1. Introduction
Cutaneous squamous cell carcinoma (cSCC) is one of the most common forms of skin cancer worldwide. The exact incidence rate cannot be ascertained, as most national cancer registries do not record this diagnosis [
1]. cSCC traditionally accounted for the second highest incidence among skin cancer, although a recent study cited a 1:1 ratio between basal cell carcinoma and cSCC [
2]. While the majority of cSCC are early stage and can be cured with local treatments, 3-5% of patients develop advanced disease that is not amenable to surgery or radiotherapy [
3,
4,
5]. Moreover, as the average lifespan increases, so is the chance of encountering patients with unresectable or metastatic cSCC.
In the pre-immune checkpoint inhibitor (ICI) era, patients with advanced cSCC had poor long-term outcomes to palliative chemotherapy and epidermal growth factor receptor (EGFR) inhibitors [
6]. The ICIs targeting programmed cell death protein 1 (PD-1) have revolutionized the treatment of advanced cSCC, a highly immunogenic tumor featuring high mutational burden likely resultant from UV radiation-induced DNA damage [
4,
7].
The PD-1 inhibitors cemiplimab and pembrolizumab demonstrated an objective response rate of 35 to 58% and have not reached the median duration of response in phase I/II clinical trials [
8,
9,
10,
11,
12,
13,
14,
15]. Based on these findings, the U.S. Food and Drug Administration (FDA) approved cemiplimab in September 2018, and pembrolizumab in June 2020 for advanced cSCC.
Due to the recent FDA approval of those therapies, limited data is available about the ICI efficacy and safety in real-world cohorts, especially related to patients with poor ECOG performance status or with chronic immune suppression (e.g., use of immunosuppressant drugs for active autoimmune diseases, solid organ transplant recipients, or concomitant hematological malignancies), who are usually excluded from clinical trials. In this study, we investigated the clinical outcomes of patients with advanced cSCC treated with anti-PD-1 ICI outside clinical trials at a Canadian Comprehensive Cancer Centre. We aimed to assess efficacy and safety according to age, performance status, comorbidities of interest, and immune-related adverse events (irAE).
2. Materials and Methods
2.1. Study Design and Patient Cohort
In this single-center retrospective cohort study, we included all non-trial patients with incurable locoregionally advanced (defined as technically unresectable or not clinically suitable for surgery, or not amenable to radiation therapy with curative intent based on multidisciplinary tumor board discussions) or metastatic cSCC (defined as patients with disease beyond regional nodal involvement) treated with at least one dose of anti-PD-1 between June 2017 and July 2022 at a Comprehensive Cancer Centre in Canada. To identify this cohort, the institutional electronic pharmacy record was queried by diagnosis cSCC, and the name of ICI received (cemiplimab, pembrolizumab or nivolumab). Demographics and clinicopathologic features were collected from the electronic medical records in a protected dataset. Patients were staged following the 8th edition of the American Joint Committee on Cancer staging system for cSCC of the head and neck. Treatment history, outcomes, and comorbidities of special interest (immunosuppressive condition [autoimmune disease on immunosuppressant drug, HIV, solid organ transplant recipient, hematological malignancy], and genetic syndrome predisposing to cSCC [e.g., epidermolysis bullosa]) were recorded. The data cut-off for analysis was February 15, 2023. This project was carried out with the approval of the University Health Network Research Ethics Board.
2.2. Efficacy and Safety Outcomes
The best overall response (BOR) was determined by investigator´s assessment of clinical and radiological parameters and was defined as the best response recorded from the start of the treatment until disease progression. Complete response (CR) was defined as complete regression of the lesion(s); partial response (PR) as a clinical/radiological tumor reduction with persistence of detectable tumor; progression of disease (PD) as clinical/radiological increase in lesion(s) or the appearance of a new lesion; stable disease (SD) as neither response nor PD. Response rate was defined as CR + PR. Disease control rate was defined as CR + PR + SD.
Adverse events were assessed using Common Terminology Criteria in Solid Tumors (CTCAE) version 5.0.
Overall survival (OS) and progression-free survival (PFS) were defined, respectively, as the times from the first anti-PD-1 dose to death from any cause, and the time until first documentation of disease progression or death from any cause, whichever occurred first. OS and PFS were censored at the date of last follow-up.
Reason for discontinuation of treatment was also recorded: maximum benefit achieved (if treatment was stopped earlier than 2 years without any toxicities justifying treatment discontinuation and no evidence of progressive disease, at the medical oncologist´s discretion), maximum number of doses (2 years of treatment; 35 doses for cemiplimab or pembrolizumab), discontinuation due to toxicities, progression of disease or death.
For the purposes of answering our research question, we grouped patients with a disease compromising the immune system with patients on immunosuppression needs (those on immunosuppressant drug), to whom we will refer as immunocompromised.
2.3. Statistical Analysis
Demographic characteristics of our cohort of patients were analyzed by descriptive statistics, as number of cases and percentages for discrete variables, and mean ± standard deviation or median (range) for continuous variables. Chi-square, Fisher’s exact, and Mann-Whitney U tests were used to assess differences in categorical and continuous variables among subgroups of interest, respectively. OS and PFS were estimated using the Kaplan–Meier method and the log-rank test, and expressed as median with 95% confidence interval (CI). Univariable Cox proportional hazards model were fitted to evaluate the impact of clinical variables on survival. Multivariable analysis was not performed due to the low number of events in our cohort. Median follow-up was estimated using the Kaplan-Meier reverse method. All statistical tests were two-sided, and p value <0.05 was deemed significant. We performed all the statistical analysis in RStudio Version 2023.03.0+386.
3. Results
3.1. Patients
Our cohort included 35 patients treated with cemiplimab and one with pembrolizumab; both drugs were administered every 3 weeks, as per standard of care. We did not identify any patients treated with nivolumab. Baseline patient characteristics are reported in
Table 1.
At the time of ICI initiation, the median age was 75.4 years (range from 27.9 to 100.1), 36.1% of the cohort was 80 years of age or older, and 27.8% had an ECOG performance status equal to two or higher. The majority of patients were male (75%) and had another skin cancer (58%). The most common primary site of disease was the head and neck (68.6%). Sixteen of 36 (44.4%) patients had a comorbidity of interest: two with an autoimmune condition on immunosuppressant treatment, two were solid organ transplant recipients (both kidney transplant recipients), ten had hematological malignancies and two epidermolysis bullosa. None of our patients had immunosuppression related to HIV or a known HIV-positive history.
Primary treatment included surgery alone for 58.3% of patients, surgery followed by adjuvant radiation therapy for 25%, radiation therapy alone for 8.3%, and systemic therapy for 8.3%. Of those, two patients received treatment with anti-PD-1, and one with Cetuximab, an EGFR inhibitor. At ICI start, most patients had an unresectable locally advanced disease (72.2%), and 27.8% had distant metastasis. Nearly all patients had a recurrent disease (91.7%), with an 8.3% of patients presenting with advanced disease from the beginning.
All patients received single-agent anti-PD-1 in the first line setting for advanced or metastatic disease, except for one patient that received cemiplimab after progressing to two cycles of cetuximab. Eight patients underwent radiation therapy immediately before or concurrent to ICI, either at the beginning of treatment or for treatment of oligoprogression. Median treatment duration was 10.2 months, for a median of 12 infusions (interquartile range [IQR] of 6 to 26.5), and up to a maximum of 38 cycles. Fourteen patients received ICI beyond 12 months. At the data cut off, 10 patients (27.7%) were receiving ongoing treatment. Following anti-PD-1 discontinuation due to progression of disease or intolerance, only two patients received a further line of systemic treatment (cetuximab). Reason for treatment discontinuation is shown in
Table 2.
3.2. Effectiveness Outcomes
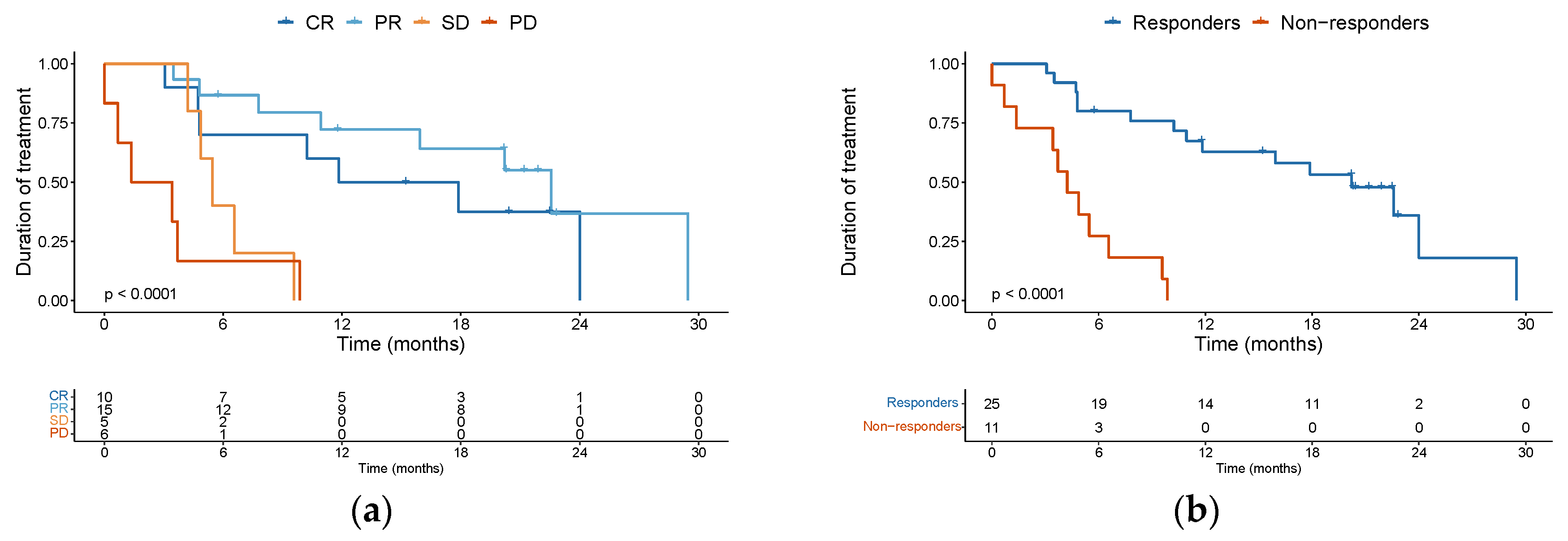
Investigator-assessed BOR of the complete cohort of patients was CR in 10 patients (27.8%), PR in 15 (41.7%), and SD in 5 (13.9%), with a disease control rate (DCR) of 83.4%. Six patients (16.7%) presented PD as best overall response. The median treatment duration was 14.85 months for complete responders, 22.57 months for partial responders, 5.47 months for patients achieving SD as BOR, and 2.4 months for patients who presented PD as BOR (
Figure 1a). When comparing the clinical activity of anti-PD1 therapy amongst various subgroups of patients, the response rate did not statistically differ according to age (age ≥75 versus <74), ECOG status (ECOG 0-1 versus ECOG ≥2) or immune status. For immune status, we compared the rate of response in patients immunocompromised (characterized by the presence of autoimmune disease on immune suppressive drugs, solid organ transplant recipient or hematological malignancy) versus immunocompetent patients that did not have these comorbidities. Interestingly, contrary to expectation, we observed significant clinical activity of the ICI therapy in patients with some immunosuppressive comorbidity. For instance, of the ten patients with hematological malignancy included in our study, four attained a CR and six achieved a PR. Similarly, for the two patients with an autoimmune disease on immune suppressive medication, one of them had a CR and the other a PR. The first patient was a 92-year-old lady with rheumatoid arthritis on methotrexate who discontinued ICI after achieving a CR. The second patient with an autoimmune disease was an 84-year-old lady with giant cell arteritis and polymyalgia rheumatica being treated with low-dose prednisone (less than 15 mg daily). She has an ongoing PR and continues on ICI treatment. Regarding our solid organ transplant recipients, one of them had a PR, and the other one PD as BOR. The patient with PR developed an aggressive pancreatic cancer and passed away. The patient with PD passed away as a consequence of cSCC progression. Overall, we observed impressive clinical activity of ICI therapy irrespective of age, ECOG or immune status.
Additionally, many of the responses observed proved to be durable. When comparing responders (CR + PR) versus non-responders (SD + PD), the median duration of treatment was 20.2 months (95%CI 11.83 – non-evaluable [NE]) versus 4.23 months (95%CI 3.43 – NE) respectively (
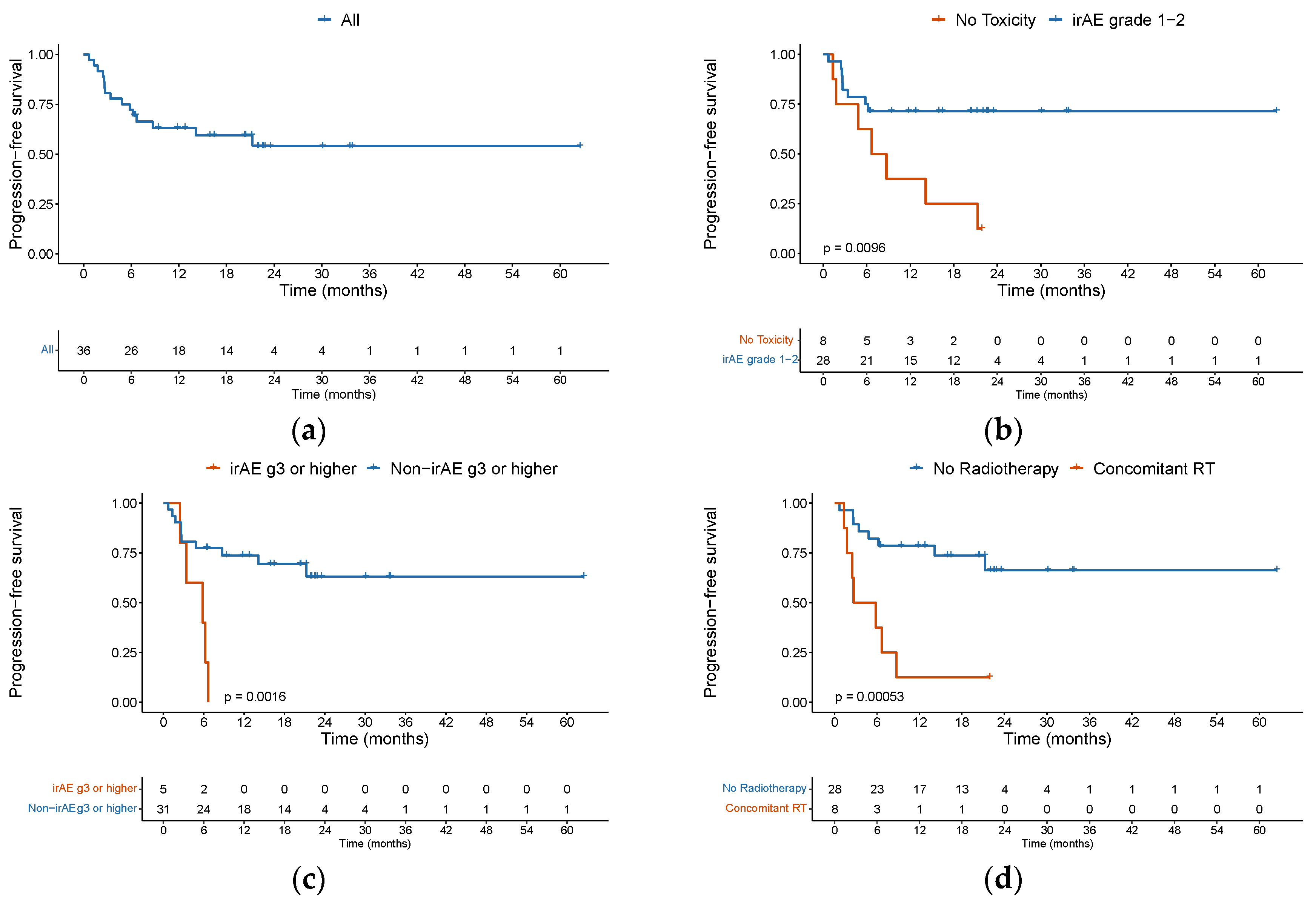
Figure 1b). Among the 10 complete responders, however, 4 patients did ultimately progress during follow-up. Eight patients were treated beyond first evidence disease progression, with none later achieving a further response. With a median follow-up of 21.9 months (95%CI 20.2 to 23.5) among the entire cohort, 6-month PFS was 72.2% (95%CI 59 – 88) and 1-year PFS was 63.1% (95%CI 49 – 81) (
Figure 2a).
The median PFS was not reached. Of 15 patients who progressed, 66.7% had disease progression in the first 6 months, with only 2 patients progressing after 1 year. We found that patients who presented a grade 1 or 2 irAE had a higher PFS with a HR of 0.284 (95%CI 0.103 – 0.785, p = 0.0152) (
Figure 2b), but this did not have an impact on OS (p = 0.7). Conversely, patients with grade 3 or more irAE had a worse PFS and OS, although this was only statistically significant for PFS (p=0.0016) (
Figure 2c). We observed that patients who underwent concomitant radiation therapy had a worse PFS, with a median PFS of 4.25 months versus NA (hazard ratio [HR] 5.2, 95% confidence interval [CI] 1.84 to 14.6, p = 0.002) (
Figure 2d). The univariable analysis for PFS is depicted in
Table 3.
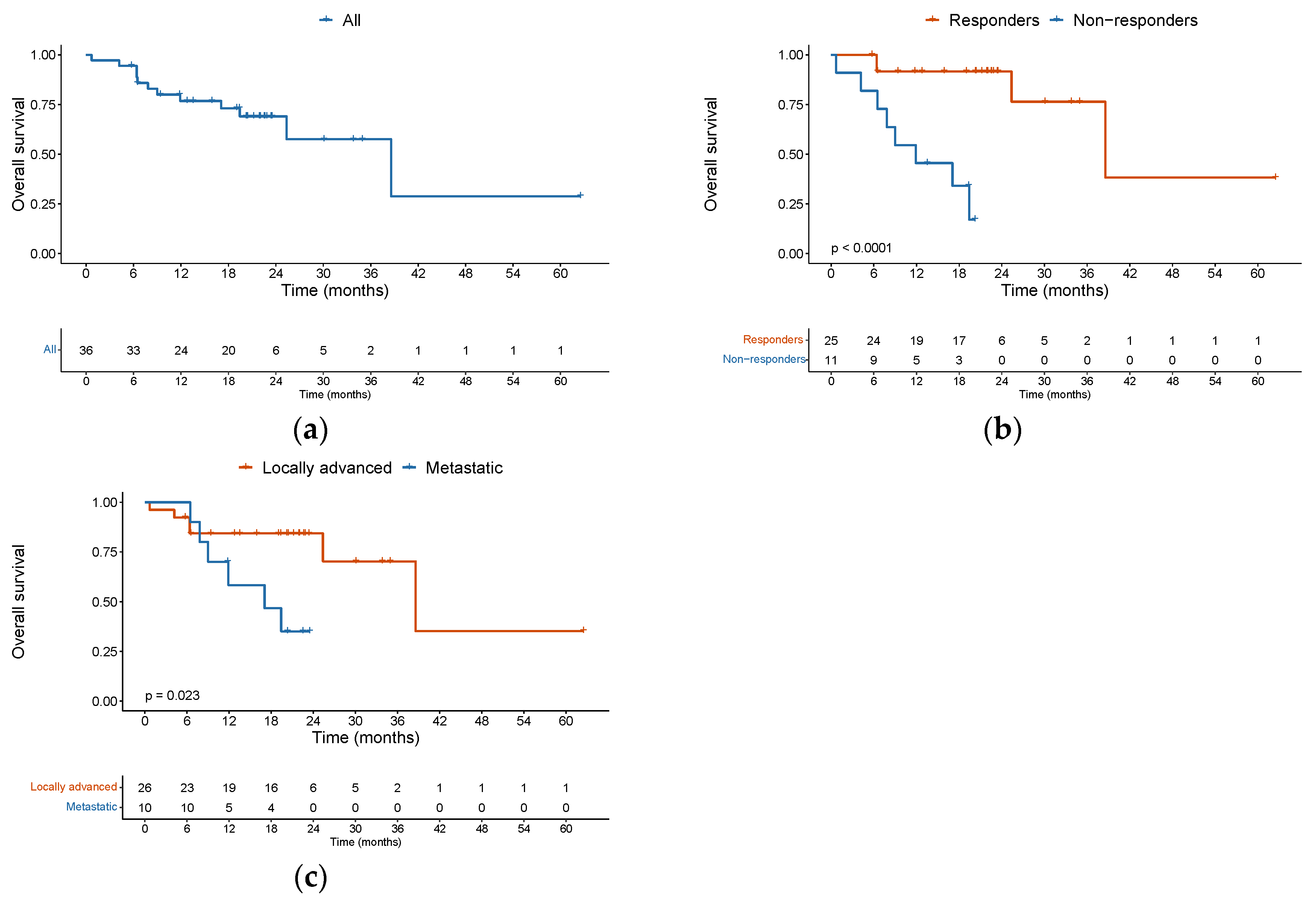
The median OS was 38.6 months (95%CI 25.4 – NA) among the entire cohort. Twelve patients died during the follow-up period, of whom 8 (66.7%) passed away in the first year. The 1-year OS was 76.7% (95%CI 0.64 – 0.92). For those patients who responded to ICI (CR + PR), median OS was significantly improved, as compared with non-responders (SD + PD) (38.6 versus 7.8 months, HR: 0.08, 95% CI 0.016 – 0.375, p = 0.00145) (
Figure 3b). Patients with metastatic disease had a median OS of 17.1 months versus 38.6 months for patients with a locally advanced disease (HR: 3.4, 95%CI 1.1 to 13.9, p = 0.0358 (
Figure 3c).
PFS and OS were not significantly associated with age groups ≥ 75 years versus < 75 years, ECOG performance status 0-1 versus 2-3, or patients with or without comorbidities of interest. The presence of distant metastatic disease increased the risk of death (HR: 3.9, 95% CI 1.1 to 13.9, p = 0.036). The univariable analysis for OS is depicted in
Table 4.
3.3. Safety
PD-1 inhibition was overall well tolerated among the entire cohort, with only 5 patients (13.9%) developing a grade 3 or higher immune related adverse event (irAE), which included grade 3 rash (n = 2), grade 4 lipase increase (n = 1), grade 3 fatigue (n = 1) and grade 3 diarrhea (n = 1). Toxicities led to treatment discontinuation in 5 patients (19.2%), and were as follows: in the first patient, grade 2 fatigue and grade 1 persistent peripheral neuropathy; in the second patient, grade 3 rash; in the third patient, grade 2 pneumonitis, in the fourth patient, grade 2 hepatitis and colitis; and in the fifth patient, grade 2 polymyalgia rheumatica. Two patients died while still receiving ICI; in both cases, death was considered not related to ICI, and explained by progression of disease. At time of data cut-off, 10 patients were still on active treatment. The main reason for ICI discontinuation was disease progression for 8 patients (30.8%) (
Table 2).
Out of ten patients with hematological malignancy, only one of them presented an irAE grade 3 or higher (grade 4 increase in lipase) that resolved, and the patient continues on active treatment with ICI. Our two solid organ transplant recipients (kidney transplant) did not present any safety concerns, and none of them presented allograft loss. Regarding our patients with autoimmune disease, only one of them presented a flare of her disease (polymyalgia rheumatica); this was treated with an increase of her baseline dose of prednisone, and at the moment of data cut-off her ICI treatment was ongoing. Grade 1-2 irAE (p=0.1) and grade 3 or more irAE (p=0.35) were similar between immunocompromised and immune-competent patients.
4. Discussion
Multi-disciplinary management is critical to obtain the best clinical outcomes for patients with advanced cSCC. Patients with advanced cSCC that are deemed not suitable for surgery or radiation therapy with curative intent should undergo systemic treatment with anti-PD-1 as the new standard of care. Our study confirms the efficacy and safety of anti-PD-1 inhibition in patients with advanced cSCC reported in phase I and II clinical trials [
8,
10,
11,
12,
13,
14,
15,
16] in a real-world setting. In contrast to previous phase I and II clinical trials [
8,
13,
14,
15,
16], we observed a higher ORR (69.5% vs. 35 - 58%), despite our cohort consisting of patients with both locoregional recurrent disease and distant metastasis, as opposed to some trials that also included patients with locally advanced disease only. We were surprised to observe a CR rate of 27.8% among our cohort, which is also higher than that reported in the aforementioned trials (0 – 16.7%). There might be several factors contributing to these differences. First, our study had a small sample size; second, the subjective nature of the investigator-assessed response; and third, nearly all our patients (97.2%) had not received any previous systemic cancer treatment. Other real-world studies have reported ORR between 42% and 76.7%, and CR rates ranging from 20% to 33% [
4,
17,
18,
19,
20,
21,
22,
23,
24], which is closer to our outcomes. A recent meta-analysis that included a total of 13 studies (seven randomized clinical trials and six real-world studies) with 930 patients, reported a pooled objective response rate of 47.2% [
25]. We acknowledge the subjectiveness of investigator-assessed response as a main limitation of our study, which could be responsible for an overestimated response rate.
It is also important to notice that 50% of our patients would have been excluded from clinical trials; ten patients for hematological malignancy, two patients for autoimmune disease on immune suppressive drug, two patients for being recipients of solid organ transplant, and four patients for ECOG performance status ≥2. Our two patients with epidermolysis bullosa would also probably have been excluded from trials. Immunocompromised patients represent a uniquely challenging cohort within the population of cSCC patients. It is appreciated that immune suppression is an adverse prognostic factor in developing cSCC. However, neither response rate nor survival outcomes differed according to immune status (compromised versus competent). Other real-world studies have described similar findings. Haist et al. described similar response rates without significantly increased toxicities among immunocompromised patients with advanced cSCC treated with ICI, although the remissions were often short-lived [
17]. Hober e.t al reported similar response rate, PFS and OS, between immunocompromised and immune competent patients [
18], as well as did Hanna et al. for OS [
22]. Taken together, we believe that ICI therapy may offer a promising treatment approach for immunocompromised advanced cSCC patients. An ongoing study is being conducted to explore the safety of PD-1 inhibition in patients with auto-immune disease and advanced metastatic or unresectable cancer (NCT03816345), and another clinical trial is studying the efficacy of combined ICIs with Tacrolimus in kidney transplant recipients with advanced melanoma and non-melanoma skin cancers (NCT03816332). The reporting from these ongoing trials will hopefully provide further clarity as how to optimally use ICI therapy in conjunction with immune-suppressive therapies for these challenging patient populations.
The median age of our cohort was 75.4 years old, which is similar to the aforementioned phase I-II clinical trials and real-world studies. The response rate and survival outcomes were similar between patients ≥ 75 years old versus younger. Even though immunosenescence may reduce the capacity of elderly patients to mediate antitumor responses [
26], several studies have also shown similar response rated to ICIs among 65 years old or older [
22,
27,
28]. High tumor mutational burden, associated with increased immunogenicity, is commonly observed in the tumors of older patients [
29], which might be a factor contributing to the favorable response to ICIs.
Eight patients in our cohort received concomitant radiation therapy in different settings: (1) concurrent at ICI start (n=3), (2) completed in the 2 weeks prior to ICI initiation (n=1), or (3) concurrent for oligoprogression of disease (n=4). The evidence supporting the strategy of combining radiation therapy with immunotherapy is growing. Radiation therapy may act as an “accelerant” by killing tumor cells and triggering a systemic immune response, being the abscopal effect the most significant example [
30]. We observed however that the patients who underwent concomitant radiation therapy had a worse PFS. This might be biased and reflect the fact that in our center, patients with a more locally aggressive disease at presentation are usually offered RT concomitant to cemiplimab at the beginning of their treatment. The use of concomitant RT to ICI in these 8 patients from our cohort was safe, although this combination warrants further investigation. A retrospective study assessed the efficacy of pembrolizumab concurrent with RT in 4 patients with advanced unresectable cSCC; 2 patients presented a CR and 2 PD, with a median PFS of 14.4 months [
31]. An ongoing trial is exploring the efficacy of cemiplimab in combination with RT in patients with locally advanced cSCC (NCT05574101).
In terms of safety, ICIs were well tolerated in our patient cohort, with only 5 patients (13.9%) discontinuing therapy because of toxicity. Four of the five patients that discontinued ICI due to toxicities, were older than 75 years old, with only one of them presenting a grade 3 toxicity (rash). Probably all these patients could have been rechallenged with ICI, but given their age, the occurrence of toxicities is usually less tolerated. Roughly 14% of the patients developed grade 3 or higher irAE, which is consistent with what has been previously reported in trials (5.7% – 13.9%) [
9,
11,
12,
13,
14]. There were no adverse events resulting in death. Despite the infrequent incidence of grade 3 or higher irAE, it is of critical significance given that advanced cSCC primarily occurs in geriatric or immunocompromised patients, where the benefits and risks of any systemic therapy necessitates careful individualized consideration. We would also like to point out that despite our aging cohort, with 47.2 % of patients being older than 70 years old and 13.9% more than 90, ICIs were well tolerated. This is particularly important to note, as other forms of systemic therapy like chemotherapy may lead to increased toxicity in the elderly population. These safety results should be interpreted with caution given the retrospective nature of this study. It is important to note that the recording of adverse events in the medical chart may not have been exhaustive, which could have an impact on the accuracy of the findings. Multiple retrospective and prospective studies [
32,
33,
34,
35,
36,
37,
38,
39,
40] have suggested an association between irAE and the effectiveness to ICIs, in terms of response and survival outcomes. In our cohort, we found an improved PFS in patients that presented an irAE grade 1 or 2, but not for those who presented an irAE grade 3 or more. For patients with grade 1 or 2 irAE, there was a trend toward better OS, but it did not meet statistical significance.
Since its approval by the FDA in 2018 and 2020 for cemiplimab and pembrolizumab, respectively, both anti-PD-1 inhibitors have become preferred systemic treatment options for patients with unresectable, recurrent or metastatic cSCC according to the National Comprehensive Cancer Network Guidelines (version 1.2023). Surgery remains the cornerstone of treatment as the primary curative option for resectable patients. The therapeutic activity of neoadjuvant cemiplimab was demonstrated in a recent phase II non-randomized trial, that showed a 51% of pathological complete response [
41]. This result is encouraging, although a longer follow-up is needed to demonstrate if this finding translates into a longer disease-free survival, as it has in patients with melanoma and non-small cell lung cancer [
42,
43,
44]. The next step will be to elucidate the role of anti-PD-1 in the adjuvant setting (NCT03969004). Finally, how to overcome primary and secondary resistance to ICI are active areas of research for many different cancer types. There are not many systemic options for patients with cSCC that progress to ICI. A phase II nonrandomized trial aiming to revert the resistance to pembrolizumab in patients with locally advanced or metastatic cSCC patients that presented SD or PD, administered cetuximab in addition to pembrolizumab until progression [
45]. This study reported a response rate of 44% with the combination strategy, although grade 3-4 treatment-related adverse events occurred in 35% of patients [
45]. The two patients in this study that presented acquired resistance to pembrolizumab, presented PR when introducing cetuximab [
45]. In our cohort, two patients received cetuximab after progression to cemiplimab. One of them had primary resistance to cemiplimab (receiving only 3 cycles) and had a PR to cetuximab. The second patient had PR to cemiplimab (receiving 12 cycles) and presented a CR to cetuximab. Both patients however progressed to cetuximab in less than a year. A phase II trial assessing the efficacy of avelumab in combination with cetuximab in patients with advanced cSCC is currently ongoing (NCT03944941). Other ongoing trials are exploring nivolumab in combination with talimogene lapherparepvec (NCT02978625), and pembrolizumab in combination with the C5a Antibody IFX-1 (NCT04812535).
While we acknowledge the small sample size and retrospective methodology of our study, we also recognize the lack of clinical data on older patients or with immune suppressive conditions, which are precisely the patients with the diagnosis of advanced cSCC that we encounter in real-world scenarios. We hope to provide additional data about PD-1 inhibition from a real-world cohort of advanced cSCC patients to help clinicians in decision making, especially for patients not represented in clinical trials.
5. Conclusions
Our single-centre institutional experience with anti-PD-1 in locally advanced or metastatic cSCC patients demonstrated its effectiveness and safety in the real-world setting, regardless of age or ECOG performance status. More important, our data suggest that patients with immunosuppressive conditions, such as active autoimmune diseases, hematological malignancies and solid organ transplant recipients, also benefit from this treatment. This is the first study to report outcomes of ICI in patients with advanced cSCC in Canada, and our findings support its use as first-line treatment.
Author Contributions
Conceptualization, Erica Koch Hein, Maysa Vilbert and David Hogg; Data curation, Erica Koch Hein; Formal analysis, Erica Koch Hein and Maysa Vilbert; Investigation, Erica Koch Hein and Maysa Vilbert; Methodology, Erica Koch Hein and Maysa Vilbert; Project administration, Erica Koch Hein, Maysa Vilbert and Samuel Saibil; Resources, Erica Koch Hein; Software, Maysa Vilbert; Supervision, Samuel Saibil; Validation, Maysa Vilbert; Visualization, Erica Koch Hein; Writing – original draft, Erica Koch Hein, Maysa Vilbert and Samuel Saibil; Writing – review & editing, Erica Koch Hein, Maysa Vilbert, Ian Hirsch, Mauricio Ribeiro, Thiago Muniz, Khaled Abdulalem, Erick Figueiredo Saldanha, Erika Martinez, Anna Spreafico, David Hogg, Marcus Butler and Samuel Saibil.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Princess Margaret Cancer Centre (protocol code: 21-6056; approval date: from January 1st 2009, until November 1st 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of data collection from the medical chart. Most patients were no longer attending the clinic on a regular practice; hence, trying to obtain an informed consent would have been impractical. Patient data was anonymized.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to protection of patient information.
Acknowledgments
We thank the Alamos Gold inc. for supporting research and the Medical Oncology Fellowship at Princess Margaret Cancer Centre.
Conflicts of Interest
E. C. Koch Hein: Advisory role: Novartis, MSD; Speaker´s Bureau: Novartis, MSD; Research Funding: funding paid to Dr. Koch Hein Institution for support of a melanoma registry in Chile; travel, accommodations, expenses: Pfizer, Novartis, Roche Pharma AG. A. Spreafico: Advisory Board: Merck, Bristol-Myers Squibb, Oncorus, Janssen; Research Grant, Funding paid to Dr. Spreafico Institution to support clinical trials: Novartis, Bristol-Myers Squibb, Symphogen AstraZeneca/Medimmune, Merck, Bayer, Surface Oncology, Northern Biologics, Janssen Oncology/Johnson & Johnson, Roche, Regeneron, Alkermes, Array Biopharma/Pfizer, GSK, Treadwell. M.O. Butler: Advisory Board: Merck, BMS, Novartis, GlaxoSmithKline, IOVANCE, Instil Bio, Sanofi, Pfizer, Adaptimmune, GSK, Immunocore, EMD Serono, Sun Pharma, Immunovaccine; Invited Speaker: Merck, BMS, Novartis, Sanofi; Research Grant: Merck, Takara Bio. S. D. Saibil: Advisory Board: Janssen, Novartis, Sanofi genzyme. All other authors have declared no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA: A Cancer Journal for Clinicians 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatology 2015, 151, 1081. [Google Scholar] [CrossRef] [PubMed]
- Brantsch, K.D.; Meisner, C.; Schonfisch, B.; Trilling, B.; Wehner-Caroli, J.; Rocken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008, 9, 713–720. [Google Scholar] [CrossRef] [PubMed]
- In, G.K.; Vaidya, P.; Filkins, A.; Hermel, D.J.; King, K.G.; Ragab, O.; Tseng, W.W.; Swanson, M.; Kokot, N.; Lang, J.E.; et al. PD-1 inhibition therapy for advanced cutaneous squamous cell carcinoma: a retrospective analysis from the University of Southern California. Journal of Cancer Research and Clinical Oncology 2021, 147, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Schmults, C.D.; Karia, P.S.; Carter, J.B.; Han, J.; Qureshi, A.A. Factors Predictive of Recurrence and Death From Cutaneous Squamous Cell Carcinoma. JAMA Dermatology 2013, 149, 541. [Google Scholar] [CrossRef]
- Hillen, U.; Leiter, U.; Haase, S.; Kaufmann, R.; Becker, J.; Gutzmer, R.; Terheyden, P.; Krause-Bergmann, A.; Schulze, H.J.; Hassel, J.; et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns-Results of a non-interventional study of the DeCOG. Eur J Cancer 2018, 96, 34–43. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational Landscape of Aggressive Cutaneous Squamous Cell Carcinoma. Clinical Cancer Research 2014, 20, 6582–6592. [Google Scholar] [CrossRef]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. The Lancet Oncology 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer 2020, 8. [Google Scholar] [CrossRef]
- Rischin, D.; Khushalani, N.I.; Schmults, C.D.; Guminski, A.; Chang, A.L.S.; Lewis, K.D.; Lim, A.M.; Hernandez-Aya, L.; Hughes, B.G.M.; Schadendorf, D.; et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Grob, J.J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J Clin Oncol 2020, 38, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.G.M.; Munoz-Couselo, E.; Mortier, L.; Bratland, A.; Gutzmer, R.; Roshdy, O.; Gonzalez Mendoza, R.; Schachter, J.; Arance, A.; Grange, F.; et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol 2021, 32, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.J.; Dreno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II Study of Pembrolizumab As First-Line, Single-Drug Therapy for Patients With Unresectable Cutaneous Squamous Cell Carcinomas. J Clin Oncol 2020, 38, 3051–3061. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, R.R.; Nader-Marta, G.; De Camargo, V.P.; Queiroz, M.M.; Cury-Martins, J.; Ricci, H.; De Mattos, M.R.; De Menezes, T.A.F.; Machado, G.U.C.; Bertolli, E.; et al. A phase 2 study of first-line nivolumab in patients with locally advanced or metastatic cutaneous squamous-cell carcinoma. Cancer 2022, 128, 4223–4231. [Google Scholar] [CrossRef]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Lewis, K.D.; Schmults, C.D.; Hernandez-Aya, L.; Meier, F.; Schadendorf, D.; Guminski, A.; Hauschild, A.; et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol 2020, 21, 294–305. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Lang, B.M.; Tsochataridou, A.; Salzmann, M.; Mohr, P.; Schadendorf, D.; Ugurel, S.; Placke, J.-M.; Weichenthal, M.; et al. Response to First-Line Treatment with Immune-Checkpoint Inhibitors in Patients with Advanced Cutaneous Squamous Cell Carcinoma: A Multicenter, Retrospective Analysis from the German ADOReg Registry. Cancers 2022, 14, 5543. [Google Scholar] [CrossRef]
- Hober, C.; Fredeau, L.; Pham-Ledard, A.; Boubaya, M.; Herms, F.; Celerier, P.; Aubin, F.; Beneton, N.; Dinulescu, M.; Jannic, A.; et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Guillaume, T.; Puzenat, E.; Popescu, D.; Aubin, F.; Nardin, C. Cemiplimab-rwlc in advanced cutaneous squamous cell carcinoma: real-world experience in a French dermatology department. Br J Dermatol 2021, 185, 1056–1058. [Google Scholar] [CrossRef]
- Strippoli, S.; Fanizzi, A.; Quaresmini, D.; Nardone, A.; Armenio, A.; Figliuolo, F.; Filotico, R.; Fucci, L.; Mele, F.; Traversa, M.; et al. Cemiplimab in an Elderly Frail Population of Patients With Locally Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Center Real-Life Experience From Italy. Front Oncol 2021, 11, 686308. [Google Scholar] [CrossRef]
- Baggi, A.; Quaglino, P.; Rubatto, M.; Depenni, R.; Guida, M.; Ascierto, P.A.; Trojaniello, C.; Queirolo, P.; Saponara, M.; Peris, K.; et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur J Cancer 2021, 157, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Ruiz, E.S.; LeBoeuf, N.R.; Thakuria, M.; Schmults, C.D.; Decaprio, J.A.; Silk, A.W. Real-world outcomes treating patients with advanced cutaneous squamous cell carcinoma with immune checkpoint inhibitors (CPI). Br J Cancer 2020, 123, 1535–1542. [Google Scholar] [CrossRef]
- Salzmann, M.; Leiter, U.; Loquai, C.; Zimmer, L.; Ugurel, S.; Gutzmer, R.; Thoms, K.M.; Enk, A.H.; Hassel, J.C. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: real-world data of a retrospective, multicenter study. Eur J Cancer 2020, 138, 125–132. [Google Scholar] [CrossRef]
- Rios-Vinuela, E.; Alvarez, P.; Lavernia, J.; Serra-Guillen, C.; Requena, C.; Bernia, E.; Diago, A.; Llombart, B.; Sanmartin, O. Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience in a Monographic Oncology Center. Actas Dermosifiliogr 2022, 113, T610–T615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, A.; Chen, J. Immune checkpoint inhibitors in advanced cutaneous squamous cell carcinoma: A systemic review and meta-analysis. Skin Research and Technology 2023, 29. [Google Scholar] [CrossRef]
- Rodriguez, J.E.; Naigeon, M.; Goldschmidt, V.; Roulleaux Dugage, M.; Seknazi, L.; Danlos, F.X.; Champiat, S.; Marabelle, A.; Michot, J.-M.; Massard, C.; et al. Immunosenescence, inflammaging, and cancer immunotherapy efficacy. Expert Review of Anticancer Therapy 2022, 22, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Poropatich, K.; Fontanarosa, J.; Samant, S.; Sosman, J.A.; Zhang, B. Cancer Immunotherapies: Are They as Effective in the Elderly? Drugs & Aging 2017, 34, 567–581. [Google Scholar] [CrossRef]
- Daste, A.; Domblides, C.; Gross-Goupil, M.; Chakiba, C.; Quivy, A.; Cochin, V.; de Mones, E.; Larmonier, N.; Soubeyran, P.; Ravaud, A. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017, 82, 155–166. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Medicine 2017, 9. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduction and Targeted Therapy 2022, 7. [Google Scholar] [CrossRef]
- Lavaud, J.; Blom, A.; Longvert, C.; Fort, M.; Funck-Brentano, E.; Saiag, P. Pembrolizumab and concurrent hypo-fractionated radiotherapy for advanced non-resectable cutaneous squamous cell carcinoma. Eur J Dermatol 2019, 29, 636–640. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. Journal for ImmunoTherapy of Cancer 2019, 7. [Google Scholar] [CrossRef]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.-M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. Journal of Clinical Oncology 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- Rogado, J.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Leví, A.; Arranz, R.; Lorenzo, A.; Gullón, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo. JAMA Oncology 2020, 6, 519. [Google Scholar] [CrossRef] [PubMed]
- Bastacky, M.L.; Wang, H.; Fortman, D.; Rahman, Z.; Mascara, G.P.; Brenner, T.; Najjar, Y.G.; Luke, J.J.; Kirkwood, J.M.; Zarour, H.M.; et al. Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact Upon Anti-Tumor Efficacy: A Real World Analysis. Front Oncol 2021, 11, 749064. [Google Scholar] [CrossRef]
- Serna-Higuita, L.M.; Amaral, T.; Forschner, A.; Leiter, U.; Flatz, L.; Seeber, O.; Thomas, I.; Garbe, C.; Eigentler, T.K.; Martus, P. Association between Immune-Related Adverse Events and Survival in 319 Stage IV Melanoma Patients Treated with PD-1-Based Immunotherapy: An Approach Based on Clinical Chemistry. Cancers 2021, 13, 6141. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nature Communications 2022, 13. [Google Scholar] [CrossRef]
- Watson, A.S.; Goutam, S.; Stukalin, I.; Ewanchuk, B.W.; Sander, M.; Meyers, D.E.; Pabani, A.; Cheung, W.Y.; Heng, D.Y.C.; Cheng, T.; et al. Association of Immune-Related Adverse Events, Hospitalization, and Therapy Resumption With Survival Among Patients With Metastatic Melanoma Receiving Single-Agent or Combination Immunotherapy. JAMA Network Open 2022, 5, e2245596. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Nishio, M.; Mok, T.S.K.; Reck, M.; Finley, G.G.; Kaul, M.D.; Yu, W.; Paranthaman, N.; et al. Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non–Small Cell Lung Cancer. JAMA Oncology 2023, 9, 527. [Google Scholar] [CrossRef]
- Gross, N.D.; Miller, D.M.; Khushalani, N.I.; Divi, V.; Ruiz, E.S.; Lipson, E.J.; Meier, F.; Su, Y.B.; Swiecicki, P.L.; Atlas, J.; et al. Neoadjuvant Cemiplimab for Stage II to IV Cutaneous Squamous-Cell Carcinoma. N Engl J Med 2022, 387, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Uprety, D.; Mandrekar, S.J.; Wigle, D.; Roden, A.C.; Adjei, A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J Thorac Oncol 2020, 15, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. New England Journal of Medicine 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Bossi, P.; Alberti, A.; Bergamini, C.; Resteghini, C.; Locati, L.D.; Alfieri, S.; Cavalieri, S.; Colombo, E.; Gurizzan, C.; Lorini, L.; et al. Immunotherapy followed by cetuximab in locally advanced/metastatic (LA/M) cutaneous squamous cell carcinomas (cSCC): The I-TACKLE trial. Journal of Clinical Oncology 2022, 40, 9520–9520. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).