Submitted:
05 August 2023
Posted:
07 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Experimental Process
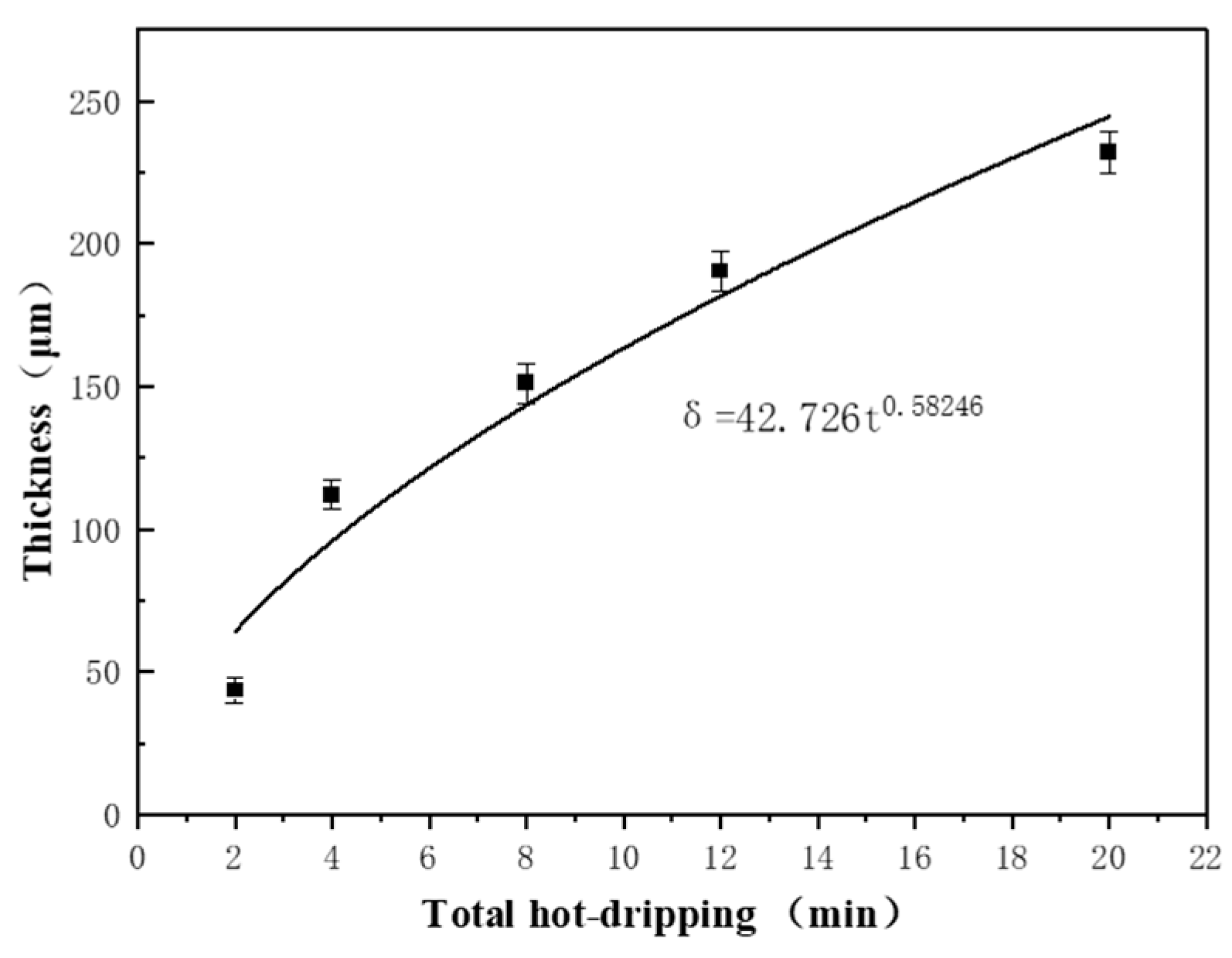
2.2.1. Pre-Processing
2.2.2. Hot Dip Plating
2.3. Microstructure and Corrosion Resistance Test
2.3.1. Microstructure Characterization Methods
2.3.2. Corrosion Resistance
3. Results and Discussion
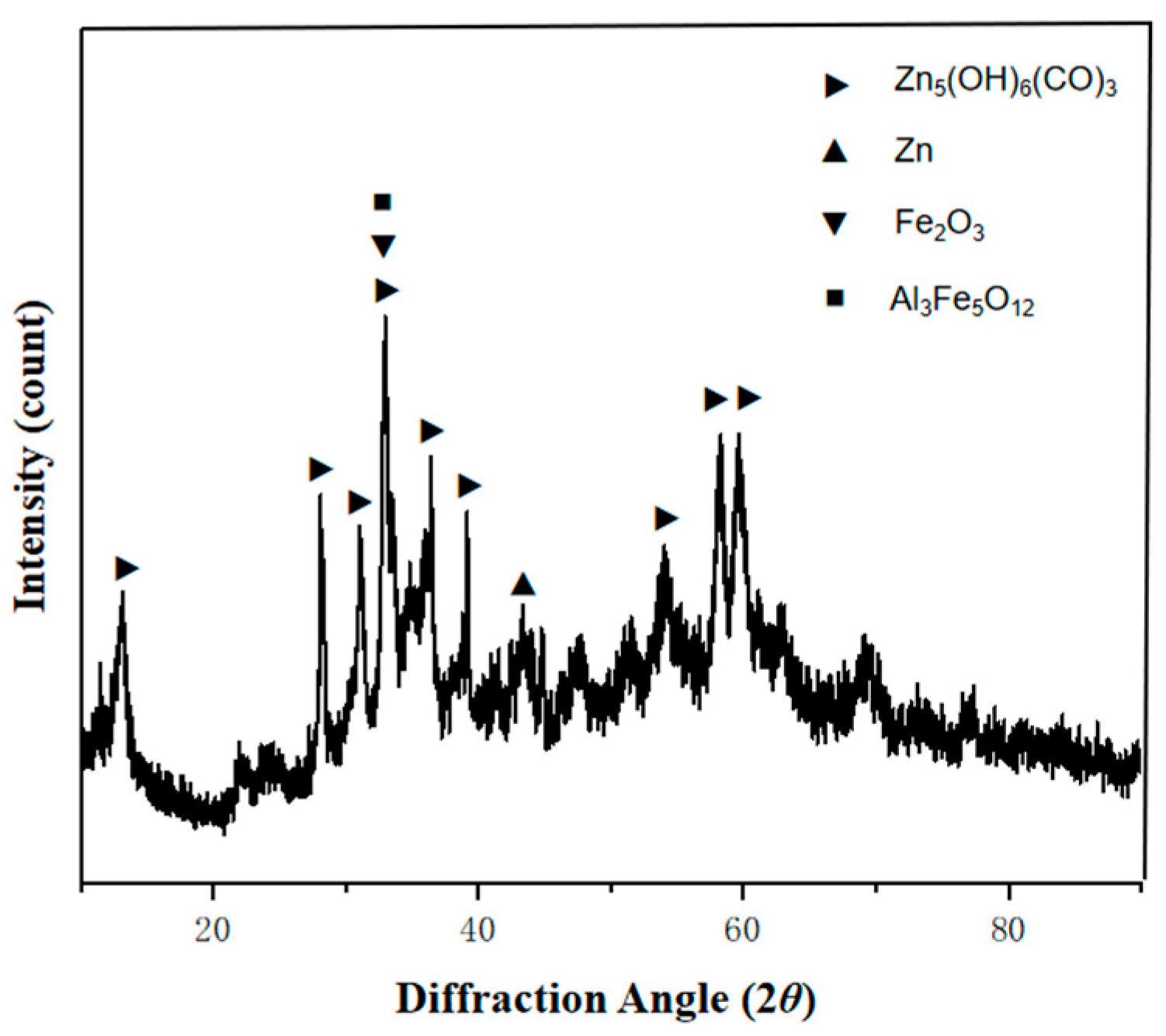
3.1. Microstructure of the Coating
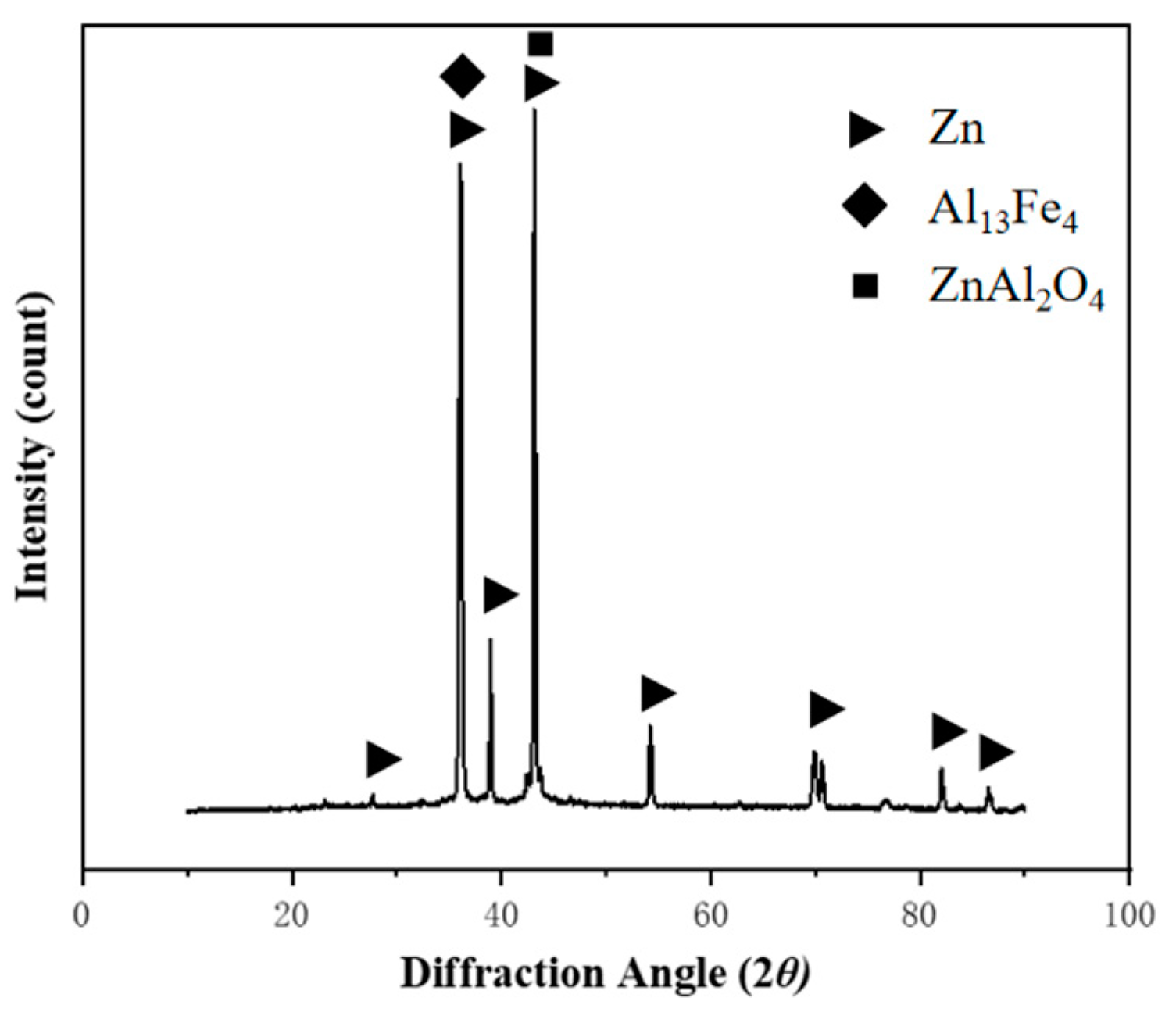
3.2. Analysis of Corrosion Resistance of Plated Layer
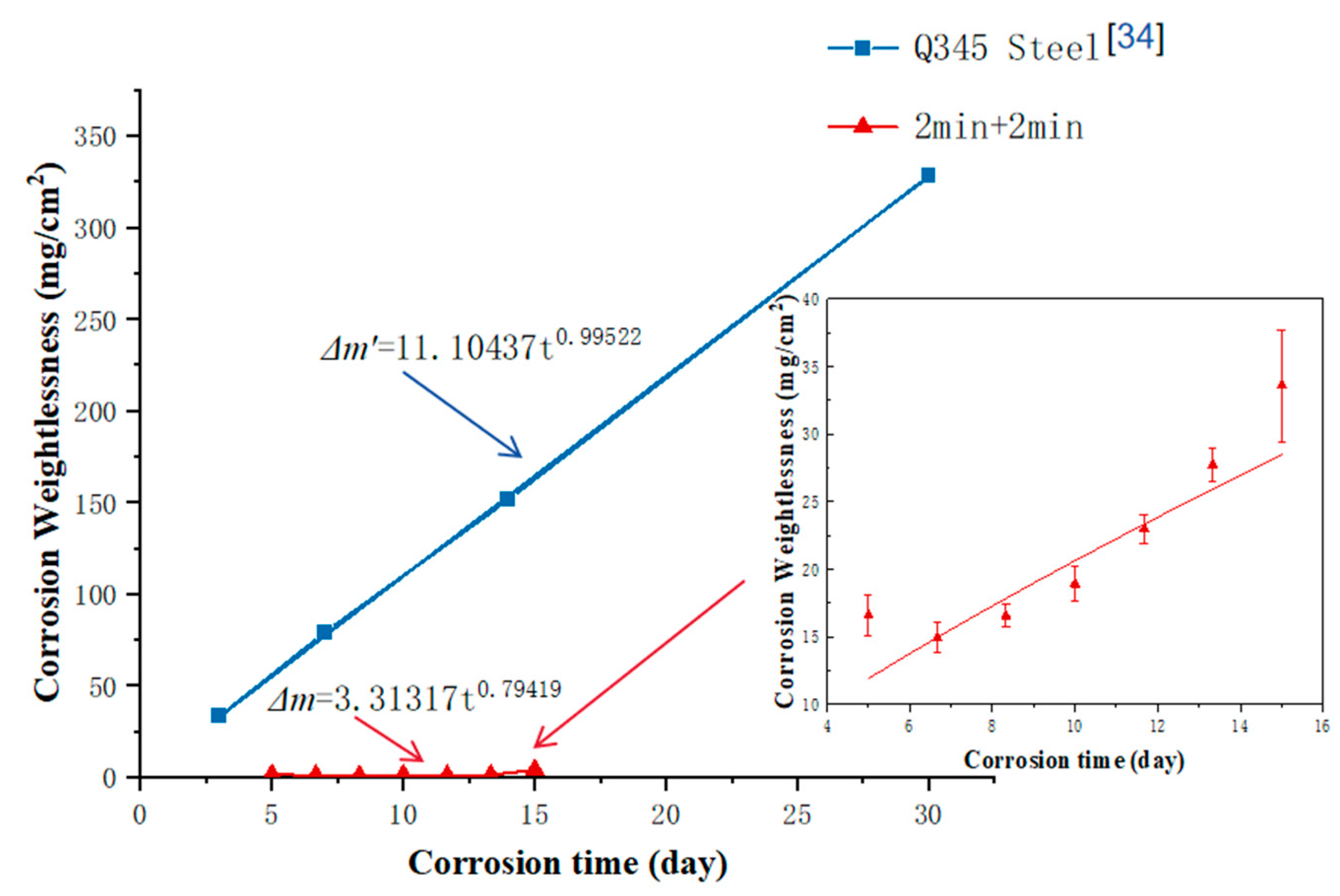
3.2.1. Neutral Salt Spray Test Results
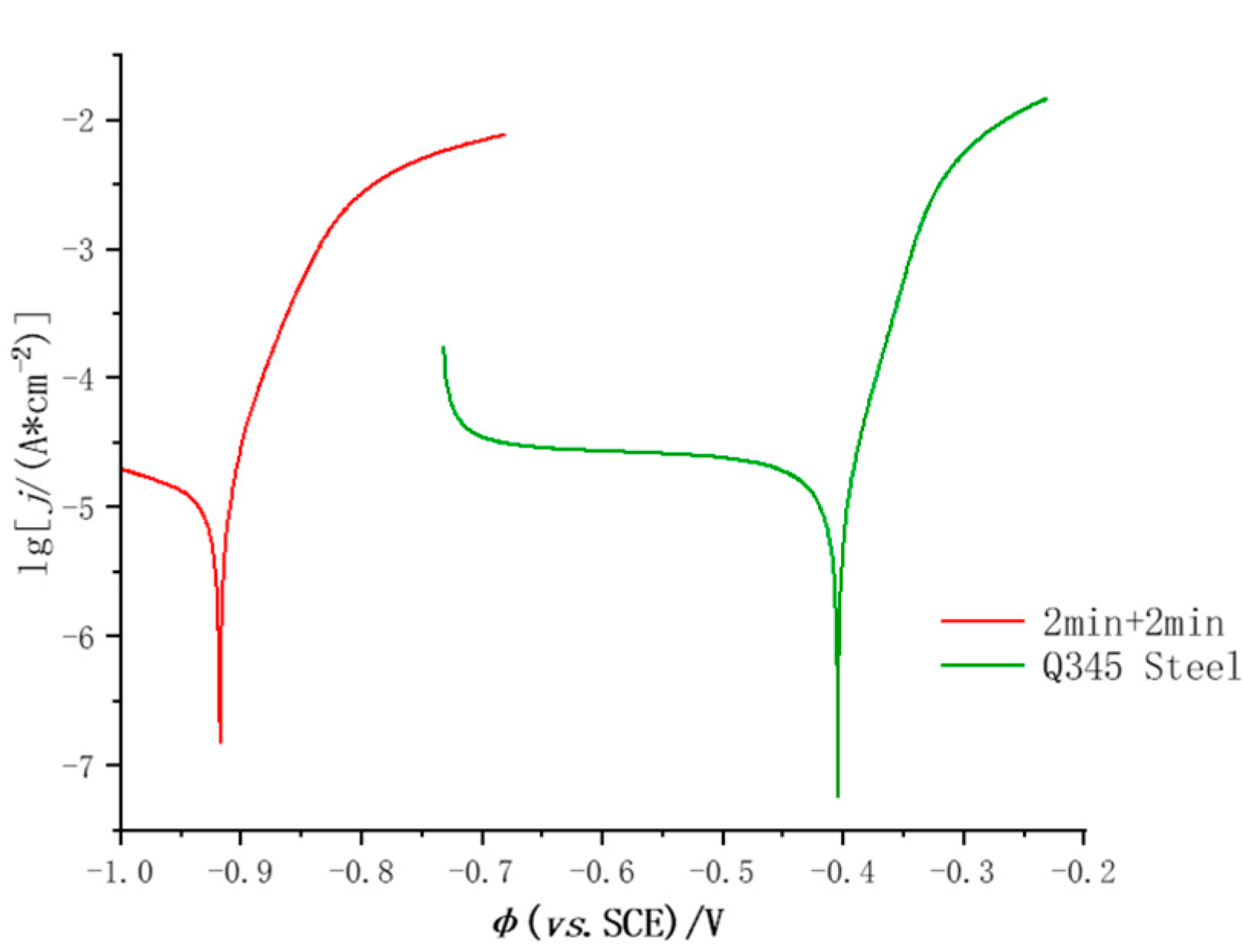
3.2.2. Tafel Polarization Curves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Z.; Wang, W.; Wang, Z. Effect of cold-form and tensile strain rate on mechanical properties of Q345 steel at elevated temperatures. J. Constr. Steel Res. 2022, 191, 107192. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Wang, S.M.; Zhao, X.J.; Dang, J.W.; et al. Research status of the process mechanism of batch hot-dip galvanizing. Surf. Technol. 2016, 45, 19–25. [Google Scholar]
- Shih, H.C.; Hsu, J.W.; Sun, C.N.; et al. The lifetime assessment of hot-dip 5% Al–Zn coatings in chloride environments. Surf. Coat. Technol. 2002, 150, 70–75. [Google Scholar] [CrossRef]
- Sa-nguanmoo, R.; Nisaratanaporn, E.; Boonyongmaneerat, Y. Corros, Sci. 2011, 53, 122.
- Liu, X.; Liu, C.L.; Yu, W.G.; et al. Effects of alloying elements on the platability and corrosion resistance of a new Zn–Mg–Ni–V–Al alloy coating. Corros. Prot. 2018, 39, 924–929. [Google Scholar]
- Feng, G.; Hou, J.; Zhang, L. Effects of steel composition and adding elements on microstructure and properties of hot-dip galvanizing. Hot Work. Technol. 2011, 40, 118–121. [Google Scholar]
- Sandlin, R.W. Galvanizing characteristics of different types of steel. Wire Wire Prod. 1941, 16, 28–35. [Google Scholar]
- Mertens, A.; Bellhouse, E.M.; McDermid, J.R. Microstructure and mechanical properties of a mixed Si–Al TRIP-assisted steel subjected to continuous galvanizing heat treatments. Mater. Sci. Eng. A 2014, 608, 249–257. [Google Scholar] [CrossRef]
- Ryabov, V.R. Aluminizing of Steel (Translated from the Russian Work: Alitirovanie Stali); Amerind Publishing Co. Pvt. Ltd.: New Delhi, India, 1985. [Google Scholar]
- Bahadur, A.; Mohanti, O.N. Structural studies of hot dip aluminized coatings on mild steel. Mater. Trans. JIM 1991, 32, 1053–1061. [Google Scholar] [CrossRef]
- Heumann, T.; Dittrich, N.A. Structure character of the Fe2Al5 intermetallics compound in hot dip aluminizing process. Z. Met. 1959, 50, 617–623. [Google Scholar]
- Eggeler, G.; Auer, W.; Kaesche, H. On the influence of silicon on the growth of the alloy layer during hot dip aluminizing. J. Mater. Sci. 1986, 21, 3348–3350. [Google Scholar] [CrossRef]
- Lainer, D.I.; Kurakin, A.K. Mechanism of the effect of silicon in aluminum on the process of reactive diffusion of iron into aluminum. Fiz. Metal. Metalloved. 1964, 18, 145. [Google Scholar]
- Zarei, F.; Nuranian, H.; Shirvani, K. Effect of Si addition on the microstructure and oxidation behaviour of formed aluminide coating on HH309 steel by cast-aluminizing. Surf. Coat. Technol. 2020, 394, 125901. [Google Scholar] [CrossRef]
- Wang, C.J.; Chen, S.M. The high-temperature oxidation behavior of hot-dipping Al–Si coating on low carbon steel. Surf. Coat. Technol. 2006, 200, 6601–6605. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, K.; Fang, X.; Xiong, Z.; Wei, L.; Zhu, P.; Li, X. Oxidation behavior of 316L austenitic stainless steel in high temperature air with long-term exposure. Mater. Res. Express 2020, 7, 066517. [Google Scholar] [CrossRef]
- Abro, M.A.; Hahn, J.; Lee, D.B. High temperature oxidation of hot-dip aluminized T92 steels. Met. Mater. Int. 2018, 24, 507–515. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, F.; Lu, G.; Xiang, X.; Tang, T.; Wang, X. Fabrication of Al 2 O 3/FeAl coating as tritium permeation barrier on tritium operating component on quasi-CFETR scale. J. Fusion Energy 2018, 37, 317–324. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Hua, P.; Wang, C.; Chen, K.; Wu, Y.; Zhou, W. Growth of inter-metallic compound layers on CLAM steel by HDA and preparation of permeation barrier by oxidation. Fusion Eng. Des. 2017, 125, 57–63. [Google Scholar] [CrossRef]
- Cheng, W.J.; Wang, C.J. Study of microstructure and phase evolution of hot-dipped aluminide mild steel during high-temperature diffusion using electron backscatter diffraction. Appl. Surf. Sci. 2011, 257, 4663–4668. [Google Scholar] [CrossRef]
- Badaruddin, M. Improvement of high temperature oxidation of low carbon steel exposed to ethanol combustion product at 700 ℃ by hot-dip aluminizing coating. Makara J. Technol. 2012, 15, 137–141. [Google Scholar] [CrossRef]
- Yan Lei, Cheng Shumao. Technology analysis of Steel wire hot-dip galvanized aluminum alloy. Wire Cable 2018, 17–19, 31. [Google Scholar]
- TACHIBANA K, MORINAGA Y, MAYUZUMI M. Hot dip fine Zn and Zn-Al alloy double coating for corrosion resistance at coastal area. Corros. Sci. 2006, 49, 149–157. [Google Scholar]
- Xiong Ziliu, ZHANG Yunfei, Jiang Tao. Properties, characteristics and development status of Zn-Al alloy coating. Hebei Metall. 2012, 8–11, 24. [Google Scholar]
- Lu Jintang, Jiang Aihua, Che Chunshan; et al. Research progress of hot dipping Zn-Al alloy coatings. Mater. Prot. 2008, 41, 47–51. [Google Scholar]
- Yan, L.; Cheng, S.M. The introduction of hot dipped zinc–aluminum alloy technology for steel wire. Electric Wire Cable 2018, 17–19, 31. [Google Scholar]
- Peng, H.P.; Su, X.P.; Wang, J.H.; et al. Interface reaction mechanism for galvanizing in Zn–Al baths. Chin. J. Nonferrous Met. 2012, 22, 3168–3175. [Google Scholar]
- Wang, Q.Y.; Zhang, L.; Lu, J.Y.; et al. Microstructure and corrosion resistance of zinc–aluminum alloy coating prepared by a two-step hot dipping process. Electroplat. Finish. 2020, 39, 392–398. [Google Scholar]
- Qiao, H.; Li, Z.; Yang, J.; et al. Effect of Si content on microstructure and corrosion resistance of hot-dip Zn–Al alloy coating. Heat Treat. Met. 2018, 43, 178–183. [Google Scholar]
- Zhang, Y.Y.; Zhang, Y.L.; Li, Q.; et al. Influencing mechanism of alloy elements on structure and performance of Zn–Al hot dip coating. Chin. J. Process Eng. 2009, 9 (Suppl.1), 465–472. [Google Scholar]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Han, W. Effect of Si on the growth kinetics of Fe2Al5 phase during Fe-Al reaction; Xiangtan University: Xiangtan, China, 2009. [Google Scholar]
- Qiang, Z.; Hua, D.F.; Yi, S.; et al. Microstructure and salt spray Corrosion Behavior of surfacing Joint of Q345C Steel for bogies. Electr. Weld. Mach. 2016, 46, 107–111. [Google Scholar]
- Liu, W.; Li, H.; Wu, H.; et al. Corrosion behavior of 6061-T4 aluminum alloy in salt spray environment. Mater. Prot. 2022, 11, 37–43. [Google Scholar]
- Lan, Y.J. Electrochemical corrosion behavior of Q235 steel in sodium chloride contaminated silt; Taiyuan University of Technology: Taiyuan, China, 2022. [Google Scholar]
- Wang, Z.; Jin, L.; Liang, X. Effect of surface state on electrochemical corrosion behavior of Q235 steel in solutions with different chloride ion concentrations. Electroplat. Finish. 2019, 42, 6–12. [Google Scholar] [CrossRef]
- Yuan, M.; Luo, X.Y.; Yao, Z.J. Influence of addition of Mg, RE(La, Ce) on corrosion resistance of hot dipping 55% Al-Zn -- 1.6%Si coating. J. Nanjing Univ. Aeronaut. Astronaut. 2015, 47, 696–701. [Google Scholar]
- Tan Chengyu, WEI Xiuyu, Chen Zhun. Study on corrosion resistance of Zn-5%Al-RE coating. Electroplat. Environ. Prot. 2002, 22, 29–32. [Google Scholar]
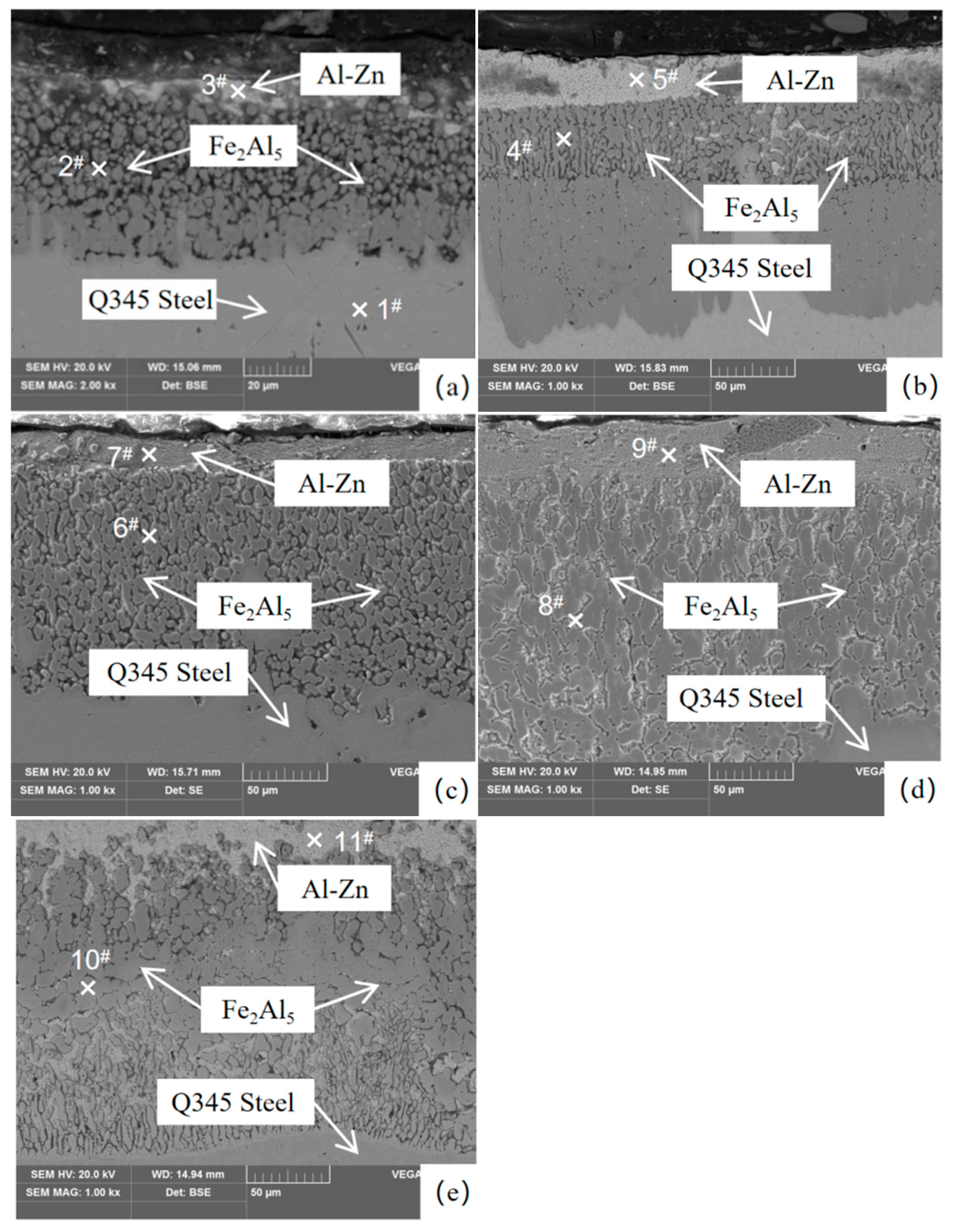
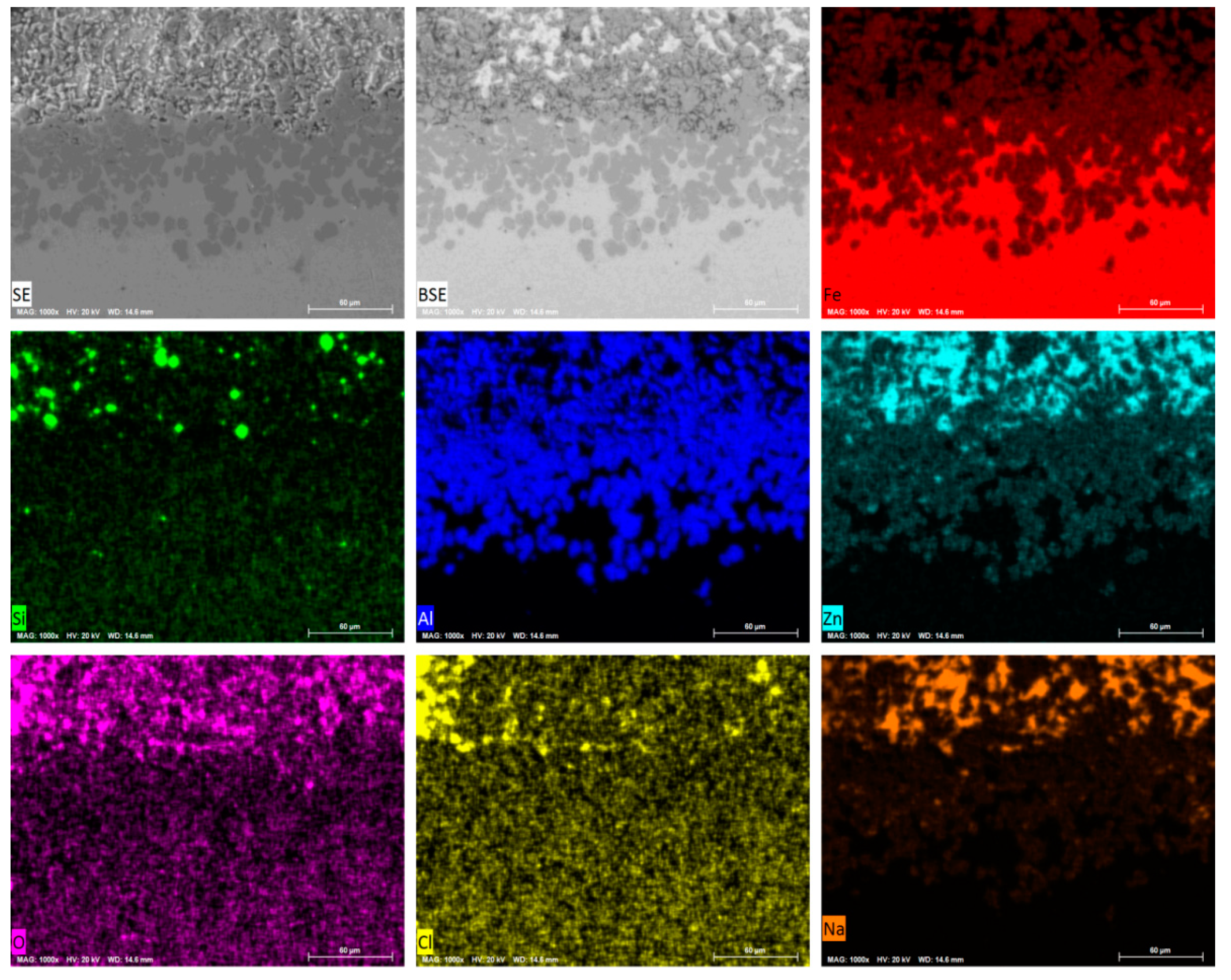
| Analysis Region | Al | Fe | Zn | Phase |
|---|---|---|---|---|
| 1# | 0 | 99.99 | 0 | Fe |
| 2# | 64.28 | 27.43 | 8.28 | Fe2Al5 |
| 3# | 22.60 | 66.57 | 10.83 | Al-Zn |
| 4# | 64.87 | 26.52 | 8.60 | Fe2Al5 |
| 5# | 23.28 | 65.60 | 11.12 | Al-Zn |
| 6# | 64.35 | 26.78 | 8.87 | Fe2Al5 |
| 7# | 21.75 | 65.89 | 12.36 | Al-Zn |
| 8# | 64.42 | 27.26 | 8.32 | Fe2Al5 |
| 9# | 21.70 | 63.71 | 14.59 | Al-Zn |
| 10# | 64.74 | 26.85 | 8.41 | Fe2Al5 |
| 11# | 22.34 | 63.52 | 14.14 | Al-Zn |
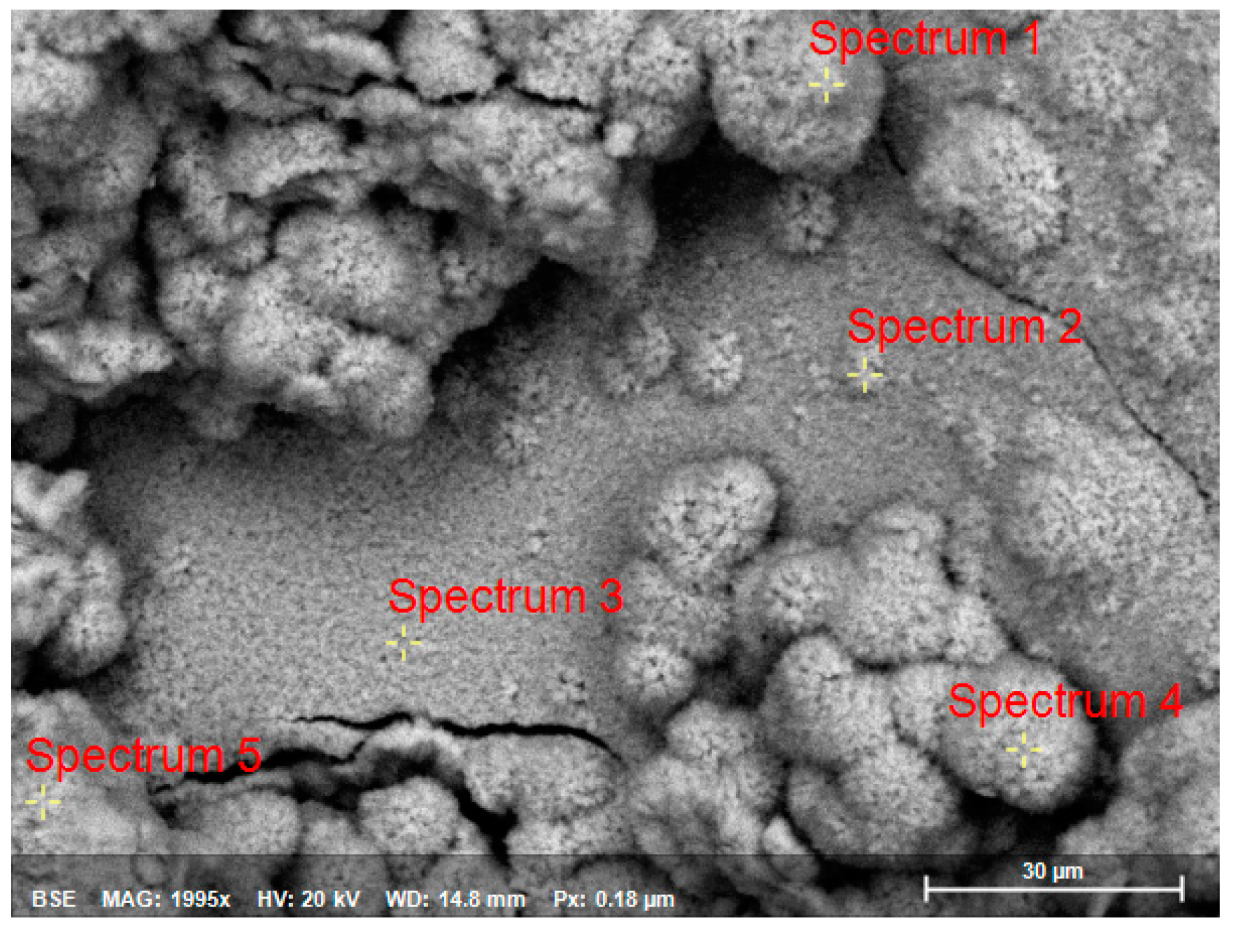
| Spectrum | O | Al | Cl | Fe | Zn |
|---|---|---|---|---|---|
| Spectrum1 | 70.75 | 0 | 0 | 0 | 29.25 |
| Spectrum2 | 73.33 | 0 | 0 | 0 | 26.67 |
| Spectrum3 | 10.46 | 1.79 | 1.14 | 1.04 | 85.57 |
| Spectrum4 | 66.60 | 0 | 0 | 0 | 32.40 |
| Spectrum5 | 67.35 | 0 | 0 | 0 | 32.65 |
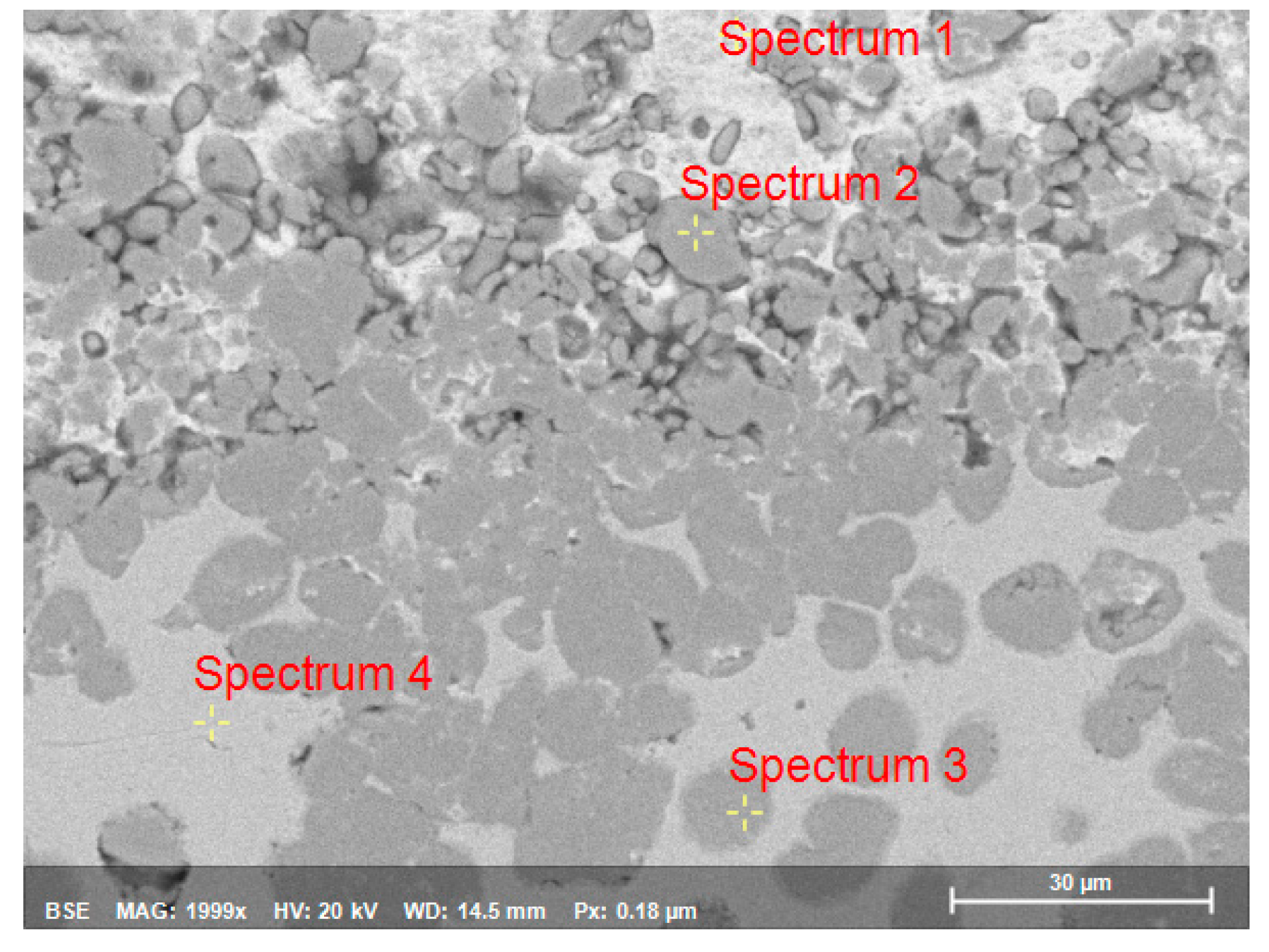
| Spectrum | O | Na | Al | Cl | Fe | Zn |
|---|---|---|---|---|---|---|
| Spectrum1 | 16.70 | 0 | 2.43 | 1.18 | 1.65 | 78.04 |
| Spectrum2 | 1.67 | 0 | 62.16 | 0 | 26.12 | 10.06 |
| Spectrum3 | 0 | 0 | 63.35 | 0 | 27.68 | 8.97 |
| Spectrum4 | 5.24 | 2.30 | 0 | 0 | 92.46 | 0 |
| Specimen | φcorr(vs. SCE)/ V | Lg[jcorr / (μA·cm-2)] |
|---|---|---|
| 2min+2min | -0.917 | -6.812 |
| Q345 Steel | -0.405 | -7.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





