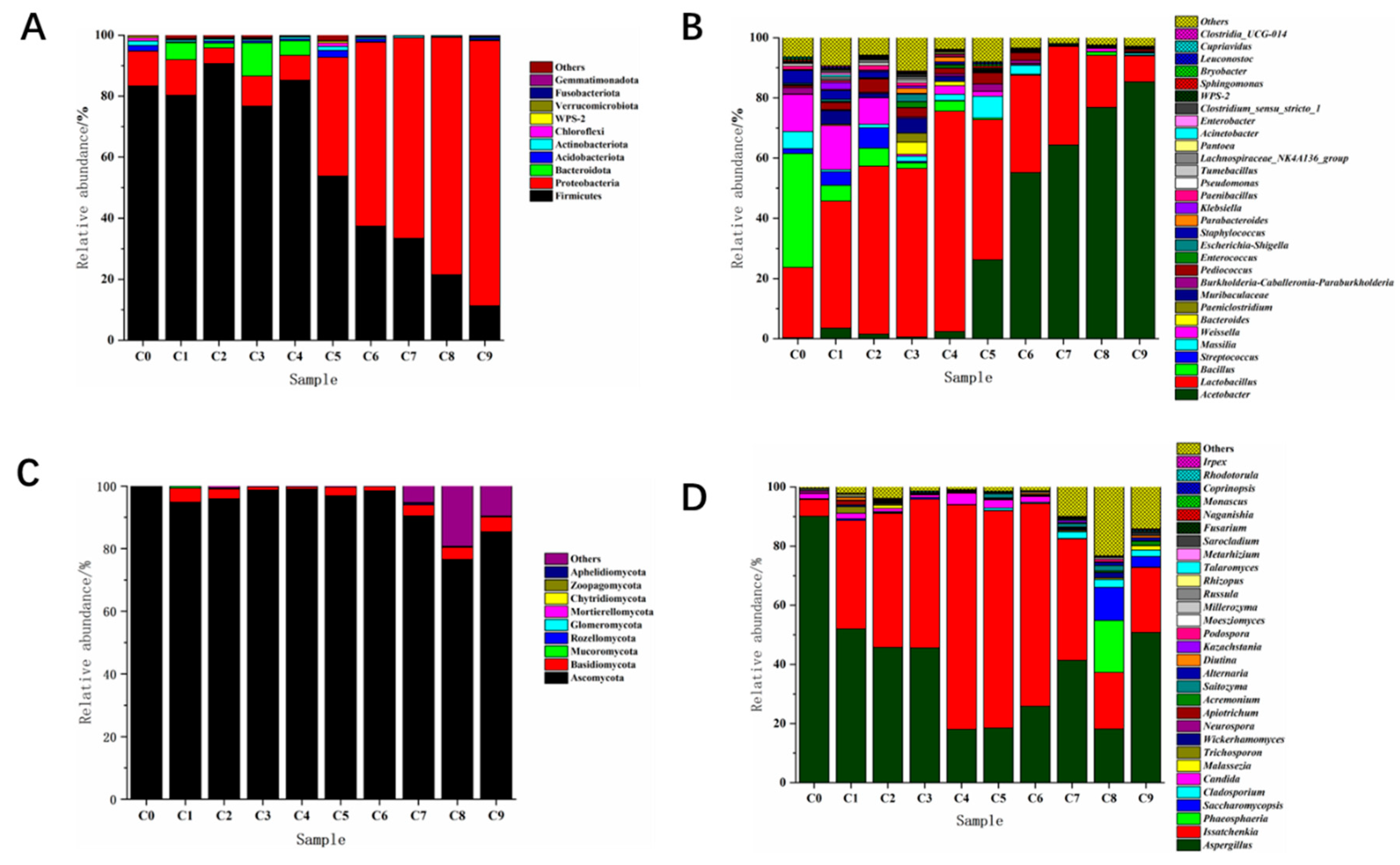
From
Figure 4 (A), it can be seen that Firmicutes and Proteobacteria have always existed in the phylum based bacterial community, whether in Cuqu samples (C0) or vinegar mash samples (C1~C9), and the relative abundance of these two phyla in each sample has reached over 85.0 ± 1.5%. Among them, Firmicutes has always dominated the alcohol fermentation stage (C1~C4), and the relative abundance of Firmicutes reaches its maximum value at C2 stage, which is 96.5 ± 1.9%. Proteobacteria shows little overall variation during the alcohol fermentation stage. Firmicutes and Proteobacteria exhibit opposite trends during the acetic acid fermentation stage (C5~C9), with Firmicutes showing a downward trend, while Proteobacteria continues to rise and become the final dominant phylum, with a relative abundance of 86.8 ± 3.6%. The succession of bacterial communities based on genus level is shown in
Figure 4 (B). The dominant species in vinegar yeast (C0) are
Bacillus,
Lactobacillus, and
Weissella, with relative abundances of 37.9 ± 2.6%, 23.3 ± 1.1%, and 12.3 ± 1.8%, respectively. During the alcohol fermentation stage (C1~C4), the
Weissella showed a decreasing trend, with relative abundance decreasing from the initial 14.6 ± 2.7% to 2.8 ± 0.8%, while the
Lactobacillus gradually became the dominant bacterial group, with relative abundance increasing from 42.2 ± 3.6% to 73.2 ± 2.1%.
Acetobacter,
Bacillus,
Streptococcus and
Staphylococcus were also detected at this stage, and the overall variation of relative abundance was small, indicating that these bacteria were in a relatively stable state at this stage. During the acetic acid fermentation stage (C5~C9), the relative abundance of
Lactobacillus showed a continuous decreasing trend, from 46.5 ± 3.1% to the final 8.6 ± 1.8%. Although there are fluctuations in the
Weissella at this stage, the overall trend is decreasing. The relative abundance of
Acetobacter increased with the fermentation, and finally rose to 85.4 ± 2.2% at C9 stage, becoming the dominant genus of the whole system.
Weissella produces β- The characteristic of glucosidase is its ability to degrade cellulose in fermentation materials[
19]. At the same time, it also has characteristics such as producing lactic acid and antibacterial substances. Wheat is one of the main raw materials in Shanxi aged Cuqu production, rich in starch and cellulose which creates favorable conditions for the growth and reproduction of the
Weissella genus[
20].
Bacillus can endow Daqu with rich enzyme systems, such as α-Amylases, proteases, etc. will also participate in fermentation together with acetic acid bacteria, which has a certain role in improving the yield of vinegar and the formation of flavor[
21].
Lactobacillus is mainly concentrated within Daqu during the fermentation process, and its metabolite lactic acid can improve and regulate the flavor of aged vinegar and regulate the microbial community structure[
22]. In the stage of alcohol fermentation, except for the contact with air on the surface, the interior is basically in an anaerobic state, suitable for the growth and reproduction of
Lactobacillus. In addition, with the continuous ethanol fermentation, the whole system is in a high ethanol environment[
23], which promotes the death of bacteria that are not resistant to high ethanol environment.
Lactobacillus is the absolute advantage bacteria at this stage. After the end of alcohol fermentation, the vinegar mash (starter mash) from the third day of fermentation will be added to each fermentation tank. In the following days, only a small area of the mash will be flipped, and the interior will still be in a relatively anaerobic environment.
Lactobacillus can still grow, so the relative abundance will show a short-term increase. After the introduction of fermented grains, a large area of flipping will be carried out to ensure that the grains are loose and can pass through oxygen. At the same time, the acidity value in the fermentation broth continues to decrease, inhibiting the growth of
Lactobacillus and reducing its relative abundance.
Acetobacter is a kind of nutritive bacteria. The acetic acid produced by its metabolism is the main component of organic acid in Shanxi aged vinegar. The daily fermentation of fermented grains with oxygen is conducive to its growth and metabolism[
24], so its relative abundance gradually increases in the acetic acid fermentation stage, becoming the dominant bacteria affecting the quality of aged vinegar.
Staphylococcus is the most common kind of pathogenic bacteria, which can not only cause a series of human infectious diseases such as septicemia, Endocarditis and blood infection[
25], but also have certain pathogenicity to animals. The most common is Mastitis of cows[
26]. The analysis results show that, with the progress of fermentation, its relative abundance eventually drops to 0.8 ± 0.1%, which is extremely low. Some pathogenic bacteria in Acinetobacter can exist in medical environment and devices for a long time. After infecting human brain, respiratory tract and other parts, they can cause meningitis, pneumonia and other related diseases[
27,
28]. Some non pathogenic bacteria can secrete vitamins, phospholipids and other nutrients[
29]. There is a small number of pathogenic bacteria in the genus
Leuconostoc cause diseases such as hemophagocytic syndrome in humans after infection[
30].
3.2.5. Analysis of Fungal Community Structure Succession
Figure 4 (C) shows the succession changes of fungal community structure at the phylum level. Nine fungal phyla were mainly detected in the whole process. Ascomycota has always been in the absolute advantage position in the Cuqu and fermentation process, with the highest content in the Cuqu (C0), and the relative abundance reached 99.7 ± 0.9%. Basidiomycota continued to decline during the alcohol fermentation stage, from 4.5 ± 0.5% to 0.7 ± 0.1%. During the acetic acid fermentation stage, its relative abundance fluctuated between 1.3 ± 0.3% and 4.6 ± 0.8%. Although the relative abundance of Mucormycota is relatively low during the fermentation process, it remains stable throughout the fermentation process, indicating that this fungus can adapt to the brewing environment of aged vinegar and have an impact on the brewing of aged vinegar. The fungal community structure based on genus level is shown in
Figure 4 (D). In Cuqu (C0), the main genera are
Aspergillus and
Issatchenkia, with relative abundance of 90.2 ± 1.5% and 5.6 ± 0.5% respectively. In the alcohol fermentation stage (C1~C4),
Issacchenkia and
Aspergillus are still the main dominant genera. The relative abundance of
Issatchenkia increased from 36.7 ± 3.8% to 75.8 ± 3.0%. The relative abundance of
Aspergillus decreased from 52.1 ± 1.3% to 18.1 ± 0.5% during this stage. In the acetic acid fermentation stage (C5~C9),
Issatchenkia is the main genus of bacteria in the C5~C7 stage. The relative abundance of
Issatchenkia decreased in the C8 and C9 stages, and was the highest in C5 samples, 73.4 ± 2.2%. The relative abundances of
Phaeosphaeria and
Saccharomycosis reached the peak in C8 samples, which were 17.6 ± 2.9% and 11.2 ± 3.6%, respectively.
Aspergillus is the main genus at the end of fermentation, with a relative abundance of 50.9 ± 1.2%. In addition, some genera with relatively low abundance were detected during the whole fermentation process, such as
Millerozyma,
Cladosporium,
Trichosporon,
Candida, etc.
Issatchenkia is a relatively important non Saccharomyces cerevisiae, which is resistant to ethanol, acid and high temperature, and produces ethanol and ethyl acetate by metabolism [
31]. In the early stage of fermentation, the raw materials are relatively sufficient, which is conducive to yeast metabolism. Therefore, this genus of yeast rapidly multiplies. With the progress of fermentation, microorganisms adapt to the environment of the system, and will tend to be stable [32, 33].
Aspergillus can produce saccharifying enzymes and amylases [34, 35], which play a good role in saccharifying starch in raw materials. During the alcohol fermentation stage, it is related to the production of flavor substances such as isoamyl alcohol, isobutanol, and ethyl acetate [
36]. Due to the cell wall structure of molds being: outer layer β-Glucan, middle layer glycoprotein, inner layer chitin; The cell wall structure of yeast is: the inner glucan layer, the middle layer is mainly composed of proteins, and the outer mannan layer. Under acidic conditions, this “sandwich” cell wall structure can still exist stably without being damaged[
37]. Therefore,
Issatchenkia and
Aspergillus can also exist in the acetic acid fermentation stage. Some low abundance bacteria also played a role in fermentation, for example,
Cladosporium can produce cellulase[
38],
Candida has good fermentation ability and alcohol tolerance, and can reduce alcohol content with water, secrete glycosides Enzymatic hydrolysis of aromatic glycosides, release terpenes, and improve the aroma of wine[
39]. In addition, the genus
Russula is only detected during the acetic acid fermentation stage. In addition to being an edible fungus with multiple nutrients, the genus
Russula is also a medicinal fungus with anti tumor and antioxidant effects. In recent years, it has attracted attention due to its good development value[
40].
3.2.6. Beta Diversity Analysis
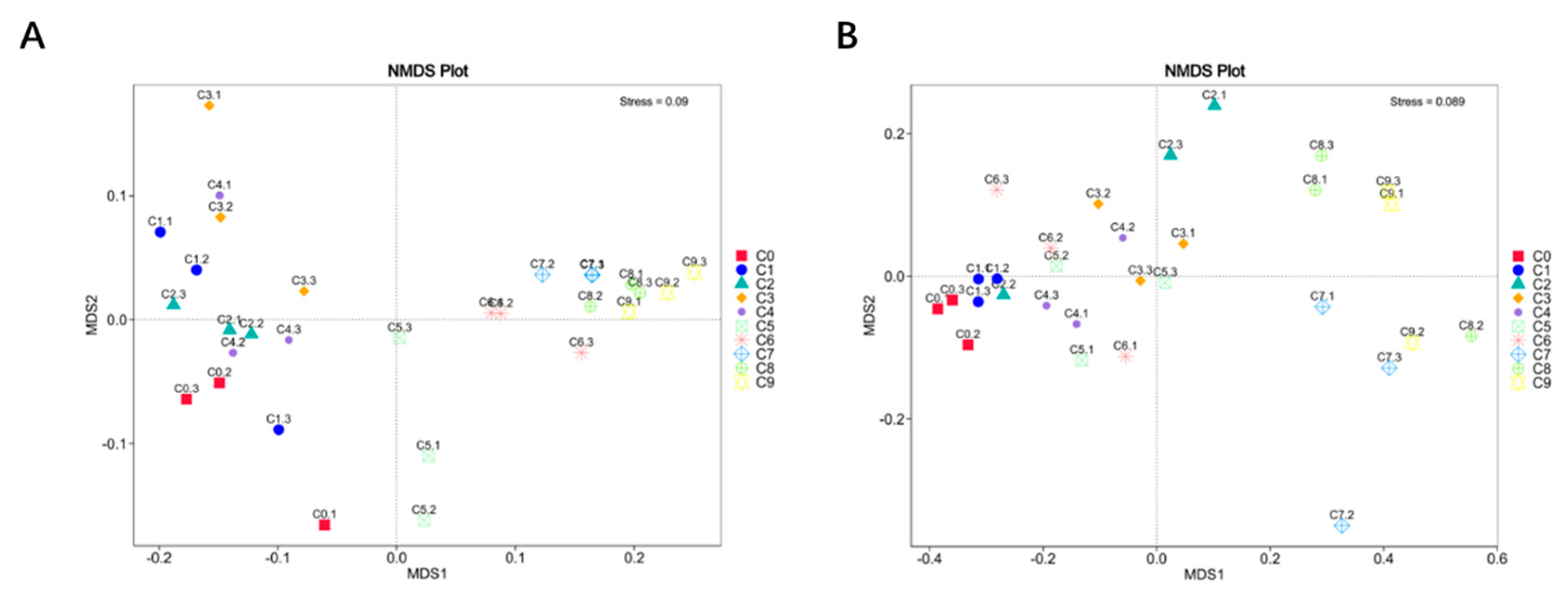
Beta diversity can be used to characterize the compositional differences between different samples. NMDS analysis was selected to reflect the differences in microbial community structure among different samples. The analysis results are shown in
Figure 5, with bacterial and fungal Stress values of 0.09 and 0.089, respectively. This indicates that the 10 samples are significantly separated from each other, indicating significant differences in bacterial and fungal microbial communities between each sample. As shown in
Figure 5 (A), the four samples from the alcohol fermentation stage (C1~C4) are distributed in the second and third quadrants, while the five samples from the acetic acid fermentation stage (C5~C9) are distributed in the first and fourth quadrants, indicating differences in bacterial community structure between the two stages. The parallel samples within the bacterial and fungal groups are also far apart, possibly due to uneven sampling
[51].
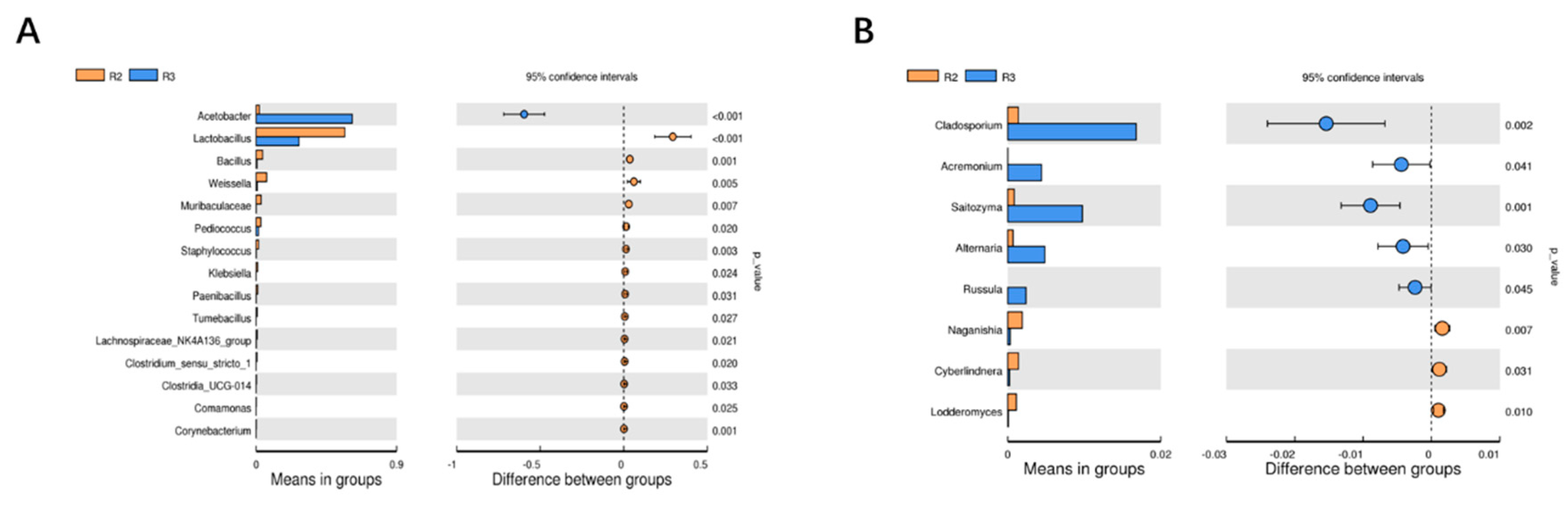
3.2.7. Screening of differential bacterial genera in two fermentation stages
There is currently limited research on the potential differential genera of bacteria between the alcohol fermentation stage and the acetic acid fermentation stage[
41]. In response to this critical issue, this study conducted inter group T-test analysis to further identify the potentially significant differences in bacterial genera between these two major brewing stages. It can be seen from
Figure 6 that 23 bacteria genera with significant differences are found at the genus level in the two fermentation stages, including 15 bacterial genera, including
Acetobacter,
Lactobacillus,
Bacillus,
Weissella, and 8 fungal genera, including
Cladosporium,
Acremonium,
Saitozyma, and
Alternaria.
3.3. Correlation analysis
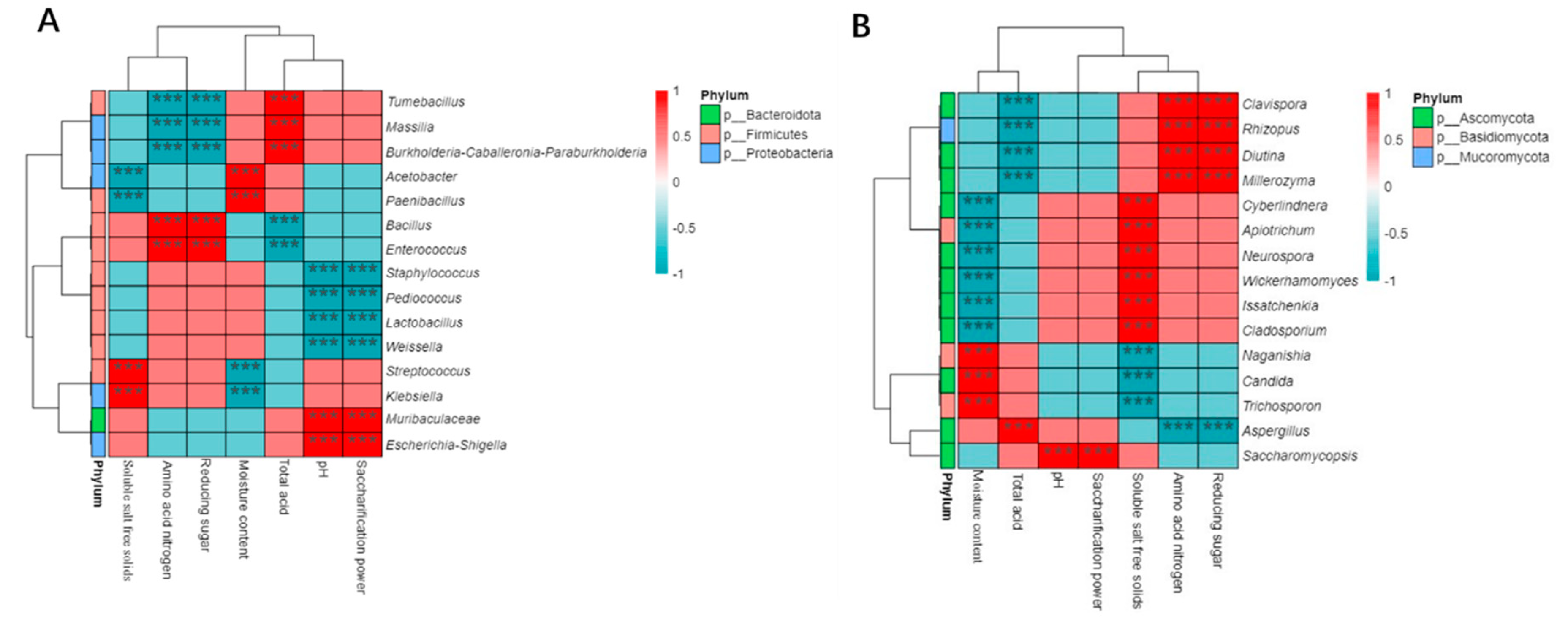
3.3.1. Correlation analysis between the physicochemical characteristics of Cuqu and microorganisms
Select the top 15 bacterial and fungal microorganisms with relative abundance of Cuqu at the genus level, and explore the correlation between these microorganisms and the physicochemical characteristics of Cuqu based on the Sperman correlation coefficient. As shown in
Figure 7, 15 bacterial and 11 fungal genera have significant correlation with the physicochemical characteristics of Cuqu.
Acetobacter and
Paenibacillus were positively correlated with moisture content, but negatively correlated with soluble salt free solids. The bacterial genera that are significantly positively and negatively correlated with reducing sugars and amino acid nitrogen are the same, while the bacterial genera that are significantly positively and negatively correlated with pH and saccharification power are the same. The same genus of fungi with significant negative correlation with soluble salt free solids, total acid and amino acid nitrogen are
Trichosporon,
Candida,
Neurospora and
Apiotrichum.
Issatchenkia is only positively correlated with reducing sugar, but not with other physicochemical characteristics.
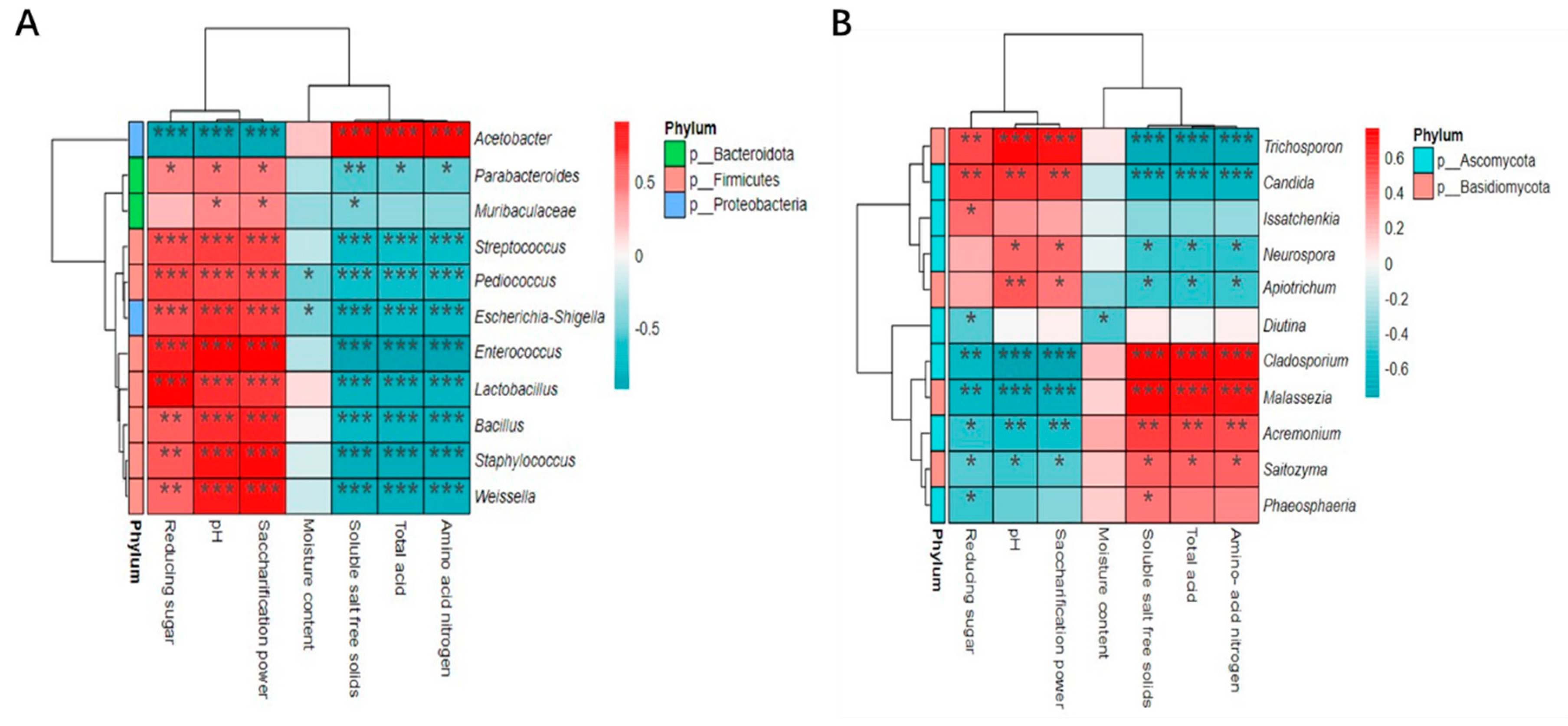
3.3.2. Correlation analysis between physicochemical characteristics of fermentation process and microorganisms
Select the top 15 bacteria and fungal microorganisms with relative abundance during the fermentation process at the genus level, and explore the correlation between these microorganisms and their physicochemical characteristics based on the Sperman correlation coefficient. As shown in
Figure 8, 11 bacterial and fungal genera have a significant correlation with the physicochemical characteristics of vinegar mash. PH and saccharifying power were significantly positively correlated with 10 bacterial genera, including
Enterococcus,
Lactobacillus,
Staphylococcus,
Bacillus,
Weissella, and 4 fungal genera, including
Trichosporon,
Candida, and
Neurospora. These 10 bacterial genera, in addition to
Muribaculaceae, also have a significant positive correlation with reducing sugars, and these 4 fungal genera have a significant negative correlation with soluble salt free solids, total acids, and amino acid nitrogen.
Acetobacter was negatively correlated with soluble salt free solids, total acid and amino acid nitrogen, but positively correlated with pH, saccharifying power and reducing sugar. The four fungal genera
Cladosporium,
Malassezia,
Acremonium, and
Saitozyma have significant negative correlations with pH, saccharification capacity, and reducing sugars, but are significantly positively correlated with soluble salt free solids, total acids, and amino acid nitrogen. There was a significant negative correlation between moisture content and the genera
Pediococcus,
Escherichia coli-Shigella, and
Diutina, but no significant positive correlation was found between them. This study found that during the fermentation process, the overall correlation between fungal communities and physicochemical characteristics is weaker compared to bacteria. This indicates that the bacterial community is the fundamental factor affecting the quality of aged vinegar fermentation, which is similar to the results of Yang and Yanli et al.’s study[42, 43].
4. Conclusion
In this study, the main physicochemical characteristics of Cuqu and aged vinegar during the brewing process were detected and analyzed, and the 16S rDNA V3~V4 region of the sample bacteria and the fungal ITS1 region were detected by high-throughput sequencing technology, so as to analyze the microbial diversity and structural composition in the brewing process of Shanxi aged vinegar, and explore the correlation between microorganisms and physicochemical characteristics. The following conclusion has been drawn:
In Cuqu (R1), the main bacterial genera are Bacillus, Lactobacillus and Weissella, and the main fungal genera are Aspergillus and Issatchenkia. Among them, Bacillus and Weissella have endowed vinegar yeast with α-Rich fungal strains such as amylase and protease play an important role in the formation of koji aroma. In the alcohol fermentation stage (R2), Lactobacillus and Weissella showed a trend of growth and decline. In the later stage of fermentation, Lactobacillus gradually became the dominant bacterial genus, and Issatchenkia also showed an upward trend, making it finally become the absolute advantage fungal genus. In the acetic acid fermentation stage (R3), the absolute advantage of the bacteria is changed from the original Lactobacillus to Acetobacter. Issacchenkia is the main bacteria in the C5~C7 period, Aspergillus is the main bacteria at the end of the fermentation, and Cladosporium, Candida and other bacteria exist in the whole fermentation process although their relative abundance is low. Through T-test analysis, it was found that there are 23 significantly different bacterial genera between R2 and R3, including 15 bacterial genera and 8 fungal genera.
Correlation analysis showed that in Cuqu, Acetobacter and Paenibacillus were significantly positively correlated with moisture content, but negatively correlated with soluble salt free solids. The bacterial genera that are significantly positively and negatively correlated with reducing sugars and amino acid nitrogen are the same, while the bacterial genera that are significantly positively and negatively correlated with pH and saccharification power are the same. During the fermentation process, there is a significant correlation between 11 bacterial and 11 fungal genera and the physicochemical characteristics of vinegar mash. pH, reducing sugar, and saccharification ability are mainly positively correlated with bacterial genera. Acetobacter was negatively correlated with soluble salt free solids, total acid and amino acid nitrogen, but positively correlated with pH, saccharifying power and reducing sugar. The four fungal genera of Cladosporium, Malassezia, Acremonium, and Saitozyma have significant negative correlations with pH, saccharification capacity, and reducing sugar, while they are significantly positively correlated with soluble salt free solids, total acids, and amino acid nitrogen. There is a significant negative correlation between water content and the genera Pediococcus, Escherichia Shigela, and Diutina. There is no significant positive correlation between bacterial genera and water content. In summary, during the fermentation process, compared to bacteria, the overall correlation between fungal communities and physicochemical characteristics is weak, and bacterial communities are the fundamental reason affecting the physicochemical characteristics of aged vinegar.
Author Contributions
Z.H: Investigation, Funding acquisition, Writing—review and editing, Formal analysis. H.X.: Conceptualization, Writing—original draft, Formal analysis. S.F.: Funding acquisition. T.B: Resources. J.Z.: Writing—review and editing, Supervision, Funding acquisition. M.C,Y.Y: Writing—review and editing, Supervision, Funding acquisition, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the General Youth Fund Project of Shanxi Province (20210302124511), Xinghuacun College Open Project Foundation (XCSXU-KF-202201, XCSXU-KF-202203, XCSXU-KF-202208, XCSXU-KF-202211)
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
This study was supported by the General Youth Fund Project of Shanxi Province (20210302124511) and Xinghuacun College Open Project Foundation (XCSXU-KF-202201, XCSXU-KF-202203, XCSXU-KF-202208, XCSXU-KF-202211).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zong-Min, W., L. Zhen-Ming, S. Jin-Song, et al., Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Scientific reports 2016, 6, 26818. [Google Scholar] [CrossRef]
- Gao, Y.; Jo, Y.; Chung, N.; Gu, S.-Y.; Jeong, Y.-J.; Kwon, J.-H. Physicochemical Qualities and Flavor Patterns of Traditional Chinese Vinegars Manufactured by Different Fermentation Methods and Aging Periods. Prev. Nutr. Food Sci. 2017, 22, 30–36. [Google Scholar] [CrossRef]
- Jizhong, Z., H. Zhili, Y. Yunfeng, et al., High-throughput metagenomic technologies for complex microbial community analysis: open and closed formats. mBio 2015, 6, 10–1128. [CrossRef]
- Aiguo, L., Y. Niutian, Y. Jing, et al., Effects of microbial interspecies relationships and physicochemical parameters on volatile flavors in sorghum-based fermented grains during the fermentation of Shanxi light-flavored liquor. Food Science & Nutrition 2022, 11, 1452–1462. [CrossRef]
- Peng, W.-X.; Marchal, J.; van der Poel, A. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed. Sci. Technol. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Tesfaye, W.; Morales, M.; Callejón, R.; Cerezo, A.B.; González, A.; García-Parrilla, M.; Troncoso, A. Descriptive sensory analysis of wine vinegar: tasting procedure and reliability of new attributes. J. Sens. Stud. 2010, 25, 216–230. [Google Scholar] [CrossRef]
- Katarína, V. and H. Viera, Starch degradation by glucoamylase Glm from Saccharomycopsis fibuligera IFO 0111 in the presence and absence of a commercial pullulanase. Chemistry & biodiversity 2007, 4, 874–880. [CrossRef]
- Rong, K., L. Min, X. Junde, et al., Exploring of seasonal dynamics of microbial community in multispecies fermentation of Shanxi mature vinegar. Journal of Bioscience and Bioengineering, 2022, 133, 375–381. [CrossRef] [PubMed]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2018, 275, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, J.; Li, F.; Tian, H.; Ma, X. Characterization of Chinese rice wine taste attributes using liquid chromatographic analysis, sensory evaluation, and an electronic tongue. J. Chromatogr. B 2015, 997, 129–135. [Google Scholar] [CrossRef]
- Chunjuan, L., G. Xiangwei, Z. Guan, et al., Liquor Flavour Is Associated With the Physicochemical Property and Microbial Diversity of Fermented Grains in Waxy and Non-waxy Sorghum (Sorghum bicolor) During Fermentation. Frontiers in Microbiology 2021, 12, 618458. [CrossRef] [PubMed]
- Entani, E.; Ohmori, S.; Masai, H.; Suzuki, K.-I. Acetobacter polyoxogenes sp. nov., a new species of an acetic acid bacterium useful for producing vinegar with high acidity. J. Gen. Appl. Microbiol. 1985, 31, 475–490. [Google Scholar] [CrossRef]
- Joyeux, A.; Lafon-Lafourcade, S.; Ribéreau-Gayon, P. Evolution of acetic Acid bacteria during fermentation and storage of wine. Applied and environmental microbiology 1984, 48, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Wei, R., L. Junli, L. Pengliang, et al., Dynamics of Microbial Communities, Flavor, and Physicochemical Properties during Ziziphus jujube Vinegar Fermentation: Correlation between Microorganisms and Metabolites. Foods 2022, 11, 3334. [CrossRef] [PubMed]
- Babayan, T.L.; Bezrukov, M.G.; Latov, V.K.; Belikov, V.M.; Belavtseva, E.M.; Titova, E.F. Induced autolysis ofSaccharomyces cerevisiae: Morphological effects, rheological effects, and dynamics of accumulation of extracellular hydrolysis products. Curr. Microbiol. 1981, 5, 163–168. [Google Scholar] [CrossRef]
- Haruta, S.; Ueno, S.; Egawa, I.; Hashiguchi, K.; Fujii, A.; Nagano, M.; Ishii, M.; Igarashi, Y. Succession of bacterial and fungal communities during a traditional pot fermentation of rice vinegar assessed by PCR-mediated denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2006, 109, 79–87. [Google Scholar] [CrossRef]
- Comelli, R.N.; Seluy, L.G.; Isla, M.A. Performance of several Saccharomyces strains for the alcoholic fermentation of sugar-sweetened high-strength wastewaters: Comparative analysis and kinetic modelling. New Biotechnol. 2016, 33, 874–882. [Google Scholar] [CrossRef]
- Min, Z., C. Zhuo, L.H. Bo, et al., Study of the Phase Characteristics of Sichuan Bran Vinegar Fermentation Based on Flavor Compounds and Core Bacteria. Journal of the American Society of Brewing Chemists 2021, 79, 201–211. [CrossRef]
- Young, K.S., O.C. Geun, L.Y. Joo, et al., Sequence analysis of a cryptic plasmid pKW2124 from Weissella cibaria KLC140 and construction of a surface display vector. Journal of microbiology and biotechnology 2013, 23, 545–554. [CrossRef]
- Zheng, X.-W.; Yan, Z.; Nout, M.R.; Smid, E.J.; Zwietering, M.H.; Boekhout, T.; Han, J.-S.; Han, B.-Z. Microbiota dynamics related to environmental conditions during the fermentative production of Fen-Daqu, a Chinese industrial fermentation starter. Int. J. Food Microbiol. 2014, 182-183, 57–62. [Google Scholar] [CrossRef]
- Wu, J.J., Y.K. Ma, F.F. Zhang, et al., Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiology 2012, 30, 289–297. [CrossRef] [PubMed]
- Yu, Z., M. Jun, N. Jiwei, et al., Succession sequence of lactic acid bacteria driven by environmental factors and substrates throughout the brewing process of Shanxi aged vinegar. Applied microbiology and biotechnology 2018, 102, 2645–2658. [CrossRef] [PubMed]
- Signore, A.D. Chemometric analysis and volatile compounds of traditional balsamic vinegars from Modena. Journal of Food Engineering 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Haghshenas, B.; Nami, Y.; Abdullah, N.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Anticancer impacts of potentially probiotic acetic acid bacteria isolated from traditional dairy microbiota. Lwt 2015, 60, 690–697. [Google Scholar] [CrossRef]
- Mahato, S.; Mistry, H.U.; Chakraborty, S.; Sharma, P.; Saravanan, R.; Bhandari, V. Identification of Variable Traits among the Methicillin Resistant and Sensitive Coagulase Negative Staphylococci in Milk Samples from Mastitic Cows in India. Front. Microbiol. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Reyher, K.; Dufour, S.; Barkema, H.; Côteaux, L.D.; DeVries, T.; Dohoo, I.; Keefe, G.; Roy, J.-P.; Scholl, D. The National Cohort of Dairy Farms—A data collection platform for mastitis research in Canada. J. Dairy Sci. 2011, 94, 1616–1626. [Google Scholar] [CrossRef]
- Freire, M.; Garcia, D.d.O.; Garcia, C.; Bueno, M.C.; Camargo, C.; Magri, A.K.; Francisco, G.; Reghini, R.; Vieira, M.; Ibrahim, K.; et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin. Microbiol. Infect. 2015, 22, 352–358. [Google Scholar] [CrossRef]
- Lob, S.H.; Hoban, D.J.; Sahm, D.F.; Badal, R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2016, 47, 317–323. [Google Scholar] [CrossRef]
- José, L.N.-B., H. Jiménez-Islas, E. Botello-Alvarez, et al., An optimization study of solid-state fermentation: xanthophylls extraction from marigold flowers. Applied microbiology and biotechnology 2004, 65, 383–390. [CrossRef]
- Xinfeng, L., J. Qilong, L. Jiduo, et al., Leuconostoc pseudomesenteroides -associated hemophagocytic syndrome: A case report. Experimental and therapeutic medicine 2018, 15, 184–188.
- Dhaliwal, S.S., H.S. Oberoi, S.K. Sandhu, et al., Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresource Technology 2011, 102, 5968–5975. [CrossRef]
- Stenuit, B.; Agathos, S.N. Deciphering microbial community robustness through synthetic ecology and molecular systems synecology. Curr. Opin. Biotechnol. 2015, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Hirokazu, T., A.M. S, I. Chiharu, et al., Scoring Species for Synthetic Community Design: Network Analyses of Functional Core Microbiomes. Frontiers in microbiology 2020, 11, 1361. [CrossRef]
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Gan, X.; Luo, L.; Lin, W.-T. Effect of bioaugmented inoculation on microbiota dynamics during solid-state fermentation of Daqu starter using autochthonous of Bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiol. 2017, 61, 83–92. [Google Scholar] [CrossRef]
- Huang, Y.; Yi, Z.; Jin, Y.; Huang, M.; He, K.; Liu, D.; Luo, H.; Zhao, D.; He, H.; Fang, Y.; et al. Metatranscriptomics Reveals the Functions and Enzyme Profiles of the Microbial Community in Chinese Nong-Flavor Liquor Starter. Front. Microbiol. 2017, 8, 1747. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Okada, S. Monitoring the lactic acid bacterial diversity during shochu fermentation by PCR-denaturing gradient gel electrophoresis. J. Biosci. Bioeng. 2005, 99, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: new structure and new challenges. Journal of bacteriology, 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Abrha, B.; Gashe, B.A. Cellulase production and activity in a species ofCladosporium. World journal of microbiology & biotechnology 1992, 8, 164–166. [Google Scholar]
- Lanlan, H., W. Jia, J. Xueao, et al., Selection of non- Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. Journal of food science and technology 2018, 55, 4001–4012. [CrossRef]
- Shuang, Z., Z. Yongchang, L. Shuhong, et al., A novel lectin with highly potent antiproliferative and HIV-1 reverse transcriptase inhibitory activities from the edible wild mushroom Russula delica. Glycoconjugate journal 2010, 27, 259–265. [CrossRef]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y., W.Y. Na, O. Erdenebat, et al., Correlation analysis between microbial diversity and physicochemical indices of Koumiss. Food Bioscience 2022, 49, 101922. [CrossRef]
- Yanli, Y., F. Ying, L. Ting, et al., Microbial composition and correlation between microbiota and quality-related physiochemical characteristics in chongqing radish paocai. Food Chemistry 2022, 369, 130897. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).